Key Points
Question
Do 18F-fludeoxyglucose–positron emission tomography with computed tomography (FDG-PET/CT) and staging laparoscopy provide benefit in patients with locally advanced gastric cancer?
Findings
In this multicenter cohort study comprising 394 patients, FDG-PET/CT detected metastatic disease in 3% of patients and staging laparoscopy detected metastatic or noncurable disease in 19% of patients with locally advanced gastric cancer. Treatment intent changed from curative to palliative in 16% of the patients.
Meaning
These findings suggest that FDG-PET/CT has limited additive value, but staging laparoscopy adds considerably to the staging process in patients with locally advanced gastric cancer.
Abstract
Importance
The optimal staging for gastric cancer remains a matter of debate.
Objective
To evaluate the value of 18F-fludeoxyglucose–positron emission tomography with computed tomography (FDG-PET/CT) and staging laparoscopy (SL) in addition to initial staging by means of gastroscopy and CT in patients with locally advanced gastric cancer.
Design, Setting, and Participants
This multicenter prospective, observational cohort study included 394 patients with locally advanced, clinically curable gastric adenocarcinoma (≥cT3 and/or N+, M0 category based on CT) between August 1, 2017, and February 1, 2020.
Exposures
All patients underwent an FDG-PET/CT and/or SL in addition to initial staging.
Main Outcomes and Measures
The primary outcome was the number of patients in whom the intent of treatment changed based on the results of these 2 investigations. Secondary outcomes included diagnostic performance, number of incidental findings on FDG-PET/CT, morbidity and mortality after SL, and diagnostic delay.
Results
Of the 394 patients included, 256 (65%) were men and mean (SD) age was 67.6 (10.7) years. A total of 382 patients underwent FDG-PET/CT and 357 underwent SL. Treatment intent changed from curative to palliative in 65 patients (16%) based on the additional FDG-PET/CT and SL findings. FDG-PET/CT detected distant metastases in 12 patients (3%), and SL detected peritoneal or locally nonresectable disease in 73 patients (19%), with an overlap of 7 patients (2%). FDG-PET/CT had a sensitivity of 33% (95% CI, 17%-53%) and specificity of 97% (95% CI, 94%-99%) in detecting distant metastases. Secondary findings on FDG/PET were found in 83 of 382 patients (22%), which led to additional examinations in 65 of 394 patients (16%). Staging laparoscopy resulted in a complication requiring reintervention in 3 patients (0.8%) without postoperative mortality. The mean (SD) diagnostic delay was 19 (14) days.
Conclusions and Relevance
This study’s findings suggest an apparently limited additional value of FDG-PET/CT; however, SL added considerably to the staging process of locally advanced gastric cancer by detection of peritoneal and nonresectable disease. Therefore, it may be useful to include SL in guidelines for staging advanced gastric cancer, but not FDG-PET/CT.
This diagnostic study compares the use of 18F-fludeoxyglucose–positron emission tomography with computed tomography vs staging laparoscopy in addition to the initial staging in patients with locally advanced gastric cancer.
Introduction
Gastric cancer is the third leading cause of cancer-related death worldwide and accounted for more than 1 million patients with newly diagnosed gastric cancer in 2018.1 In Western countries, the recommended treatment with curative intent is subtotal or total gastrectomy with lymphadenectomy, with perioperative chemotherapy in case of locally advanced tumors.2,3 Prognosis mainly depends on tumor stage; recurrences occur in up to 60% of patients after surgery,4 with the peritoneum most frequently involved.5,6 For detecting noncurable disease, the accuracy of staging using gastroscopy and computed tomography (CT) of the thorax and abdomen is limited.7,8 As a result, some patients incorrectly undergo treatment with curative intent, exposing them to the risk of complications of surgery and perioperative chemotherapy. If noncurable disease could be detected accurately before initiation of treatment, more tailored and less toxic palliative treatment can be offered.9,10,11
To accurately detect noncurable gastric cancer, the role of other preoperative staging modalities, such as 18F-fludeoxyglucose–positron emission tomography with CT (FDG-PET/CT) and staging laparoscopy (SL) has increased over the years. A study in patients with locally advanced gastric cancer reported that FDG-PET/CT detected additional distant metastases in 10% of patients, whereas SL detected peritoneal metastases in 19%, preventing futile treatment and improving quality of life of patients and cost-effectiveness.12 As a result, several international guidelines now advise to perform FDG-PET/CT and SL in patients with locally advanced gastric cancer in addition to initial staging with CT and gastroscopy.2,3,13 Although the evidence for performing SL is strong in Asian populations, the evidence for both SL and FDG-PET/CT in Western populations is limited. Therefore, the aim of the present study (Evaluation of FDG-PET/CT and Laparoscopy in Staging Advanced Gastric Cancer [PLASTIC], a Dutch multicenter prospective study) was to evaluate the value of FDG-PET/CT and SL in addition to initial staging in patients with locally advanced gastric cancer.
Methods
Study Design
The protocol of this multicenter prospective, observational cohort study has been published.13 Inclusion criteria consisted of patients with a histologically proven adenocarcinoma of the stomach or gastroesophageal junction (Siewert type III); patients having undergone a CT scan of the thorax/abdomen; patients with locally advanced gastric cancer, defined either as transmural and invading the outer layer of the stomach or involving at least 1 lymph node, as reported on CT (≥cT3 and/or N+, M0 category according to the seventh edition of the American Joint Committee on Cancer TNM staging system)13,14; surgically resectable gastric cancer (<cT4b); and patients considered fit for treatment with curative intent (surgery with or without chemotherapy), as determined by the multidisciplinary team (MDT). In all centers in the Netherlands with patients included in the study, MDTs are composed of upper gastrointestinal surgeons, radiologists/nuclear medicine physicians, medical oncologists, gastroenterologists, radiation oncologists, and pathologists. Patients in whom it was not possible to make a clear distinction between cT2 and cT3 cancer based on CT scan or endoscopic ultrasonographic findings were also included. Data on race and ethnicity were not included in the electronic case report forms.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1964 and later versions.15 Because this study does not allocate patients to interventions other than standard of care according to national guidelines, this study does not fall within the Medical Research Involving Human Subjects Act (WMO). A non-WMO declaration (METC 16-633/C) has been obtained from the Medical Ethical Review Board of the University Medical Center Utrecht, Utrecht, the Netherlands. In addition, the trial was approved by the institutional review board in each of 18 participating centers (eAppendix in Supplement 1). Because questionnaires and financial hospital data were required for a side study, written informed consent was obtained. Patients did not receive financial compensation.
Patients who met the inclusion criteria as determined by the MDT were invited to participate in the study in 1 of the 18 Dutch centers. All 18 centers participated in a regional MDT including at least 1 surgical high-volume center (>20 procedures). Surgical procedures were performed in 13 high-volume centers. In the Netherlands, centralization of gastric cancer treatment was initiated in 2013, meaning that only hospitals in which at least 20 gastrectomies are performed annually are considered sufficiently competent to perform this type of surgery.
Procedures
After written informed consent was obtained, patients were enrolled in the study and underwent FDG-PET/CT and/or SL as standard of care according to Dutch national guidelines. Ideally, FDG-PET/CT was performed first, followed by SL if no distant metastases were found on FDG-PET/CT. Staging laparoscopy was performed as a separate procedure in patients scheduled for neoadjuvant chemotherapy or otherwise at the onset of gastrectomy. The protocol for performing FDG-PET/CT and SL is summarized in the eMethods in Supplement 1 and was previously published.13 The FDG avidity of the primary tumor and lymph nodes and the presence of distant metastases were scored as yes, equivocal, or no, and distant metastases were scored as suspicious, equivocal, or no at the discretion of the nuclear medicine physician. Staging laparoscopy reported the location and extent of peritoneal metastases and local resectability, and it was recommended to perform peritoneal lavage with cytologic testing. Based on the results of both investigations, the final treatment strategy was determined at the subsequent MDT meeting.
Outcomes
The primary outcome of the study was the number of patients in whom the treatment intent was changed from curative to palliative based on the results of the FDG-PET/CT or SL. Secondary outcomes were the diagnostic performance (sensitivity and specificity) of both modalities, number of incidental findings on FDG-PET/CT, morbidity of SL, diagnostic delay, quality of life, and cost-effectiveness. All patient data were prospectively registered using electronic case report forms.13
Statistical Analysis
Factors associated with FDG avidity were evaluated using the χ2 test or Fisher exact test when appropriate. For modality-specific performance, sensitivity and specificity with 95% CIs were calculated. By means of cross tabulation of the index test results against those of the reference standard, the sensitivity and specificity of the index test were estimated.16,17 A priori–determined subgroup analyses for specific patient and tumor characteristics were performed as described in the study protocol.13 For FDG-PET/CT, the reference standard for positivity was biopsy or additional imaging, and for negativity, clinical follow-up of 6 months. For SL, biopsy findings from macroscopically suspicious lesions were the reference standard for positivity, and false-negative findings were defined as peritoneal metastases found at the onset of gastrectomy or within 6 months after an initially negative SL result. Members of the study team were not blinded to the results. All statistical analyses were performed using SPSS, version 25.0 (IBM Corp), and a 2-sided, unpaired P value <.05 was considered statistically significant.
Results
Study Population
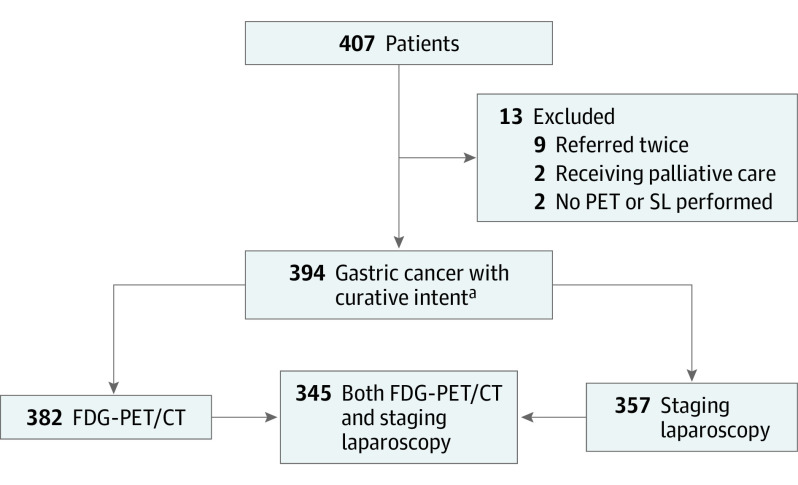
Between August 1, 2017, and February 1, 2020, a total of 407 patients with locally advanced gastric cancer were included in 18 centers in the Netherlands. Two centers included more than 40 patients, 6 centers included 20 to 40 patients, and the remaining centers included fewer than 20 patients. In total, 13 patients were excluded: 9 patients were registered twice (in the referring and tertiary hospitals), a palliative intent was already decided for 2 patients during the first MDT meeting, and 2 patients were excluded because neither FDG-PET/CT nor SL was performed (Figure). Of the 394 included patients, 256 (65%) were men and 138 (35%) were women; mean (SD) age was 67.6 (10.7) years; other patient characteristics are presented in Table 1.
Figure. Flowchart of Inclusion and Exclusion Criteria.
FDG-PET/CT indicates 18F-fludeoxyglucose–positron emission tomography/computed tomography; PET, positron emission tomography; and SL, staging laparoscopy.
acT3 and/or N+, M0 category.
Table 1. Baseline Characteristics of 394 Included Patientsa.
| Characteristic | No. (%) |
|---|---|
| Patients | |
| Age, mean (SD), y | 67.6 (10.7) |
| Missing values | 0 |
| Sex | |
| Men | 256 (65) |
| Women | 138 (35) |
| Missing values | 0 |
| Diagnostic tests | |
| Gastroscopy | 394 (100) |
| Missing values | 0 |
| CT thorax/abdomen | 394 (100) |
| Missing values | 0 |
| Endoscopic ultrasonography | 70 (18) |
| Missing values | 1 (0.2) |
| Tumors | |
| cT category | |
| T1 | 0 |
| T2 | 28 (7) |
| T3 | 301 (76) |
| T4 | 56 (14) |
| Tx | 9 (2) |
| Missing values | 0 |
| cN category | |
| N0 | 177 (45) |
| N1 | 126 (32) |
| N2 | 72 (18) |
| N3 | 13 (3) |
| Nx | 6 (2) |
| Missing values | 0 |
| Location | |
| Cardia | 94 (24) |
| Corpus | 110 (28) |
| Antrum | 125 (32) |
| Pylorus | 33 (8) |
| Diffuse | 26 (7) |
| Missing values | 6 (2) |
| Lauren classification | |
| Intestinal | 118 (30) |
| Diffuse | 135 (34) |
| Mixed | 19 (5) |
| Unknown | 122 (31) |
| Missing values | 0 |
| Differentiation | |
| Well | 15 (4) |
| Moderate | 92 (23) |
| Poor | 108 (27) |
| Undifferentiated | 3 (1) |
| Missing values | 176 (45) |
| ERBB2 statusb | |
| Positive | 18 (5) |
| Negative | 115 (29) |
| Missing values | 261 (66) |
Abbreviation: CT, computed tomography.
Percentages may not total 100% owing to rounding.
Formerly HER2/neu.
FDG-PET/CT Scan
Of the 394 patients, 382 patients underwent FDG-PET/CT, revealing an FDG-avid primary tumor in 302 patients (79%). A more frequent association was noted between FDG avidity and male sex, positive lymph nodes on CT imaging, gastroesophageal junction tumor location, and intestinal type tumor (Table 2).
Table 2. FDG-PET/CT Avidity of Primary Tumor and Tumor Characteristics.
| Variablea | FDG avidity, primary tumor, No. (%)b | P value | ||
|---|---|---|---|---|
| Yes | Equivocal | No | ||
| Sex (n = 381) | ||||
| Male | 206 (83) | 33 (13) | 9 (4) | .001 |
| Female | 96 (72) | 18 (14) | 19 (14) | |
| Site (n = 375) | ||||
| Gastroesophageal junction | 90 (97) | 3 (3) | 0 | <.001 |
| Corpus | 75 (71) | 17 (16) | 14 (13) | |
| Antrum | 96 (81) | 15 (13) | 8 (7) | |
| Pylorus | 23 (74) | 7 (23) | 1 (3) | |
| Diffuse | 16 (62) | 6 (23) | 4 (15) | |
| cT category (n = 373) | ||||
| cT2 | 20 (71) | 5 (18) | 3 (11) | .42 |
| cT3 | 232 (79) | 39 (13) | 21 (7) | |
| cT4 | 47 (89) | 4 (8) | 2 (4) | |
| cN category (n = 376) | ||||
| cN0 | 122 (72) | 31 (18) | 16 (9) | .03 |
| cN1 | 98 (80) | 17 (14) | 8 (7) | |
| cN2 | 67 (94) | 1 (1) | 3 (4) | |
| cN3 | 11 (85) | 2 (15) | 0 | |
| Lauren classification (n = 261) | ||||
| Intestinal | 106 (91) | 6 (5) | 4 (3) | <.001 |
| Diffuse | 81 (63) | 28 (22) | 19 (15) | |
| Mixed | 14 (82) | 1 (6) | 2 (12) | |
| Differentiation (n = 212) | ||||
| Well | 12 (86) | 2 (14) | 0 (0) | .02 |
| Moderate | 84 (92) | 4 (4) | 3 (3) | |
| Poor | 75 (72) | 17 (16) | 12 (12) | |
| Undifferentiated | 3 (100) | 0 | 0 | |
Abbreviation: FDG-PET/CT, 18F-fludeoxyglucose–positron emission tomography with computed tomography.
For this analysis, only patients in whom the designated tumor characteristics were registered were taken into account.
Percentages may not total 100% owing to rounding.
FDG-PET/CT results were suspicious for distant metastases in 16 patients (4%) and equivocal in 22 patients (6%). Metastatic disease was confirmed in 12 patients (3%); findings were suspicious on FDG-PET/CT in 10 patients and equivocal in 2 patients. These metastases were located in distant lymph nodes (n = 4), liver (n = 3), peritoneum (n = 3), uterus (n = 1), and bone (n = 1). Of these 12 patients with confirmed M1 category, 11 also had clinically positive locoregional lymph nodes and 2 patients had a cT4 tumor.
The sensitivity of FDG-PET/CT for detection of distant metastases was 33% (95% CI, 17%-53%), and for specificity, 97% (95% CI, 94%-99%) (eTable, A in Supplement 1). In the subgroup of patients with an FDG-avid primary tumor, sensitivity was 31% (95% CI, 14%-55%), and for specificity, 98% (95% CI, 95%-99%) (eTable, B in Supplement 1). In subgroup analyses for patients with peritoneal metastases, cT4 tumors or cN+ status, the diagnostic accuracy of FDG-PET/CT did not improve (eTables, C-E in Supplement 1).
Of the total cohort of 382 patients, a clinically relevant lesion was found in 83 (22%) of 132 patients with suspected relevant secondary findings, resulting in additional investigations in 60 patients (Table 3). In 7 of 83 patients (8%) a second primary tumor was confirmed (3 colon, 2 lung, and 2 prostate cancers), but in most of these 83 patients, follow-up was not reported.
Table 3. FDG-PET/CT Results in 382 Patients With Advanced (≥cT3 and/or N+) Gastric Adenocarcinoma With Curative Intent.
| Variable | No. (%)a |
|---|---|
| FDG avidity of primary tumor | |
| No | 28 (7) |
| Equivocal | 51 (13) |
| Yes | 302 (79) |
| Missing values | 1 (<1) |
| Maximum SUV, median (SD) | 10.5 (8.0) |
| Missing values | 270 (68) |
| Lymph nodes avid | |
| No | 225 (60) |
| Equivocal | 20 (5) |
| Yes, location | 132 (35) |
| Lesser curvature | 98 (26) |
| Greater curvature | 13 (3) |
| Paracardial | 12 (3) |
| Locoregional NOS | 45 (12) |
| Missing values | 5 (1) |
| Suspicion of metastatic disease | |
| No | 344 (90) |
| Equivocal | 22 (6) |
| Yes, location | 16 (4) |
| Liver | 8 (2) |
| Lung | 8 (2) |
| Bone | 4 (1) |
| Peritoneum | 4 (1) |
| Distant lymph nodes | 15 (4) |
| Corpus uteri | 1 (<1) |
| Missing values | 0 |
| cM1 category confirmed (n = 38) | |
| No | 26 (7) |
| Yes | 12 (3) |
| Missing values | 0 |
| Secondary findings | |
| No | 249 (65) |
| Yes | |
| Clinically irrelevantb | 49 (13) |
| Clinically relevant, location | 83 (22) |
| Pulmonary | 8 (2) |
| Gastrointestinal | 38 (10) |
| ENT | 5 (1) |
| Thyroid | 10 (3) |
| Soft tissue | 4 (1) |
| Adrenal | 1 (2) |
| Prostate | 3 (1) |
| HPB | 5 (1) |
| Other | 9 (2) |
| Additional examination, yesc | 60 (16) |
| Missing values | 1 (<1) |
Abbreviations: cM1, clinically M category; ENT, ear, nose, and throat; FDG-PET/CT, 18F-fludeoxyglucose–positron emission tomography with computed tomography; HPB, hepato-pancreato-biliary; NOS, not otherwise specified; SUV, standardized uptake value.
Percentages may not total 100% owing to rounding.
Clinically irrelevant secondary findings, such as hepatic cysts or adrenal adenomas.
Excluding 2 patients with clinically irrelevant secondary findings in whom additional examination was conducted.
Staging Laparoscopy
Of the 394 patients, 357 underwent SL and 264 (74%) also underwent peritoneal lavage for cytologic testing. Staging laparoscopy identified suspicious peritoneal lesions in 62 patients (17%), with metastatic disease confirmed in 44 of 357 patients (12%) patients: in 31 by histologic testing, 2 by cytologic testing, and 11 by both tests. Of 295 patients with no or low clinical suspicion during SL, biopsies were performed in 54 patients (18%) and peritoneal lavage was performed in 223 patients (76%). Metastatic disease was still found in 25 patients: in 6 by histologic characteristics of biopsy samples that were not expected by the surgeon to be metastases, 17 by cytologic examination, and in 2 by both tests. Of all 357 patients who underwent SL, a nonresectable tumor was identified in 13 patients: 4 patients solely because of a T4b tumor and 9 patients who also had peritoneal metastases. Altogether, SL findings were positive in 73 patients (19%). Positive SL findings were significantly associated with cT4 tumor category (39% vs 17% cT3 tumors; P = .001) and diffuse-type tumors (21% vs 14% intestinal types; P = .006).
Staging laparoscopy results were false-negative in 11 patients. Hence, the sensitivity of SL for detection of macroscopically peritoneal metastases was 82% (95% CI, 70%-91%) and specificity was 78% (95% CI, 73%-83%) (eTable, F in Supplement 1).
Staging laparoscopy resulted in postoperative complications in 3 patients (0.8%): luxation of a simultaneously placed feeding jejunostomy, a wound hematoma and bilateral adrenal bleeding, and a trocar incisional hernia with obstruction of the small intestine. All complications required surgical reintervention. No perioperative mortality was observed (Table 4).
Table 4. Staging Laparoscopy Results of 357 Patients Who Were Diagnosed With Advanced (≥cT3 and/or N+) Gastric Adenocarcinoma With Curative Intent.
| Variable | No. (%)a |
|---|---|
| Performed staging | |
| Surgeon | 215 (60) |
| Resident in presence of surgeon | 97 (27) |
| Resident | 37 (10) |
| Missing values | 8 (2) |
| Operation time, mean (SD), min | 34.6 (25.8) |
| Missing values | 4 (1) |
| All quadrants scored | |
| No | 14 (4) |
| Yes | 256 (72) |
| Missing values | 87 (24) |
| Ascites | |
| No | 237 (66) |
| Yes | 30 (8) |
| Missing values | 90 (25) |
| Adhesions | |
| No | 230 (64) |
| Yes | 56 (16) |
| Missing values | 71 (20) |
| Bursa opened | |
| No | 202 (57) |
| Yes | 72 (20) |
| Missing values | 83 (23) |
| Suspicion of peritoneal metastases | |
| No | 293 (82) |
| Yes | 62 (17) |
| PCI score, median (IQR) | 3 (1-8) |
| Missing values | 5 (1) |
| Missing values | 2 (1) |
| Histologic examination performed | |
| No | 237 (66) |
| Yes | 115 (32) |
| Positive | 50 (14) |
| Missing values | 5 (1) |
| Peritoneal washing performed | |
| No | 88 (25) |
| Yes | 264 (74) |
| Positive | 32 (9) |
| Missing values | 5 (1) |
| Missing values | 5 (1) |
| Locally resectable | |
| No (cT4b) | 13 (4) |
| Yes | 331 (93) |
| Missing values | 13 (4) |
| Positive SLb | |
| No | 284 (80) |
| Yes | 73 (20) |
| Missing values | 0 |
| SL performed | |
| As separate procedure | 342 (96) |
| At the onset of gastrectomyc | 15 (4) |
| Missing values | 0 |
| Complicated postoperative course | |
| No | 325 (91) |
| Yesd | 3 (0.8) |
| Surgical intervention | 3 (0.8) |
| Missing values | 29 (8) |
| Hospital stay, median (IQR), d | 0 (0-1) |
| Missing values | 79 (22) |
Abbreviations: PCI, peritoneal cancer index; SL, staging laparoscopy.
Percentages may not total 100% owing to rounding.
Positive SL is defined as positive cytologic test findings, positive histologic test findings, or nonresectable disease.
In 1 patient, peritoneal as well as nonresectable disease was found. In this patient, the procedure was interrupted and no resection was performed.
In a patient with a wound hematoma and bilateral adrenal bleeding, the hospital stay was prolonged to 30 days.
Treatment Changes
The combination of FDG-PET/CT and SL detected metastatic disease in 78 of 394 patients (20%), metastases in 12 patients (3%) were detected by FDG-PET/CT, metastases in 73 patients (19%) were detected by SL, and metastases in 7 patients (2%) were identified by both examinations. Theoretically, this finding should have resulted in a change of treatment intent in all these patients. All confirmed positive FDG-PET/CT findings (12 of 394 [3%]) resulted in a change from curative to palliative treatment intent. After positive SL findings, intent of treatment was changed to palliative in 60 of 73 patients (60 of 394 [15%]). Of the remaining 13 patients, 3 did not undergo resection owing to death during or shortly after neoadjuvant chemotherapy (n = 2) or progression of disease (n = 1). The other 10 patients had limited peritoneal metastases (n = 3) or only positive cytologic test results (n = 7) and underwent perioperative chemotherapy or chemoradiotherapy and surgical resection. Overall, the number of patients in whom treatment strategy changed from a curative to palliative intent was 65 of 394 (16%).
Diagnostic Delay
Performing only FDG-PET/CT resulted in a mean (SD) of 17 (20) additional days, and performing only SL resulted in 17 (8) additional days until the second MDT meeting. When the investigations were performed consecutively, the delay was 19 (14) days if FDG-PET/CT had been initially performed and 18 (12) days if SL was performed first.
Discussion
This multicenter prospective, observational cohort study evaluated the outcomes associated with adding FDG-PET/CT and SL to the staging process of locally advanced gastric cancer. We found that FDG-PET/CT identified distant metastatic disease in 12 of 394 patients (3%) and SL identified noncurable disease in 73 patients (19%). In all 12 patients with positive FDG-PET/CT results, the finding of metastatic disease resulted in a change of treatment strategy from curative to palliative intent. In the 73 patients with positive SL findings, treatment strategy was changed to palliative intent in 60 patients (15%), with an overlap of 7 patients (2%) who also had a positive FDG-PET/CT. These results suggest a limited additional role of FDG-PET/CT and what appears to be a considerable benefit of SL on the staging process of gastric cancer.
Retrospective studies reported a possible additional role of FDG-PET/CT in the identification of distant metastatic disease in gastric cancer being positive in 6% to 16% of patients,18,19,20,21,22 but limited additional value in detecting other noncurable disease.18,19,21,23,24,25,26,27,28 The present study found a much lower detection rate of 3% for distant metastases. Moreover, in 7 of 12 patients with positive FDG-PET/CT findings, metastatic disease was detected by SL, resulting in a negligible value of FDG-PET/CT. A possible reason for this difference may be that some patients with positive FDG-PET/CT results may not have been included in this study because regional centers may not have referred them to a participating center. To reduce this risk of this bias, multidisciplinary consultation lists were checked, and centers were asked to discuss all FDG-PET/CTs in the MDT meeting. In addition, the study by Smyth et al12 applied the sixth edition of the TNM classification system, whereas our staging was based on the seventh and, when available, the eighth edition. The T3 and T4 tumors according to TNM-6, included by Smyth et al, correspond to category T4a and T4b tumors according to TNM-7 and TNM-8. Therefore, we included lower T-category tumors (T3, T4a, and T4b) than Smyth et al. However, the accuracy of PET/CT did not increase in subgroup analyses with T4 tumors. In our study, 79% of tumors were FDG avid, which is comparable to other studies,8,12,24,29,30 but less than has been reported in other types of cancer, such as esophageal cancer.18 FDG avidity of the primary gastric tumor has previously been reported to be associated with male sex, intestinal type tumors, gastroesophageal junction tumors, and larger tumor size and depth.12,18,19,21,23,24,25,30,31,32 Determining FDG avidity of diffuse-type gastric cancer is challenging in clinical practice, because it may be interpreted as physiological uptake. Moreover, because FDG avidity of this type of tumor is generally lower, FDG-PET/CT will also be less sensitive for metastases of these tumors.18,19,23,33
The limited number of metastases detected by FDG-PET/CT alone, the additional waiting time of at least 17 days, and the high number of incidental findings leading to additional investigations raise questions regarding the routine use of FDG-PET/CT in patients with gastric cancer. Cost-effectiveness and quality-of-life analyses of our data will be performed after additional follow-up and may identify a subset of patients (eg, those with gastroesophageal junction or intestinal type tumors) that benefits from additional staging by FDG-PET/CT. In addition, patients with high FDG avidity of the primary tumor may have an increased risk of distant metastasis. Models to estimate the probability of this outcome, based on histopathological and other tumor characteristics, have been developed, such as the model reported by Kaneko et al.34 However, this model has limited predictive value and may benefit from further optimization.
By detecting noncurable disease in 19% of patients, SL was found to have a significant and clinically relevant added value in the staging of locally advanced gastric cancer. This finding supports the results of previous, mostly retrospective studies, reporting a yield of SL of 8% to 53%.35 Treatment was not changed to a palliative approach in all 73 patients in the present study with a positive SL outcome; instead, some patients with limited peritoneal metastases and positive cytologic test findings were treated with curative intent. In line with previous studies,36,37,38 the present study detected positive cytologic characteristics in 9% of the patients. Although positive cytologic findings are regarded as metastatic disease by the American Joint Committee on Cancer TNM-8 classification system39 and some international guidelines,2,40 no instructions exist on how to treat the patients with only positive cytologic findings. Some studies have reported a survival benefit when patients in whom a repeat SL showed a change from positive to negative cytologic findings following neoadjuvant chemotherapy undergo gastrectomy (hazard ratio, 0.42; 95% CI, 0.31-0.57; P < .001).38,41,42 Adjuvant chemotherapy could also be considered in these patients, as other studies reported a survival gain in patients with positive cytologic test results who receive postoperative chemotherapy after a surgical resection compared with no chemotherapy (hazard ratio, 4.17; 95% CI, 3.01-5.78; P = .01).43 Moreover, some studies have evaluated hyperthermic intraperitoneal chemotherapy in patients with positive cytologic findings, but no high-level evidence is yet available.44,45 The PERISCOPE-II trial is evaluating a possible survival benefit of hyperthermic intraperitoneal chemotherapy and cytoreductive surgery after systemic chemotherapy, including both patients with limited peritoneal disease and those with solely positive cytologic test results of peritoneal fluid or peritoneal washing.46
Regarding the risks of SL, research has suggested that the morbidity of SL does not outweigh the benefits.29 The present study noted metastatic disease in 19% of the patients, with a postoperative morbidity rate of 0.8%. This morbidity rate of less than 1% is in line with previously reported rates of 0% to 3%,35 and, in our opinion, supports the additional value of SL. Despite these advantages of adding SL to the staging process, there is room for an improvement of the logistics because SL resulted in extra time in the diagnostic process.
Limitations
A limitation of this study is that the sensitivity and specificity of both FDG-PET/CT and SL could not be completely adequately assessed. Because follow-up of most patients who underwent FDG-PET/CT was lacking, the number of metastases detected at 6 months’ follow-up is most likely underreported, resulting in an underestimation of sensitivity and specificity. Regarding sensitivity and specificity of SL, positive cytologic test results were not included in this analysis because peritoneal lavage was not repeated at the beginning of the gastrectomy and its clinical relevance is unclear, thereby precluding the adequate identification of true- and false-negative findings. Therefore, the sensitivity and specificity values reported herein should be interpreted with caution. In addition, no data on histopathological assessment of the resected specimens were collected, preventing examination of findings on FDG-PET/CT associated with tumor stage, nodal involvement, and metastatic status. Nevertheless, to our knowledge, the present study is the largest prospective study on the outcome of FDG-PET/CT and SL in patients with locally advanced gastric cancer in Western countries. The secondary outcomes (quality of life and cost-effectiveness) will be reported after the required 1-year follow-up for these end points has been reached.
Conclusions
In this study, FDG-PET/CT had limited value for detecting metastatic disease in patients with locally advanced gastric cancer. In contrast, SL detected metastatic or nonresectable disease in a considerable proportion of patients, resulting in a treatment change from curative to palliative intent. These findings suggest that it may be beneficial to include SL in guidelines for staging advanced gastric cancer, but not FDG-PET/CT.
eAppendix. Participating Centers
eMethods. Detailed Methods
eTable. FDG-PET/CT Sensitivity and Specificity
Nonauthor Collaborators. The PLASTIC Study Group
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(August)(suppl 5):v38-v49. doi: 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 3.Integraal Kankercentrum Nederland . Diagnostiek, behandeling en follow-up van het maagcarcinoom 2016. Published 2017. Accessed September 20, 2021. https://richtlijnendatabase.nl/gerelateerde_documenten/f/16316/IKNL%20richtlijn%20Maagcarcinoom.pdf
- 4.Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer. 2006;9(2):74-81. doi: 10.1007/s10120-006-0360-0 [DOI] [PubMed] [Google Scholar]
- 5.Koemans WJ, Luijten JCHBM, van der Kaaij RT, et al. The metastatic pattern of intestinal and diffuse type gastric carcinoma—a Dutch national cohort study. Cancer Epidemiol. 2020;69:101846. doi: 10.1016/j.canep.2020.101846 [DOI] [PubMed] [Google Scholar]
- 6.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7(32):52307-52316. doi: 10.18632/oncotarget.10740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwee RM, Kwee TC. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J Gastroenterol. 2015;21(37):10502-10509. doi: 10.3748/wjg.v21.i37.10502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seevaratnam R, Cardoso R, McGregor C, et al. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? a meta-analysis. Gastric Cancer. 2012;15(suppl 1):S3-S18. doi: 10.1007/s10120-011-0069-6 [DOI] [PubMed] [Google Scholar]
- 9.Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst. 2016;108(10):1-13. doi: 10.1093/jnci/djw166 [DOI] [PubMed] [Google Scholar]
- 10.Dijksterhuis WPM, Verhoeven RHA, Slingerland M, et al. Heterogeneity of first-line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: a real-world evidence study. Int J Cancer. 2020;146(7):1889-1901. doi: 10.1002/ijc.32580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang Y-J, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 12.Smyth E, Schöder H, Strong VE, et al. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012;118(22):5481-5488. doi: 10.1002/cncr.27550 [DOI] [PubMed] [Google Scholar]
- 13.Brenkman HJF, Gertsen EC, Vegt E, et al. ; PLASTIC Study Group . Evaluation of PET and Laparoscopy in Staging Advanced Gastric Cancer: a multicenter prospective study (PLASTIC-study). BMC Cancer. 2018;18(1):450. doi: 10.1186/s12885-018-4367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077-3079. doi: 10.1245/s10434-010-1362-z [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Initiative S. STROBE statement—checklist of items that should be included in reports of observational studies (STROBE initiative). Int J Public Health. 2008;53(1):3-4. doi: 10.1007/s00038-007-0239-9 [DOI] [PubMed] [Google Scholar]
- 17.Equator Network. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. September 9, 2021. Accessed July 10, 2021. https://www.equator-network.org/reporting-guidelines/stard
- 18.Smyth EC, Shah MA. Role of 18F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol. 2011;17(46):5059-5074. doi: 10.3748/wjg.v17.i46.5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch KD, Chicklore S, Cook GJ, et al. Staging FDG PET-CT changes management in patients with gastric adenocarcinoma who are eligible for radical treatment. Eur J Nucl Med Mol Imaging. 2020;47(4):759-767. doi: 10.1007/s00259-019-04429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Findlay JM, Antonowicz S, Segaran A, et al. Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol. 2019;29(5):2490-2498. doi: 10.1007/s00330-018-5904-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103(11):2383-2390. doi: 10.1002/cncr.21074 [DOI] [PubMed] [Google Scholar]
- 22.Serrano OK, Love C, Goldman I, et al. The value of FDG-PET in the staging of gastric adenocarcinoma: a single institution retrospective review. J Surg Oncol. 2016;113(6):640-646. doi: 10.1002/jso.24190 [DOI] [PubMed] [Google Scholar]
- 23.Kitajima K, Nakajo M, Kaida H, et al. Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci. 2017;79(4):527-543. doi: 10.18999/nagjms.79.4.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9(3):192-196. doi: 10.1007/s10120-006-0374-7 [DOI] [PubMed] [Google Scholar]
- 25.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28(3):247-253. doi: 10.1007/s00268-003-7191-5 [DOI] [PubMed] [Google Scholar]
- 26.Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46(10):1582-1588. [PubMed] [Google Scholar]
- 27.Yoshioka T, Yamaguchi K, Kubota K, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003;44(5):690-699. [PubMed] [Google Scholar]
- 28.Lim JS, Kim MJ, Yun MJ, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7(4):249-256. doi: 10.3348/kjr.2006.7.4.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JFM, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35(5):449-455. doi: 10.1016/j.ejso.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 30.Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30(2):288-295. doi: 10.1007/s00259-002-1029-5 [DOI] [PubMed] [Google Scholar]
- 31.Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20(9):597-604. doi: 10.1007/BF02984657 [DOI] [PubMed] [Google Scholar]
- 32.Wu CX, Zhu ZH. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol. 2014;20(16):4574-4585. doi: 10.3748/wjg.v20.i16.4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donswijk ML, Hess S, Mulders T, Lam MGEH. [18F]fluorodeoxyglucose PET/computed tomography in gastrointestinal malignancies. PET Clin. 2014;9(4):421-441, v-vi. doi: 10.1016/j.cpet.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Kaneko Y, Murray WK, Link E, Hicks RJ, Duong C. Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer. J Nucl Med. 2015;56(4):523-529. doi: 10.2967/jnumed.114.150946 [DOI] [PubMed] [Google Scholar]
- 35.Fukagawa T. Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg. 2019;3(5):496-505. doi: 10.1002/ags3.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groh EM, Gupta S, Brown ZJ, et al. Staging laparoscopy is underutilized in the management of gastric adenocarcinoma. Ann Surg Oncol. 2020;27(5):1473-1479. doi: 10.1245/s10434-019-08077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikoma N, Blum M, Chiang YJ, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23(13):4332-4337. doi: 10.1245/s10434-016-5409-7 [DOI] [PubMed] [Google Scholar]
- 38.Jamel S, Markar SR, Malietzis G, Acharya A, Athanasiou T, Hanna GB. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018;21(1):10-18. doi: 10.1007/s10120-017-0749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 40.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1-19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15(10):2684-2691. doi: 10.1245/s10434-008-0055-3 [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto M, Kawano H, Yamaguchi S, et al. Comparison of neoadjuvant chemotherapy to surgery followed by adjuvant chemotherapy in Japanese patients with peritoneal lavage cytology positive for gastric carcinoma. Anticancer Res. 2015;35(9):4859-4863. [PubMed] [Google Scholar]
- 43.Yamaguchi T, Takashima A, Nagashima K, et al. Efficacy of postoperative chemotherapy after resection that leaves no macroscopically visible disease of gastric cancer with positive peritoneal lavage cytology (cy1) or localized peritoneum metastasis (p1a): a multicenter retrospective study. Ann Surg Oncol. 2020;27(1):284-292. doi: 10.1245/s10434-019-07697-x [DOI] [PubMed] [Google Scholar]
- 44.Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20(s1)(suppl 1):128-134. doi: 10.1007/s10120-016-0684-3 [DOI] [PubMed] [Google Scholar]
- 45.Badgwell B, Blum M, Das P, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24(11):3338-3344. doi: 10.1245/s10434-017-6047-4 [DOI] [PubMed] [Google Scholar]
- 46.Koemans WJ, van der Kaaij RT, Boot H, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19(1):420. doi: 10.1186/s12885-019-5640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Participating Centers
eMethods. Detailed Methods
eTable. FDG-PET/CT Sensitivity and Specificity
Nonauthor Collaborators. The PLASTIC Study Group