Abstract
Exercise can reduce the incidence of stress-related mental diseases, such as depression and anxiety. Control group was neither exposed to CVMS nor TRE (noCVMS/noTRE). Females were tested and levels of serum17-beta-oestradiol (E2), estrogen receptors α immunoreactive neurons (ERα-IRs), estrogen receptors β immunoreactive neurons (ERβ-IRs) and oxytocin immunoreactive neurons (OT-IRs) were measured. The results showed there’s increased anxiety-like behaviors for mice from CVMS/noTRE, CVMS/higher speed TRE (CVMS/HTRE) and noCVMS/HTRE groups when they were put in open field and elevated maze tests. They had lower serum E2 levels than mice from CVMS/low-moderate speed TRE (CVMS/LMTRE), noCVMS/LMTRE and noCVMS/noTRE groups. The three groups of CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE mice had more ERα-IRs and less ERβ-IRs in the medial preoptic area (mPOA), bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA), hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON). The number of OT-IRs in PVN and SON of CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE mice was also lower than that of mice from CVMS/LMTRE, noCVMS/LMTRE and noCVMS/noTRE groups. Interestingly, CVMS/LMTRE and noCVMS/LMTRE mice were similar to noCVMS/noTRE mice in that they did not show anxiety, while CVMS/HTRE and noCVMS/HTRE mice did not, which were similar to the mice in CVMS/noTRE. We propose that LMTRE instead of HTRE changes the serum concentration of E2. ERβ/ERα ratio and OT level in the brain may be responsible for the decrease in anxiety-like behavior in female mice exposed to anxiety-inducing stress conditions.
Abbreviations: ERα-IRs, estrogen receptors αimmunoreactive neurons; ERβ-IRs, estrogen receptor β immunoreactive neurons; OT-IRs, Oxytocin immunoreactive neurons; BNST, bed nucleus of the stria terminalis; ELISA, enzyme-linked immunosorbent assay; EPM, elevated plusmazetest; HPA, hypothalamic–pituitary–adrenal; HRP, horseradishperoxidase; MeA, medial amygdaloid nucleus; mPOA, medial preopticarea; OF, open field test; CVMS, chronic variable moderate stress; TRE, treadmill exercise; HTRE, higher speed TRE; LMTRE, low-moderate speed TRE; E2, 17-beta-oestradiol; PBS, phosphatebufferedsolution; PVN, paraventricular nucleus; SON, supraoptic nucleus
Keywords: Estrogen receptor α (ERα), Estrogen receptor β (ERβ), Oxytocin (OT), Chronic variable moderate stress (CVMS), Treadmill exercise (TRE)
Highlights
-
•
CVMS/LMTRE did not show anxiety.
-
•
noCVMS/LMTRE did not show anxiety.
-
•
ERβ/ERα ratio decreas anxiety.
-
•
OT decreas anxiety.
Introduction
Exercise can reduce symptoms of depression and anxiety in humans the occurrence of stress-related mood disorders, such as depression and anxiety (Lloyd et al., 2017). Studies haves showed that the relationship between physical activity intensity and mood effect is U-shaped, which means neither low-intensity nor high-intensity physical activity can get the emotional benefits of exercise, and only moderate-intensity physical activity can get the best emotional benefits. However, some studies have found that low-intensity physical activity also plays an important role in reducing symptoms of mood disorders (Conradsson et al., 2010, Catellier and Yang, 2013, Dunn et al., 2005). At present, there is not enough evidence to indicate the upper and lower limits of the intensity of physical activity as a moderator of anxiety, and there is little evidence of the interaction between activity types, exercise intensity and training frequency (Jiang and Chen, 2014). Therefore, the relationship between exercise intensity and emotional benefit needs to be further -studied.
Estrogens have profound effects on the expression of anxiety in humans and rodents. However, the directionality of these effects varies considerably within both clinical and preclinical literature, estrogen has both anxiogenic and anxiolytic effects (Kalandakanond-Thongsong et al., 2012). Discrepancies of estrogens’ effects on anxiety are attributable to differential effects of specific estrogen receptor (ER) subtypes-alpha (ERα) and beta (ERβ) estrogen receptors (Fedotova, 2013). Anxiogenic effects are mediated by activation of ERα; ERβ is predominantly involved in anxiolytic effects (Sharma and Thakur, 2015). For example, ERα activation increases fear and anxiety (Toufexis et al., 2007), and ERβ activation decreases anxiety like behavior in mice and rats (Kudwa et al., 2014). Potential brain regions that mediate mood include the medial preoptic area (mPOA), the bed nucleus of stria terminalis (BNST) and the medial nucleus of the amygdale (MeA) (Furuta et al., 2013, McHenry et al., 2015). These three brain regions participate in anxiety-related behavior and constitute a distributed network of interconnected structures controlling anxiety in rodents and humans (Lee et al., 2007, Adhikari, 2014, He, 2014).
In central nervous system, neuropeptide oxytocin (OT) is mainly expressed in magnocellular neurons of hypothalamus paraventricular nucleus (PVN) and supraoptic nucleus (SON). Release of OT via the neurohypophysis into bloodstream and by extrahypothalamic fibres projecting into the brain exerts a wide spectrum of central and peripheral effects (Le Mevel et al., 1993). OT is an important mediator of anxiety symptoms in both humans and rodents. Plasma OT levels are negatively correlated with anxiety symptoms in depressed patients (Scantamburlo et al., 2007), and intranasal OT administration attenuates emotional fear reactivity in patients with social anxiety disorder (Labuschagne et al., 2010). Estradiol has been found to increase levels of plasma OT (Amico et al., 1984), presenting an additional mechanism by which estrogens are capable of mediating anxiety (Borrow and Handa, 2017).
We know that E2 in serum, expression of ERα, ERβ and OT in brain regions are associated with anxiety, but is the reduction of anxiety through exercise is moderated by E2 in serum, expression of ERα, ERβ and OT in brain region? Exercise as a valid treatment for anxiety and depression, did not change the estrogen levels in serum of male and ovariectomized (OVX) rats (Selakovic et al., 2019, Rauf et al., 2015). It is unclear that whether decreased normal female anxiety through exercise is related to serum estrogen. Four weeks of swimming combined with intraperitoneal injection of ERα and/or ERβ agonists can reduce anxiety like behaviors in OVX rats (Bulut et al., 2016). However, whether the change of anxiety like behaviors is related to ERα and/or ERβ or not remains to be further studied. Some studies showed that exercise significantly increased OT mRNA or OT receptor expression (Higa-Taniguchi et al., 2009, Michelini, 2007), but whether expression of OT is related to anxiolytic effect still needs to be further studied.
This study aimed to investigate anxiety-like behaviors of adult female c57BL/6 J mice exposed to chronic unpredictable mild stress (CVMS), and the effects of four weeks treadmill exercise (TRE) on anxiety-like behaviors and estrogen receptors (ERs) and OT in mice. We hypothesized that the interaction of E2, ERα, ERβ, OT and treadmill exercise has a certain effect on anxiety-like behaviors that are caused by the CVMS.
Experimental procedures
Animals
60 healthy adult female C57BL/6 J mice (purchased from Xi'an Jiao tong University Medical College, 3 months old, 25–30 g). Mice were individually housed in a clear plastic cages and kept in a photoperiod of 14: 10 h and at 24–26 °C, had free access to food and water. All study protocols were approved by the Institutional Animal Care and Use Committee, Xi’an University. Estrous cycle of female mice was monitored by daily examining vaginal smears. The materials were collected at the same time every day for 10 days. Approximately 10 μl 0.9% saline was gently flushed into the vagina with the tip of a plastic pipette three times, and the final flushing fluid was placed on a glass slide and observed under 10 × objective of optical microscope. The determination of the estrous cycle phase was based on the proportion among these cell types: predominance of leukocytes (diestrous), predominance of nucleated epithelial cells (proestrous), predominance of cornified epithelial cells (estrous), and a mix of cell types with a predominance of leukocytes and a few nucleated epithelial and/or cornified squamous epithelial cells (Metestrus) (Sharma et al., 2015). The estrous cycle phase was checked to ensure that all behavior experiments were conduct for those animals in diestrus after the chronic unpredictable mild stress (CVMS).
Experiment design
Mice subjected to CVMS procedure (further described in details) were called stressed mice (CVMS). Unstressed mice were not subjected to CVMS (noCVMS). At the beginning of the experiments, mice were randomly divided into two different groups (30 mice in each group), namely group I consisted of unstressed control (noCVMS group); group II comprised stressed mice (CVMS group). noCVMS group (n = 30) was further divided into three groups: noCVMS without treadmill exercise (noCVMS/noTRE, n = 10, further described to TRE in TRE Procedure), noCVMS with from low to medium speed treadmill exercise (noCVMS/LMTRE, n = 10, further described to LMTRE in details) and noCVMS with high speed treadmill exercise (noCVMS/HTRE, n = 10, further described to HTRE in TRE Procedure). The CVMS group (n = 30) was also further divided into three groups: CVMS without treadmill exercise (CVMS/noTRE, n = 10), CVMS with from low to medium speed treadmill exercise (CVMS/LMTRE, n = 10) and CVMS with high speed treadmill exercise (CVMS/HTRE, n = 10). These six groups were individually housed in identical cages.
CVMS procedure
The CVMS protocol was performed as described with minor modifications (Biala et al., 2017). Mice were subjected to different kinds of mild stressors, which varied from day to day to make the stress procedure unpredictable. These stressors were randomly scheduled over a one–week period and repeated for 4 weeks. Specifically, the stressors were as follows: (1) restricted activities for 2 h (placing mice in a closed narrow transparent plastic pipe); (2) cage vibration for 2 h (high-speed cylindrical roller bearings); (3) light on overnight; (4) moist sawdust overnight; (5) food deprivation overday and overnight; (6) 90 dB electric buzzer for 5 mins; (7) inclinated cage at 45 °C for 2 h. Unstressed mice including noCVMS/noTRE, noCVMS/LMTRE and noCVMS/HTRE groups were left undisturbed in their home cage.
TRE procedure
Twenty-four hours after the end of the CVMS protocol, the mice of exercise groups including the noCVMS/LMTRE, CVMS/LMTRE, noCVMS/HTRE and CVMS/HTRE were exposed to treadmill exercise. The TRE protocol was performed as described with some modifications (Rauf et al., 2015). The protocol consisted of two periods, adaptation period and exercise period. Mice from noCVMS/LMTRE, CVMS/LMTRE, noCVMS/HTRE and CVMS/HTRE groups were adapted to the exercise protocol and treadmill apparatus (Huaibei Zhenghua Biological Instrument Equipment Co., Ltd., China) in a training room for 2 weeks. During the adaptation period, running speed, treadmill slope, and duration of exercise were increased gradually. Speed was increased from 5 m/min for 10 mins, 8 m/min for 10 mins up 18 m/min for 20 mins; slope was increased from 0° up to 5°. Mice were later carried out with formal exercise for 4 weeks, and were conducted the treadmill exercise at a slope of 5° for a total duration of 20 min after 14:00 per day: both the noCVMS/LMTRE and CVMS/LMTRE groups carried out treadmill exercise from slow to medium speed (low-medium intensity): 5 m/ min for 5 mins, 8 m/min for 5 mins and 12 m/min for 10 mins; both the noCVMS/HTRE and CVMS/HTRE groups carried out higher speed (higher intensity) treadmill exercise: 18 m / min for 20 mins. The exercises were performed five times per week (Mondays, Tuesdays, Wednesdays, Fridays, and Saturdays) with two rest days (Thursdays and Sundays). The treadmill used in the experiments have electrical shock system to keep animals running. noCVMS/noTRE and CVMS/noTRE moved to the training room at the same time only when these groups performed exercise(Fig. 1).
Fig. 1.
Behavioral testing schedule for experimental mice subjected to unstressed (noCVMS, n = 30) or stressed treatment group (CVMS, n = 30); noCVMS group was further divided into noCVMS/noTRE (n = 10), noCVMS/LMTRE (n = 10), noCVMS/HTRE group (n = 10), and CVMS group was further divided into CVMS/noTRE (n = 10), CVMS/LMTRE (n = 10) and CVMS/HTRE group (n = 10).
Open field test (OF)
Twenty-four hours after the end of the TRE protocol, anxiety-like behaviors were examed in an open field test (OF, 50 × 50 ×50 cm) illuminated by six 60 W lamps mounted 2 m above the apparatus (Ernsberger et al., 1983, Cullen et al., 2013). The OF was divided into 25 squares (nine central and 16 peripherals). Female mice were placed individually in the centre of the OF for 5 mins. The time spent in the central and peripheral zones, total distance covered during the experiment and the number of crossings between squares was automatically recorded by a TSE digital system. Anxiety related indicators, including frequency and time of entry into the central area, total distance and number of crossings, were examed by PC-compatible software (TSE, VideoMot 2, Germany). The apparatus was rinsed with 70% ethanol after each test. The interval between each experiment was 30 min
Elevated plus maze test (EPM)
On the day after all OF experiments were completed, elevated plus maze tests (EPM) was carried out. Open arm and closed arm of EPM apparatus were placed 55 cm above the floor with movable pulley in the bottom. Female mice were moved out from the cage and placed at the connection of the open arm and closed arm, and their activities in the EPM were recorded by an overhead video camera for 5 min and coded later by a blind observer. Time and frequency of activities in the open and closed arms and total distance were analyzed as indicators of anxiety-related behaviors. Mice were returned to their cage when the experiment was completed. The apparatus was rinsed with 70% ethanol after each animal, and the interval between each vole was 30 min (Rodgers and Dalvi, 1997, He et al., 2018).
Enzyme-linked immunosorbent assay (ELISA)
All samples (n = 60) were used for ELISA in diestrus. Animals were euthanized terminally after behavioral tests, Mice were deeply anesthetized and perfused with 0.1 M phosphate-buffered solution (PBS, pH 7.4) and 4% paraformaldehyde in 0.1 M PBS. Blood samples were collected from the retro-orbital sinus of anesthetized female mice (using pentobarbital sodium dose of 4 mg/kg of body weight) between 8:00 and 10:00 h two days after behavior tests to avoid any acute effects of these tests, enzyme-linked immunosorbent assay (ELISA) was performed as described (He et al., 2018). Thyrotropin-releasing hormone (TRH) was found to antagonize pentobarbital-induced sleeping time and hypothermia (Breese et al., 1975). However, no effect on estrogen in serum has been found, so E2 in serum was measured using a species-specific ELISA (CEA461Ge, Cloud-Clone, USA), the quantitative range of E2 ELISA kit was 3.75–120pmol/L. The optical density was measured at 450 nm using a microplate reader (Bio-Tek, Winooski, USA), and the blank was set as zero. Variation between duplicate values was less than 5%. All mice were anesthetized and their brains quickly removed and bisected.
Immunohistochemistry test
All samples were used for immunohistochemistry assays in diestrus. Brains of all animals were obtained at the same time as blood. Brains were collected within 3 min and placed in 4% paraformaldehyde overnight. After dissection, brains were immersed in 30% sucrose until saturated. Coronal sections (40 µm) were cut on a cryostat, and consecutive sections were collected in three vials containing 0.01 M PBS for three different immunohistochemical stainings. ERα (MC-20: sc-542, Santa Cruz, CA, USA) was an affinity purified rabbit polyclonal antibody raised against a peptide mapping at the C-terminus of ERα of mouse origin. Antibody specificity tests were performed using as control antibody for ERα siRNA (h): sc-29305 and ERα siRNA (m): sc-29306. ERβ (H-150: sc-8974, Santa Cruz, CA, USA) was a rabbit polyclonal antibody raised against amino acids 1–150 of ERβ human origin, antibody specificity tests were performed using as control antibody for ERβ siRNA (h) sc-35325, ERβ siRNA (m): sc-35326, ERβ siRNA (r): sc-77356. OT (AB911; Upstate Biotechnology, Lake Placid, NY, USA, 0.4 mg/ml) was an unpurified rabbit polyclonal antibody, antibody specificity tests were performed by cross-reactivity with arginine vasopressin to be less than 1%. Floating sections were processed using primary antibody and streptavidin and peroxidase methods (Paxinos and Franklin, 1997). The sections were incubated for 7 min with 3% H2O2 and then washed twice for 10 mins with distilled water. The tissue was shrunk in 0.01 M PBS. Sections were blocked for 1.5 h with normal goat serum (SP-0023) and incubated at 4 °C overnight with the primary antibody (both of ERα and ERβ antibody 1: 100; OT antibody 1: 5000,) diluted by antibody dilution buffer (0.01 M PBS containing 20% bovine serum albumin and 1.7% Triton X-100). The next day, sections were washed four times for 5 min each with 0.01 M PBS and incubated for 1 h in a 37 °C water bath with biotinylated goat anti-rabbit secondary antibody (SP-0023, Boster Company, ready to use – no dilution required), followed by another round of four washes with 0.01 M PBS (5 min each). After 60 min of incubation with S-A/HRP (Boster Company, ready to use – no dilution required) and four washes for 10 mins each with 0.01 M PBS, sections were stained with 3,30-diaminobenzidinetetrahydrochloride (DAB) to visualize immunoreactivity. The reaction product appears as a brownish yellow punctate, nuclear stain. Because ERα, ERβ and OT are included in nuclei, stained nuclei were counted using an Olympus microscope (Tokyo, Japan).
Slides were randomized and coded prior to quantitative analysis to ensure that counters were blind to experimental treatment. The number of immunoreactive cells was randomly quantified by eye per standard area (200 × 200 µm2) using grid sampling. The number of ERα-IRs and ERβ-IRs in the BNST, mPOA, MeA, PVN and SON, and OT-IRs in the PVN and SON in 200 × 200 µm2 was counted. These brain regions were selected because the expressions of ERα and ERβ are found in abundance in the BNST, mPOA, MeA, PVN and SON (Krezel et al., 2001, He et al., 2012). OT is released from the PVN and SON via neurohypophysis into the bloodstream or by extrahypothalamic fibres projecting into the brain including the BNST, mPOA and MeA (Dong et al., 2001) and exerts a wide range of central and peripheral effects (Le Mevel et al., 1993). For example, OT is found in abundance in the PVN and SON, areas involved in anxiety (Hellemans et al., 2010, He, 2014). Various brain regions were determined based on Nissl stained brain sections from mice and a stereotaxic atlas of the mice brain (Paxinos and Franklin, 1997).
For each brain nucleus, three representative sections from anterior to posterior and anatomically matched between subjects were chosen and counted to minimize variability. Individual mean values for each animal were obtained by counting positive neurons (brownish yellow punctates, nuclear stain) bilaterally in three sections from each nucleus. Counts were performed separately for each hemisphere, and results were averaged between hemispheres. Sections were chosen by correspondence to the reference atlas plate instead of the level or intensity of ERα-IRs, ERβ-IRs or OT-IRs labelling. All immunohistochemistry procedures included negative controls (primary antibody was not added). An observer blind to experimental conditions performed the entire analysis. Chosen sections were photographed with a Nikon camera (Tokyo, Japan) attached to a Nikon microscope (He et al., 2018).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software (San Diego, CA, USA). All results are expressed as the means ± standard error (SE). We used two-way ANOVA plus Tukey test. Probability values of P < 0.05 were considered statistically significant among various groups.
Results
Behavioral testing 1: OF
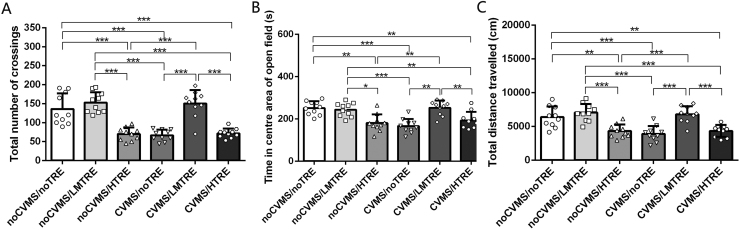
In the OF, the two-way ANOVA showed that there’s a significant effect on chronic variable moderate stress (CVMS) in the time which the mice spent when engaging in the center of the chamber (F(1, 59) = 25.603, P < 0.001), number of crossings (F(1, 59)= 28.566, P < 0.001) and total distance (F(1, 59) = 15.370, P < 0.001). However, treadmill exercise (TRE) did not changed significantly for time spent in the central area (F(1, 59) = 2.820, P = 0.071), number of crossings (F(1, 59) = 2.221, P = 0.082) and total distance (F(1, 59) = 1.375, P = 0.101). The interaction between both factors was significant for time spent in the central area (F(5, 55) = 7.171, P < 0.001), number of crossings (F(5, 55) = 9.303, P < 0.001) and total distance (F(5, 55) = 4.899, P = 0.002).
According to Tukey post hoc tests, CVMS/noTRE, CUMS/HTRE and noCVMS/HTRE spent less time in the central area, made fewer crossings and covered less distance in the OF test compared with all other three groups. There were no significant differences among noCVMS/noTRE, noCVMS/LMTRE and CVMS/LMTRE groups for any measure of OF performance (Table 1, Fig. 2).
Table 1.
Behavior testing analysis in open field by Tukey post hoc (n = 10).
| Behavior ariable |
|||
|---|---|---|---|
| Groups | Time spent in the central area | Number of crossings | Total distance |
| noCVMS/noTRE | 260.4 ± 26.7 | 135.9 ± 40.9 | 6378.8 ± 1558.7 |
| noCVMS/LMTRE | 249.5 ± 30.6 | 152.8 ± 27.4 | 7034.7 ± 1291.2 |
| noCVMS/HTRE | 180.2 ± 20.2 * *# | 69.6 ± 16.5 * **### | 4304.6 ± 972.7 * *### |
| CVMS/noTRE | 170.0 ± 18.4 * **### | 66.7 ± 14.9 * **### | 3891.5 ± 1152.5 * **### |
| CVMS/LMTRE | 253.5 ± 31.9^^&& | 149.9 ± 35.3^^^&&& | 6767.6 ± 1231.3^^&&& |
| CVMS/HTRE | 190.4 ± 35.5 * *##$$ | 71.7 ± 12.4 * **###$$$ | 4332.1 ± 872.2 * *##$$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * *: P < 0.01, * ** : P < 0.001; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, #: P < 0.05, ##: P < 0.01, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^^: P < 0.01, ^^^: P < 0.001; Comparison between CVMS/LMTRE and CVMS/noTRE, &&: P < 0.01, &&&: P < 0.001; Comparison between CVMS/HTRE and CVMS/LMTRE, $$: P < 0.01, $$$: P < 0.001.
Fig. 2.
Behavior in the open field test (n = 60). (A) Bar + scatter graph with mean ± SEM, the mice spent time in the center of the chamber. (B) Bar + scatter graph with mean ± SEM, mice crossed total number in open field. (C) Bar + scatter graph with mean ± SEM, the mice travelled total distance. Two-way ANOVA plus Tukey test was used. P > 0.05). * : P < 0.05, * *: P < 0.01, * ** : P < 0.001.
Behavioral testing 2: EPM
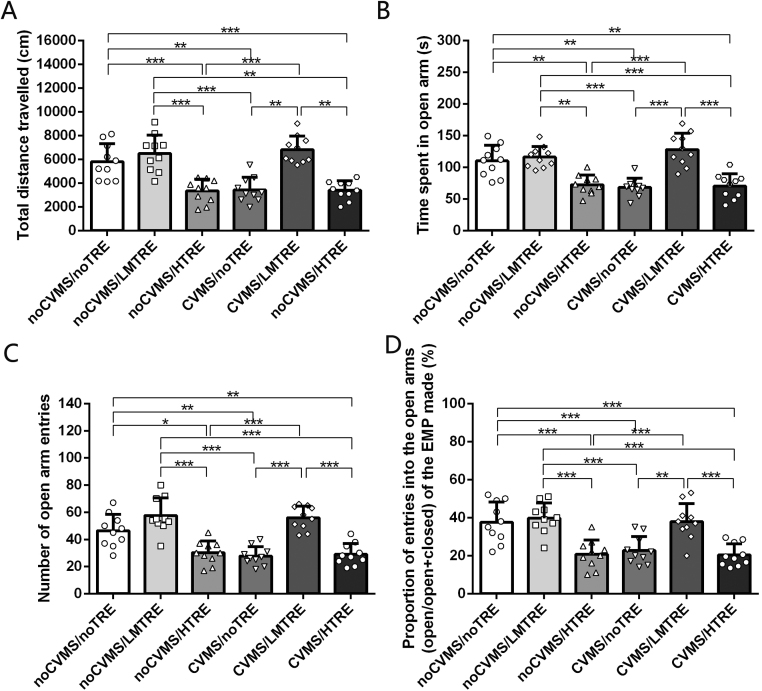
In EPM, CVMS had significant effect on total distance (F(1, 59) = 22.761, P < 0.004), time spent in the open arm (F(1, 59) = 26.632, P < 0.001), number of entries to the open arm (F(1, 59) = 26.227, P < 0.001), and proportion of number of open arms (%) (F(1, 59) = 16.607, P < 0.001). TRE did not significantly change total distance (F(1, 59) = 2.920, P = 0.061), time spent in the open arm (F(1, 59) = 2.622, P = 0.075), number of entries to the open arm (F(1, 59) = 2.775, P = 0.072), and proportion of number of open arms (%) (F(1, 59) = 1.820, P = 0.097). The interaction between CVMS and TRE was significant for total distance (F(5, 55) = 6.318, P < 0.001), time spent in the open arm (F(5, 55) = 9.228, P < 0.001), number of entries to the open arm (F(5, 55) = 7.916, P < 0.001), and proportion of number of open arms (%) (F(5, 55) = 5.245, P = 0.001).
According to Tukey post hoc tests, the CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE group had a smaller total distance, spent less time in the open arm, made fewer entries to the open arm and made fewer entries to open arms as a proportion of entries to all arms, compared with all other groups. There were no significant differences between the noCVMS/noTRE, noCVMS/LMTRE and CVMS/LMTRE groups for any measure of EPM performance (Table 2, Fig. 3).
Table 2.
Behavior testing analysis in elevated plus maze test by Tukey post hoc (n = 10).
| Behavior ariable |
||||
|---|---|---|---|---|
| Groups | Total distance | Time spent in the open arms | Number of entries to the open arm | Proportion of number of open arms (%)@ |
| noCVMS/noTRE | 5811.1 ± 1514.3 | 110.2 ± 24.7 | 46.4 ± 8.8 | 37.6 ± 10.6 |
| noCVMS/LMTRE | 6496.9 ± 1546.8 | 116.0 ± 16.5 | 57.6 ± 13.0 | 39.6 ± 8.0 |
| noCVMS/HTRE | 3339.7 ± 986.0 * **### | 72.4 ± 15.2 * *## | 30.5 ± 8.4 *### | 20.7 ± 6.5 * **### |
| CVMS/noTRE | 3426.7 ± 1055.8 * *### | 68.2 ± 14.5 * *### | 27.8 ± 6.9 * *### | 22.7 ± 7.4 * **### |
| CVMS/LMTRE | 6806.6 ± 1145.8^^^&& | 127.8 ± 20.6^^^&&& | 56.0 ± 8.8^^^&&& | 37.8 ± 6.8^^^&& |
| CVMS/HTRE | 3369.6 ± 809.1 * **##$$ | 70.4 ± 12.6 * *###$$$ | 29.0 ± 8.0 * *###$$$$ | 20.4 ± 5.9 * **###$$$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * *: P < 0.01, * ** : P < 0.001; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, ##: P < 0.01, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^^^: P < 0.001; Comparison between CVMS/LMTRE and CVMS/noTRE, &&: P < 0.01, &&&: P < 0.001; Comparison between CVMS/HTRE and CVMS/LMTRE, $$: P < 0.01, $$$: P < 0.001.
@ : Formula = the % of open/(open+closed) arm frequency.
Fig. 3.
Behavior in the elevated plus-maze test (n = 60). (A) Bar + scatter graph with mean ± SEM, the mice travelled total distance. (B) Bar + scatter graph with mean ± SEM, the mice spent time in open arm. (C) Bar + scatter graph with mean ± SEM, the mice crossed number of open arm entries. (D) Bar + scatter graph with mean ± SEM, the mice had a percentage for open/(open + closed) arm frequency. P > 0.05). Two-way ANOVA plus Tukey test was used. * : P < 0.05, * *: P < 0.01, * ** : P < 0.001.
E2 in serum
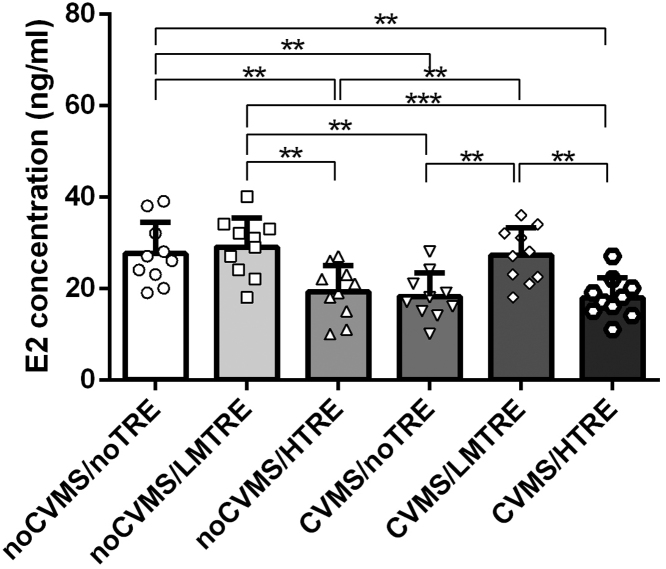
Two-way ANOVA revealed a significant effect of CVMS for E2 levels (F(1, 59) = 11.615, P < 0.001). However, TRE did not change significantly for E2 levels (F(1, 59) = 2.120, P = 0.088). The interaction between both factors was significant for E2 levels (F(5, 55) = 4.318, P = 0.008).
According to Tukey post hoc tests, the serum E2 levels of the CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE group were lower than all other groups. There were no significant differences between the noCVMS/ noTRE, noCVMS/LMTRE and CVMS/LMTRE groups for E2 levels (Table 3, Fig. 4).
Table 3.
E2 level in serum analysis by Tukey post hoc (n = 10).
| Groups | E2 level in serum |
|---|---|
| noCVMS/noTRE | 27.6 ± 6.3 |
| noCVMS/LMTRE | 29.0 ± 5.4 |
| noCVMS/HTRE | 19.2 ± 3.2 * *## |
| CVMS/noTRE | 18.2 ± 3.5 * *## |
| CVMS/LMTRE | 27.3 ± 6.0^^&& |
| CVMS/HTRE | 17.9 ± 4.5 * *###$$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * *: P < 0.01; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, ##: P < 0.01, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^^: P < 0.01; Comparison between CVMS/LMTRE and CVMS/noTRE, &&: P < 0.01; Comparison between CVMS/HTRE and CVMS/LMTRE, $$: P < 0.01.
Fig. 4.
Effects of chronic unpredictable mild stress and treadmill exercise on oestradiol levels in plasma in female mice (n = 60). A Bar + scatter graph with mean ± SEM, P > 0.05). Two-way ANOVA plus Tukey test was used. * *: P < 0.01, * ** : P < 0.001.
Immunohistochemistry
CVMS had an effect on the number of ERα-IRs in the BNST (F(1, 59) = 14.276, P < 0.001), mPOA (F(1, 59) = 38.582, P < 0.001), MeA (F(1, 59) = 35.835, P < 0.001), PVN (F(1, 59) = 30.04, P < 0.001) and SON (F(1, 59) = 10.207, P < 0.001). TRE did not have such an effect in the BNST (F(1, 59) = 1.976, P = 0.095), mPOA (F(1, 59) = 1.776, P = 0.099), MeA (F(1, 59) = 2.423, P = 0.078), PVN (F(1, 59) = 2.521, P = 0.077) and SON (F(1, 59) = 2.620, P = 0.076). The interaction both factors was significant for the number of ERα-IRs in the BNST (F(5, 55) = 4.287, P < 0.001), mPOA (F(5, 55) = 12.257, P < 0.001), MeA (F(5, 55) = 11.258, P < 0.001), PVN (F(5, 55) = 10.457, P < 0.001), and SON (F(5, 55) = 5.757, P < 0.001). Tukey Post hoc tests showed that the number of ERα-IRs in the BNST, mPOA, MeA, PVN and SON in the CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE group was greater compared with all other groups. However, there were no significant differences in the number of ERα-IRs in the MeA, mPOA, BNST, PVN and SON in the noCVMS/noTRE compared with the noCVMS/LMTRE and CVMS/LMTRE groups for all five brain regions (Table 4, Fig. 5).
Table 4.
ERα-IRs in five brain regions analysis in by Tukey post hoc (n = 10).
| Brain regions |
|||||
|---|---|---|---|---|---|
| Groups | BNST | mPOA | MeA | PVN | SON |
| noCVMS/noTRE | 20.8 ± 3.6 | 28.2 ± 3.3 | 19.8 ± 2.9 | 19.1 ± 3.1 | 16.8 ± 2.9 |
| noCVMS/LMTRE | 18.5 ± 4.1 | 26.5 ± 2.9 | 17.8 ± 2.9 | 18.1 ± 3.1 | 17.7 ± 2.9 |
| noCVMS/HTRE | 30.3 ± 4.6 * **### | 38.2 ± 4.3 * **### | 30.8 ± 3.5 * **### | 28.3 ± 2.8 * **### | 22.7 ± 3.0 * *## |
| CVMS/noTRE | 28.6 ± 5.4 * *### | 40.1 ± 4.5 * **### | 31.6 ± 5.2 * **### | 29.2 ± 3.6 * **### | 23.8 ± 2.9 * **### |
| CVMS/LMTRE | 20.6 ± 5.5^^^&& | 27.1 ± 3.7^^^&&& | 20.8 ± 2.9^^^&&& | 19.0 ± 3.1^^^&&& | 18.8 ± 2.9^&& |
| CVMS/HTRE | 28.1 ± 3.7 * *###$$ | 36.2 ± 3.3 * **###$$$ | 30.4 ± 2.9 * **###$$$ | 27.1 ± 3.1 * **###$$$ | 23.3 ± 3.0 * **##$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * *: P < 0.01, * ** : P < 0.001; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, ##: P < 0.01, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^: P < 0.05, ^^^: P < 0.001; Comparison between CVMS/LMTRE and CVMS/noTRE, &&: P < 0.01, &&&: P < 0.001; Comparison between CVMS/HTRE and CVMS/LMTRE, $: P < 0.05, $$: P < 0.01, $$$: P < 0.001.
Fig. 5.
Mean ( ± SEM) expression of ERα-IRs after chronic unpredictable mild stress and treadmill exercise (n = 60). (A) Scatter graph with mean ± SEM, the number of ERα-IRs was expressed in the MeA, mPOA, BNST, PVN and SON in the mice. Two-way ANOVA plus Tukey test was used. * *: P < 0.05, * *: P < 0.01, * ** : P < 0.001. (B) Position of ERα-IRs expressed in the MeA, mPOA, BNST, PVN and SON of the mice. ERα-IRs in 200 × 200 µm2 counted. Bar = 200 µm.
CVMS had an effect on the number of ERβ-IRs in the BNST (F(1, 59) = 26.123, P < 0.001), mPOA (F(1, 59) = 46.793, P < 0.001), MeA (F(1, 59) = 29.397, P < 0.001), PVN (F(1, 59) = 14.104, P < 0.001) and SON (F(1, 59) = 5.939, P < 0.001). TRE did not have such an effect in BNST (F(1, 59) = 1.977, P = 0.095), mPOA (F(1, 59) = 2.420, P = 0.079), MeA (F(1, 59) = 2.520, P = 0.077), PVN (F(1, 59) = 2.820, P = 0.071) and SON (F(1, 59) = 2.885, P = 0.063). The interaction between CVMS and TRE was significant for the number of ERβ-IRs in the BNST (F(5, 55) = 7.255, P < 0.001), mPOA (F(5, 55) = 14.267, P < 0.001), MeA (F(5, 55) = 10.248, P < 0.001), PVN (F(5, 55) = 4.656, P = 0.003), and SON (F(5, 55) = 3.857, P = 0.039). Tukey post hoc test showed that the number of ERβ-IRs in the BNST, mPOA, MeA, PVN and SON in the CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE animals was reduced compared to all other groups. However, there were no significant differences in the number of ERβ-IRs in the MeA, mPOA, BNST, PVN and SON in the noCVMS/noTRE compared with the noCVMS/LMTRE and CVMS/LMTRE groups for all five brain regions (Table 5, Fig. 6).
Table 5.
ERβ-IRs in five brain regions analysis in by Tukey post hoc (n = 10).
| Brain regions |
|||||
|---|---|---|---|---|---|
| Groups | BNST | mPOA | MeA | PVN | SON |
| noCVMS/noTRE | 40.6 ± 3.8 | 48.5 ± 3.6 | 49.5 ± 3.9 | 20.1 ± 3.8 | 12.6 ± 3.9 |
| noCVMS/LMTRE | 41.6 ± 4.1 | 50.5 ± 5.9 | 51.5 ± 3.6 | 21.1 ± 3.8 | 13.4 ± 4.0 |
| noCVMS/HTRE | 30.6 ± 3.8 * **### | 33.8 ± 5.0 * **### | 31.5 ± 3.6 * **### | 13 ± 3.6 * *### | 6.6 ± 3.0 * *## |
| CVMS/noTRE | 31.6 ± 3.8 * **### | 31.1 ± 4.5 * **### | 30.6 ± 3.2 * **### | 12.2 ± 3.6 * **### | 7.4 ± 3.6 *## |
| CVMS/LMTRE | 42.7 ± 5.5^^^&&& | 49.5 ± 3.6^^^&&& | 50.8 ± 5.9^^^&&& | 19.0 ± 3.8^^&& | 12.0 ± 3.4^& |
| CVMS/HTRE | 32.1 ± 3.5 * **###$$$ | 32.8 ± 5.0 * **###$$$ | 29.4 ± 3.5 * **###$$$ | 11.1 ± 3.7 * **###$$$ | 7.1 ± 3.2 *##$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * : P < 0.05, * *: P < 0.01, * ** : P < 0.001; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, ##: P < 0.01, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^: P < 0.05, ^^: P < 0.01, ^^^: P < 0.001; Comparison between CVMS/LMTRE and CVMS/noTRE, &: P < 0.05, &&: P < 0.01, &&&: P < 0.001; Comparison between CVMS/HTRE and CVMS/LMTRE, $: P < 0.05, $$$: P < 0.001.
Fig. 6.
Mean ( ± SEM) expression of ERβ-IRs after chronic unpredictable mild stress and treadmill exercise (n = 60). (A) Scatter graph with mean ± SEM, the number of ERβ-IRs was expressed in the MeA, mPOA, BNST, PVN and SON in the mice. Two-way ANOVA plus Tukey test was used. * *: P < 0.05, * *: P < 0.01, * ** : P < 0.001. (B) Position of ERβ-IRs expressed in the MeA, mPOA, BNST, PVN and SON of the mice. ERβ-IRs in 200 × 200 µm2 counted. Bar = 200 µm.
We did not find OT-IRs in the MeA, mPOA, BNST. Two-way ANOVA revealed a significant effect of CVMS for OT-IRs in the PVN (F(1, 59) = 15.097, P < 0.001) and SON (F(1, 59) = 26.571, P < 0.001). However, TRE did not change significantly for OT-IRs in the PVN (F(1, 59) = 2.421, P = 0.078) and SON (F(1, 59) = 1.970, P = 0.096). The interaction between CVMS and LMTRE was significant for OT-IRs in the PVN (F(5, 55) = 5.257, P < 0.001) and SON (F(5, 55) = 9.182, P < 0.001). Tukey post hoc test showed that the number of OT-IRs in the PVN and SON in the CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE group was reduced compared to all other groups. There were no significant differences in the number of OT-IRs in the PVN and SON in the noCVMS/noTRE compared with the noCVMS/LMTRE and CVMS/LMTRE groups in these two brain regions (Table 6, Fig. 7).
Table 6.
OT-IRs in two brain regions analysis in by Tukey post hoc (n = 10).
| Brain regions |
||
|---|---|---|
| Groups | PVN | SON |
| noCVMS/noTRE | 19.3 ± 2.9 | 16.4 ± 3.5 |
| noCVMS/LMTRE | 22.7 ± 3.4 | 17.5 ± 3.6 |
| noCVMS/HTRE | 14.7 ± 3.5 *### | 6.4 ± 2.0 * **### |
| CVMS/noTRE | 13.6 ± 3.4 * *### | 8.5 ± 2.5 * **### |
| CVMS/LMTRE | 21.7 ± 3.5^^^&&& | 16.3 ± 2.6^^^&&& |
| CVMS/HTRE | 14.9 ± 3.2 * *###$$$ | 7.5 ± 2.1 * **###$$$ |
Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/noTRE, * : P < 0.05, * *: P < 0.01, * ** : P < 0.001; Comparison between noCVMS/HTRE, CVMS/noTRE, CVMS/HTRE and noCVMS/LMTRE, ###: P < 0.001; Comparison between CVMS/LMTRE and noCVMS/HTRE, ^^^: P < 0.001; Comparison between CVMS/LMTRE and CVMS/noTRE, &&&: P < 0.001; Comparison between CVMS/HTRE and CVMS/LMTRE, $$$: P < 0.001.
Fig. 7.
Mean ( ± SEM) expression of OT-IRs after chronic unpredictable mild stress and treadmill exercise (n = 60). (A) Scatter graph with mean ± SEM, the number of OT-IRs was expressed in the PVN and SON in the mice. Two-way ANOVA plus Tukey test was used. * *: P < 0.05, * *: P < 0.01, * ** : P < 0.001. (B) Position of OT-IRs expressed in the PVN and SON of the mice. OT-IRs in 200 × 200 µm2 counted.
Discussion
Compared with noCVMS/noTRE group, mice from CVMS/noTRE group took less time in the center of the OF and had less activity during this test. To further analyze anxiety-like behaviors of mice treated with CVMS, an EPM was conducted (He et al., 2015). Our results show that mice in CVMS/noTRE group took less time in the open arms of the EPM, spent fewer entries to the open arms, the % of open/(open + closed) arm frequency and covered shorter distance. These results are indicative of increased anxiety-like behavior only the chronic unpredictable mild stress. Our results were consistent with male mice, rats and female prairie voles in CVMS-induced anxiety (Smith et al., 2013). To evaluate the stressor effects on rodents, a potentially stressful event is various CVMS, as animals are exposed to multiple stressors and cannot predict or control the changes (i.e. restricted activities, deprived of water/food, placed in moist sawdust) in their environment for 3–4 weeks (Mineur et al., 2006, Pothion et al., 2004),
Compared with CVMS/noTRE animals, mice from noCVMS/LMTRE and CVMS/LMTRE groups took more time in the center of the OF and had more activity during this test, and spent more time in the open arms of the EPM, showed more entries to the open arms, had higher % of open/(open + closed) arm frequency and longer distance in movement. These results indicated that anxiety-like behaviors of CVMS/LMEG and CVMS/LMTRE mice were reduced. Appropriate physical activities help reduce negative emotions. Treadmill exercise from low to medium speed might have a positive influence in regulating anxiety. Regular physical activity might be an effective way to reduce negative emotion and emotional disorders symptoms. Anxiety-like behavior improvements following exercise were decreases in tension, depression, anger, and confusion (Yik et al., 1999). These results were similar to when rates were allowed access to a running wheel for 3 weeks and exhibited anxiolytic-like behaviors after exposed to a repeated stress (Sciolino and Holmes, 2012). Conversely, mice from noCVMS/HMTRE did not exhibited anxiolytic-like behaviors, while increased anxiety-like behaviors compared with noCVMS/LMTRE and CVMS/LMTRE animals, the result indicated that treadmill exercise with higher speed might act as a new stressor to stimulate animals, causing mice to produce anxiety-like behaviors. Our study is consistent with the conclusion that low-moderate intensity exercise had the best emotional benefits and had better effect on relieving anxiety than high-intensity exercise (Basso and Suzuki, 2017, Guan, 2003). Frequent stress for a long time can increase cortisol level, which will cause down-regulation of HPA axis function leading to anxiety-like behaviors (Gunnar and Quevedo, 2007). Stressors activate hypothalamic–pituitary–adrenal (HPA) axis resulting in the release of glucocorticoids (cortisol in humans and corticosterone in rodents) from the adrenal gland that bind to mineralocorticoid (MR) and glucocorticoid receptors (GR) in the brain. Through coordinated actions, MR and GR activation prepares the organism, both physiologically and psychologically, to deal with the stressor and return the activated systems to a homeostatic level of functioning (Chester et al., 2014). Although noCVMS/HTRE females are without CVMS, fast treadmill exercise (18 m / min for 20 mins) might still be a stressor for them, because during the experiments, we found that when they were placed on the treadmill, they were more afraid and had more tension (There were more feces and urine of mice, which was a manifestation of emotional tension. However, the exact amounts were not measured). Since the treadmill used in the experiments have electrical shock system to keep animals running, these mice were worried that they were going to be thrown off from the fast -moving running belt on the treadmill, so they did not feel the joy of exercise. Higher speed exercise did not alleviate negative emotions (Netz et al., 2005), it could also produce anxiogenic-like and null effects (Fuss et al., 2010, Pietropaolo et al., 2006, Helgadóttir et al., 2015). Discrepancies between LMTRE and HTRE on anxiety-like behaviors were likely due to a variety of internal and/or external variables that ultimately influence the impact of stressors on the organism (Sciolino and Holmes, 2012). For example, external variables such as the speed of the treadmill as environmental stressors, and internal variables such as changes in stress hormones because of worry and fear of falling off the treadmill. Interestingly, apart from CVMS effect, the increased anxiety-like behaviors are likely due to high speed treadmill. There are only few studies investigating the specific effects of high intensity exercise as a stressor, particularly the effects on mood disturbances (Guan, 2003). With respect to the interactions between exercise and stress, it has been shown that both high-intensity treadmill exercise and CVMS enhanced unfavorable effects on behavioral function including those on anxiety-related behavioral alterations in the OF and EPM tests. Currently, there’s limited evidence for the upper and lower limits of movement intensity with emotional regulation effect. Therefore, the relation between exercise intensity and emotional benefits needs to be further explored.
We found that serum E2 levels in CVMS/noTRE group was lower than that of noCVMS/noTRE, CVMS/LMTRE and noCVMS/LMTRE group. A negative correlation between CVMS and serum E2 levels is not consistent with a study on female rats, in which serum E2 levels increased 10 min after an acute restraint stress (Liu et al., 2011). There are three reasons for the difference. First, the serum measurement times are different. Rats were measured after 10 min of stress; mice were measured after 2 days of behavior test (behavior test lasted for 1 week after stress). The serum hormones varied greatly in different periods, and this is related to different physiological cycles (Holder and Blaustein, 2014). Second, the stress methods are different, rats were subject to short-term acute restraint stress (1 h / day for only 1 day), and did not reach a state of anxiety, while the mice in our study were subject to long-term chronic unpredictable repeated stress (24 h/day for 4 weeks), and had anxiety-like behaviors. Last but not least, it may also because of the different types of experiment animals.
In the present study, serum E2 levels of the CVMS/HTRE groups was lower than that of noCVMS/noTRE, CVMS/LMTRE and noCVMS/LMTRE. This may be related to prolonged high-intensity exercise that may cause excessive fatigue in the body, which may lead to HPG axis dysfunction and reduce E2 in the serum of female mice (Schmitz et al., 2015). When level of estrogen in women's body is low, stress response is enhanced levels of estrogen is negatively correlated with stress response (Chen et al., 2011). Therefore, high-intensity exercise may be a stress in mice to change E2 levels in the serum, thereby increasing anxiety levels.
We found that the number of ERα-IRs in five brain regions including BNST, mPOA, MeA, PVN and SON was increased in the CVMS/noTRE group, while ERβ-IRs decreased in the five brain regions in the CVMS/noTRE group. These findings are similar to other studies in mice and rats, and our previous studies in mandarin vole, whereby enhanced ERα expression and a concomitant declined in ERβ expression are relevant with increased anxiety and depression-like behaviors in an EPM and OF (He et al., 2018). Amygdala receives signals from cerebral cortex and sub-cortex regions, and integrates information from other brain regions, including BNST, mPOA, PVN and SON, which ultimately brings about anxious expression of animals (Lee, 2007; Le Doux, 2000). MeA expresses ERα and the reduction of ERα in MeA decreased anxiety, demonstrating that anxiety is regulated by ERα in amygdale (Spiteri et al., 2010). Both BNST and mPOA were closely involved in modulating anxiety-like behaviors (Davis, 2006). It is possible that the changes of ERα and ERβ expression in BNST and mPOA associated with CVMS lead to changes in the responses of mice with respect to anxiety. The distribution of ERα in PVN and SON in CVMS/noTRE group was increased in our experiment, and the distribution suggests an indirect mode of estradiol action on PVN and SON responses to stress (Handa et al., 2012a). ERα is known to regulate the activity of corticotropin-releasing factor. Increased number of ERα-IRs in these brain regions makes corticotropin-releasing factor disorderly increase, and it may affect the activity of the HPA axis and lead to anxiety (He et al., 2015). However, ERβ is declined in the five brain regions including the BNST, mPOA, PVN and SON in the CVMS/noTRE group. The five pivotal brain areas have high levels of ERβ distribution, and ERβ inhibits HPA responsiveness and reduces the anxiety behavior of rodents (Bulut et al., 2016). Therefore, alterations in ERβ-IRs in these areas would likely result in anxiety-like behaviors in mice. Despite the fact that estradiol feedback to control reproduction occurs principally through ERα-dependent mechanisms, modulatory roles for ERβ also exist. The roles of ERα and ERβ within a particular neural network may be synergistic or antagonistic. Examples of the latter include the role of ERα to enhance, and ERβ to suppress anxiety-like and aggressive behaviors. We speculate that decreased E2 probably causes downregulation of ERβ in these brain areas because their expression levels may reflect estradiol exposure of relevance to estradiol replacement therapy (Handa et al., 2012b), and it is consistent with the fact that ERβ is activated by elevated circulating estrogen (Giguere et al., 1998). Taken together, estradiol through ERα and ERβ signaling pathways regulates HPA axis function and has opposite effects on emotion (Handa and Weiser, 2014), the increase in ERα expression and concomitant decrease in ERβ expression are associated with increased anxiety-like behavior.
The number of OT-IRs in PVN and SON was lower in mice exposed to CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE. Reduced numbers of OT-IRs in certain brain regions may be one of the causes of anxiety-like behaviors. OT knockouts present an anxious phenotype indicating involvement of endogenous OT (Mantella et al., 2003). Endogenous OT is also directly involved in anxiolysis in females and males (Bosch and Neumann, 2012, Waldherr and Neumann, 2007). In rats and mice, OT administered peripherally or centrally attenuates anxiety (Ayers et al., 2011). OT is primarily expressed in the PVN and SON, release of OT via neurohypophysis into the brain exerts a wide spectrum of central effects (Neumann, 2008). Therefore, reduced numbers of OT-IRs in the PVN and SON may be one of the causes of anxiety-like behavior in CVMS/noTRE, CVMS/HTRE and noCVMS/HTRE females.
The number of OT-IRs in the PVN and SON was higher in mice exposed to CVMS/LMTRE and noCVMS/LMTRE, and both CVMS/LMTRE and noCVMS/LMTRE were similar to noCVMS/noTRE OT can also reverse some of the anxiogenic effects of treadmill exercise. For example, when accompanied with exercise, OT can inhibit anxiety in OVX rats (Bulut et al., 2016). Exercise training was demonstrated to increase hypothalamic OT and OT receptor (OTR) expressions in rats (Martins et al., 2005), and were normalized or enhanced by exercise training in rats (Bulut et al., 2016). In rats, OT in the brain was positively correlated with serum estrogen (McCarthy et al., 1996). It plays an important role in regulating anxiety through E2 and ERβ in specific brain regions. ERβ is co-expressed with OT in neurons of the PVN, an ERβ-selective agonist would decrease stress induced HPA reactivity and anxiety-like behaviors via an OTergic pathway (Kudwa et al., 2014), suggesting the interaction of OT and ERβ in modulating anxiety-like behaviors (Kudwa et al., 2014). However, OT-IRs in male prairie voles that had undergone voluntary running-wheel exercises were not only increased, but also decreased in comparison with non-exercise group, and anxiety-related behaviors were no different from those from non-exercise group (Kenkel and Carter, 2016). It shows that the changes in animal anxiety-like behaviors caused by exercise are not only related to animal species, but may also be related to the different mechanisms of gender differences in anxiety-like behaviors (Sciolino and Holmes, 2012, Munive et al., 2016).
In summary, this study examined the complexity of the combined effect of CVMS and treadmill exercise speed on female mice. Our data indicated that female mice were subjected to CVMS demonstrate increased anxiety in OF and EPM tests, and this is related to (i) lower serum E2 levels; (ii) increased expression of ERα in BNST, mPOA, MeA, PVN and SON; (iii) decreased expression of ERβ in BNST, mPOA, MeA, PVN and SON; (iv) decreased expression of OT in PVN and SON. Only the low-moderate speed treadmill exercise was similar to the control group and did not exhibit anxiety, and higher speed treadmill exercise increased anxiety. The low-moderate treadmill exercise reversed CVMS-induced changes in serum E2 concentration and changed expression of ERα, ERβ and OT in the brain. The interaction among appropriate exercise, differences in serum E2 among female mice, and an increase in the ratios of ERβ/ERα and OT may be responsible for the observed decrease in anxiety-like behavior in female mice.
CRediT authorship contribution statement
He Feng-Qin:conceived and designed the study, and acquisition of data, and analysis and interpretation of data; Yan Bing-Jie, Tian Zhen, Fan Mei-Yang and Hui Yu-Nan: performed the experiments and participated in drafting the article; Lai Rui-Juan and Chen Xin: reviewed and edited the manuscript; Yang Ming-Juan and Cheng Xiao-Xia: performed analysis and interpretation of data; Wang Zi-Jian and Yu Bin: performed behavioral experiment analysis and statistics. All authors read and approved the manuscript.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (NSFC81972883; 321000389), Natural Science Basic Research Project of Shaanxi Province (2020JM-617; 2017JQ8040), Shaanxi Provincial Department of Education" Special Project for Emergent Public Health Safety" (20JG025); Chang'an District Science and Technology Project of Xi’an City (the second batch of JC1701 in 2017); National University Student Innovation and Entrepreneurship Training Program (S202111080010); Shanxi Province University Student Innovation and Entrepreneurship Training Program (S202111080038).
References
- Adhikari A. Distributed circuits underlying anxiety. Front. Behav. Neurosci. 2014;8:1–6. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico J.A., Seitchik J., Robinson A.G. Studies of oxytocin in plasma of women during hypocontractile labor. J. Clin. Endocrinol. Metab. 1984;58:274–279. doi: 10.1210/jcem-58-2-274. [DOI] [PubMed] [Google Scholar]
- Ayers L.W., Missig G., Schulkin J., Rosen J.B. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacol. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso J.C., Suzuki W.A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Brain Plast. 2017;2:127–152. doi: 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G., Pekala K., Boguszewska-Czubara A., Michalak A., Kruk-Slomka M., Budzynska B. Behavioral and biochemical interaction between nicotine and chronic unpredictable mild stress in mice. Mol. Neurobiol. 2017;54:904–921. doi: 10.1007/s12035-016-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow A.P., Handa R.J. Estrogen receptors modulation of anxiety-like behavior. Vitam. Horm. 2017;103:27–52. doi: 10.1016/bs.vh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch O.J., Neumann I.D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Breese G.R., Cott J.M., Cooper B.R., Prange J.A.J., Lipton M.A., Plotnikoff N.P. Effects of Thyrotropin-Releasing Hormone (TRH) on the actions of pentobarbital and other centrally acting drugs. J. Pharmacol. Exp. Ther. 1975;193:11–22. [PMC free article] [PubMed] [Google Scholar]
- Bulut E.C., Abueid L., Ercan F., Süleymanoğlu S., Ağırbaşlı M., Yeğen B.C. Treatment with oestrogen-receptor agonists or oxytocin in conjunction with exercise protects against myocardial infarction in ovariectomized rats. Exp. Physiol. 2016;101:612–627. doi: 10.1113/EP085708. [DOI] [PubMed] [Google Scholar]
- Catellier J.R.A., Yang Z.J. The role of affect in the decision to exercise: does being happy lead to a more active lifestyle? Psychol. Sport Exerc. 2013;14:275–282. [Google Scholar]
- Chen C.P., Cheng D.Z., Luo Y.J. Estrogen impacts on emotion: psychological, neuroscience and endocrine studies. Sci. Sin. Vitae. 2011;41:1049–1062. [Google Scholar]
- Chester J.A., Kirchhoff A.M., Barrenha G.D. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addict. Biol. 2014;19:663–675. doi: 10.1111/adb.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradsson M., Littbrand H., Lindelof N., Gustafson Y., Rosendahl E. Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: a cluster-randomized controlled trial. Aging Mental Health. 2010;14:565–576. doi: 10.1080/13607860903483078. [DOI] [PubMed] [Google Scholar]
- Cullen C.L., Burne T.H.J., Lavidis N.A., Moritz K.M. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Dong H.W., Petrovich G.D., Swanson L.W. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Le Doux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Dunn A.L., Trivedi M.H., Kampert J.B., Clark C.G., Chambliss H.O. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ernsberger P., Azar S., Lwai J. Open-field behavior in two models of genetic hypertension and the behavioral effects of salt excess. Behav. Neural Biol. 1983;37:46–60. doi: 10.1016/s0163-1047(83)91061-0. [DOI] [PubMed] [Google Scholar]
- Fedotova J. Anxiolytic-like effect of quinpirole in combination with a low dose of 17b-estradiol in ovariectomized rats. Acta Physiol. Hung. 2013;100:211–223. doi: 10.1556/APhysiol.100.2013.2.8. [DOI] [PubMed] [Google Scholar]
- Furuta M., Numakawa T., Chiba S., Ninomiya M., Kajiyama Y., Adachi N., Akema T., Kunugi H. Estrogen, predominantly via estrogen receptor a, attenuates postpartum-induced anxiety- and depression-like behaviors in female rats. Endocrinology. 2013;154:3807–3816. doi: 10.1210/en.2012-2136. [DOI] [PubMed] [Google Scholar]
- Fuss J., Ben Abdallah N.M., Vogt M.A., Touma C., Pacifici P.G., Palme R., Witzemann V., Hellweg R., Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6 J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Giguere V. , Tremblay A. , Tremblay GB (1998) Estrogen receptor b: reevaluation.
- Guan J.H. Effect of aerobic exercise on estrogen level in female climacteric syndrome. Chin. J. Phys. Med. Rehabil. 2003;25:536–537. [Google Scholar]
- Gunnar M., Quevedo K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Handa R.J., Mani S.K., Uht R.M. Estrogen receptors and the regulation of neural stress responses. Neuroendocrino. 2012;96:111–118. doi: 10.1159/000338397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R.J., Ogawa S., Wang J.M., Herbison A.E. Roles for oestrogen receptor β in adult brain function. J. Neuroendocrinol. 2012;24(1):160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R.J., Weiser M.J. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F. The relationship of prenatal ethanol exposure and anxiety related behaviors and central androgen receptor and vasopressin expression in adult male mandarin voles. Neuroscience. 2014;266:224–234. doi: 10.1016/j.neuroscience.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Helgadóttir B., Forsell Y., Ekblom Ö. Physical activity patterns of people affected by depressive and anxiety disorders as measured by accelerometers: a cross-sectional study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans K.G.C., Sliwowska J.H., Verma P., Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rrv. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.Q., Fang G., Wang B., Guo X.J., Guo C. Perinatal stress effects on later anxiety and hormone secretion in male Mandarin voles. Behav. Neurosci. 2015;129:789–800. doi: 10.1037/bne0000094. [DOI] [PubMed] [Google Scholar]
- He F., Wang Z., Guo G. Postnatal separation prevents the development of prenatal stress-induced anxiety in association with changes in oestrogen receptor and oxytocin immunoreactivity in female mandarin vole (Microtus mandarinus) offspring. Eur. J. Neurosci. 2018;47:95–108. doi: 10.1111/ejn.13788. [DOI] [PubMed] [Google Scholar]
- He F.Q., Zhang J., Guo X. Prenatal ethanol exposure increased adult depressive-like behaviors and central ERα and OT expression in female Mandarin Voles (Microtus mandarinus) Zool. Stud. 2012;51:1–11. [Google Scholar]
- Higa-Taniguchi K.T., Felix J.V., Michelini L.C. Brainstem oxytocinergic modulation of heart rate control in rats: effects of hypertension and exercise training. Exp. Physiol. 2009;94:1103–1113. doi: 10.1113/expphysiol.2009.049262. [DOI] [PubMed] [Google Scholar]
- Holder M.K., Blaustein J.D. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front. Neuroendocrinol. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.H., Chen T.T. The effect of physical activity on affect. Adv. Psychol. Sci. 2014;22:1889–1898. [Google Scholar]
- Kalandakanond-Thongsong S., Daendee S., Srikiatkhachorn A. Effect of the acute and chronic estrogen on anxiety in the elevated T-maze. Physiol. Behav. 2012;105:357–363. doi: 10.1016/j.physbeh.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Kenkel W.M., Carter C.S. Voluntary exercise facilitates pair-bonding in male prairie voles. Behav. Brain Res. 2016;296:326–330. doi: 10.1016/j.bbr.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W., Dupont S., Krust A., Chambon P., Chapman P.F. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa A.E., McGivern R.F., Handa R.J. Estrogen receptor b and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol. Behav. 2014;129:287–296. doi: 10.1016/j.physbeh.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., Angstadt M., Chua P., Heinrichs M., Stout J.C., Nathan P.J. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmaco. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.R., Brady D.L., Shapiro R.A., Dorsa D.M., Koenig J.I. Prenatal stress generates defificits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Hu P., Qi X.R., Meng F.T., Kalsbeek A., Zhou J.N. Acute restraint stress increases intrahypothalamic oestradiol concentrations in conjunction with increased hypothalamic oestrogen receptor beta and aromatase mRNA expression in female rats. J. Neuroendocrinol. 2011;23:435–443. doi: 10.1111/j.1365-2826.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- Lloyd B.A., Hake H.S., Ishiwata T., Farmer C.E., Loetz E.C., Fleshner M., Bland S.T., Greenwood B.N. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav. Brain. Res. 2017;323:56–67. doi: 10.1016/j.bbr.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella R.C., Vollmer R.R., Li X., Amico J.A. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- Martins A.S., Crescenzi A., Stern J.E., Bordin S., Michelini L.C. Hypertension and exercise training differentially affect oxytocin and oxytocin receptor expression in the brain. Hypertension. 2005;46:1004–1009. doi: 10.1161/01.HYP.0000175812.03322.59. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., McDonald C.H., Brooks P.J., Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol. Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McHenry J.A., Rubinow D.R., Stuber G.D. Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: putative circuits for regulating anxiety and reward. Front. Neuroendocrinol. 2015;38:65–72. doi: 10.1016/j.yfrne.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mevel J.C., Pamantung T.F., Mabin D., Vaudry H. Effects of central and peripheral administration of arginine vasotocin and related neuropeptides on blood pressure and heart rate in the conscious trout. Brain Res. 1993;610:82–89. doi: 10.1016/0006-8993(93)91220-m. [DOI] [PubMed] [Google Scholar]
- Michelini L.C. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin. Exp. Pharmacol. Physiol. 2007;34:369–376. doi: 10.1111/j.1440-1681.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- Mineur Y.S., Belzung C., Crusio W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Munive V., Santi A., Torres-Aleman I.A. Concerted action of estradiol and insulin like growth factor i underlies sex differences in mood regulation by exercise. Sci. Rep. 2016;6:25969. doi: 10.1038/srep25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz Y., Wu M.J., Becker B.J., Tenenbaum G. Physical activity and psychological well-being in advanced age: a meta-analysis of intervention studies. Psychol. Aging. 2005;20:272–284. doi: 10.1037/0882-7974.20.2.272. [DOI] [PubMed] [Google Scholar]
- Neumann I.D. Brain oxytocin: a key regulator of emotional and social behaviors in both females and males. J. Neuroendocrinol. 2008;63:335–339. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. second ed. Academic Press; San Diego, California: 1997. pp. 85–109. [Google Scholar]
- Pietropaolo S., Feldon J., Alleva E., Cirulli F., Yee B.K. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav. Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- Pothion S., Bizot J.C., Trovero F., Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Rauf S., Soejono S.K., Partadiredja G. Effects of treadmill exercise training on cerebellar estrogen and estrogen receptors, serum estrogen, and motor coordination performance of ovariectomized rats. Iran. J. Basic Med. Sci. 2015;18:587–592. [PMC free article] [PubMed] [Google Scholar]
- Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plusmaze. Neurosci. Biobehav. Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Scantamburlo G., Hansenne M., Fuchs S., Pitchot W., Marechal P., Pequeux C., Ansseau M., Legros J.J. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrino. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schmitz K.H., Williams N.I., Kontos D., Domchek S., Morales K.H., Hwang W.T., Grant L.L., DiGiovanni L., Salvatore D., Fenderson D., Schnall M., Galantino M.L., Stopfer J., Kurzer M.S., Wu S., Adelman J., Brown J.C., Good J. Dose–response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast Cancer Res. Treat. 2015;154:309–318. doi: 10.1007/s10549-015-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino N.R., Holmes P.V. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 2012;36:1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selakovic D., Joksimovic J., Jovicic N., Mitrovic S., Mihailovic V., Katanic J., Milovanovic D., Pantovic S., Mijailovic N., Rosic G. The impact of hippocampal sex hormones receptors in modulation of depressive-like behavior following chronic anabolic androgenic steroids and exercise protocols in rats. Front. Behav. Neurosci. 2019;13:19. doi: 10.3389/fnbeh.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H.R., Thakur M.K. Correlation of ERα/ERβ expression with dendritic and behavioral changes in CUMS mice. Physiol. Behav. 2015;145:71–83. doi: 10.1016/j.physbeh.2015.03.041. [DOI] [PubMed] [Google Scholar]
- Smith A.S., Lieberwirth C., Wang Z. Behavioral and physiological responses of female prairie voles (Microtus ochrogaster) to various stressful conditions. Stress. 2013;16:531–539. doi: 10.3109/10253890.2013.794449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T., Musatov S., Ogawa S., Ribeiro A., Pfaff D.W., Agmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res. 2010;210:211–220. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Toufexis D.J., Myers K.M., Bowser M.E., Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor α (ERα) and ERβ. J. Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldherr M., Neumann I.D. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl. Acad. Sci. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik M.S.M., Russell J.A., Barrett L.F. Structure of self reported current affect: Integration and beyond. J. Pers. Soc. Psychol. 1999;77:600–619. [Google Scholar]