Abstract
Reduced daily stepping in stroke survivors may contribute to decreased functional capacity and increased mortality. We investigated the relationships between clinical and biomechanical walking measures that may contribute to changes in daily stepping activity following physical interventions provided to participants with subacute stroke. Following ≤40 rehabilitation sessions, 39 participants were categorized into three groups: responders/retainers increased daily stepping >500 steps/day post-training (POST) without decreases in stepping at 2–6 month follow-up (F/U); responders/non-retainers increased stepping at POST but declined >500 steps/day at F/U; and, non-responders did not change daily stepping from baseline testing (BSL). Gait kinematics and kinetics were evaluated during graded treadmill assessments at BSL and POST. Clinical measures of gait speed, timed walking distance, balance and balance confidence were measured at BSL, POST and F/U. Between-group comparisons and regression analyses were conducted to predict stepping activity from BSL and POST measurements. Baseline and changes in clinical measures of walking demonstrated selective associations with stepping, although kinematic measures appeared to better discriminate responders. Specific measures suggest greater paretic vs non-paretic kinematic changes in responders with training, although greater non-paretic changes predicted greater gains (i.e., smaller declines) in stepping in retainers at F/U. No kinetic variables were primary predictors of changes in stepping activity at POST or F/U. The combined findings indicate specific biomechanical assessments may help differentiate changes in daily stepping activity post-stroke.
Keywords: Stroke, Gait kinematics, Walking function, Walking ability
1. Introduction
While over 80% of stroke survivors regain some form of ambulation (Butler and Evenson, 2014), persistent impairments in postural stability (Schmid et al., 2012), strength (Patterson et al., 2007), and cardiorespiratory capacity (Michael et al., 2005) contribute to reduced ambulatory function. Many physical interventions can mitigate these impairments (Reisman et al., 2013), which may result in improvements in walking function, such as gait speed and timed distance (Holleran et al., 2014; Leddy et al., 2016). However, improvements in clinical outcomes often do not translate to changes in community walking, measured as daily stepping activity (#steps/day) (Bowden et al., 2013; Nicholson et al., 2017).
Previous research has identified variables that can mediate the relationship between clinical measures of walking and daily stepping activity. Specific variables include neuromuscular and cardiovascular impairments (Danks et al., 2016b), strength and balance (Fulk et al., 2010), as well as socio-demographics (Vahlberg et al., 2018) and psychological variables (Nicholson et al., 2017). However, these variables individually are only able to explain 10–30% of patients’ stepping activity. Few studies have evaluated specific variables that may contribute to changes in stepping activity following interventions (Danks et al., 2016a; Wright et al., 2018). A better understanding of factors that can predict changes in stepping activity following physical rehabilitation may help identify patients in whom more focused interventions are required.
Other variables that may contribute to daily stepping activity include gait biomechanical parameters. Substantial literature has delineated how specific biomechanical variables contribute to locomotor function in controlled (i.e., laboratory) conditions (Awad et al., 2015b; Roelker et al., 2018), although studies detailing their contributions to community activity are sparse (Ciprandi et al., 2017; Danks et al., 2016b; Dawe et al., 2017; Egerton et al., 2017). The biomechanical strategies utilized post-stroke may be important, as some patients can adopt similar strategies used prior to their stroke and can better accommodate to the demands of daily stepping. Conversely, others may adopt different biomechanical strategies, such as greater non-paretic limb use, to compensate for specific gait deficits (Levin et al., 2009). Such compensatory strategies may temporarily enable patients to improve walking function but could lead to reduced walking efficiency and increased gait asymmetry (Awad et al., 2015a; Sanchez and Finley, 2018), which can discourage daily stepping activity. Continued use of compensatory patterns may lead to reduced use of the paretic limb that can attenuate the influence of physical interventions. To date, few studies have evaluated the potential influence of biomechanical gait parameters on changes in community mobility with specific interventions.
The overall goals were two-fold: (1) to identify clinical, demographic, or biomechanical parameters at pre-training (baseline, BSL) that contribute to changes in daily stepping; and, (2) to identify biomechanical or clinical variables following rehabilitation that influence daily stepping. The primary outcomes were short- and long-term changes in daily stepping activity, evaluated following ≤40 training sessions (POST) and at 2–6 months follow-up (F/U). Patients were divided into three groups: (1) patients who demonstrated greater daily stepping at POST and remained active at F/U (responders/retainers); (2) patients who increased stepping at POST but could not sustain changes at F/U (responder/nonretainer); and (3) those who could not achieve greater stepping activity at POST (non-responders). Between-group comparisons were then conducted to identify any pre- and/or post-rehabilitation differences in demographics, clinical and gait biomechanical parameters.
2. Materials and methods
2.1. Patients
The present investigation represents a secondary analysis from two separate studies including a randomized controlled trial (RCT) comparing high intensity stepping training with conventional strategies (Hornby et al., 2016), and a pilot experimental training study that served as the basis for the RCT (Holleran et al., 2014). Inclusion criteria were similar between studies as follows: 1–6 months post-stroke; 18–75 years of age; ability to follow 3-step commands; ability to sit unsupported for at least 30 s; and, the ability to ambulate at least 10 m over ground with no more than moderate physical assistance at a self-selected walking velocity of <1.0 m/s. During walking assessments, bracing below the knee and assistive devices were utilized as needed. Patients were excluded if they had any additional central or peripheral nervous system conditions, cardiorespiratory, metabolic or orthopedic disease that limits walking, and the inability to walk at least 150 feet without assistance prior to their stroke. To evaluate gait biomechanics, an additional inclusion criterion for this analysis was the ability to walk at least 0.1 m/s on a motorized treadmill without weight support but use of a handrail as needed.
Details of the interventions have been described elsewhere (Hornby et al., 2016). The goal of experimental training sessions was to provide focused stepping practice in multiple environments. Each 60-minue training session included 25% forward treadmill walking, 25% variable walking on the treadmill, 25% over ground variable walking, and 25% stair climbing. Subjects who received the experimental training practiced stepping while maintaining cardiovascular intensities between 70 and 80% of heart rate (HR) reserve or ratings of perceived exertion (RPE) (Borg, 1982) of 15–17 on the 6–20 Borg scale. Subjects who received the conventional intervention practiced traditional therapy activities, including balance and strengthening exercises, transfers, and walking practice, with the training intensity maintained at 30–40% of their HR reserve (RPEs of 11–13).
2.2. Data collection
The primary outcomes were changes in daily stepping activity from BSL to POST and POST to 2–6 month F/U, and their potential associations with biomechanical variables collected during treadmill walking assessments performed at BSL and POST. An accelerometer (StepWatch, Modus Inc, Washington, DC) was used to quantify patients’ stepping activity as the average number of steps per day. Daily stepping activity was recorded one week prior to the start of training, for one week immediately after training and at 2–6 months following their enrollment. Patients were instructed to wear the accelerometer on their paretic ankle for 7 consecutive days (up to 90% of waking hours) (Tudor-Locke et al., 2005). At BSL, POST and F/U, clinical assessments included self-selected (SSV) and fastest possible (FV) velocities during over ground walking (Gait-Mat II, Equitest, Chalfont, PA), the 6-minute walk test (6MWT) with instructions to walk at participants’ SSV, the Berg Balance Scale, and the Activities-specific Balance Confidence (ABC) scale (Pang et al., 2007).
Participants performed a graded treadmill test at BSL and POST on a motorized split-belt treadmill embedded with force plates (Bertec Corporation, Columbus, OH), surrounded by an eight-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA) and 32 retroreflective markers were placed bilaterally on the lower limbs using a modified Cleveland Clinic marker set. The graded treadmill test began at 0.1 m/s and increased by 0.1 m/s increments every 2 min until the participants reached 85% age-predicted maximum HR, had evidence of gait instability, or refused to continue. Data were collected for one continuous minute at each speed, beginning 30 sec after increases in speed to allow for speed adjustments. The highest treadmill speed that patients could walk for at least 1 min was considered the peak treadmill speed (TM) for which gait kinematics and kinetics were analyzed.
2.3. Data analyses
Marker and ground reaction force data were filtered (low-pass, 2nd order Butterworth filter, cutoff frequency 10 Hz). Sagittal ankle, knee, and hip joint angles and moments were calculated using Visual3D (C-Motion Incorporated, Germantown, MD). Sagittal plane joint powers were calculated as the product of joint moment and angular velocity. Kinetic data were normalized to body weight for each subject. Kinetic and kinematic data were normalized to percentage of gait cycle (GC) and average step cycle profiles were created.
All biomechanical variables at the peak TM speed were compared between groups at BSL and POST assessments, but also at post-training speeds matched to the peak TM speed at BSL (i.e., MATCH). This was conducted to collect speed-matched trials prior to and following the training and to address the influence of speed on biomechanical variables. Specific kinematic variables included sagittal peak angles and excursions of bilateral hip, knee and ankle joints. Hip-knee joint coordination was also defined as the cyclogram of hip versus knee joint angles and quantified in terms of its stride-to-stride consistency. Consistency was calculated as the angular component of the coefficient of correspondence (ACC) (Field-Fote and Tepavac, 2002; Leech et al., 2016). The ACC ranges from 0 to 1, with higher values indicating greater hip-knee kinematic consistency during walking. Specific kinetic variables included average positive (concentric) hip, knee and ankle joint powers across the entire gait cycle (Fig. 1). Average positive (concentric) powers were focused as these are the primary determinants of locomotor function post-stroke (Jonkers et al., 2009; Nadeau et al., 1999). To evaluate the degree of relative use of the paretic vs non-paretic limb, we also calculated power asymmetry index, determined using the following equation (Hornby et al., 2008):
Fig. 1.
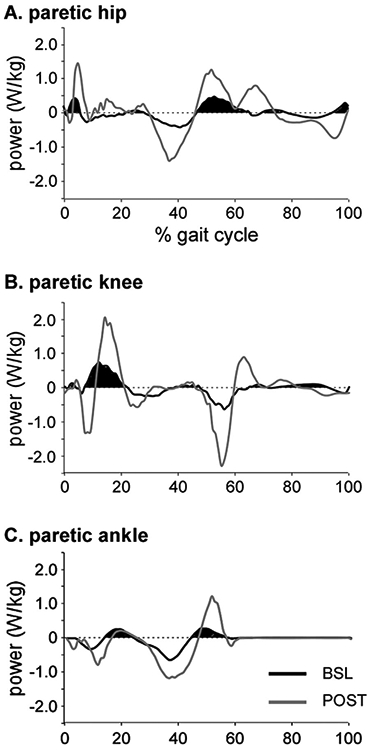
Single-participant example of hip (A), knee (B), and ankle (C) powers throughout the gait cycle at baseline (BSL; black) and post-training (POST; gray). Given the primary contributions of positive (i.e., concentric) joint powers to locomotor function, only average positive powers were used (shaded area). Data is identical to Fig. 1 in Ardestani et al., 2018 NNR.
Given the typically larger power generation of the non-paretic vs paretic powers, values of asymmetry index are normally between 0 and 1, with 0 indicating perfect symmetry.
2.4. Statistical analysis
Participants were initially categorized into two groups; “responders” were patients who increased their walking activity by >500 steps/day at POST (POST-BSL ≥ 500 steps/day). And non-responders did not increase stepping above this threshold. A threshold of 500 steps/day was set to reflect the observed differences in stepping activity following various interventions as reported in other studies. More directly, the average changes in stepping activity following higher intensity walking interventions range from 900 to 1200 steps/day, whereas average changes observed following conventional physical interventions range from 0 to 500 steps/day (Holleran et al., 2014; Hornby et al., 2016; Moore et al., 2010). Without validated estimates of minimum clinically important differences (MCIDs) for stepping activity poststroke, thresholds of 500 steps/day was set as an approximate median for the average highest and lowest changes observed. Responders were further divided into two groups: “responders/retainers” included those who increased daily stepping ≥500 steps/-day post-training (POST) with no decreases in stepping at 2–6 month follow-up (F/U) (F/U-POST ≥ −500 steps/day) and “responders/non-retainers” included those who increased stepping at POST but declined > 500 steps/day at F/U (F/U-POST < −500 steps/day).
One-way analysis of variance (ANOVA) was used for between-group comparisons of stepping activity, clinical measures and gait biomechanics. To avoid type I error, between-group comparisons were limited to initial measurements (BSL) and the observed changes following the training (ΔPOST-BSL and ΔFU-POST). Considering the relatively small size of our study population and the inherent large inter-patient variability in gait biomechanics, between-group comparisons were focused on “responders” vs “non-responders”, and “retainers” vs “non-retainers”. Considering that biomechanical variables (e.g., joint excursions and powers) are speed-dependent (Ardestani et al., 2016), within-group changes were calculated from speed-matched trials and speed was considered as a co-variate for between-group comparisons. Each patient received only one of the two training interventions; i.e., high-intensity training or conventional therapy. To compare the distribution of training paradigm amongst the three groups, Chi-square with 2 degrees of freedom (conventional vs high-intensity) was used. Chi-square was also used to compare the distribution of other categorical variables including the use of AFO (yes or no), gender (male or female) and the affected side (left or right).
Separate multiple-linear regression analyses were also conducted. The first two regressions attempted to predict changes in daily stepping after training (POST-BSL) from clinical measures and gait biomechanics at BSL (GaitBSL → ΔStepping(POST–BSL)). Subsequent analyses focused on the relations between changes in walking function and gait biomechanics (POST-BSL) and changes in daily stepping from BSL to POST (ΔGait(POST–BSL) → ΔStepping(POST–BSL)) and from POST to F/U (ΔGait(POST–BSL) → ΔStepping(FU–POST)). Stepwise linear regressions were utilized for all analyses, with α = 0.05 and variance inflation factors <3.0 to minimize collinearity.
3. Results
Of the 39 participants that met the inclusion criteria (Table 1), 17 were classified as “non-responders” (POST-BSL < 500 steps/day) and the remaining 22 were labeled as responders, with an average increase of 1958 ± 987 steps/day from BSL to POST. Of this latter group, 12 participants maintained their elevated daily stepping at F/U and labeled as “responder/retainers” while 10 demonstrated >500 steps/day decreases in walking activity at F/U (“responder/non-retainers”). There were no significant differences in demographics (Table 1). The number of individuals who received experimental vs conventional training were not significantly different between responder vs non-responder groups (X2 = 1.28, p = 0.26).
Table 1.
Comparison of basic demographics among the three groups.
| Responder/retainer | Responder/non-retainer | Non-responder | p | |
|---|---|---|---|---|
| # Patients (M/F) | 12 (7/5) | 10 (9/1) | 17 (12/5) | |
| Age | 51.58 ± 15.68 | 57.90 ± 9.37 | 59.65 ± 8.19 | 0.168 |
| BMI | 26.51 ± 5.5 | 24.89 ± 3.34 | 29.30 ± 7.78 | 0.340 |
| Days post-stroke | 104.8 ± 50 | 106.3 ± 55 | 94.3 ± 53 | 0.831 |
| Affected side (L/R) | 6/6 | 7/3 | 9/8 | 0.452 |
| AFO (yes/no) | 7/5 | 9/1 | 14/3 | 0.102 |
| Training (exp/con) | 9/3 | 6/4 | 8/8 | 0.260 |
Exp: experimental training (high-intensity training).
Con: Conventional training.
3.1. Stepping activity and clinical variables
All 3 groups demonstrated similar levels of walking function and stepping activity at BSL (Table 2). Following training (POST), stepping activity was significantly improved in both responder/retainers and responder/non-retainers (Δsteps/day = 2047 ± 895 and 1851 ± 1156, respectively), but decreased in non-responders (Δsteps/day = −328 ± 772). Both responder groups showed significantly greater gains in their clinical measures of walking compared to non-responders, including SSV (POST-BSL = 0.30 ± 0.22 vs. 0.12 ± 0.13 m/s, p<0.01), FV (0.44 ± 0.40 vs. 0.15 ± 0.15 m/s, p < 0.01) and 6MWT (120 ± 119 vs. 56 ± 60 m, p = 0.03). Changes in BBS and ABC were not different between responders and non-responders following training (Table 2).
Table 2.
Comparison of patient’s daily stepping activity, clinical walking function and other clinical measures (balance and balance confidence) prior to the training (BSL), immediately after 40 sessions of training (POST) and 6-month following the training (F/U).
| BSL |
Post |
F/U |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Responder/ retainer |
Responder/non- retainer |
Non- responders |
p | Responder/ retainer |
Responder/non- retainer |
Non- responders |
p | Responder/ retainer |
Responder/non- retainer |
Non- responders |
p | |
| Steps/day | 2123 ± 1618 | 1999 ± 1950 | 2185 ± 1810 | 0.96 | 4171 ± 2235 | 3850 ± 2481 | 1856 ± 1600 | 0.00 | 4918 ± 2598 | 2575 ± 1653 | 2387 ± 2276 | 0.01 |
| SSV | 0.38 ± 0.26 | 0.39 ± 0.23 | 0.31 ± 0.24 | 0.61 | 0.75 ± 0.31 | 0.62 ± 0.35 | 0.42 ± 0.28 | 0.02 | 0.78 ± 0.33 | 0.67 ± 0.40 | 0.43 ± 0.28 | 0.02 |
| FV | 0.57 ± 0.41 | 0.50 ± 0.30 | 0.41 ± 0.35 | 0.48 | 1.10 ± 0.57 | 0.87 ± 0.68 | 0.56 ± 0.41 | 0.04 | 1.08 ± 0.61 | 0.89 ± 0.60 | 0.55 ± 0.39 | 0.03 |
| 6MWD | 139.78 ± 99.34 | 147.15 ± 90.02 | 110.66 ± 98.33 | 0.54 | 275.67 ± 143.05 | 253.29 ± 174.04 | 166.58 ± 114.44 | 0.10 | 263.52 ± 128.73 | 262.60 ± 158.86 | 147.35 ± 113.51 | 0.04 |
| BBS | 35.83 ± 15.66 | 33.50 ± 10.90 | 31.41 ± 15.33 | 0.70 | 43.92 ± 9.26 | 40.90 ± 10.34 | 37.35 ± 12.52 | 0.29 | 44.91 ± 7.06 | 42.10 ± 8.88 | 38.81 ± 11.74 | 0.29 |
| ABC | 57.77 ± 20.78 | 58.57 ± 26.01 | 48.96 ± 15.23 | 0.39 | 72.61 ± 21.75 | 75.21 ± 26.19 | 63.67 ± 18.79 | 0.37 | 74.42 ± 30.90 | 71.66 ± 18.78 | 63.25 ± 16.38 | 0.48 |
SSV: Self-selected normal velocity over-ground.
FV: Fast walking velocity over-ground.
6MWD: Six-minute test walking distance.
BBS: Berg balance scale.
ABC: Activities-specific Balance Confidence.
At F/U, changes in stepping activity between the two responder groups varied (p = 0.003); non-retainers reduced their daily stepping at F/U (FU-POST = −1275 ± 1306 steps/day) while retainers increased their stepping activity (746 ± 984 steps/day). However, there were no differences in SSV (FU-POST = 0.03 ± 0.10 vs 0.05 ± 0.20 m/s, p = 0.58), FV (0.02 ± 0.13 vs. 0.01 ± 0.22 m/s, p = 0.94), and 6MWT (11 ± 23 vs. 9.3 ± 56 m, p = 0.92) between responder/retainer and responder/non-retainer groups, respectively.
3.2. Biomechanical assessments during treadmill testing
3.2.1. Baseline gait kinematics
During graded treadmill testing at BSL, nearly two-fold differences in peak TM speed were observed between responders and non-responders (0.60 ± 0.33 vs 0.36 ± 0.30 m/s, p = 0.04). Evaluation of specific biomechanical variables also revealed significant differences between groups (Table 3). After controlling for speed differences, responders vs. non-responders showed greater non-paretic peak plantarflexion (−3.1 ± 8.2° vs 3.3 ± 6.0°; p = 0.02) and non-paretic knee excursion (58.4 ± 5.3° vs. 54.1 ± 5.6°; p = 0.04). In addition, differences in paretic hip extension (−9.1 ± 9.5° vs. 4.4 ± 15.2°, p < 0.01) and knee extension (−3.4 ± 7.6° vs. −10.9 ± 11.2°; p = 0.03) were observed in responders vs non-responders.
Table 3.
Summary of gait biomechanics prior to training (BSL). Statistically significant between-group differences are marked with *.
| BSL | |||||||
|---|---|---|---|---|---|---|---|
| Responders | Non-responders | p | Retainers | Non-retainers | p | ||
| Paretic | TM (m/sec) | 0.60±0.33 | 0.36±0.30 | 0.040 | 0.68±0.33 | 0.50±0.33 | 0.220 |
| Ankle excursion(°) | 9.76±7.69 | 14.53±10.06 | 0.154 | 7.08±4.73 | 14.24±9.94 | 0.059 | |
| Ankle dorsiflexion(°) | 14.61±4.51 | 15.85±7.26 | 0.548 | 16.04±3.24 | 13.0±5.35 | 0.151 | |
| Ankle plantar flexion(°) | 4.98±4.79 | 6.50±5.25 | 0.400 | 4.92±6.05 | 5.04±3.48 | 0.962 | |
| Knee excursion(°) | 34.01±13.01 | 42.42±12.50 | 0.083 | 31.21±8.82 | 38.68±18.04 | 0.281 | |
| Knee extension(°) | −3.40±7.60 | −10.90±11.20 | 0.030* | −3.52±8.32 | −3.19±7.36 | 0.930 | |
| Knee flexion(°) | −41.96±11.43 | −39.82±14.35 | 0.637 | −41.68±9.66 | −42.27±13.74 | 0.915 | |
| Hip excursion(°) | 28.51±8.65 | 34.83±9.44 | 0.066 | 27.35±8.96 | 30.44±8.54 | 0.509 | |
| Hip extension(°) | −9.10±9.50 | 4.40±15.20 | 0.004* | −7.35±8.30 | −11.03±10.87 | 0.416 | |
| Hip flexion(°) | 22.90±11.18 | 27.00±14.26 | 0.360 | 22.93±11.53 | 22.86±11.47 | 0.990 | |
| ACC | 0.83±0.11 | 0.80±0.15 | 0.556 | 0.82±0.13 | 0.83±0.08 | 0.837 | |
| Ankle power(W/kg) | 0.034±0.016 | 0.023±0.025 | 0.192 | 0.035±0.018 | 0.034±0.013 | 0.947 | |
| Knee power(W/kg) | 0.062±0.029 | 0.041±0.035 | 0.131 | 0.051±0.023 | 0.078±0.033 | 0.118 | |
| Hip power(W/kg) | 0.078±0.05 | 0.042±0.040 | 0.096 | 0.067±0.050 | 0.095±0.050 | 0.342 | |
| Non-paretic | Ankle excursion(°) | 21.02±10.54 | 17.28±10.89 | 0.329 | 19.30±11.00 | 22.93±10.30 | 0.470 |
| Ankle dorsiflexion(°) | 18.81±7.59 | 23.09±5.56 | 0.072 | 18.63±5.50 | 19.03±9.78 | 0.913 | |
| Ankle plantar flexion(°) | −3.10±8.20 | 3.30±6.00 | 0.020* | −2.41±10.31 | −3.90±5.47 | 0.705 | |
| Knee excursion(°) | 58.40±5.30 | 54.10±5.60 | 0.040* | 57.12±6.50 | 59.68±3.82 | 0.325 | |
| Knee extension(°) | −7.90±6.83 | −9.45±6.18 | 0.508 | −6.40±8.54 | −9.57±4.12 | 0.327 | |
| Knee flexion(°) | −65.80±8.58 | −62.56±7.5 | 0.268 | −62.69±9.86 | −69.25±5.55 | 0.097 | |
| Hip excursion(°) | 41.76±6.28 | 39.54±9.60 | 0.448 | 40.45±7.63 | 43.07±4.66 | 0.392 | |
| Hip extension(°) | −10.58±12.42 | −6.71±15.05 | 0.424 | −10.41±12.04 | −10.17±13.57 | 0.952 | |
| Hip flexion(°) | 30.71±11.17 | 30.94±12.98 | 0.958 | 29.28±10.47 | 32.30±12.34 | 0.572 | |
| ACC | 0.90±0.11 | 0.91±0.09 | 0.88±0.13 | 0.92±0.06 | 0.358 | ||
| Ankle power (W/kg) | 0.153±0.132 | 0.097±0.064 | 0.216 | 0.174±0.156 | 0.118±0.085 | 0.480 | |
| Knee power (W/kg) | 0.100±0.111 | 0.077±0.068 | 0.565 | 0.096±0.126 | 0.107±0.097 | 0.867 | |
| Hip power (W/kg) | 0.143±0.071 | 0.083±0.087 | 0.077 | 0.124±0.060 | 0.174±0.083 | 0.231 | |
ACC: Angular component of the coefficient of correspondence.
3.2.2. Changes in gait kinematics following training
Following training, changes in peak TM speed were not different between responders vs non-responders (POST-BSL = 0.42 ± 0.35 vs 0.31 ± 0.24 m/s; p = 0.27). However, gait biomechanics changed differently between the two groups (Table 4). Specific between-group differences included greater increases in non-paretic ankle excursion (p = 0.04), greater changes in paretic hip extension (p = 0.03) and greater, but non-significant, paretic hip flexion (p = 0.07) in responders vs non-responders. Further differences were observed between paretic vs non-paretic limbs within each group. For example, between-limb comparisons in the responder group indicated greater paretic vs non-paretic changes in joint excursions (Fig. 2A) and ACC (Table 4). Changes in paretic ACC were significantly greater than changes in non-paretic ACC (POST-BSL = 0.08 ± 0.10 vs. 0.05 ± 0.11; p = 0.02). Conversely, between-limb comparisons in non-responders showed greater improvements in non-paretic vs paretic joint excursions (Fig. 2B, Table 4), with changes primarily in non-paretic vs paretic ankle excursion (POST-BSL = 6.7 ± 5.2° vs. 2.6 ± 4.7°, p = 0.02).
Table 4.
Comparison of changes in gait biomechanics from BSL to POST between responders and non-responders, and between retainers versus non-retainers.
| ΔPOST- BSL | ΔPOST- BSL | ||||||
|---|---|---|---|---|---|---|---|
| Responders | Non-responders | p | Retainer | Non-retainer | p | ||
| Paretic | TM (m/sec) | 0.43±0.36 | 0.31±0.24 | 0.255 | 0.50±0.39 | 0.35±0.32 | 0.310 |
| Ankle excursion (°) | 2.81±4.59 | 2.60±4.70 | 0.902 | 1.15±3.10 | 5.56±5.59 | 0.060 | |
| Ankle dorsiflexion(°) | −0.48±8.29 | 0.49±3.02 | 0.676 | 0.83±10.74 | −1.90±4.49 | 0.479 | |
| Ankle plantar flexion(°) | −4.41±7.58 | −0.85±4.15 | 0.123 | −4.05±8.68 | −4.82±6.65 | 0.833 | |
| Knee excursion(°) | 5.48±9.02 | 1.05±7.16 | 0.152 | 5.73±8.95 | 5.06±9.98 | 0.891 | |
| Knee extension(°) | −0.98±8.20 | 2.98±6.61 | 0.150 | −2.20±9.25 | 0.37±7.35 | 0.514 | |
| Knee flexion(°) | −3.32±10.75 | −0.45±5.71 | 0.330 | −5.63±12.04 | −0.75±9.11 | 0.337 | |
| Hip excursion(°) | 8.18±9.56 | 5.85±6.50 | 0.448 | 11.14±10.10 | 3.24±6.63 | 0.081 | |
| Hip extension(°) | 0.50±10.77 | −9.15±13.09 | 0.030* | −3.90±11.17 | 5.41±8.31 | 0.054 | |
| Hip flexion(°) | 7.18±8.10 | 0.10±14.00 | 0.070* | 5.15±7.72 | 9.43±8.42 | 0.268 | |
| ACC | 0.08±0.10 | 0.069±0.12 | 0.730 | 0.095±0.12 | 0.068±0.07 | 0.588 | |
| Ankle power(W/kg) | −0.006±0.023 | −0.006±0.015 | 0.940 | 0.06±0.07 | 0.01±0.03 | 0.175 | |
| Knee power(W/kg) | −0.015±0.028 | −0.004±0.031 | 0.394 | 0.08±0.09 | 0.10±0.10 | 0.774 | |
| Hip power(W/kg) | −0.009±0.030 | −0.015±0.028 | 0.683 | 0.07±0.10 | 0.14±0.12 | 0.273 | |
| Non-paretic | Ankle excursion(°) | 3.08±4.35 | 6.70±5.20 | 0.040* | 3.70±4.31 | 2.46±4.55 | 0.566 |
| Ankle dorsiflexion(°) | −0.30±4.450 | −0.21±4.85 | 0.965 | −0.57±3.60 | 0.022±5.56 | 0.783 | |
| Ankle plantar flexion(°) | −3.79±4.37 | −6.51±6.27 | 0.151 | −4.99±4.74 | −2.44±3.72 | 0.214 | |
| Knee excursion(°) | 2.17±8.75 | 4.23±6.80 | 0.498 | 3.71±11.33 | 0.63±5.40 | 0.472 | |
| Knee extension(°) | 0.25±7.00 | 1.45±8.54 | 0.660 | −0.21±6.80 | 0.78±7.58 | 0.766 | |
| Knee flexion(°) | −2.87±9.35 | −2.21±8.66 | 0.838 | −5.58±11.04 | 0.14±6.35 | 0.190 | |
| Hip excursion(°) | 6.48±9.33 | 9.00±9.08 | 0.472 | 8.41±6.34 | 4.55±11.67 | 0.396 | |
| Hip extension(°) | −0.86±8.76 | −7.34±14.18 | 0.115 | −4.55±9.66 | 3.24±5.63 | 0.050 | |
| Hip flexion(°) | 5.25±10.42 | 0.21±16.91 | 0.299 | 2.96±7.60 | 7.79±12.87 | 0.327 | |
| ACC | 0.05±0.11 | 0.035±0.050 | 0.604 | 0.084±0.12 | 0.015±0.07 | 0.184 | |
| Ankle power(W/kg) | −0.023±0.155 | −0.033±0.056 | 0.852 | 0.05±0.30 | 0.21±0.30 | 0.382 | |
| Knee power(W/kg) | −0.016±0.152 | −0.032±0.072 | 0.744 | −0.05±0.13 | 0.10±0.28 | 0.200 | |
| Hip power(W/kg) | −0.022±0.052 | −0.026±0.037 | 0.837 | 0.06±0.09 | 0.16±0.10 | 0.093 | |
ACC: Angular component of the coefficient of correspondence.
Fig. 2.
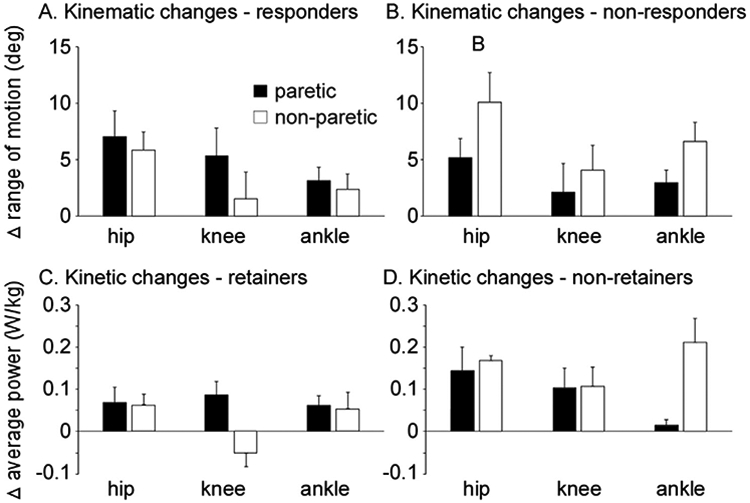
Changes in paretic and non-paretic kinematics in responders (A) and non-responders (B) following rehabilitation interventions, and changes in paretic and non-paretic kinetics in responders/retainers (C) and responders/non-retainers (D).
3.2.3. Changes in gait kinetics following training
Following training (POST), changes in joint kinetics were not different between responders and non-responders, although kinetic variables were altered differentially between the responder/retainers vs. responder/non-retainers. Surprisingly, the primary findings were that non-retainers vs retainers tended to generate larger improvements in non-paretic joints. For example, greater gains in non-paretic ankle (0.21 ± 0.3 vs 0.05 ± 0.30 W/Kg), knee (0.10 ± 0.28 vs −0.05 ± 0.13 W/Kg), and hip power (0.16 ± 0.10 vs 0.06 ± 0.09 W/kg) were observed in non-retainers vs retainers at POST (Table 4). Conversely, greater changes in paretic ankle power was observed in retainers vs. non-retainers (0.06 ± 0.07 vs 0.01 ± 0.03 W/Kg), although these differences were not significant (Table 4). Between-limb comparisons in the retainer group indicated greater kinetic changes in paretic vs non-paretic joints (Fig. 2C, Table 4). Conversely, between-limb comparisons in non-retainers showed greater improvements in non-paretic vs paretic joint powers (Fig. 2D, Table 4). Accordingly, post-training interlimb asymmetry changed in opposite directions in retainer vs non-retainer groups. Retainers showed improved symmetrical power generation (i.e., less asymmetry and smaller asymmetry index) at the ankle (BSL = 0.68 + 0.34 vs POST = 0.27 ± 0.93) while non-retainers increased ankle asymmetry (BSL = 0.58 ± 0.22 vs POST = 0.83 ± 0.15). Between group differences confirmed that non-retainers vs retainers became significantly asymmetric in terms of ankle concentric power (POST-BSL = 0.25 ± 0.35 vs. −0.41 ± 0.6; p = 0.03) with negative changes indicating a reduction in asymmetry (improvement in symmetry).
3.3. Association between gait biomechanics and stepping activity
Multiple linear regression analyses were utilized to estimate short- (POST-BSL) and long-term (FU-POST) changes in stepping activity from clinical measures of walking and gait biomechanics. Regression analyses highlighted a significant association between peak TM speed at BSL and improvements in stepping activity at POST (r = 0.39, p = 0.02; Eq. (1.1), explaining 16% of the variance in changes in daily stepping, with no other BSL clinical variables contributing significantly to the regression. Conversely, incorporation of gait biomechanical variables into a separate stepwise regression revealed that kinematic parameters explained 55% of the variance. Specific contributors included a positive association with non-paretic knee joint excursion, and negative associations with BSL paretic ankle joint excursion and non-paretic plantar flexion (Eq. (1.2)). There were no significant associations between BSL gait kinetics and changes in daily stepping from BSL to POST training.
| (1.1) |
| (1.2) |
Following training, changes in specific clinical waking measures, including SSV and 6MWT, explained up to 33% of changes in stepping activity (Eq. (2.1)). However, changes in gait biomechanics explained up to 86% of the variance in altered daily stepping, with specific positive associations with paretic hip extension and total excursion, and negative correlations with non-paretic hip flexion (Eq. (2.2)). Again, no kinetic variables assisted in the prediction of daily stepping outcomes at POST.
| (2.1) |
| (2.2) |
We also evaluated changes in clinical and biomechanical variables with training that predicted changes in daily stepping from POST to F/U. Regression analyses revealed that changes in clinical measures were not significantly related to stepping changes from POST to F/U. In contrast, daily stepping was negatively associated with paretic ankle excursion, and positively associate with non-paretic knee excursion, explaining 60% of changes in stepping activity from POST to F/U. Changes in individual joint powers were not associated with retention of changes in stepping at F/U.
| (3) |
The combined regression analyses are presented in Table 5.
Table 5.
Summary of regression analyses highlighting changes in clinical and biomechanical variables that predict stepping activity following training.
| Clinical variables | Biomechanical variables | |
|---|---|---|
| GaitBSL → ΔStepping(POST–BSL) | ||
| ↑Peak treadmill speed | ↑Non-paretic knee ROM ↓Paretic ankle ROM ↓Non-paretic peak plantarflexion |
|
| ΔGait(POST–BSL) → ΔStepping(POST–BSL) | ||
| ↑Δself-selected velocity ↓Δ6 min walk distance |
↑Δparetic hip extension ↓Δparetic hip ROM ↓Δnon-paretic hip flexion |
|
| ΔGait(POST–BSL) → ΔStepping(FU–POST) | ||
| N/A | ↓Δparetic ankle ROM ↑Δnon-paretic knee ROM |
4. Discussion
Previous studies have investigated changes in gait biomechanics following stroke and whether physical rehabilitation can positively influence these changes (Mahtani et al., 2016; Reisman et al., 2013). Most studies focus on regaining mobility and community activity often do not detail the potential biomechanical strategies underlying walking, and those factors that influence changes in physical activity (Danks et al., 2016a; Danks et al., 2016b; Fulk et al., 2010; Wright et al., 2018). Studies investigating the relationship between gait biomechanics and physical activity are scarce (Ciprandi et al., 2017; Egerton et al., 2017) and no previous study has identified biomechanical variables of prognostic value. This pilot investigation provides evidence that selected gait kinematics may facilitate our understanding of patient-specific variables that contribute to enhanced stepping activity following rehabilitation.
Consistent with other studies (Bowden et al., 2013; Danks et al., 2016b), baseline or changes in walking capacity as measured by standard clinical outcomes appear to contribute to gains in daily stepping. For example, greater peak treadmill speed at BSL was able to differentiate patients’ stepping changes, despite similarities in other BSL clinical measures. Changes in other clinical walking outcomes were also important, however, and were significantly different between responders and non-responders. With responders at F/U, however, both retainers and non-retainers achieved similar improvements in clinical walking measures from BSL to POST, but demonstrated a marked divergence in daily stepping from POST to F/U. Measures of gait kinematics and kinetics, including the relatively greater use of paretic vs non-paretic limbs, appeared to explain some of these differences in changes in daily stepping activity at POST and F/U assessments, although regression analyses suggest greater contributions for gait kinematics, emphasizing the potential role of specific biomechanical variables on community mobility.
The specific kinematic variables that contributed to changes in stepping at POST and F/U are of additional interest. Attempts to predict changes in daily stepping at POST indicate greater paretic limb extension and reduced non-paretic hip flexion, suggesting greater use of strategies consistent with recovery vs compensation. However, calculated regressions using BSL kinematics to predict changes at POST with training, and changes in kinematics to predict altered stepping at F/U, indicate greater non-paretic knee ROM and reduced paretic ankle ROM both play significant roles. An explanation for these findings are not entirely clear but suggest that training-induced changes may require greater paretic limb use to achieved increased daily stepping. However, predictive models of long-term changes may suggest that those individuals who use selected compensatory strategies (greater non-paretic vs paretic limb use) may be able to better accommodate to the community stepping demands. This interpretation is speculative, and further work in additional populations is needed to confirm the findings.
While the relative contributions of the paretic vs non-paretic limb kinematics to daily stepping are of interest, many other studies suggest joint kinetics, rather than kinematics, are greater contributors to locomotor function. Differences in kinetic patterns were certainly observed between selected population subgroups, but the lack of significant contributions of kinetics in the regression analyses were surprising. One potential explanation is that community stepping activity, while dependent on gait kinetics, does not precisely measure the same construct as other measures of walking function (speed) that are more related to joint kinetics. The relatively greater importance of gait kinematics may represent an increased ability to navigate various task- or environment-dependent barriers or conditions, although precise explanations are uncertain.
Limitations of this study include the small sample size and absence of a blinded clinical examiner during treadmill assessments, although all tests utilized standardized criteria. Prospective analysis of a greater sample size is required to further investigate the relative contributions of clinical and/or biomechanical factors on changes in stepping activity post-stroke. A related limitation is the combination of interventions utilized to achieve this sample size, as therapies provided may have contributed to non-significant differences in walking outcomes that may have influenced the physical activity of individual participants. An additional limitation is the use of a specific threshold of 500 steps/day to classify participants. This threshold was an approximate median of changes in stepping activity following other studies and from changes from BSL to POST training observed in the present study (Macko et al., 2005). The primary concerns are the lack of validation that 500 steps represent either clinically important difference to patients post-stroke, or a statistically relevant difference for stepping activity, particularly given the tremendous variability in stepping activity observed across studies. Despite this limitation, the regression analyses were conducted using continuous variables and the chosen threshold does not affect the regression results.
Additional limitations include data collection on a motorized TM with use of handrails, which may alter selected locomotor strategies as compared with over ground walking, even with encouragement to minimize handrail use. Another limitation is including patients from two separate training paradigms, which differed in both tasks practiced and cardiovascular intensity. These factors contributed to differences in clinical measures of walking function but not differences in daily stepping but may have contributed to differences in selected patients. Further data is required to differentiate potential contributions of training strategies on daily stepping.
In summary, patients may adopt different walking strategies to regain their walking function throughout the training. The use of compensatory strategies (greater use of non-paretic vs paretic limb) may temporarily enable patients regaining walking function but it may not necessarily enhance daily stepping activity in long-term. Gait biomechanics seems to regulate the relation between walking function and stepping activity.
Acknowledgement
Funding for this study was provided by National Institute on Disability and Rehabilitation Research grants H133B031127 and H133B140012 and the Bullock Foundation.
Footnotes
Conflicts of interest statement
Authors do not have any conflict of interest to declare.
References
- Ardestani MM, Ferrigno C, Moazen M, Wimmer MAJG, 2016. From normal to fast walking: impact of cadence and stride length on lower extremity joint moments. Gait Posture 46, 118–125. [DOI] [PubMed] [Google Scholar]
- Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS, 2015a. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehab. Neural Re 29, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DSJN, 2015b. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehab. Neural Re 29, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G, 1982. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med 3, 153–158. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA, 2013. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Arch. Phys. Med. Rehabil 94, 856–862. [DOI] [PubMed] [Google Scholar]
- Butler EN, Evenson KR, 2014. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Top. Stroke Rehab 21, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciprandi D, Bertozzi F, Zago M, Ferreira CLP, Boari G, Sforza C, Galvani C, 2017. Study of the association between gait variability and physical activity. Eur. Rev. Aging Phys. Act 14, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks KA, Pohlig R, Reisman DS, 2016a. Combining fast-walking training and a step activity monitoring program to improve daily walking activity after stroke: a preliminary study. Arch. Phys. Med. Rehabil 97, S185–S193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks KA, Pohlig RT, Roos M, Wright TR, Reisman DS, 2016b. The relationship between walking capacity, biopsychosocial factors, self-efficacy and walking activity in individuals post stroke. J. Neurol. Phys. Ther.: JNPT 40, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe RJ, Leurgans SE, Yang J, Bennett JM, Hausdorff JM, Lim AS, Gaiteri C, Bennett DA, Buchman AS, 2017. Association between quantitative gait and balance measures and total daily physical activity in community-dwelling older adults. J. Gerontol.: Ser. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton T, Paterson K, Helbostad JL, 2017. The association between gait characteristics and ambulatory physical activity in older people: a cross-sectional and longitudinal observational study using generation 100 data. J. Aging Phys. Act 25, 10–19. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Tepavac D, 2002. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys. Ther 82, 707–715. [PubMed] [Google Scholar]
- Fulk GD, Reynolds C, Mondal S, Deutsch JE, 2010. Predicting home and community walking activity in people with stroke. Arch. Phys. Med. Rehab 91, 1582–1586. [DOI] [PubMed] [Google Scholar]
- Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG, 2014. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehab. Neural Re 28, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR, 2008. Enhanced gait-related improvements after therapist-versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 39, 1786–1792. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, Woodward J, Mahtani G, Lovell L, Roth EJ, 2016. Variable intensive early walking poststroke (VIEWS) a randomized controlled trial. Neurorehab. Neural Re 30, 440–450. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Delp S, Patten C, 2009. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture 29, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy AL, Connolly M, Holleran CL, Hennessy PW, Woodward J, Arena RA, Roth EJ, Hornby TG, 2016. Alterations in aerobic exercise performance and gait economy following high intensity dynamic stepping training in persons with subacute stroke. J. Neurol. Phys. Ther.: JNPT 40, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech KA, Kinnaird CR, Holleran CL, Kahn J, Hornby TG, 2016. Effects of locomotor exercise intensity on gait performance in individuals with incomplete spinal cord injury. Phys. Ther 96, 1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL, 2009. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehab. Neural Re 23, 313–319. [DOI] [PubMed] [Google Scholar]
- Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP, 2005. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke 36, 2206–2211. [DOI] [PubMed] [Google Scholar]
- Mahtani GB, Kinnaird CR, Connolly M, Holleran CL, Hennessy PW, Woodward J, Brazg G, Roth EJ, Hornby TG, 2016. Altered sagittal-and frontal-plane kinematics following high-intensity stepping training versus conventional interventions in subacute stroke. Phys. Ther 97, 320–329. [DOI] [PubMed] [Google Scholar]
- Michael KM, Allen JK, Macko RF, 2005. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch. Phys. Med. Rehabil 86, 1552–1556. [DOI] [PubMed] [Google Scholar]
- Moore JL, Roth EJ, Killian C, Hornby TG, 2010. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke 41, 129–135. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D, 1999. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin. Biomech 14, 125–135. [DOI] [PubMed] [Google Scholar]
- Nicholson S, Greig C, Sniehotta F, Johnston M, Lewis S, McMurdo M, Johnston D, Scopes J, Mead G, 2017. Quantitative data analysis of perceived barriers and motivators to physical activity in stroke survivors. J. Roy. College Phys. Edinburgh [DOI] [PubMed] [Google Scholar]
- Pang MY, Eng JJ, Miller WC, 2007. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Phys. Ther 87, 282–291. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, Macko RF, 2007. Determinants of walking function after stroke: differences by deficit severity. Arch. Phys. Med. Rehabil 88, 115–119. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Kesar TM, Perumal R, Roos MA, Rudolph KS, Higginson J, Helm E, Binder-Macleod S, 2013. Time course of functional and biomechanical improvements during a gait training intervention in persons with chronic stroke. J. Neurol. Phys. Ther.: JNPT 37, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelker SA, Bowden MG, Kautz SA, Neptune RR, 2018. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: a review. Gait Posture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez N, Finley JM, 2018. Individual differences in locomotor function predict the capacity to reduce asymmetry and modify the energetic cost of walking poststroke. Neurorehab. Neural Re 32, 701–713. [DOI] [PubMed] [Google Scholar]
- Schmid AA, Van Puymbroeck M, Altenburger PA, Dierks TA, Miller KK, Damush TM, Williams LS, 2012. Balance and balance self-efficacy are associated with activity and participation after stroke: a cross-sectional study in people with chronic stroke. Arch. Phys. Med. Rehabil 93, 1101–1107. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Burkett L, Reis J, Ainsworth B, Macera C, Wilson D, 2005. How many days of pedometer monitoring predict weekly physical activity in adults? Prev. Med 40, 293–298. [DOI] [PubMed] [Google Scholar]
- Vahlberg B, Bring A, Hellström K, Zetterberg L, 2018. Level of physical activity in men and women with chronic stroke. Physiother. Theory Pract, 1–9 [DOI] [PubMed] [Google Scholar]
- Wright H, Wright T, Pohlig RT, Kasner SE, Raser-Schramm J, Reisman D, 2018. Protocol for promoting recovery optimization of walking activity in stroke (PROWALKS): a randomized controlled trial. BMC Neurol. 18, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]


