Abstract
Introduction
Abemaciclib is an oral, selective small-molecule CDK 4 and 6 inhibitor. In preclinical models, abemaciclib synergized with programmed cell death protein-1 blockade to enhance antitumor efficacy. Here, we report the safety and anticancer activity of abemaciclib plus pembrolizumab in two cohorts with NSCLC.
Methods
This nonrandomized, open-label, phase 1b study included patients with previously untreated programmed death-ligand 1–positive, KRAS-mutant nonsquamous metastatic NSCLC (cohort A); squamous NSCLC after one previous platinum-containing chemotherapy regimen for metastatic disease (cohort B); and two breast cancer cohorts (disclosed separately). Patients received 150 mg abemaciclib every 12 hours plus 200 mg pembrolizumab intravenously on day 1 every 21 days. The primary objective was safety; secondary objectives included objective response rate, disease control rate, progression-free survival, and overall survival. Clinical Trial Number: NCT02779751.
Results
Each cohort enrolled 25 patients. Grades greater than or equal to 3 treatment-emergent adverse events in cohorts A and B were reported by 20 (80%) and 19 patients (76%), respectively. Six patients in cohort A (24.0%) and two patients in cohort B (8.0%) had a confirmed partial response; disease control rate was 56% and 64%, respectively. Median progression-free survival was 7.6 months (95% confidence interval [CI]: 1.6–not estimable) and 3.3 months (95% CI: 1.4–5.2); median overall survival was 27.8 months (95% CI: 9.9–not estimable) and 6.0 months (95% CI: 3.7–13.1) in cohorts A and B, respectively.
Conclusions
The combination of abemaciclib and pembrolizumab in stage IV NSCLC resulted in greater toxicity compared with that previously reported for each individual treatment. Risk-benefit profile does not warrant further evaluation of the combination in this population.
Keywords: Abemaciclib, KRAS-mutant, PD-L1 positive non–small cell lung cancer, Pembrolizumab, Squamous
Introduction
A hallmark of cancerous growth is uncontrolled cell division. Constitutive or deregulated activity of cell cycle regulatory proteins including CDK leads to rapid progression through the cell cycle.1 CDK 4 and 6 participate in a complex with D-type cyclins to initiate the transition through the G1 restriction point.1 Inhibiting CDK 4 and 6 may prevent cell cycle progression, thus arresting tumor growth.1,2 In addition to cell cycle deregulation, cancer cells have the ability to escape immune surveillance. One such approach is through the immune checkpoint including the programmed cell death protein-1 (PD-1) or programmed death-ligand 1 (PD-L1) pathway to inhibit immune activation.3 Thus, inhibition of PD-1 and PD-L1 can lead to restoration of antitumor immunity.
Lung cancer is the second most often diagnosed cancer and the leading cause of cancer death in the world.4 NSCLC represents 80% to 85% of all lung cancer cases, of which approximately 20% is squamous cell carcinoma.5,6 Treatment options for squamous NSCLC present challenges and have poorer outcomes because of the lack of driver mutations responding to approved agents.7,8 For patients with advanced NSCLC without an oncogenic driver mutation, platinum-based therapy or immune checkpoint inhibitor–containing regimens are the standard first-line therapy. Nevertheless, not all patients respond to first-line therapy. Furthermore, KRAS is the most often mutated oncogene in NSCLC and has long been associated with poor prognoses.10, 11, 9 Hence, developing treatment for patients with advanced NSCLC remains a considerable unmet medical need.12
Abemaciclib is an oral, potent, and selective small-molecule inhibitor of CDK 4 and 613,14 and has been found to have activity in multiple human xenograft models, including NSCLC.1,15 Synthetic lethal interaction between KRAS mutation and CDK 4 inhibition indicates a potential therapeutic application for CDK 4 and 6 inhibitors in KRAS-mutant NSCLC.16 Several preclinical studies conducted with KRAS-mutant NSCLC models revealed increased activity either alone or in combination with other cytotoxic or targeted agents suggesting increased sensitivity to abemaciclib in the presence of activated KRAS oncogenes.1,15,16
Pembrolizumab is a humanized immunoglobulin G4 monoclonal antibody with high specificity of binding to the PD-1 receptor.17 Pembrolizumab is approved for treatment of metastatic NSCLC in a variety of settings.17,18 Abemaciclib and pembrolizumab each have single-agent activity and acceptable safety profiles in metastatic NSCLC with minimal overlapping toxicity.17,19 Preclinical and preliminary clinical data support the investigation of whether the combination of abemaciclib and pembrolizumab could provide treatment options for patients with stage IV NSCLC.
In part E of the I3Y-MC-JPBJ study, which enrolled and treated 20 patients with stage IV NSCLC with abemaciclib and pembrolizumab, the maximum tolerated dose of abemaciclib (150 mg twice daily) and pembrolizumab (200 mg infused intravenously on day 1 of 21-d cycles) was determined.20 This study was designed to further evaluate the safety and preliminary anticancer activity of this combination in patients with stage IV NSCLC or HR+, HER2− metastatic breast cancer (MBC).
Here, we report the safety and efficacy results from a phase 1b study of abemaciclib plus pembrolizumab as initial treatment in patients with KRAS-mutant, PD-L1–positive nonsquamous NSCLC (cohort A) or in pretreated squamous NSCLC (cohort B). Results of the MBC cohorts will be reported separately.
Materials and Methods
Study Design and Treatment
JPCE was a multicenter, nonrandomized, open-label, phase 1b study of abemaciclib in combination with pembrolizumab. The study was conducted using a parallel design in four tumor-specific cohorts, which are the following: stage IV KRAS-mutant, PD-L1–positive (PD-L1 tumor proportion score [TPS] ≥ 1%), nonsquamous NSCLC (cohort A); stage IV squamous NSCLC previously treated by one platinum-containing chemotherapy regimen (cohort B); and two cohorts of HR+, HER2− patients with MBC who were treatment naive or who were pretreated for metastatic disease. Here, we report the safety and efficacy in cohorts A and B.
Patients received abemaciclib (150 mg orally twice daily) on days 1 to 21 in combination with pembrolizumab (200 mg infused intravenously in approximately 30 min) on day 1 of a 21-day cycle. Abemaciclib dose reduction was required for drug-related hematologic toxicity that was recurrent grade 3 or grade 4 or that required administration of blood cell growth factors; persistent or recurrent grade 2 or grade 3 to 4 diarrhea or any-grade diarrhea requiring hospitalization; persistent or recurrent grade 2 or grade 3 alanine aminotransferase (ALT) or aspartate aminotransferase (AST) increased without total bilirubin greater than 2 times from the upper limit of normal; grade 2 interstitial lung disease (ILD) or pneumonitis; or any grade 3 to 4 nonhematologic toxicity not mentioned previously. Abemaciclib was discontinued for grade 3 ALT increased with total bilirubin greater than 2 times from the upper limit of normal or grade 4 ALT increased and for recurrent grade 2 or grade 3 to 4 ILD or pneumonitis.
Pembrolizumab was withheld (dose reductions were not permitted) for prespecified drug-related toxicities and severe or life-threatening adverse events (AEs) including permanent discontinuation for the following: grade 4 diarrhea or colitis; grade 3 to 4 AST, ALT, or increased bilirubin; grade 4 hyperthyroidism; grade 3 to 4 infusion reaction; grade 3 to 4 or recurrent grade 2 pneumonitis; grade 3 to 4 renal failure or nephritis; grade 3 to 4 myocarditis; and grade 4 or recurrent grade 3 immune-related toxicities and for any severe or grade 3 (grade 2 for pneumonitis) drug-related AE that recurs or any life-threatening event.
Treatment continued until patients experienced disease progression, unacceptable toxicity, or another criterion for discontinuation. Study completion occurred after the final analysis of overall survival (OS), approximately 1 year after the last patient entered treatment in the study. Patients who remained on the study treatment at the time of study completion could continue receiving the study treatment if they were experiencing clinical benefit with no undue risks.
The study protocol was approved by institutional review boards and ethics committees before initiation and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Study Objectives
The primary objective was to characterize the safety profile of abemaciclib in combination with pembrolizumab. Secondary objectives were to evaluate the preliminary anticancer activity of abemaciclib in combination with pembrolizumab defined as objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS), according to the Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v.1.1)21 and OS.
Safety and Efficacy End Points
Safety outcomes were evaluated by physical examination results, clinical laboratory findings, and vital signs. AE terms and severity grades were assigned by the investigator using Common Terminology Criteria for Adverse Events version 4.0 and coded using the Medical Dictionary for Regulatory Activities. End points included treatment-emergent AEs (TEAEs), defined as an event that first occurred or worsened in severity after baseline, and serious AEs (SAEs).
Efficacy end points included ORR, DCR, and PFS according to RECIST v.1.1 and OS. On the basis of the definition of target lesions at baseline, tumor response was evaluated by the investigator, according to RECIST v.1.1 every 6 weeks (±7 d) from cycle 1, day 1 for 48 weeks, and then every 9 weeks thereafter. The same methods of assessment and technique were to be used at baseline and for each consecutive assessment. A designation of partial response (PR) or complete response (CR) required the changes in tumor measurements to be confirmed by repeat assessments that were performed no less than 4 weeks after the criteria for response were met. Best overall response (BOR) was the best response recorded from the start of treatment until disease progression. ORR was defined as the proportion of treated patients achieving a BOR of PR or CR. DCR was defined as the proportion of treated patients achieving a BOR of CR plus PR plus stable disease. PFS was defined as the time from the date of first treatment until the date of radiographic documentation of progression (as defined by RECIST v.1.1) on the basis of investigator assessment or the date of death owing to any cause, whichever occurred earlier. At the data cutoff date, living patients for whom no disease progression was observed had a PFS censoring date corresponding to the last tumor evaluation date. OS was defined as time from the date of first treatment to the date of death from any cause. For each patient who was not known to have died as of the data cutoff date, OS was censored at the date of last contact date before the cutoff date.
Patient Population
Patients accrued in cohort A were previously untreated, had metastatic nonsquamous NSCLC with a KRAS mutation, and had a tumor PD-L1 TPS greater than or equal to 1%. Patients accrued in cohort B had metastatic squamous NSCLC and must have received only one previous therapy containing platinum-based chemotherapy for advanced or metastatic NSCLC. Previous therapy in the neoadjuvant or adjuvant setting was considered as a previous line of systemic chemotherapy if the disease of the patient progressed less than or equal to 6 months since last dose.
Patients in both cohorts were at least 18 years old and were required to have measurable disease as defined by RECIST v.1.1, an Eastern Cooperative Oncology Group performance status of less than or equal to 1,22 and adequate organ functions.
Baseline PD-L1 protein expression was evaluated by an immunohistochemistry assay in tumor tissue samples using DAKO PD-L1 22C3 immunohistochemistry PharmDx (catalog #SK006, Dako, Agilent Technologies, Carpinteria, CA) performed at NeoGenomics Laboratories.17,23 Baseline PD-L1 TPS greater than or equal to 50% was defined as strong TPS, and a PD-L1 TPS of 1% to 49% was defined as weak TPS. Allele-specific polymerase chain reaction or next-generation sequencing was used to locally determine KRAS mutation status.
Key exclusion criteria included a history of or current pneumonitis or ILD, an active autoimmune disease, or previous treatment with an anti–PD-1 or PD-L1 or any CDK 4 and 6 inhibitor.
Statistical Analyses
This was a nonrandomized, open-label, phase 1b study of abemaciclib in combination with pembrolizumab with safety as the primary end point. A sample size of 25 patients per cohort was planned to enroll in the study.
The safety and efficacy analyses were conducted on the safety population, which included all patients who received at least one dose of study treatment (abemaciclib or pembrolizumab). Baseline characteristics and safety data were summarized by each cohort. ORR and DCR were summarized by cohort and included exact 95% confidence intervals (CIs) using Clopper-Pearson method. Median OS and PFS were estimated for each cohort with 95% CIs using the Kaplan-Meier method.24 Individual changes in tumor burden over time are presented graphically (as waterfall plots).
Results
Patient Demographics, Treatment, and Disposition
A total of 25 patients with NSCLC from six countries (n = 4 Belgium, n = 2 Spain, n = 4 France, n = 5 Italy, n = 2 Turkey, n = 8 United States) were enrolled in cohort A and 25 patients from seven countries (n = 2 Belgium, n = 4 Spain, n = 7 France, n = 1 Italy, n = 4 Turkey, n = 5 Taiwan, n = 2 United States) were enrolled in cohort B. All patients received treatment with abemaciclib in combination with pembrolizumab (safety population). Most patients from both cohorts were white (n = 33; 66.0%) and male (n = 32; 64.0%) with an Eastern Cooperative Oncology Group performance status of 1 (n = 33; 66%) (Table 1). The most frequent KRAS mutation was G12C (52%) followed by G12V (20%) in cohort A. Most patients in cohort B (21 [84.0%]) received chemotherapy in the metastatic setting (Table 1), and four patients had disease progression within 6 months on chemotherapy in the (neo)adjuvant setting.
Table 1.
Baseline Patient Demographics and Disease Characteristics
| Characteristics | Cohort A, KRAS-mt NSCLC (N = 25) | Cohort B, Squamous NSCLC (N = 25) |
|---|---|---|
| Sex, n (%) | ||
| Male | 11 (44.0) | 21 (84.0) |
| Female | 14 (56.0) | 4 (16.0) |
| Age, y | ||
| Median (range) | 62 (36–80) | 60 (42–73) |
| Race, n (%) | ||
| White | 21 (84.0) | 12 (48.0) |
| Asian | 0 | 5 (20.0) |
| Black or African American | 0 | 1 (4.0) |
| Not reported | 4 (16.0) | 7 (28.0) |
| ECOG PS, n (%) | ||
| 0 | 9 (36.0) | 8 (32.0) |
| 1 | 16 (64.0) | 17 (68.0) |
| Smoking status, n (%) | ||
| Current | 7 (28.0) | 4 (16.0) |
| Past | 17 (68.0) | 21 (84.0) |
| Never | 1 (4.0) | 0 |
| Duration of disease from initial diagnosis, mo | ||
| Median (range) | 1.9 (0.7–27.1) | 7.4 (2.0–50.9) |
| Previous systemic therapy, n (%) | NA | 25 (100.0) |
| Metastatic disease, n (%) | NA | 21 (84.0)a |
| PD-L1 status, n (%) | ||
| Strong (≥50%) | 13 (52.0) | 2 (8.0) |
| Weak (1%–49%) | 12 (48.0) | 3 (12.0) |
| Negative | 0 | 16 (64.0) |
| NA | 0 | 4 (16.0) |
| KRAS mutation status | ||
| G12C | 13 (52.0) | NA |
| G12V | 5 (20.0) | NA |
| Others | 7 (28.0) | NA |
ECOG PS, Eastern Cooperative Oncology Group performance status; KRAS-mt, KRAS-mutant; n, number of subjects in the specified category; N, number of subjects in safety population; NA, not applicable; PD-L1, programmed death-ligand 1.
Four patients had disease progression within 6 months since the last dose of chemotherapy in the (neo)adjuvant setting.
In cohort A and B, the median duration of abemaciclib treatment was 9.7 weeks (range: 0.6–112.6) and 12.9 weeks (range: 0.3–159.0), respectively. The median duration of pembrolizumab treatment was 12.0 weeks (range: 3.0–112.6) and 13.1 weeks (range: 1.3–159.0) in cohort A and B, respectively. In both cohorts, all patients completed greater than or equal to 1 cycle. In cohort A, the patients received a median of three cycles (range: 1–38 cycles) of abemaciclib and a median of three cycles (range: 1–35) of pembrolizumab. In cohort B, the patients received a median of three cycles (range: 1–53) of abemaciclib and a median of four cycles (range: 1–35) of pembrolizumab.
At the time of data cutoff (August 19, 2020), 24 patients (96.0%) in each cohort had discontinued study treatment.
In cohort A, the main reasons for treatment discontinuation were AE (n = 7, 28.0%), progressive disease (PD) (n = 7; 28.0%), physician decision (n = 5; 20.0%), and withdrawal by patient’s decision (n = 4; 16.0%). In cohort B, the most common reason for treatment discontinuation was PD (n = 17; 68.0%) followed by AE or death (n = 2 each).
Safety
An overview of TEAEs by cohort is presented in Table 2. A total of 13 patients (52.0%) in each cohort (A and B) had greater than or equal to 1 SAE, six (24.0%) and four (16.0%) of which were deemed related to treatment, respectively. In cohort A, eight patients (32%) discontinued study treatment owing to an AE, three (12%) owing to AST or ALT increased, and two (8%) owing to pneumonitis. In cohort B, four patients (16%) discontinued study treatment owing to an AE, one (4%) each owing to cardiogenic shock, dyspnea, acute hepatitis, and thrombocytopenia. In cohort A, one patient experienced a study treatment-related death of pneumonitis. In cohort B, three patients (12.0%) died owing to TEAEs while on study treatment or within 30 days of discontinuation, none of which were considered related to the treatment.
Table 2.
Safety Overview
| Number of Patients,a n (%) | Cohort A, KRAS-mt NSCLC (N = 25) | Cohort B, Squamous NSCLC (N = 25) |
|---|---|---|
| Patients with ≥1 TEAE | 25 (100.0) | 25 (100.0) |
| Related to study treatmentb | 22 (88.0) | 23 (92.0) |
| Patients with ≥1 grade ≥ 3 TEAE | 20 (80.0) | 19 (76.0) |
| Related to study treatmentb | 16 (64.0) | 13 (52.0) |
| Patients with ≥1 SAE | 13 (52.0) | 13 (52.0) |
| Related to study treatmentb | 6 (24.0) | 4 (16.0) |
| Patients on treatment | 1 (4.0) | 1 (4.0) |
| Patients who discontinued study treatment owing to AE | 8 (32.0) | 4 (16.0) |
| Related to study treatmentb | 6 (24.0) | 2 (8.0) |
| Patients who died owing to AE on study treatment or within 30 d of discontinuation from study treatmentc | 2 (8.0) | 3 (12.0) |
| Related to study treatmentb | 1 (4.0) | 0 |
AE, adverse event; KRAS-mt, KRAS-mutant; n, number of subjects in the specified category; N, number of subjects in safety population; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Patients may be counted in more than one category.
Included events that were considered related to study treatment as judged by the investigator.
Deaths also included as SAEs and discontinuations owing to AEs.
In cohort A, the most frequent all-grade TEAEs regardless of causality that occurred in greater than or equal to 20% of patients were diarrhea (n = 15; 60.0%); abdominal pain, vomiting, and nausea (n = 10 each; 40.0%); decreased appetite and fatigue (n = 9 each; 36.0%); and pneumonitis, AST increased, and dyspnea (n = 8 each; 32.0%) (Table 3). Grade greater than or equal to 3 TEAEs were reported in 20 patients (80.0%) with the most common being ALT increased (n = 6; 24.0%), followed by cardiac disorders (atrial fibrillation, cardiac arrest, pericardial effusion, n = 1 each) and diarrhea, AST increased, pneumonitis, and neutropenia (n = 3 each; 12.0%).
Table 3.
TEAEs (All-Causality) in Greater Than or Equal to 20% Patients (in Either Cohort, Any Grade)
| MedDRA Preferred Term | Cohort A KRAS-mt NSCLC (N = 25), n (%) |
Cohort B Squamous NSCLC (N = 25), n (%) |
||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| Patients with ≥1 TEAE | 25 (100) | 20 (80) | 25 (100) | 19 (76) |
| Diarrhea | 15 (60) | 3 (12) | 16 (64) | 0 |
| Vomiting | 10 (40) | 2 (8) | 5 (20) | 1 (4) |
| Abdominal pain | 10 (40) | 1 (4) | 2 (8) | 0 |
| Nausea | 10 (40) | 2 (8) | 8 (32) | 0 |
| Decreased appetite | 9 (36) | 1 (4) | 10 (40) | 0 |
| Fatigue | 9 (36) | 0 | 15 (60) | 2 (8) |
| Pneumonitis | 8 (32) | 3 (12) | 1 (4) | 0 |
| AST increased | 8 (32) | 3 (12) | 1 (4) | 0 |
| Dyspnea | 8 (32) | 0 | 11 (44) | 3 (12) |
| ALT increased | 7 (28) | 6 (24) | 1 (4) | 0 |
| Neutropenia | 6 (24) | 3 (12) | 3 (12) | 0 |
| Lung infection | 6 (24) | 2 (8) | 4 (16) | 3 (12) |
| Anemia | 6 (24) | 1 (4) | 5 (20) | 0 |
| Leukopenia | 5 (20) | 1 (4) | 1 (4) | 0 |
| Muscular weakness | 5 (20) | 1 (4) | 4 (16) | 1 (4) |
| Pruritus | 5 (20) | 1 (4) | 8 (32) | 0 |
| Dizziness | 5 (20) | 0 | 0 | 0 |
| Pain | 5 (20) | 0 | 0 | 0 |
| Pain in extremity | 5 (20) | 0 | 0 | 0 |
| Cough | 4 (16) | 0 | 9 (36) | 0 |
| Thrombocytopenia | 4 (16) | 0 | 8 (32) | 3 (12) |
| Headache | 4 (16) | 0 | 5 (20) | 0 |
| Hypophosphatemia | 1 (4) | 1 (4) | 6 (24) | 3 (12) |
| Myalgia | 1 (4) | 0 | 5 (20) | 2 (8) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; KRAS-mt, KRAS-mutant; MedDRA, Medical Dictionary for Regulatory Activities; n, number of subjects in the specified category; N, number of subjects in safety population; TEAE, treatment-emergent adverse event.
In cohort B, the most frequent all-grade TEAEs regardless of causality were diarrhea (n = 16; 64.0%); fatigue (n = 15; 60.0%); dyspnea (n = 11; 44.0%); decreased appetite (n = 10; 40%); cough (n = 9; 36.0%); and nausea, thrombocytopenia, and pruritis (n = 8 each; 32.0%) (Table 3). Grade greater than or equal to 3 TEAEs were reported in 19 patients (76.0%) with the most common being dyspnea, lung infection, thrombocytopenia, and hypophosphatemia (n = 3 each; 12.0%) (Table 3).
In cohort A, abemaciclib and pembrolizumab dose adjustments occurred for 16 (64.0%) and 13 patients (52.0%), respectively. Abemaciclib dose adjustments included dose reductions (n = 9; 36.0%) and dose omissions (n = 15; 60.0%). Pembrolizumab dose adjustments included dose delays (n = 10; 40.0%) and dose omissions (n = 8; 32.0%). In cohort B, abemaciclib and pembrolizumab dose adjustments occurred for 15 (60.0%) and 12 patients (48.0%), respectively. Abemaciclib dose reductions occurred for seven patients (28.0%) and dose omissions for 14 patients (56.0%). Pembrolizumab dose adjustments included dose delays for 10 patients (40.0%) and dose omissions for two patients (8.0%). The most common reason for abemaciclib and pembrolizumab dose adjustments was having AEs in both cohorts.
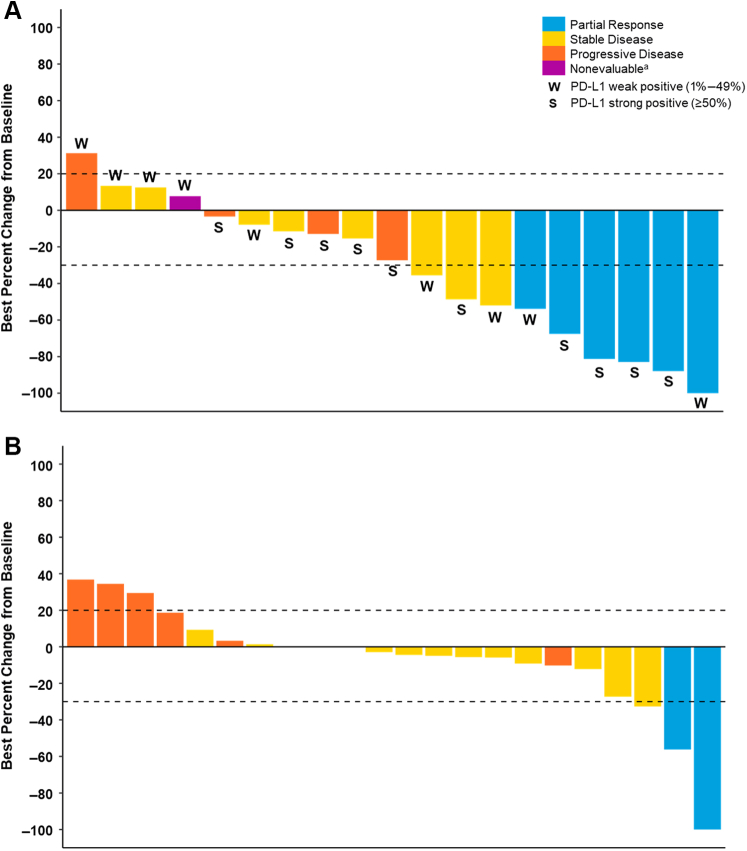
Anticancer Activity
In cohort A, six patients (24.0%) had a BOR of PR and eight patients had a BOR of stable disease (32.0%) for a DCR of 56% (Table 4). Two patients (8.0%) achieved stable disease that persisted for more than or equal to 6 months. The best percent change in tumor size (according to RECIST v.1.1 criteria for treated patients in cohort A) is illustrated in Figure 1A. Anticancer activity in cohort A was also evaluated by baseline PD-L1 TPS. The median PFS was 7.6 months (95% CI: 1.6–not estimable), and the 12-month PFS rate was 44.6% (95% CI: 21.2–65.7). At the time of data cutoff, the median follow-up time for OS was 34.7 months. The median OS was 27.8 months (95% CI: 9.9–not estimable), and the 12-month survival rate was 69.6% (95% CI: 46.6–84.2).
Table 4.
Summary of Anticancer Activity (Safety Population)
| Response | Cohort A KRAS-mt NSCLC (N = 25) n (%) |
Cohort B Squamous NSCLC (N = 25) n (%) |
||
|---|---|---|---|---|
| n (%) | 95% CIa | n (%) | 95% CIa | |
| Best overall response | ||||
| CR | 0 | NA | 0 | NA |
| PR | 6 (24.0) | 9.4–45.1 | 2 (8.0) | 1.0–26.0 |
| Stable disease | 8 (32.0) | 15.0–53.5 | 14 (56.0) | 34.9–75.6 |
| PD | 5 (20.0) | 6.8–40.7 | 7 (28.0) | 12.1–49.4 |
| Nonevaluable | 6 (24.0) | 9.4–45.1 | 2 (8.0) | 1.0–26.0 |
| Overall response rate (CR + PR) | 6 (24.0) | 9.4–45.1 | 2 (8.0) | 1.0–26.0 |
| Disease control rate (CR + PR + stable disease) | 14 (56.0) | 34.9–75.6 | 16 (64.0) | 42.5–82.0 |
| PFS, median mo (95% CI) | 7.6 (1.6–NE) | 3.3 (1.4–5.2) | ||
| OS, median mo (95% CI) | 27.8 (9.9–NE) | 6.0 (3.7–13.1) | ||
CI, confidence interval; CR, complete response; KRAS-mt, KRAS-mutant; n, number of subjects in the specified category; N, number of subjects in safety population; NA, not applicable; NE, not estimable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response.
CIs were based on the Clopper-Pearson method.
Figure 1.
Best percentage change in tumor size from baseline according to RECIST version 1.1. Best percentage change in tumor size from baseline is presented for the safety populations in (A) cohort A and (B) cohort B. The PD-L1 TPS in cohort A was evaluated by an IHC assay in tumor tissue samples (see the Materials and Methods section). Strong PD-L1 TPS was greater than or equal to 50%, and weak PD-L1 TPS was 1% to 49%. Best overall responses presented here are confirmed responses. Note: Patients without any postbaseline data are not included in the graphs. aThe patient did not meet stable disease criteria. IHC, immunohistochemistry; PD-L1, programmed death-ligand 1; RECIST, Response Evaluation Criteria In Solid Tumors; TPS, tumor proportion score.
The subgroup of patients in cohort A with strong PD-L1 TPS (n = 13) had an ORR of 30.8% (95% CI: 9.1–61.4). Patients with weak PD-L1 TPS (n = 12) had an ORR of 16.7% (95% CI: 2.1–48.4). Patients with strong PD-L1 TPS had a PFS and OS of 14.8 months (95% CI: 1.4–not estimable) and 25.9 months (95% CI: 5.0–not estimable), respectively; those with weak PD-L1 TPS had a PFS and OS of 5.5 months (95% CI: 0.4– not estimable) and 27.8 months (95% CI: 2.4–not estimable), respectively. The subgroup of patients with KRAS G12C mutation (n = 13) had an ORR of 23.1% (95% CI: 5.0–53.8) and a PFS and OS of 15.9 months (95% CI: 0.39–not estimable) and 25.9 months (95% CI: 3.7–not estimable), respectively. The analyses of patients with other KRAS mutations were limited owing to the small sample size.
In cohort B, two patients (8.0%) had a BOR of PR and 14 had stable disease (56.0%) (Table 4). In four patients (16.0%), stable disease persisted for more than or equal to 6 months. ORR and DCR were 8.0% and 64.0%, respectively (Table 4). The median PFS was 3.3 months (95% CI: 1.4–5.2), and the 12-month PFS survival rate was 13.8% (95% CI: 3.5–31.0). The best percentage change in tumor size according to RECIST v.1.1 criteria for treated patients in cohort B is illustrated in Figure 1B. At the time of data cutoff, the median follow-up time for OS was 35.5 months. The median OS was 6.0 months (95% CI: 3.7–13.1), and the 12-month survival rate was 35.3% (95% CI: 17.0–54.3).
Discussion
This phase 1b study evaluated the safety and preliminary efficacy of abemaciclib in combination with pembrolizumab for patients with metastatic NSCLC or HR+, HER2− MBC. Reported here are the results for the two cohorts of patients with either PD-L1–positive, KRAS-mutant, previously untreated, nonsquamous stage IV NSCLC (cohort A) or with previously treated squamous NSCLC (cohort B). Abemaciclib in combination with pembrolizumab was associated with a higher rate of transaminase elevation and pneumonitis in cohort A than has previously been reported for either drug alone. Evidence of anticancer activity was observed in both cohorts.
The overall safety profile of abemaciclib in combination with pembrolizumab in cohort A was similar to the single-agent safety profiles, with exceptions for transaminase elevations and ILD or pneumonitis. Compared with JUNIPER, a phase 3 study that evaluated monotherapy abemaciclib versus erlotinib in pretreated patients with stage IV NSCLC with a KRAS mutation, in the study presented here, we observed a higher incidence of pneumonitis (32%), AST increased (32%), and ALT increased (28%) in cohort A (versus <10% from JUNIPER).25 Observed pneumonitis and transaminase elevation incidences were higher when compared with monotherapy pembrolizumab for patients with untreated advanced NSCLC in the KEYNOTE-042 study (ALT increased, 7%; AST increased, 6%; and pneumonitis, 8%).26 This contributed to the higher discontinuation rate owing to AE observed in cohort A when compared with that of JUNIPER and KEYNOTE-042 (32% versus 12% and 9%, respectively).25,26 These compared similarly to the data reported previously in the phase 1b study JPBJ (NCT02079636) part E in which 10 of 20 patients (50%) experienced an SAE, five (25%) of which were treatment-related events of hepatic toxicity, hepatitis, pneumonitis, ALT or AST increased, and neutropenia (n = 1 each).20 Similarly, the NEWFLAME trial, which investigated abemaciclib in combination with another anti–PD-1 immune checkpoint inhibitor, nivolumab, plus endocrine therapy in patients with MBC, reported similar safety findings to this study, including high rates of treatment discontinuation, elevated liver function tests, and ILD or pneumonitis.27
The ORR observed from this study in cohort A (24%, 95% CI: 9.4–45.1) compares well with that observed with monotherapy pembrolizumab versus platinum-based chemotherapy (KEYNOTE-042) as first-line treatment for patients with advanced NSCLC and PD-L1 TPS greater than or equal to 1% (27% for pembrolizumab).26 An exploratory analysis from KEYNOTE-042 suggests that the subgroup of patients with KRAS-mutant NSCLC had a higher response rate of 56.7% (95% CI: 37.4–74.5) with pembrolizumab versus 18% (95% CI: 7.5–33.5) in the chemotherapy arm.28 In the study presented here, patients with KRASG12C mutation had greater PFS but similar ORR and OS relative to the intent-to-treat population. It is noteworthy that this exploratory analysis is based on a small sample size. The most frequent oncogenic driver mutation in NSCLC is in the KRAS gene. Recently, a KRASG12C inhibitor (sotorasib) has been found to have a tolerable safety profile and encouraging anticancer activity in pretreated patients with NSCLC.29 This expanded phase 1 study accrued 59 patients suffering from KRASG12C-mutant NSCLC who experienced PD although having been heavily pretreated and receiving single-drug daily sotorasib (dose escalation up to 960 mg): the ORR, DCR, and median PFS were 32%, 88%, and 6.3 months, respectively. These promising features deserved an ongoing phase 3 trial comparing sotorasib with docetaxel (ClinicalTrials.gov identifier: NCT04303780). In a mouse model, it has been found that CDK 4 inhibition could induce selective death of KRAS-mutant cancer cells.16 Taking into account the acceptable safety profile of both abemaciclib and sotorasib, the combination of a CDK 4 and 6 inhibitor with a KRASG12C inhibitor may provide a potential therapeutic opportunity for patients with NSCLC harboring the KRASG12C mutation.
A prespecified exploratory analysis from KEYNOTE-042 revealed that patients in the pembrolizumab arm with a PD-L1 TPS of greater than or equal to 50% had an ORR of 39% versus 33% in patients with PD-L1 TPS of greater than or equal to 20%.30 This is consistent with the higher response rates observed here in the subgroup of patients with tumors that had a strong PD-L1 TPS versus weak PD-L1 TPS in cohort A.
In cohort B of this study, patients with pretreated squamous NSCLC had a low response rate of 8% (95% CI: 1.0–26.0) and a median OS of 6.0 months (95% CI: 3.7–13.1), which aligns with the subgroup analyses of OS from the KEYNOTE-010 study. KEYNOTE-010 enrolled patients with previously treated nonsquamous and squamous NSCLCs with PD-L1 expression of at least 1% of tumor cells who were treated with different doses of pembrolizumab (2 mg/kg or 10 mg/kg) or docetaxel. Although the study reported a significant OS benefit for the intent-to-treat population and the subgroup of patients with nonsquamous NSCLC, the post hoc exploratory subgroup analyses of OS from patients with squamous NSCLC did not reveal a statistically significant survival benefit in the pooled pembrolizumab arms versus docetaxel (OS hazard ratio = 0.74, 95% CI: 0.50–1.09).17 Nevertheless, CheckMate 017 revealed a survival benefit with nivolumab versus docetaxel for previously treated patients with squamous cell NSCLC.31
This study was limited by the small sample size making it difficult to extrapolate results to the clinical population. In addition, this phase 1b study was a single-arm trial without a randomized control arm. Data should be hypothesis generating, and results should be interpreted with caution.
In conclusion, the combination of abemaciclib and pembrolizumab resulted in a higher rate of transaminase elevations, pneumonitis, and treatment discontinuation owing to AE in PD-L1–positive, KRAS-mutant, nonsquamous NSCLC. Although evidence of anticancer activity was observed in both treatment naive nonsquamous and pretreated squamous NSCLC cohorts, further evaluation of the combination of abemaciclib and pembrolizumab for the treatment of patients with stage IV NSCLC is not supported by the totality of the current benefit-risk analysis.
CRediT Authorship Contribution Statement
Jean-Louis Pujol, Johan Vansteenkiste, Luis Paz-Ares Rodríguez, Vanesa Gregorc, Julien Mazieres, Mark Awad, Pasi A. Jänne, J. Thaddeus Beck: Investigation, Writing—review and editing.
Michael Chisamore: Supervision, Writing—review and editing.
Anwar M. Hossain: Software, Validation, Formal analysis, Writing—review and editing, Visualization.
Yanyun Chen: Supervision, Writing—original draft, Writing—review and editing.
Acknowledgments
This work was funded by Eli Lilly and Company. The authors thank the patients and their caregivers for their participation in this study, the study investigators and their staff, and the JPCE (NCT02779751) clinical trial team. Pembrolizumab was provided by Merck & Co., Inc., Kenilworth, New Jersey. Medical writing support, funded by Eli Lilly and Company, was provided by Andrea D. Humphries and Anchal Sood, and editorial support was provided by Cynthia Rae Abbott of Syneos Health.
Footnotes
Disclosure: Vansteenkiste reports receiving fees or services for medical education lectures from Eli Lilly and Company. Rodríguez reports receiving fees for scientific advice from Merck Sharp & Dohme, Roche, Eli Lilly and Company, Merck, Novartis, Amgen, Takeda, Blueprint, Bayer, PharmaMar, and Pfizer; receiving grants and fees for scientific advice from Bristol-Myers Squibb and AstraZeneca; and serving as an external board member for Genomica and a founder and board member for Altum Sequencing, outside of the submitted work. Mazieres is on the advisory boards for Merck, Roche, AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, Pfizer, Hengrui, Daiichi, and Boehringer, and Pierre Fabre and reports receiving research grants from Roche, AstraZeneca, and Pierre Fabre, outside of the submitted work. Awad reports receiving grants and personal fees from Genentech, Bristol-Myers Squibb, and AstraZeneca; grants from Eli Lilly and Company; and personal fees from Merck, Maverick, Blueprint Medicines, Syndax, Ariad, Nektar, Gritstone, ArcherDX, Mirati, NextCure, Novartic, and EMD Serono, outside of the submitted work. Dr. Jänne reports receiving grants and consulting fees from Eli Lilly and Company during the conduct of the study, and consulting fees from Mirati Therapeutics, Pfizer, Roche/Genentech, Chugai Pharmaceuticals, Ignyta, Novartis, Voronoi, SFJ Pharmaceuticals, Biocartis, Loxo Oncology, Sanofi, Transcenta, Silicon Therapeutics, and AbbVie; grants and consulting fees from Boehringer Ingelheim, AstraZeneca, Takeda Oncology, Puma, and Daiichi Sankyo; and grants from Astellas Pharmaceuticals outside of the submitted work. Dr. Jänne reports receiving postmarketing royalties on a DFCI-owned patent on EGFR mutations licensed to Lab Corp. Chisamore is an employee of Merck & Co. Chen reports having full-time employment for Eli Lilly and Company. Hossain reports having full-time employment and stock ownership for Eli Lilly and Company. Beck reports receiving grants from Novartis Pharmaceuticals paid to their institution. Pujol and Gregorc declare no conflict of interest.
Cite this article as: Pujol JL, Vansteenkiste J, Rodríguez LPA, et al. Abemaciclib in combination with pembrolizumab for stage IV KRAS-mutant or squamous NSCLC: a phase 1B study. JTO Clin Res Rep. 2021;2:100234.
References
- 1.Gelbert L.M., Cai S., Lin X. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asghar U., Witkiewicz A.K., Turner N.C., Knudsen E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disis M.L. Immune regulation of cancer. J Clin Oncol. 2010;28:4531–4538. doi: 10.1200/JCO.2009.27.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger D.S., Wood D.E., Aisner D.L. Non-small cell lung cancer version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 6.Travis W.D. Pathology of lung cancer. Clin Chest Med. 2011;32:669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Garon E.B., Ciuleanu T.E., Arrieta O. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 8.Simon G.R. nab-paclitaxel for the treatment of advanced squamous none-small-cell lung cancer: a comprehensive update. Clin Lung Cancer. 2014;15:391–397. doi: 10.1016/j.cllc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Karachaliou N., Mayo C., Costa C. KRAS mutations in lung cancer. Clin Lung Cancer. 2013;14:205–214. doi: 10.1016/j.cllc.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Slebos R.J., Kibbelaar R.E., Dalesio O. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 11.Nadal E., Chen G., Prensner J.R. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. 2014;9:1513–1522. doi: 10.1097/JTO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 12.FDA approves first KRAS inhibitor: sotorasib. Cancer Discov. 2021;11:OF4. doi: 10.1158/2159-8290.CD-NB2021-0362. [DOI] [PubMed] [Google Scholar]
- 13.Patnaik A., Rosen L.S., Tolaney S.M. LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol. 2014;32(suppl 15) 534–534. [Google Scholar]
- 14.Scott S.C., Lee S.S., Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol. 2017;44:385–394. doi: 10.1053/j.seminoncol.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey J.A., Chan E.M., Burke T.F. LY2835219, a selective inhibitor of CDK4 and CDK6, inhibits growth in preclinical models of human cancer. Cancer Res. 2013;73(suppl) Abstract nr LB-122. [Google Scholar]
- 16.Puyol M., Martin A., Dubus P. A synthetic lethal interaction between K-Ras oncogenes and CDK4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1 positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network Non-small cell lung cancer. http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf version 3.2020.
- 19.Patnaik A., Rosen L.S., Tolaney S.M. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 20.Goldman J.W., Provencio M., Jalal S. P3.02c-102 safety and tolerability of abemaciclib combined with LY3023414 or with pembrolizumab in patients with stage IV NSCLC. J Thorac Oncol. 2017;12(suppl):S1341–S1342. [Google Scholar]
- 21.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 23.Arkenau H.T., Martin-Liberal J., Calvo E. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF) Oncologist. 2018;23 doi: 10.1634/theoncologist.2018-0044. 1407–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Asso. 1958;53:457–481. [Google Scholar]
- 25.Goldman J.W., Mazieres J., Barlesi F. A randomized phase III study of abemaciclib versus erlotinib in patients with stage IV non-small cell lung cancer with a detectable KRAS mutation who failed prior platinum-based therapy: JUNIPER. Front Oncol. 2020;10:578756. doi: 10.3389/fonc.2020.578756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok T.S.K., Wu Y.L., Kudaba I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 27.Masuda J., Tsurutani J., Masuda N. Phase II study of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR+, HER2- metastatic breast cancer: WJOG11418B NEWFLAME trial. Cancer Res. 2021;81(suppl) doi: 10.1136/jitc-2023-007126. Abstract nr PS12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbst R.S., Lopes G., Kowalski D.M. LBA4 association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann Oncol. 2019;30(suppl 11):xi63–xi64. [Google Scholar]
- 29.Hong D.S., Fakih M.G., Strickler J.H. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talmadge J.E. Immune cell infiltration of primary and metastatic lesions: mechanisms and clinical impact. Semin Cancer Biol. 2011;21:131–138. doi: 10.1016/j.semcancer.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]