Abstract
Background
Few studies have investigated whether hospital utilization patterns of cancer sufferers are associated with their suicide. This study aims to explore whether clinical profiles and healthcare utilization patterns are related to suicide among cancer sufferers.
Methods
Verified suicide cases with cancer (2012-2016) were identified. Each case was matched with two non-suicide controls suffering cancer, by birthyear, sex, and admission year. Cancer-related information, physical and psychiatric comorbidities, opioid-based painkiller usage, the number and length of inpatient admissions, and the number of outpatient and Accident & Emergency (A&E) attendances, in the six months leading up to the suicide, were identified. Conditional logistic regression models were constructed to explore the influence of clinical profiles and hospital utilization on suicide. These models were stratified by age and cancer stage.
Outcomes
383 cases and 766 controls were included in the analyses. Overall, younger age, metastasis/recurrent status, suffering head and neck cancer, having psychiatric comorbidities, using opioid-based painkillers, and high frequency of A&E attendances and inpatient admissions increased the odds of suicide. Being diagnosed with liver cancer, consuming high numbers of outpatient attendances, and high numbers of inpatient days decreased the odds of suicide. Stratified analyses confirmed the influence of young age and metastatic/recurrent cancer status on risk of suicide.
Interpretations
Suicidal cancer sufferers had distinctive clinical profiles and hospital utilization patterns. Detecting and mitigating suicidal risk should be incorporated as an important component in treatment of cancer sufferers in the clinical setting.
Funding
Li Ka Shing Foundation and Hong Kong Research Grants Council
Keywords: Psycho-oncology, Suicide, Suicide prevention
1. Introduction
Cancer sufferers are at higher risk of suicide than the general population. [1] Factors associated with this elevated risk included having received a cancer diagnosis, and suffering unbearable pain. [2] Optimizing physical and mental well-being among cancer sufferers is key to suicide prevention. Cancer sufferers frequently visit hospitals for medical treatment, symptom control and/or follow-up checks, thus it seems strategic to provide them with suicide prevention support in these clinical settings.
Suicide prevention programmes have been implemented in healthcare settings around the world, for example, “Zero Suicide” in outpatient behavioural clinics in the United States, [3] and suicide prevention programmes in Iranian primary care. [4] Currently in Hong Kong (HK), there is a guideline for doctors regarding assessing and managing potentially suicidal patients. [5] However, this guideline is intended for general use, and some strategies might not be effective for cancer sufferers. The guideline recommends that people are screened for depression prior to implementing suicide prevention strategies. However, previous research suggested that psychiatric illnesses were not contributing factors for cancer inpatient sufferers’ suicidal behaviours. [6] Exploring their clinical profiles and hospital utilization patterns might identify their specific needs and assist healthcare providers with early identification of suicidal risk, implementation of hospital-based suicide prevention strategies, and development of a safe home discharge plan to reduce tragedies. [7]
A study exploring hospital utilization patterns of people who died by suicide, one year prior to the suicide event, found different patterns for different subgroups, as the suicide event approached. [7] The elderly had more hospital visits compared to young people, [8] as did people whose suicide was motivated by physical illnesses. [9] Cancer sufferers with severe disease had more frequent but less regular contact with hospitals, [10] however, there was no evidence as to whether hospital utilization patterns predicted cancer-related suicides.
Our study aimed to examine whether clinical profiles and hospital utilization patterns were associated with suicide among cancer sufferers in HK. This might provide insights into how effective suicide prevention strategies might be offered in HK hospital settings for cancer sufferers, and raising awareness of family members or carers about their suicidal risk.
2. Methods
Ethics approval was obtained from the Institutional Review Boards of the University of Hong Kong, and the Hong Kong Hospital Authority (HKHA) West Cluster (UW19-568).
2.1. Study design
Retrospective case-control study
2.2. Timeframe
2012 to 2016
2.3. Setting
HKHA public hospitals
2.4. Case identification
Verified suicide death cases between 2012 and 2016 were identified from HK Coroner's Court records. Suicides with a history of cancer were flagged, and their demographic information was retrieved, including birthday and sex, date of suicide, and brief medical history. Their personal identification number was then entered into the Electronic Patient Records (ePR) from the HKHA Clinical Data Analysis and Reporting System (CDARS), and their detailed medical history was retrieved. CDARS contains all medical records from the public hospitals managed by HKHA. HKHA has divided all public hospitals into seven clusters based on their geographical locations. [11] These hospitals provide diagnosis and treatment to 90% of new cancer cases in HK. [12] The ePR system also includes detailed clinical notes written by medical professionals for cancer sufferers’ inpatient admissions and outpatient attendances. Cancer suicide cases with no records in the ePR system, or with no information related to cancer, were excluded.
2.5. Control selection
All sufferers who had cancer specific diagnosis in the ePR system with at least one CDARS record of an inpatient admission, outpatient and/or Accident & Emergency (A&E) attendance from HK West cluster or New Territories West cluster in each year between 2012 and 2016 were identified. For each year, one hospital record with information on identification (reference) number, birthday, and sex was retained for each individual, becoming the control selection pools. Suicides (cases) were excluded from the pools.
2.6. Matching
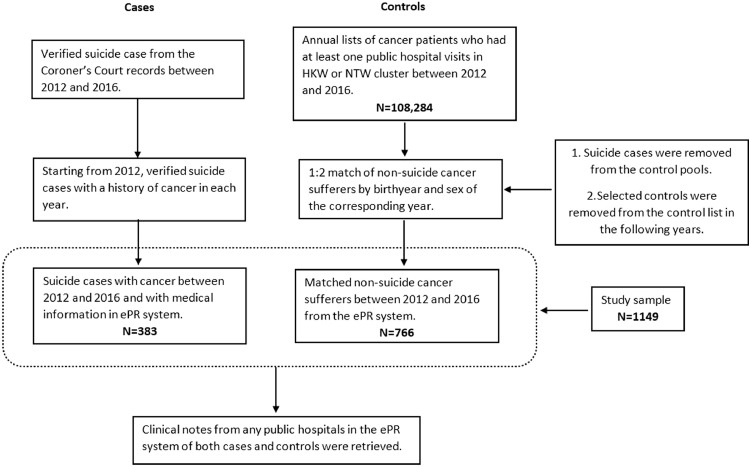
Starting from 2012, each case (cancer-related suicide) within that year was then matched with two cancer-related controls from the control pool of the corresponding year by birthyear and sex using nearest neighbour (NN) matching function in “matchIt” package in R. [13] The controls selected were excluded from the control pools in the following years. After all controls were selected, their reference number was entered into ePR system for detailed medical history retrieval. Figure 1 illustrates the selection process of cases and controls.
Figure 1.
Flow chart of case and control selection.
2.7. Study variables
For the cases and controls, all the medical records, clinical notes from medical professionals, and hospital attendance from any public hospital were available from the ePR system. Clinical information including date of cancer diagnosis, age at diagnosis, cancer type, metastasis/recurrence status, and psychiatric or physical comorbidities was manually retrieved. Cancer was classified into 14 categories (lung, colorectal, liver, pancreas, stomach, breast, prostate, head and neck (H&N), oesophagus, lymphoma, leukaemia, and others). Individuals may have multiple cancer diagnoses, thus each cancer type was binarily coded. Having psychiatric illness was coded as positive if the records reported a clinical diagnosis of any psychiatric disorder, or any psychiatric treatment. Physical comorbidities other than cancer were also binarily coded (myocardial infarction, chronic heart failure, peripheral vascular disease, stroke, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer, liver disease, diabetes, hemiplegia or moderate to severe chronic kidney disease).
To understand the influence of hospital utilization patterns on cancer-related suicide, the study time frame was set for six months. The period was chosen following the study window suggested in previous research investigating hospital utilizations and pain management among people who died from cancer, [14], [15], [16] and it reflected the usual time frame of palliative care referral in late-stage cancer sufferers. [17] For cases, this was the six months prior to their date of suicide. For controls, the starting date of the six-month study period was randomly chosen between 2012 and 2016 post-dating their cancer diagnosis date. For all subjects, the end of their study period was either death or the end of the six-month timeframe. The number of inpatient admissions, and outpatient and A&E attendances within each subject's study period, and number of days of inpatient admissions within the study period were summed for each subject. Whether subjects used opioid-based painkillers (Morphine, Fentanyl, Methadone, Oxycodone or Oxycontin) within the study period was also binary coded.
2.8. Statistical analysis
To ensure the representativeness of the control group, the distribution of cancer types among control group was compared with the annual incidence and mortality of each cancer type reported by the Hong Kong Cancer Registry. [18] We reported frequency and percentage for categorical variables, mean (standard deviation [s.d.]) for age at diagnosis, and median and interquartile range (IQR) for hospital utilization-related variables. Independent t-tests and Chi-squared tests compared the clinical profiles of cancer sufferers who killed themselves, or not. Wilcoxon signed-rank tests compared the medians of hospital utilization-related variables by suicide status among cases. This was because the number of hospital visits and admission days had significant skewness, violating the normality distribution assumption for independent t-tests. To reduce inflated false positive rates from multiple testing, a correction method proposed by Benjamini and Hochberg was used to adjust for the p-values from bivariate analyses. [19] To account for the potential clustering from the matching process, multivariable conditional logistic regressions were conducted to identify factors independently associated with suicide using “survival” package in R. [20] Conditional logistic regression was often used for matched case-control studies. [21] We tested an overall model (unadjusted for cancer type) and then models for the specific cancer types which were identified as significant predictors from bivariate analyses. Further stratified analyses were performed to examine the impact of clinical profiles and hospital utilization on cancer-related suicides, by cancer stage (primary cancer; metastasis/recurrent) and age group (younger than 60 years old; 60 years or older). Previous research has found that cancer sufferers in advanced stage had higher risk of suicide, [22] and those with metastatic cancer had different patterns of hospital utilization compared to those without metastasis. [23] The 60-year age threshold was established from research which had highlighted the critical value of 58 years, from a non-linear relationship between age and suicidal/self-harm behaviours among cancer sufferers in inpatient services. [6] The stratification did not take into account the matching process. Therefore, sex variable was put into all the stratified analyses to control for potential confounding. The multicollinearity assumption was checked for each model. Adjusted odds ratios (aORs) (95% confidence interval [95% CI]) were reported. Significance was set at p<0.05 with two-sided p-values reported. All analyses were performed with R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
2.9. Role of the funding source
Funding sources was not involved in the study design; collection, analysis or interpretation of the data; and in writing of the manuscript or in the decision to submit the manuscript for publication.
3. Results
Between 2012 and 2016, 458 cancer sufferers killed themselves, with 383 cases’ ePR records were available from CDARS for analysis and 766 matched controls. The mean difference in distance for the matched controls was -0•00015 (s.d.=0•02). The distribution of cancer subtypes in our control group generally represented the cancer subtypes in HK. Clinical profiles and hospital utilization for cases and controls are reported in Table 1. Among suicide cases, 65•54% were male. Suicide mean age was 64•83 years (s.d.=13•61), while control mean age was significantly older (66•16 years, s.d.=12•95) (p<•05). Approximately 60% cases had significantly higher frequency of metastasis/recurrent status compared with controls (52•76%) (p<•05). Lung and colorectal cancers had the highest frequencies in both groups, whilst H&N cancer was the third most-common cancer in cases (15•40%), which was significantly higher than controls (9•40%) (p<•05). There was a significantly lower percentage with liver cancer among cases (3•92%) than controls (10•44%) (p<•05). The proportion of cases with a history of any physical illness (32•90%) was also significantly lower than controls (39•69%) (p<•05). Only 4•83% of the controls reported a history of psychiatric illness, compared with cases (16•45%) (p<•05). Nearly 30% cases had used opioid-based painkillers within the study period (significantly more than controls (18•67%) (p<•05)). Compared with cases, controls had fewer A&E visits and inpatient admissions (p<•05), and fewer days of inpatient admission (p<•05) but had more outpatient visits within the study period (p<•05).
Table 1.
The comparisons of clinical profiles and hospital utilization between cancer sufferers with and without suicide.
| Suicides | Non suicides | |||
|---|---|---|---|---|
| N=383 | N=766 | |||
| N (%) / Mean (±S.D.) | N (%) / Mean (±S.D.) | Z / Cramer's V / Cohen's D | Adjusted p-value | |
| Clinical profiles | ||||
| Age at diagnosis | 64•83 (±13•61)* | 66•16 (±12•95)* | -0•100 | 0•037 |
| Cancer type | ||||
| Lung | 82 (21•41) | 134 (17•49) | 0•047 | 0•171 |
| Colorectal | 83 (21•67) | 152 (19•84) | 0•021 | 0•572 |
| Liver | 15 (3•92) | 80 (10•44) | -0•112 | 0•0007 |
| Pancreatic | 10 (2•61) | 17 (2•22) | 0•012 | 0•712 |
| Stomach | 27 (7•05) | 46 (6•01) | 0•020 | 0•572 |
| Breast | 31 (8•09) | 72 (9•40) | -0•022 | 0•572 |
| Prostate | 18 (4•70) | 50 (6•53) | -0•037 | 0•317 |
| Head & neck | 59 (15•40) | 72 (9•40) | 0•089 | 0•009 |
| Esophagus | 15 (3•92) | 24 (3•13) | 0•020 | 0•572 |
| Lymphoma | 9 (2•35) | 35 (4•57) | -0•055 | 0•110 |
| Leukaemia | 2 (0•52) | 18 (2•35) | -0•066 | 0•048 |
| Brain | 6 (1•57) | 9 (1•17) | 0•016 | 0•639 |
| Others | 59 (15•40) | 1118 (15•40) | 0•000 | 1•000 |
| Metastasis/recurrent status | 233 (61•80) | 402 (52•76) | 0•086 | 0•009 |
| History of any physical illness | 126 (32•90) | 304 (39•69) | -0•066 | 0•048 |
| History of any psychiatric illness | 63 (16•45) | 37 (4•83) | 0•194 | 0•0004 |
| Whether used painkiller in 6 months | 113 (29•50) | 143 (18•67) | 0•123 | 0•0004 |
| Hospital utilization | ||||
| Number of A&E visits within 6 months | ||||
| 0 | 88 (23•04) | 386 (50•46) | ||
| 1-3 | 229 (59•95) | 300 (39•22) | ||
| 4-7 | 60 (15•71) | 70 (9•15) | ||
| 8+ | 5 (1•31) | 9 (1•18) | ||
| Median (IQR) | 1•00 (1•00-3•00) | 0•00 (0•00-2•00) | 7•663 | 0•0004 |
| Number of outpatient visits within 6 months | ||||
| 0 | 53 (14•10) | 25 (3•27) | ||
| 1-3 | 152 (40•43) | 257 (33•64) | ||
| 4-7 | 90 (23•94) | 238 (31•15) | ||
| 8+ | 81 (21•54) | 244 (31•94) | ||
| Median (IQR) | 3•00 (1•00-7•00) | 5•00 (3•00-8•00) | -7•197 | 0•0004 |
| Number of inpatient visits within 6 months | ||||
| 0 | 83 (21•73) | 321 (41•91) | ||
| 1-3 | 216 (56•54) | 325 (42•43) | ||
| 4-7 | 68 (17•80) | 109 (14•23) | ||
| 8+ | 15 (3•93) | 11 (1•44) | ||
| Median (IQR) | 2•00 (1•00-3•00) | 1•00 (0•00-3•00) | 5•578 | 0•0004 |
| Number of days in inpatient services within 6 months | ||||
| 0 | 99 (26•40) | 313 (43•05) | ||
| 1-15 | 100 (26•67) | 123 (16•92) | ||
| 16-45 | 100 (26•67) | 113 (15•54) | ||
| 46-90 | 55 (14•67) | 105 (14•44) | ||
| 91+ | 21 (5•60) | 73 (10•04) | ||
| Median (IQR) | 12•00 (0•00-39•00) | 4•00 (0•00-45•00) | 2•918 | 0•010 |
S.D.: Standard deviation
Italic indicates that the p-value is significant (i.e. p < 0•05).
Mean and S.D. were shown.
Table 2 reports outputs of the conditional logistic regression models. Seventy participants were listwise deleted in the analyses due to missing values. The missing cases were evenly distributed among cases and controls, and they had mostly similar characteristics to those included in the analyses (see Appendix I). The multicollinearity assumption was not violated in any model, with variance inflation factors (VIF) less than 3•0 for all variables (see Appendix II). Considering the overall model, for every year increase in age, the odds of suicide decreased by 6% with all other variables held constant. In metastasis/recurrent cancer stage (OR=1•76, 95% CI=1•26-2•46), having history of psychiatric illness (OR=4•60, 95% CI=2•65-8•00), and using opioid-based painkillers in the study period (OR=2•02, 95% CI=1•33-3•06) increased the odds of suicide. For hospital utilization, with all other variables held constant, with every additional visit to A&E, or inpatient admission, the odds of suicide increased by 18% (OR=1•18, 95% CI=1•05-1•32) and 25% (OR=1•25, 95% CI=1•12-1•40) respectively. For every additional visit to outpatient services, the odds of suicide decreased by 14% (OR=0•86, 95% CI=0•83-0•90); and for every additional day in inpatient admission, the odds of suicide reduced by 1% (OR=0•99, 95% CI=0•98-0•99). Similar results were observed for subjects with liver, and H&N cancers, which were further explored. Although leukaemia was also significantly associated with suicide in the bivariate analysis, further regression analyses could not be performed due to low numbers of cases (N=2) and controls (N=18). Holding all other variables constant, and compared to all cancer types, having liver cancer reduced the odds of suicide by 64% (OR=0•36, 95% CI=0•18-0•70), while suffering H&N cancer increased the odds of suicide by 87% (OR=1•87, 95% CI=1•14-3•05).
Table 2.
Adjusted ORs and 95% CI for the association between variables of interest and suicide among cancer sufferers of overall and cancer-specific models.
| Overall | Cancer type | ||
|---|---|---|---|
| Liver | Head & Neck | ||
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Clinical profiles | |||
| Age at diagnosis | 0•94 (0•90-0•97)a | 0•94 (0•90-0•98)b | 0•94 (0•90-0•97)b |
| Cancer type (other types as reference) | NA | 0•36 (0•18-0•70)b | 1•87 (1•14-3•05)c |
| Metastasis/recurrent status | 1•76 (1•26-2•46)b | 1•87 (1•33-2•64)a | 1•82 (1•30-2•57)a |
| History of physical illness | 0•74 (0•53-1•03) | 0•85 (0•60-1•20) | 0•75 (0•54-1•05) |
| History of psychiatric illness | 4•60 (2•65-8•00)a | 4•35 (2•50-7•59)a | 4•49 (2•57-7•84)a |
| Whether used painkiller in 6 months | 2•02 (1•33-3•06)a | 1•92 (1•26-2•92)b | 2•08 (1•37-3•17)a |
| Hospital utilization | |||
| Number of A&E visits within 6 months | 1•18 (1•05-1•32)b | 1•19 (1•06-1•34)b | 1•17 (1•04-1•31)b |
| Number of outpatient visits within 6 months | 0•86 (0•83-0•90)a | 0•86 (0•83-0•90)a | 0•86 (0•82-0•89)a |
| Number of inpatient visits within 6 months | 1•25 (1•12-1•40)a | 1•24 (1•11-1•40)a | 1•25 (1•12-1•41)a |
| Number of days in inpatient service within 6 months | 0•99 (0•98-0•99)a | 0•98 (0•98-0•99)a | 0•99 (0•98-0•99)a |
AOR: Adjusted odds ratio
95% CI: 95% Confident interval
NA: Not applicable
Bold and italic indicate that the p-value is significant (i.e. p < 0•05).
p-value less than 0•001.
p-value between 0•001 and 0•01.
p-value between 0•01 and 0•05.
The relationships between clinical profiles, hospital utilization, and suicide in cancer sufferers varied by cancer stage and age. The cancer stage- and age-stratified results are summarized in Table 3. For cases in primary cancer stage, after controlling for all covariates, the odds of suicide were higher among those with a history of psychiatric disorder (OR=7•25, 95% CI=2•39-21•95). No other variable influenced the odds of suicide. For subjects in metastasis/recurrent cancer stage, the odds of suicide increased in younger people (OR=0•89, 95% CI=0•81-0•97), those with a history of psychiatric disorder (OR=3•24, 95% CI=1•20-8•74), those without a history of physical illness (OR=0•53, 95% CI=0•30-0•95) and those using opioid-based painkillers in the study period (OR=3•16, 95% CI=1•65-6•06). Regarding hospital utilization, higher numbers of A&E visits (OR=1•23, 95% CI=1•01-1•50), lower numbers of outpatient visits (OR=0•86, 95% CI=0•81-0•91), and fewer days of inpatient admission (OR=0•99, 95% CI=0•98-0•99) increased the odds of suicide.
Table 3.
Adjusted ORs and 95% CI for the association between variables of interest and suicide among cancer sufferers stratified by cancer stage and age.
| Cancer stage | Age group | |||
|---|---|---|---|---|
| Primary | Metastasis/recurrent | Younger than 60 | 60 or above | |
| N=514 | N=645 | N=359 | N=790 | |
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Clinical profiles | ||||
| Age at diagnosis | 0•93 (0•86-1•00) | 0•89 (0•81-0•97)b | 0•92 (0•82-1•03) | 0•89 (0•84-0•95)a |
| Sex (male as reference) | 0•21 (0•04-1•17) | 0•24 (0•05-1•20) | 0•33 (0•04-2•80) | 0•31 (0•09-1•01) |
| Metastasis/recurrent status | NA | NA | 2•12 (0.97-4•66) | 1•58 (1•05-2•38)c |
| History of physical illness | 0•86 (0•45-1•64) | 0•53 (0•30-0•95)c | 1•05 (0•39-2•84) | 0•66 (0•45-0•96)c |
| History of psychiatric illness | 7•25 (2•39-21•95)a | 3•24 (1•20-8•74)c | 5•58 (1•89-16•46)b | 4•21 (2•01-8•81)a |
| Whether used painkiller in 6 months | 1•06 (0•39-2•87) | 3•16 (1•65-6•06)a | 2•52 (0•96-6•65) | 2•16 (1•29-3•60)b |
| Hospital utilization | ||||
| Number of A&E visits within 6 months | 1•15 (0•90-1•47) | 1•23 (1•01-1•50)c | 1•48 (1•04-2•09)c | 1•09 (0•95-1•25) |
| Number of outpatient visits within 6 months | 0•96 (0•89-1•03) | 0•86 (0•81-0•91)a | 0•76 (0•68-0•85)a | 0•89 (0•85-0•93)a |
| Number of inpatient visits within 6 months | 1•18 (0•91-1•54) | 1•18 (0•99-1•40) | 1•21 (0•97-1•52) | 1•33 (1•14-1•55)a |
| Number of days in inpatient service within 6 months | 0•99 (0•97-1•01) | 0•99 (0•98-0•99)a | 0•99 (0•98-1•00) | 0•98 (0•98-0•99)a |
AOR: Adjusted odds ratio
95% CI: 95% Confident interval
NA: Not applicable
Bold and italic indicate that the p-value is significant (i.e. p < 0•05).
p-value less than 0•001.
p-value between 0•001 and 0•01.
p-value between 0•01 and 0•05.
For young cancer sufferers, age at cancer diagnosis, sex, cancer stage, having a history of physical illness, using opioid-based painkillers, frequency of inpatient admissions, and number of inpatient days were not associated with the odds of suicide. For older cancer sufferers, the results were similar to the overall model, except that having a history of physical illness decreased the odds of suicide (OR=0•66, 95% CI=0•45-0•96). The impact of A&E visits was insignificant.
4. Discussion
To the best of our knowledge, this is the first study exploring the patterns of hospital utilization in the six months prior to cancer-related suicide. The findings provide valuable insights for the provision of targeted suicide prevention strategies in hospital settings for HK cancer sufferers.
Our findings concurred with previous research on risk factors for suicides by cancer sufferers, for instance, the predominance of men, younger age, advanced stage of cancer, history of psychiatric illnesses, experiencing pain and suffering from H&N cancer. [2,22,[24], [25], [26], [27]] New findings from our study were the elevated risks of suicide for cancer sufferers who used opioid-based painkillers. Suffering H&N cancer influences appearance, and capacity to speak, taste, and breathe, thus diminishing quality of life. [28] Close monitoring and better support (both in hospital and at home) should be made available to them. On the other hand, people suffering with liver cancer had lower odds of suicide compared to other cancer types. This finding concurred with research which found that people with lung, colorectal, and H&N cancers were at higher risk of suicide compared to the general population, while the risk of suicide was no different for people with liver cancer, and the general population. [2]
Although previous studies have identified that many physical illnesses such as diabetes and hypertension increased the risk of suicide, [29] our research found no elevated risk. One possible explanation is that our study treated physical comorbidities in binary form, and the study sample was cancer sufferers. Research is required to further investigate relationships between chronic disease status, cancer diagnoses and suicide.
The increased odds of suicide for cancer sufferers who were frequent A&E attendees, or were often admitted to hospital may reflect poorly-managed symptom crises, or difficult personal situations. In HK between 2012 and 2016, there was constrained community support for cancer sufferers, and thus those with high symptom burdens may have received suboptimal community care and support and hence relied on unplanned attendances at hospitals for crisis and pain management. On the other hand, frequent visits to outpatient services, and longer inpatient stays were associated with lower risk of suicide. Regular attendance at outpatient services may indicate cancer sufferers’ motivation to self-manage with available ambulatory supports and they were in a more stable condition, whilst longer hospital stays suggests that consistent medical and nursing attention and monitoring received in hospital reduce the risk of suicide.
There was significant impact of cancer stage on clinical profiles and hospital utilizations, and on suicide risk. Among primary stage cancer sufferers, only having a history of psychiatric illness increased the odds of suicide. Painkiller usage and the frequency of hospital admissions were not associated with suicide, indicating that primary stage cancer sufferers may have fewer risks (such as unmanaged pain, worse prognosis, poor physical condition). However, people with metastases or cancer recurrence may be deteriorating physically, have more severe and perhaps poorly managed pain, and poorer prognosis. This may be devastating, especially to younger people. Their frequent use of A&E services for crisis management may exacerbate (rather than manage) their health problems, and lead to higher risk of suicide.
There were few age-related differences in the impact of clinical profiles and hospital utilization patterns on the risk of suicide in cancer sufferers. However, older cancer sufferers with other physical illnesses had lower odds of suicide. The presence of physical illnesses means that they may receive better care and support from healthcare providers and family, thus reducing their risk of suicide. Moreover, physical deterioration due to physical comorbidities may reduce their capacity to end their lives.
4.1. Limitations
There are several limitations in this study. First is the public sector-only coverage of CDARS data on HK cancer sufferers’ hospital utilization patterns. Those cases which only received care in the private sector would not be captured, and no information related to private hospitals is included in CDARS. Thus, our data may have underestimated the actual service usage. Nevertheless, more than 90% of the cancer sufferers are treated in public sector. Second, this study considered cancer sufferers’ utilization of hospital inpatient, outpatient and A&E services in general terms. There was no information on service specialty (e.g., psychiatry, oncology, etc.). A greater focus is needed into the types of medical care consumed by those cancer sufferers who killed themselves, and controls, to provide better insights into which areas to target for implementing effective suicide prevention for cancer sufferers. Third, since the data were from clinical records, there was little information on sufferers’ socioeconomic background and insurance status. However, in our study, the participants were drawn from public hospitals. In the HK public hospital system, the medical cost is affordable with a daily charge at around US$20.0. [30] Residents with difficulties paying the medical cost can apply for discount or full waive of the payment. [31] Therefore, the medical cost for cancer management will be a less of concern in HK. Due to the limitation and the availability of the data, other factors including the type of cancer treatments and palliative care cannot be accessed in the current study. Future studies may be conducted to look at these factors with the use of other datasets. Last, this study retrospectively compared hospital utilization information between cases in the six-months leading up to their suicide, and a randomly chosen six-month time period for controls. Future research might explore hospital service utilization patterns in other time periods preceding the suicide event to better understand precursor events.
4.2. Implications
This study provides valuable insights into suicide prevention for cancer sufferers. Since cancer sufferers have frequent medical contacts, even when they are suicidal, improving suicide identification and implementing suicide prevention strategies in hospital settings may reduce suicide risk.
Current mental health screening strategies in healthcare settings should be maintained and strengthened. Healthcare professionals need to be made more aware of suicide risk in cancer sufferers, and the importance of risk identification and suicide prevention. Special attention should be paid to cancer sufferers with poor prognosis and who are in pain. Healthcare professionals should be trained to better understand the difficulties faced by cancer sufferers and observe signs of self-harm or suicide during conversation, so as to provide timely interventions or refer patients to social workers for further assistance. Palliative care has been found to decrease the risk of suicide among cancer patients. [32] Therefore, palliative or end-of-life care should be promoted for terminally ill patients to reduce the A&E admission and optimize the quality of end-of-life.
Cancer sufferers may show early warning signals of suicide in terms of hospital service utilization. Paying attention to changes in service utilization patterns may assist healthcare professionals to more accurately detect suicidality among cancer sufferers for early interventions. If a person's physical condition deteriorates and there is an increased frequency of unplanned visits to A&E, or inpatient admissions, or he/she starts to miss outpatient appointments, flags for potential suicidality should be raised. Targeted follow-up services should be considered such as calling them to remind them about regular visits and asking them about their recent physical and mental well-being. If cancer sufferers expressed concerns or psychological needs, they should be referred to social workers and psychiatric services for timely assistance.
Inpatient facilities are the ideal place to implement suicide prevention strategies. When performing ward rounds, nurses and healthcare professionals should pay attention to cancer sufferers’ physical and mental conditions. Cancer sufferers are usually co-located. Sudden deterioration or death of others in the same location may result in severe mental distress among other sufferers. Therefore, counselling or careful conversations should be arranged afterwards. Meanwhile, emotional support should be continued after discharge. Follow-up phone calls after discharge to check on peoples’ physical and mental health has been promoted in the United States as an effective and cost-efficient suicide prevention strategy. [3]
Promoting suicide prevention only in hospital settings may not be sufficient due to severe shortage of manpower. Because of the COVID-19 pandemic, new arrangements and policy changes of not allowing any visitation have a huge impact on cancer sufferers. Dramatic reductions in social contact arising from the quarantine measures during COVID-19 has made it even more challenging to appropriately support cancer sufferers who have been disproportionately affected. More innovative ways are desperately needed to ensure social connectedness while practicing physical distancing. [33] Brochures and pamphlets with information about available resources should be designed and distributed to cancer sufferers and their carers, and carers should be better supported in providing physical and mental health support to sufferers.
5. Conclusion
This study found that cancer sufferers who killed themselves had distinctly different age and clinical profiles, and hospital service utilizations patterns, from non-suicidal cancer sufferers, in the six months leading up to their death. Hospitals can be important sites for opportunities for cancer-related suicide prevention programmes because these sites are frequented by cancer sufferers. Effective targeted suicide prevention strategies could be provided in these settings, by tailoring them to the specific needs of cancer sufferers. Formal and informal care support network in both hospital and home settings need to be established for cancer sufferers for reducing suicidal risk.
Authors’ Contributions
Y. Men: conceptualisation, formal analysis, methodology, validation, writing – original draft, and writing – review & editing. T-C. Lam: conceptualisation, data curation, methodology, resources, validation, writing – original draft, and writing – review & editing. C. Y. Yeung: formal analysis, methodology, validation, and writing – review & editing. P. S. F. Yip: funding acquisition, methodology, supervision, validation, and writing – review & editing.
Research in context
Evidence before this study
Cancer sufferers has a higher suicide risk compared to the general population. A MEDLINE search was conducted for research articles published between January 1, 2001, and May 31, 2021, using the search terms [(suicid*)] AND [(cancer)* OR (tumor)* OR (carcinoma)* OR (neoplas)* OR (oncolog)* OR (metastas)* OR (malign)*]. Previous research mainly focused on calculating incidence rate of suicide among cancer sufferers and investigating sociodemographic and clinical risk factors of suicide. Few studies conducted stratified analysis despite evidence that the risk of suicide among cancer sufferers varies by factors such as age and metastasis status. Further search on MEDLINE adding additional keywords “AND [(healthcare utilization) OR (hospital utilization) OR (hospital visit)]” suggested that no studies have examined the association between hospital service utilization patterns (inpatient admissions, and outpatient and Accident and Emergency (A&E) attendances) and suicide by cancer sufferers, even though cancer sufferers commonly use hospital services for treatment and symptom control.
Added value of this study
This study provides new insights on the patterns of public hospital service use in the six months prior to suicide by cancer sufferers. After controlling for cancer stage, and physical and psychiatric comorbidities, high numbers of A&E visits and inpatient admissions appeared to be associated with suicidal risk by cancer sufferers, while frequent attendance at outpatient clinics and longer inpatient stays attenuated their risk of suicide. Higher suicide risks were found for people with metastases or recurrent cancers. This study highlights the opportunity of using hospital settings for suicide prevention activities targeted to specific cancer-related risk factors.
Implications of all the available evidence
This study suggested that cancer sufferers may have distinct patterns of healthcare utilization before they killed themselves. Hospital settings are valuable sites to provide suicide prevention for cancer sufferers. Change in patterns of healthcare utilization by cancer sufferers should raise the alarm among healthcare professionals for potential downstream suicidal behaviours. Depression screening in the clinical setting should be continued. Additional supports should be provided to people with a poor prognosis even if no mental distress is observed.
Data sharing statement
The data that support the findings of this study are available from Hong Kong Hospital Authority and Hong Kong Coroner's Court. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from Dr. Tai-Chung Lam with the permission of Hong Kong Hospital Authority and Prof. Paul Siu Fai Yip with the permission of Hong Kong Coroner's Court.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgements
This work was supported by the Li Ka Shing Foundation (grant number: AR180055) and Hong Kong Research Grants Council General Research Fund (GRF) (grant number 17103620). We thank Hong Kong Hospital Authority and Hong Kong Coroner's Court for support in accessing the data. We would like to acknowledge the great contribution of medical and health professionals and carers who have been doing an excellent job to look after cancer sufferers, especially during the COVID-19.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100298.
Appendix. Supplementary materials
References
- 1.Du L, Shi H-Y, Yu H-R, Liu X-M, Jin X-H, Yan-Qian Incidence of suicide death in patients with cancer: A systematic review and meta-analysis. J Affect Disord. 2020;276:711–719. doi: 10.1016/j.jad.2020.07.082. [DOI] [PubMed] [Google Scholar]
- 2.Henson KE, Brock R, Charnock J, Wickramasinghe B, Will O, Pitman A. Risk of suicide after cancer diagnosis in England. JAMA Psychiatry. 2019;76:51–60. doi: 10.1001/jamapsychiatry.2018.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labouliere CD, Vasan P, Kramer A, Brown G, Green K, Rahman M, et al. “Zero Suicide” - A model for reducing suicide in United States behavioral healthcare. Suicidologi. 2018; 23: 22–30. [PMC free article] [PubMed]
- 4.Malakouti SK, Nojomi M, Poshtmashadi M, Hakim Shooshtari M, Mansouri Moghadam F, Rahimi-Movaghar A. Integrating a suicide prevention program into the primary health care network: A field trial study in Iran. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/193729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung SL. Assessing and Managing Potentially Suicidal Patients: Practical Guidelines for Doctors. 2015. Available from: https://csrp.hku.hk/wp-content/uploads/2015/06/DoctorGuidelines.pdf (Accessed 10th July 2021).
- 6.Men VY, Emery CR, Lam T-C, Yip PSF. Suicidal/self-harm behaviors among cancer patients: a population-based competing risk analysis. Psychol Med. 2020;23:1–10. doi: 10.1017/S0033291720004250. [DOI] [PubMed] [Google Scholar]
- 7.Yim PH, Yip PS, Li RH, Dunn EL, Yeung WS, Miao YK. Suicide after discharge from psychiatric inpatient care: a case-control study in Hong Kong. Aust N Z J Psychiatry. 2004;38:65–72. doi: 10.1177/000486740403800103. [DOI] [PubMed] [Google Scholar]
- 8.Cho J, Kang DR, Moon KT, Suh M, Ha KH, Kim C. Age and gender differences in medical care utilization prior to suicide. J Affect Disord. 2013;146:181–188. doi: 10.1016/j.jad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Cho J, Lee WJ, Moon KT, Suh M, Sohn J, Ha KH. Medical care utilization during 1 year prior to death in suicides motivated by physical illnesses. J Prev Med Public Heal. 2013;46:147–154. doi: 10.3961/jpmph.2013.46.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNaughton CH, Horst M, Gehron E, Sivendran S, Nguyen J, Holliday R. Patterns of support service, emergency department, and hospital utilization in patients with advanced cancer: A descriptive study. J Palliat Care. 2020;35:34–39. doi: 10.1177/0825859719851492. [DOI] [PubMed] [Google Scholar]
- 11.Hong Kong Hospital Authority. Clusters, Hospitals & Institutions. Published 2020. Available from https://www.ha.org.hk/visitor/ha_visitor_text_index.asp?Content_ID=10084&Lang=ENG&Dimension=100. (Accessed 30th August 2021).
- 12.Ko TP. Cancer Treatment Services in Public Hospitals. 2018. Available from: https://hkacs.org.hk/ufiles/1025_PS_CancerTreatmentServicesinPublicHospitals_DrTonyKo.pdf (Accessed 10th July 2021).
- 13.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 14.Huang J, Boyd C, Tyldesley S, Zhang-Salomons J, Groome PA, Mackillop WJ. Time spent in hospital in the last six months of life in patients who died of cancer in Ontario. J Clin Oncol. 2002;20:1584–1592. doi: 10.1200/JCO.2002.20.6.1584. [DOI] [PubMed] [Google Scholar]
- 15.Lowe JR, Yu Y, Wolf S, Samsa G, LeBlanc TW. A cohort study of patient-reported outcomes and healthcare utilization in acute myeloid leukemia patients receiving active cancer therapy in the last six months of life. J Palliat Med. 2018;21:592–597. doi: 10.1089/jpm.2017.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolnick SJ, Jackson J, Nelson WW, Butani A, Herrinton LJ, Hornbrook M. Pain management in the last six months of life among women who died of ovarian cancer. J Pain Symptom Manage. 2007;33:24–31. doi: 10.1016/j.jpainsymman.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Lam T-C, Chan S-K, Choi C-W, Tsang K-C, Yuen K-K, Soong I. Integrative palliative care service model improved end-of-life care and overall survival of advanced cancer patients in Hong Kong: A review of ten-year territory-wide cohort. J Palliat Med. 2021;24:1314–1320. doi: 10.1089/jpm.2020.0640. [DOI] [PubMed] [Google Scholar]
- 18.Hong Kong Hospital Authority. Cancer Statistics Query Systems. 2016. Available from https://www3.ha.org.hk/cancereg/allages.asp. (Accessed 15th September 2021).
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 20.Therneau T. A package for survival analysis in S. Version 3.2-11. 2020. Available from https://cran.r-project.org/web/packages/survival/survival.pdf (Accessed 10th July 2021).
- 21.Pearce N. Analysis of matched case-control studies. BMJ. 2016:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendal WS. Suicide and cancer: a gender-comparative study. Ann Oncol. 2007;18:381–387. doi: 10.1093/annonc/mdl385. [DOI] [PubMed] [Google Scholar]
- 23.Seal B, Sullivan SD, Ramsey SD, Asche CV., Shermock K, Sarma S. Comparing hospital-based resource utilization and costs for prostate cancer patients with and without bone metastases. Appl Health Econ Health Policy. 2014;12:547–557. doi: 10.1007/s40258-014-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anguiano L, Mayer DK, Piven ML, Rosenstein D. A literature review of suicide in cancer patients. Cancer Nurs. 2012;35:1250–1258. doi: 10.1097/NCC.0b013e31822fc76c. [DOI] [PubMed] [Google Scholar]
- 25.Robson A, Scrutton F, Wilkinson L, MacLeod F. The risk of suicide in cancer patients: a review of the literature. Psychooncology. 2010;19:1250–1258. doi: 10.1002/pon.1717. [DOI] [PubMed] [Google Scholar]
- 26.McFarland DC, Walsh L, Napolitano S, Morita J, Jaiswal R. Suicide in patients with cancer: Identifying the risk factors. Oncology. 2019;33:221–226. [PubMed] [Google Scholar]
- 27.Osazuwa-Peters N, Simpson MC, Zhao L, Boakye EA, Olomukoro SI, Deshields T. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer. 2018;124:4072–4079. doi: 10.1002/cncr.31675. [DOI] [PubMed] [Google Scholar]
- 28.Zeller JL. High Suicide risk found for patients with head and neck cancer. JAMA. 2006;296:1716. doi: 10.1001/jama.296.14.1716. [DOI] [PubMed] [Google Scholar]
- 29.Bolton JM, Walld R, Chateau D, Finlayson G, Sareen J. Risk of suicide and suicide attempts associated with physical disorders: a population-based, balancing score-matched analysis. Psychol Med. 2015;45:495–504. doi: 10.1017/S0033291714001639. [DOI] [PubMed] [Google Scholar]
- 30.Hong Kong Hospital Authority. Fees and Charges. 2021. Available from https://www.ha.org.hk/visitor/ha_visitor_index.asp?Parent_ID=10044&Content_ID=10045&Ver=HTML. (Accessed 15th September 2021).
- 31.Hong Kong Hospital Authority. Waiving of Medical Charges (For Eligible Persons). 2021. Available from https://www.ha.org.hk/visitor/ha_visitor_index.asp?Parent_ID=10047&Content_ID=259365&Ver=HTML. (Accessed 15th September 2021).
- 32.Sullivan DR, Forsberg CW, Golden SE, Ganzini L, Dobscha SK, Slatore CG. Vol. 15. Ann Am Thorac Soc; 2018. pp. 1357–1359. (Incidence of suicide and association with palliative care among patients with advanced lung cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yip PSF, Chau PH. Physical distancing and emotional closeness amidst COVID-19. Crisis. 2020;41:153–155. doi: 10.1027/0227-5910/a000710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.