Abstract
Mutations in the thymidine kinase (TK) gene of herpes simplex virus (HSV) have been associated with resistance to acyclovir (ACY) and possible recognition of neurotropic strains. We sequenced a 335-bp segment of the TK gene to determine the frequency of mutations in HSV strains recovered from dermal, genital, and cerebrospinal fluid (CSF) specimens (n = 200; 102 HSV type 1 [HSV-1] 98 HSV-2 strains). Four polymorphic sites were detected in HSV-1 strains; C513T, A528G, C575T, and C672T. Among the polymorphisms, only C575T resulted in a change of amino acid sequence (residue 192, Ala→Val). For HSV-2 strains, only one polymorphism (G420T) which resulted in an amino acid substitution (residue 139, Leu→Phe) was detected. Phenotypic determination of resistance to ACY by a plaque reduction assay of 48 HSV isolates was not correlated with the sequence results of 11 strains in that 7 of these with genotypic polymorphisms were susceptible to the drug in vitro. In addition, of 32 ACY-resistant HSV strains, 28 (87.5%) had no polymorphisms detected in the 335-bp amplicon of the TK gene. There was no statistical difference in the frequency of polymorphisms according to the source of the specimens. We conclude that the detection of nucleic acid polymorphisms in a previously implicated 335-bp segment of the TK gene cannot be interpreted as indicative of either ACY resistance or neurotropism of HSV strains from dermal, genital, and CSF sources.
Herpes simplex virus (HSV) is the most frequently detected virus in the clinical virology laboratory; the virus causes a wide spectrum of disease, including primary and recurrent infections of mucous membranes, neonatal infections, visceral infections of the immunocompromised host, and encephalitis in all age groups (17). The clinical spectrum of the central nervous system (CNS) manifestations of this infection is expanding with laboratory diagnosis by PCR (4, 9, 16). Acyclovir (ACY) is considered the drug of choice for treatment of these infections (3). Several reports have documented the essential role of activation of ACY by strains of HSV that are genotypically competent for thymidine kinase (TK) activity (3, 5, 6, 7, 18). Conversely, the function of this enzyme has been compromised by mutations in the gene resulting in a deficiency in TK production or in altered enzyme specificity in which the enzyme is capable of phosphorylating thymidine, the usual substrate, but does not phosphorylate ACY. Recently, Gaudreau et al., in a genotypic analysis of the TK gene of 30 phenotypically ACY-resistant strains of HSV, reported that 14 (46.7%) isolates had an insertion or deletion of 1 or 2 nucleotides (12). In addition, 16 (53.3%) of the 30 ACY-resistant isolates had point mutations in regions of the TK gene.
Amplification of HSV DNA from cerebrospinal fluid (CSF) has provided an abundant source of the viral genome for genetic analysis; previously, only strains that were rarely recovered from CSF or brain biopsy specimens were available for such comparisons with isolates from different anatomic sources. Routine molecular testing of CSF specimens from patients with CNS disease has provided our laboratory with source specimens containing PCR-amplified HSV DNA (16). First, our goal was to determine if distinct nucleotide polymorphisms (genotypes) existed in a 335-bp segment of the TK gene in HSV strains from dermal, genital, and CSF (PCR product) sources. Second, we wanted to correlate nucleotide polymorphisms (genotypes) in HSV PCR products with antiviral resistance of viral strains to ACY in vitro (phenotypes).
MATERIALS AND METHODS
Samples.
HSV DNA was extracted from virus recovered in MRC-5 cell cultures from dermal (n = 41) and genital (n = 43) sources. CSF (68) specimens, known to contain HSV DNA and stored at −20°C, were reamplified in preparation for sequence analysis (16, 18). In addition, 48 strains of HSV (HSV-1 strains, n = 10; HSV-2 strains, n = 38) characterized as susceptible (n = 16) or resistant (n = 32) to ACY by plaque reduction assay were obtained from ViroMed Laboratories, Inc., Minneapolis, Minn. These strains were subsequently sequenced for analysis of the presence of nucleotide polymorphisms at the Mayo Clinic.
Serotype determination of HSV strains.
HSV strains recovered in cell cultures were serotyped with monoclonal antibodies in an immunofluorescence assay in shell vials (Syva Corp., Palo Alto, Calif.) (13).
Antiviral susceptibility assay.
Plaque reduction assay was used to determine phenotypic resistance to ACY measured by 50% inhibitory concentration of the drug. Resistance to ACY was defined as an IC50 of >2 μg/ml. Briefly, HSV was isolated from swabs of vesicular and ulcerative lesions in tube cultures of rabbit kidney cells (ViroMed Laboratories). Once cultures exhibiting typical virus-induced cytopathic effect reached a cytopathic effect of 4+ (76 to 100% of cells affected), samples of the infected cells were processed as described above to determine virus serotype (HSV-1 or HSV-2) and the remainder of the culture was aliquoted and frozen at less than −70°C.
A plaque reduction assay was performed with Vero cells (ViroMed Laboratories) in a six-well plate format. Each drug concentration was tested in duplicate. Control viruses included wild-type, ACY-susceptible HSV-2 (MS-2), and an ACY-resistant TK-negative clinical isolate of HSV-2 (15486) obtained from Glaxo Wellcome (Research Triangle Park, N.C.). For each virus tested, 100 μl of undiluted virus (70 to 100 PFU) from a frozen aliquot was placed in all wells of two culture plates, gently mixed, and allowed to adsorb to the cells for 1 h in a humidified CO2 incubator at 36 to 38°C. After the 1-h adsorption period, 3.0 ml of culture medium (Eagle’s minimal essential medium [E-MEM] supplemented with 5% heat-inactivated fetal bovine serum [FBS], penicillin, gentamicin, amphotericin B, HEPES, and NaHCO3; ViroMed Laboratories) containing 0.8% human immune serum globulin (Michigan Department of Public Health, Lansing) and the appropriate concentration of ACY was added to each well, and the plates were returned to the incubator for 3 days. After 3 days, the cells were fixed with formalin-crystal violet (1 part formalin to 9 parts crystal violet), washed with deionized water, and dried. Plaques were counted in each well, and the calculation of dose-response curve for the virus controls and clinical isolates was performed with a Microsoft Excel spreadsheet module.
DNA extraction.
HSV DNA was extracted with an IsoQuick nucleic acid extraction kit (Orca Research, Inc., Bothell, Wash.). A total volume of 200 μl of HSV-infected cell cultures (dermal or genital specimens) or CSF was used for extraction. Twenty micrograms of glycogen or Pellet Paint coprecipitant (Novagen, Madison, Wis.) was added to each extracted sample as a carrier during the isopropanol precipitation step in the IsoQuick technique. Extracted nucleic acids were suspended in Tris-EDTA buffer or RNase-free water. Preparation of the master mix, specimen extraction and addition, and analysis steps were performed in separate air-controlled rooms.
PCR.
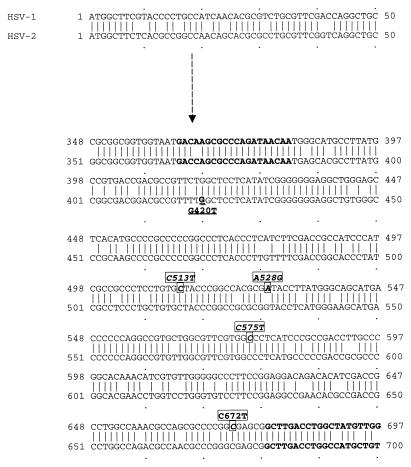
The PCR mixture contained the following: 200 μM (each) deoxyribonucleoside triphosphate, 10× buffer (500 mM KCl, 100 mM Tris-Cl [pH 8.3], 15 mM MgCl2, 2.5 mg of bovine serum albumin per ml), 50 pmol each of primer (see Table 4), and 1.25 U of Taq polymerase. Each reaction tube received 45 μl of the reaction mixture, 2 drops of mineral oil, and 5 μl of target DNA. Reaction mixtures for dermal or genital specimens were placed in a thermal cycler (model 480; Perkin-Elmer Cetus) programmed for a three-step protocol: 2 min at 94°C for one cycle and then 1 min of denaturation at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C for 40 cycles. The primers were selected to amplify a 335-bp portion of the TK locus which contains the nucleoside-binding site (nucleotides 502 to 528) but not the ATP-binding site (nucleotides 151 to 189) (Fig. 1). The 335-bp amplicon was detected by gel electrophoresis (3%; 1.5% NuSieve–1.5% SeaKem [FMC, Rockland, Maine]).
TABLE 4.
Polymorphisms among 48 strains tested for ACY susceptibility by plaque reduction assay
| Type | No. of strains with ACY phenotype
|
|
|---|---|---|
| Susceptible | Resistant | |
| HSV-1 | ||
| Total | 6 | 4 |
| Wild | 2 | 1 |
| With polymorphisms | ||
| C513T | 2 | 1 |
| A528G, C575T, C672T | 2 | 2 |
| HSV-2 | ||
| Total | 10 | 28 |
| Wild | 7 | 27 |
| With G420T polymorphism | 3 | 1 |
FIG. 1.
Nucleotide sequence alignment of a 335-bp segment of the TK gene of HSV-1 (KOS) with that of HSV-2 (333). Upstream and downstream primers for amplification of a 335-bp amplicon and individual polymorphisms are designated in boldface type. Polymorphisms associated with HSV-1 are indicated by boxes, and the single HSV-2 polymorphism is indicated by underlining.
Sequencing for the TK locus.
Primers for forward and reverse sequencing of the 335-bp TK locus were the same as those used for PCR. Taq FS polymerase and d-rhodamine terminator (Perkin-Elmer Applied Systems) were used for sequencing PCR products. Data were compared with the known 335-bp sequence of the TK gene from HSV-1 (KOS) mutant KG 111 (GenBank accession no. J04327) and HSV-2 (333) (GenBank accession no. X01712). Nucleotide sequences and polymorphism numerical positions were aligned with the ATG (methionine) codon as published by Gaudreau et al. (12). Target DNA amplified by PCR was purified by using the QIAquick purification kits (Qiagen Inc., Chatsworth, Calif.) prior to sequencing. A reaction mixture including approximately 200 ng of purified DNA fragment and 3.2 pmol of primer was used for cycle sequencing both DNA strands of each HSV strain using an automated instrument (model 377; Applied Biosystems). Multiple sequence alignments were carried out with the software program Wisconsin Package version 9.1 (Genetics Computer Group, Madison, Wis.).
RESULTS
Nucleotide sequencing of the 335-bp amplicon within the TK gene of HSV-1 (KOS) mutant KG111 and HSV-2 (333) revealed 51 positions that accurately identified the two type strains of the virus. For dermal (n = 41; 30 HSV-1 and 11 HSV-2 strains) and genital (n = 43; 30 HSV-1 and 13 HSV-2 strains) sources in which HSV was initially recovered, the genotype results were identical to those determined by monoclonal antibodies specific for the HSV-1 or -2 serotype. Genotyping of the HSV strains from CSF yielded 32 HSV-1 and 36 HSV-2 strains.
Overall, four polymorphic sites were detected among HSV-1 strains: C513T, A528G, C575T, and C672T (Table 1; Fig. 1). Among the polymorphisms, only C575T (GCC→GTC) resulted in a change of amino acid sequence (residue 192, Ala→Val). This mutation occurred in 16 of 46 (17.4%) strains with polymorphisms; this nucleotide change was always found in combination with A528G and C672T. Combinations of the four polymorphisms were detected in 15 of 30 (50%) dermal, 11 of 30 (36.7%) genital, and 20 of 32 (62.5%) CSF specimens. Sixteen of 46 (34.8%) HSV-1 strains had three nucleotide polymorphisms; 26 (56.5%) of these had only a single nucleotide change (C513T). For HSV-2 strains, only one mutation (G420T; TTG→TTT) which resulted in a change of amino acid sequence (residue 139, Leu→Phe) was detected (Tables 2 and 3). Polymorphism rates were 2 of 11 (18.2%), 3 of 13 (23.1%), and 7 of 36 (19.4%) for dermal, genital, and CSF specimens, respectively. There were no statistical differences in HSV-1 and -2 strains with specific polymorphisms that were unique for dermal, genital, or CSF sources (Fisher’s exact test).
TABLE 1.
Polymorphic sites detected in HSV-1 strains and their frequencies according to the source of specimens
| Strain type | Polymorphic sites
|
No. of specimens
|
Total no. (%) of specimens | |||||
|---|---|---|---|---|---|---|---|---|
| C513T | A528G | C575Ta | C672T | Dermal | Genital | CSF | ||
| Wild type | C | A | C | C | 15 | 19 | 12 | 46 (50.0) |
| Strains with polymorphisms | T | 9 | 5 | 12 | 26 (28.3) | |||
| G | T | T | 5 | 5 | 6 | 16 (17.4) | ||
| G | 0 | 1 | 1 | 2 (2.2) | ||||
| T | T | 1 | 0 | 0 | 1 (1.1) | |||
| G | T | 0 | 0 | 1 | 1 (1.1) | |||
| Total | 30 | 30 | 32 | 92 (100) | ||||
Mutation of C575T resulted in a change of amino acid sequence (Ala→Val), and others were silent polymorphisms.
TABLE 2.
Polymorphic sites detected in HSV-2 strains and their frequencies according to the source of specimens
| Strain type | Polymorphic site (G420Ta) | No. of specimens
|
Total no. (%) of specimens | ||
|---|---|---|---|---|---|
| Dermal | Genital | CSF | |||
| Wild type | G | 9 | 10 | 29 | 48 (80.0) |
| Strains with polymorphisms | T | 2 | 3 | 7 | 12 (20.0) |
| Total | 11 | 13 | 36 | 60 (100) | |
Mutation of G420T resulted in a change of amino acid sequence (Leu→Phe).
TABLE 3.
Summary of polymorphisms identified in the TK gene corresponding to amino acid sequence between residues 122 and 232
| Virus type | Nucleotide substitution | Amino acid changea | Reference |
|---|---|---|---|
| HSV-1 | C513T (TGC→TGT) | −(residue 171) | This study |
| A528G (CGA→CGG) | −(residue 176) | 15 | |
| C575T (GCC→GTC) | Ala→Val (residue 192) | This study | |
| C672T (GGC→GGT) | −(residue 224) | 18 | |
| HSV-2 | G420T (TTG→TTT) | Leu→Phe (residue 139) | This study |
−, deletion.
DNA was extracted and the TK target of HSV was amplified from an additional 48 strains (HSV-1, n = 10; HSV-2, n = 38) characterized as susceptible (n = 16) or resistant (n = 32) to ACY by plaque reduction assay (Table 4). Of 11 (22%) strains with polymorphisms, 4 (36.3%) had nucleotide changes at three sites (A528G, C575T, and C672T) and 7 (63.6%) had only a single C513T (HSV-1) or G420T (HSV-2) locus change. Of the four strains resistant to ACY, two had 3 polymorphic nucleotide base changes. Overall, of the 11 strains with polymorphisms, 7 were phenotypically susceptible (5 with one polymorphism) to ACY.
DISCUSSION
We initially developed and implemented a PCR test for the detection of a conserved target (UL30) of the polymerase gene of HSV in CSF specimens. Tests based on this target have become the accepted standard of practice for laboratory diagnosis of this viral infection involving the CNS (1, 4, 16, 19). Technically, this locus satisfied several criteria as a target for an ideal PCR-based test. Most importantly, HSV, especially serotype 1, has been only rarely recovered from CSF specimens; however, our PCR detected as few as 50 copies of HSV DNA in CSF, thereby resulting in a rapid laboratory diagnosis of CNS infections due to this virus (16). Nevertheless, enzyme restriction sites to differentiate HSV-1 from HSV-2 and informative polymorphisms for possible markers of ACY resistance or neurotropism have not been described in the UL30 gene. Therefore, for these reasons, we felt that target DNA within the TK gene would be more informative than the DNA polymerase target.
Overall, of the 200 strains for which we determined the nucleotide sequences, 69 (34.2%) had polymorphisms detected in the 335-bp region of the TK gene. More HSV strains from the Mayo Clinic (58 of 152; 38.2%) had polymorphisms than those from ViroMed (11 of 48; 23%) (P = 0.053). This may reflect differences in specimen referral patterns. To our knowledge, detection of polymorphic sites C515T and C578T have not been reported even though they were present in 50 of 53 (94.3%) of the HSV-1 strains with nucleotide substitutions. Similarly, G420T has not been reported for HSV-2 (Table 3).
Our results can be directly compared to recent data reported by Gaudreau et al. Type 1 (KOS) and type 2 (333) strains of the virus were used as the genotype templates for sequence analysis of our data and their results (12). Further verification and number designations of nucleotide positions beginning with the start codon (ATG) for methionine were ascertained by the locations of six and four homopolymer runs in HSV-1 and -2, respectively. Gaudreau et al. found distinct mutations in the coding region of the TK gene in all 30 phenotypically ACY-resistant strains that they tested (12). For example, 13 of 30 HSV ACY-resistant strains in their study had insertions or deletions in four homopolymer runs of HSV-1 and -2 in the 335-bp portion of the TK gene that we sequenced; however, none of these nucleotide polymorphisms or mutations were identified in the 200 strains that we analyzed. We detected four polymorphisms that were present singly, or in combination, in 69 (34.5%) of the 335-bp amplicons that we sequenced. More importantly, the occurrence of these polymorphisms was not statistically related to phenotypically resistant HSV strains. Yet, of 32 ACY-resistant phenotypes of HSV, 28 (87.5%) (HSV-1, n = 1; HSV-2, n = 27) had no polymorphisms (genotypic wild type).
Our initial goal in this study was to determine whether any unique polymorphisms were present in the 335-bp PCR product within the TK gene among HSV strains that infect the CNS compared to those that produce genital or other dermal lesions. Thus, we selected random CSF, dermal, or genital specimens in which HSV was detected. Certainly, a few strains of the virus could have been resistant, but they were not selected by any biological criterion. Conversely, HSV strains from ViroMed were selected with a bias for a tendency to be resistant to ACY since the initial specimens were submitted to that laboratory for antiviral susceptibility testing. Nevertheless, our genotyping results yielded identical polymorphisms with 200 HSV strains from the Mayo Clinic and ViroMed.
Perhaps the striking differences between the results obtained in our report and those of Gaudreau et al. were due to the differences in study design and the selection of HSV strains for analysis. Importantly, three-fourths of the strains we analyzed (152 of 200, 76%) were unselected with regards to ACY phenotype; in contrast, 30 of 35 (85.7%) strains in the Gaudreau et al. study were included because they were phenotypically resistant to ACY.
In this manuscript, we have described the sequence analysis of a 335-bp segment of the TK gene of 200 HSV isolates (or PCR products; i.e., from CSF). Because of our unique findings that did not agree with those of Gaudreau et al. (12), we sequenced a subset of these strains (n = 10; 6 phenotypically susceptible and 4 resistant to ACY) by using primers directed to the upstream (nucleotides 1 to 335) and downstream (nucleotides 701 to 1121) portions of the TK gene. We obtained very similar results with these 10 strains (in both the upstream and downstream portions from the 335-bp product) as we did with the comprehensive 200-strain analysis. At least one polymorphism was identified in each of the 10 strains (6 susceptible and 4 resistant to ACY by phenotypic assay).
Sequence analysis readily differentiated HSV-1 from HSV-2 genotypes based on 51 strain-specific nucleotide positions within the 335-bp product of the amplified TK locus. Interestingly, amplified HSV DNA from 68 CSF specimens yielded 36 HSV-2 and 32 HSV-1 types by genotypic analysis. Previous studies have relied on HSV recovery from CSF or brain tissue to yield strains in cell culture that could be serotyped. These reports likely select for patients with severe forms of CNS disease due to HSV in which the level of virus is high and invasive biopsy specimens are obtained. Alternatively, by testing CSF specimens by PCR, HSV-2, rather than HSV-1, may be involved in relatively mild forms of CNS disease (such as Mollaret’s meningitis) associated with this genotype.
ACY is the drug of choice for the treatment of HSV infection (3). The virus-specific mechanism of action of ACY involves the virus-encoded enzymes, DNA polymerase and TK. HSV resistance due to the effect of ACY on the gene coding for DNA polymerase has been associated clinically with point mutations in strains that resulted in alteration of a single amino acid. Conversely, HSV strains have been generated experimentally with mutation sites that result in amino acid alterations which did not affect ACY resistance (18).
Alternatively, most ACY-resistant HSV strains have been associated with the failure to phosphorylate the drug, resulting in mutant strains that express little or no functional TK activity (11, 13). Compromised TK activity may be a consequence of a chain-terminating mutation or TK-altered mutants that phosphorylate thymidine, the usual substrate for the enzyme, but do not phosphorylate ACY (2, 7). Phenotypically, these ACY-resistant strains express reduced or no TK activity; however, these enzymatic deficiencies may not be recognized readily by point mutations in selected genomic loci. Rather, ACY resistance may involve TK mutants with substrate specificities that involve a nucleotide binding pocket, an ATP-binding site, and a thymidine-binding site, as suggested by Darby et al. (8). Our data are consistent with a more-complex, rather than simpler, explanation of TK resistance, in that single or even multiple polymorphisms in 11 HSV strains (i.e., 7 strains with polymorphisms were phenotypically susceptible to ACY) did not correlate with plaque reduction assays that demonstrate resistance to ACY. Obviously, functional relationships among other HSV genes with TK activity may influence expression of the enzyme phenotypically. ACY-resistant strains may not be consistently associated with and recognized by specific locus mutations within open reading frames that span a 1,128-bp stretch of the gene (14, 15).
Collectively, our results and the report by Gaudreau et al. (12) have indicated that several polymorphisms or mutations can be recognized by nucleotide sequence analysis of the TK gene. However, we have demonstrated the occurrence of polymorphisms in 58 (38.2%) of 152 unselected HSV strains and, more importantly, the lack of association of any recognized polymorphisms with phenotypic ACY resistance among another 48 strains. Our data suggests that studies comparing phenotypic and genotypic assays for characteristics such as antiviral resistance need to be expanded to recognize unique nucleotide changes beyond those that naturally occur as background polymorphisms in HSV strains.
REFERENCES
- 1.Aslanzadeh J, Osmon D R, Wilhelm M P, Espy M J, Smith T F. A prospective study of the polymerase chain reaction for detection of herpes simplex virus in cerebrospinal fluid submitted to the clinical virology laboratory. Mol Cell Probes. 1992;6:367–373. doi: 10.1016/0890-8508(92)90029-w. [DOI] [PubMed] [Google Scholar]
- 2.Balfour H H., Jr Resistance of herpes simplex to acyclovir. Ann Intern Med. 1983;98:404–406. doi: 10.7326/0003-4819-98-3-404. [DOI] [PubMed] [Google Scholar]
- 3.Balfour H H., Jr Acyclovir and other chemotherapy for herpes group viral infection. Annu Rev Med. 1984;35:279–291. doi: 10.1146/annurev.me.35.020184.001431. [DOI] [PubMed] [Google Scholar]
- 4.Cinque P, Vago L, Marenzi R, Giudici B, Weber T, Corradini R, Ceresa D, Lazzarin A, Linde A. Herpes simplex virus infections of the central nervous system in human immunodeficiency virus-infected patients: clinical management by polymerase chain reaction assay of cerebrospinal fluid. Clin Infect Dis. 1998;27:303–309. doi: 10.1086/514657. [DOI] [PubMed] [Google Scholar]
- 5.Coen D M. Acyclovir-resistant, pathogenic herpes viruses. Trends Microbiol. 1994;2:481–484. doi: 10.1016/0966-842x(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 6.Collins P, Ellis M N. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J Med Virol. 1993;1(Suppl.):58–66. doi: 10.1002/jmv.1890410512. [DOI] [PubMed] [Google Scholar]
- 7.Crumpacker C S, Schnipper L E, Chartrand P, Knopf K W. Genetic mechanisms of resistance to acyclovir in herpes simplex virus. Am J Med. 1982;73:361–368. doi: 10.1016/0002-9343(82)90123-1. [DOI] [PubMed] [Google Scholar]
- 8.Darby G, Larder B A, Inglis M M. Evidence that the ‘active center’ of the herpes simplex thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986;67:753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- 9.Dominque R B, Lakeman F D, Pannuti C S, Fink M C D, Tsanaclis A M C. Advantage of polymerase chain reaction in the diagnosis of herpes simplex encephalitis: presentation of 5 atypical cases. Scand J Infect Dis. 1997;29:229–231. doi: 10.3109/00365549709019033. [DOI] [PubMed] [Google Scholar]
- 10.Englund J A, Zimmerman M E, Swierkosz E M, Goodman J L, Scholl D R, Balfour H H., Jr Herpes simplex virus resistant to acyclovir. Ann Intern Med. 1990;112:416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- 11.Field A K, Biron K K. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin Microbiol Rev. 1994;7:1–13. doi: 10.1128/cmr.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudreau A, Hill E, Balfour H H, Jr, Erice A, Boivin G. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J Infect Dis. 1998;178:297–303. doi: 10.1086/515626. [DOI] [PubMed] [Google Scholar]
- 13.Gleaves C A, Wilson D J, Wold A D, Smith T F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985;21:29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kit S, Kit M, Qavi H, Trkula D, Otsuka H. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim Biophys Acta. 1983;741:158–170. doi: 10.1016/0167-4781(83)90056-8. [DOI] [PubMed] [Google Scholar]
- 15.McKnight S L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell P S, Espy M J, Smith T F, Toal D R, Rys P N, Berbari E F, Osmon D R, Persing D H. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997;35:2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlesinger Y, Tebas P, Graudreault-Keener M, Buller R S, Storch G A. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin Infect Dis. 1995;20:842–848. doi: 10.1093/clinids/20.4.842. [DOI] [PubMed] [Google Scholar]
- 18.Suzutani T, Koyano S, Takada M, Yoshida I, Azuma M. Analysis of the relationship between cellular thymidine kinase activity and virulence of thymidine kinase-negative herpes simplex virus types 1 and 2. Microbiol Immunol. 1995;39:787–794. doi: 10.1111/j.1348-0421.1995.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 19.Whitley R J, Roizman B. Herpes simplex virus. In: Richman D D, Whitley R J, Hayden F C, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 375–410. [Google Scholar]