Highlights
-
•
The synergistic bactericidal effect had produced by ultrasound (US) and chlorogenic acid (CA).
-
•
US plus CA changed the morphology of S. aureus cells..
-
•
US plus CA led the leakage of internal constituents.
-
•
US plus CA reduced the concentration of polysaccharide in biofilms.
-
•
US plus CA reduced S. aureus in mutton by 1.14 log CFU/g.
Keywords: S. aureus, Planktonic cells, Biofilm, Ultrasound, Chlorogenic acid
Abstract
This study aimed to investigate the mechanism of different treatments, namely, ultrasound (US), chlorogenic acid (CA), and ultrasound combined with chlorogenic acid (US plus CA) on the inactivation of Staphylococcus aureus planktonic and biofilm cells. Results showed that the combined treatment of US and CA exhibited remarkable synergistic antibacterial and antibiofilm effects. Scanning electron microscopy images indicated that the combined treatment of US and CA caused the most serious damage to the cell morphology. Confocal laser scanning microscopy images revealed that the combined treatment led to sharp increase and severe damage to the permeability of the cell membrane, causing the release of ATP and nucleic acids and decreasing the exopolysaccharide contents in S. aureus biofilm. Finally, the combined treatment of US plus 1% CA for 60 min inactivated S. aureus cells by 1.13 lg CFU/g on mutton. Thus, the combined treatment of US and CA had synergistic effect against S. aureus under planktonic, biofilm, and food systems.
1. Introduction
Mutton is well received because of its low fat and high protein contents, abundant vitamins, and presence of trace elements [1], [2]. However, mutton is easily perishable during preservation because its high protein content provides nutrients for the growth of bacterial cells [1]. Staphylococcus aureus is a Gram-positive zoonotic pathogen that causes lower respiratory tract site infections, surgical site infections, cardiovascular infections, and pneumonia in humans and animals [3], [4]. Meat products, such as mutton, chicken, pork, and beef, are easily contaminated by S. aureus [5], [6], [7]. Additionally, S. aureus has strong ability to form biofilm on surfaces of food, food processing equipment, and water [8], [9], [10]. Biofilm is a three-dimensional dense network structure that comprises, proteins, nucleic acids, and polysaccharides [3], [7], [11]. Bacterial cells in biofilm would be protected by the extracellular matrix from the interference of external environment challenges [9], [12], [13]. Therefore, developing an effective and efficient bactericidal technology to inactivate S. aureus planktonic and biofilm cells is of great significance in the food industry.
Ultrasound (US) is an environment-friendly, non-thermal, and non-destructive bactericidal technology used in the food industry. However, US treatment alone exhibits weak antibacterial and antibiofilm activities for the inactivation of bacterial cells [14]. Therefore, many researchers considered synergistic sterilization as a substitutable method to improve the efficiency of sterilization. Guo et al., (2020) reported that the combined treatment of US and sodium hypochlorite had synergistic effect against Escherichia coli planktonic cells [15]. Bi et al., (2019) found that ultrasound combined with lysozyme effectively inactivated Salmonella typhimurium [16]. Huu et al., (2021) revealed that the combined treatment of ultrasound and propyl gallate had higher bactericidal efficiency to inactivate Listeria innocua and E. coli O157:H7 cells than single treatment [17]. Chlorogenic acid (CA) is an ester abundant in fruits and vegetables and has a wide range of antibacterial activities [18]. CA exhibits strong antibacterial activity against many kinds of microorganisms, including E. coli, Pseudomonas aeruginosa, Listeria monocytogenes, and S. aureus [19]. Lou et al., (2011) reported that CA effectively inhibited the growth of bacterial cells by destroying the integrity of the cell membrane [20]. However, the synergistic effect of US and CA on the inactivation of S. aureus planktonic and biofilm cells has not been reported yet.
This study aimed to (1) determine the effect of chlorogenic acid (CA), ultrasound (US), and ultrasound combined with chlorogenic acid (US plus CA) on S. aureus planktonic and biofilm cells and (2) investigate the bactericidal activity and mechanism of these treatments against S. aureus under planktonic, biofilm, and food systems
2. Materials and methods
2.1. Cultivation of microorganisms
The strain of S. aureus was obtained from the Institute of Agricultural Products Processing, Jiangsu Academy of Agricultural Sciences (Nanjing, China). Prior to the assay, a loopful of S. aureus cells were streaked on brain–heart infusion (BHI) agar (Qingdao Hope Bio-Technology Co., Ltd.) and incubated at 37 ℃ for 24 h. One single colony was transferred into 5 mL of BHI broth (Qingdao Hope Bio-Technology Co., Ltd.). S. aureus cells were cultured to exponential phase under shaking at 200 rpm (37 °C) for 24 h. The concentration of S. aureus cells in the exponential phase reached approximately 9 1 g CFU/mL [21], [22].
2.2. US, CA, and combined US and CA
The bacterial suspension and biofilm were subjected to US (400 W, 50 kHz), CA (0.5%, 1%, and 2%), and US plus CA. CA powder was diluted in deionized water to produce 0.5%, 1%, and 2% solutions. Treatments with US and US plus CA were carried out in an ultrasonic cleaning machine (50 kHz, 800 W) (Kunshan Ultrasonic, Inc, Suzhou, China). The untreated bacterial suspension and biofilm were placed at room temperature and used as negative control.
2.3. Inactivation of S. aureus planktonic cells
S. aureus cells in the exponential phase were harvested by centrifugation at 5000 g and 4 °C for 10 min. The bacterial pellets were washed with 0.85% NaCl solution. S. aureus planktonic cells were treated with control, US, CA, and US plus CA. For US treatment, the bacterial pellets were mixed with 0.85% NaCl solution and placed in the ultrasonic cleaning machine for 5, 10, 20, 30, and 60 min. For CA treatment, 0.5%, 1%, and 2% CA was added into the bacterial pellets for 5, 10, 20, 30, and 60 min. For combined treatment, the bacterial pellets were mixed with 0.5%, 1%, and 2% CA and then placed into the ultrasonic cleaning machine for 5, 10, 20, 30, and 60 min. After treatment, 1 mL of the bacterial suspension was neutralized with 9 mL of 0.1 mol/L PBS to terminate the antibacterial action of CA. The neutralized solution was serially diluted tenfold with 0.85% NaCl solution. Microbiological analysis was conducted according to our previous paper [23].
2.4. Inactivation of S. aureus biofilm cells
For biofilm formation, approximately 109 CFU/mL of S. aureus cells (1 mL) were inoculated into 100 mL of BHI to obtain the final concentration of S. aureus cells (107 CFU/mL). Biofilms were formed on the 24-well polystyrene microliter plates (Costar, Corning, USA) after incubation for 72 h at 37 ℃ in an incubator. BHI broth was replaced with fresh broth every 24 h. After incubation, the broth was discarded, and the biofilm was washed twice with 0.01 mol/L PBS buffer. The S. aureus biofilm cells were treated with control, US, CA, and US plus CA and subjected to similar steps in section 2.3 [23] .
2.5. Scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) analyses
SEM and CLSM analyses were conducted according to our previous paper [23], [24], [25]. S. aureus planktonic and biofilm cells were treated with US, 2% CA, and US plus 2% CA for 30 min prior to analyses. Additionally, S. aureus planktonic and biofilm cells without any treatment were placed at room temperature and used as negative control.
In the SEM analysis, the treated and untreated bacterial suspension were centrifuged at 5000 g and 4 ℃ for 10 min, and the bacterial pellets were left. The pellets were fixed with glutaraldehyde (2.5%, v/v) at 4 ℃ for 12 h. Approximately 107 CFU/mL of S. aureus cells (400 µL) were added to each well of eight-well chamber slides (Nunc™ Lab-Tek™, Thermo Fisher Scientific), and the biofilm was incubated according to Section 2.4. After different treatments, the plates were cut into small squares and fixed with 2.5% glutaraldehyde at 4 °C for 12 h. Finally, the bacterial cells were observed by EVO-LS10 SEM (Carl Zeiss AG, Jena, Germany).
In the CLSM analysis, the treated and untreated bacterial suspensions were centrifuged at 5000 g and 4 ℃ for 10 min. The bacterial pellets were dyed by the LIVE/DEAD BacLight viability kit (Molecular Probes; Life Technologies, Eugene, OR). S. aureus biofilm used for CLSM analysis was prepared according to the methods of SEM. After different treatments, the S. aureus biofilm was dyed by the LIVE/DEAD BacLight viability kit. Finally, the CLSM images were observed under a Leica Ultra View VOX CLSM (Leica Microsystems, Ltd., Wetzlar, Germany).
2.6. Exopolysaccharide (EPS) content analyses
The S. aureus biofilm was incubated according to Section 2.4, and the contents of EPS weredetermined according to our previous paper [25]. The S. aureus biofilms were treated by US, 0.5% CA, 1% CA, 2% CA, US plus 0.5% CA, US plus 1% CA, and US plus 2% CA for 30 min. The treated and untreated biofilms were collected into different tubes. The biofilm was centrifuged at 5000 g and 4 ℃ for 30 min, and the precipitate was collected and resuspended in 10 mL of 0.85% NaCl solution (including 0.22% formaldehyde) for determination of the content of insoluble polysaccharides. The supernatant was obtained to determine the content of soluble polysaccharides. The contents of soluble and insoluble polysaccharides were measured using phenol–sulfuric acid method [26].
2.7. Release of intracellular ATP and nucleic acids
The release of intracellular ATP and nucleic acids in S. aureus planktonic and biofilm cells was determined according to our previous paper [27]. The bacterial suspension and biofilms were treated with US, 0.5% CA, 1% CA, 2% CA, US plus 0.5% CA, US plus 1% CA, and US plus 2% CA. The supernatant was collected by centrifugation at 3000 g and 4 °C for 10 min and used to measure the release of nucleic acids. The concentration of nucleic acids was determined at 260 nm on a UV–VIS Spectrophotometer (Mapada, Shanghai, China).
For determining the concentration of extracellular ATP, 1 mL of untreated or treated S. aureus planktonic and biofilm cells were fixed with 9 mL of 0.1 mol/L PBS buffer. After that, the suspension was centrifuged at 10,000 × g at 0 °C for 1 min, the supernatants were used to measure the release of intracellular ATP by following the instructions of the ATP detection kit (Beyotime, Shanghai, China).
2.8. Inactivation of S. Aureus cells on mutton
2.8.1. Inoculation of mutton
Mutton was bought from a supermarket (Nanjing, China) and cut into 10 g sample in the laboratory. About 1 mL of each 107-108 CFU/mL S. aureus suspension was inoculated into the mutton. The concentration of S. aureus in mutton was approximately 6 lg CFU/g.
2.8.2. US in combination with CA treatment
Inoculated mutton was treated by US plus 1% CA for different durations of 5, 10, 20, 30, and 60 min. The mutton was immediately mixed with 90 mL of 0.1 mol/L PBS to neutralize the pH. About l mL of neutralizing solution was serially diluted in 9 mL of 0.01 PBS. Appropriate dilutions of bacterial suspension (l mL) were added into plate containing 15 mL of S. aureus Chromogenic Medium (Qingdao Hope Bio-Technology Co., Ltd.). All of the plates were incubated at 37 ℃ for 24 h.
2.9. Statistical analysis
All samples in the experiment were prepared in triplicate. ANOVA in SPSS version 26.0 was used to analyze significant difference (p < 0.05) between the control and experimental groups.
3. Results and discussion
3.1. Inactivation of S. aureus planktonic and biofilm cells
Table 1, Table 2 show the inactivation of S. aureus planktonic and biofilm cells with different treatment times of 5, 10, 20, 30 and 60 min in the presence of US or CA alone and their combination (US plus CA). US by itself was not effective to inactivate S. aureus planktonic and biofilm cells. US treatment alone for 30 min only inactivated 0.31 and 0.22 lg CFU/mL of S. aureus planktonic and biofilm cells, respectively. Similarly, He et al [28] reported that US treatment alone for 9 min only inactivated 0.36 lg CFU/mL of S. aureus planktonic cells. Yu et al [29] reported that US alone for 10 min only caused 0.09 lg CFU/cm2 reduction in S. aureus biofilm cells. Treatments with 0.5% CA, 1% CA, and 2% CA for 30 min inactivated 0.9, 1.78, and 3.4 lg CFU/mL of S. aureus planktonic cells, respectively, while treatments with US plus 0.5% CA, US plus 1% CA, and US plus 2% CA for 30 min achieved 2.18, 4.52, and 6.9 lg CFU/mL reduction in S. aureus planktonic cells. After treatment with 0.5% CA, 1% CA, and 2% CA for 30 min, the viable bacterial counts of S. aureus biofilm cells were reduced by 1.08, 1.80, and 2.77 lg CFU/ mL, respectively. The combinations of US plus 0.5% CA, US plus 1% CA, and US plus 2% CA for 30 min achieved 1.40, 4.42, and 5.53 lg CFU/mL reduction in S. aureus biofilm cells. Hence, the combined treatment (US plus CA) exhibited significantly (p < 0.05) stronger antibacterial and antibiofilm efficacy than US or CA alone to inactivate S. aureus planktonic and biofilm cells.
Table 1.
Inactivation of S. aureus planktonic cells by different treatment.
| Treatments | S. aureus planktonic cells (lg CFU/mL) at min: | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 60 | |
| Control | 9.40 ± 0.08A | 9.36 ± 0.12A | 9.34 ± 0.09A | 9.42 ± 0.02A | 9.38 ± 0.12A |
| US | 9.27 ± 0.09A | 9.26 ± 0.09A | 9.13 ± 0.04B | 9.11 ± 0.04B | 9.03 ± 0.02B |
| 0.5% CA | 9.07 ± 0.07B | 8.99 ± 0.06B | 8.77 ± 0.09C | 8.52 ± 0.12C | 6.47 ± 0.03C |
| 1% CA | 8.97 ± 0.04BC | 8.75 ± 0.06CD | 8.46 ± 0.10D | 7.64 ± 0.13D | 4.94 ± 0.06E |
| 2% CA | 8.86 ± 0.04CD | 8.67 ± 0.05DE | 7.05 ± 0.13E | 6.02 ± 0.08F | 3.35 ± 0.20D |
| US plus 0.5% CACA | 9.00 ± 0.05BC | 8.87 ± 0.04BC | 8.64 ± 0.02C | 7.24 ± 0.13E | 3.71 ± 0.10F |
| US plus 1% CA | 8.90 ± 0.03CD | 8.75 ± 0.02CD | 7.01 ± 0.09F | 4.90 ± 0.06G | 3.00 ± 0.20G |
| US plus 2% CA | 8.80 ± 0.13D | 8.62 ± 0.07E | 5.84 ± 0.07G | 2.52 ± 0.09H | ≤1.4 |
Different lower cases indicate significant differences among treatments (p < 0.05).
Table 2.
Inactivation of S. aureus biofilm cells by different treatments.
| Treatments | S. aureus biofilm cells (lg CFU/mL) at min: | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 30 | 60 | |
| Control | 9.43 ± 0.02A | 9.42 ± 0.04A | 9.42 ± 0.08A | 9.43 ± 0.05A | 9.42 ± 0.10A |
| US | 9.35 ± 0.04B | 9.31 ± 0.02B | 9.28 ± 0.01B | 9.21 ± 0.04B | 9.00 ± 0.05B |
| 0.5% CA | 9.30 ± 0.04B | 9.21 ± 0.11C | 9.08 ± 0.06C | 8.35 ± 0.04C | 6.75 ± 0.10C |
| 1% CA | 9.25 ± 0.06C | 9.12 ± 0.02C | 8.34 ± 0.10D | 7.63 ± 0.16E | 4.60 ± 0.12D |
| 2% CA | 9.09 ± 0.03D | 8.95 ± 0.02D | 6.99 ± 0.09F | 6.68 ± 0.05F | 4.21 ± 0.06E |
| US plus 0.5% CACA | 9.03 ± 0.03D | 8.85 ± 0.04E | 8.20 ± 0.02E | 8.03 ± 0.20D | 3.83 ± 0.15F |
| US plus 1% CA | 8.98 ± 0.05E | 8.69 ± 0.05F | 6.90 ± 0.13F | 5.02 ± 0.07G | 3.57 ± 0.08G |
| US plus 2% CA | 8.86 ± 0.08F | 8.55 ± 0.06G | 6.06 ± 0.06G | 3.90 ± 0.23H | 3.10 ± 0.11H |
Different lower cases indicate significant differences among treatments (p < 0.05).
S. aureus is a Gram-positive bacterium that has a thick peptidoglycan layer in the cell wall. This layer helps S. aureus cells to become resistant to US, so US treatment alone was insufficient to inactivate S. aureus planktonic and biofilm cells [28], [30]. Therefore, to obtain higher bactericidal efficacy, many researchers explored the synergistic effect of US and chemical agent to inhibit the growth of bacterial cells as an alternative method. Bi et al., (2020) reported that US treatment alone only inactivated 3.31 1og CFU/mL of Salmonella typhimurium planktonic cells, whereas the combination of lysozyme and US inactivated 4.26 1 g CFU/mL of bacterial cells; this finding indicated the synergistic relationship between lysozyme and ultrasound [16]. Zhang et al., (2020) reported that ultrasound in combination with carvacrol, citral, cinnamic acid, geraniol, gallic acid, lactic acid, or limonene had great synergistic effect against E. coli K12 and Listeria innocua cells [31]. Luo et al., (2016) reported that the combination of slightly acidic electrolyzed water, US, and mild heat had synergistic effect on the inactivation of L. monocytogenes and S. typhimurium [32]. US passes through the cavitation activity to destroy the cell membrane and cell wall, thereby facilitating the penetration of the chemical agent into the cell membrane and cell wall and resulting in the synergistic effect between US and the chemical agent [33].
3.2. Scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) analyses
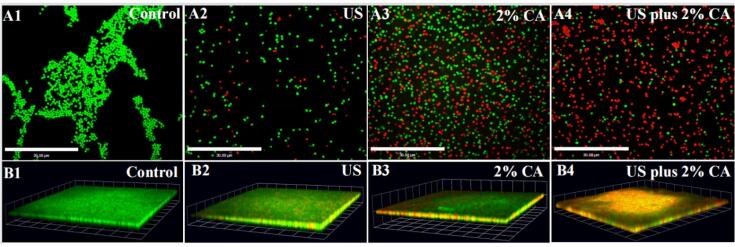
SEM was used to analyze the effect of US, CA, and US plus CA treatments on morphological changes in S. aureus planktonic and biofilm cells. The untreated S. aureus planktonic and biofilm cells possessed a complete and smooth surface. The bacterial cells in the biofilm were closely arranged and surrounded by a large amount of EPS (Fig. 1 A1, B1). After US treatment for 30 min, slight morphological destruction appeared on the surface of S. aureus planktonic and biofilm cells, only a small part of the cell surface was wrinkled, and most S. aureus planktonic and biofilm cells still had intact surface (Fig. 1 A2, B2). This finding is in accordance with the report of He et al., (2021). After treatment with US alone for 30 min, some pores appeared on the cell membrane, but most S. aureus cells still had cell membrane [28]. Yu et al., (2021) revealed that high-intensity ultrasound destroyed the structure of S. aureus biofilm, but the bacterial cells in the biofilm still had intact shape [29]. After treatment with 2% CA for 30 min, S. aureus planktonic and biofilm cells became shriveled, and many wrinkles and gullies appeared on the cell surface (Fig. 1 A3, B3). As shown in Fig. 1 A4 and B4, the combined treatment of US and CA led to the complete collapse of S. aureus planktonic and biofilm cells. Moreover, the combined treatment of US and agent could cause more serious damage to the morphology of bacterial cells because US could damage the integrity of the cell membrane and promote the entry of the chemical agent into the bacterial cells. Li et al., (2021) revealed that the combined treatment of US and slightly acid electrolytic water could severely damage the cell wall [33]. Guo et al., (2021) reported the complete collapse of E. coli O157:H7 cells after the combined treatment of US and thyme essential oil nanoemulsion [34].
Fig. 1.
SEM images of S. aureus planktonic and biofilm cells treated with control, US, 2% CA, and US plus 2% CA.
CLSM was used to estimate the effect of US, CA, and US plus CA on the permeability of the cell membrane. S. aureus planktonic and biofilm cells after treatment with control, US, CA, and their combination (US plus CA) were examined by CLSM (Fig. 2). As shown in Fig. 2 A1 and B1, the control S. aureus planktonic and biofilm cells emitted green fluorescence, indicating that all of the cells were alive and the permeability of the cell membrane did not increase. After treatment with US alone for 30 min, few S. aureus planktonic and biofilm cells emitted red fluorescence, indicating that the permeability of the cell membrane slightly increased, but most of the cells remained viable (Fig. 2 A2, B2). This finding is in accordance with a published work by Guo et al., (2020). The CLSM images indicated that a small proportion of E. coli planktonic cells emitted red fluorescence after treatment with US alone, meaning that most E. coli cells were alive [34]. Li et al., (2017) revealed that very few S. aureus planktonic cells emitted red fluorescence after US treatment for 15 min [14]. For 2% CA treatment for 30 min, the proportion of S. aureus planktonic and biofilm cells emitting red fluorescence significantly increased (Fig. 2 A3, B3), indicating that CA was more effective than US to increase the permeability of the cell membrane. Fig. 2 A4, B4 shows that the proportion of S. aureus planktonic and biofilm cells emitting red fluorescence after the combined treatment of US and 2% CA sharply increased, indicating that the combined treatment sharply increased the permeability of the cell membrane. US could damage the integrity of the cell membrane, helping CA penetrate the membrane and causing the sharp increase in the permeability of the cell membrane.
Fig. 2.
CLSM images of S. aureus planktonic and biofilm cells treated with control, US, 2% CA, and US plus 2%CA.
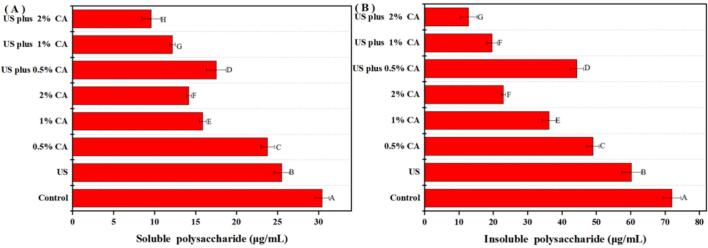
3.3. Exopolysaccharide (EPS) content analysis
Changes in the EPS content in S. aureus biofilms after US or CA alone and their combination (US plus CA) are shown in Fig. 3. The concentration of soluble and insoluble polysaccharides in the control biofilm were 30.44 and 71.95 μg/mL, respectively. After treatment with US, 0.5% CA, 1% CA, and 2% CA, the concentration of soluble polysaccharides in S. aureus biofilms were 25.51, 23.78, 15.86, and 14.19 μg/mL, respectively. Meanwhile, the contents of insoluble polysaccharides in S. aureus biofilms decreased to 60.22, 48.99, 26.27, and 22.94 μg/mL, respectively. The concentrations of soluble and insoluble polysaccharides in the biofilm decreased to 17.53, 12.18, and 9.61 μg/mL after treatment with US plus 0.5% CA, US plus 1% CA, and US plus 2% CA, respectively. Meanwhile, the insoluble polysaccharide contents in S. aureus biofilms decreased to 44. 30, 19.60, and 12.81 μg/mL, respectively. Thus, the combined treatment of US and CA was more effective than US or CA alone in decreasing the thickness of S. aureus biofilms by reducing the contents of EPS.
Fig. 3.
Soluble and insoluble polysaccharide contents in S. aureus biofilms after different treatments. Different lower case letters indicate significant differences among treatments (p < 0.05).
S. aureus biofilm cells were more difficult to be inactivated than planktonic cells because bacterial cells in the biofilm were protected by the matrix of glycoproteins, EPS, and other compounds [25]. In the cells treated with the combination of US and CA, US could destroy the structure of the extracellular matrix, promoting the penetration of CA into the biofilm barrier; thus, the combined treatment of US and CA was more effective than US or CA alone in inactivating S. aureus cells in the biofilm. Yu et al., (2021) reported that US via mechanical oscillation promoted ClO2 penetration into the S. aureus biofilm, thereby enhancing the bactericidal rate to inactivate S. aureus biofilm cells [29].
3.4. Release of intracellular ATP and nucleic acids
The effects of US or CA alone and their combination (US plus CA) on the leakage of ATP from S. aureus planktonic and biofilm cells are shown in Table 3. The intracellular ATP levels of control S. aureus planktonic and biofilm cells were 16.71 and 8.96 nmol/OD, respectively. After treatment with US alone for 30 min, the concentration of intracellular ATP in S. aureus planktonic and biofilm cell significantly increased to 27.91 and 20.61 nmol/OD, respectively (p < 0.05). After treatment with 0.5% CA, 1% CA, and 2% CA, the concentration of intracellular ATP in S. aureus planktonic cells significantly increased to 40.97, 50.87, and 102.97 nmol/OD, respectively (p < 0.05). Meanwhile, the concentration of intracellular ATP in S. aureus biofilm cells significantly increased to 38.65, 58.96, and 145.74 nmol/OD, respectively (p < 0.05). After the combined treatment of US plus 0.5% CA, US plus 1% CA, and US plus 2% CA, the concentration of intracellular ATP in S. aureus planktonic cells significantly increased to 81.31, 142.4, and 205.5 nmol/OD, respectively (p < 0.05). Meanwhile, the concentration of intracellular ATP in S. aureus biofilm cells significantly increased to 55.85, 116.21, and 189.46 nmol/OD, respectively (p < 0.05). Obviously, the combined treatment of US and CA was more effective than US or CA alone in destroying the integrity of the cell membrane, causing the leakage of ATP.
Table 3.
Measurements of ATP released from S. aureus planktonic and biofilm cells after treatment with US, CA, and US plus CA.
| Treatments | ATP concentration (nmol/ OD) | |
|---|---|---|
| S. aureus planktonic cells | S. aureus biofilm cells | |
| Control | 16.71 ± 2.75A | 8.96 ± 0.85A |
| US | 27.91 ± 2.60B | 20.61 ± 2.30B |
| 0.5% CA | 40.97 ± 3.60C | 38.65 ± 4.90C |
| 1% CA | 50.87 ± 3.13D | 58.96 ± 9.43D |
| 2% CA | 102.97 ± 3.16F | 145.74 ± 3.29F |
| US plus 0.5% CA | 81.31 ± 9.15E | 55.85 ± 5.52D |
| US plus 1% CA | 142.40 ± 9.07G | 116.21 ± 6.19E |
| US plus 2% CA | 205.5 ± 5.53H | 189.46 ± 6.62G |
Different lower-case letters indicate significant differences among treatments (p < 0.05).
The effects of US or CA alone and their combination (US plus CA) on the leakage of nucleic acids at 260 nm from S. aureus planktonic and biofilm cells are shown in Table 4. The OD260 of the control S. aureus planktonic and biofilm cells were 0.31 and 0.12. After treatment with US, 0.5% CA, 1% CA, and 2% CA, the OD260 of the S. aureus planktonic cells significantly increased to 0.40, 0.57, 0.69, and 1.42, respectively (p < 0.05). Meanwhile, the OD260 of S. aureus biofilm cells significantly increased to 0.17, 0.23, 0.57, and 0.68, respectively (p < 0.05). In addition, the OD260 of S. aureus planktonic cells treated by US plus 0.5% CA, US plus 1% CA, and US plus 2% CA significantly increased to 0.87, 1.56, and 2.24 respectively (p < 0.05). Meanwhile, the OD260 of S. aureus biofilm cells treated by US plus 0.5% CA, US plus 1% CA, and US plus 2% CA significantly increased to 0.55, 0.88, and 1.10, respectively (p < 0.05).
Table 4.
Measurements of nucleic acids released from S. aureus planktonic and biofilm cells after treatment with US, CA, and US plus CA.
| Treatments | OD260 | |
|---|---|---|
| S. aureus planktonic cells | S. aureus biofilm cells | |
| Control | 0.31 ± 0.022A | 0.12 ± 0.022A |
| US | 0.40 ± 0.005B | 0.17 ± 0.012B |
| 0.5% CA | 0.57 ± 0.010C | 0.23 ± 0.010C |
| 1% CA | 0.69 ± 0.005D | 0.57 ± 0.001E |
| 2% CA | 1.42 ± 0.009F | 0.68 ± 0.003F |
| US plus 0.5% CA | 0.87 ± 0.005E | 0.55 ± 0.002D |
| US plus 1% CA | 1.56 ± 0.009G | 0.88 ± 0.003G |
| US plus 2% CA | 2.24 ± 0.043H | 1.10 ± 0.030H |
Different lower-case letters indicate significant differences among treatments (p < 0.05).
The release of intracellular ATP and nucleic acids in S. aureus planktonic and biofilm cells after the combined treatment of US and CA was significantly higher than US or CA treatment alone, indicating the synergistic relationship between US and CA in destroying the integrity of the cell membrane, resulting in the leakage of nucleic acids and ATP. Li et al., (2021) reported that the leakage of nucleic acids and protein in Rhizopus stolonifer after treatment with US combined with slightly acid electrolytic water was significantly higher than the individual treatment [33]. In fact, US could damage the outer cell membrane, which could help CA penetrate into the membrane and cell wall, thereby enhancing the ability of CA to damage the cell membrane and cause the leakage of ATP and nucleic acids [35], [36].
3.5. Inactivation of S. aureus in mutton by the combined treatment of US plus 1% CA
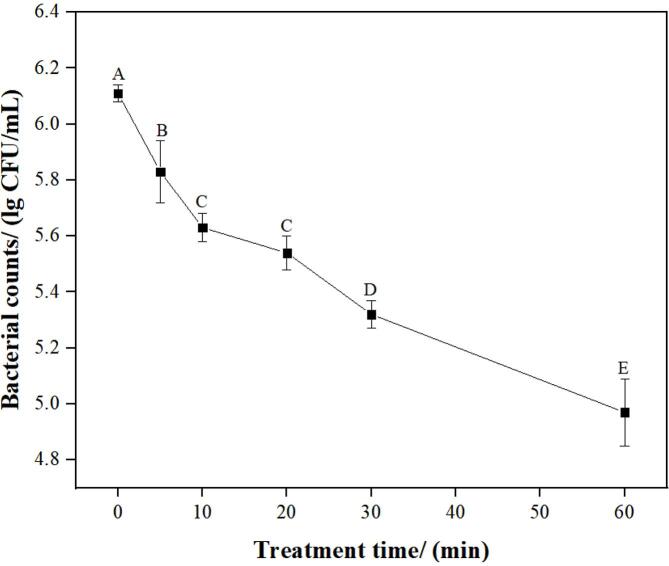
The survival of S. aureus in mutton after treatment by US plus 1% CA for 5, 10, 20, 30, and 60 min are presented in Fig. 4. The count of S. aureus cells in untreated mutton was 6.11 lg CFU/mL. After treatment with US plus 1% CA for 5, 10, 20, 30, and 60 min, the counts of S. aureus cells in mutton significantly decreased to 5.83, 5.63, 5.54, 5.32, and 4.97 lg CFU/mL, respectively. With increasing treatment time, the counts of S. aureus cells in mutton significantly decreased (p < 0.05). In recent years, many researchers reported the application of US combined with agent against bacterial cells in foods. Li et al., (2021) reported that US combined with slightly acid electrolytic water significantly controlled the growth of R. stolonifer in sweet potato [33]. Yoon et al., (2021) reported that the combined treatment of 3% malic acid, 0.1% nisin, and 40 kHz US for 20–30 min significantly decreased the counts of L. monocytogenes in king oyster mushrooms to lower than the detection level (≤1.4 lg CFU/mL) within 30 min [37]. He et al., (2021) reported that US and thyme essential oil nanoemulsions had remarkable synergistic effect on inhibiting the growth of E. coli O157:H7 on cherry tomatoes [38].
Fig. 4.
Survival of S. aureus in mutton after treatment with US plus 1% CA. Different lower case letters indicate significant differences among treatments (p < 0.05).
4. Conclusion
The bactericidal value of US combined with CA was greater than the sum of US and CA treatment alone, indicating that US combined with CA treatment had great synergistic effects on inactivating the growth of S. aureus planktonic and biofilm cells. Furthermore, the combined treatment of US and CA showed great synergistic effect on decreasing the concentration of polysaccharides in biofilm. The combined treatment of US and CA was also more effective in destroying the integrity of the cell membrane, causing the leakage of ATP and nucleic acids. In addition, the US plus 1% CA could inactivate 1.14 lg CFU/mL of S. aureus on mutton after 60 min treatment.
CRediT authorship contribution statement
Jinyue Sun: Methodology, Writing – original draft. Debao Wang: Software, Writing – original draft. Zhilan Sun: . Fang Liu: Conceptualization, Funding acquisition, Writing – review & editing. Lihui Du: Conceptualization, Methodology, Writing – review & editing. Daoying Wang: Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (31871866), and North Jiangsu Science and Technology Special Project of Jiangsu Province Policy Guidance Program (XZ-SZ202012).
Contributor Information
Fang Liu, Email: fangliu82@163.com.
Lihui Du, Email: ddabc_2000@163.com.
References
- 1.Song W.L., Du Y.F., Yang C.X., Li L., Wang S.J., Liu Y.B., Wang W. Development of PVA/EVA-based bilayer active film and its application to mutton. LWT - Food Sci. Technol. 2020;133(3) doi: 10.1016/j.lwt.2020.110109. [DOI] [Google Scholar]
- 2.Guo X., Wang Y.Q., Lu S., Wang J.Y. Changes in proteolysis, protein oxidation, flavor, color and texture of dry-cured mutton ham during storage. LWT- Food Sci. Technol. 2021;149 doi: 10.1016/j.lwt.2021.111860. [DOI] [Google Scholar]
- 3.Kang J., Jin W.Y., Wang J.F., Sun Y.Y., Wu X.X., Li L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus, LWT- Food Sci. Technol. 2019;101:639–645. doi: 10.1016/j.lwt.2018.11.093. [DOI] [Google Scholar]
- 4.Hu W., Li C.Z., Dai J.M., Cui H.Y., Lin L. Antibacterial activity and mechanism of Listeria cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crops Prod. 2019;2019(130):34–41. doi: 10.1016/j.indcrop.2018.12.078. [DOI] [Google Scholar]
- 5.Ali S.S., Moawad M.S., Hussein M.A., Azab M., Abdelkarim E.A., Badr A., Sun J.Z., Khalil M. Efficacy of metal oxide nanoparticles as novel antimicrobial agents against multi-drug and multi-virulent Staphylococcus aureus isolates from retail raw chicken meat and giblets. Int. J. Food Microbiol. 2021;344 doi: 10.1016/j.ijfoodmicro.2021.109116. [DOI] [PubMed] [Google Scholar]
- 6.Tegegnea H.A., Kolácková I., Florianová M., Gelbícová T., Madec J.Y., Haenni M., Karpíšková R. Detection and molecular characterization of methicillin-resistant Staphylococcus aureus isolated from raw meat at the retail market. J. Global Antimicrob. Resist. 2021;26:233–238. doi: 10.1016/j.jgar.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang H.W., Wang H.H., Liang L.J., Xu X.L., Zhou H. Prevalence, genetic characterization and biofilm formation in vitro of staphylococcus aureus isolated from raw chicken meat at retail level in Nanjing, China. Food Control. 2017;86:11–18. doi: 10.1016/j.foodcont.2017.10.028. [DOI] [Google Scholar]
- 8.Ziuzina D., Han L., Cullen P.J., Bourke P. Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli. Int. J. Food Microbiol. 2015;210:53–61. doi: 10.1016/j.ijfoodmicro.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Kim W.J., Kim S.H., Kang D.H. Thermal and non-thermal treatment effects on Staphylococcus aureus biofilms formed at different temperatures and maturation periods. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109432. [DOI] [PubMed] [Google Scholar]
- 10.Wang W., Liu F., Zulqarnain B., Zhang C.S., Ma K., Peng Z.X., Yan S.F., Hu Y.J., Gan X., Dong Y.P., Bai Y., Li F.Q., Yan X.M., Ma A.G., Xu J. Genotypic characterization of methicillin-resistant Staphylococcus aureus isolated from pigs and retail foods in China. Biomed. Environ. Sci. 2017;30(8):570–580. doi: 10.3967/bes2017.076. [DOI] [PubMed] [Google Scholar]
- 11.Cui H.Y., Zhang C.H., Li C.Z., Lin L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT - Food Sci. Technol. 2020;122 doi: 10.1016/j.lwt.2020.109057. [DOI] [Google Scholar]
- 12.Kaneko H., Nakaminami H., Ozawa K., Wajima T., Noguchi N. In vitro anti-biofilm effect of anti-methicillin-resistant Staphylococcus aureus (MRSA) agents against the USA300 clone. J. Global Antimicrob. Resist. 2021;24:63–71. doi: 10.1016/j.jgar.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Hamid M., Romeih E., Saporito P., Osman A., Mateiu R.V., Mojsoska B., Jenssen H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control. 2020;111 doi: 10.1016/j.foodcont.2019.107056. [DOI] [Google Scholar]
- 14.Li J., Suo Y.J., Liao X.Y., Ahn J., Liu D.H., Chen S.G., Ye X.Q., Ding T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017;39:101–110. doi: 10.1016/j.ultsonch.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Guo L.P., Sun Y.C., Zhu Y.L., Wang B.W., Xu L., Li Y.F., Sun J.X. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2019;129 doi: 10.1016/j.foodres.2019.108887. [DOI] [PubMed] [Google Scholar]
- 16.Bi X.F., Wang X.Q., Chen Y., Chen L.Y., Xing Y.G., Che Z.M. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2019;60 doi: 10.1016/j.ultsonch.2019.104763. [DOI] [PubMed] [Google Scholar]
- 17.Huu C.N., Rai R., Yang X., Tikekar R.V., Nitin N. Synergistic inactivation of bacteria based on a combination of low frequency, low-intensity ultrasound and a food grade antioxidant. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee B., Dong G.L. Depletion of reactive oxygen species induced by chlorogenic acid triggers apoptosis-like death in Escherichia coli. Free Radical Res. 2018;52(5):1–11. doi: 10.1080/10715762.2018.1456658. [DOI] [PubMed] [Google Scholar]
- 19.Fu S.L., Wu C.H., Wu T.T., Yu H.X., Yang S.B., Hu Y.Q. Preparation and characterization of chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017;221:657–663. doi: 10.1016/j.foodchem.2016.11.123. [DOI] [PubMed] [Google Scholar]
- 20.Lou Z.X., Wang H.X., Zhu S., Ma C.Y., Wang Z.P. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011;76(6):398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu F., Jin P.P., Sun Z.L., Du L.H., Wang D.Y., Zhao T., Doyle M.P. Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food Control. 2020;119 doi: 10.1016/j.foodcont.2020.107473. [DOI] [Google Scholar]
- 22.Qi M.Y., Zhao R.Q., Liu Q.Y., Yan H.Y., Zhang Y., Wang S.Y., Yuan Y. Antibacterial activity and mechanism of high voltage electrostatic field (HVEF) against Staphylococcus aureus in medium plates and food systems. Food Control. 2021;120 doi: 10.1016/j.foodcont.2020.107566. [DOI] [Google Scholar]
- 23.Liu F., Du L., Zhao T., Zhao P., Doyle M.P. Effects of phenyllactic acid as sanitizing agent for inactivation of Listeria monocytogenes biofilms. Food Control. 2017;78:72–78. doi: 10.1016/j.foodcont.2017.02.050. [DOI] [Google Scholar]
- 24.Liu F., Sun Z.L., Wang F.T., Liu Y.W., Zhu Y.Z., Du L.H., Wang D.Y., Xu W.M. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2019;86 doi: 10.1016/j.fm.2019.103344. [DOI] [PubMed] [Google Scholar]
- 25.Liu F., Tang C., Wang D.B., Sun Z.L., Du L.H., Wang D.Y. The synergistic effects of phenyllactic acid and slightly acid electrolyzed water to effectively inactivate Klebsiella oxytoca planktonic and biofilm cells. Food Control. 2020;20 doi: 10.1016/j.foodcont.2020.107804. [DOI] [Google Scholar]
- 26.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350. doi: 10.1021/ac60111a017. [DOI] [PubMed] [Google Scholar]
- 27.Liu F., Wang F.T., Du L.H., Zhao T., Doyle M.P., Wang D.Y., Zhang X.X., Sun Z.L., Xu W.M. Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control. 2018;84:442–448. doi: 10.1016/j.foodcont.2017.09.004. [DOI] [Google Scholar]
- 28.He Q., Zhang L.J., Song L.Y., Zhang X.H., Liu D.H., Hu Y.Q., Guo M.M. Inactivation of Staphylococcus aureus using ultrasound in combination with thyme essential oil nanoemulsions and its synergistic mechanism. LWT - Food Sci. Technol. 2021;337 doi: 10.1016/j.lwt.2021.111574. [DOI] [Google Scholar]
- 29.Yu H., Liu Y., Yang F., Xie Y., Guo Y., Cheng Y., Yao W. Synergistic efficacy of high-intensity ultrasound and chlorine dioxide combination for Staphylococcus aureus biofilm control. Food Control. 2021;122:107822. doi: 10.1016/j.foodcont.2020.107822. [DOI] [Google Scholar]
- 30.Piñon M.I., Alarcon-Rojo A.D., Renteria A.L., Carrillo-Lopez L.M. Microbiolgical properties of poultry breast meat treated with high-intensity ultrasound. Ultrasonics. 2020;102 doi: 10.1016/j.ultras.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H.C., Wang S.Y., Goon K., Gilbert A., Huu C.N., Walsh M., Nitin N., Wrenn S., Tikekar R.V. Experimental data for Inactivation of foodborne pathogens based on synergistic effects of ultrasound and natural compounds during fresh produce washing. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104983. [DOI] [PubMed] [Google Scholar]
- 32.Luo K., Oh D.H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016;53:165. doi: 10.1016/j.fm.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Mu T.H., Zhang M. Contribution of ultrasound and slightly acid electrolytic water combination on inactivating Rhizopus stolonifer in sweet potato. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M., Zhang L., He Q., Arabi S.A., Zhao H., Chen W., Ye X., Liu D. Synergistic Antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochem. 2020;66:104988. doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- 35.Shu Q., Lou H.H., Wei T.Y., Zhang X.L., Chen Q.H. Synergistic antibacterial and antibiofilm effects of ultrasound and MEL-A against methicillin-resistant Staphylococcus aureus. Ultrason. Sonochem. 2020;72 doi: 10.1016/j.ultsonch.2020.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.J. M. Dai, M. Bai, C. Z. Li, H. Y. Cui, L. Lin, Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry, Trends Food Sci. Technol, 2020 105 (4) (2020) 211-222, 10.1016/j.tifs.2020.09.016.
- 37.Yoon J.H., Jeong D.Y., Lee S.B., Choi S.Y., Jeong M.I., Lee S.Y., Kim S.R. Decontamination of Listeria monocytogenes in king oyster mushrooms (Pleurotus eryngii) by combined treatments with organic acids, nisin, and ultrasound. LWT - Food Sci. Technol. 2021;144 doi: 10.1016/j.lwt.2021.111207. [DOI] [Google Scholar]
- 38.He Q., Guo M.M., Jin T., Arabi A. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 2021;337 doi: 10.1016/j.ijfoodmicro.2020.108936. [DOI] [PubMed] [Google Scholar]