Abstract
Introduction
The 2018 updated molecular testing guidelines for patients with advanced lung cancer incorporated ALK immunohistochemistry (IHC) analysis as an equivalent to fluorescence in situ hybridization (FISH) method recommended in 2013. Nevertheless, no specific recommendation for alternative methods was proposed owing to insufficient data. The aim of this study was to compare the results of ALK IHC, FISH, RNA next-generation sequencing (NGS), and RNA in situ hybridization (ISH) with available clinical data.
Methods
A search for lung carcinomas with ALK testing by greater than or equal to one modality (i.e., ALK IHC, FISH, NGS) was performed; a subset underwent RNA ISH. When available, clinical data were recorded.
Results
The results were concordant among all performed testing modalities in 86 of 90 cases (95.6%). Of the four discordant cases, two were ALK positive by FISH but negative by IHC, RNA NGS, and RNA ISH. The remaining two cases failed RNA NGS testing, one was IHC negative, FISH positive, RNA ISH negative and the second was IHC positive, FISH positive, RNA ISH equivocal. RNA NGS identified one rare and one novel ALK fusion. Sufficient therapy data were available in 10 cases treated with tyrosine kinase inhibitors; three had disease progression, including one with discordant results (FISH positive, RNA NGS negative, IHC negative, RNA ISH negative) and two with concordant ALK positivity among all modalities.
Conclusions
Our results reveal high concordance among IHC, RNA NGS, and RNA ISH. In cases of discordance with available RNA NGS, FISH result was positive whereas IHC and ISH results were negative. On the basis of our data, multimodality testing is recommended to identify discrepant results and patients (un)likely to respond to tyrosine kinase inhibitors.
Keywords: ALK, Fluorescent in situ hybridization (FISH), Next-generation sequencing (NGS), RNA in situ hybridization (RNA ISH), Archer, Anchored multiplex
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. In the past, systemic treatment for advanced-stage disease tended to be quite circumscribed—patients with small cell carcinoma received one regimen and those with NSCLC another. After the discovery of EGFR driver mutations in NSCLC and results of the 2009 Iressa Pan-Asia Study trial,1 which correlated a specific genotype to greatest response with a tyrosine kinase inhibitor (TKI), the management of NSCLC transformed dramatically.
From this landmark study emerged a change in paradigm. The current standard of care includes companion diagnostic biomarker testing to determine eligibility for targeted therapy. In 2007, EML4–ALK fusion in the short (p) arm of chromosome two was first described in NSCLC by Soda et al.,2 and in 2011, crizotinib was approved after response in ALK-positive patients.3 ALK rearrangements are present in approximately 3% to 8%4 of lung adenocarcinomas and tend to occur more frequently in younger patients who are never or light smokers. Nevertheless, similar clinical characteristics may be found in patients with other genetic alterations, such as in EGFR, ERBB2, and RET. Although signet ring cytomorphology, mucinous cribriform pattern, and psammoma bodies5 have been described in ALK-rearranged adenocarcinomas, these patterns are not uniformly present. Consequently, no treatment is based solely on (cyto)morphological pattern or clinical characteristics.
In 2013, the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology (CAP/IASLC/AMP)6 set forth molecular testing guidelines to select patients eligible for TKI therapy on the basis of the presence of EGFR mutations or ALK fusions. For ALK, testing with fluorescence in situ hybridization (FISH) using dual-color break-apart probe was recommended, and appropriately validated immunohistochemistry (IHC) could serve as a screening modality. In 2018, the guidelines were updated, and strong granular cytoplasmic ALK IHC was deemed an equivalent alternative to FISH on the basis of multiple studies.7 Meanwhile, there were insufficient data to support nucleic acid-based assays for detection of ALK fusions, though two DNA next-generation sequencing (NGS) assays (Foundation One [Foundation Medicine, Cambridge, MA] and Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets [New York, NY]) that provide comprehensive diagnostic testing were approved by the Food and Drug Administration (FDA) in 2017.
The aim of this study was to compare the ALK detection methodologies currently recommended by CAP/IASLC/AMP with anchored multiplexed (AMP) RNA-based RNA NGS and RNA in situ hybridization (ISH), which have not previously been evaluated in parallel. These findings were correlated with therapy outcomes to evaluate for response in specific ALK variants.
Materials and Methods
Case Selection: ALK Positive and Negative
After institutional review board approval (#AAAD7936) and waiver of consent as part of a retrospective review, a search for lung carcinomas that had previously undergone testing for ALK by greater than or equal to one testing modality was performed. The search comprised primary and metastatic lung carcinomas diagnosed on cytology, small biopsy, and surgical resection specimens.
After the search, the cases were divided into two study groups. Group 1 (n = 66) consisted of positive and negative ALK samples that had successfully undergone testing with all the following methods: ALK FISH, ALK IHC, targeted RNA NGS, and DNA NGS. Group 2 (n = 7) consisted of only resection samples before the availability of RNA NGS in the laboratory but had tested positive for ALK rearrangement by FISH. Group 2 was selected to include additional ALK-rearranged cases and to evaluate success or failure of RNA ISH and RNA NGS on archived specimens. For group 2, previous DNA multiple single-gene analyses (SGAs) and IHC were recorded when available and targeted RNA NGS was subsequently performed on all samples in addition to IHC, if previously unavailable. Additional negative controls (n = 17) defined as lacking ALK by RNA NGS and/or having a non-ALK driver mutation by DNA NGS were identified.
Case Selection: RNA ISH
RNA ISH testing was performed on a total of 51 of 90 cases. More specifically, RNA ISH testing was performed on all group 1 and group 2 cases, which had tested positive for ALK rearrangement by more than one testing modality, nine randomly selected group 1 negative cases, and 17 additional negative controls.
Case Selection: Anchored Multiplexed RNA NGS
In keeping with standard practice at our institution, targeted RNA NGS was conducted only on cases that lacked an identifiable driver mutation in the DNA NGS panel in group 1 and all cases from group 2.
Laboratory Protocols
Per laboratory protocol, all primary and metastatic lung adenocarcinomas, or when this diagnosis could not be excluded (e.g., large cell carcinoma, NSCLC not otherwise specified, squamous cell carcinoma in a small biopsy/cytology of a never or light smoker), were reflexively submitted for testing to identify driver mutations. The tests and platforms varied with guidelines and the contemporaneous methods available in the laboratory. At the outset, ALK FISH was the gold standard and IHC was variably used for screening; subsequently, all specimens were subject to ALK IHC and FISH. Initially, multiple single-gene DNA analyses were performed; subsequently, NGS was adopted (with SGA reserved only for instances of insufficient DNA) to identify driver mutations. More recently, RNA NGS was adopted and performed (1) reflexively only on cases in which DNA NGS failed to identify a driver mutation (i.e., KRAS, EGFR, BRAF, MET, ERBB2) and (2) in addition to and independent of results of ALK IHC and ALK FISH.
Laboratory Protocol: Detection of ALK Rearrangement by FISH and ALK Protein Expression by IHC
FISH analysis for ALK rearrangement was performed using the FDA-approved Vysis ALK break-apart probe kit (Abbott Molecular, Des Plaines, IL) and interpreted in accordance with the manufacturer’s instructions. IHC was performed using ALK (D5F3) rabbit monoclonal primary antibody (Ventana, Tucson, AZ) on BenchMark ULTRA system with OptiView detection kit and interpreted using manufacturer’s criteria; the stain was interpreted independently before performing and availability of FISH and RNA results.
Laboratory Protocol: Detection of ALK Fusion by RNA NGS
After internal laboratory validation as described previously,8 RNA NGS was adopted to identify specific fusions and mutations (e.g., ALK, MET, RET, ROS1, NTRKs) in lung carcinomas; this was performed in addition to IHC and FISH. From formalin-fixed, paraffin-embedded (FFPE) samples, the slides were cut and evaluated for tumor content, and when below approximately 20%, both macrodissection and manual microdissection (using a needle under a microscope) were used. RNA was isolated using the ALLPrep DNA/RNA kit (Qiagen) and quantified using the Qubit fluorometer. A custom NGS panel using AMP technology (Archer Dx, Boulder, CO), which targets 17 genes, was performed as previously described.9 Results were interpreted after minimum quality control measures set forth by manufacturer’s guidelines. A positive result “fusion” required (1) a minimum of five unique reads (2) with at least 10% of reads greater than the wild-type transcript and (3) at least three unique start sites. Results of RNA NGS were considered gold standard on the basis of its reported high sensitivity10, 11, 12 and 100% specificity compared with other methods.12
Laboratory Protocol: Detection of ALK Rearrangement by RNA ISH
RNA ISH testing was performed using RNAScope 2.5 LS Probe-Hs-ALK (Advanced Cell Diagnostics, Hayward, CA) on the Leica Bond III automated staining platform with RNA Scope DAB kit. The probes target the region of exons 19 to 29 (kinase domain and adjacent sequences). Recommended RNA scope strategies, which detect exuberant expression (rather than abnormal ALK protein), were used. Increase in the number of ALK signals, which preferentially occur in ALK-rearranged cells, was evaluated. A positive result by RNA ISH was defined as two signals per cell in at least 5% of cancer cells and at least one cancer cell with greater than or equal to four signals. A positive RNA control (ubiquitin) was performed on a serial section from each specimen. If the positive control slide was positive (two–three signals per cell), then a negative result on the target slide was interpreted as a true negative. If the positive control slide was negative or had minimal staining, ALK results were interpreted as equivocal.
Laboratory Protocol: DNA SGA and NGS
Similar to RNA NGS testing, sections were cut at 5 μm, reviewed for tumor content, and macrodissected or microdissected when tumor content was below the required threshold. Testing was performed using Sanger sequencing for multiple SGA and TruSeq (Illumina, San Diego, CA) or a custom panel (Pillar Biosciences) for NGS, which contained mutational hotspots in several genes, including, EGFR, KRAS, BRAF, ERBB2, and MET, among others. A positive result required an average coverage depth of at least 500 reads and minimum 5% variant allele fraction.
Clinical Follow-Up
When available, clinical data, including response to therapy and progression-free survival (PFS), defined as a new metastatic lesion or unequivocal growth of existing lesion(s), were recorded.
Statistical Analysis and Concordance and Discordance
The sensitivity and specificity of each testing modality were analyzed using RNA NGS as the gold standard. Two cases of RNA NGS testing failure were not included in calculations. RNA ISH sensitivity and specificity calculation were based on 32 cases (discluded 17 negative control cases lacking corresponding RNA NGS testing). IHC and FISH sensitivity and specificity calculation were based on 71 cases (all cases with available results for IHC, FISH, and RNA NGS). Concordance and discordance across the various testing modalities were detailed.
Results
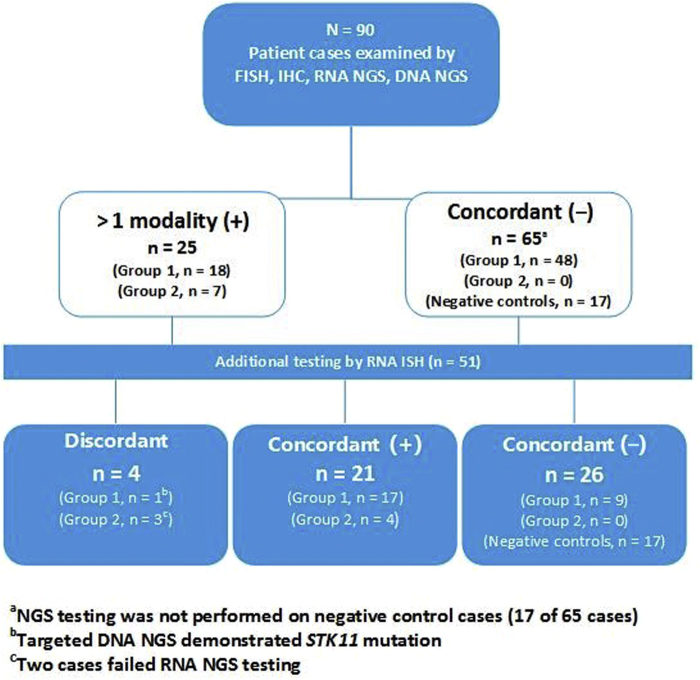
The study consisted of 90 lung adenocarcinomas from 46 women and 44 men (median age = 69 y; range: 29–90 y) who underwent testing for an ALK gene rearrangement with greater than one testing modality. Specimen types evaluated included resections (n = 60, 66.7%), small biopsies (n = 23, 25.6%), and cytology (pleural effusion [n = 1, 1.1%]; fine-needle aspiration [n = 6, 6.7%]). The patients were subdivided into group 1 (n = 66), group 2 (n = 7), and RNA ISH-negative controls (n = 17) on the basis of previously detailed criteria (Table 1). Overall, 25 patient cases were positive by at least one modality and 65 patient cases were negative by all modalities (Fig. 1A–E). Results of all studies are summarized in Figure 2.
Table 1.
Clinical Characteristics
| Group 1 | Group 2 | Negative Controls | Overall | |
|---|---|---|---|---|
| Total cases | 66 | 7 | 17 | 90 |
| Male, n (%) | 33 (50.0) | 3 (42.9) | 8 (47.1) | 44 (48.9) |
| Female, n (%) | 33 (50.0) | 4 (57.1) | 9 (52.9) | 46 (51.1) |
| Average age, y | 72 | 60 | 73 | 69 |
| Median age (age range) | 69.5 (29–90) | 58 (45–71) | 72 (62–87) | 69 (29–90) |
Figure 1.
Images of testing modalities in one ALK-positive concordant case. Image (A) reveals cribriform pattern frequently encountered in, though not diagnostic of, ALK-rearranged NSCLC on H&E-stained sample. Image (B) reveals strong homogenous staining with ALK IHC. Image (C) reveals positive RNA ISH study; positivity is defined by two signals per cell in at least 5% of cancer cells and at least one cancer cell with four or more signals. Image (D) reveals corresponding FISH study: fused (yellow arrowhead) signal represents unrearranged gene and red arrows represent rearranged ALK with loss of 5′ ALK (green signal). Image (E) reveals the RNA NGS (Archer) results: schematic of the EML4 (exon 13)–ALK (exon 20) fusion. The chromosomal breakpoint of the fusion is chr2:42522656, chr2:29446394. FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin; IHC, immunohistochemistry; ISH, in situ hybridization; NGS, next-generation sequencing.
Figure 2.
Summary of the results. A total of 90 cases (group 1, n = 66; group 2, n = 7; negative controls, n = 17) underwent testing for ALK gene rearrangement. RNA ISH was performed on 51 cases, including all cases positive by greater than one testing modality, 17 negative control cases, and nine randomly selected concordant negative cases from group 1. FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; ISH, in situ hybridization; NGS, next-generation sequencing.
Concordance and Discordance
Using RNA NGS as the gold standard, the sensitivity and specificity of ALK IHC, FISH, and RNA ISH are as follows: 100% and 100%, 100% and 96%, and 100% and 100%, respectively.
There was complete concordance in all but four cases. Two discordant cases had all testing modalities completed for comparison. In both cases, FISH results were positive for ALK rearrangement whereas IHC, RNA NGS, and RNA ISH were negative (Fig. 3A–D). The remaining two cases, designated as case A and case B and both from group 2, failed RNA NGS testing. The completed testing modalities in these cases had the following: case A: IHC negative, FISH positive, RNA ISH negative and case B: IHC positive, FISH positive, RNA ISH equivocal. Cases A and B were both resection specimens from 8 and 13 years before, respectively. Evaluation of corresponding hematoxylin and eosin slides revealed a relatively low tumor content (∼20% of slide content) and a marked lymphocyte infiltrate in both cases and background fibrosis in case B. Among the remaining four cases in group 2, testing was successfully performed on FFPE samples retrieved from up to 10 years before, though with comparatively higher tumor content (∼90% of slide content) and no considerable lymphoid component.
Figure 3.
H&E and FISH studies of discrepant cases. A high-powered H&E-stained image of a tumor present in one discordant case (Table 2; case 9) is illustrated in image (A). The corresponding FISH is illustrated in image (B): fused (yellow arrowhead) signal represents unrearranged gene and red arrows represent rearranged ALK with loss of 5′ ALK (green signal). FISH positivity was found in 88% of the diploid and tetraploid cells. A high-powered H&E image of a tumor present in the second discordant case is illustrated in image (C). The corresponding FISH is illustrated in image (D): fused (yellow arrowhead) signals represent unrearranged gene, and red and white arrows represent rearranged ALK. FISH positivity was found in 68% of the cells. FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin.
RNA NGS: Novel and Rare Fusion Partners
With targeted RNA NGS, one rare and one novel ALK fusion partner, TFG (exon 6) and SLMAP (exon 12 and exon 13 isoforms), respectively, were identified. The remaining 19 positive cases had ALK exon 20 with the most often described fusion, EML4 (variable) (Fig. 4). The two most frequent variants detected by RNA NGS were variant 1 (11 of 20; 55%) and variant 3a/b (5 of 20; 25%).
Figure 4.

ALK gene fusion variants observed. Variant 1 = EML4 (ex 13); variant 2 = EML4 (ex 20); variant 3a/b = EML4 (ex 6); TFG (ex 6); SLMAP (ex 12/13). All indicated variants represent fusions with ALK ex 20. For one positive case evaluated, ex data were unavailable. ex, exon.
Immunohistochemistry
IHC studies were reviewed for all positive cases and a subset of negative cases. All positive cases had some degree of moderate to strong staining intensity; all negative cases reviewed revealed no staining.
DNA SGA and NGS
Because driver mutations are mutually exclusive, targeted DNA SGA and NGS served as controls. In all concordant ALK-positive cases and the four discordant cases, no driver mutation was detected by targeted DNA NGS. In the one discordant case, STK11: c.1027G>A, p.D343N, a variant of uncertain siginificance (VUS) was detected. This mutation was reported 8 times in ClinVar as a VUS and is therefore not likely a driver of the tumor. Among the concordant negative cases, no mutation was detected by DNA NGS in 32 of 48 cases (66.7%), KRAS mutation was present in six cases (12.5%), MET mutation in three cases (6.3%), EGFR mutation in three cases (6.3%), STK11 mutation in three cases (6.3%), and BRAF mutation in one case (2.1%).
Clinical Follow-Up
Clinical data were sought for all cases with an ALK rearrangement detected by greater than one testing modality. A total of 10 cases with sufficient clinical data after initiation of TKI therapy were identified. Of cases with available clinical data, three had progression on first-line therapy. Specifically, among patients with concordant positive findings among all ALK studies, one case with variant 3a/b had progression on alectinib at 4 months and one case with variant 2 had progression on crizotinib at 5 months followed by response on ceritinib at 21-month follow-up. The third case of documented progression was a previously described discordant case (IHC negative, FISH positive, RNA ISH negative, RNA NGS negative), in which disease progression was noted at 3-month follow-up (Table 2).
Table 2.
Therapy Outcomes
| Case | IHC | FISH | RNA NGS | Variant | DNA NGS | RNA ISH | First-Line Therapy | Documented Progression | Second-Line Therapy | Follow-Up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | P | EML4 (ex 13)–ALK (ex 20) | 1 | N | P | Alectinib | N | — | 4 |
| 2 | P | P | EML4 (ex 13)–ALK (ex 20) | 1 | N | P | Alectinib | N | — | 9 |
| 3 | P | P | EML4 (ex 6)–ALK (ex 20) | 3 a/b | N | P | Alectinib | N | — | 10 |
| 4 | P | P | EML4 (ex13)- ALK (ex20) | 1 | N | P | Alectinib | N | — | 10 |
| 5 | P | P | EML4 (ex 13)–ALK (ex 20) | 1 | N | P | Alectinib | N | — | 6 |
| 6 | P | P | EML4 (ex 6)–ALK (ex 20) | 3 a/b | F1174L | P | Alectinib | @ 4 mo, 12 d | Lorlatinib | 6 |
| 7 | P | P | EML4 (ex 13)–ALK (ex 20) | 1 | N | P | Alectinib | N | — | 13 |
| 8 | P | P | EML4 (ex 20)–ALK (ex 20) | 2 | Na | P | Crizotinib | @ 5 mo | Ceritinib | 21 |
| 9 | N | P | N | — | STK11 | N | Alectinib | @ 3 mo, 5 d | Pembrolizumab Carboplatin-pemetrexed |
18 |
| 10 | P | P | SLMAP (ex 12/13)–ALK (ex 20) | — | N | P | Crizotinibb | N | — | 24 |
@, at the rate; ex, exon; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; N, negative; NGS, next-generation sequencing; P, positive; RNA ISH, RNA in situ hybridization; SGA, single-gene analysis.
SGA KRAS/EGFR.
Patient initially received cisplatin and pemetrexed for four cycles, after RNA NGS testing ALK fusion was identified and patient was switched to crizotinib.
Discussion
To date, the FDA has approved rearrangement by FISH or reactivity with IHC as primary testing methods to identify ALK fusions, and the updated CAP/IASLC/AMP guidelines consider the two equivalents. Additional methodologies to detect ALK fusions have been developed, investigated, or variably adopted, and first, we evaluated two of these—RNA NGS and RNA ISH—against the currently available FISH and IHC standards. Second, we correlated results of the various methods, including specific variants identified by RNA NGS, with clinical outcomes.
Concordant and Discordant Cases
Our study reveals discordance among ALK IHC, FISH, RNA ISH, and RNA NGS in a subset of cases. Among the “positives,” all four methods were concordant in 21 of 23 cases (91.3%). If RNA NGS is considered to be the gold standard, greatest correlation was equally found with IHC and RNA ISH methods, whereas there were discrepancies in a small subset of cases with FISH—the currently accepted gold standard. Meanwhile, the “negatives” were concordant among IHC, FISH, and RNA NGS results in 48 of 48 cases (100%); nine such cases with available RNA ISH were all negative.
Among the four cases in which there were discordances, two had all testing modalities successfully completed for comparison and were found to be FISH positive and otherwise ALK negative with IHC, RNA ISH, and RNA NGS. DNA NGS and SGA results evaluated in these two cases had STK11 mutation in one case (Table 2; case 9) and no KRAS or EGFR mutations in the second case. Clinical follow-up was available for case 9 who had disease progression on TKI therapy at 3 months.
IHC and FISH
Previous studies have compared FISH and IHC with and without other ALK detection methods—DNA NGS and several non-Archer, RNA-based techniques—and illustrated concordance in most but not all cases.13, 14, 15 Discordances may be due to interpretation of borderline FISH cases (i.e., positivity on the recommended ≥15% cutoff) or heterogeneity in ALK FISH patterns (i.e., [1] fusion and split green signals, [2] fusion and single orange, and [3] single orange or green signals). The discrepancies between various methods in our case can also be due to complex biological events, including transcriptional and post-translocation events, rather than technical issues that could modulate gene expression and potentially affect response to therapy. Such discrepant cases have been reported previously.15,16 Furthermore, among FISH-negative, IHC-positive and FISH-positive, IHC-negative15,17, 18, 19 cases, studies have revealed response13,17,20 and worse outcome in both scenarios.21 Marchetti et al.17 in a review of the literature found that among FISH-positive, IHC-negative discordant cases, there was only a 46% response rate to therapy. Although this response is not insignificant, a shift away from FISH as the gold standard15,18,22,23 and incorporation of NGS as standard clinical practice has been proposed.24 Our study data support this proposal and have the added strength of additional testing modalities for comparison. Nevertheless, discordance and disease progression require further investigation and optimization of current protocols to segregate eligible patients from those unlikely to respond to TKIs.
Targeted RNA NGS
In our cohort, RNA NGS was particularly advantageous in three scenarios. These encompass, first, identification of a novel gene fusion, second, confirmation (or lack thereof) of canonical and noncanonical ALK partners, and third, identification of specific rearrangements with potential impact on susceptibility to TKIs.
In the first scenario, RNA NGS uncovered SLMAP-ALK, a novel partner, and the results were concordant with IHC, FISH, RNA ISH, and DNA NGS (no hotspot mutations detected). In the second scenario, a FISH-positive result (IHC negative) prompted treatment with alectinib but without response leading to discontinuation. This case had a VUS in the STK11 gene that is unlikely a driver mutation. In general, pathogenic STK11 mutations are associated with poor prognosis in NSCLC and have been reported rarely with an ALK rearrangement25; as a VUS, it may not be consistently reported.26,27 More recent studies have used RNA NGS AMP.11,28 Similar to our analysis, others have uncovered (1) rare and (2) novel fusions28 and noted discordance between the current methods and RNA NGS.11
In the third scenario, variable response to TKIs was noted among nine patients with positive concordance among ALK IHC, FISH, RNA ISH, and RNA NGS. Currently, there are only rare studies suggesting differences in response to ALK fusion variants.25, 26, 27 Emerging data reveal that specific ALK variants may be inherently aggressive25, 26, 27, 28 or responsive. An analysis of 54 tumors revealed that compared with variant 3a/b or 5a, variants 1/2/others have higher PFS.29 Most cases in our cohort had PFS as of most recent clinical evaluation. One exception is case 8 with variant 2, which revealed progression at 5 months; the reported 2-year PFS is 76% in crizotinib-treated patients.27 The second exception was in cases 3 and 6 both with variant 3a/b—one (case 3) responded to alectinib and one (case 6) with a known ALK resistance mutation (F1174L) that progressed.30 These suggest that ALK positivity across all testing modalities may still portend suboptimal TKI response. We postulate, similar to previous reports, that there may be either variable response to specific variants or an underlying resistance mutation, akin to EGFR exon 20 mutations that have been found to convey de novo resistance to approved TKIs. Nevertheless, additional studies using RNA NGS are warranted to confirm the extent of correlation between ALK variant and clinical response after TKIs.
Several RNA-based methods are available, and we used AMP Archer FusionPlex.12 First, a major advantage to this method is the ability to identify specific fusion partners, including novel ones. In contrast to DNA NGS and other RNA methods, RNA NGS (AMP) uses gene-specific primers known to occur adjacent to fusion breakpoints and permits detection of rearrangements, including those in noncoding regions and novel ones, with a single primer. This is relevant as ALK rearrangements are heterogeneous and some may predict (greater) responsivity than others as described previously. Second, RNA NGS has the ability to multiplex and identify other targetable fusions/alterations (e.g., MET exon 14 skipping, RET, ROS1, and NTRK fusions) without additional cost or tumor tissue for each additional test. Third, our ability to successfully perform Archer in most archived FFPE samples for the past 5 years makes RNA degradation a less likely hurdle when a sufficient contemporaneous sample is available. Nevertheless, RNA NGS (AMP) is neither widely available nor widely implemented. Although DNA NGS and reverse transcriptase-polymerase chain reaction are alternatives, they can only identify known variants.
RNA ISH
In the two cases that failed RNA NGS, owing possibly to low quantity/quality of RNA or inadequate tumor cellularity, RNA ISH was negative in one case and equivocal in the other. In the remaining cohort, RNA ISH correlated 100% with the results of RNA NGS testing. To the best of our knowledge, only a single study has evaluated ALK RNA ISH. In it, Nakajima et al.31 describe 16 cases with complete correlation between RNA ISH and either IHC or FISH positivity; NGS methods (DNA, RNA) were not performed.
Relative to RNA NGS, RNA ISH is more accessible and has a shorter turnaround time. In addition, RNA ISH analysis can provide quantitative scoring of gene expression by enumerating the number of punctuate dots present within each cell. This process provides an objective means of evaluation of positivity (in contrast to IHC) and can also be automated using RNAScope software.32 Similar to RNA NGS, the ability to successfully perform ISH on older paraffin-embedded samples, at least 5 years before, makes RNA degradation a less likely possibility, when sufficient sample is available. A noteworthy challenge in evaluation of RNA ISH is localizing the brown signal at low magnification when present focally and distinguishing it from anthracotic pigment or entrapped fine air bubbles. An amplified and red chromogen that provides sharper contrast would address both concerns.
Proposed Optimized Algorithm
To capture all (in)eligible patients, we propose an algorithm that incorporates greater than one ALK testing modality (Fig. 533). In this algorithm, we suggest ALK IHC followed by RNA NGS when no driver mutation is identified by DNA NGS, and sufficient tumor and resources are available to identify novel ALK fusion partners and other potential targetable fusion/rearrangements (e.g., MET, RET, ROS1, NTRKs). This combination provides a reportable result in a short turnaround time (initial IHC), followed by confirmation with a second testing method (RNA NGS) that has the advantage of identifying the specific ALK fusion partner. It is acknowledged that the overall turnaround time and cost of both testing modalities (IHC and RNA NGS) may be slightly increased in comparison to performing FISH alone or FISH and IHC; however, the improvement in specificity and avoidance of treating false positives or potential specific variants with ineffective therapy greatly outweigh this shortcoming. In addition, RNA ISH may serve as an alternative secondary testing method in the following three scenarios: (1) confirmation of IHC results in cases with insufficient material to conduct RNA NGS testing; (2) clarity in cases of equivocal staining by IHC; and (3) inaccessibility to RNA NGS (complementary techniques [DNA NGS and RNA ISH] serve as alternatives). Although IHC (FDA-approved Ventana clone D5F3) is recommended in the optimal algorithm, FISH is a standard and FDA-approved alternative. A negative result by RNA NGS or RNA ISH in an archived specimen may require further investigation. Finally, in scenarios with negative ALK IHC (and/or FISH) and insufficient tissue for DNA NGS and RNA NGS, the 2018 CAP/IASLC/AMP minimum testing guidelines for lung adenocarcinomas include EGFR and ROS1 (in addition to ALK).
Figure 5.
Proposed algorithm for lung NSCLC (i.e., adenocarcinoma, large cell carcinoma, NSCLC, NOS): ALK testing. Optimal algorithm (light gray background, right): Perform ALK IHC and targeted DNA NGS testing for lung NSCLC in parallel. If a positive result is obtained by IHC (in the absence of other driver mutations—EGFR, KRAS, BRAF, ERBB2, and MET), follow with RNA NGS, when sufficient tumor material and resources are available. Alternative algorithm (dark gray background, left): RNA ISH may serve as an alternative secondary testing method for the confirmation of IHC results in cases without sufficient material for NGS or for clarity in cases of equivocal staining by IHC. If FISH is the predominant testing modality, additional confirmatory testing, which includes parallel IHC and/or NGS testing followed by RNA ISH in cases of equivocal IHC or insufficient material for NGS, can exclude a potential false-positive result. Note: The proposed algorithm is for first-line ALK testing; its application for identifying acquired ALK rearrangements that may develop after treatment with TKI was not evaluated.33 ∗RNA NGS is performed on ALK IHC-negative cases without driver mutations on DNA NGS to identify other potential targetable rearrangements (e.g., MET, RET, ROS1, NTRKs). FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; ISH, in situ hybridization; NGS, next-generation sequencing; NOS, not otherwise specified; TKI, tyrosine kinase inhibitor.
Strengths and Limitations
Strengths of this study are evaluation and comparison of four ALK testing modalities and the greatest numbers of positive and negative cases compared with previous studies evaluating AMP RNA NGS and RNA ISH. Though the results were compared with clinical outcomes, a limitation is that not all patients received targeted therapy or had available follow-up to evaluate response to various rearrangements.
Conclusions
This study highlights that a small subset of results of IHC, FISH, RNA ISH, and RNA NGS are discordant and gold standard designation for FISH necessitates reassessment. An alternative to the current recommendations is IHC followed by RNA NGS, but it requires further investigation.
CRediT Authorship Contribution Statement
Carleigh R. Canterbury: Data curation, Writing—original draft, Writing—review and editing.
Helen Fernandes: Investigation, Resources, Writing—review and editing.
John P. Crapanzano, Vundavalli V. Murty, Mahesh M. Mansukhani: Investigation, Writing—review and editing.
Catherine A. Shu: Data curation, Writing—review and editing.
Matthias Szabolcs: Methodology, Resources, Writing—review and editing.
Anjali Saqi: Conceptualization, Investigation, Writing—original draft, Writing—review and editing.
Acknowledgments
Leonore Peruyero, the clinical research coordinator, is acknowledged for technical expertise in performing RNA in situ hybridization.
Footnotes
Cite this article as: Canterbury CR, Fernandes H, Crapanzano JP, et al. ALK gene rearrangements in lung adenocarcinomas: concordance of immunohistochemistry, fluorescent in situ hybridization, RNA in situ hybridization, and RNA next-generation sequencing testing. JTO Clin Res Rep. 2021;2:100223.
Disclosure: The authors declare no conflict of interest.
References
- 1.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Soda M., Choi Y.L., Enomoto M. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Kwak E.L., Bang Y.J., Camidge D.R. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devarakonda S., Morgensztern D., Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015;16:e342–e351. doi: 10.1016/S1470-2045(15)00077-7. [DOI] [PubMed] [Google Scholar]
- 5.Pareja F., Crapanzano J.P., Mansukhani M.M., Bulman W.A., Saqi A. Cytomorphological features of ALK-positive lung adenocarcinomas: psammoma bodies and signet ring cells. Cancer Cytopathol. 2015;123:162–170. doi: 10.1002/cncy.21507. [DOI] [PubMed] [Google Scholar]
- 6.Lindeman N.I., Cagle P.T., Beasley M.B. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindeman N.I., Cagle P.T., Aisner D.L. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13:323–358. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Barua S., Wang G., Mansukhani M., Hsiao S., Fernandes H. Key considerations for comprehensive validation of an RNA fusion NGS panel. Pract Lab Med. 2020;21 doi: 10.1016/j.plabm.2020.e00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagan C., Barua S., Hsiao S.J. Targeting SLMAP-ALK—a novel gene fusion in lung adenocarcinoma. Cold Spring Harb Mol Case Stud. 2019;5:a003939. doi: 10.1101/mcs.a003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benayed R., Offin M., Mullaney K. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen D., Hondelink L.M., Solleveld-Westerink N. Optimizing mutation and fusion detection in NSCLC by sequential DNA and RNA sequencing. J Thorac Oncol. 2020;15:1000–1014. doi: 10.1016/j.jtho.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Z., Liebers M., Zhelyazkova B. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum J.N., Bloom R., Forys J.T. Genomic heterogeneity of ALK fusion breakpoints in non-small-cell lung cancer. Mod Pathol. 2018;31:791–808. doi: 10.1038/modpathol.2017.181. [DOI] [PubMed] [Google Scholar]
- 14.Vollbrecht C., Lenze D., Hummel M. RNA-based analysis of ALK fusions in non-small cell lung cancer cases showing IHC/FISH discordance. BMC Cancer. 2018;18:1158. doi: 10.1186/s12885-018-5070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scattone A., Catino A., Schirosi L. Discordance between FISH, IHC, and NGS analysis of ALK status in advanced non-small cell lung cancer (NSCLC): a brief report of 7 cases. Transl Oncol. 2019;12:389–395. doi: 10.1016/j.tranon.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camidge D.R., Kono S.A., Flacco A. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010;16:5581–5590. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti A., Di Lorito A., Pace M.V. ALK protein analysis by IHC staining after recent regulatory changes: a comparison of two widely used approaches, revision of the literature, and a new testing algorithm. J Thorac Oncol. 2016;11:487–495. doi: 10.1016/j.jtho.2015.12.111. [DOI] [PubMed] [Google Scholar]
- 18.Gao X., Sholl L.M., Nishino M., Heng J.C., Jänne P.A., Oxnard G.R. Clinical implications of variant ALK FISH rearrangement patterns. J Thorac Oncol. 2015;10:1648–1652. doi: 10.1097/JTO.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallander M.L., Geiersbach K.B., Tripp S.R., Layfield L.J. Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med. 2012;136:796–803. doi: 10.5858/arpa.2011-0321-OA. [DOI] [PubMed] [Google Scholar]
- 20.Shan L., Jiang P., Xu F. BIRC6-ALK, a novel fusion gene in ALK break-apart FISH-negative lung adenocarcinoma, responds to crizotinib. J Thorac Oncol. 2015;10:e37–e39. doi: 10.1097/JTO.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 21.Thunnissen E., Lissenberg-Witte B.I., van den Heuvel M.M. ALK immunohistochemistry positive, FISH negative NSCLC is infrequent, but associated with impaired survival following treatment with crizotinib. Lung Cancer. 2019;138:13–18. doi: 10.1016/j.lungcan.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Pekar-Zlotin M., Hirsch F.R., Soussan-Gutman L. Fluorescence in situ hybridization, immunohistochemistry, and next-generation sequencing for detection of EML4-ALK rearrangement in lung cancer. Oncologist. 2015;20:316–322. doi: 10.1634/theoncologist.2014-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilie M.I., Bence C., Hofman V. Discrepancies between FISH and immunohistochemistry for assessment of the ALK status are associated with ALK ‘borderline’-positive rearrangements or a high copy number: a potential major issue for anti-ALK therapeutic strategies. Ann Oncol. 2015;26:238–244. doi: 10.1093/annonc/mdu484. [DOI] [PubMed] [Google Scholar]
- 24.Dacic S., Villaruz L.C., Abberbock S., Mahaffey A., Incharoen P., Nikiforova M.N. ALK FISH patterns and the detection of ALK fusions by next generation sequencing in lung adenocarcinoma. Oncotarget. 2016;7:82943–82952. doi: 10.18632/oncotarget.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Z., Zhang J., Lu X. Coexistent genetic alterations involving ALK, RET, ROS1 or MET in 15 cases of lung adenocarcinoma. Mod Pathol. 2018;31:307–312. doi: 10.1038/modpathol.2017.109. [DOI] [PubMed] [Google Scholar]
- 26.Pécuchet N., Laurent-Puig P., Mansuet-Lupo A. Different prognostic impact of STK11 mutations in non-squamous non-small-cell lung cancer. Oncotarget. 2017;8:23831–23840. doi: 10.18632/oncotarget.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shire N.J., Klein A.B., Golozar A. STK11 (LKB1) mutations in metastatic NSCLC: prognostic value in the real world. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vendrell J.A., Taviaux S., Béganton B. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep. 2017;7:12510. doi: 10.1038/s41598-017-12679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo C.G., Seo S., Kim S.W. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol. 2017;28:791–797. doi: 10.1093/annonc/mdw693. [DOI] [PubMed] [Google Scholar]
- 30.Shaw A.T., Solomon B.J., Besse B. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima N., Yoshizawa A., Kondo K. Evaluating the effectiveness of RNA in-situ hybridization for detecting lung adenocarcinoma with anaplastic lymphoma kinase rearrangement. Histopathology. 2017;71:143–149. doi: 10.1111/his.13198. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Su N., Wang L.C. Quantitative ultrasensitive bright-field RNA in situ hybridization with RNAscope. Methods Mol Biol. 2014;1211:201–212. doi: 10.1007/978-1-4939-1459-3_16. [DOI] [PubMed] [Google Scholar]
- 33.Offin M., Somwar R., Rekhtman N. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR-mutant lung cancers. JCO Precis Oncol. 2018;2 doi: 10.1200/PO.18.00126. PO.18.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]