Abstract
A SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) is a rapidly progressing subtype of lung cancer with a poor prognosis and causes early postoperative recurrence among operable patients. In this study, we present a case of SMARCA4-UT with vertebral and chest wall invasion that successfully underwent conversion surgery after treatment with atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin. The surgical specimen comprised SMARCA4-deficient and SMARCA2-positive adenocarcinoma, confirming intratumor heterogeneity. Gene panel analysis revealed no substantial differences in mutant gene profiles among tumors and no differences in SMARCA2 mutations. Furthermore, no recurrence occurred for 9 months after surgery. Thus, this case illustrates the possibility of multidisciplinary treatment including neoadjuvant therapy with immunotherapy and conversion surgery for SMARCA4-UT.
Keywords: SMARCA4-deficient undifferentiated tumor, Conversion surgery, Neoadjuvant therapy, Immunotherapy, Case report
Introduction
Thoracic SMARCA4-deficient undifferentiated tumor (SMARCA4-UT) is a lung cancer newly classified in the fifth edition of the WHO classification of thoracic tumors published in 2021. Initially published as SMARCA4-deficient thoracic sarcoma, it was later classified as a subtype of undifferentiated lung cancer of pulmonary epithelial origin. Most cases of SMARCA4-UT exhibit advanced stage at presentation, with poor prognosis and median survival time of less than 6 months even after surgery, radiotherapy, and chemotherapy.1 No clear guidelines have been established for treating SMARCA4-UT; however, anti–programmed cell death protein-1 antibody drugs were effective2; moreover, atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin (ABCP) treatment was effective for a longer period than the conventional therapy.3
Here, we present a patient with advanced-stage SMARCA4-UT having vertebral invasion who successfully underwent conversion surgery after six courses of ABCP treatment.
Case Presentation
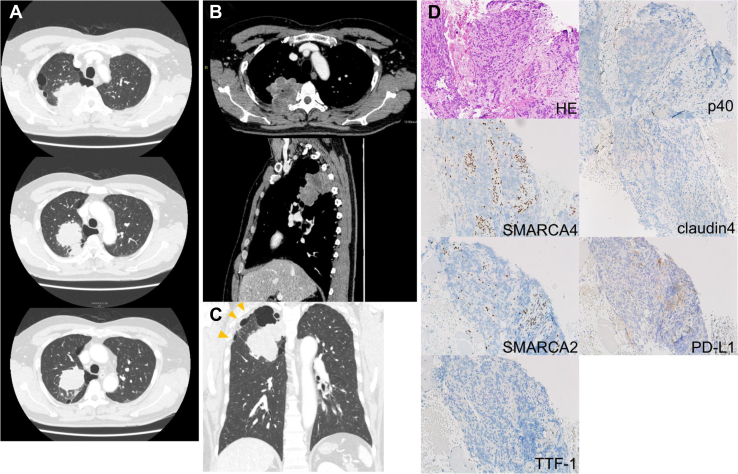
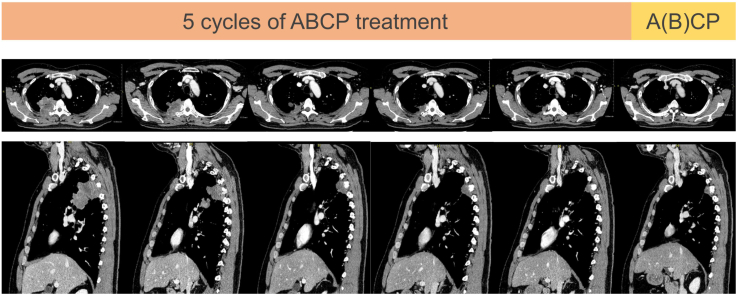
A 51-year-old Japanese man presented with persistent back pain. He was a current 22.5 pack-year smoker. Chest radiograph and computed tomography (CT) scan revealed an 8-cm mass in the lung right upper lobe with soft tissue and vertebral invasion (Fig. 1A and B). A coronal image of CT scan suggested pleural dissemination (Fig. 1C). Positron emission tomography–CT imaging revealed abnormal uptake in the mass, pleura around the mass, and a right hilar lymph node, and no uptake in other organs. An enhanced brain magnetic resonance imaging indicated no brain metastases. CT-guided biopsy and histopathologic analysis revealed undifferentiated NSCLC (Fig. 1D). The patient was diagnosed as SMARCA4-UT with clinical T4N1M1a stage IVA with no targetable driver mutations, and with a programmed death ligand-1 tumor proportion score of 0%. ABCP was administered as first-line chemotherapy at doses of atezolizumab 1200 mg/body, bevacizumab 15 mg/kg body weight, paclitaxel 175 mg/m2 body surface area, and carboplatin at an area under the concentration-time curve of 6 mg/mL/min, respectively. Pegfilgrastim reduced the risk of febrile neutropenia at every course. After completing six courses of ABCP treatment, the tumor shrunk to a maximum diameter of 2 cm and was no longer invasive to the spine, and the image suggestive of pleural dissemination disappeared (Fig. 2). Therefore, conversion surgery was planned. Five courses of ABCP treatment were administered without dose reduction, and the sixth course was administered without bevacizumab considering its impact on surgery.
Figure 1.
Chest plain enhanced CT images and histopathological analysis of CT-guided biopsy specimen. (A) Transverse chest CT images depict lung mass and parenchymal emphysema. (B) Transverse and sagittal enhanced CT images reveal tumor invasion into vertebrae and ribs. (C) Coronal CT image suggests pleural dissemination (yellow arrowheads). (D) Immunohistopathologic analysis of the biopsy specimen. HE staining presents undifferentiated round to plasmacytoid cells with prominent nucleoli and overall monomorphism. Immunohistochemical features indicate the complete loss of SMARCA4 and SMARCA2 in tumor cells with retained expression in normal inflammatory and stromal cells, lack of TTF-1, p40, and Claudin-4. The PD-L1 TPS using the 22C3 pharmDx assay is 0%. COSMIC, Catalogue Of Somatic Mutations In Cancer; CT, computed tomography; HE, hematoxylin and eosin; PD-L1, programmed death ligand-1; TPS, tumor proportion score.
Figure 2.
Changes in transverse and sagittal enhanced CT images of the chest during the ABCP treatment. ABCP, atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin; CT, computed tomography.
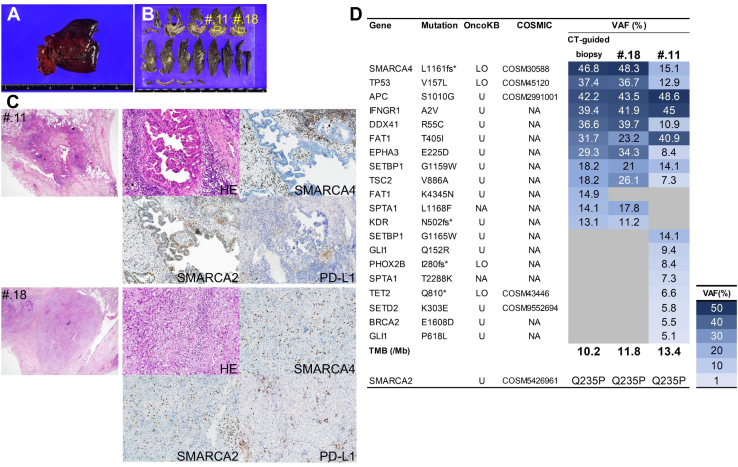
In addition to right upper lobectomy with mediastinal lymph node dissection (ND2a), the third, fourth, and fifth ribs and fourth transverse processes were resected. During surgery, gross tumor invasion of the vertebral body or pleural malignant nodule was not observed as in the preoperative images. Histopathologic analysis revealed that tumor resection was done with adequate margins with no viable tumor cells found at the resection margins or in all resected lymph nodes (Fig. 3A and B). In addition to residual SMARCA4-UT, SMARCA4-deficient, SMARCA2-retained, and TTF-1–positive adenocarcinoma (SMARCA4-deficient adenocarcinoma [SMARCA4-DA]) were found at a distant site. The programmed death ligand-1 tumor proportion score of both components was 0% (Fig. 3C).
Figure 3.
Macroscopic illustrations of the (A) right upper lobectomy with ribs and (B) cut surfaces of the formalin-fixed tumor. (C) Immunohistopathologic analysis of the two sections (#.11 and #.18) from the fixed tumor. In the #.11 section, the low-power field (×20) indicates the papillary configuration of the tumor gland and the high-power field (×200) reveals complete loss of SMARCA4 and expression of SMARCA2. PD-L1 TPS is 0%. In the #.18 section, the low-power field indicates the undifferentiated tumor and the high-power field (×200) presents a complete loss of SMARCA4 and SMARCA2. PD-L1 TPS is 0%. The result of the TSO500 panel and the whole-exome sequence of SMARCA2 is summarized in (D). The left column lists the mutated genes with the corresponding amino acid changes. The annotations of OncoKB and COSMIC databases are presented. If the mutation is registered in COSMIC, the COSMIC ID is appended. Heat map of the mutations detected in each sample. #, number; COSMIC, Catalogue Of Somatic Mutations In Cancer; CT, computed tomography; HE, hematoxylin and eosin; ID, identification; LO, likely oncogenic; Mb, Megabase; NA, not annotated; PD-L1, programmed death ligand-1; TMB, tumor mutational burden; TPS, tumor proportion score; U, unknown; VAF, variant allele fraction.
Genomic analysis of SMARCA4-UT specimen and two surgical resected specimens of SMARCA4-UT and SMARCA4-deficient adenocarcinoma was performed using the Illumina TruSight Oncology 500 (TSO500) panel (Supplementary Method) (Fig. 3D). Similar mutations were detected in SMARCA4 L1161fs∗ and in TP53 V157L in all three specimens. The oncogenic mutations of TET2 and PHOX2B were less than 10% in variant allele frequency. Moreover, the whole-exome sequence of the SMARCA2 gene was performed separately from the TSO500 panel, and the SMARCA2 Q235P mutation was detected from all three samples.
After surgery, the patient was followed up for 9 months, but no recurrence was observed.
Discussion
SMARCA4-UT often occurs in advanced stages, presenting early postoperative recurrence and poor prognosis among operable patients.1 In the present case, the patient was diagnosed with clinical stage IVA with vertebral invasion and pleural dissemination; however, ABCP treatment markedly reduced the tumor and paved the way for conversion surgery. Postoperative adjuvant therapy was not performed, and no recurrence was observed for 9 months.
SMARCA4-UT is caused by heavy smoking, and mutations such as STK11, KEAP1, ARID1A, KRAS, and NF1 may occur as comutations after the TP53 mutation.1 In the present case, TP53 V157L mutation was recognized in addition to the SMARCA4 L1161fs∗ mutation, and the oncogenic mutation was not recognized otherwise. Mutations in STK11 and KEAP1 have been implicated for resistance to immunotherapy,4 and the absence of these mutations may exhibit an excellent response to ABCP therapy. Moreover, no major adverse events were associated with ABCP treatment, and the ability to complete six courses without dose reduction may have contributed to the successful conversion surgery.
SMARCA4 mutations and loss of expression occur in approximately 5% of NSCLC.5 Although it is unclear whether SMARCA2 deletion affects pathomorphologic differences between undifferentiated carcinoma and adenocarcinoma, the hypothesis that SMARCA4-UT is formed by additional SMARCA2 deletion has been proposed.1 In the present case, we confirmed the heterogeneity of SMARCA4-UT and SMARCA4-DA in the resected specimen. In this study, we investigated SMARCA2 mutations by adding the whole-exome sequence of SMARCA2. The detected SMARCA2 Q235P mutation is a nonsynonymous mutation registered in the Catalogue Of Somatic Mutations In Cancer database,6 but its oncogenicity is unknown, and it is also detected in the components of SMARCA2-positive SMARCA4-DA. Therefore, it is highly unlikely that it is involved in the loss of SMARCA2 function. Although SMARCA2 is rarely included in the mutations to be analyzed in gene panels and its mutations are rarely evaluated, analysis in small cell carcinoma of the ovary, hypercalcemic type, which is characterized by SMARCA4 inactivating mutations, revealed that SMARCA4 and SMARCA2 are rarely mutated together.7 Both SMARCA4 and SMARCA2 are subunits of the BAF complex and their conformational analysis suggests that they exist in combination,8 and SMARCA2 deletion may occur by a mechanism that is not mediated by gene mutation, such as a loss of SMARCA4 by inactivating mutation resulting in an inability to maintain SMARCA2 conformationally.
Analysis of the TSO500 panel revealed no substantial difference in the mutation profile between SMARCA4-UT and SMARCA4-DA. Although PHOX2B I280fs∗ and TET2 Q810∗ mutations were detected as new oncogenic mutations in SMARCA4-DA, their variant allele frequencies were less than 10%, and it is unclear whether they are major drivers mutations or not. The present analysis was performed on surgical resection specimens after ABCP therapy, and it is unclear whether these differences occurred as a result of the treatment or during the development of SMARCA4-UT. The results of the TSO500 panel analysis, together with the results of the SMARCA2 sequencing, did not reveal a mechanism for the differentiation of SMARCA4-DA to SMARCA4-UT.
Clinical studies of neoadjuvant therapy with immunotherapy are in progress.9 Although careful follow-up is necessary, we believe that this case illustrates the potential application of ABCP and multidisciplinary treatment including surgery for SMARCA4-UT.
Conclusions
SMARCA4-UT is a rapidly progressing subtype of lung cancer with a poor prognosis. In many cases, SMARCA4-UT is in an advanced stage at the time of diagnosis and is not amenable to surgical treatment; furthermore, it is said that it often recurs even if surgery is performed. Immunotherapy including ABCP treatment may lead to conversion surgery, as in this case. The effects of immunotherapy and the possibility of conversion surgery in SMARCA4-UT need to be evaluated in the future.
CRediT Authorship Contribution Statement
Kei Kunimasa: Conceptualization, Methodology, Investigation, Writing—original draft.
Jiro Okami: Investigation, Supervision, Writing—review & editing.
Takahisa Kawamura, Takako Inoue, Motohiro Tamiya, Hanako Kuhara, Kazumi
Nishino, Satoshi Takenaka: Investigation.
Shigenori Nagata,Keiichiro Honma: Pathologic Investigation.
Yoji Kukita: Next-generation sequencing, Investigation, Software.
Hideaki Tahara, Toru Kumagai: Supervision.
Acknowledgments
Informed consent was obtained from the patient. This study was approved by the institutional review board at Osaka International Cancer Institute (#19205-3). This study was supported by The Japan Society for the Promotion of Science (grant number JP19K176974) Grants-in-aid for scientific research (KAKENHI) early-career scientists (Dr. Kunimasa), Takeda Science Foundation (Dr. Kunimasa), and the Osaka Medical Research Foundation for Intractable Diseases.
Footnotes
Disclosure: Dr. Kunimasa reports receiving honoraria as a lecturer from AstraZeneca, Chugai Pharma, and Novartis. Dr. Nishino reports receiving a grant from Nippon Boehringer Ingelheim and honoraria as a lecturer from Chugai Pharma, AstraZeneca, Nippon Boehringer Ingelheim, Eli Lilly Japan, Roche Diagnostics, Novartis, Pfizer, and Merk. Dr. Tamiya reports receiving grants from Ono Pharmaceutical, Bristol-Myers Squibb, and Boehringer Ingelheim; and honoraria as a lecturer from Taiho Pharmaceutical, Eli Lilly, Asahi Kasei Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, and Bristol-Myers Squibb. Dr. Kumagai reports receiving grants from Ono Pharmaceutical, Merck Sharp & Dohme K.K., Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Takeda Pharmaceutical Company Limited. Regeneron Pharmaceuticals, Inc., Merck Serono Co., Ltd., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., AbbVie GK., Delta-Fly Pharma, Inc., and The Osaka Foundation for The Prevention of Cancer and Life-style related Diseases (Public Interest Incorporated Foundation); and personal fees from Ono Pharmaceutical, AstraZeneca K.K., Taiho Pharmaceutical Co. Ltd., Merck Sharp & Dohme K.K., Teijin Pharma Limited, Novartis Pharma K.K., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Pfizer, Inc., Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb K.K. The remaining authors declare no conflict of interest.
Cite this article as: Kunimasa K, Okami J, Takenaka S, et al. Conversion surgery for advanced thoracic SMARCA4-deficient undifferentiated tumor with atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin treatment: a case report. JTO Clin Res Rep. 2021;2:100235.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100235.
Supplementary Data
References
- 1.Rekhtman N., Montecalvo J., Chang J.C. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15:231–247. doi: 10.1016/j.jtho.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henon C., Blay J.Y., Massard C. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol. 2019;30:1401–1403. doi: 10.1093/annonc/mdz160. [DOI] [PubMed] [Google Scholar]
- 3.Kawachi H., Kunimasa K., Kukita Y. Atezolizumab with bevacizumab, paclitaxel and carboplatin was effective for patients with SMARCA4-deficient thoracic sarcoma. Immunotherapy. 2021;13:799–806. doi: 10.2217/imt-2020-0311. [DOI] [PubMed] [Google Scholar]
- 4.Marinelli D., Mazzotta M., Scalera S. KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Ann Oncol. 2020;31:1746–1754. doi: 10.1016/j.annonc.2020.08.2105. [DOI] [PubMed] [Google Scholar]
- 5.Cancer genome atlas research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate J.G., Bamford S., Jubb H.C. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jelinic P., Mueller J.J., Olvera N. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46:424–426. doi: 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S., Wu Z., Tian Y. Structure of nucleosome-bound human BAF complex. Science. 2020;367:875–881. doi: 10.1126/science.aaz9761. [DOI] [PubMed] [Google Scholar]
- 9.Chaft J.E., Rimner A., Weder W., Azzoli C.G., Kris M.G., Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547–557. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.