Abstract
Background
Pancreatic adenocarcinoma (PDAC) is highly lethal. Surgery offers the only chance of cure, but 5‐year overall survival (OS) after surgical resection and adjuvant therapy remains dismal. Adjuvant trials were mostly conducted in the West enrolling fit patients. Applicability to a general population, especially Asia has not been described adequately.
Aim
We aimed to evaluate the clinical outcomes, prognostic factors of survival, pattern, and timing of recurrence after curative resection in an Asian institution.
Methods and Results
The clinicopathologic and survival outcomes of 165 PDAC patients who underwent curative resection between 1998 and 2013 were reviewed retrospectively. Median age at surgery was 62.0 years. 55.2% were male, and 73.3% had tumors involving the head of pancreas. The median OS of the entire cohort was 19.7 months. Median OS of patients who received adjuvant chemotherapy was 23.8 months. Negative predictors of survival include lymph node ratio (LNR) of >0.3 (HR = 3.36, P = .001), tumor site involving the body or tail of pancreas (HR = 1.59, P = .046), presence of perineural invasion (PNI) (HR = 2.36, P = .018) and poorly differentiated/undifferentiated tumor grade (HR = 1.86, P = .058). The median time to recurrence was 8.87 months, with 66.1% and 81.2% of patients developing recurrence at 12 months and 24 months respectively. The most common site of recurrence was the liver.
Conclusion
The survival of Asian patients with resected PDAC who received adjuvant chemotherapy is comparable to reported randomized trials. Clinical characteristics seem similar to Western patients. Hence, geographical locations may not be a necessary stratification factor in RCTs. Conversely, lymph node ratio and status of PNI ought to be incorporated.
Keywords: pancreatic adenocarcinoma, prognostic factors, resected
1. INTRODUCTION
Pancreatic adenocarcinoma (PDAC) is a highly lethal malignancy. It is the eighth leading cause of cancer‐related deaths in men and the ninth leading cause of cancer‐related deaths in women worldwide. 1 PDAC often presents in advanced stages due to its aggressive biology and non‐specific symptoms.
The prognosis is poor even among patients with resectable disease, with a 5‐year survival of 10% to 30%. 2 Current staging and prognostic tools rely on the American Joint Committee of Cancer (AJCC) TNM staging system eighth edition. 3 Numerous other prognostic factors have been identified to better prognosticate patients with resected PDAC such as neutrophil/lymphocyte ratio, 4 lymph node ratio, 5 presence of lymphovascular invasion (LVI) or perineural invasion (PNI), 6 and resection margin status. 7 , 8 The standard operation for tumors of the pancreatic head is a pancreaticoduodenectomy (Whipple procedure), whereas tumors of the body or tail can be resected using a distal pancreatectomy. 9 These procedures are associated with high operative mortality and morbidity. 9 Advancement in surgical technique and perioperative management of patients has led to a reduction in the morbidity and mortality associated with the above‐mentioned surgeries. Moreover, with the improvement of imaging technique and the employment of a multi‐disciplinary team approach, better selection of suitable patients for surgery could be done. 10 Surgical outcomes at high‐volume centers have been shown to be superior compared to outcomes at low‐volume centers. In spite of that, many patients relapse at both local and distant sites after resection. Hence, adjuvant chemotherapy is crucial in the management of these patients as demonstrated in multiple randomized controlled trials (RCT). 11 , 12 , 13 , 14 , 15 , 17 , 18 , 38 Often, these trials stratify patients by geographical locations, resection margins, T‐stage and lymph node status. Adjuvant chemotherapy or chemoradiotherapy was conducted primarily in West enrolling fit patients with preserved organ functions and good performance status. Applicability to a general population especially in an Asian population has been inadequately described.
Pattern, timing, and predictors of recurrence after curative resection have been described primarily in Western populations.
We aimed to evaluate the clinical outcomes, prognostic factors of survival, pattern, and timing of recurrence after curative resection in an Asian institution.
We also compared the resected PDAC series from both Asian and Western populations.
2. METHODS
Patients who underwent resection with curative intent in our center between 1998 and 2013 were identified from a retrospective database. Patients eventually noted to have R2 resection or stage 4 disease were excluded. We collected clinicopathological and operative data of 165 patients. Follow‐up and data collection extended to December 2015.
Following surgery, all specimens underwent histopathological review, and features such as histology subtype, pathological AJCC stage and grade, resection margin status, tumor size, LVI and PNI. Resection margin involvement was defined according to the Royal College of Pathologists guidelines, with microscopic evidence of tumor within 1 mm of a resection margin (RM) being classified as R1. 19 Laboratory parameters such as CA 19‐9 and carcinoembryonic antigen (CEA) were measured preoperatively and postoperatively (patients without tests done within 3 months before or after the surgery was excluded from the analysis). The development of a hypointense mass in the resection site was considered as evidence of local recurrence. Similarly, detection of a new hypointense nodule/mass in the liver, lung, or peritoneum was considered evidence of distant recurrence. No biopsies were performed in this series to confirm the diagnosis of recurrent cancer. If the CT findings were non‐specific, a follow‐up CT would be performed, and the date of recurrence will be taken as the date of the follow‐up CT that demonstrate enlargement of the nodule or mass. Our study was approved by the Centralized Institutional Review Board of our institution.
2.1. Statistical analysis
Continuous variables were summarized using median and range. Categorical variables were summarized using frequency and percentage. Overall survival (OS) was calculated as the time from surgery to death from all causes. Patients who were alive at last follow‐up were censored at date of last follow‐up. Median OS was estimated using the Kaplan‐Meier method. Differences in survival curves were tested using the log‐rank test. Univariable and multivariable analyses were performed using the Cox proportional hazards model. For multivariable analysis, variable selection was performed using a forward selection procedure. All variables, regardless of significance in univariable analysis, were entered as candidate variables in the forward selection procedure. Only variables with more than 10% missing data were excluded. The proportional hazards assumption was tested on the final multivariable model using a test based on Schoenfeld residuals. A P‐value of less than .05 was taken as statistically significant in the univariable analyses. For the forward selection procedure, a P‐value of less than .10 was used for addition of variables into the multivariable model. P‐values for Cox models were calculated using the likelihood ratio test. All analyses were performed in Stata 15.0 (StataCorp, College Station, Texas).
3. RESULTS
3.1. Study population characteristics
Our study population consisted of 165 patients with resected pancreatic ductal adenocarcinoma. Median age at surgery was 62.0 (41‐84) years. 55.2% were male and 44.8% were female. The ethnic proportion of our study population was 77.6% Chinese, 4.8% Malay, 4.2% Indian, and 12.7% of other races. The median follow‐up time was 15.5 months. Regarding grade of differentiation, 10.9% had well differentiated, 75.2% moderately differentiated, 12.1% poorly differentiated, and 0.6% undifferentiated histology. Majority (73.3%) of patients had tumors involving the head of pancreas. Whipple operation or pylorus‐preserving pancreaticoduodenectomy (PPPD) was the most common form of surgery (73.3%) followed by distal pancreatectomy in 22.4%, and total pancreatectomy in 2.4%. The institution's surgical outcomes and details were previously published. 20 , 21 Only 50.9% of patients who underwent curative resection eventually received adjuvant therapy. Of these, 55 (33.3%) received adjuvant chemoradiotherapy, 33 (20.0%) received only adjuvant chemotherapy and 1 (0.6%) received only adjuvant radiotherapy. No patients received neoadjuvant chemotherapy or chemoradiotherapy. All patients who received adjuvant chemotherapy received gemcitabine or 5‐fluorouracil (5‐FU)/oral capecitabine monotherapy. Patients receiving adjuvant chemoradiotherapy received either concurrent radiotherapy with radiosensitizing 5‐FU or gemcitabine followed by gemcitabine or 5‐FU monotherapy. Patient demographic and clinicopathologic characteristics of the cohort are detailed in Table 1.
TABLE 1.
Patient demographics and clinical characteristics
| Characteristic | Frequency | Percentage |
|---|---|---|
| Total number of patients | 165 | 100 |
| Age at surgery (years) | ||
| Median (Range) | 62 (41–84) | |
| Gender | ||
| Male | 91 | 55.2 |
| Female | 74 | 44.8 |
| Race | ||
| Chinese | 128 | 77.6 |
| Malay | 8 | 4.8 |
| Indian | 7 | 4.2 |
| Others | 21 | 12.7 |
| Unknown | 1 | 0.6 |
| Smoking status | ||
| Never | 86 | 52.1 |
| Ex | 30 | 18.2 |
| Current | 10 | 6.1 |
| Unknown | 39 | 23.6 |
| Alcohol consumption | ||
| Never | 96 | 58.2 |
| Ex | 9 | 5.5 |
| Current | 19 | 11.5 |
| Unknown | 41 | 24.8 |
| Charlson comorbidities index | ||
| Median (Range) | 3 (1–9) | |
| Symptoms | ||
| Loss of weight | 42 | 25.5 |
| Loss of appetite | 27 | 16.4 |
| Fever | 4 | 2.4 |
| Abdominal pain | 48 | 29.1 |
| Abdominal distension | 5 | 3.0 |
| Diarrhea | 4 | 2.4 |
| Jaundice | 84 | 50.9 |
| Malaena | 1 | 0.6 |
| Tumor site | ||
| Head involved | 121 | 73.3 |
| Head not involved | 44 | 26.7 |
| AJCC TNM stage | ||
| IA | 5 | 3.0 |
| IB | 16 | 9.7 |
| IIA | 45 | 27.3 |
| IIB | 90 | 54.5 |
| III | 9 | 5.5 |
| T stage | ||
| T1 | 4 | 2.4 |
| T2 | 30 | 18.2 |
| T3 | 123 | 74.5 |
| T4 | 8 | 4.8 |
| N stage | ||
| N0 | 69 | 41.8 |
| N1 | 95 | 57.6 |
| NX | 1 | 0.6 |
| Histological grade | ||
| Well differentiated | 18 | 10.9 |
| Moderately differentiated | 124 | 75.2 |
| Poorly differentiated | 20 | 12.1 |
| Undifferentiated | 1 | 0.6 |
| Not stated/not determined | 2 | 1.2 |
| Type of surgery | ||
| Whipples operation or Pylori preserving pancreaticoduodenectomy (PPPD) | 121 | 73.3 |
| Pancreatectomy, distal or subtotal | 37 | 22.4 |
| Pancreatectomy, total | 4 | 2.4 |
| Pancreatectomy, NOS | 3 | 1.8 |
| Resection margins | ||
| R0 | 80 | 48.5 |
| R1 | 85 | 51.5 |
| Perineural invasion | ||
| No | 14 | 8.5 |
| Yes | 135 | 81.8 |
| Indeterminate | 6 | 3.6 |
| Unknown | 10 | 6.1 |
| Lymphovascular invasion | ||
| No | 80 | 48.5 |
| Yes | 62 | 37.6 |
| Indeterminate | 13 | 7.9 |
| NA | 10 | 6.1 |
| Lymph node resected | ||
| Median (Range) | 9 (0‐36) | |
| Lymph node ratio (No. positive/No. resected) | ||
| Median (Range) | 0.08 (0–1) | |
| Unknown (no LN resected) | 5 | 3.0 |
| Tumor size (largest diameter) (cm) | ||
| Median (Range) | 3.0 (0.8‐18.0) | |
| Not Reported | 18 | 10.9 |
| Posterior margins involved | ||
| No | 102 | 61.8 |
| Yes | 36 | 21.8 |
| Unknown | 27 | 16.4 |
| Type of adjuvant treatment | ||
| No adjuvant treatment | 76 | 46.1 |
| Radiotherapy only | 1 | 0.6 |
| Chemotherapy only | 33 | 20.0 |
| Chemoradiotherapy | 55 | 33.3 |
| Pre‐op CEA (ng/mL) a | ||
| Median (range) | 3.3 (0.5‐61.8) | |
| Unknown | 87 | 52.7 |
| Post‐op CEA (ng/mL) a | ||
| Median (range) | 2.2 (0.7‐14.9) | |
| Unknown | 124 | 75.2 |
| Pre‐op CA19‐9 (U/mL) a , b | ||
| Median (range) | 187.0 (<0.6‐>10 000) | |
| Unknown | 73 | 44.2 |
| Post‐op CA19‐9 (U/mL) a , b | ||
| Median (range) | 25.2 (<0.6‐6825) | |
| Unknown | 66 | 40.0 |
| Pre‐op albumin (g/L) a | ||
| Median (range) | 34 (16‐48) | |
| Unknown | 34 | 20.6 |
| Post‐op albumin (g/L) a | ||
| Median (range) | 24 (14‐47) | |
| Unknown | 26 | 15.8 |
| Pre‐op neutrophil/lymphocyte ratio a | ||
| Median (range) | 2.9 (0.6‐36.5) | |
| Unknown | 29 | 17.6 |
| Post‐op neutrophil/lymphocyte ratio a | ||
| Median (range) | 12.3 (0.8‐49.6) | |
| Unknown | 23 | 13.9 |
Abbreviation: NOS, Not otherwise specified.
Taken within 90 days before or after surgery.
Values of <0.6, < 2.0, > 5000, and >10 000 were taken as 0.6, 2.0, 5000, and 10 000, respectively, for the calculation of median.
3.2. Recurrence pattern
After median follow‐up of 15.5 months, 112 patients (67.9%) developed recurrence. The median time to recurrence was 8.87 months. 66.1% and 81.2% of patients developed recurrence at 12 and 24 months, respectively. (Figure S1).
Majority of patients developed distant recurrence as the first site of relapse. Seventy‐three (44.2%) had recurrence in a distant site, 20 (12.1%) had both local (defined as resection bed) and distant recurrences and 19 (11.5%) had solely local recurrence.
The most common site of recurrence was the liver (n = 58; 35.2%), followed by local recurrence (n = 39; 23.6%), distant lymph nodes (n = 31, 18.8%), peritoneum (n = 22, 13.3%), and lungs (n = 19; 11.5%).
3.3. Univariable analysis of OS
The median OS of the entire patient cohort was 19.7 months (95%CI: 16.9‐23.7). Median OS of patients who did not receive adjuvant therapy after curative resection was 15.7 months (95%CI: 11.7‐26.9). Median OS of patients who received adjuvant chemoradiotherapy or chemotherapy were 20.1 months (95%CI: 15.7‐28.2) and 23.8 months (95%CI: 19.1‐31.5) respectively. 1‐, 3‐, and 5‐year OS rates were 73.1% (95%CI: 65.1‐79.5), 28.0% (95%CI: 20.3‐36.1), and 14.8% (95%CI 7.6‐22.0), respectively.
Factors which conferred a poorer prognosis on OS by univariable analysis were: poorly differentiated/undifferentiated tumor (HR 2.15, 95% CI: 1.24‐3.74, P = .013), non‐pancreatic head tumors (HR 1.54, 95% CI: 1.04‐2.29, P = .037), N1 nodal status (HR 1.84, 95% CI: 1.24‐2.72, P = .002), lymph node ratio (LNR) of >0‐0.3 (HR 1.68, 95% CI: 1.09‐2.58, P = .001), LNR > 0.3 (HR 3.06, 95% CI: 1.75‐5.37, P = .001), presence of PNI (HR 2.62, 95% CI: 1.20‐5.73, P = .006), LVI (HR 1.52, 95% CI: 1.01‐2.29, P = .045), pre‐op CA 19‐9 (>75 U/mL) (HR 2.39, 95% CI 1.23‐4.63, P = .005), post‐op CA 19‐9 (>75 U/mL) (HR 2.61, 95% CI: 1.56‐4.38, P = .001). (Table 2).
TABLE 2.
Univariable and multivariable analysis of overall survival
| No. of events/patients | Median OS, months (95% CI) | Log‐rank P‐value | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Cox model P‐value | Hazard ratio (95% CI) | Cox model P‐value | ||||
| All patients | 111/165 | 19.7 (16.9, 23.7) | 98/146 | ||||
| Age at surgery (years) | |||||||
| <65 | 66/97 | 19.7 (16.9, 24.4) | 1 | ||||
| ≥65 | 45/68 | 20.1 (14.1, 24.9) | .389 | 1.18 (0.81, 1.74) | .392 | ||
| Gender | |||||||
| Male | 59/91 | 19.7 (15.5, 24.4) | 1 | ||||
| Female | 52/74 | 20.0 (16.8, 31.0) | .463 | 0.87 (0.60, 1.26) | .463 | ||
| Race | |||||||
| Chinese | 93/128 | 20.1 (17.4, 24.1) | 1 | ||||
| Non‐Chinese | 18/36 | 13.5 (10.6, 31.5) | .198 | 1.40 (0.84, 2.33) | .216 | ||
| Smoking status | |||||||
| Never | 58/86 | 21.4 (17.9, 31.0) | 1 | ||||
| Former | 21/30 | 15.7 (12.8, 19.6) | 1.73 (1.04, 2.88) | ||||
| Current | 6/10 | 31.6 (24.9, UD) | .027 | 0.58 (0.25, 1.36) | .032 | ||
| Alcohol consumption | |||||||
| Never | 64/96 | 20.0 (17.2, 28.2) | 1 | ||||
| Former | 7/9 | 12.3 (6.9, 28.8) | 1.70 (0.77, 3.73) | ||||
| Current | 14/19 | 26.4 (16.9, 36.1) | .406 | 1.02 (0.57, 1.84) | .465 | ||
| Charlson comorbidities index | |||||||
| 1–2 | 46/63 | 19.7 (15.4, 28.8) | 1 | ||||
| >2 | 65/102 | 19.7 (15.7, 23.7) | .463 | 1.16 (0.78, 1.71) | .462 | ||
| Tumor site | |||||||
| Head involved | 74/121 | 21.1 (17.9, 24.9) | 1 | 1 | |||
| Head not involved | 37/44 | 15.4 (11.4, 24.4) | .031 | 1.54 (1.04, 2.29) | .037 | 1.59 (1.02, 2.48) | .046 |
| AJCC TNM stage | |||||||
| I | 15/21 | 23.7 (11.4, 50.2) | 1 | ||||
| II | 90/135 | 19.6 (15.7, 23.7) | 1.16 (0.67, 2.02) | ||||
| III | 6/9 | 26.9 (8.9, UD) | .730 | 0.89 (0.34, 2.31) | .719 | ||
| T stage | |||||||
| T1/T2 | 26/34 | 19.7 (11.4, 30.2) | 1 | ||||
| T3/T4 | 85/131 | 19.7 (16.6, 24.4) | .505 | 0.86 (0.55, 1.34) | .511 | ||
| N stage | |||||||
| N0 | 42/69 | 28.8 (20.4, 45.4) | 1 | ||||
| N1 | 68/95 | 15.5 (13.2, 19.7) | .002 | 1.84 (1.24, 2.72) | .002 | ||
| Histological grade | |||||||
| Well/moderately differentiated | 95/142 | 21.1 (17.4, 24.9) | 1 | 1 | |||
| Poorly differentiated/Undifferentiated | 15/21 | 11.2 (7.6, 20.0) | .005 | 2.15 (1.24, 3.74) | .013 | 1.86 (1.02, 3.38) | .058 |
| Type of surgery | |||||||
| Whipples operation or PPPD | 77/121 | 20.1 (17.4, 24.1) | 1 | ||||
| Pancreatectomy, distal or subtotal | 29/37 | 17.6 (11.4, 31.6) | 1.29 (0.84, 1.98) | ||||
| Pancreatectomy, total | 3/4 | 4.3 (3.1, UD) | 7.24 (2.22, 23.60) | ||||
| Pancreatectomy, NOS | 2/3 | 14.2 (14.2, UD) | .002 | 1.31 (0.32, 5.37) | .057 | ||
| Resection margins | |||||||
| R0 | 52/80 | 19.7 (16.9, 26.9) | 1 | ||||
| R1 | 59/85 | 19.7 (14.2, 24.1) | .612 | 1.10 (0.76, 1.60) | .611 | ||
| Perineural invasion | |||||||
| No | 7/14 | 50.2 (17.2, UD) | 1 | 1 | |||
| Yes | 94/135 | 19.1 (15.5, 22.6) | .013 | 2.62 (1.20, 5.73) | .006 | 2.36 (1.07, 5.23) | .018 |
| Lymphovascular invasion | |||||||
| No | 54/80 | 23.7 (17.7, 35.4) | 1 | ||||
| Yes | 42/62 | 16.6 (11.7, 20.1) | .042 | 1.52 (1.01, 2.29) | .045 | ||
| Lymph node ratio | |||||||
| 0 | 38/64 | 31.0 (20.1, 45.4) | 1 | 1 | |||
| >0–0.3 | 49/70 | 17.9 (14.1, 22.0) | 1.68 (1.09, 2.58) | 1.58 (1.00, 2.49) | |||
| >0.3 | 20/26 | 12.3 (7.5, 19.6) | <.001 | 3.06 (1.75, 5.37) | .001 | 3.36 (1.83, 6.16) | .001 |
| Tumor size (largest diameter) (cm) | |||||||
| ≤3 | 50/78 | 23.7 (17.9, 28.8) | 1 | ||||
| >3 | 51/69 | 14.1 (11.5, 21.1) | .017 | 1.61 (1.09, 2.38) | .018 | ||
| Posterior margins involved | |||||||
| No | 68/102 | 19.7 (15.7, 26.4) | 1 | ||||
| Yes | 24/36 | 18.5 (10.8, 31.6) | .797 | 1.06 (0.67, 1.70) | .798 | ||
| Adjuvant treatment | |||||||
| None | 48/76 | 15.7 (11.7, 26.9) | 1 | ||||
| Chemotherapy only | 19/33 | 23.8 (19.1, 31.5) | 0.74 (0.43, 1.26) | ||||
| Chemoradiotherapy | 43/55 | 20.1 (15.7, 28.2) | .528 | 0.89 (0.59, 1.35) | .520 | ||
| Pre‐op CEA (ng/ml) | |||||||
| ≤5 | 27/44 | 22.0 (17.6, 44.6) | 1 | ||||
| >5 | 28/34 | 14.1 (10.0, 24.4) | .110 | 1.54 (0.90, 2.61) | .114 | ||
| Post‐op CEA (ng/ml) | |||||||
| ≤5 | 27/34 | 21.8 (14.7, 30.2) | 1 | ||||
| >5 | 6/7 | 21.4 (3.1, UD) | .330 | 1.55 (0.64, 3.80) | .356 | ||
| Pre‐op CA19‐9 (U/ml) | |||||||
| ≤75 | 13/28 | 55.5 (14.0, 74.4) | 1 | ||||
| >75 | 51/64 | 19.1 (15.3, 22.0) | .008 | 2.39 (1.23, 4.63) | .005 | ||
| Post‐op CA19‐9 (U/ml) | |||||||
| ≤75 | 48/72 | 22.6 (18.5, 30.2) | 1 | ||||
| >75 | 22/27 | 13.2 (8.4, 19.4) | <.001 | 2.61 (1.56, 4.38) | .001 | ||
| Pre‐op albumin (g/L) | |||||||
| >35 | 38/54 | 22.0 (14.2, 31.6) | 1 | ||||
| ≤35 | 50/77 | 17.9 (14.1, 23.7) | .870 | 1.04 (0.68, 1.58) | .869 | ||
| Post‐op albumin (g/L) | |||||||
| >35 | 11/17 | 24.4 (17.6, 36.0) | 1 | ||||
| ≤35 | 83/122 | 18.5 (14.7, 22.6) | .300 | 1.39 (0.74, 2.62) | .283 | ||
| Pre‐op NLR | |||||||
| ≤5 | 77/109 | 19.1 (15.4, 26.4) | 1 | ||||
| >5 | 16/27 | 19.4 (12.8, 24.1) | .363 | 1.29 (0.74, 2.24) | .377 | ||
| Post‐op NLR | |||||||
| ≤5 | 14/18 | 22.6 (13.2, 50.0) | 1 | ||||
| >5 | 83/124 | 19.4 (15.4, 24.1) | .861 | 1.05 (0.60, 1.86) | .861 | ||
Abbreviations: NLR, neutrophil‐lymphocyte ratio; PPPD, pylori preserving pancreaticoduodenectomy; UD, undefined.
Note: For the multivariable analysis, only variables with less than 10% missing data were considered in the forward selection procedure. The criterion for variable addition was P < .10.
3.4. Multivariable analysis of OS
The final multivariable model for OS revealed that LNR > 0‐0.3 (HR 1.58, 95%CI: 1.00‐2.49, P < .001), lymph node ratio > 0.3‐1 (HR 3.36, 95%CI: 1.83‐6.16, P = .001), non‐pancreatic head tumors (HR 1.59, 95%CI: 1.02‐3.38, P = .046), presence of PNI (HR 2.36 95%CI: 1.07‐5.23, P = .018), and poorly differentiated or undifferentiated tumor grade (HR 1.86, 95%CI: 1.02‐3.38, P = .058) were negative predictors of survival. (Table 2) The Kaplan‐Meier plot of the OS for the above‐mentioned prognostic factors can be found in Figure 1.
FIGURE 1.
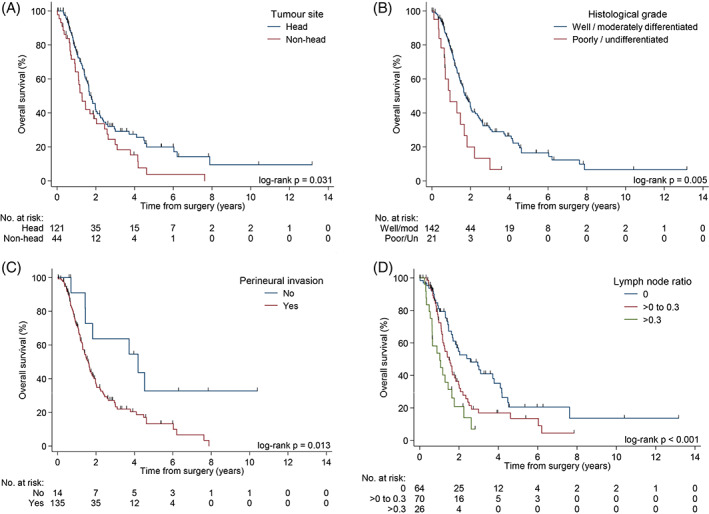
Kaplan‐Meier curves of OS by tumor site, A, tumor grade, B, PNI, C, LNR, D
4. DISCUSSION
The median survival of patients in this study was 19.7 months (95%CI: 16.9‐23.7) with a 5‐year OS of 14.8% (95%CI: 7.6‐22). This is comparable to the experience of major centers in both Western and Asian series with a median survival ranging from 13 to 24 months and a 5‐year OS ranging from 4% to 27%. The patient characteristics and prognostics factors described in both Western and Asian series are also similar (Table 3).
TABLE 3.
Series of resected pancreatic adenocarcinoma in Asian and Western Centers
| Series (Author, country) | Year | Number of Patients (N) | Median age | Gender (%) | Tumour site (%) | Stage (%) | Tumour | Lymph node (%) | Differentiation (%) | Adjuvant tx (%) | Median OS (mth) | 1‐year OS (%) | 3‐year OS (%) | 5‐year OS (%) | Prognostic Factors (multivariable analysis) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asian | |||||||||||||||
| Liu et al, China 22 | 2007‐2015 | 1223 | 62 | M: 57 |
Head/Body: 56.9 Tail: 43.1 |
IA: 9.4 IB: 30.9 IIA: 15.8 IIB: 33.7 III: 10.2 |
Mean: 3.77 cm | N1: 43.9 |
WD: 65.0 MD/PD: 35.0 |
82.5 | 18.7 | 64.0 | 28.7 | NR |
Tumor grade Post‐operative (post‐op) CA 19–9 |
| Yamamoto et al, Japan 23 | 2001‐2015 | 100 | 70 | M: 58 | NR |
IA: 5 IB: 3 IIA: 38 IIB 52 III: 1 IV: 1 |
Median: 2.6 cm | N1: 53 | NR | 64.0 | NR | 79.7 | 40.9 | 22.6 | NR |
| You et al, Korea 5 | 2005‐2017 | 351 | 63.3 | M: 57.8 |
Head: 64.1 Non‐head: 32.5 Overlapping: 3.4 |
I: 19.1 II: 67.0 III: 12.8 IV: 1.1 |
NR | N1: 57.3 |
WD: 8.0 MD: 82.3 PD: 8.8 UD: 0.9 |
100.0 a | 31.7 | NR | NR | NR |
LNR Tumor grade Lymphovascular invasion (LVI) Perineural invasion (PNI) Tumor stage (T‐stage) Pre‐operative (pre‐op) CA 19‐9 |
| Xu et al, China 24 | 2010‐2014 | 353 | 61 | M: 56.9 |
Head/body: 57.5 Tail: 42.5 |
I: 24.4 IIA: 29.7 IIB: 45.9 |
Mean: 4.13 cm | N1: 45.9 |
WD/MD: 63.7 PD: 36.3 |
Chemo (82.4) ChemoRT (31.7) |
18.1 | 62.2 | 27.1 | ‐ |
Those with elevated post‐op serum CA19‐9: Tumour size, no adjuvant chemoradiotherapy (chemoRT), post‐op CA125, no decrease in CA19‐9 from pre‐op Those with normal post‐op serum CA19‐9: No adjuvant chemoradiotherapy, post‐op CA125, post‐op CEA |
| Haruki et al, Japan 25 | 2001‐2011 | 113 | 66.8 (mean) | M: 61.9 | NR |
0:6.2 I: 2.7 II: 16.8 III: 48.7 IV: 25.7 |
NR | NR | NR | NR | NR | NR | NR | 24.1 |
CRP/albumin ratio Higher TNM stage |
| Shin et al, Korea 26 | 2000‐2007 | 528 | 61 | M: 60.2 |
Head: 74.4 Non‐head: 25.6 |
≤3 cm: 51.3 >3 cm: 48.7 |
N1: 42.0 |
WD/MD: 78.0 PD: 15.2 Missing 6.8 |
NR | NR | NR | NR | 15.5 |
Tumor size Tumor grade Node‐stage (N‐stage) PNI LVI Portal/mesenteric vein invasion |
|
| Western | |||||||||||||||
| Sohn et al, USA 27 | 1984‐1999 | 616 | 64.3 (mean) | M: 54 | NR | NR | Mean: 3.2 cm | N1: 72.0 |
WD/MD: 64.0 PD: 36.0 |
74.0 | 17.0 | 63.0 | 25.0 | 17.0 |
Resection Margin Tumor size Intra‐op blood loss Tumor grade Post‐op chemoRT |
| Katz et al, USA 10 | 1990‐2002 | 329 | 64 | M: 58 |
Head: 92 Non‐head: 8 |
NR | Mean: 3.0 cm | N1: 52.0 | NR | 91.0 | 24.0 | NR | NR | 27.0 |
N‐stage Prior attempts at resection |
| Schnelldorfer et al, USA 28 | 1981‐2001 | 357 | 65 (mean) | M: 54 | Head: 100 |
IA: 7.6 IB: 14.9 IIA: 27.4 IIB: 48.5 III: 0.8 IV: 0.8 |
Mean: 3.2 cm | N1: 49.4 |
WD: 0 MD: 21.9 PD: 55.8 UD: 22.3 |
77.0 | 17.0 | NR | NR | 18.0 |
Tumour size N‐stage |
| Winter et al, USA 29 | 1990‐1999 | 399 | NR | NR | NR | NR | NR | NR | NR | NR | 25.6 | 68.0 | NR | 20.0 | ‐ |
| Winter et al, USA 29 | 2000‐2009 | 625 | NR | NR | NR | NR | NR | NR | NR | NR | 24.5 | 68.0 | NR | 8.0 | ‐ |
| Lewis et al, USA 6 | 2001–2011 | 424 | 67 | M: 50.5 | NR |
IA: 3.8 IB: 6.8 IIA: 19.3 IIB 64.9 III: 2.4 IV: 2.6 |
NR | N1: 68.4 |
WD: 10.8 MD: 49.5 PD 39.7 |
76.4 | 21.3 | 76.0 | 34.0 | 23.0 |
T‐stage N‐stage LNR Tumor size Tumor grade LVI PNI Resection margin Adjuvant treatment Pre‐op physiology |
| Konstantinidis et al, USA 30 | 1993‐2008 | 517 | 67 | M: 47.2 | NR | NR | Median: 3.0 cm | N1: 31.5 |
WD: 3.5 MD: 54.5 PD: 39.7 UD: 2.3 |
NR | 19.7 | NR | NR | 17 |
Size of tumour Tumor grade LVI PNI Resection margin LNR |
Abbreviation: CRP, C‐reactive protein; LNR, Lymph Node Ratio; LVI, Lymphovascular invasion; M, Male; MD, Moderately differentiated; NR, Not reported; PD, Poorly differentiated; PNI, Perineural invasion; UD, Undifferentiated; WD, Well differentiated
Only patients who received adjuvant chemotherapy are included in this study.
Despite the benefits of adjuvant chemotherapy, only 50.9% of our patients received adjuvant treatment, which was comparable with other institutions and large series reporting rates of approximately 35% to 60%. 31 , 32 , 33 , 34 There are numerous reasons why patients do not receive adjuvant chemotherapy. These include post‐operative complications leading to poor performance status post‐surgery, tumor recurrence or metastases detected prior to initiation of adjuvant chemotherapy, and patient's preferences. 35 , 36 , 37 Patients who received adjuvant chemotherapy in our series had an OS of 23.8 months as compared to 15.7 months for those who did not receive adjuvant chemotherapy. This is comparable to that of the Phase 3 trials evaluating the efficacy of these regimes, 11 , 12 , 14 , 38 and consistent with real‐world data described by other authors. 34 , 37 Given the low rates of receipt of adjuvant chemotherapy and early dissemination of disease in PDAC, a neoadjuvant approach may be advantageous. 39 , 40 Studies exploring this approach have conflicting results. The Phase 3 PREOPANC‐1 trial randomized patients to preoperative chemoradiotherapy followed by surgery and four courses of adjuvant gemcitabine or to immediate surgery and six courses of adjuvant gemcitabine. There was no difference in the OS by intention to treat in both groups. 63 The Prep‐02/JSAP‐05 randomized Phase 2/3 trial randomized 362 patients with resectable PDAC to neoadjuvant gemcitabine and S‐1 followed by surgery and adjuvant S‐1 or initial surgery and adjuvant S‐1. There was a significant benefit of neoadjuvant gemcitabine and S‐1 followed by surgery and adjuvant S‐1 compared with initial surgery and adjuvant S‐1 therapy (median OS: 36.7 vs 26.6 months, HR 0.72 (95%CI: 0.55‐0.94), P = .015). 64 However, this was done exclusively in Japanese patients and the generalizability of these data is debatable. The SWOG S1505 Phase 2 randomized trial randomized patients with resectable PDAC to perioperative FOLFIRINOX or perioperative gemcitabine and nab‐paclitaxel. The primary outcome was 2‐year OS. Each arm was compared against the historical threshold of 40%. The 2‐year OS was 41.6% with mFOLFIRINOX (P = .42) and 48.8% with gemcitabine/nab‐paclitaxel (P = .12).65 There are multiple other trials examining this question including the randomized Phase 2/3 NEPAFOX trial (ClinicalTrials.gov identifier: NCT02172976) which is evaluating neoadjuvant FOLFIRINOX, surgery, and adjuvant FOLFIRINOX compared with surgery and adjuvant gemcitabine in patients with resectable and borderline resectable pancreatic cancer. There is also the randomized Phase 2 NEONAX trial (ClinicalTrials.gov identifier: NCT02047513) which compares neoadjuvant gemcitabine and nab‐paclitaxel followed by surgery and adjuvant gemcitabine and nab‐paclitaxel compared with initial surgery and adjuvant gemcitabine and nab‐paclitaxel. While no patients in our series received neoadjuvant treatment, it is a promising approach worth considering and we await the results of ongoing trials.
The pattern of recurrence in our series of patients is similar to that reported in the literature. 10 Most of the recurrences occurred within the first year after surgery as demonstrated in Figure S1. The most common sites of recurrence are the liver, local recurrence, distant lymph nodes, lungs, and peritoneum. 61.3% of patients in our study developed recurrence within 1 year after curative resection; this is reflective of the aggressive disease biology and presence of micrometastases at diagnosis.
In this study consisting of Asian patients, we identified four prognostic factors associated with poor prognosis: LNR > 0.3, poorly differentiated/undifferentiated tumor grade, location of tumor at the body or tail and the presence of PNI.
LNR has been found to be an independent prognostic factor in various studies. 5 , 41 , 42 Different groups have used different cutoffs for the LNR. Valsangkar et al demonstrated that increasing values of LNR of 0.2, 0.20 to 0.30 and ≥0.30 were associated with poor prognosis, 41 Huebner et al showed that a LNR of ≥0.17 had poorer prognosis. 42 We found that a LNR ≥0.30 was associated with a poorer prognosis. Patients with LNR of 0, >0 to 0.3 and > 0.3 had median OS of 31.0, 17.9, and 12.3 months, respectively. Total number of lymph nodes examined (TLN) may be of prognostic significance, especially in patients with pN0 disease. Slidell et al found that patients with pN0 disease could be further stratified based on the number of lymph nodes evaluated, with those with 11 or less LN examined having a poorer prognosis. 43 Another study showed that those with <12 TLN had a poorer prognosis, but this did not reach statistical significance. 44 In our study, however, we did not find that the TLN was a prognostic factor in patients with pN0 disease or in our entire cohort. While nodal status is incorporated as a stratification in a large proportion of randomized adjuvant trials in pancreatic cancer, 12 , 13 , 15 , 18 LNR could be a better stratification factor. LNR did not feature as a stratification factor in any of the randomized trials (Table 4). The only randomized trial, which included LNR in its patients' clinic‐pathological characteristics, was JASPAC‐01 trial. 15 Tumor grade is a known prognostic factor found in many studies, including various RCTs. 5 , 6 , 11 , 14 , 18 , 22 , 26 , 27 , 30 , 48 , 49 (Tables 3 and 4) Our study confirmed this finding. While Brennan et al found that tumors located at the head are associated with a worse prognosis, our results are contrary to this. 50 We found that patients with tumors at the body or tail had poorer prognosis. Multiple studies have suggested that the anatomical site is a prognostic factor; however, studies have been conflicting regarding which site is associated with a better prognosis. 51 , 52 , 53 , 54 Artinyan et al and Watanabe et al reported that patients with body/tail PDAC are more likely to be have unresectable or metastatic disease at presentation and consequently have poorer OS. This is attributed to the earlier onset of symptoms (eg, jaundice) in patients with head lesions. 52 , 53 Body/tail lesions were found to be a poorer prognostic factor compared with head lesions even in patients who had undergone surgical resection. 53 This may potentially be due to more aggressive tumor biology for lesions arising from the body/tail. 55 However, Lau et al, which utilized the Surveillance, Epidemiology, and End Results (SEER) registry, found that patients with local‐stage pancreatic body/tail cancer had higher OS compared with local‐stage pancreatic head cancer. 51
TABLE 4.
Phase 3 randomized clinical trials evaluating efficacy of adjuvant treatment in resected pancreatic adenocarcinoma
| Randomized controlled trials | Arms | N | Stratifications | Clinico‐pathological features described in patient characteristics Y: Yes, N: No | Median OS (mths) | 5‐year‐OS (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | T‐status | Nodal status | LNR | Resection status | Grade | Baseline CA19‐9 | Post‐operative CA19‐9 | Site of primary | PNI | LVI | ||||||
| ESPAC 1 11 |
Observation (Obs) 5‐FU ChemoRT ChemoRT followed by 5‐FU |
289 |
Country Resection margin |
Y | Y | Y | Y | N | Y | Y | N | N | N | N | N |
Obs: 16.9 5‐FU: 20.1 ChemoRT: 15.9 ChemoRT followed by 5‐FU: 19.9 |
Obs: 11 5‐FU: 29 ChemoRT: 7 ChemoRT followed by 5‐FU: 13 |
| CONKO‐001 12 |
Gemcitabine (Gem) Observation |
368 |
Tumour stage: T1‐2 vs T3‐4 Nodal status: N0 vs N1 Resection margin: R0 vs R1 |
Y | Y | Y | Y | N | Y | Y | N | N | N | N | N |
Gem: 22.8 Obs: 20.2 |
Gem: 20.7 Obs: 10.4 |
| RTOG 9704 13 , 45 |
5‐FU‐RT Gem‐RT |
451 |
Tumor diameter: <3 cm vs ≥3 cm Nodal status: N0 vs N1 Surgical margins: R0 vs R1 vs unknown |
Y | Y | Y | Y | N | Y | Y | Y | N | Y | N | N |
5‐FU‐RT: 16.9 Gem‐RT: 20.6 |
5FU: 18 Gem: 22 |
| ESPAC 3 14 |
Gem 5‐FU |
1008 |
Country Surgical margins: R0 vs R1 |
Y | Y | Y | Y | N | Y | Y | N | Y | N | N | N |
Gem:23.6 5‐FU:23.0 |
‐ |
| JASPAC‐01 15 |
Gem TS‐one |
377 |
Study site Surgical margin: R0 vs R1 Nodal status: N0 vs N1 |
Y | Y | Y | Y | Y | Y | N | Y | N | N | N | N |
Gem:25.5 TS‐one: 46.5 |
Gem: 24.4 TS‐one: 44.1 |
| ESPAC‐4 38 , 46 N |
Gem Gem/Cape |
730 |
Country R0 vs R1 |
Y | Y | Y | Y | N | Y | Y | Y | Y | N | N | N |
Gem: 25.5 Gem/Cape: 28.0 |
Gem: 20.0 Gem/Cape: 28.0 |
| PRODIGE‐24 18 |
Gem mFFX |
493 |
Study site Surgical margin: R0 vs R1 Nodal status: N0 vs N1 Post‐op CA19‐9 (≤90 U/mL vs 91‐180 U/mL) |
Y | Y | Y | N | N | Y | Y | N | Y | Y | Y | Y |
Gem: 35.0 mFFX: 54.4 |
‐ |
| APACT 17 , 47 |
Gem Gem/nab‐paclitaxel |
866 |
Country Surgical margin: R0 vs R1 Nodal status: N0 vs N1 |
Y | Y | Y | Y | N | Y | Y | Y | N | N | N | N |
Gem: 37.7 Gem/nab‐paclitaxel: 41.8 |
‐ |
Abbreviations: 5‐FU, 5‐Flurouracil; Cape, Capecitabine; Gem, Gemcitabine; mFOLFIRINOX, modified 5‐FU, leucovorin, oxaliplatin, irinotecan; nab‐Paclitaxel, nanoparticle albumin‐bound paclitaxel; Obs, Observation; TS‐one, tegafur, gimeracil, oteracil.
Chatterjee et al found that the presence of PNI and LVI correlated with poorer outcomes. We found that the presence of PNI but not LVI was associated with poor prognosis. PNI is the presence of cancer cells along nerves and/or within the epineurial, perineurial, and endoneurial spaces of the neuronal sheath and is commonly found in PDAC. 56 The presence of PNI has been demonstrated as a negative prognostic factor in multiple studies. 5 , 6 , 26 , 30 (Table 3).
While the previously described factors are well described in the literature to be prognostic, the prognostic value of the resection margin remains controversial. 57
Margin status has been identified as prognostic factor in multiple studies. 58 , 59 However, other studies have demonstrated no relationship between the resection margin and OS. 60 , 61 Conflicting results have also been found for the posterior resection margin. 58 , 62 Our study found that resection margin status (R0 vs R1) and the posterior resection margin status (R0 vs R1) were not independently associated with OS in the multivariable analysis. There are numerous postulations for the conflicting results. First, the definition of microscopic margin positivity differs from study to study. 19 , 60 Second, there are wide variability in the way different centers handle and sample the resection tissue. 57 Third, the definition of the posterior margin is also not standardized in multiple studies. 57
Taking the above together, our study showed that our cohort had similar prognostic factors, recurrence patterns, and survival as other Western and Asian institutions. 5 , 6 , 10 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 (Table 3) In the APACT trial which recruits both Western and Asian patients, country was used as a stratification factor. 17 Given the similarity in clinical characteristics in Western and Asian patients with PDAC, using country as a stratification factor may not be necessary. On the other hand, LNR and presence of PNI have consistently been found to be a significant prognostic factor in RCTs or large series from high‐volume centres 5 , 6 , 11 , 14 , 16 , 18 , 22 , 26 , 27 , 30 , 48 , 49 (Tables 3 and 4) and should perhaps be used as a stratification factor instead.
Our study has several limitations. While we managed to demonstrate applicability of adjuvant therapy in a general Asian population consistent with what has been reported in RCT, all the patients in this cohort received single agent systemic therapy (gemcitabine or 5FU). A number of RCT has since been reported providing evidence for doublet and triplet combination therapies. 17 , 18 Future population‐based studies are needed to clarify its applicability to a general population. As this study is retrospective in nature, there may be recall bias. Furthermore, the study sample size is modest, perhaps explaining for lack of statistical significance in previously reported prognostic factors (eg, resection margins and presence of LVI). Finally, incomplete capture of variables may introduce bias in survival analysis.
In conclusion, the survival of Asian patients with resected PDAC who received adjuvant chemotherapy is comparable to reported randomized trials. Clinical characteristics of Asian patients with resected PDAC are similar to datasets described among patients from the West. Hence, geographical locations/country of origin may not be a necessary stratification factor in RCTs. Conversely, LNR and status of PNI ought to be incorporated.
CONFLICT OF INTEREST
Su Pin Choo has received research funding and speaking fees from Bristol‐Myers Squibb (BMS) speaking fees from Lilly, research funding from Sirtex, and has participated on advisory boards for BMS, Sirtex, Lilly, Norvatis, Eisai, Bayer, Celgene. David Tai has received research funding for BMS and Sirtex, honorarium from Bayer and has participated on advisory boards for Eisai, Bayer, and Ipsen. Joycelyn Jie Xin Lee has received research funding from Bayer, honorarium from BMS and Ipsen, and has participated on advisory boards for Bayer and Ipsen.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, K.Y.Y.N., E.W.X.C., D.T.; Methodology, K.Y.Y.N., E.W.X.C., D.T.; Investigation, K.Y.Y.N., E.W.X.C., B.J.; Formal Analysis, K.Y.Y.N., E.W.X.C., C.L.; Resources, D.T.; Writing ‐ Original Draft, K.Y.Y.N., E.W.X.C., D.T.; Writing ‐ Review & Editing, All authors: Visualization, K.Y.Y.N., E.W.X.C., D.T.
ETHICAL STATEMENT
Our study was approved by the Centralized Institutional Review Board of our institution.
Supporting information
Figure S1. Recurrence pattern.
ACKNOWLEDGEMENTS
Fun Loon Leong for assisting with the maintenance of the database. We thank the patients and their families.
Ng KYY, Chow EWX, Jiang B, et al. Resected pancreatic adenocarcinoma: An Asian institution's experience. Cancer Reports. 2021;4:e1393. 10.1002/cnr2.1393
Kennedy Yao Yi Ng and Edwin Wei Xiang Chow contributed equally to the manuscript.
Contributor Information
Kennedy Yao Yi Ng, Email: kennedy.ng@mohh.com.sg.
David Tai, Email: david.tai.w.m@singhealth.com.sg.
DATA AVAILABILITY STATEMENT
The unidentified dataset is available upon reasonable requests made to the corresponding author.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Allen PJ, Kuk D, Castillo CF, et al. Multi‐institutional validation study of the American joint commission on cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265(1):185‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845‐847. [DOI] [PubMed] [Google Scholar]
- 4. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil‐lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. You MS, Lee SH, Choi YH, et al. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment. BMC Cancer. 2019;19(1):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB. 2013;15(1):49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta‐analysis of randomized controlled trials. Arch Surg. 2008;143(1):75‐83.discussion 83. [DOI] [PubMed] [Google Scholar]
- 8. Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27(17):2855‐2862. [DOI] [PubMed] [Google Scholar]
- 9. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846‐4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz MHG, Wang H, Fleming JB, et al. Long‐term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200‐1210. [DOI] [PubMed] [Google Scholar]
- 12. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long‐term outcomes among patients with resected pancreatic cancer: the CONKO‐001 randomized trial. JAMA. 2013;310(14):1473‐1481. [DOI] [PubMed] [Google Scholar]
- 13. Regine WF, Winter KA, Abrams R, et al. Fluorouracil‐based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5‐year analysis of the U.S. intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18(5):1319‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus Folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073‐1081. [DOI] [PubMed] [Google Scholar]
- 15. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248‐257. [DOI] [PubMed] [Google Scholar]
- 16. Neoptolemos JP, Palmer DH, Ghaneh P, et al. ESPAC‐4: a multicenter, international, open‐label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma: five year follow‐up. J Clin Oncol. 2020;38(15_suppl):4516‐4516. [Google Scholar]
- 17. Reni M, Riess H, O'Reilly EM, et al. Phase III APACT trial of adjuvant nab‐paclitaxel plus gemcitabine (nab‐P + gem) versus gemcitabine (gem) alone for patients with resected pancreatic cancer (PC): outcomes by geographic region. J Clin Oncol. 2020;38(15_suppl):4515‐4515. [Google Scholar]
- 18. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395‐2406. [DOI] [PubMed] [Google Scholar]
- 19. Campbell F, Foulis AK, Verbeke CS. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. 2 Carlton House Terrace, London, SW1Y 5AF: The Royal College of Pathologists; 2010. https://www.rcpath.org/uploads/assets/34910231‐c106‐4629‐a2de9e9ae6f87ac1/G091‐Dataset‐for‐histopathological‐reporting‐of‐carcinomas‐of‐the‐pancreas‐ampulla‐of‐Vater‐and‐common‐bile‐duct.pdf
- 20. Goh BKP, Tan Y‐M, Chung Y‐FA, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and Management of the Postoperative Pancreatic Fistula: a 21‐year experience at a single institution. Arch Surg. 2008;143(10):956‐965. [DOI] [PubMed] [Google Scholar]
- 21. Goh BKP, Tan YM, Cheow PC, et al. Outcome of distal pancreatectomy for pancreatic adenocarcinoma. Dig Surg. 2008;25(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 22. Liu L, Xu HX, He M, et al. A novel scoring system predicts postsurgical survival and adjuvant chemotherapeutic benefits in patients with pancreatic adenocarcinoma: implications for AJCC‐TNM staging. Surgery. 2018;163(6):1280‐1294. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto T, Uchida Y, Terajima H. Clinical impact of margin status on survival and recurrence pattern after curative‐intent surgery for pancreatic cancer. Asian J Surg. 2019;42(1):93‐99. [DOI] [PubMed] [Google Scholar]
- 24. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19‐9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373‐384. [DOI] [PubMed] [Google Scholar]
- 25. Haruki K, Shiba H, Shirai Y, et al. The C‐reactive protein to albumin ratio predicts long‐term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. 2016;40(9):2254‐2260. [DOI] [PubMed] [Google Scholar]
- 26. Shin SH, Kim SC, Hong SM, et al. Can statistically determined prognostic factors predict the long‐term survival of patients with pancreatic ductal adenocarcinoma following surgical resection?: clinicopathological analysis of 82 long‐term survivors. Pancreas. 2014;43(4):571‐577. [DOI] [PubMed] [Google Scholar]
- 27. Sohn TA, Yeo CJ, Lillemoe KD, et al. Resected adenocarcinoma of the pancreas ‐ 616 patients: results, outcome and prognostic indicators. Gastroenterology. 2000;118(4, Part 1):A1059. [DOI] [PubMed] [Google Scholar]
- 28. Schnelldorfer T, Ware AL, Sarr MG, et al. Long‐term survival after pancreatoduodenectomy for pancreatic adenocarcinoma. Ann Sur. 2008;247(3):456‐462. 10.1097/sla.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 29. Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169‐175. [DOI] [PubMed] [Google Scholar]
- 30. Konstantinidis IT, Deshpande V, Zheng H, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg. 2010;14(2):261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parmar AD, Vargas GM, Tamirisa NP, Sheffield KM, Riall TS. Trajectory of care and use of multimodality therapy in older patients with pancreatic adenocarcinoma. Surgery. 2014;156(2):280‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital‐Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kutlu OC, Vega EA, Salehi O, et al. Laparoscopic pancreatectomy for cancer in high volume centers is associated with an increased use and fewer delays of adjuvant chemotherapy. HPB. 2020.(23 .(4):625‐632. [DOI] [PubMed] [Google Scholar]
- 34. Altman AM, Wirth K, Marmor S, et al. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26(12):4108‐4116. [DOI] [PubMed] [Google Scholar]
- 35. Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372‐377. [DOI] [PubMed] [Google Scholar]
- 36. Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma ‐ a population‐based cohort study. Acta Oncol. 2016;55(3):265‐277. [DOI] [PubMed] [Google Scholar]
- 37. Chikhladze S, Lederer A‐K, Kousoulas L, et al. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: retrospective real‐life data. World J Surg Oncol. 2019;17(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011‐1024. [DOI] [PubMed] [Google Scholar]
- 39. Tzeng C‐WD, Cao HST, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18(1):16‐25. [DOI] [PubMed] [Google Scholar]
- 40. O'Reilly EM, Ferrone C. Neoadjuvant or adjuvant therapy for resectable or borderline resectable pancreatic cancer: which is preferred? J Clin Oncol. 2020;38(16):1757‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valsangkar NPBD, Michaelson JS, Ferrone CR, Wargo JA, Lillemoe KD. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17(12):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huebner MKM, Reid‐Lombardo KM, Que F, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16(15):920‐926. [DOI] [PubMed] [Google Scholar]
- 43. Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large. Population‐Based Anal. 2007;15(1):165. [DOI] [PubMed] [Google Scholar]
- 44. Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610‐618. [DOI] [PubMed] [Google Scholar]
- 45. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil‐based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019‐1026. [DOI] [PubMed] [Google Scholar]
- 46. Jones RP, Psarelli E‐E, Jackson R, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC‐4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019;154(11):1038‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open‐label, randomized trial of adjuvant nab‐paclitaxel plus gemcitabine (nab‐P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15_suppl):4000. [Google Scholar]
- 48. Rochefort MM, Ankeny JS, Kadera BE, et al. Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Ann Surg Oncol. 2013;20(13):4322‐4329. [DOI] [PubMed] [Google Scholar]
- 49. Wasif N, Ko CY, Farrell J, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17(9):2312‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240(2):293‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population‐based study in the United States. Pancreas. 2010;39(4):458‐462. [DOI] [PubMed] [Google Scholar]
- 52. Watanabe I, Sasaki S, Konishi M, et al. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas. 2004;28(2):160‐165. [DOI] [PubMed] [Google Scholar]
- 53. Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JDI, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB. 2008;10(5):371‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tomasello G, Ghidini M, Costanzo A, et al. Outcome of head compared to body and tail pancreatic cancer: a systematic review and meta‐analysis of 93 studies. J Gastrointest Oncol. 2019;10(2):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dreyer SB, Jamieson NB, Upstill‐Goddard R, et al. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg. 2018;105(2):e183‐e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695‐707. [DOI] [PubMed] [Google Scholar]
- 57. Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol. 2016;113(3):283‐288. [DOI] [PubMed] [Google Scholar]
- 58. Ghaneh P, Kleeff J, Halloran CM, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg. 2019;269(3):520‐529. [DOI] [PubMed] [Google Scholar]
- 59. Tummers WS, Groen JV, Sibinga Mulder BG, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg. 2019;106(8):1055‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kato K, Yamada S, Sugimoto H, et al. Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: influence of resection margin status on survival. Pancreas. 2009;38(6):605‐612. [DOI] [PubMed] [Google Scholar]
- 62. Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreatico‐duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251(6):1003‐1010. [DOI] [PubMed] [Google Scholar]
- 63. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized Phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763‐1773. 10.1200/jco.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S‐1 versus upfront surgery for resectable pancreatic cancer (Prep‐02/JSAP‐05). J Clin Oncol. 2019;37(4_suppl):189‐189. 10.1200/jco.2019.37.4_suppl.189. [DOI] [PubMed] [Google Scholar]
- 65. Sohal D, Duong MT, Ahmad SA, et al. SWOG S1505: Results of perioperative chemotherapy (peri‐op CTx) with mfolfirinox versus gemcitabine/nab‐paclitaxel (Gem/nabP) for resectable pancreatic ductal adenocarcinoma (PDA). J Clin Oncol. 2020;38(15_suppl):4504‐4504. 10.1200/jco.2020.38.15_suppl.4504. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recurrence pattern.
Data Availability Statement
The unidentified dataset is available upon reasonable requests made to the corresponding author.


