Abstract
Clostridoides difficile infection (CDI) is the leading cause of antibiotic related diarrhea therapy and may associate high morbidity and mortality. Providing a potential biomarker to assess disease severity may help physicians in choosing the right treatment. Methods. This was a prospective, single-centre cohort study which included patients diagnosed with CDI which were assessed by fecal calprotectin (FC). Results. Patients included had a mean of 69.29 years of age, 54.23% of male gender. Patients diagnosed with mild CDI had a mean ATLAS score of 3.39 (±1.24), statistically lower (p<0.001) than patients with severe CDI who had a mean ATLAS score of 7.33 (±0.77). Fecal calprotectin concentrations were significantly higher (p<0.001) in the severe CDI patients (615.14μg/g; IQR, 403.62-784.4μg/g) than in the mild CDI patients (195.42μg/g; IQR, 131.12-298.59μg/g). We suggest a cut-off of 290.09μg/g for the predictive marker of fecal calprotectin, which permitted to identify patients with severe and mild CDI, having 100% sensitivity and 76% specificity. Conclusions. Our findings point out the potential that FC might have, as a biomarker for disease severity. However, future multicentre studies and in larger cohort need to validate the results.
Keywords: Clostridoides difficile infection, fecal calprotectin, predictive value
Introduction
Clostridoides difficile infection (CDI) still ranks as the most encountered nosocomial infection, with an increased incidence over the past years [1].
Most of the cases appear after antibiotic exposure during admissions, but there is also a community spread especially in young patients [2].
CDI is responsible for membranous colitis and is considered a major cause of diarrhea in adult patients. The main risk factors which have been related to CDI, besides antibiotic exposure are older age, hospitalization, proton pump inhibitors (PPI), end-stage kidney disease, cirrhosis, cancer [3].
Disease severity depends on the patient’s vulnerability and may even lead to a fulminant colitis with megacolon, low blood presure and shock which is associated to a high mortality rate [4].
Several guidelines tried to define CDI severity by using several parameters such as age, temperature, albumin, white blood count, end-stage kidney disease [5,6,7].
However, potential use of specific biomarkers might help differentiate patients with severe colitis thus providing more data to the decision-making process. On the other hand, by using a biomarker to determine disease evolution after CDI therapy might help assess the therapeutic response.
Fecal calprotectin (FC) is a non-invasive biomarker used to assess disease severity of inflammatory bowel disease [8].
However, it may be also used for other causes of intestinal inflammation as it is released during an inflammatory response. Few studies reported a higher level of calprotectin in patients diagnosed with CDI when compared to other causes of diarrhea [9,10].
However, there are not many studies that differentiate CDI disease severity which might be very helpful to assess patient’s prognosis and response to treatment.
Our objective was to consider FC as predictive biomarker to identify patients with CDI severe disease and differentiate from mild disease, which might help to prevent possible complication.
Methods
This was a prospective, single-centre cohort study which included patients diagnosed with CDI within the University County Hospital of Craiova, Romania, from January 1, 2017 to December 31, 2019.
All patients who agreed to join the study signed an informed consent form, conforming to the Declaration of Helsinki, 1967.
The study was approved by the University of Medicine and Pharmacy of Craiova Ethics Commission no. 88/2020.
Study design
CDI diagnosis
CDI was diagnosed by an enzyme immunoassay for detecting glutamate dehydrogenase according to European Society of Clinical Microbiology and Infectious Disease (ECSMID).
Patients were tested positive for CDI by A/B stool assay and received vancomycin +/-metronidazole according to available guidelines.
Patient’s characteristics
We included the following variables medical records: age, gender, comorbidities, admission in the ICU, previous antibiotic use (<14 days), biological findings with focus on complete blood count, urea, creatinine, serum albumin, CRP.
To assess disease severity, we also performed the Atlas score which consists of age, systemic antibiotics during CDI therapy, leukocyte count, albumin, creatinine.
Fecal calprotectin was tested by Elisa technique with normal below <50 microg/g.
We divided the patients into two groups with mild and severe disease activity according to Zar et al. [11].
Patients with colorectal cancer and IBD were excluded.
Statistical analysis
To investigate categorical variables, we used Fisher’s Exact Test.
For continuous variables, we used non-parametric Mann-Whitney U Test, due to the small number of the patients, to determine whether clinical characteristics differed between dead and living patients.
ROC curve and Youden’s index was analyzed to identify the optimal cut-off values for fecal calprotectin in severity CDI diagnosis.
The significance level is 0.05. Statistical analyses were performed by using GraphPad Prism 9.1.2.
Results
A total of 59 patients were included, 41 patients had mild CDI and 18 patients had severe CDI.
The mean age of the patients was 69.29 years (±8.95, range 43-89), with more male 32 (54.23%) than female.
Baseline characteristics for every group of patients are shown in Table 1.
Table 1.
Characteristics and outcomes of the study CDI patients
|
|
Mild n=41 |
Severe n=18 |
p-value |
|
Age, years |
64.9 (±6.28) 65 (62-69) |
79.28 (±5.32) 81 (76-82) |
<0.0001 |
|
Gender, Male |
23 (56.1%) |
9 (50%) |
0.779 |
|
Mortality, Yes |
6 (14.6%) |
11 (61.1%) |
0.001 |
|
Cirrhosis, Yes |
8 (19.5%) |
2 (11.1%) |
0.708 |
|
Hypertension, Yes |
23 (56.1%) |
16 (88.9%) |
0.017 |
|
Diabetes, Yes |
22 (53.7%) |
13 (72.2%) |
0.252 |
|
Cardiovascular disease, Yes |
18 (43.9%) |
13 (72.2%) |
0.053 |
|
Lung disease, Yes |
5 (12.2%) |
8 (44.4%) |
0.014 |
|
Renal failure, Yes |
13 (31.7%) |
12 (66.7%) |
0.021 |
|
Malignancy, Yes |
9 (22.0%) |
6 (33.3%) |
0.517 |
|
Intensive care unit, Yes |
4 (9.8%) |
18 (100%) |
<0.0001 |
|
Antibiotics<14 days, Yes |
33 (80.5%) |
18 (100%) |
0.092 |
|
Leucocytes |
13,477.24 (±2,428.69) 13,300 (11,750-15,250) |
22,305.00 (±4,737.61) 22,325 (18,688-25,445) |
<0.0001 |
|
Neutrophils |
81.36 (±8.66) 79.8 (78.5-88.55) |
95.89 (±2.65) 96.25 (94.88-98.2) |
<0.0001 |
|
Hemoglobin |
11.18 (±1.33) 11.1 (10.1-12.25) |
11.00 (±1.29) 11.15 (9.78-12.15) |
0.723 |
|
Hematocrit |
39.12 (±4.54) 41 (37.65-42.3) |
36.56 (±5.48) 38.15 (31.39-41.38) |
0.074 |
|
Thrombocytes |
198,279.02 (±81,413.0) 190,000 (160,500-218,450) |
181,833.33 (±38,097.78) 170,000 (152,000-197,250) |
0.370 |
|
Creatinine |
1.35 (±0.53) 1.32 (0.8-1.74) |
2.95 (±0.76) 3.18 (2.2-3.5) |
<0.0001 |
|
Urea |
91.44 (±42.25) 88 (58.5-117.5) |
210.11 (±75.2) 204 (143.5-262) |
<0.0001 |
|
Albumin |
3.4 (±0.33) 3.5 (3.2-3.6) |
2.65 (±0.32) 2.6 (2.4-2.83) |
<0.0001 |
|
CRP |
29.32 (±13.41) 26 (20.5-34) |
163.06 (±89.77) 129 (101-243) |
<0.0001 |
|
ATLAS |
3.39 (±1.24) 3 (2-4.5) |
7.33 (±0.77) 7 (7-8) |
<0.0001 |
|
Calprotectin (μg/g) |
217.92 (±106.05) 195.42 (131.12-298.59) |
629.16 (±254.59) 615.14 (403.62-784.4) |
<0.0001 |
Comorbidities in both groups were not similar: more patients with hypertension, lung disease or renal failure were in the group of severe CDI.
No differences were found between the two groups for malignancy examination (p=0.517), even if a higher percentage was found for severe CDI patients (33.3%) than for mild CDI patients (22%).
All the severe CDI patients were treated in the intensive care unit, whereas only 9.8% mild CDI patients were hospitalized in the intensive care unit (p<0.0001).
There was no statistically significant difference in receiving antibiotic therapy within 14 days (p=0.092).
The median leucocytes, neutrophils, creatinine, urea, and CRP were significantly higher in the severe CDI group than in the mild CDI group, whereas albumin level was lower (p<0.0001).
Fecal calprotectin concentrations were higher (p<0.001) in the severe CDI patients (615.14μg/g; IQR, 403.62-784.4μg/g) than in the mild CDI patients (195.42μg/g; IQR, 131.12-298.59μg/g).
Data are presented as mean (±S.D.) and median (interquartile range) for continuous characteristics or count (percentage) for categorical characteristics.
The distribution of ATLAS and Fecal calprotectin scores among mild and severe CDI patients can be seen in Figure 1.
Figure 1.
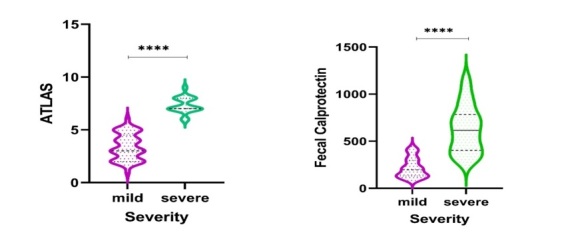
Violin plots showing the distribution of values for ATLAS score and Fecal calprotectin by severity CDI patients
Their comparations demonstrated that patients with severe CDI tended to have higher values than mild CDI patients, with skewed distribution.
The AUC was 0.953 (95% CI, 0.903-1.0) for CDI severity (p-value<0.001), as in Figure 2.
Figure 2.
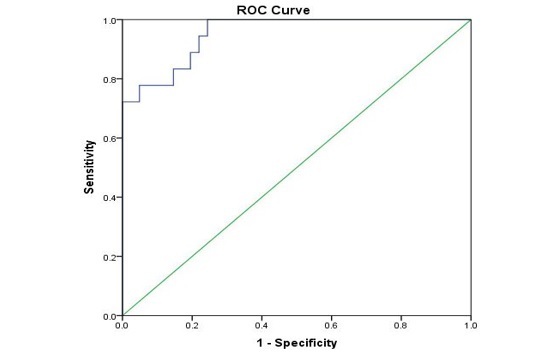
ROC curve analysis of fecal calprotectin concentrations by severity CDI
The optimum FC value of 290.09μg/g permits to identify patients with severe and mild CDI, having 100% sensitivity and 76% specificity.
Discussions
Identifying patients that might develop severe CDI colitis or fulminant colitis is of major importance as it may help choose the proper treatment.
Metronidazole and vancomycin are recommended as first line of treatment, whereas fidaxomicin, and fecal microbiota transplantation have been suggested for refractory or severe disease [12].
Potential biomarkers, such as fecal calprotectin and lactoferrin have been suggested for diagnosis and CDI severity assessment, as they may corelate with colonic inflammation.
Providing a specific biomarker for risk stratification in CDI could decrease morbidity and mortality and may reduce the resources used to treat these patients.
Our study focused on FC to differentiate between mild and severe CDI colitis.
Even though it is currently included for IBD diagnosis, treatment response and disease relapse, it might also be considered as an alternative for CDI assessment.
This biomarker has been tested even for irritable bowel syndrome and colorectal cancer but with lower sensitivity and specificity [13].
Basically, calprotectin is calcium binding protein derived from neutrophils cytoplasm and epithelial cells which provides information on the inflammatory status of the intestinal lumen [14].
FC was first used as a diagnosis biomarker by differentiating its level in CDI patients to other patients whom presented with bacterial diarrhea.
Even though its sensibility might be high for CDI diagnosis, specificity proved to be rather low which might not promote it as a reliable tool for diagnosis purposes.
On the other hand, if patients are already diagnosed with CDI a similar profile might be suggested as in IBD.
Studies have proven its correlation with IBD disease activity, and this might also be a valid argument even for CDI.
Thus, a more severe disease might be directly related to a higher value.
However, there is no threshold to determine what value is necessary to differentiate from mild to severe disease.
Our findings showed a major difference between patients whom developed severe CDI and the one with mild disease activity.
Peretz et al. [15] also tried to correlate disease severity to FC levels and hypervirulent ribotype 027 strain.
Their results proved that patients who had this hypervirulent strain also had higher values of cFC 811.9ug/g compared to others which had a mean value of 331.4ug/g.
We also found a good correlation between FC and Atlas score to assess disease severity.
These may be important tools for the physician to differentiate from complicated disease and to choose the right treatment.
A recent study highlighted that FC should not be used for treatment indication but as a tool for the physicians to make the therapeutic decision [16].
Thus, FC might extend its indications even to CDI severity assessment.
Our study has several limitations. Firstly, the sample number of patients is rather small and we did not exclude patients that already started metronidazole or vancomycin before enrollment.
Also, we did not provide a follow-up or response to treatment FC assessment as we only focused on disease severity.
Conclusions
Providing a new biomarker for CDI disease assessment could be essential for disease management.
Our study results point out the potential that FC might have as a biomarker for disease severity.
However, future multicentre studies and in larger cohort should be performed to validate the results.
Conflict of interests
None to declare.
References
- 1.Martin JS, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol. 2016;13(4):206–216. doi: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- 2.Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014;8(1):1–6. doi: 10.5009/gnl.2014.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song JH, Kim YS. Recurrent Clostridium difficile Infection: Risk Factors, Treatment, and Prevention. Gut Liver. 2019;13(1):16–24. doi: 10.5009/gnl18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313(4):398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare epidemiology of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 6.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance docu¬ment for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(2S Suppl1):S1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 7.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 8.Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis. 2018;27(3):299–306. doi: 10.15403/jgld.2014.1121.273.pti. [DOI] [PubMed] [Google Scholar]
- 9.Mari A, Baker FA, Mahamid M, Yacoob A, Sbeit W, Khoury T. Clinical utility of fecal calprotectin: potential applications beyond inflammatory bowel disease for the primary care physician. Ann Gastroenterol. 2019;32(5):425–430. doi: 10.20524/aog.2019.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shastri YM, Bergis D, Povse N, Schafer V, Shastri S, Weindel M, Ackermann H, Stein J. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med. 2008;121:1099–1106. doi: 10.1016/j.amjmed.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 12.Ooijevaar RE, van Beurden YH, Terveer EM, Goorhuis A, Bauer MP, Keller JJ, Mulder CJJ, Kuijper EJ. Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol Infect. 2018;24(5):452–462. doi: 10.1016/j.cmi.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Mari A, Baker FA, Mahamid M, Yacoob A, Sbeit W, Khoury T. Clinical utility of fecal calprotectin: potential applications beyond inflammatory bowel disease for the primary care physician. Ann Gastroenterol. 2019;32(5):425–430. doi: 10.20524/aog.2019.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 pro¬tein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 15.Peretz A, Tkhawkho L, Pastukh N, Brodsky D, Halevi CN, Nitzan O. Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect Dis. 2016;16:309–309. doi: 10.1186/s12879-016-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez-Carantoña C, Rodriguez-Torres A, Viteri-Noel A, Pintado V, Garcia-Fernandez S, Mora-Pimentel D, Escudero-Sanchez R, Martin-Jusdado F, Moreno S, Cobo J. Usefulness of Fecal Calprotectin in the Management of Patients with Toxigenic Clostridoides diffcile. J Clin Med. 2021;10(8):1627–1627. doi: 10.3390/jcm10081627. [DOI] [PMC free article] [PubMed] [Google Scholar]


