Abstract
We have found that 17β-estradiol induces bcl-2 transcription in human breast cancer MCF-7 cells. To identify cis-acting elements involved in this regulation, we have analyzed hormone responsiveness of transiently transfected reporter constructs containing the bcl-2 major promoter (P1). Hormone inducibility was observed only when either of two sequences, located within the bcl-2 coding region and showing one and two mutations with respect to the consensus estrogen-responsive element, were inserted downstream from the P1 promoter. Both sequences behaved as enhancers exclusively in cells expressing the estrogen receptor and were able to bind this receptor in in vitro assays. Transfections into MCF-7 cells of plasmids carrying a bcl-2 cDNA fragment which included these two elements revealed that their simultaneous presence resulted in an additive effect on reporter gene activity, whose size resembled the increase of endogenous bcl-2 mRNA level observed in untransfected cells after hormone treatment. Moreover, the identified elements were able to mediate up-regulation of bcl-2 expression by 17β-estradiol, since exogenous bcl-2 mRNA was induced by hormone challenge of MCF-7 cells transiently transfected with a vector containing the bcl-2 coding sequence cloned under the control of a non-estrogen-responsive promoter. Finally, we show that hormone prevention of apoptosis, induced by incubating MCF-7 cells with hydrogen peroxide, was strictly related to bcl-2 up-regulation. Our results indicate that the bcl-2 major promoter does not contain cis-acting elements directly involved in transcriptional control by 17β-estradiol and that hormone treatment inhibits programmed cell death in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements located within its coding region.
Estrogens control cell proliferation in normal and transformed mammary epithelial cells, where they induce expression of immediate and delayed hormone-responsive genes, important for cell cycle progression (2, 7, 12, 54). However, factors which stimulate cellular proliferation have interplay with the molecular mechanisms that control programmed cell death (PCD), a physiological cell suicide mechanism induced by several stimuli and inhibited by the Bcl-2/Ced-9 family of proteins (39, 55). Bcl-2 has been shown, in fact, to prevent apoptosis after several treatments, including hydrogen peroxide (H2O2) (19), as its overexpression has been reported to inhibit plasma membrane blebbing, nuclear condensation, and DNA endonucleolytic cleavage, which are all classical features of PCD (20). Estrogens inhibit PCD in the mammary gland, which cyclically undergoes apoptosis at the end of the menstrual cycle (15), and in human breast cancer MCF-7 cells, expressing functional estrogen receptor (ER), in which an increase of bcl-2 mRNA level has been observed (29, 51).
Transcription of the bcl-2 gene is controlled by two promoters (45). The major promoter (P1), where 90 to 95% of transcripts initiate, is located approximately 1.6 kb upstream of the coding region (57). It is a TATA-less, GC-rich promoter, with multiple transcription start sites resembling the promoters of housekeeping genes such as that of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (8, 40). Several Sp1 binding sites and a cyclic AMP (cAMP)-responsive element (CRE) have been identified in the P1 sequence (45, 56). A minor promoter (P2) is located 1.3 kb downstream from the first one and includes a CCAAT and a TATA box (35). For this promoter, two transcription-inhibitory elements and an open reading frame, which behaves as a posttranscriptional down-regulator, have been characterized (17).
Estrogen activity is mediated by its cognate receptor (ER): occupancy by the hormone induces ER conformational changes which allow its interaction with specific enhancers known as estrogen-responsive elements (EREs) and with general transcription factors (5). To date, two isoforms of the ER (α and β) have been identified; however, even though both ER subtypes are able to bind to DNA as homo- or heterodimers, it has been shown previously that, in MCF-7 cells, ERα represents the largely predominant form, while ERβ is only barely detectable (37, 47).
The consensus ERE sequence is a 13-bp palindrome with 5-bp stems and a 3-bp spacer which was first discovered in the Xenopus laevis vitellogenin A2 gene, thus being referred to as vit-ERE (GGTCAcagTGACC) (25). However, most of the EREs so far identified show one or more mutated nucleotides compared to the consensus palindrome (6, 11, 13, 49), and several genes contain multiple half-sites (24). Although ERE-like sequences are mainly located in promoters of target genes, EREs have also been identified in other regions (9, 21, 22, 28, 30, 33).
We have investigated the mechanism by which 17β-estradiol up-regulates the bcl-2 mRNA level in MCF-7 cells, and we have found that the hormone induces bcl-2 gene transcription via two EREs located within the coding region. In addition, we show that hormone induction of bcl-2 expression mediates prevention of PCD observed in the same cells upon 17β-estradiol challenge.
MATERIALS AND METHODS
Materials.
Reagents and Rous sarcoma virus-lacZ plasmid were purchased from Sigma. Nonfat dry milk and the kit for determination of protein concentration were obtained from Bio-Rad. Actinomycin D was from U.S. Biochemical. Enzymes and random-primed DNA labeling kits used for probes were purchased from Roche. [α-32P]dATP (3,000 Ci/mmol), [γ-32P]dATP (6,000 Ci/mmol), d-threo-[14C]chloramphenicol (55 mCi/mmol), nitrocellulose and nylon filters, peroxidase-linked anti-rabbit antibody, and Western blotting detection reagents (ECL) were from Amersham. Synthetic oligonucleotides were from Primm. Triphosphate nucleotides were obtained from Perkin-Elmer. BA-85 nitrocellulose filters were purchased from Schleicher & Schuell. For autoradiography, Kodak XAR-5 films have been used with Du Pont Cronex intensifying screens (Du Pont). For thin-layer chromatography, Polygram plastic sheets were from Macherey-Nagel. All plasmids have been purified by use of Qiagen plasmid kits purchased from Qiagen Inc. For sequencing, the T7 sequencing kit from Pharmacia has been used. Monoclonal anti-ER antibody Ab 314 was obtained as previously described (1). Rabbit polyclonal antibody to poly(ADP-ribose) polymerase (PARP) H-250, mouse monoclonal anti-Bcl-2 antibody 100, rabbit polyclonal anti-ER antibody HC-20, and goat polyclonal antiactin antibody C-11 were purchased from Santa Cruz. The annexin V-CY3 apoptosis detection kit made by M.B.L. International Co. was obtained from Eppendorf. The pCAT- promoter and pCAT-basic plasmids were from Promega Corp. The CRE-chloramphenicol acetyltransferase (CAT) plasmid was kindly provided by V. E. Avvedimento (Naples, Italy). The pcDNA3 and pcDNA3.1/Myc-HisA vectors were from Invitrogen. The pcDNA3 construct with bcl-2 coding region was a gift from E. Crescenzi (Naples, Italy). The pEGFP-C1 vector was purchased from Clontech. The pSG5-HEGO plasmid expressing human ER was kindly provided by P. Chambon (Strasbourg, France).
Cells.
Human breast cancer MCF-7 cells and COS cells were routinely grown at 37°C in a humidified atmosphere composed of 95% air and 5% CO2, in Dulbecco's modified Eagle's medium (DMEM) supplemented with phenol red, l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (50 μg/ml), insulin (6 ng/ml), hydrocortisone (3.75 ng/ml), and 5% fetal calf serum (FCS). Cells were provided with fresh medium every 3 days. To evaluate the effect of estrogen challenge, MCF-7 cells were grown in phenol red-free DMEM containing 5% dextran–charcoal-stripped FCS for 5 to 7 days, whereas COS cells were cultured in the same medium with 0.5% dextran–charcoal-stripped FCS for 2 to 3 days, before being incubated with 10 nM 17β-estradiol for 48 h.
Nuclear extracts.
Nuclear extracts from 17β-estradiol-challenged MCF-7 or COS cells were prepared as previously described, with minor modifications (14). Both cell lines were treated with the hormone (10 nM) for 48 h, in order to get maximal protein-DNA complex formation. In brief, cells were washed with Dulbecco's modified phosphate-buffered saline (PBS) without Mg2+ and Ca2+ at pH 7.4, harvested, and suspended in 5-pellet volumes of 0.3 M sucrose–2% Tween 40 in buffer A. After freezing, the cells were thawed and gently homogenized; the suspension was layered onto 1.5 M sucrose in buffer A and centrifuged at 25,000 × g. Nuclei were washed with 0.3 M sucrose in buffer A, and nuclear proteins were extracted with 2.5 volumes of buffer B. Extracts were centrifuged at 100,000 × g for 1 h, and the supernatant was dialyzed for 4 h at 4°C against buffer C prior to use in electrophoretic mobility shift assays (EMSAs).
EMSAs.
EMSAs were performed as described previously (18). The 23-mer double-stranded synthetic oligonucleotides used as probes were end labeled with [γ-32P]dATP and T4 polynucleotide kinase. Nuclear extracts (4 to 5 μg) were incubated for 20 min on ice in a 20-μl reaction volume containing 15% glycerol, 20 mM HEPES, 0.1 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenymethylsulfonyl fluoride, 2 mg of leupeptin per ml, 2 mg of pepstatin A per ml, and 1 μg of poly(dI-dC). Approximately 1.5 fmol of 32P-labeled probe (roughly 60,000 cpm) was added, and the incubation was continued for an additional 20 min at room temperature. Where indicated, unlabeled double-stranded oligonucleotides, used as competitors, were also incubated with the extract for 20 min on ice prior to probe addition. Protein-DNA complexes were subjected to electrophoresis on 5% native polyacrylamide gels at 160 V in 1× Tris-borate-EDTA buffer at 4°C. In experiments using antibody to the ER (Ab 314), nuclear extracts were incubated with the antibody in the same 20-μl reaction volume for 30 min at 4°C before addition of the probe and processing as described above. Gels were then dried and autoradiographed.
CAT chimeric plasmids.
The P1 promoter was obtained as a PCR product using equimolar amounts of two synthetic 150-mer oligonucleotides with 37 overlapping bases at their 3′ ends. The upstream oligonucleotide was from −1637 to −1488 relative to the translation start site; the downstream oligonucleotide was from −1375 to −1524. The 263-bp PCR product was digested with PstI and HindIII restriction enzymes using artificially added sites. The resulting 234-bp fragment, which represented the bcl-2 promoter and spanned from −1623 to −1390 bp, was cloned in the correct orientation into pCAT-basic plasmid (P1 construct). Next, synthetic vit-ERE and several bcl-2 ERE-like oligonucleotides (E-a and E-3 to E-7), as well as mutants of the E-4 sequence (M-1, M-2, and M-3), were separately inserted into the P1 plasmid at the BamHI site located roughly 1.7 kb downstream from the P1 promoter (P1/vit, P1/E-a, P1/E-3 to P1/E-7, and P1/M-1 to P1/M-3 constructs), whereas E-b and E-c oligonucleotides were cloned at the XbaI site immediately 3′ to the promoter (P1/E-b and P1/E-c plasmids). Vit-ERE oligonucleotide was also cloned at the BamHI site into pCAT-basic plasmid without the bcl-2 promoter (vit plasmid). In a parallel series of constructs, vit-ERE, E-3, and E-4 oligonucleotides were inserted at the same site into the pCAT-promoter plasmid, approximately 1.6 kb downstream from the simian virus 40 (SV40) promoter. The 104-bp fragment from the bcl-2 coding region, which included the sequences reported as E-3 and E-4, was also obtained as a PCR product using 80-mer oligonucleotides with 28 overlapping bases at their 3′ ends. The 132-bp product was then digested with BamHI using artificial sites inserted on both ends, and the resulting fragment (from nucleotide 190 to 293) was cloned into the P1 vector (P1/104). Synthetic 80-mer oligonucleotides with appropriate mutations were used as primers and processed as described above, in order to obtain 104-bp fragments with either E-3 (P1/mut3) or E-4 (P1/mut4) or both mutated sequences (P1/2mut). Plasmids were sequenced according to the method of Sanger et al. (42) in order to verify presence, orientation, and number of cloned fragments, and appropriate constructs were selected for transfections, according to experimental needs.
Transfections and transient expression analysis.
MCF-7 and COS cells were grown to 80 to 90% confluence in 90-mm-diameter plates. MCF-7 cells were then shifted to DMEM without phenol red plus 5% dextran–charcoal-stripped FCS for 5 to 7 days, whereas COS cells were grown in 0.5% dextran–charcoal-stripped FCS for 2 to 3 days before transfection. Both cell lines were then plated in 60-mm-diameter dishes, and approximately 3 × 105 to 5 × 105 cells were transfected using a calcium phosphate protocol (41). In brief, cells were washed with PBS and incubated at 37°C with 0.5 ml of HEPES-buffered saline solution containing, for MCF-7 cells, 9 μg of the specific CAT construct and, for COS cells, either 2 μg of pSG5-HEGO plasmid expressing human ER and 7 μg of carrier DNA or 9 μg of carrier DNA for cells used as negative controls. In all reported experiments, transfection efficiency was evaluated using 1 μg of Rous sarcoma virus-lacZ plasmid. After 16 to 18 h, cells were incubated with fresh medium, in the absence or presence of 10 nM 17β-estradiol for 48 h, prior to being collected. MCF-7 cells were also subjected to a 1-min shock (15% glycerol in HEPES-buffered saline solution), before incubation with fresh medium. In experiments in which the effect of cAMP levels on CAT activity was evaluated, hormone-starved MCF-7 cells were treated with 0.5 mM 8Br-cAMP for 6 h, prior to being harvested. CAT assays were performed as previously described (41), and results were normalized to β-galactosidase activity. In order to evaluate expression of exogenous bcl-2 or c-myc by Northern blot analysis, MCF-7 cells were transfected with 2 μg of pcDNA3 containing the bcl-2 coding region (pc3/bcl-2) or of pcDNA3.1/Myc, together with 7 μg of carrier plasmid, respectively, and treated with the hormone as described above, prior to RNA extraction. In experiments in which apoptosis was investigated upon detection of PARP cleavage or the annexin V binding assay, MCF-7 cells were transfected and processed as described above, with minor modifications aimed at increasing transfection efficiency. In brief, cells were washed with PBS and incubated at 37°C with 0.5 ml of BES (N,N-bis[2-hydroxyethyl]-2-amino-ethanesulfonic acid)-buffered solution containing, respectively, 2.5 μg of pcDNA3 or of pc3/bcl-2 vector, together with 0.5 μg of pEGFP-C1 plasmid expressing green fluorescent protein and 7 μg of carrier DNA. After 16 to 18 h, cells were incubated with fresh medium in the absence or presence of 10 nM 17β-estradiol for 48 h, prior to being processed according to experimental needs. Both starved and hormone-challenged cells were treated or not with 5 mM hydrogen peroxide (H2O2) for 90 min, prior to being washed and incubated with fresh medium for 6 h.
Northern blot analysis.
Total RNA was isolated by the guanidinium thiocyanate-acid phenol procedure (41). To analyze endogenous bcl-2 mRNA level, hormone-depleted MCF-7 cells were incubated in the absence or presence of 17β-estradiol for 18 h, alone or with 25 μg of cycloheximide per ml or 1 μg of actinomycin D per ml, prior to being harvested by scraping. Incubation of cells with actinomycin D for 18 h was carried out according to preliminary tests in which more than 70% of MCF-7 cells appeared to be still alive after being trypsinized and plated following treatment with the transcription inhibitor. In experiments performed to determine mRNA stability, cells challenged or not with the hormone for 24 h were incubated with 2 μg of actinomycin D per ml for 4, 8, 12, and 16 h, before being collected. RNA samples (20 μg) were resuspended in 20 μl of a denaturing solution (48% formamide, 7% formaldehyde, 1× morpholinepropanesulfonic acid [MOPS], 5% glycerol) and run on a 1% agarose gel with 2% formamide, before being blotted on nylon filters. In experiments to evaluate expression of exogenous bcl-2, RNA samples (10 μg) from cells transfected with pcDNA3-derived constructs were processed as indicated above, before being hybridized with the respective probe. Prehybridization (60°C, 1 h), hybridization (60°C, 16 h), and stringent washes were carried out as already reported (41). Fragments of bcl-2 or c-myc coding regions were labeled with the random-primed DNA labeling kit and used as probes. After hybridization, filters were autoradiographed. Time of exposure varied, in different experiments, from 6 h for transfected bcl-2 to 15 days for endogenous bcl-2 detection. Quantitative analysis was performed with a PhosphorImager. Normalization was accomplished using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA as a reference probe.
Electrophoresis and immunoblotting.
To detect the presence of the ER, 20 μg of proteins from COS nuclear extracts was subjected to sodium dodecyl sulfate (SDS)–15% polyacrylamide gel electrophoresis (PAGE). Gels were then electrophoretically transferred to nitrocellulose filters at 100 V, at room temperature for 50 min, in transfer buffer (50 mM Tris, 380 mM glycine, 0.1% SDS, 20% methanol). After blocking with 10 mM Tris-HCl–150 mM NaCl–0.05% Tween 20, pH 8.0 (TBST), containing 3% nonfat dry milk for 1 h, filters were incubated for 2 h with rabbit polyclonal anti-ER antibody HC-20. They were then washed and incubated with peroxidase-linked anti-rabbit antibody (1:2,000 in TBST buffer) for 1 h, at room temperature. Protein-antibody complexes were revealed using Western blotting detection reagents (ECL), according to the manufacturer's instructions.
Assessment of apoptosis by annexin V binding assay and fluorescence microscopy.
Approximately 2.5 × 105 MCF-7 cells were transfected with 2.5 μg of bcl-2-expressing vector (pc3/bcl-2) or control vector (pcDNA3) and 7 μg of carrier DNA; 0.5 μg of pEGFP-C1 plasmid, expressing green fluorescent protein, was also added to identify transfected cells and to measure transfection efficiency, which approximated 30%. After being incubated or not with 5 mM H2O2, according to the experimental design, and processed as described above, cells were subjected to rhodamine-labeled annexin V binding assay, performed according to the manufacturer's instructions. Cells present in randomly selected areas of the 60-mm-diameter dish were then counted, by fluorescence or light microscopy, or photographed.
Assessment of apoptosis by PARP immunodetection.
MCF-7 cells transfected with pc3/bcl-2 or pcDNA3 vectors and incubated or not with 5 mM H2O2 as described above were washed twice with ice-cold PBS prior to being harvested and lysed in 100 μl of ice-cold lysis buffer (1 mM EDTA, 0.2% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml). Cells were then centrifuged at 13,000 × g for 10 min, at 4°C, and the protein concentration of the supernatant was assayed using a Bio-Rad kit. Equal amounts of proteins from MCF-7 lysates (40 μg) were subjected to SDS–8% PAGE and electrophoretically transferred to a nitrocellulose membrane at 100 V for 70 min, at room temperature. Detection of PARP cleavage was accomplished by incubating membranes with polyclonal anti-PARP antibody H-250 for 45 min and proceeding as described above. To evaluate Bcl-2 levels, protein samples (20 μg) from the same MCF-7 lysates were electrophoresed by SDS–15% PAGE and transferred to nitrocellulose filters at 100 V for 40 min at room temperature, prior to immunodetection using monoclonal anti-Bcl-2 antibody 100. Actin levels, recognized by polyclonal antiactin antibody C-11, were used as the internal control. All filters were incubated with suitable peroxidase-labeled secondary antibodies and then analyzed using the ECL detection kit, according to the manufacturer's instructions. In the two experiments performed, transfection efficiency was evaluated upon expression of cotransfected pEGFP-C1 and ranged from 35 to the 50% obtained in the experiment reported in Results.
RESULTS
17β-Estradiol (E2) induces bcl-2 expression in MCF-7 cells.
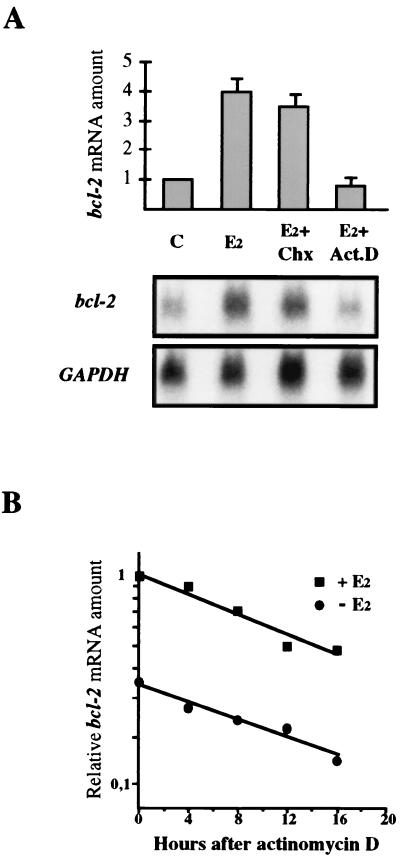
17β-Estradiol has been reported to increase bcl-2 mRNA level in breast cancer MCF-7 cells. Since this induction can be determined by transcriptional or posttranscriptional mechanisms, we have investigated whether the hormone effect requires de novo protein synthesis or is obtained by modulating gene transcription. To address this issue, we have evaluated the effect of the protein synthesis inhibitor cycloheximide and of actinomycin D on bcl-2 mRNA level measured in MCF-7 cells challenged with the hormone. We show that 17β-estradiol up-regulates bcl-2 transcription independently by new protein synthesis, since the fourfold increase in mRNA induced by the hormone was prevented only by addition of the transcription inhibitor (Fig. 1A), and treatment with 17β-estradiol did not affect bcl-2 mRNA stability (Fig. 1B).
FIG. 1.
Effect of cycloheximide and actinomycin D on bcl-2 mRNA up-regulation by 17β-estradiol. (A) On top are graphically represented the results of Northern blot analysis carried out with MCF-7 cells depleted by the hormone for 7 days (C) or treated for 18 h with 10 nM 17β-estradiol alone (E2) or with 25 μg of cycloheximide per ml (E2+Chx) or 1 μg of actinomycin D per ml (E2+Act.D). Amounts of bcl-2 mRNA are reported relative to that measured in starved cells (C), set to 1. Twenty micrograms of total RNA was loaded onto each lane and run on a 1% agarose gel before blotting and hybridization. bcl-2 mRNA was quantified by phosphorimaging analysis and normalized to GAPDH. The error bars represent the standard errors of two different experiments. The observed fourfold increase of bcl-2 mRNA induced by the hormone was inhibited only by actinomycin D treatment. At the bottom is reported the result of one experiment after a 15-day exposure. (B) Hormone-depleted MCF-7 cells were incubated with or without 10 nM 17β-estradiol for 24 h; actinomycin D (2 μg/ml) was then added for the reported times prior to RNA extraction. Twenty micrograms of total RNA per sample was then processed as described above. RNA amounts are represented as fractions of that assayed at time zero in hormone-challenged MCF-7 cells, which was given the arbitrary value of 1. Treatment with 17β-estradiol did not affect bcl-2 mRNA stability since, after 16 h of actinomycin D addition, roughly 40% of the initial RNA amount was measured in both hormone-challenged and -depleted cells.
No EREs are located within the bcl-2 promoter.
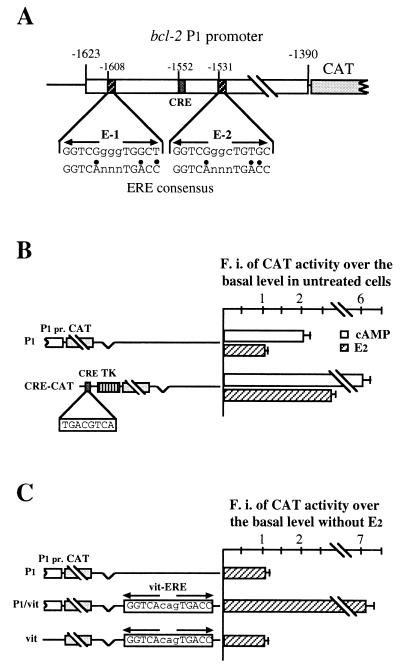
To identify cis-acting elements involved in hormone control of bcl-2 expression, we have made a reporter construct in which the bcl-2 P1 promoter, from 1,623 to 1,390 bp upstream of the translation start site, was cloned 5′ to the CAT gene (P1 construct). We have, then, assessed whether P1 insertion conferred hormone inducibility on CAT activity, in transiently transfected MCF-7 cells. Sequence analysis of the bcl-2 major promoter revealed, in fact, two potential EREs with 70% homology to the consensus site, located 56 bp upstream and 21 nucleotides downstream, respectively, from the already characterized CRE (TGACGTTA) (Fig. 2A).
FIG. 2.
(A) Simplified structure of the 234-bp fragment including the P1 promoter (from 1,623 to 1,390 bp upstream of the bcl-2 translation start site), in which have been evidenced the CRE (TGACGTTA) and the two selected sequences showing 70% homology to the consensus ERE (designated E-1 and E-2). Mutant nucleotides in E-1 and E-2 sequences are indicated by black dots. Numbers on top of each box mark the position of the 5′-most nucleotide. (B) Analysis of CAT activity reported as fold induction measured in transfected MCF-7 cells challenged with 10 nM 17β-estradiol for 48 h, or 0.5 mM 8Br-cAMP for 6 h, versus that assayed in starved cells transfected with the same construct, arbitrarily set to 1. On the left are schematically represented constructs used in transfections: P1 indicates the plasmid in which the bcl-2 P1 promoter was joined upstream of the CAT reporter gene, as detailed in Materials and Methods; CRE-CAT is the plasmid containing the somatostatin CRE sequence 5′ to the thymidine kinase (TK) promoter. 17β-estradiol challenge induces CAT activity only in cells transfected with the CRE-CAT construct. (C) Estrogen responsiveness of CAT activity assayed in MCF-7 cells separately transfected with the constructs represented on the left. P1 is used as described for panel B; P1/vit and vit are plasmids in which one copy of a 23-bp oligonucleotide representing the consensus vit-ERE was cloned into the P1 construct (P1/vit) or into the pCAT-basic (Promega) vector (vit), as described in Materials and Methods. Insertion of the consensus ERE sequence confers hormone responsiveness on the P1 construct. MCF-7 cells were hormone depleted for 5 to 7 days before transfection. CAT activity was normalized to β-galactosidase. The results reported in the figure represent the means of three to five different experiments. F.i., fold induction.
As shown in Fig. 2B, although treatment of transfected MCF-7 cells with 8Br-cAMP for 6 h roughly doubled CAT activity, confirming that the P1 construct contains an active bcl-2 promoter, challenge of the same cells with 17β-estradiol for 48 h failed to induce reporter gene activity. This result clearly indicates that the P1 promoter does not contain EREs. Moreover, since it is known that the hormone increases intracellular cAMP levels in MCF-7 cells, we also asked whether failure of estrogen responsiveness might be due to an impairment of this effect in transfected cells. To answer this question, we measured CAT activity in 17β-estradiol-challenged cells transfected with a reporter plasmid containing the consensus CRE fused 5′ to the thymidine kinase promoter (CRE-CAT). Incubation with 8Br-cAMP produced an effect which was fivefold greater than that evidenced in cells transfected with the P1 construct, and more interestingly, treatment with the hormone induced, in CRE-CAT-transfected cells, reporter gene activity by 2.5-fold (Fig. 2B). On the basis of these data, we can argue that, even though 17β-estradiol induces cAMP levels in MCF-7 cells, bcl-2 up-regulation by the hormone is not CRE dependent.
Two sequences able to confer hormone inducibility on CAT activity are present in the bcl-2 coding region.
To assess whether bcl-2 promoter activity could be enhanced by an ERE, even when present at a distance, we have inserted a 23-bp oligonucleotide, containing the consensus ERE sequence (vit-ERE), into the P1 plasmid, at a site located approximately 1.7 kb downstream from the P1 promoter (P1/vit) (Fig. 2C). Of note, a CAT activity induced sevenfold by hormone was evidenced in MCF-7 cells transfected with the P1/vit construct.
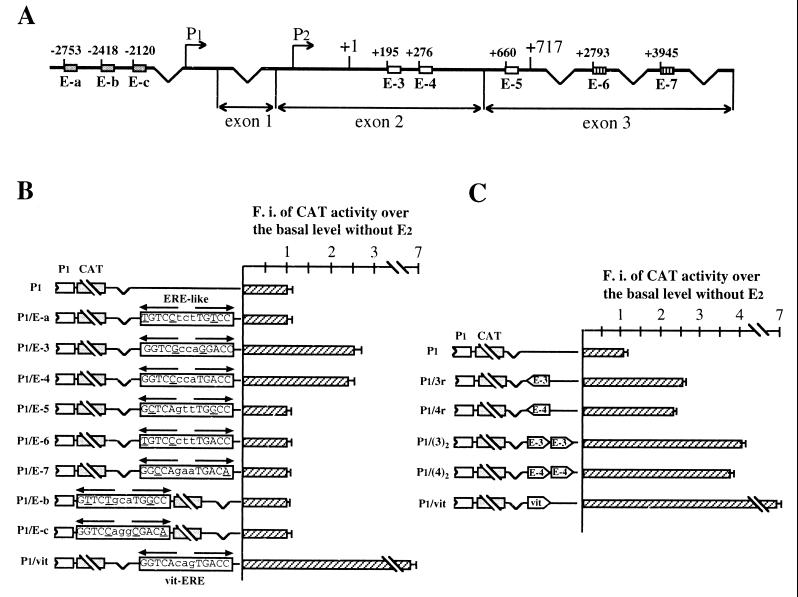
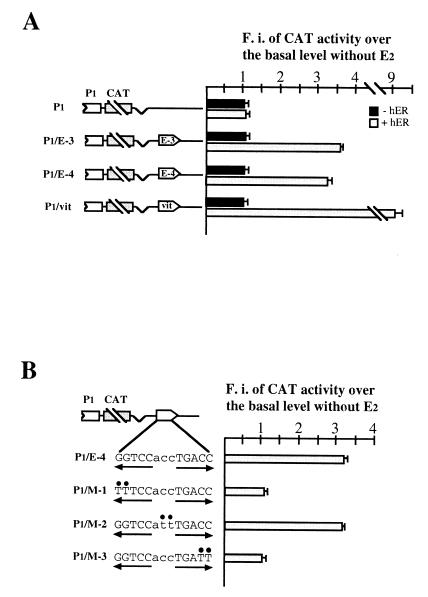
This result and consideration of the fact that EREs are not exclusively located in promoters of hormone-regulated genes convinced us to search for potential responsive elements in bcl-2 5′-flanking and complete cDNA sequences. Upon this analysis, eight sequences were selected from which 23-bp oligonucleotides were synthesized, with no less than 70% homology to the consensus ERE and a perfect half-site or at least five of the six G's present in the canonical palindrome (Fig. 3A). One copy of each oligonucleotide was then separately cloned into the P1 vector, at a distance from the bcl-2 promoter not greater than that existing in the endogenous gene, and the resulting constructs were then transfected into MCF-7 cells. As shown in Fig. 3B, two plasmids (P1/E-3 and P1/E-4) displayed a 2.5- and 2.3-fold hormone induction of CAT activity. These constructs contain, respectively, the E-3 and the E-4 sequences, which are both located within the bcl-2 coding region (Fig. 3A).
FIG. 3.
(A) Schematic structure of the human bcl-2 gene and location of putative EREs. Eight sequences have been identified: three in the 1.2-kb region 5′ to the P1 promoter (E-a to E-c, grey boxes), three within the 717-bp coding region (E-3 to E-5, white boxes), and two within the 3′ untranslated region (E-6 and E-7, boxes with vertical lines). The broken arrows indicate the 5′ ends of P1 and P2 promoters. Numbers on top of each box identify the position of the first nucleotide. Exons 2 and 3 are separated by a 225-kb intron. (B) Analysis of CAT activity measured in MCF-7 cells separately transfected with the constructs schematically represented on the left. Each construct contains one of the potential ERE-like sequences, cloned either 1.7 kb downstream from (P1/E-a and P1/E-3 to P1/E-7) or just 3′ to (P1/E-b and P1/E-c) the P1 promoter. P1 and P1/vit constructs were included as negative and positive controls, respectively. Opposite-pointing arrows on top of each sequence denote the ERE half-palindromes. Mutant nucleotides compared to consensus ERE are underlined. Insertion of E-3 and E-4 sequences confers hormone sensitivity on reporter constructs. (C) Estrogen responsiveness of CAT activity measured in MCF-7 cells transfected with the plasmids schematically represented on the left and containing, respectively, one copy of E-3 or E-4 sequence in reverse orientation (P1/3r and P1/4r) or two copies of each sequence in forward orientation, cloned as described for panel B. P1 and P1/vit constructs were also included as negative and positive controls, respectively. As for panel B, reporter gene activities are as detailed in the Fig. 2 legend; the error bars reflect the standard errors of three separate assays. Both E-3 and E-4 oligonucleotides behave as classical enhancer sequences. Transfections of 5- to 7-day hormone-starved MCF-7 cells were performed as described in Materials and Methods. F.i., fold induction.
The identified responsive elements, presenting one and two mutant nucleotides compared to the consensus ERE sequence, were also shown to behave as classical enhancers, since an almost identical result was obtained when they were cloned in reverse orientation, and a double effect was measured with reporter constructs in which two copies of the respective ERE-like sites had been inserted (Fig. 3C). Moreover, they conferred identical hormone inducibility even when cloned approximately 1.6 kb downstream from the SV40 promoter in the pCAT promoter plasmid, indicating that their enhancer activity could be evidenced even in the presence of a different promoter context (data not shown).
Both bcl-2 EREs bind the ER in vitro.
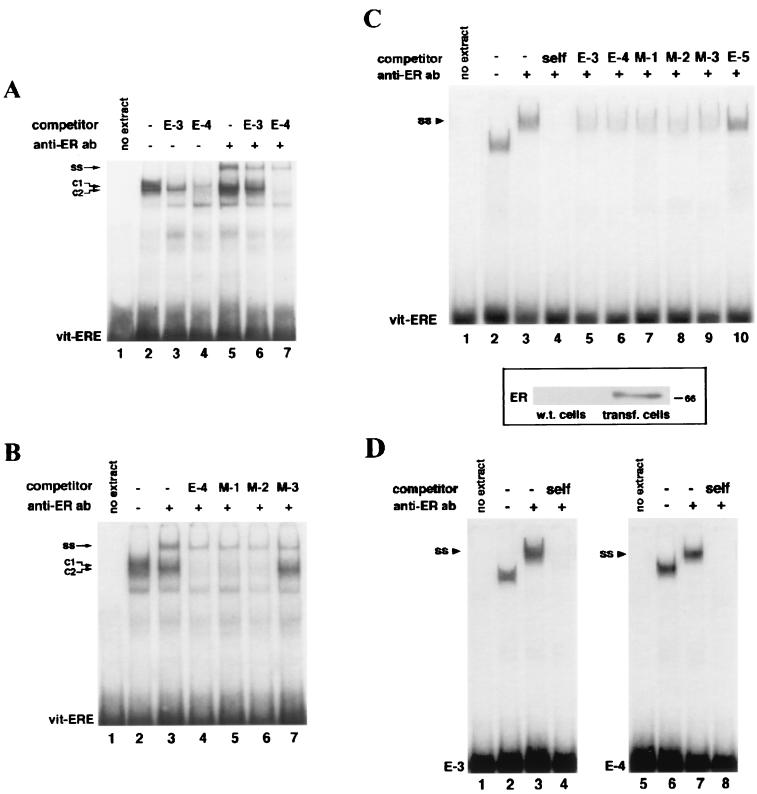
In order to assess whether the identified bcl-2 estrogen-responsive sequences were able to bind in vitro the ER expressed in MCF-7 cells, competitive EMSAs were carried out with nuclear extracts from these cells and consensus vit-ERE as a probe. As shown in Fig. 4A, two protein-DNA complexes, with almost identical migrations, could be seen (C1 and C2). This pattern, which was obtained with a crude nuclear extract, closely resembled that of previously reported gel shift assays in which ER-enriched protein extracts had been used. However, while addition of the E-4 oligonucleotide resulted in competition of both complexes (Fig. 4A, lane 4), the E-3 competitor titrated only the slower (C1) band (Fig. 4A, lane 3). Moreover, when the anti-ER antibody Ab 314 was added to the binding reaction, the mobility of the faster band (C2) was not affected, suggesting that this complex might involve other nuclear receptors and/or the ER in a conformation not recognized by the antibody (Fig. 4A, lane 5). On the other hand, even in the presence of Ab 314, the competition pattern of E-3 and E-4 sequences closely paralleled that described above. The former titrated, in fact, only the supershifted C1 complex, whereas E-4 also competed the faster band (Fig. 4A, lanes 6 and 7). However, the supershifted complex (SS) was titrated by both competitors with similar affinities.
FIG. 4.
(A) EMSA in which labeled vit-ERE oligonucleotide was incubated with MCF-7 nuclear extracts, in the absence (lanes 2 to 4) or presence (lanes 5 to 7) of the monoclonal anti-ER antibody Ab 314. Where indicated, a 500× molar excess of cold E-3 or E-4 oligonucleotides was added. C1 and C2 denote, respectively, the slower- and faster-migrating complexes. Addition of E-4 competitor titrated both complexes (lane 4), whereas the E-3 oligonucleotide competed only the slower-migrating (C1) band (lane 3). Anti-ER antibody addition resulted in a supershift (SS) of only the C1 complex (lane 5). However, formation of the supershifted complex was prevented by both competitors (lanes 6 and 7). (B) EMSA in which was tested the ability of a 500× excess of 23-bp mutants derived from the E-4 sequence to compete formation of protein-DNA complexes formed by vit-ERE probe and MCF-7 nuclear extracts. C1, C2, and SS are used as described for panel A. While M-1 (TTTCCaccTGACC) and M-2 (GGTCCattTGACC) oligonucleotides maintained the ability to compete both SS and C2 complexes (lanes 5 and 6), mutations in the right arm of the E-4 palindrome (M-3; GGTCCaccTGATT) abolished titration of the faster complex (lane 7). Mutant nucleotides in reported sequences are underlined. All mutants, however, competed the supershifted band, mimicking wild-type E-4, in this respect. (C) On top, EMSA in which nuclear extracts from COS cells expressing human ER were incubated with the vit-ERE probe. Addition of anti-ER Ab 314 resulted in a complete supershift (SS) of the retarded complexes which were specifically titrated by incubation with a 250× excess of E-3 and wild-type or mutant E-4 competitors (lanes 5 to 9 versus lane 10). At the bottom is reported the result of an immunoblot of nuclear extracts from COS cells transfected with carrier DNA (wild-type [w.t.] cells) or with pSG5-HEGO plasmid expressing human ER (transfected [transf.] cells). Electrophoresis and immunoblotting were done as described in Materials and Methods. (D) EMSAs carried out with nuclear extracts, as described for panel C, incubated with E-3 and E-4 probes. Both radiolabeled oligonucleotides formed complexes with COS nuclear extracts (lanes 2 and 6) which were also supershifted (SS) by anti-ER antibody addition (lanes 3 and 7). Nuclear extracts were prepared from MCF-7 or COS cells depleted by the hormone as described in Materials and Methods and then challenged with 17β-estradiol for 48 h, in order to obtain maximal protein-DNA complex formation. EMSAs and transfections were performed as detailed in Materials and Methods.
To explain the different behaviors of E-3 and E-4 oligonucleotides when used as competitors of vit-ER binding and to further investigate the role of specific nucleotides in the ER-ERE interaction, we performed competitive EMSAs using 23-bp competitors presenting half-sites with abolished affinity for the ER, since doublet mutations were inserted, respectively, in the 5′ stem (M-1), the spacer sequence (M-2), or the 3′ stem (M-3) of the E-4 palindrome. As shown in Fig. 4B, mutations in the E-4 5′ half-site, as well as in the spacer nucleotides, had no effect on the overall competition pattern (lanes 5 and 6). On the other hand, the 3′ half-palindrome appeared to be necessary for recognition of proteins present in the C2 complex, since the M-3 oligonucleotide failed to compete this band (Fig. 4B, lane 7). We speculate that the 3′ stem of the E-4 sequence, representing a canonical half-site, can then account for the ability of that sequence to titrate proteins present in the C2 complex and bound to the consensus vit-ERE probe. However, since both M-1 and M-3 oligonucleotides competed the supershifted band, we can argue that the ER is able to bind even one arm of the E-4 palindrome.
In order to confirm the ability of E-3 and E-4 sequences to bind the ER, we transfected the pSG5-HEGO plasmid expressing human ER into the ER-negative COS cells and performed EMSAs with nuclear extracts from these cells, in which the presence of human ER was detected (Fig. 4C). Only one retarded band appeared in these assays (Fig. 4C, lane 2), presumably due to the enrichment of receptor protein in the extracts and/or the absence of other proteins in the nuclei of COS cells bound by the vit-ERE probe. The gel shift assay shown in Fig. 4C also displays a complete supershift of the retarded complex when the anti-ER antibody was incubated with the reaction mixture, indicating that it was formed exclusively by the ER (Fig. 4C, lane 3). Moreover, E-3 and E-4 sequences reproduced the competition behavior shown with MCF-7 nuclear extracts, since both oligonucleotides titrated the complex formed in the presence of the anti-ER antibody (Fig. 4C, lanes 5 and 6). Of note, mutants of the E-4 palindrome, used as competitors, also showed an identical pattern, confirming that the ER is able to bind the E-4 half-palindrome (Fig. 4C, lanes 7 to 9).
Finally, when E-3 and E-4 oligonucleotides were used as probes in EMSAs with COS nuclear extracts expressing transfected human ER, a direct binding was evidenced, definitely proving the ability of bcl-2 EREs to bind the ER in vitro (Fig. 4D).
Intact palindrome is necessary for E-4 enhancer activity.
Thereafter, P1/E-3 and P1/E-4 plasmids were transfected into COS cells, in order to assess whether the identified responsive elements required the presence of the ER to display their enhancer effect. CAT activity measured in ER-negative COS cells was not increased by hormone challenge, whereas a 3.6- and a 3.2-fold hormone induction was observed in cells expressing cotransfected human ER (Fig. 5A). These data indicate that the enhancer activity of both bcl-2 EREs is mediated by the ER; on the other hand, the observed higher effect of 17β-estradiol treatment in these cells, evidenced even with the P1/vit construct, might be due to the constitutive expression of the ER or to the different cellular environment.
FIG. 5.
(A) Analysis of CAT activity in ER-negative COS cells cotransfected or not with human ER-expressing plasmid. Cells depleted by the hormone for 2 to 3 days were separately transfected with the constructs schematically represented on the left and were challenged or not with 10 nM 17β-estradiol for 48 h. The presence of the ER appears necessary to evidence estrogen inducibility of reporter gene activity in transfected constructs. (B) Estrogen responsiveness of CAT activities measured in COS cells expressing human ER, transfected with the constructs represented on the left. Only P1/M-2 seems to have the hormone inducibility of the P1/E-4 plasmid, suggesting that both E-4 half-palindromes are necessary to form transcriptionally active complexes with the ER. CAT activities have been reported as detailed in the Fig. 2 legend. As for panel A, the results represent the means of three separate experiments. Transfections were carried out as described in Materials and Methods. F.i., fold induction.
We have also analyzed whether the complex formed by the ER with the E-4 mutant oligonucleotides (M-1, M-2, and M-3) was transcriptionally active. For this purpose, we separately cloned one copy of each E-4-derived sequence into the P1 plasmid, at the same site used for insertion of the E-4 oligonucleotide (P1/M-1 to P1/M-3 [Fig. 5B]). The results of CAT assays performed with COS cells transiently transfected with these constructs revealed that the ER required the presence of both wild-type half-palindromes to trans activate P1 promoter activity, since P1/M-1 and P1/M-3, carrying mutant half-sites, failed to respond to hormone challenge (Fig. 5B). On the other hand, P1/M-2 paralleled the estrogen responsiveness of the P1/E-4 construct, suggesting that, at least in this experimental system, the spacer nucleotides do not play an essential role in the formation of a functional trans-activating complex (Fig. 5B).
The identified EREs mediate hormone induction of bcl-2 transcription with an additive effect.
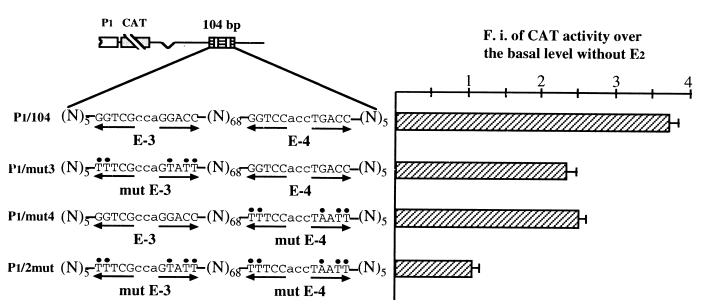
Based on these findings, we have investigated whether the simultaneous presence of E-3 and E-4 sequences within the same plasmid resulted in a hormone responsiveness which approximated the observed fourfold induction of endogenous bcl-2 mRNA measured in 17β-estradiol-challenged MCF-7 cells (Fig. 1A). To address this issue, we have cloned into the P1 construct a 104-bp sequence corresponding to the fragment of the bcl-2 coding region which included both EREs (P1/104). The 3.7-fold hormone induction observed in MCF-7 cells transfected with the P1/104 construct largely corresponds to the sum of the enhancer effects shown by E-3 and E-4 oligonucleotides when cloned separately and indicates that the activity of these responsive elements is additive (Fig. 6). Moreover, the observed up-regulation appears to be in good agreement with the increase of the endogenous bcl-2 mRNA level evidenced in MCF-7 cells challenged with 17β-estradiol. On the other hand, the hormone response of constructs containing a 104-bp fragment with mutations in either one or the other ERE-like site (P1/mut3 and P1/mut4) closely resembled that measured with P1/E-3 and P1/E-4 plasmids, confirming that the enhancer activity of the identified elements was additive (Fig. 6).
FIG. 6.
Analysis of CAT activities assayed in 17β-estradiol-challenged MCF-7 cells separately transfected with the constructs represented on the left. P1/104 represents the construct in which a 104-bp bcl-2 coding fragment, including wild-type E-3 and E-4 sequences, was cloned into the P1 vector. The same 104-bp fragments carrying either mutant E-3 (P1/mut3) or E-4 (P1/mut4) or both mutated sequences (P1/2mut) were also inserted, at the same site, into the P1 vector. Mutant nucleotides are indicated by black dots on top. (N)68 indicates the number of nucleotides which separate the two bcl-2 ERE sequences; (N)5 indicates the flanking 5 bp on both sides. Opposite-pointing arrows are placed under each ERE half-palindrome. Insertion into the P1 vector was at the same BamHI site located 1.7 kb downstream from the P1 promoter and already used for E-3 and E-4 cloning. The presence of wild-type EREs resulted in an additive effect on hormone-induced CAT activity, reported as detailed in the Fig. 2 legend. The error bars represent the standard errors of three separate assays. Transfections were carried out as detailed in Materials and Methods. F.i., fold induction.
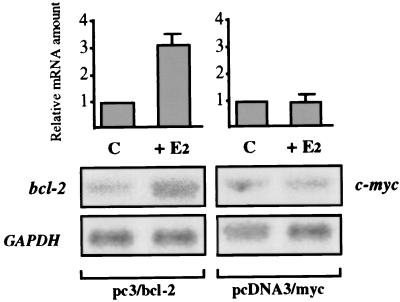
Thereafter, in order to definitely prove that the bcl-2 coding region could confer hormone inducibility on its own transcription, we have transfected MCF-7 cells with a construct containing the complete bcl-2 coding sequence cloned under the control of the cytomegalovirus (CMV) promoter, which does not per se contain EREs (pc3/bcl-2). As shown in Fig. 7, evaluation of the expression of exogenous bcl-2, whose mRNA of 1.1 kb could be easily distinguished by the endogenous transcript (8.5 kb), indicates that hormone challenge of MCF-7 cells gave rise to a specific 3.1-fold increase of transfected bcl-2 mRNA level. The size of this effect almost equals the induction of CAT activity observed in cells transfected with the P1/104 construct and strongly suggests that it may be attributed to the EREs identified in the bcl-2 coding region.
FIG. 7.
Northern blot analysis of transfected bcl-2 mRNA levels expressed in MCF-7 cells. Seven-day hormone-starved cells were separately transfected with pcDNA3 vectors containing either the complete bcl-2 coding region or a fragment of the c-myc coding sequence, cloned under the control of the CMV promoter. MCF-7 cells were then challenged (+E2) or not (C) with 10 nM 17β-estradiol for 48 h, prior to RNA extraction. Hormone treatment resulted in a 3.1-fold induction of transfected bcl-2 mRNA level, whereas expression of exogenous c-myc remained unchanged. Ten micrograms of total RNA per lane was run on a 1% agarose gel and blotted onto nylon filters, prior to being hybridized with the respective probes and quantified by phosphorimaging analysis. Normalization was accomplished using GAPDH as an internal control. The results shown at the bottom of the figure represent a 6-h autoradiography. The error bars reflect the standard errors of three experiments.
Hormone induction of transfected bcl-2 protects MCF-7 cells from apoptosis.
Finally, we asked whether the inhibitory effect of 17β-estradiol on the apoptotic process in MCF-7 cells was actually mediated by its up-regulation of bcl-2 expression. To answer this question, we have challenged with the hormone MCF-7 cells transfected with pc3/bcl-2 plasmid and in which apoptosis was induced by incubation with 5 mM hydrogen peroxide (H2O2) for 90 min. We reasoned that, if 17β-estradiol prevented apoptosis through up-regulation of bcl-2 expression, we should observe the best protection in pc3/bcl-2-transfected cells treated with the hormone, compared either to starved cells or to untransfected cells challenged with the estrogen. We also based our speculation on the results that we have reported above, which demonstrate that hormone treatment of pc3/bcl-2-transfected cells induced, by approximately threefold, exogenous bcl-2 expression (Fig. 7). PCD was, then, assessed by the annexin V assay. This protein binds, in fact, to the phosphatidylserine molecules which translocate from the inner to the outer leaflet of cell membrane during the early steps of PCD and allows identification of apoptotic cells by fluorescence microscopy. We have also cotransfected MCF-7 cells with the pEGFP-C1 vector, expressing green fluorescent protein, in order to measure the percentage of apoptotic cells within the transfected population (red-green versus green cells). Furthermore, upon examination of green fluorescent cells, we could also directly measure transfection efficiency by comparing green cells to the number of total cells, present in the same randomly chosen areas and evaluated by light microscopy.
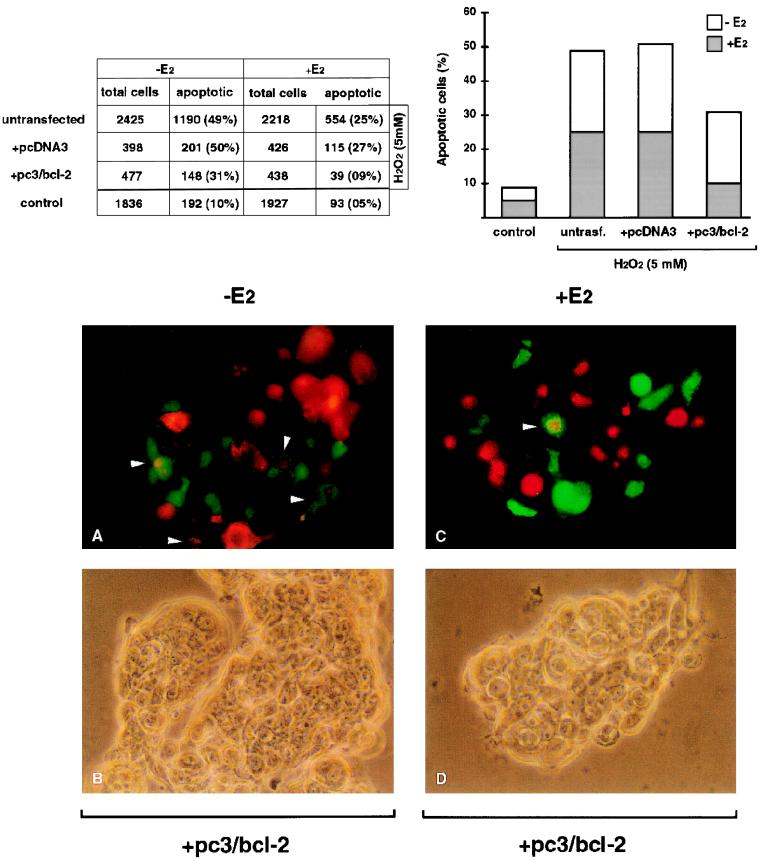
According to data reported in the table of Fig. 8, we can calculate that roughly 30% of total cells were transfected, as shown by the ratio between the number of transfected cells (875, i.e., 398 + 477) and that of total counted cells (3,300, i.e., 2,425 + 875). Moreover, it appears that approximately 50% of parental cells, as well as those transfected with the pcDNA3 control vector, underwent apoptosis after H2O2 treatment and that hormone challenge prevented apoptosis in a further 25% of cells, in both cases. Interestingly, evaluation of PCD within cells transfected with the pc3/bcl-2 construct revealed that the fraction of apoptotic cells was lowered to 30% simply due to the presumably increased bcl-2 expression after pc3/bcl-2 transfection and that 17β-estradiol still further inhibited apoptosis, detected in less than 10% of hormone-treated cells. Of note, this value could be considered the basal apoptosis, since it mimicked the percentage calculated for untransfected cells not incubated with H2O2 (last value of the second column).
FIG. 8.
(Top) Evaluation of the percentage of apoptotic MCF-7 cells by fluorescence microscopy. Cells transfected with the pc3/bcl-2 construct or the pcDNA3 control vector were treated with 5 mM hydrogen peroxide (H2O2) for 90 min and subjected to annexin V binding assay. Untransfected cells were incubated with H2O2 to estimate the percentage of apoptosis in parental cells. Basal apoptosis was evaluated in non-H2O2-treated parental MCF-7 cells (control). The results of cell counting, reported in the table, are also graphically represented on the right. (Bottom) Two randomly chosen areas are shown, in which transfected cells may be recognized by green fluorescence, apoptotic cells are indicated by rhodamine-labeled annexin binding, and apoptotic transfected cells are indicated by mixed fluorescence (also indicated by arrowheads). Transfection of the bcl-2-expressing construct results in inhibition of apoptosis in a further 20% of hormone-depleted cells, and 17β-estradiol challenge still elicits protection approaching the value measured with non-H2O2-treated parental cells. Transfections and incubation with H2O2, as well as the annexin binding assay, were performed as detailed in Materials and Methods.
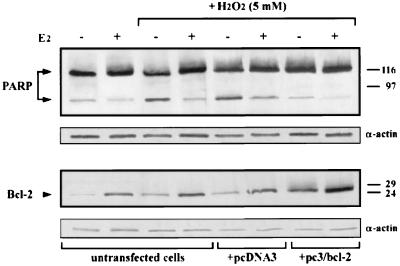
On the basis of these data and in order to more directly correlate the inhibitory effect of 17β-estradiol treatment on the apoptotic process with its up-regulation of bcl-2 expression, we have compared cleavage of PARP by the Ced/ICE proteases, used as an apoptotic marker, to the Bcl-2 protein level detected in the same cells. Immunodetection of the distinctive 85-kDa PARP fragment, concomitantly with assessment of Bcl-2 protein in untransfected cells, as well as in cells transfected with different constructs, revealed an inverse correlation between the presence of the PARP fragment and Bcl-2 level in all examined cells. Notably, Bcl-2 appeared to be overexpressed in pc3/bcl-2-transfected cells, and hormone treatment of the same cells resulted in a further Bcl-2 increase, concomitantly with a decrease of PARP fragment to a level which strictly resembled that present in untransfected cells in the absence of hydrogen peroxide treatment and, then, considered the marker of basal PCD (Fig. 9).
FIG. 9.
Western blot analyses of PARP cleavage and Bcl-2 levels. Cell extracts prepared as described in Materials and Methods were subjected to SDS-PAGE and transferred electrophoretically to nitrocellulose filters. Proteins were visualized by reaction with peroxidase-labeled secondary antibody and chemiluminescence, as detailed in Materials and Methods. Relative positions of the molecular weight markers are reported on the right (molecular weights are in thousands). The 117-kDa PARP and its 85-kDa fragment are indicated by the arrows. p26Bcl-2 is also indicated by the arrowhead. To induce apoptosis, cells were treated with 5 mM H2O2 for 90 min. Assessment of PARP cleavage in non-H2O2-treated cells was carried out to evaluate basal apoptosis. Actin levels were also detected as an internal control of loaded proteins. Bcl-2 protein appears to be overexpressed in pc3/bcl-2-transfected cells. However, hormone treatment of the same cells results in a further increase of Bcl-2 level, accompanied by a concomitant decrease of the PARP fragment, whose level approaches that evidenced in cells not incubated with the proapoptotic agent. In the reported experiment, transfection occurred in approximately 50% of cells, as determined upon evaluation of green fluorescent protein expressed by the pEGFP-C1 vector.
According to these results, we can conclude that bcl-2 up-regulation by 17β-estradiol is responsible for hormone-induced protection from apoptosis in MCF-7 breast cancer cells.
DISCUSSION
Estrogens stimulate growth of hormone-responsive breast cancer cells, through complex and still incompletely characterized mechanisms. Identification of estrogen-regulated genes and analysis of their expression appear, then, useful approaches to understanding tumor progression in mammary tissue. Recent studies have focused on hormone effects in modulating the activity of cyclins and cyclin-dependent kinase inhibitors which occur at different levels of the cell cycle and can directly affect the expression of genes important in the control of cell differentiation and proliferation (36). However, growing evidence has accumulated in recent years supporting the role of estrogen hormones in the prevention of PCD in human breast cancer. For instance, it has been suggested that antiestrogens, opposing the hormone effect, induced PCD in mammary tumor MCF-7 cells. This hypothesis is based on two different observations made with MCF-7 cells, including morphological changes and inhibition of growth evidenced in the same cells overexpressing the cyclin D1 gene after antiestrogen treatment (4, 38). Likewise, rapid regression of MCF-7-derived breast cancers in nude mice, following estrogen ablation, was attributed either to arrest of cell proliferation or to PCD activation (29). The finding that hormone protection from apoptosis was accompanied in MCF-7 cells by an increase of bcl-2 mRNA level (51), and the results of a study which revealed expression of the ER in 80% of the bcl-2-positive mammary tumors and in only 30% of the bcl-2-negative group (31), suggested that inhibition of PCD by estrogens was mediated by the bcl-2 gene, which is able to prevent apoptotic death in multiple contexts (27).
We have demonstrated that 17β-estradiol up-regulates bcl-2 transcription in MCF-7 cells, showing that its effect is mediated by two cis-acting elements present in the bcl-2 coding region, as revealed by the observation that the hormone induced expression of transfected bcl-2 cloned downstream from the non-estrogen-responsive CMV promoter. Interestingly, the two identified EREs are located near the junction of exon 2 with the 225-kb-long intron, a region in which a DNase I-hypersensitive site, due to a particular chromatin structure associated with high levels of bcl-2 transcription, has been already identified in a human pre-B-cell line (57).
The inability of the bcl-2 P1 promoter to induce reporter gene activity after hormone challenge of transfected MCF-7 cells also indicates that, in our experimental system, 17β-estradiol does not control bcl-2 expression through the CRE present in its promoter. It has been reported, in fact, that the hormone up-regulates adenylate cyclase activity in MCF-7 cells, inducing transcription of genes under the control of consensus somatostatin CRE (3). Although we observed such an effect in cells transfected with a CRE-CAT plasmid, the P1 promoter barely responded to only direct 8Br-cAMP addition. This apparently contradictory result may be due to the noncanonical sequence of the bcl-2 CRE or to the modulating activity of its flanking nucleotides. The observation that cAMP treatment of MCF-7 cells produced a fivefold-higher effect on CAT activity regulated by the somatostatin CRE than that measured with the P1 construct raises this hypothesis. Moreover, failure of bcl-2 CRE activation by increased cAMP levels, even though it occurred in a B-lymphoma cell line, has been already reported (56).
The EREs located within the bcl-2 coding region conferred hormone responsiveness on the CAT gene when cloned in our constructs at a distance from the promoter very similar to that present in the endogenous gene, suggesting that they could enhance P1 promoter activity even in their natural context. The ability of bcl-2-responsive sequences to act over a long distance is not surprising, since EREs have been shown to be still active when cloned 2.4 kb downstream from the SV40 promoter (43). Moreover, hormone-responsive elements located far from promoters have been already reported, and formation of a DNA loop, which allows the distal and proximal trans-acting factors to come into close proximity with one another, has been hypothesized in order to explain how distant proteins may interact (16, 44). The observation that the distal enhancer and the proximal promoter of the rat prolactin gene revealed an estrogen-induced DNase I hypersensitivity, even though the responsive element is located only in the distal region, further confirms this mechanism (46).
Our data also exclude the possibility that EREs located in the P1 promoter, silent in the absence of other regulatory elements, might have contributed to hormone inducibility of CAT expression, as has been suggested for the EREs located in the 5′-flanking and coding regions of the rat progesterone receptor gene (28). The almost identical induction obtained with constructs containing bcl-2 ERE sequences inserted 1.6 kb 3′ to the SV40 promoter (data not shown) or 1.7 kb downstream from the P1 promoter is inconsistent, in fact, with this hypothesis.
Both bcl-2 EREs were able to bind in vitro the ER, as revealed by EMSAs. The incomplete titration of protein-DNA complexes between nuclear extracts and consensus ERE sequence, observed when the identified EREs were added to the binding reaction, may be attributed to the increased affinity for the receptor shown by the vit-ERE oligonucleotide in the presence of the anti-ER Ab 314, as previously reported (1). Moreover, data from EMSAs carried out with mutants of the E-4 sequence indicate that even a single stem of that palindrome was able to bind the ER. Although the ability of receptor monomers to bind in vitro half-ERE sequences has already been described (34), we cannot confirm this hypothesis, on the basis of reported gel shift assays. However, transfections of constructs carrying E-4 mutated sequences (P1/M-1 to P1/M-3) into COS cells expressing human ER revealed that formation of a transcriptionally active complex with the ER required both wild-type half-palindromes, whereas at least in our experimental system, the spacer nucleotides appeared to play a secondary role in this respect. Whether this may be considered a general requirement needs further investigation.
Like all imperfect EREs, the effect of the identified bcl-2 elements on CAT activity was weaker than that of the consensus palindrome. However, noncanonical hormone-responsive enhancers are present in the great majority of target genes, which compensate for the lower affinity of these elements for cognate receptors presenting more than one responsive sequence (52). For instance, it has been shown that the glucocorticoid-responsive element identified in the TAT gene failed to confer hormone regulation when cloned far upstream of its TATA box, whereas insertion of two copies in the same region restored high inducibility (50). This finding sustains the hypothesis that a second mechanism, besides a strong binding to one responsive element, may be involved in transcriptional activation.
The identified bcl-2 EREs did not display a synergistic effect, and this is not unexpected, since they are separated by 81 bp, which does not represent an integer number of helical turns, whereas an optimal distance is required for synergy between EREs located far from the promoter (32, 43). However, we observed a fourfold increase of endogenous bcl-2 mRNA level in MCF-7 cells challenged with 17β-estradiol; this induction was comparable to previously reported data (51) and similar to the fold induction of CAT activity measured in cells transfected with the P1/104 construct and of exogenous bcl-2 mRNA evidenced in cells transfected with the pc3/bcl-2 plasmid. These results strongly suggest that, even though we cannot exclude the involvement of other upstream regulatory sequences, bcl-2 transcriptional control by the hormone is mediated by the two elements that we have identified.
It is generally accepted that Bcl-2 inhibits PCD by protecting cells from oxidative stress (53), even though several studies have shown protection at very low oxygen levels, suggesting a mechanism other than inhibition of reactive oxygen substances (23, 48). The most plausible role proposed for Bcl-2, in fact, is that it counteracts its twin protein Bax, also functioning as a trap for reactive free radicals (19, 26). We have demonstrated that bcl-2 up-regulation by estrogens inhibits hydrogen peroxide (H2O2)-induced PCD in MCF-7 cells. This statement is based on the finding that overexpression of transfected bcl-2 in hormone-starved cells mimicked per se the protective effect of 17β-estradiol from PCD in parental cells and, moreover, that hormone challenge further induced Bcl-2 synthesis resulting in additional protection from apoptosis of transfected cells in which PCD was lowered to the basal level.
On the other hand, the observation that hormone challenge of MCF-7 cells expressing exogenous bcl-2 lowered the percentage of apoptosis to the level observed in parental cells untreated with H2O2 suggests that, in our experimental system, involvement of other genes in inhibition of the apoptotic process was not elicited.
In summary, although complete comprehension of the molecular mechanism by which estrogen hormones control PCD in human breast cancer cells needs to be addressed in more detail, we believe that data that we have reported may represent good grounds for future studies aimed at developing new therapeutic approaches in the treatment of hormone-responsive mammary tumors.
ACKNOWLEDGMENTS
We are grateful to V. E. Avvedimento for very helpful discussions and criticism. We also thank M. Berardone for partial artwork.
This work was supported by a grant from the Agenzia Spaziale Italiana (ASI, Rome, Italy) and, in part, from the Associazione Italiana per la Ricerca sul Cancro (A.I.R.C., Milan, Italy).
REFERENCES
- 1.Abbondanza C, De Falco A, Nigro V, Medici N, Armetta I, Molinari A M, Moncharmont B, Puca G A. Characterization and epitope mapping of a new panel of monoclonal antibodies to estradiol receptor. Steroids. 1993;58:4–12. doi: 10.1016/0039-128x(93)90011-b. [DOI] [PubMed] [Google Scholar]
- 2.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 3.Aronica S M, Kraus W L, Katzenellenbogen B S. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardon S, Vignon F, Montocourrier P, Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987;47:1441–1448. [PubMed] [Google Scholar]
- 5.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 6.Berry M, Nunez A M, Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfect palindromic sequence. Proc Natl Acad Sci USA. 1989;86:1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthois Y, Dong X F, Martin P M. Regulation of epidermal growth factor receptor by estrogen and antiestrogen in human breast cancer cell line, MCF-7. Biochem Biophys Res Commun. 1989;159:126–131. doi: 10.1016/0006-291x(89)92413-3. [DOI] [PubMed] [Google Scholar]
- 8.Bifulco M, Perillo B, Saji M, Laezza C, Tedesco I, Kohn L D, Aloj S M. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in FRTL-5 cells. J Biol Chem. 1995;270:15231–15236. doi: 10.1074/jbc.270.25.15231. [DOI] [PubMed] [Google Scholar]
- 9.Buckley M F K, Sweeney K J E, Hamilton J A, Sini R L, Manning D L, Nicholson R I, de Fazio A, Watts C K W, Musgrove A, Sutherland R L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 10.Cavailles V, Augereau P, Rochefort H. Cathepsin D gene of human MCF-7 cells contains estrogen-responsive sequences in its 5′ proximal flanking region. Biochem Biophys Res Commun. 1991;174:816–824. doi: 10.1016/0006-291x(91)91491-t. [DOI] [PubMed] [Google Scholar]
- 11.Chang T C, Nardulli A M, Lew D, Shapiro D J. The role of estrogen response elements in expression of the Xenopus laevis vitellogenin B1 gene. Mol Endocrinol. 1992;6:346–354. doi: 10.1210/mend.6.3.1584211. [DOI] [PubMed] [Google Scholar]
- 12.Chiu R, Boyle W J, Meek J, Smeal T, Hunter T, Karin M. The c-fos protein interacts with c-jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988;54:541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 13.Darwish H, Krisinger J, Furlow J D, Smith C, Murdoch F E, De Luca H F. An estrogen-responsive element mediates the transcriptional regulation of calbindin D-9K gene in rat uterus. J Biol Chem. 1991;266:551–558. [PubMed] [Google Scholar]
- 14.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson D J P, Anderson T J. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981;44:177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gothard L Q, Hibbard J C, Seyfred M A. Estrogen-mediated induction of rat prolactin gene transcription requires the formation of a chromatin loop between the distal enhancer and proximal promoter regions. Mol Endocrinol. 1996;10:185–195. doi: 10.1210/mend.10.2.8825558. [DOI] [PubMed] [Google Scholar]
- 17.Harigai M, Miyashita T, Hanada M, Reed J C. A cis-acting element in the bcl-2 gene controls expression through translational mechanisms. Oncogene. 1996;12:1369–1374. [PubMed] [Google Scholar]
- 18.Hennighausen L, Lubon H. Interaction of protein with DNA in vitro. Methods Enzymol. 1987;152:721–735. doi: 10.1016/0076-6879(87)52076-6. [DOI] [PubMed] [Google Scholar]
- 19.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 20.Hockenbery D M, Nunez G, Milliman C L, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 21.Hyder S M, Nawaz Z, Chiappetta C, Yokoyama K, Stancel G M. The protooncogene c-jun contains an unusual estrogen-inducible enhancer within the coding sequence. J Biol Chem. 1995;270:8506–8513. doi: 10.1074/jbc.270.15.8506. [DOI] [PubMed] [Google Scholar]
- 22.Hyder S M, Stancel G M, Nawaz Z, McDonnell D P, Loose-Mitchell D S. Identification of an estrogen response element in the 3′-flanking region of the murine c-fos protooncogene. J Biol Chem. 1992;267:18047–18054. [PubMed] [Google Scholar]
- 23.Jacobson M D, Raff M C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Tora L, Yamauchi J, Masushige S, Bellard M, Chambon P. A far upstream oestrogen-response element of the ovalbumin gene contains several half palindromic 5′-TGACC-3′ motifs acting synergistically. Cell. 1992;68:731–742. doi: 10.1016/0092-8674(92)90148-6. [DOI] [PubMed] [Google Scholar]
- 25.Klein-Hitpass L, Ryffel G U, Heitlinger E, Cato A C B. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988;16:647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korsmeyer S J. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693–1700. [PubMed] [Google Scholar]
- 27.Korsmeyer S J. Bcl-2 initiates a new category of oncogenes: regulation of cell death. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- 28.Kraus W L, Montano M M, Katzenellenbogen B S. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- 29.Kyprianou N, English H F, Davidson N E, Isaacs J T. Programmed cell death during regression of the MCF-7 human breast cancer cell following estrogen ablation. Cancer Res. 1991;51:162–166. [PubMed] [Google Scholar]
- 30.Lee J H, Kim J, Shapiro D J. Regulation of Xenopus laevis estrogen receptor gene expression is mediated by an estrogen response element in the protein coding region. DNA Cell Biol. 1995;14:419–430. doi: 10.1089/dna.1995.14.419. [DOI] [PubMed] [Google Scholar]
- 31.Leek R D, Kaklamanis L, Pezzella F, Gatter K C, Harris A L. Bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994;69:135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez E, Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989;8:3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurer R A, Notides A C. Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol Cell Biol. 1987;7:4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medici N, Nigro V, Abbondanza C, Moncharmont B, Molinari A M, Puca G A. In vitro binding of purified hormone-binding subunit of the estrogen receptor to oligonucleotides containing natural or modified sequences of an estrogen-responsive element. Mol Endocrinol. 1991;5:555–563. doi: 10.1210/mend-5-4-555. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita T, Harigai M, Hanada M, Reed J C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 36.Musgrove E A, Hamilton J A, Lee C S, Sweeney K J, Watts C K, Sutherland R L. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pace P, Taylor J, Suntharalingam S, Coombes R C, Ali S. Human estrogen receptor β binds in a manner similar to and dimerises with estrogen receptor α. J Biol Chem. 1997;272:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- 38.Pacilio C, Germano D, Addeo R, Altucci L, Petrizzi V B, Cancemi M, Cicatiello L, Salzano S, Lallemand F, Michalides J A M, Bresciani F, Weisz A. Constitutive overexpression of cyclin D1 does not prevent inhibition of hormone-responsive human breast cancer cell growth by antiestrogens. Cancer Res. 1998;58:871–876. [PubMed] [Google Scholar]
- 39.Raff M C. Social control on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds G A, Basu S K, Osborne T F, Chin D-J, Gil G, Brown M S, Goldstein J-L, Luskey K L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5′ untranslated regions. Cell. 1984;38:275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathya G, Li W, Klinge C M, Anolik J H, Hilf R, Bambara R A. Effects of multiple estrogen responsive elements, their spacing, and location on estrogen response of reporter genes. Mol Endocrinol. 1997;11:1994–2003. doi: 10.1210/mend.11.13.0039. [DOI] [PubMed] [Google Scholar]
- 44.Schild C, Claret F X, Wahli W, Wolffe A P. A nucleosome-dependent static loop potentiates estrogen-regulated transcription from the Xenopus vitellogenin B1 promoter. EMBO J. 1993;12:423–433. doi: 10.1002/j.1460-2075.1993.tb05674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seto M, Jaeger U, Hockett R D, Graninger W, Bennett S, Goldman P, Korsmeyer S J. Alternative promoters and exons, somatic mutation and deregulation of the bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seyfred M A, Gorski J. An interaction between the 5′-flanking distal and proximal regulatory domains of the rat prolactin gene is required for transcriptional activation by estrogens. Mol Endocrinol. 1990;4:1226–1234. doi: 10.1210/mend-4-8-1226. [DOI] [PubMed] [Google Scholar]
- 47.Shanmugam M, Krett N L, Maizels E T, Cutler R E, Jr, Peters C A, Smith L M, O'Brien M L, Park-Sarge O K, Rosen S T, Hunzicker-Dunn M. Regulation of protein kinase C delta by estrogen in the MCF-7 human breast cancer cell line. Mol Cell Endocrinol. 1999;148:109–118. doi: 10.1016/s0303-7207(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 48.Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374:811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 49.Slater E P, Redeuihl G, Theis K, Suske G, Beato M. The uteroglobin promoter contains a noncanonical estrogen responsive element. Mol Endocrinol. 1990;4:604–610. doi: 10.1210/mend-4-4-604. [DOI] [PubMed] [Google Scholar]
- 50.Strahle U, Klock G, Schutz G. A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proc Natl Acad Sci USA. 1987;84:7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teixeira C, Reed J C, Pratt M A C. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- 52.Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr Rev. 1993;14:459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- 53.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 54.Weisz A, Bresciani F. Estrogen regulation of proto-oncogenes coding for nuclear proteins. Crit Rev Oncog. 1993;4:361–388. [PubMed] [Google Scholar]
- 55.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Wilson B E, Mochon E, Boxer L M. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young R L, Korsmeyer S J. A negative regulatory element in the bcl-2 5′-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993;13:3686–3697. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]