We have expressed our concern about HPV vaccination coverage in Japan remaining below 1%, which will result in an increase in vaccine-targeted HPV infection rates and cervical cancer morbidity [1], [2], [3], [4]. Once again, we have been appealing for the resumption of proactive recommendations for HPV vaccination. Public funding for HPV vaccination became available for girls aged 13–16 years from 2010 in Japan and the vaccine was subsequently included in the national immunization program (NIP) for girls aged 12–16 years from April, 2013. In 2016, we calculated the risk of an HPV16/18 infection at age 20 years for birth cohorts eligible for HPV vaccination. While we predicted that HPV 16/18 infection rates would drop by about 30% in vaccinated cohorts, we also foresaw a future increase in unvaccinated cohorts due to suspension of proactive recommendations for the vaccine [3]. Vaccination coverage for Japanese girls decreased from 68.9% for those born in 1999 to 14.3% for those born in the year 2000 [5]. In 2020, girls born in the year 2000 reached the cervical screening age in line with the Japanese national cervical screening guidelines. Data from our screening program has shown that HPV 16/18 infection rates have indeed risen once again. This is the first report to document the real-world harms of the suspension of proactive recommendations for HPV vaccination in Japan in terms of increased infection of vaccine-targeted HPV types.
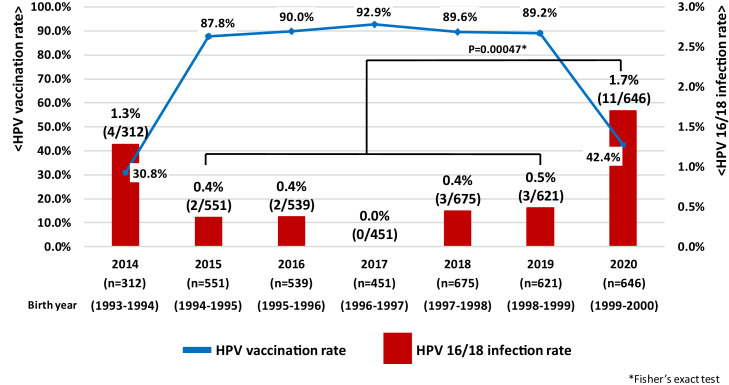
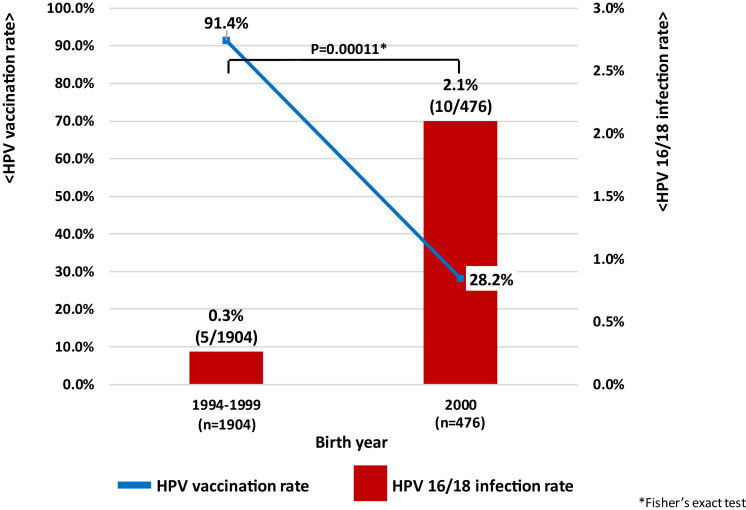
We investigated HPV infection rates in women aged 20-21 years undergoing cervical screening in Niigata City from April 2014 to March 2021 (fiscal years 2014-2020) [6] (supplementary Figure 1). HPV vaccination coverage in the target cohorts increased from 30.8% (2014) to 87.8% (2015), 90.0% (2016) and 92.9% (2017), followed by 89.6% (2018) and 89.2% (2019). However, coverage decreased sharply to 42.4% in 2020. Similarly, HPV 16/18 infection rate in the cohorts decreased significantly from 1.3% (2014) to 0.4% (2015), 0.4% (2016), and 0% (2017) as HPV vaccination coverage increased. Furthermore, the infection rates rose slightly to 0.4% in 2018 and 0.5% in 2019 and rose sharply to 1.7% in 2020. HPV 16/18 infection rates, which had previously decreased due to increased HPV vaccination coverage also increased significantly due to decreased vaccination coverage after the suspension of proactive recommendations (p = 0.00047: Figure 1a). In particular, in women aged 20 years (birth cohort year 2000), where vaccination coverage decreased to 28.2%, HPV 16/18 infection rates were 2.1% (p = 0.00011: Figure 1b) compared to the publicly funded generation for HPV vaccination (0.3%: birth cohort years 1994-1999) where vaccination coverage in Niigata prefecture exceeded 90%. According to previous survey data from Niigata, the HPV 16/18 infection rates in unvaccinated women aged 20-22 years was 2.2%, and it can be seen that the infection rate has increased again to almost the same level [7]. There was no significant difference in either “Age at sexual debut” or “Number of sexual partners” between the two groups (supplementary Figure 2, 3). Suspension of proactive recommendations for HPV vaccination has real world public health consequences. WHO made a statement in 2015 that Japan's policy decisions based on weak evidence, leading to lack of use of safe and effective vaccines, can result in real harm [8], and that has become a reality.
Figure 1.
(a) Annual trends in HPV 16/18 infection rate among Japanese women aged 20-21. (b) Changes in HPV 16/18 infection rate of Japanese 20-year-old women after "Suspension of proactive recommendations of HPV vaccination"
This result may not come as a shock to scientists, but how does the Japanese government feel about it? Although it is a radical expression, it can be said that they are conducting a real-world clinical trial on Japanese women. It goes without saying that an increase in HPV 16/18 infections in women in their 20s will lead to an increase in cervical precancers and cancers in women in their 30s and 40s in the near future. We urge the government to focus on the results and take urgent action to resume the proactive recommendations for the vaccine. However, in the current climate where safety concerns have spread among the public, it is unlikely that vaccination coverage will improve immediately due to the characteristics of Japanese people who are easily influenced by the decisions of those around them. If the vaccine crisis continues, approximately 10000 preventable deaths due to cervical cancer are estimated to occur in the next 50 years (2020–69) [1].
As Ueda and Tanaka et al. stated previously in the Lancet journal, when the Ministry of Health, Labour and Welfare resumes proactive encouragement, the following actions are required [2,9]: Enlightenment activities using a behavioral economics approach, accurate and scientific information dissemination in the media, policies for catch-up vaccination, introduction of 9-valent vaccine to NIP, and vaccination for boys to achieve herd immunity. We hope that the new Japanese Cabinet, in addition to its policy on COVID-19 infection, will significantly change its thinking about HPV vaccination to save the lives of Japanese women.
Author contributions
T. E., M. S., and Y. U. designed the study. M. S. wrote the initial draft of the manuscript. T. E. and S. H. critically reviewed the manuscript. M. Y., R. K., M. H. M. K. and E. M. contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. S. A. contributed to data collection and interpretation of data. All authors contributed to the writing of the final version of the manuscript.
Supplementary Figure 1
Target group in this study
Supplementary Figure 2
Comparison of sexual activity in Figure 1a
Supplementary Figure 3
Comparison of sexual activity in Figure 1b
Declaration of Competing Interest
Manako Yamaguchi, Yutaka Ueda, and Takayuki Enomoto received lecture fees from Merck Sharp and Dohme. Etsuko Miyagi received honoraria and lecture fees from Roche Diagnostics, Merck Sharp and Dohme, and Hologic Japan. All other authors report no potential conflicts.
Acknowledgements/Funding
We would like to thank Ms. Yuka Watanabe, Ms. Sachiko Ono, Ms. Anna Ishida, and administrator of Niigata city for their support in conducting the survey. This work was supported by the Japanese Agency for Medical Research and Development (JP15ck0106103).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100300.
Appendix. Supplementary materials
Reference
- 1.Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. The Lancet Public health. 2020;5(4):e223. doi: 10.1016/S2468-2667(20)30010-4. -e34. [DOI] [PubMed] [Google Scholar]
- 2.Ueda Y, Yagi A, Ikeda S, Enomoto T, Kimura T. Beyond resumption of the Japanese Government's recommendation of the HPV vaccine. The Lancet Oncology. 2018;19(12):1563–1564. doi: 10.1016/S1470-2045(18)30573-4. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Ueda Y, Egawa-Takata T, Yagi A, Yoshino K, Kimura T. Outcomes for girls without HPV vaccination in Japan. The Lancet Oncology. 2016;17(7):868–869. doi: 10.1016/S1470-2045(16)00147-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanley SJ, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet (London, England) 2015;385(9987):2571. doi: 10.1016/S0140-6736(15)61152-7. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa S, Ueda Y, Yagi A, Ikeda S, Hiramatsu K, Kimura T. Corrected human papillomavirus vaccination rates for each birth fiscal year in Japan. Cancer science. 2020;111(6):2156–2162. doi: 10.1111/cas.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekine M, Yamaguchi M, Kudo R. Epidemiologic Profile of Type-Specific Human Papillomavirus Infection after Initiation of HPV Vaccination. Vaccines. 2020;8(3) doi: 10.3390/vaccines8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo R, Yamaguchi M, Sekine M. Bivalent Human Papillomavirus Vaccine Effectiveness in a Japanese Population: High Vaccine-Type-Specific Effectiveness and Evidence of Cross-Protection. The Journal of infectious diseases. 2019;219(3):382–390. doi: 10.1093/infdis/jiy516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Advisory Committee on Vaccine safety Statement on Safety of HPV vaccines. 17 December 2015. https://www.who.int/vaccine_safety/committee/GACVS_HPV_statement_17Dec2015.pdf.
- 9.Tanaka Y. Time to resume active recommendation of the HPV vaccine in Japan. The Lancet Oncology. 2020;21(12):1552–1553. doi: 10.1016/S1470-2045(20)30608-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.