To the Editor:
Recipients of allogeneic hematopoietic stem cell transplantation (AlloHCT) face numerous life-threatening complications, and even more perhaps not so severe but also incapacitating complications. Among the latter, pure red blood cell aplasia (PRCA) due to persistent anti-donor hemagglutinins in the setting of major ABO-mismatched transplantation is one of the most difficult to manage by most clinicians. We thus welcomed the two splendid manuscripts published concomitantly in the April issues of Bone Marrow Transplantation by Marco-Ayala et al. [1] and British Journal of Haematology by Longval et al. [2]. The manuscript by Marco-Ayala et al. is the most extensive review on all published articles on PRCA, covering its incidence, risk factors, heterogeneous natural history, and the numerous treatments tested. The study by Longval et al. on the other hand is a multicenter study from Paris and the largest and most detailed cohort study including 631 recipients of major ABO-incompatible alloHSCT; this study, of course, was not included in the review by Marco-Ayala et al. Both articles suggest that, although major ABO-incompatible alloHSCT does not appear to have a relevant impact on overall survival, none of the treatments tested so far appeared to modify its natural history, and both articles and an accompanying editorial [3] suggested that daratumumab appears to be the most promising treatment, although based on only 4 published case reports [4–7]. We herein report two further cases of successful treatment of PRCA with daratumumab and suggest that it appears to be the best available treatment for this complication, as suggested by the characteristics of the ten successfully treated patients reported to date.
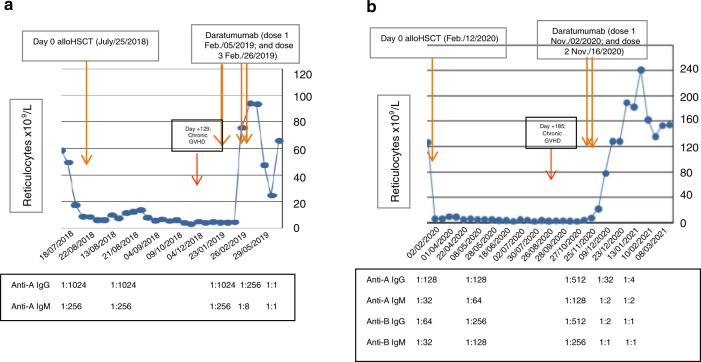
Case 1. A 77-year-old woman with secondary AML in CR received an alloRIC based on fludarabine-busulphan and sirolimus plus tacrolimus as GVHD prophylaxis [8]. Details important for this discussion are summarized in Fig. 1a. Due to major ABO mismatch (Patient 0 positive and Donor A positive with high pre-alloHSCT isohemagglutinings [IsoHG] titers), she developed post-engraftment PRCA, which was not treated until day +205, when she received treatment with daratumumab following the report by Chapuy et al. [4], as detailed in Table 1. Prompt and brisk reticulocytosis occurred 2 weeks after the first dose and one week after the second dose. Response was long-lasting (2.5 years now), and the only side effect was a 70% reduction of IgG, IgA and IgM, which required monthly IVIG treatment for 9 months.
Fig. 1. Graphic sumary of our two patients treated with daratumumab for PRCA.
a, b. Reticulocyte count shown graphically post-transplant in our two case reports with times of day 0 and treatment with daratumumab shown with arrows. The anti-donor isohemagglutinin titers in each patient post-transplant are shown below each figure (only a few representative titers are shown for the sake of simplicity). a Corresponds to case #1 and b to case #2.
Table 1.
Details of the eight case reports reported to date (August 2021) and the two cases herein summarized on the use daratumumab for PRCA after major ABO-mismatched allogeneic HSCT and some characteristics supporting the recommendations made by the authors for its potentially most optimal use.
| Reference (year) | At least > 2 months post-transplantation | Complete donor engraftment (>95%) in myeloid (and T cells > 50%) | PRCA as major component of graft dysfunction | Post-engraftment presence of high titers of anti-donor IsoHG (IgG/IgM ≥ 1/64) | Active GVHD | Dose of daratumumab after which reticulocytosis occurreda | Total number of doses given | Disappearance of IsoHG documented (timing) | Duration of response w/o further treatment | Hypogamma. reported as side effects (and details) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chapuy (2018) [4] | Yes (day +390)b | Yes (from day +25) | Yes | Yes (Anti-A titer 1:128) | NR | 2nd dose | 6 (weekly) | Undetectable at 3 months | >1 year | NR |
| Bathini (2019) [5] | Yes (day +411)b | Yes (from day +30) | Yes | Yes (Anti-A IgG titer 1:256) | NR | 1st dose | 4 (weekly) | Anti-A IgG/IgM <1:8 at 4 months | 20 monthsd | NR |
| Queralt-Salas (2020) [6] | Yes (+32 months)b | Yes (from day +30) | Yes | Yes (Anti-A titer 1:128; and anti-B titer 1:64) | NR | 6th dose | 6 (weekly) | NR | 1 year | NR |
| Rautenberg (2020) [7] | Yes (day +206)b | Yes (from day +28) | Yes | Yes (Anti-A titer 1:1024) | NR | 1st dose | 2 (10 day interval) | Undetectable 2 weeks after 2nd dose | 19 monthsd | NR |
| Jeyaraman (2021) [14] | Yes (day +163)b | Yes (from day +30) | Yes | Yes (Anti-B IgG/IgM ≥1:512; Anti-A IgG/IgM ≥1:128) | No GVHD | 3rd dose | 4 (weekly) | Undetectable but timing NR | 4 months | NR |
| Yates (2021) [15] | Yes (+13 months) | Yes (from day +25)c | Yes | Yes (Anti-A titer ≥ 1:64) | No GVHD | 1st dose | 3 (weekly) | Anti-A 1:4 after dose 1 and undetectable after dose 3 | 11 months | Requires monthly IVIG, but relationship to dara. NR |
| Bicsko (2021) [16] | Yes (day +60)b | Yes (from day +30) | Yes | Yes (Anti-A titer 1:128) | NR | 2nd dose | 4 (from +60 to +96) | Undetectable but timing NR | NR | NR |
| Henig (2021) [17] | Yes (day +320)b | Yes (from day +42) | Yes | Yes (Anti-A titer 1:512) | No GVHD | 2nd dose | 5 (weekly) | Anti-A 1:64 after dose 1 and undetectable after dose 4 | 12 months and ongoing | None. Mild COVID-19 at 6 months |
| Sant Pau case #1 | Yes (day +205)b | Yes (100% myeloid on day +30; day +155 for T-cells) | Yes | Yes (Anti-A IgG 1:1024; IgM 1:256) | Yes. Mild cGVHD from day +129 | 2nd dose (2 weeks after first and 1 week after second) | 3 (weekly) | Yes (After dose 2 Anti-A IgG 1:256; IgM 1:32; 3 weeks after dose 3 both undetectable) | 2.5 years and ongoing | Yes, IgG and IgA dropped <300 and <20 mg/dL at 3 months and normalized at 22 months, post-COVID-19 |
| Sant Pau case #2 | Yes (day +270)b | Yes (100% myeloid on day +39; day +186 for T-cells) | No. Patient also had platelet count < 30 × 109/L pre-daratumumab | Yes (Anti-A and Anti-B IgG both 1:512; and IgG 1:128 and 1:256) | Yes. Moderate cGVHD from day +185 | 2nd dose (4 weeks after first and 2 after second). Platelets also rose after second dose. | Two (2 week interval) | Yes (At 7 weeks Anti-A IgG 1:32 and Anti-B IgG 1:2; at 12 weeks 1:4 and 1:1) | 9 months and ongoing | Yes, IgG still <300 mg/dL 9 months later. IgA normal. |
GVHD graft-versus-host disease, IsoHG isohemagglutinins, Hypogamma. hypogammaglobulinemia, cGVHD chronic graft-versus-host disease, NA not reported, dara.=daratumumab.
aThe dose of daratumumab used in all cases wa 16 mg/kg intravenously daily (with standard premedication) weekly or every 2 weeks.
bPatient received (and failed) multiple treatments for PRCA before daratumumab, and all treatments were started after day +60 post transplant.
cChimerism of isolated bone marrow nucleated red blood cell precursors (CD71+ cells) showed 76% donor origin
dThese patients’ follow-up was updated by Yates et al. [15] after feedback from the authors of the original published case report.
Case 2. A 62-year-old woman with high-risk MDS received an alloRIC based on low dose thiotepa-fludarabine-busulphan and post-transplant cyclophosphamide plus tacrolimus as GVHD prophylaxis [9]. Again, relevant details for this discussion are shown in Fig. 1b. Due to major ABO mismatch (Patient 0 positive and Donor AB positive with high pre-alloHSCT IsoHG titers), she developed post-engraftment PRCA, as seen in Fig. 1b, which was not treated until day +270, when she received treatment with daratumumab (2 doses 2 weeks apart). The patient had moderate chronic GVHD requiring treatment with steroids and sirolimus and secondary thrombocytopenia, as detailed in Table 1. Prompt and brisk reticulocytosis occurred 4 weeks after the first dose and 2 weeks after the second dose, with dramatic reduction in the titers of IsoHG. Response lasts 10 months as of September 2021, and she also developed severe reduction in IgG, and moderate in IgA and IgM, which requires monthly IVIG treatment. The platelet count also rose above 100 × 109/L 6 weeks post daratumumab.
Some features that the authors of this manuscript would like to emphasize of the current two case reports and the eight other cases reported to date are shown in Table 1.
As emphasized by Marco-Ayala et al. [1] and Longval et al. [2], it is difficult for clinicians to know when PRCA requires treatment beyond transfusions or simply requires patience since spontaneous resolution may occur within few weeks. This explains why most patients are treated many months after alloHSCT, often having tried numerous other treatments and having developed severe iron overload. However, from all these reports and the current state of knowledge one can conclude the following:
The most important risk factor for PRCA and its duration beyond 2–3 months post-alloHSCT is a pre-transplant titer of IsoHG > 1:64 [1, 2].
PRCA has a “biomarker”, since the titers of IsoHG must progressively decrease until they disappear. Persistently high titers beyond 2–3 months, and, especially, rising titers (as in our two case reports) is a clear indication for therapeutic intervention. In other words, therapeutic intervention would ideally be done when we can exclude the mere presence of residual pre-alloHSCT host IsoHG which simply take time to disappear (first 2–3 months) AND suspect that there are persistent host IsoHG-producing plasma cells. In this respect, the median time to IsoHG disappearance in patients who do not develop prolonged PRCA is around 60 days, while it is beyond 90 days in prolonged PRCA [1, 2, 10, 11].
GVHD can have a strong graft-vs.-plasma cell effect and is a major predictor of disappearance of IsoHG and recovery from PRCA [1, 2, 10, 11]. Since both of our patients developed cGVHD, we expected remission of their PRCA, but this did not occur, prompting a therapeutic intervention.
PRCA can be a significant problem following major ABO-mismatched transplantation, resulting in a significant need for long-term transfusions, significant increase in the need for patient care and leads to marked iron overload.
All treatments tested in PRCA have significant potential toxicities. Withdrawal of immunosuppression can result in resolution of PRCA in many patients, but can also lead to significant GVHD. High-dose steroids, plasma exchange, bortezomib, rituximab and many others have been used, with some cases of clear response, but no true evidence of having a good efficacy-to-risk balance [1].
Patients who have severe pancytopenia and thus a poor graft function may also be candidates to treatment with daratumumab if they have IsoHG-linked PRCA. However, these patients have a poor prognosis [12], and treatment with eltrombopag or romiplostim may be of benefit [13], although eliminating high-titer IsoHG should also be a target in such cases.
From the ten cases reported to date and summarized herein, it appears that two to four doses of daratumumab can obtain prolonged remission of IsoHG-mediated PRCA, without relevant side effects except hypogammaglobulinemia, whose incidence and duration remains to be determined. The rapid response in all ten cases confirms the hypothesis that eliminating the residual recipient plasma cells leads to cure of PRCA since in a context of complete donor chimerism these residual plasma cells cannot reappear once eliminated by a short-course of daratumumab. Based on the available data, we suggest using 2–4 doses of daratumumab as the first-line treatment of PRCA, and believe that a few logical variables should be met to optimize its efficacy.
Patients should be treated at least 60 days post transplantation and 30 days post engraftment.
Complete donor chimerism should be present in myeloid cells and predominant in T cells (>50%), since recipient plasma cells could theoretically reappear in a bone marrow environment of mixed myeloid and, especially, T-cell chimerism [11].
Persistently (and even rising) high titers of anti-donor IsoHG are present (this is, in fact, the major “biomarker” for using daratumumab).
The characteristics of the ten cases shown in Table 1 support these characteristics when requesting daratumumab as compassionate use.
Author contributions
All three co-authors (RM, IG-C, and AE) contributed to the conception and execution of this brief manuscript. RM was the main author responsible for its drafting and managing its review process.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marco-Ayala J, Gómez-Seguí I, Sanz G, Solves P. Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question. Bone Marrow Transplant. 2021;56:769–78. doi: 10.1038/s41409-020-01124-6. [DOI] [PubMed] [Google Scholar]
- 2.Longval T, Galimard JE, Leprêtre AC, Suarez F, Amiranoff D, Cazaux M, et al. Treatment for pure red cell aplasia after major ABO-incompatible allogeneic stem cell transplantation: a multicentre study. Br J Haematol. 2021;193:814–26. doi: 10.1111/bjh.17463. [DOI] [PubMed] [Google Scholar]
- 3.Booth GS, Savani BN, Langston AA. Pure red blood cell aplasia: patient management pitfalls in major ABO-incompatible haematopoietic cell transplantation. Br J Haematol. 2021;193:701–2. doi: 10.1111/bjh.17465. [DOI] [PubMed] [Google Scholar]
- 4.Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for delayed red-cell engraftment after allogeneic transplantation. N Engl J Med. 2018;379:1846–50. doi: 10.1056/NEJMoa1807438. [DOI] [PubMed] [Google Scholar]
- 5.Bathini S, Holtzman NG, Koka R, Singh Z, Wilding E, Zou Y, et al. Refractory postallogeneic stem cell transplant pure red cell aplasia in remission after treatment with daratumumab. Am J Hematol. 2019;94:E216–9. doi: 10.1002/ajh.25515. [DOI] [PubMed] [Google Scholar]
- 6.Salas MQ, Alahmari A, Lipton JH. Successful treatment of refractory red cell aplasia after allogeneic hematopoietic cell transplantation with daratumumab. Eur J Haematol. 2020;104:145–7. doi: 10.1111/ejh.13343. [DOI] [PubMed] [Google Scholar]
- 7.Rautenberg C, Kaivers J, Germing U, Haas R, Ackerstaff S, Hoffmann T, et al. Daratumumab for treatment of pure red cell aplasia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2020;55:1191–3. doi: 10.1038/s41409-019-0664-4. [DOI] [PubMed] [Google Scholar]
- 8.Parody R, López-Corral L, Lopez-Godino O, Martinez C, Martino R, Solano C, et al. GvHD prophylaxis with tacrolimus plus sirolimus after reduced intensity conditioning allogeneic transplantation: results of a multicenter study. Bone Marrow Transplant. 2016;51:1524–6. doi: 10.1038/bmt.2016.163. [DOI] [PubMed] [Google Scholar]
- 9.García-Cadenas I, Awol R, Esquirol A, Saavedra S, Bosch-Vilaseca A, Novelli S, et al. Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transplant. 2020;55:1041–9. doi: 10.1038/s41409-019-0771-2. [DOI] [PubMed] [Google Scholar]
- 10.Hirokawa M, Fukuda T, Ohashi K, Hidaka M, Ichinohe T, Iwato K, et al. Efficacy and long-term outcome of treatment for pure red cell aplasia after allogeneic stem cell transplantation from major ABO-incompatible donors. Biol Blood Marrow Transplant. 2013;19:1026–32. doi: 10.1016/j.bbmt.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Alcaina PS. A review of isoagglutinin change kinetics in ABO incompatible hematopoietic stem cell transplantation. Ann Stem Cell Res Ther. 2018;2:1025–8. [Google Scholar]
- 12.Aung FM, Lichtiger B, Rondon G, Yin CC, Alousi A, Ahmed S, et al. Pure red cell aplasia in major ABO-mismatched allogeneic hematopoietic stem cell transplantation is associated with severe pancytopenia. Biol Blood Marrow Transplant. 2016;22:961–5. doi: 10.1016/j.bbmt.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busca A, Dellacasa C, Giaccone L, Manetta S, Biale L, Godio L, et al. Eltrombopag for the treatment of refractory pure RBC aplasia after major ABO incompatible hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:1765–70. doi: 10.1016/j.bbmt.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Jeyaraman P, Borah P, Rajput P, Dayal N, Pathak S, Naithani R. Daratumumab for pure red cell aplasia post ABO incompatible allogeneic hematopoietic stem cell transplant for aplastic anemia. Blood Cells Mol Dis. 2021;88:102464. doi: 10.1016/j.bcmd.2020.102464. [DOI] [PubMed] [Google Scholar]
- 15.Yates B, Molloy E, Dulau-Florea A, Braylan R, Hogan L, Hickstein BD, et al. Daratumumab for delayed RBC engraftment following major ABO mismatched haploidentical bone marrow transplantation. Transfusion. 2021;61:1041–6. doi: 10.1111/trf.16281. [DOI] [PubMed] [Google Scholar]
- 16.Bicsko RR, Magyari F, Szasz R, Illes A, Gergely L. Administration of daratumumab in the case of severe pure red cell aplasia after allogeneic transplantation. Ann Hematol Oncol. 2021;8:1331–2. [Google Scholar]
- 17.Henig I, Yehudai-Ofir D, Zohar Y, Zuckerman T. Pure Red cell aplasia following ABO-mismatched allogeneic hematopoietic stem cell transplantation: resolution with daratumumab treatment. Acta Haematol. 2021. 10.1159/000515257. [DOI] [PubMed]