Abstract
This review and commentary is the product of an invited lecture called “Autoimmunity: PANS/PANDAS” presented at the 2018 Neurobiology of Diseases in Children Symposium in Chicago, IL. The talk addressed clinical and scientific questions and recently published data. At this time, among highly experienced and respected clinicians and researchers spanning relevant disciplines, there is substantial controversy regarding a role for inflammation in producing tics and obsessive-compulsive disorder. This commentary summarizes these controversies, discusses reasons for opposing views on best clinical practices, and concludes with suggestions for pathways forward.
Keywords: tourette syndrome, obsessive compulsive disorder, autoimmune diseases, children placebo
This review and commentary is the product of an invited lecture called “Autoimmunity: PANS/PANDAS” presented at the 2018 Neurobiology of Diseases in Children Symposium in Chicago, IL. The talk addressed Pediatric Acute onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS). Both diagnoses differentiate children with acute onset from those with more gradual onset symptoms. PANDAS attributes the onset of tics or obsessive-compulsive disorder (OCD) to immune mechanisms provoked by streptococcal infection; PANS by definition specifies neither etiology nor mechanism, but an autoimmune mechanism is presumed in most cases. At this time, among highly experienced and respected clinicians and researchers spanning relevant disciplines, there is substantial controversy regarding a role for inflammation in producing tics and obsessive-compulsive disorder. Individuals with differing views will be referred to in this commentary as skeptics and advocates. This commentary summarizes these controversies, emphasizes reasons for opposing views, enumerates challenges to progress based on placebo- and nocebo-related factors and families’ prejudging of the science, and concludes with suggestions for pathways forward. More detailed summaries of the basic, epidemiologic, and clinical evidence relating to these 2 diagnoses lie outside the scope of this article but have recently been published else-where.1–4
History and Causal Pathway Models
The overarching questions involve whether and when infections and/or inflammation play a role in the production of neuropsychiatric disorders that are currently classified as idiopathic. Questions about a role for infection causing neurologic and psychiatric disorders date back many decades.5–11 Among doctors caring specifically for children with Tourette syndrome and obsessive-compulsive disorder, special interest was generated by observations of the temporal associations in the clinical presentation of acute rheumatic fever. In particular, doctors were intrigued by tics and obsessive-compulsive disorder, which often preceded, waxed during, and waned after this illness.12–15 A causal pathway16 for this illness is shown in Figure 1.
Figure 1.
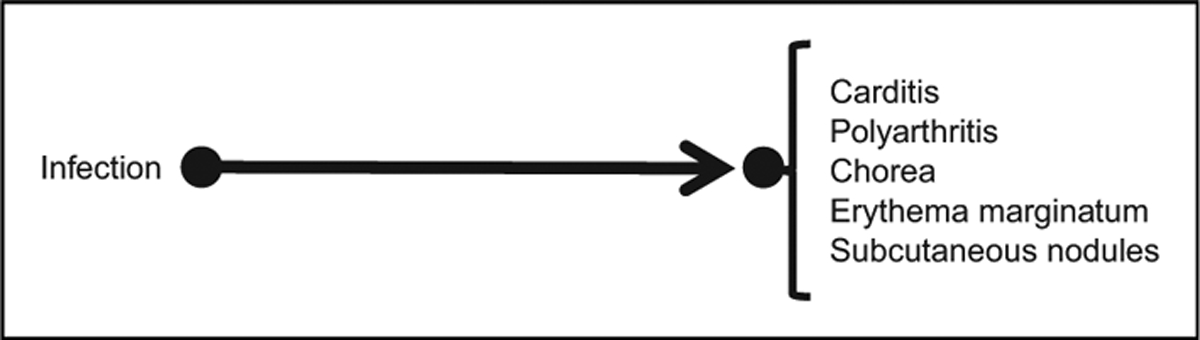
Direct causal pathway for acute rheumatic fever. Infection is with group A streptococcal bacteria. Disease manifestations are the major Jones criteria for acute rheumatic fever.
Causal pathways, as opposed to statistical correlations, make claims about a directional relationship in a pair of observed events or phenomena. For example, the causal pathway in Figure 1 does not imply that children with chorea are more likely to get infections (eg, because their involuntary movements bring their hands more often to infected surfaces and to their faces). Rather, it implies that in some individuals with chorea (or other listed findings), were it not for their infection, they would not have acquired chorea. The causal pathway in Figure 1 makes a number of testable predictions, including (1) treating with antibiotics active against streptococcal infections would diminish symptoms of the disease if the action of the infection is direct; (2) treating with antibiotics might not diminish symptoms if there is a long latency between infection and symptoms, and if an intervening, mediating factor occurs that outlasts the infection; (3) long-term antibiotics might prevent recurrences of this disease; and (4) dopamine receptor-2 blocking or dopamine-depleting agents for chorea17—treatments that do not directly address the root cause—might improve symptoms. A mainstay of management after the diagnosis of acute rheumatic fever follows prediction 3, that is, chronic treatment in childhood with penicillin, as secondary prevention.18,19 Symptomatic treatments are also often provided following prediction 4.20,21
Given the latency of weeks to months between streptococcal infections and the onset of chorea, as well as spatially separated signs and symptoms, particularly cardinal symptoms of inflammation in the joints and skin, clinicians hypothesized that the causal pathway from infection to manifestations of acute rheumatic fever might be mediated by the body’s inflammatory response, which might outlast the infection (see Figure 2).
Figure 2.
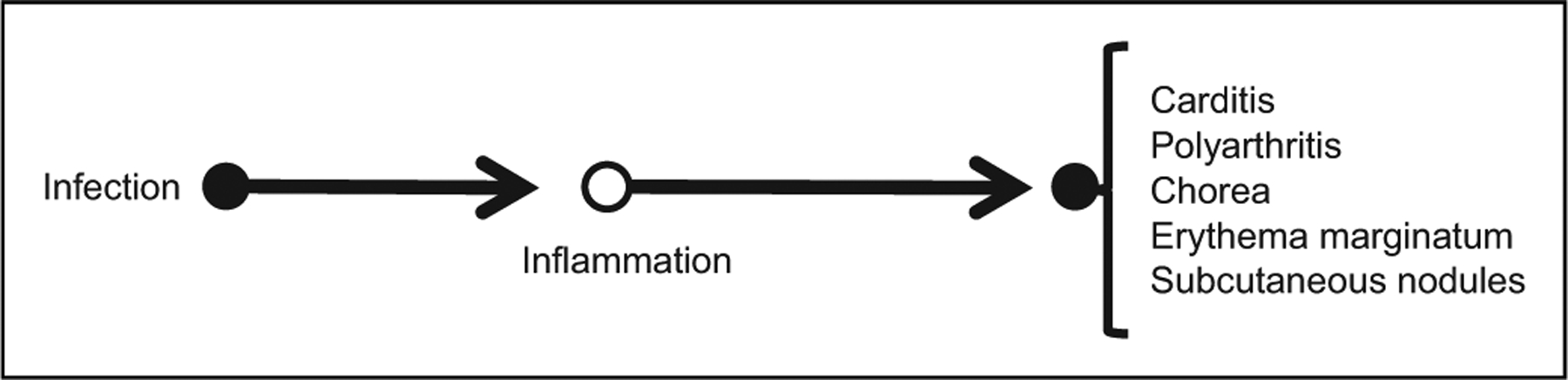
Causal pathway for acute rheumatic fever. Infection is with group A streptococcal bacteria. The infection triggers an inflammatory response, the specific elements of which may involve antibodies and molecular mimicry, but which may not be directly measured (open circle).
The pathway in Figure 2 makes a number of additional predictions, including (5) inflammatory markers (specific and/or non-specific) in the blood should be present in affected patients; and (6) anti-inflammatory drugs might reduce disease symptoms. Consistent with prediction 5, diagnostic protocols for acute rheumatic fever include testing for inflammatory markers.18,19 Based on prediction 6, Sydenham chorea may be treated with steroids.22–25
Decades of clinical observations of associations of motor and psychiatric symptoms in Sydenham chorea yielded an analogous hypothesis in the 1990s whereby group A streptococcal infections might cause tics or obsessive-compulsive disorder (see Figure 3). This proposed clinical entity was designated Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infection (PANDAS).26 The current taxonomy subsumes PANDAS into a broader category, Pediatric Acute onset Neuropsychiatric Syndrome (PANS), which is agnostic about the primary triggers but presumes an inflammatory mediator in most cases.27
Figure 3.
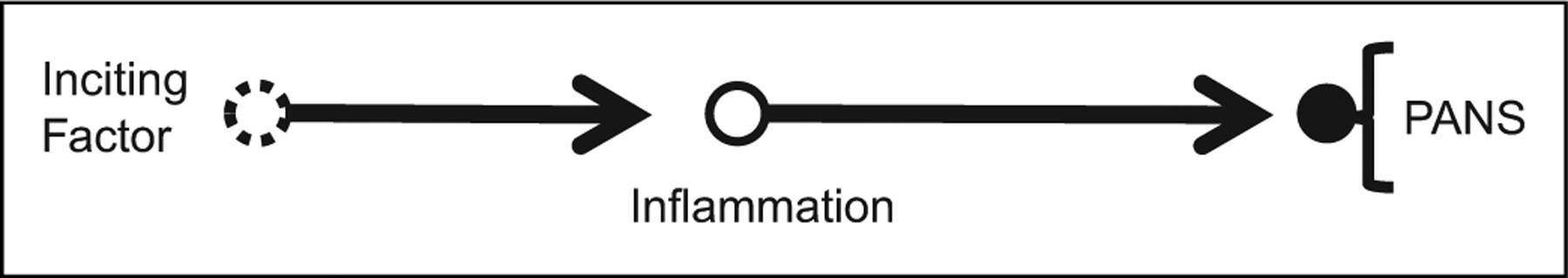
Causal pathway for Pediatric Acute Onset Neuropsychiatric Syndrome (PANS). For PANS subtype PANDAS, the inciting factor is infection with group A streptococcal bacteria. For PANS, the inciting factor is other infections or noninfectious causes that may not be identified (open circle, dashed border). This may trigger an inflammatory response, the specific elements of which may not be understood (open circle), which in turn causes 1 or more neuropsychiatric symptoms (see text).
The pathway in Figure 3 makes the same 6 predictions as the pathway in Figures 1 and 2 with the exception that other antimicrobial agents might be needed if PANS is triggered by a different microbe than group A Streptococci, that other inflammatory markers should be identified, and that other symptomatic treatments that do not directly address the root cause, for example, cognitive behavioral therapy or selective serotonin reuptake inhibitors, might improve symptoms.
These causal models and associated predictions have been sufficiently compelling to generate a great deal of basic, translational, epidemiologic, and therapeutic research. For PANS/PANDAS advocates, this has also influenced clinical decision making regarding testing and treatment. In contrast, PANS/PANDAS skeptics at most accept these as rare disorders comparable to other autoimmune encephalitides. They view the PANS/PANDAS causal model as scientifically unsubstantiated, they believe that advocates vastly overestimate its incidence, and they interpret the results of research to date as not meeting common standards of evidence for causality.2,28,29 As a result, although advocates order many tests, skeptics order few or none, and whereas advocates treat with antibiotics or anti-inflammatory drugs as well as (or in place of) symptom-based treatments, skeptics treat with only the latter. Progress depends in part on understanding reasons for these differing views and practices. Skeptics have offered pointed criticisms of methodologies of specific basic, epidemiologic, and clinical studies.4,30–32 Examples of skeptics’ questions include the following:
Prevalence and coincidental associations: Given the high prevalence of streptococcal and other infections in childhood, and the high prevalence of obsessive-compulsive disorder and Tourette syndrome, chance temporal associations are likely. Throat cultures and blood antibody tests may fail to distinguish a carrier state, unlikely to trigger a systemic response, from a new infection.33 How would a clinician determine in an individual child that a temporal association between any streptococcal infections and neuropsychiatric symptoms is causal versus coincidental? If this cannot be done precisely, how would clinicians avoid over-diagnosing PANDAS?34
Primacy of acute-onset: Many neurologic diseases, for example, epilepsy, begin suddenly and dramatically (see Table 1), and developmental milestones, for example, walking or putting words together, can seem to occur “almost overnight.” Rapid changes in the nervous system can occur in the absence of inflammation. What evidence is there to support categorizing acute-onset obsessive-compulsive disorder as inflammatory/distinct from idiopathic obsessive-compulsive disorder?
Attribution of a restricted range of symptoms to inflammation: Most inflammatory diseases of the nervous system cause some combination of seizures, continuous dyskinetic movements, altered mental status to the point of confusion, and/or central or peripheral nervous system demyelination or destruction.1 Tics, obsessive-compulsive disorder, and restricted food intake27 alone seem atypical for the disease category of autoimmune encephalitis. Why are PANS/PANDAS inflammatory with such a narrow range of symptoms?
Prevalence: Most inflammatory diseases affecting the nervous system in children are rare. How can advocates justify their claim that 1% to 2% of children have PANS/PANDAS?35 Why have no specific diagnostic tests emerged?36
Negative and small treatment studies: PANDAS was proposed more than 20 years ago.26 During that time period, randomized, placebo-controlled trials involving more than 100 patients have been published in pediatric migraine,37 absence epilepsy,38 Tourette syndrome,39 and obsessive-compulsive disorder.40 All controlled trials for PANDAS have enrolled fewer than 50 children. Why is there no large study with high-quality evidence supporting PANDAS treatments?
Table 1.
Subacute and Acute Genetic and Inflammatory Diseases of the Nervous System.
| Diagnosis | Acute/subacute-onset symptoms and signs | Infection triggering inflammation at onset? | Laboratory or other evidence of inflammation | Infectious or other cause |
|---|---|---|---|---|
| Inflammatory | ||||
| Anti–NMDA receptor encephalitis | Facial dyskinesia, confusion, dysautonomia, psychosis | No/unknown | Elevated NMDA receptor IgG antibody in CSF; EEG changes | Ovarian teratoma, post–herpes simplex encephalitis |
| Guillain-Barre syndrome | Back pain, generalized weakness, hyporeflexia | Sometimes | Elevated inflammatory proteins in the cerebrospinal fluid | Campylobacter jejuni |
| Multiple sclerosis | Symptoms/signs localizing to >1 sites in CNS | Unknown | MRI enhancement brain/spine, elevated Ig or oligoclonal bands CSF | Unknown |
| Opsoclonus myoclonus ataxia syndrome | Opsoclonus, myoclonus, ataxia, extreme irritability | No/unknown | No | Neuroblastoma |
| Sydenham chorea / rheumatic fever | Chorea | Yes | Elevated ASO, DNAseB in blood, inflammation in heart/skin/joints | Group A beta-hemolytic streptococcal bacteria |
| Genetic | ||||
| Acute chorea due to mitochondrial DNA mutation | Chorea | No | No | Mitochondrial ND5 |
| Familial hemiplegic migraine | Weakness, sudden onset, unilateral | No | No | CACNA1A, ATP1A2 |
| Status epilepticus, followed by chronic recurring seizures | Prolonged Seizure | (First seizure may be febrile) | No | SCN1A |
| Leber optic neuropathy | Unilateral blindness | No | No | Mitochondrial ND1, ND2 etc |
| Mitochondrial encephalopathy, lactic acidosis, and stroke | Weakness, sudden-onset focal or multifocal | No | No | Mitochondrial MTTL1, etc. |
Abbreviations: ASO, antistreptolysin O; CNS, central nervous system; CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; EEG, electroencephalography; IgG, immunoglobulin G; MRI, magnetic resonance imaging; NMDA, N-methyl-d-aspartate.
On the basis of their view that evidence addressing these questions is unsatisfactory, the skeptic’s view is that PANS/PANDAS are not distinct but rather that disorders like Tourette syndrome and obsessive-compulsive disorder are heterogeneous conditions resulting from multiple small genetic risks mediated by various environmental factors,41 and that these have a spectrum of time courses ranging from acute to more indolent.
This commentary addresses PANDAS and PANS controversies in terms of the following questions: (1) How do the definitions of PANDAS and PANS influence the views of both advocates and skeptics? (2) How does inflammation research compare for obsessive-compulsive disorder and tics versus more common conditions of depression and anxiety? (3) How are skeptics and advocates reasoning differently about causality? (4) How can clinicians and researchers who are skeptics and advocates move forward?
Influence of Definitions of PANS and PANDAS on Advocates and Skeptics
The causal pathways (Figures 1–3) and research definitions of PANDAS have played a fundamental role in generating research questions. Study results have been interpreted in distinct ways by advocates and skeptics.
PANDAS: Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections
In 1998, investigators at the National Institute of Mental Health (NIMH) published their seminal paper reporting a series of 50 patients, evaluated beginning in 1991, whom they had painstakingly characterized. They proposed a distinct, clinical entity, PANDAS, with 5 diagnostic criteria: (1) presence of obsessive-compulsive disorder and/or a tic disorder; (2) prepubertal symptom onset (age 3 years to the beginning of puberty); (3) episodic course characterized by acute, severe onset and dramatic symptom exacerbations; (4) temporal relationship between group A beta-hemolytic streptococcal (GABHS) infections and symptom onset and exacerbations; (5) association with neurologic abnormalities. Criteria 3, 4, and 5 support specific research questions.
Criterion 3, the acute, severe onset of symptoms.
Criterion 3 is critical to both PANDAS and PANS. Fundamental principles of medical diagnosis include (1) that the healthy body has a limited repertoire of responses to multiple pathogenic processes, and that therefore a broad differential diagnosis should be considered, and (2) that it is important to include the symptom/sign complex plus the time course of illness in refining and limiting the differential diagnosis. When considering acute or subacute presentations, the following categories generally dominate: trauma, vascular pathology, infections, and inflammatory diseases. However, recent discoveries have shown that in the nervous system genetic diseases are also important. Table 1 shows instructive examples of diagnoses with rapid onset. In some cases, specific test results such as antibodies or tumors support an inflammatory pathophysiology. In other cases, genetic discoveries have clarified our understanding of pathophysiology.
Criterion 4, infections and re-infections with group A streptococci.
Many neurologic and psychiatric conditions have a waxing and waning course. PANDAS was initially emphasized to have a distinct, extreme exacerbation pattern referred to as a “sawtooth time course.” This framework, along with the belief that group A streptococcal infection is the primary causal agent, has guided many advocate clinicians to swab a child’s throat to test for streptococci whenever tics or obsessive-compulsive disorder symptoms worsen, even in the absence of fevers or other infections symptoms or signs, and to treat with antibiotics, even in the absence of positive test results.34,42 There are parallels to this practice by practitioners who attribute neuropsychiatric symptoms to chronic Lyme disease, although chronic antibiotic treatment was recently shown, in a randomized controlled trial, to have equivalent benefits to placebo.43
Taken together, criteria 3 and 4 provided the incentive for 2 highly intensive, multicenter prospective research studies, funded by the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. The principal investigators collaborated, rigorously and prospectively applying all 5 original PANDAS criteria. Intensive scheduled and peri-illness/peri-exacerbation throat cultures and blood tests were used to attempt to validate causal pathways, namely, that streptococcal infections, possibly mediated by inflammation, cause clinical exacerbations in PANDAS. The studies enrolled prepubertal children meeting all 5 criteria for PANDAS (n = 71) matched demographically and geographically to non–acute onset / non–sawtooth course tic / obsessive-compulsive disorder controls (n = 93), tracking symptoms and test results for 2 years. Surprisingly, both groups experienced similar rates of infections and exacerbations, and the vast majority of exacerbations were not temporally associated with streptococcal infections.44,45 Skeptics conclude from these results that even if a streptococcal infection does trigger the onset of tics or obsessive-compulsive disorder in a child, this likely represents an unmasking of symptoms that would have occurred anyway and that over time, recurring infections play no demonstrable role in symptom severity. Thus skeptics test, at most, judiciously. PANS/PANDAS advocates responded by attempting to marginalize these studies, suggesting the investigators enrolled patients who “may have represented a distinctive population.”46
Criterion 5, movement disorder.
During symptom exacerbations, but not necessarily between exacerbations in obsessive-compulsive disorder–PANDAS cases, patients should have “motoric hyperactivity and adventitious movements.” These adventitious movements could include “choreiform movements” but not “frank chorea.” Although one could anticipate some confusion in the presence of symptoms due to ambiguous terminology, in cases diagnosed retrospectively, clinicians might have even more difficulty distinguishing between degrees of chorea. For minor chorea or adventitious movements, this raised the possibility of diagnostic misclassification between PANDAS and Sydenham chorea. For tics, this raised the possibility of diagnostic misclassification between PANDAS and Tourette syndrome. Of the original PANDAS series, 26 of 50 were diagnosed with tic disorder, whereas 24 of 50 were diagnosed with obsessive-compulsive disorder.26 Tics are common, and many families describe their onset as sudden.47 Skeptics believe that some PANDAS cases are mild Sydenham chorea. Advocates see these as separate diagnoses.
Net results of the PANDAS framework.
Skeptics in pediatrics, infectious diseases, immunology, and child neurology believe that the best evidence to date does not support PANDAS as a distinct neuropsychiatric diagnosis or that it is, at most, a rare condition.29,48,49 Advocates have continued to pursue evidence for the PANDAS causal models. Convinced that these children’s disorders were biologically distinct from routine obsessive-compulsive disorder, a group of clinicians and researchers convened a workshop in 2010 and developed research criteria for PANS.27
Recent PANS/PANDAS research has included epidemiologic50 or laboratory51 studies. Clinical publications have predominantly described open-label treatment.3 In contrast, to date, controlled studies of antibiotics for PANDAS have not provided strong evidence. For example, a randomized, placebo-controlled trial of the antibiotic cefdinir in children with recent-onset obsessive-compulsive disorder and/or tics was negative, and furthermore showed large symptom improvements in the placebo arm.52 The speculation that tonsillectomies might reduce or prevent tics or obsessive-compulsive disorder appears to be unfounded.53,54
In general, all controlled clinical trials for treatment of PANDAS have been negative, small, and/or had high risk of bias.3 The most recent immune modulatory study, which randomized children diagnosed with PANDAS to intravenous immunoglobulin versus placebo, failed to show differential benefit.55 Skeptics have concluded that treatment studies do not support antibiotic or immunomodulatory interventions for PANDAS.4 Advocates published treatment guidelines supporting both.46,56
PANS: Pediatric Acute-Onset Neuropsychiatric Syndrome
The criteria for PANS include (1) abrupt, dramatic onset of obsessive-compulsive disorder or severely restricted food intake and (2) concurrent abrupt onset of additional severe neuropsychiatric symptoms from at least 2 of the following 7 categories: (a) anxiety; (b) emotional lability and/or depression; (c) irritability, aggression, and/or severe oppositional behaviors; (d) behavioral (developmental) regression; (e) deterioration in school performance; (f) sensory or motor abnormalities, including heightened sensitivity to sensory stimuli, hallucinations, dysgraphia, complex motor and/or vocal tics; and (g) somatic signs and symptoms, including sleep disturbances, enuresis, or urinary frequency.27
This new formulation was applicable in some cases to children whose only brain-based symptoms were psychiatric, for example, obsessive-compulsive disorder plus anxiety and emotional lability. No precipitant was specified, and the diagnosis no longer required 2 or more episodes. However, the presumption remained that most cases were inflammatory.57
Criteria-based, initial PANS research.
Initial clinical research recapitulated the PANDAS trajectory, including presentation of a case series of 47 children.58 But taking causal research further, as done for PANDAS, has posed special problems. The lack of a specific etiology in PANS criteria provides at most limited guidance for epidemiologic or biological studies with specific hypotheses. PANS advocates therefore to date utilize methodologies such as surveys of self-selected samples. These have generated claims of triggers ranging from known neurotropic microbes to vaccines to food additives to winter weather and have even suggested that PANS might include non-acute cases.59 Perhaps unsurprisingly, then, a randomized controlled trial of the antibiotic azithromycin in 31 children diagnosed with PANS showed no statistical advantage over placebo at 4 weeks for either tic scores or obsessive-compulsive disorder scores.60
Although this ever-widening scope of PANS poses scientific challenges for researchers, it provides benefits for other stake-holders. For suffering children and families, a broader diagnosis of PANS with inflammatory mediation of symptoms mitigates the painful social stigma of psychiatric diagnoses. It also provides a more satisfying “root cause” than idiopathic psychiatric diagnoses: a biological entity rather than a categorical label. For purveyors of therapies in cash-pay clinics, this offers another opportunity to profit from desperate families by offering evaluations and treatments with no strong empirical or biological rationale. For some members of the PANS physician research community, this has provided profitable opportunities to claim that vaccines cause reimbursable suffering.61,62
For scientists, however, a result of the PANS framework is that a more productive approach is to focus less on putative triggers and more on the basic science of inflammation. To place this emphasis on inflammation in clinical context, the next section compares the state of research on inflammation in PANS to that of inflammation in major depression and includes results of research using immunomodulatory agents.
Inflammation Research in PANS/PANDAS vs Depression and Anxiety
Identifying a causative role of inflammation in generating neuropsychiatric symptoms could benefit children and adults.63 This is an active area of research,64 and PANS/PANDAS researchers’ work57,58 is part of this broad effort. A goal is the development of treatment protocols using biologically targeted medications to treat persons whose symptoms may have an inflammatory basis, and for whom standard symptomatic treatments are ineffective.
To date, has treatment research targeting inflammation in PANS/PANDAS penetrated high-level clinical journals? One brute force method for assessing the amount of research in these areas is to quantify recent publication numbers. To do this, I used the OVID platform for a PubMed search on October 1, 2018, querying inflammation using an OR search with the keywords cytokine, antibody, allergy, histamine, histamine receptors, encephalitis, and autoimmunity. I then queried separately anxiety OR depression, as these higher-prevalence mental health conditions would likely have more “hits.” Finally, I queried Obsessive Compulsive Disorder OR Tourette OR Tic Disorder. I limited this search to articles published in the past 5 years and, further, to those published in journals designated in the Abridged Index Medicus as “Core Clinical Journals.”
As seen in Table 2, to date research in inflammation appears limited to 1% to 2% of publications. In the last 5 years, strikingly, the core clinical journals, that is, high-impact journals that reflect the most rigorous science and influence clinical practice, have no inflammatory publications for obsessive-compulsive disorder / tic. For anxiety/depression, there were only 5, an average of 1 per year.
Table 2.
2018 PubMed Search of Immunology and OCD/Tics/Tourette.
| Anxiety, depression | OCD, tics, Tourette | |
|---|---|---|
| Immunity/inflammatory terms | 620 000 | |
| Psychiatric terms | 76 560 | 11 150 |
| Inflammatory and psychiatric terms | 962 (1.3%) | 193 (1.7%) |
| Limit: to last 5 years | 342 (36%) | 52 (27%) |
| Limit: to OVID “Core Clinical Journals” | 5 (1.5%) | 0 (0%) |
Abbreviation: OCD, obsessive-compulsive disorder.
Nonetheless, the observed association between psychiatric symptoms and autoimmune conditions such as Sydenham chorea remains compelling. There is also the imperative to improve outcomes in persons with severe psychiatric symptoms. These rationales have been sufficiently strong to foster randomized controlled trials. In one study, 60 adults with major depression were randomized to either the tumor necrosis factor alpha antagonist infliximab or placebo. Baseline status included 40% unemployment, a mean of 9 lifetime episodes, mean duration of greater than 15 years, and comorbid medical illnesses afflicting approximately 50%. After treatment, improvements in depressive symptoms were clinically significant. However, tremendous gains occurred equally in the placebo and treatment arms, a statistically negative result. Despite the negative clinical result, the authors chose to highlight in the abstract a variety of exploratory analyses relating treatment responses to baseline levels of inflammatory biomarkers.65 Subsequently, a meta-analysis did not provide strong support for immunologic treatments for depression, and moreover published studies had a high risk of bias.66
The possibility of inflammatory causes of children’s obsessive-compulsive disorder symptoms,57 although supported by fewer studies, has also provided a strong incentive for clinical trials of immunomodulatory agents in PANS/PANDAS. These have had negative results.55 Despite the negative results, PANS/PANDAS advocates published treatment guidelines recommending aggressive, escalating treatments with NSAIDs, steroids, intravenous immunoglobulin, plasmapheresis, rituximab, and mycophenylate.56
Concurrently, members of parent advocacy groups believe very strongly that these immunomodulatory treatments are essential, even life-saving. They have lobbied state legislatures to have these treatments reimbursed by insurance companies.67 Some have also argued for a broader clinical diagnosis that includes chronic, non–acute onset cases as well as cases with other clinical presentations, including symptoms of autism spectrum disorder.42
Reasoning About Causality in PANS/PANDAS
To better understand the contrasting views of advocates and skeptics surrounding PANS/PANDAS, it is instructive to compare causal research in PANS/PANDAS to anti–N-methyl-d-aspartate (NMDA) receptor encephalitis. In 1999, Taylor and colleagues published a case report describing a woman with an ovarian tumor and encephalomyelitis and proposed the etiology was inflammatory and paraneoplastic.68 In 2007, Dalmau and colleagues published a case series of 12 women with encephalomyelitis and proposed a biological pathway whereby ovarian teratomas induce an inflammatory process involving antibodies against the NMDA receptor, causing psychiatric symptoms, delirium, movement disorders, and seizures.69,70 Subsequent case series, including in children, in whom psychiatric presentations are less frequent,71 have shown that many cases lack tumors and some occur following herpes simplex virus encephalitis.72 Twelve years later, anti-NMDA receptor encephalitis has widely accepted diagnostic approaches and treatments, including tumor-resection surgery and aggressive and expensive immune-modulatory therapy, despite no large randomized, blinded, placebo-controlled trials.1
Are PANS/PANDAS skeptics inconsistent, or even hypocritical, in accepting anti-NMDA receptor encephalitis and rejecting PANS/PANDAS? Skeptics could pose several reasons for the difference. First, as exemplified by the conditions in Table 1, the anti-NMDA receptor disease resembled most encephalopathies in producing delirium with cognitive, continuous motor, and psychiatric symptoms, whereas this is not the case for PANS/PANDAS. Second, the early cases presenting with ovarian teratomas allowed categorization of anti-NMDA receptor encephalitis within the accepted medical framework of paraneoplastic syndromes (as is the case for opsoclonus myoclonus ataxia syndrome, where some but not all cases are associated with neuroblastomas). This is not the case for PANS/PANDAS. Third, the proposed analogous condition, acute rheumatic fever, has other organ involvement; PANDAS does not. Fourth, the anti-NMDA receptor antibodies are quantifiable, highly correlated with disease, and more prevalent in cerebrospinal fluid than in blood. Although many tests in wide use, even newborn screening tests, face some challenges regarding implementation and improving test characteristics,73,74 research published to date suggests that the anti-streptococcal antibodies and the commercialized PANS/PANDAS inflammatory diagnostic test panel have not reached a high level of specificity.33,36,75 Finally, the low incidence and high severity allowed the clinical manifestations of anti-NMDA-receptor encephalitis to be studied intensively in academic centers, whereas PANDAS/PANS researchers collect data in less reliable settings such as outpatient practices58,76 and even Web-based surveys.59
Another instructive comparison is with the 100-year-old theory that focal infections and abscesses anywhere in the body could produce psychosis.9 Vigorous promotion of an invasive surgical approach to psychosis by Dr Henry Cotton generated media attention but also fostered a ground-breaking, for its time, clinical trial. This rigorous, pseudo-randomized study, which notably enrolled more participants than any of the randomized trials of PANDAS to date, showed that surgery did not improve outcomes.77
To understand where the PANS practice guideline currently falls along a Cotton/Dalmau evidence spectrum, it is advisable to think formally about the pathway in Figure 3 as it relates to levels of reasoning about causality. Human reasoning about causality can be schematized to involve 3 tiers on a ladder of causation: (1) passive observations of connections where causal inference is based on observation of temporal association; (2) active interventions generating sequences of events that support causal inference; and (3) counterfactual causal statements, generated based on repeated or powerful life experiences and the human tendency and ability to reason about possible alternative realities.78 These processes, summarized in Table 3, are helpful for clarifying the current state of PANS/PANDAS science, reasons for disagreements between skeptics and advocates, and routes forward.
Table 3.
Ladder of Causation Applied to PANS/PANDAS.
| Tier, name, probability equation | Typical activity | Typical question | PANDAS example | PANS example |
|---|---|---|---|---|
| 3. Counterfactuals P(yx|x’, y’) | Imagining | What if I had acted differently? | If the patient had not had a strep infection, they would not have developed tics | If the patient had not been sick, they would not have developed OCD |
| 2. Intervention P(y|do(x), z) | Doing | What if I do x? | If I treat with amoxicillin, the tics will be cured | If I treat with plasmapheresis, the OCD will be cured |
| 1. Association P(y|x) | Observing | How does seeing x change my belief in y? | Tics flared up after the patient got an infection | OCD flared up after the patient got sick |
Abbreviations: OCD, obsessive-compulsive disorder; PANDAS, Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections; PANS, Pediatric Acute onset Neuropsychiatric Syndrome.
Tier 1 causality.
Scientists reading case reports or small case series routinely reject level I causal claims with phrases like “association does not imply causality” or “the plural of individual cases is not data.” PANS/PANDAS research includes not only case reports and case series but also large epidemiologic studies. However, these have not generated high odds ratios or consensus among experts.2,79 Arguments from level I can be difficult to resolve, even when both the hypothesized cause and effect are objective and measurable. The skeptical view is informed by bringing together the biological and epidemiologic data and assessing the strength of the observational causality argument using Hill criteria80 and finding this evidence for causality to be weak.4 Moving forward, additional epidemiologic studies may provide circumstantial evidence relating infections to features of PANDAS, such as obsessive-compulsive disorder, but ultimately are unlikely to be sufficiently convincing. This is because hidden biases, for example, relating to differences in health care utilization, can confound such studies. Most critically, large-scale diagnostic code–based, observational studies lack clinical detail to differentiate PANS/PANDAS from other psychiatric illnesses. It is not unreasonable to pursue such studies, but they will do little to resolve the controversies regarding appropriate treatment for affected children.
Tier 2 causality.
At the second level of causation, rigorous, controlled, blinded interventional studies with objective outcomes may be accepted by clinicians and scientists as implying causality and be sufficient to guide clinical practice. Advocates point out that PANDAS research has included clinical trials and case series of antibiotics and immunomodulatory agents from high-profile authors and medical centers. Skeptics will argue that for PANS/PANDAS, interventional studies suffer from biases, small sample sizes, subjective rating scale outcomes, and negative statistical outcomes3 and that therefore causality is also not supported. In adopting this view, the skeptics align with standard medical and scientific arguments that use rigorous standards to limit type I error81,82 and with best practice guidelines by major medical societies, published in high-impact journals, which make conservative treatment recommendations based on standards of evidence and quantitative analyses of rigorously conducted studies.83 The recent PANS guidelines for antibiotics46 and immunomodulatory treatments56 depart from this pattern of scientific arguments, accepting expert opinion based on clinical experience as sufficient for recommendations of interventions.
Moving forward, only a randomized, blinded, placebo-controlled trial with low risk of bias and conducted in a large, carefully diagnosed, generalizable group of patients with moderate to severe neuropsychiatric symptoms will support widespread acceptance of these treatment recommendations. Ideally, studies should have statistically and clinically meaningful effects, and be replicated. Further open label trials will not support causal reasoning and should be discouraged from publication, even if currently available biomarkers are utilized.
Tier 3 causality.
Tier 3 reasoning about causality supports a version of Figure 3 for anti-NMDA receptor encephalitis whereby an ovarian teratoma or an undiagnosed microbe causes the production of an inflammatory response that causes the characteristic neuropsychiatric syndrome in newly encountered patients. Clinicians and researchers, based on an accepted biological framework, strong basic science, objective clinical data, and powerful experiences of clinically meaningful treatment results then reflect on individual new cases and attempt to discern what features are necessary or sufficient to explain the neuropsychiatric syndrome. In evaluating new patients, they generate causal statements in the form of the following counter-factuals: (1) were it not for the ovarian tumor, the anti NMDA receptor antibodies would not have been produced; (2) were it not for the inflammatory response evidenced specifically by the presence of the anti-NMDA receptor antibodies in cerebrospinal fluid, the neuropsychiatric symptoms would not have occurred; and, weeks to months later, (3) were it not for early diagnosis and the tumor resection plus aggressive immunomodulatory treatment, the patient would not have improved.
Analogously, based on tier 3 causal reasoning, a large, multidisciplinary group of clinicians and scientists believe the PANS/PANDAS causal pathway should guide testing and treatment.27,79 According to the summary statement,79 the 2017 PANS/PANDAS guidelines46,56,84 are based on “clinical experience with more than 1000 children with PANS/PANDAS.” In other words, while continuing to do basic, translational, and clinical research, advocates have embraced the biological framework of autoimmune encephalitis, the supportive basic science, and the open-label clinical data. For parent advocates who are nonscientists, the embrace emanates more directly from a personal experience such as “OCD happened to my child’s brain around the time of an infection, and it got better after antibiotics (or IVIG, etc).” As a result, parallel to anti–NMDA receptor encephalitis causal reasoning, PANS/PANDAS advocates reason as follows: (1) were it not for the streptococcal (or other) infection, the inflammation / obsessive-compulsive disorder in my patient / my child would not have occurred; (2) were it not for inflammation, as evidenced by antistreptococcal antibodies or other blood inflammatory tests, the obsessive-compulsive disorder in my patient / my child would not have occurred; and (3) were it not for the early diagnosis and treatment with antibiotics and/or immunomodulatory therapies, my patient / my child would not have been rescued from his obsessive-compulsive disorder symptoms.
Thus, when PANS/PANDAS doctors say their views are based on thousands of patients59,85,86 and personal experiences in clinic, it is not that they reject good clinical science or standard forms of causal reasoning. If they did, they would not have performed placebo-controlled trials, nor would they be continuing to seek funding for basic research and larger clinical trials. But it is also the case that, to date, the level II results do not support causal conclusions. Moving forward, recent mathematical advances have resulted in algorithmic approaches that researchers can use in certain circumstances to validate counterfactual causal statements without requiring a level II, controlled experimental intervention.78 Such analyses have been employed to evaluate relationships between pesticides and cognitive decline87 and blood pressure and stroke outcomes.88 This algorithmic approach, the mathematics behind the ladder of causation, may provide a better path forward into research on PANS/PANDAS etiology. However, skeptics will still seek confirmation of treatment recommendations by rigorous randomized controlled treatment trials.
Although innate patterns of tier 3 causal thinking generally serve us well throughout life, they can also result in wrong conclusions.89 It is the role of the scientific method to provide data supporting our understanding of causal pathways such that we avoid those mistakes. But, as science is a human enterprise, this also requires humble acknowledgment that there are many ways for us as scientists and clinicians to “fool ourselves,” particularly when we as clinicians sincerely wish our patients will get better. An important exercise then is to enumerate all of the other possible causes that could explain our observations.90 With regard to PANS/PANDAS, there are 2 fundamental features that may contribute to the observed effects and where attribution of causality to inflammation may be overestimated or in error. These could be studied more comprehensively by both skeptics and advocates as a means forward. The first involves acute onset. The second involves the treatment response with particular attention to placebos, nocebos, and the current clinical practice environment where the parents obtain information from the Internet prior to seeing a physician.
Pathways Forward in the PANS/PANDAS Controversy
Acute Onset
A key feature that induces the advocate’s view that PANS/PANDAS is distinct from other psychiatric illness is the rapid change in the child. If PANS/PANDAS is as common as advocates believe, the challenges for genetic research may rival those for genetic research of other DSM5 conditions. Large-scale genetics studies of Tourette syndrome and obsessive-compulsive disorder in progress may offer insights. But genetic variation may underlie increased risk factors that are not sufficient causes, so research should also emphasize nongenetic factors. The inflammatory hypothesis emphasizes infectious triggers preceding acute clinical presentations and maintaining ongoing symptoms. However, researchers should also study factors that may amplify early symptoms or diminish the probability of expected attenuation of acute-onset symptoms.
Consider the common clinical presentation of new-onset tics. This can range from one simple facial tic to one self-injurious tic. It can range from several simple facial or phonic tics to a cascade of multiple tics in multiple body areas. Today, the more severe the presentation, or the more anxious the care-giver, the more likely the family will seek information from the Internet and then seek medical attention from a physician or from a practitioner that will recommend nonvalidated therapies.91–93
In this clinical setting, the primary physician may suggest a watch and wait approach (“Tics are common in childhood and most often a cause is unknown; fortunately, they often get better on their own.”) Alternatively, a primary clinician may provide a possible biological reason (“This started suddenly: I think this could be PANDAS”), a biological test (“Let’s do a throat swab, but remember, negative test results can be false negatives”), and a biological treatment (“We will treat with 14 days of antibiotics and see how he does”). How will previsit parental expectations, influenced by prereading from Internet sites, interact with the watch and wait vs the PANS/PANDAS approaches? The PANS/PANDAS approach may create, or validate, a parental expectation that these symptoms are to be feared because the brain is under inflammatory attack and may diminish expectations for spontaneous resolution. They may ultimately set the child up for multiple courses of antibiotics and/or immune modulatory treatments in response to natural waxing and waning of symptoms. In considering one standard treatment, a selective serotonin reuptake inhibitor, a parent who reads expert opinions that children with PANS are extrasensitive to side effects of psychiatric medications42 may come to expect those side effects.
Taking this idea a step further, Table 4 shows a variety of possible sequential scenarios. Each of those in the table are commonly seen in child neurology clinics today, and can result in substantial frustration for families and physicians.
Table 4.
Possible Trajectories of DSM5 Diagnosis Where Context and Parental Expectation May Modify Symptom Attenuation.
| Setting/ context | Information source | Information provided | Parental response; expectation | Parental behavior | Child’s perception; outcome |
|---|---|---|---|---|---|
| 1990s pre-PANDAS; parents with no anxiety or OCD | Primary physician | Tics are common in children. In tic disorders, onset of new tics can be abrupt; a watch and wait approach is acceptable because spontaneous improvement commonly occurs | Reassured; things will improve | Models coping behaviors, fosters resilience; seeks professional help, eg, behavioral intervention or medication if symptoms severe; transmits positive expectations to child, teachers | “Everything will be OK”; tics and other symptoms (anxiety, disruptive behavior) gradually attenuate (although in Tourette cases they will recur), coping skills and resilience may develop |
| 1990s pre-PANDAS; parents with anxiety or OCD | Primary physician and/or specialist | Tics are common in children. In tic disorders, onset of new tics can be abrupt; a watch and wait approach is acceptable because spontaneous improvement commonly occurs | Anxious; anxiety decreases parental tolerance of tics; not satisfied by the lack of medical explanation; not able to work with child on coping, may seek second opinion | Transmits fears to child; becomes hypervigilant around the child’s tics, which acts as a form of suggestion | “This is scary, recovery may not be possible. My parents are afraid”; remains fearful, anxious, does not learn coping skills, perpetuation of tics and disruptive behaviors more likely |
| 2000s post PANDAS | Internet first | Tics and associated behaviors are result of brain infection and/or brain inflammation | Frightened. Every tic is sign that infection/inflammation is ongoing; child is helpless victim of biological factors and cannot learn to control symptoms or emotions; antibiotics or inflammatory medications are needed | Transmits fear, negative expectations to child; attends to tics and behaviors; tells child this is not their fault/they cannot control it; inadvertently reinforces symptoms | “Tics get me more attention from anxious parent and secondary gain such as missed school or less accountability for my behavior”; Tic and other symptoms increase |
| Primary physician and/or specialist | Tics can occur suddenly, a watch and wait approach is acceptable | Physician seen as “ill-informed”; as above | |||
| 2010 s post PANS | Internet first | Vaccines may have caused behavioral change | Feels angry and violated; expects there is no way that doctors can help, symptoms may be permanent, doctors may collude to hide truth | Puts video of child with alleged vaccine injury on social media site, gets positive attention / social reward online, does not get psychological help for child, initiates litigation | “My parents are scared. I must be sick—I need strong treatment. The world is overwhelming. OCD behaviors are comforting and irresistible.” OCD and other symptoms increase |
| Internet first, then PANS doctor | PANS may be caused by anything. Many tests (blood tests, hair/stool analysis) / treatments (supplements, steroids) are needed | Feels angry, fearful, and abused by traditional health care system, testing by PANS doctor validates concerns, parent feels listened to and validated by PANS doctor; expects ongoing treatments | Requests repeated courses of antibiotic or immunomodulatory treatment, joins advocacy group |
Abbreviations: OCD, obsessive-compulsive disorder; PANDAS, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections; PANS, pediatric acute onset neuropsychiatric syndrome.
Table 4 provides questions for research that could clarify, for example, when both skeptics and advocates should consider noninflammatory factors that could amplify symptoms, even in cases triggered by infections. For example, how does parental anxiety at the time of the child’s tic onset relate to impairment 6 months later? Are symptoms more likely to amplify and/or less likely to wane in the presence of more anxious caregivers? How does a parent’s attitude toward stigmatization of mental illness at obsessive-compulsive disorder onset relate to obsessive-compulsive disorder severity or impairment 6 months later? Are symptoms more likely to persist if parents are uncomfortable with psychiatric explanations and/or believe an underappreciated external biological process must be ongoing? Finally, if there is a possibility of secondary financial gain, as in vaccine lawsuits, are parents less likely to pursue psychological or psychopharmacologic interventions?
Treatment Responses
There are a large number of ways in which expectations about treatment responses may lead to placebo and nocebo effects,94,95 as represented in Figure 4. Placebo and nocebo responses are positive and negative changes that are not explained by the mechanism of action of the treatment. In a clinical trial with informed consent and a 1:1 ratio of active to placebo treatment, the parental expectation of benefit would ideally be neutral—canceled out in a way that allows causal attribution of symptom change to biological effects of treatment. Beneficial effects in studies with substantial and equivalent treatment and placebo effects could be due to general benefits of frequent contact with the study team, or perhaps a high level of optimism that the roll of the randomization dice has been favorable, both of which might have occurred in a high-profile study of pediatric migraine in which the active treatment to placebo ratio was 4:1.37 In routine patient care, expectation of placebo and nocebo may play a large role, contributing to, augmenting, or even completely confounding the biological effects of the treatment.
Figure 4.
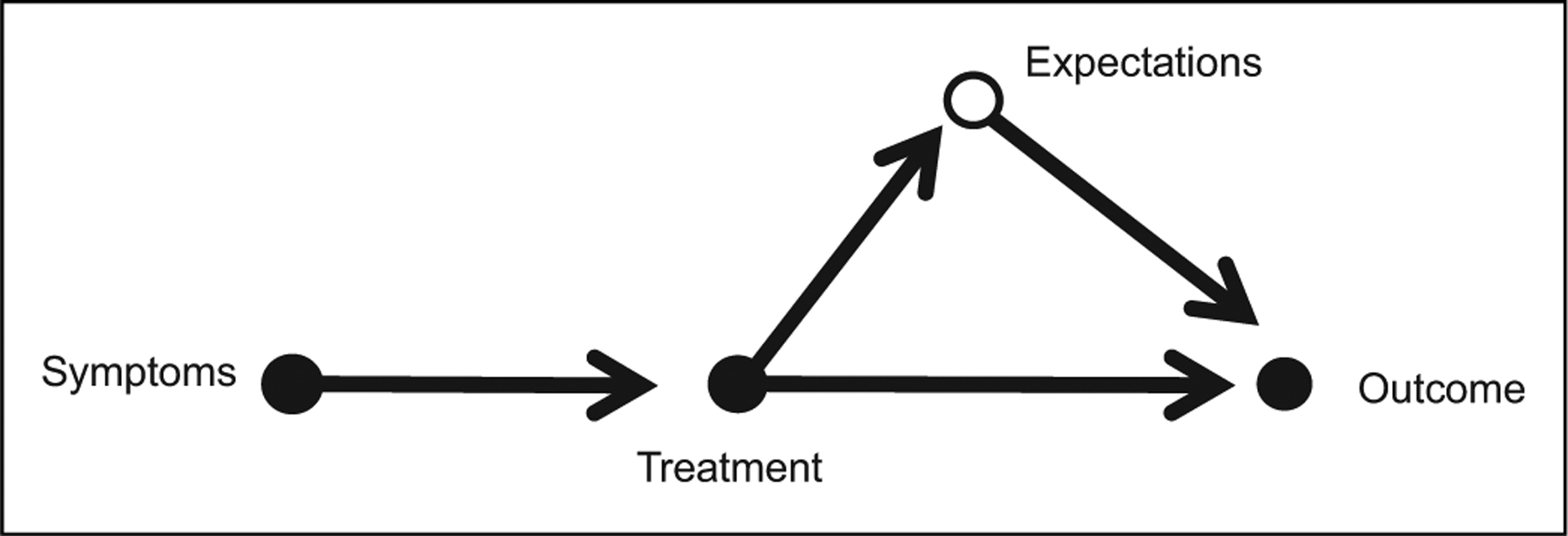
Causal pathway for PANS/PANDAS treatment. Treatment may exert a direct effect and produce the desired outcome. Alternatively, parental expectation may play a large role, with placebo or nocebo effects mediating positive or negative outcomes. In cases where the treatment is inert, all of the benefit may accrue due to parent expectation.
Source: Figure modified from Shahar et al.96
Placebo and nocebo effects may be established, reinforced, and perpetuated as conditioned responses. Examples of this, shown in Table 5, are not exhaustive but represent a variety of pathways whereby causes outside of straightforward pharmacologic ones may contribute to outcomes. In some cases, expectation may be the sole cause of the benefit. In others, expectation may mediate responses. A better understanding of the biology underlying these paths may also guide both advocates and skeptics in designing effective treatment strategies. As can be seen, a large number of combinations of events involving prior biology and prior expectations might be expected to result in PANS/PANDAS treatment seeking over an extended period of time.
Table 5.
Possible Trajectories of DSM5 Diagnosis Treatment and Response Where Patient/Parent/Clinician Expectation May Play a Role.
| True diagnosis | Parent (clinician) believed diagnosis | Responder category | Treatment offered | Treatment expectation | Treatment result | Main cause of result | Future parent (clinician) expectation |
|---|---|---|---|---|---|---|---|
| Idiopathic / familial / genetic | Same | Treatment responder | SSRI ± CBT | Positive | Positive | Pharmacology | Conditioned for future placebo responses |
| Idiopathic / familial / genetic | Same | Treatment nonresponder | SSRI ± CBT | Positive | Positive | Patient expectation | Conditioned for future placebo responses |
| Idiopathic / familial / genetic | Same | Treatment nonresponder | SSRI ± CBT | Positive | Negative—no benefit | Wrong treatment | May need alternative treatment |
| Idiopathic / familial / genetic | Same | Treatment nonresponder | SSRI ± CBT | Positive | Negative—adverse events | Pharmacology | Conditioned for future nocebo responses |
| Idiopathic / familial / genetic | PANS | Nocebo responder | SSRI | Negative | Negative—adverse events | Patient expectation | Conditioned for future nocebo responses |
| Idiopathic / familial / genetic | PANS | Placebo responder | abx ± immuno | Positive | Positive | Patient expectation | Conditioned for future placebo responses |
| Idiopathic / familial / genetic | PANS | Placebo nonresponder | abx ± immuno | Positive | Negative | Wrong treatment | Depends on strength of initial belief |
| Idiopathic / familial / genetic | PANS relapse | Conditioned placebo responder | abx ± immuno | Positive | Positive | Patient expectation | Conditioned for future placebo responses |
| Idiopathic / familial / genetic | PANS relapse | Treatment responder | abx ± immuno | Positive | Negative | Wrong treatment | Depends on strength of initial belief |
Abbreviations: Abx, antibiotics; CBT, cognitive behavioral therapy; immune, immunomodulatory (eg, intravenous immunoglobulin); PANS, pediatric acute onset neuropsychiatric syndrome; SSRI, selective serotonin reuptake inhibitor.
Summary
Skeptics and advocates, employing the same definitions of PANS/PANDAS, currently argue for opposing diagnostic and treatment practices. Both use the same definitions, but, after both designed and participated in the most intensive prospective studies,44,45 collaborations have been limited. Advocates have leap-frogged the published literature in even more prevalent conditions such as depression and promulgated treatment recommendations despite negative clinical trials to date. Advocates employ causal reasoning similar to that used for anti–NMDA receptor encephalitis, but skeptics rightly note that objective, quantifiable data about critical nodes in causal pathways is lacking for PANS/PANDAS.
A better understanding of factors that may mediate symptom acceleration and chronicity, or limit symptom attenuation, could yield improved treatment strategies for these patients. Relevant research questions include: Are there current physician practices that increase nocebo effects? Can patients most prone to nocebo effects be identified, and strategies for mitigation be tested? Could the placebo effect’s biological mechanisms be identified and utilized to improve the outcomes of children with acute-onset obsessive-compulsive disorder or tics? Are placebo effects causal in isolation or are they mediating pharmacologic benefits? Are there safer, less costly treatments that would be equally effective?
The surest path forward for PANS/PANDAS science to improve the care of affected children is not epidemiologic studies and not open-label treatment studies. Rather, as in other DSM5 conditions, if PANS/PANDAS is really common, the best approach will have to be large, randomized, placebo-controlled trials. In addition, it would behoove skeptics and advocates to collaborate in pursuing both good science and sound patient care, while eschewing pseudoscientific approaches and calling out profit-seeking behaviors such as cash-pay clinics, Internet diagnoses, and expert witness testimony. Using the current controversy as an opportunity to understand placebo and nocebo effects may also yield substantial benefit.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests
The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Gilbert has received honoraria and/or travel support from the Tourette Association of America / Centers for Disease Control and Prevention, the American Academy of Pediatrics, the Child Neurology Society, the American Academy of Neurology, and the Texas Neurological Society. He has received compensation for expert testimony for the US National Vaccine Injury Compensation Program, through the Department of Health and Human Services, and for the US Armed Services / US Attorney’s Office of Virginia. Dr Gilbert has received research support from the NIH (NIMH). He has received funding for work as a clinical trial site-investigator from Ecopipam Pharmaceuticals (clinical trial, Tourette syndrome) and EryDel (clinical trial, Ataxia Telangiectasia). He has received book royalties from Elsevier and Wolters Kluwer.
References
- 1.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4): 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer HS. Autoantibody-associated movement disorders in children: proven and proposed. Semin Pediatr Neurol. 2017;24(3): 168–179. [DOI] [PubMed] [Google Scholar]
- 3.Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev. 2018;86: 51–65. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert DL, Mink JW, Singer HS. A pediatric neurology perspective on pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection and pediatric acute-onset neuropsychiatric syndrome. J Pediatr. 2018;199: 243–251. [DOI] [PubMed] [Google Scholar]
- 5.Smith W Case of insanity depending on syphilitic infection. Br Med J. 1868;2(393):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheadle WB. The various manifestations of the rheumatic state as exemplified in childhood and early life. Lancet. 1889;1(821): 921–927. [Google Scholar]
- 7.Hobbs A The role of wound infection as a factor in the causation of insanity. Am J Psychiatry. 1899;56(1):89–94. [Google Scholar]
- 8.Briggs LV. A consideration of auto-intoxication and auto-infection as cause of various mental disorders. Boston Med Surg J. 1905;152(1):1–5. [Google Scholar]
- 9.Cotton HA. The Defective, Delinquent, and Insane; the Relation of Focal Infections to Their Causation, Treatment, and Prevention. Princeton, NJ: Princeton University Press; 1921. [Google Scholar]
- 10.French JG. Infection of the nasal sinuses in relation to insanity. Lancet. 1927;210(5418):13–14. [Google Scholar]
- 11.Selling L The role of infection in the etiology of tics. Arch Neurol Psychiatry. 1929;22:1163–1171. [Google Scholar]
- 12.Osler W On Chorea and Choreiform Affectations. Philadelphia, PA: P. Blakiston, Sons & Co; 1894. [Google Scholar]
- 13.Diefendorf AR. Mental symptoms of acute chorea. J Nerv Ment Disord. 1912;39:161–172. [Google Scholar]
- 14.Ebaugh FG. Neuropsychiatric aspects of chorea in children. JAMA. 1926;87:1083–1088. [Google Scholar]
- 15.Freeman JM, Aron AM, Collard JE, MacKay MC. The emotional correlates of Sydenham’s chorea. Pediatrics. 1965;35:42–49. [PubMed] [Google Scholar]
- 16.Shpitser I Disease models, part I: Graphical models. Medical Imaging Informatics. Boston, MA: Springer; 2010:335–369. [Google Scholar]
- 17.Coppen EM, Roos RA. Current pharmacological approaches to reduce chorea in Huntington’s disease. Drugs. 2017;77(1):29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806–1818. [DOI] [PubMed] [Google Scholar]
- 19.Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541–1551. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert DL. Acute and chronic chorea in childhood. Semin Pediatr Neurol. 2009;16(2):71–76. [DOI] [PubMed] [Google Scholar]
- 21.Axley J Rheumatic chorea controlled with haloperidol. J Pediatr. 1972;81(6):1216–1217. [DOI] [PubMed] [Google Scholar]
- 22.Aronson N, Douglas HS, Lewis JM. Cortisone in Sydenham’s chorea; report of two cases. JAMA. 1951;145(1):30–33. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz H Chorea gravis (Sydenham’s chorea) treated with cortisone and ascorbic acid. Can Med Assoc J. 1951;65(2):150–151. [PMC free article] [PubMed] [Google Scholar]
- 24.Sigwald J, Giroux M. Very grave form of Sydenham’s chorea cured in a few days by cortisone therapy [in French]. Rev Neurol (Paris). 1951;85(4):278–280. [PubMed] [Google Scholar]
- 25.Paz JA, Silva CA and Marques-Dias MJ. Randomized double-blind study with prednisone in Sydenham’s chorea. Pediatr Neurol. 2006;34(4):264–269. [DOI] [PubMed] [Google Scholar]
- 26.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264–271. [DOI] [PubMed] [Google Scholar]
- 27.Swedo SE, Leckman JF, Rose NR. From Research Subgroup to Clinical Syndrome: Modifying the PANDAS Criteria to Describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr Therapeut. 2012;2:113. [Google Scholar]
- 28.Kurlan R Tourette’s syndrome and “PANDAS”: will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology. 1998;50(6): 1530–1534. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert DL, Kurlan R. PANDAS: horse or zebra? Neurology. 2009;73(16):1252–1253. [DOI] [PubMed] [Google Scholar]
- 30.Singer HS. PANDAS and immunomodulatory therapy. Lancet. 1999;354(9185):1137–1138. [DOI] [PubMed] [Google Scholar]
- 31.Budman C, Coffey B, Dure L, et al. Regarding “antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders.” Biol Psychiatry. 2005;58(11):917; author reply 918–919. [DOI] [PubMed] [Google Scholar]
- 32.Mink JW. Intravenous immunoglobulin is not an effective treatment for pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(10):837–838. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DR, Kurlan R, Leckman J, Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis. 2010;50(4):481–490. [DOI] [PubMed] [Google Scholar]
- 34.Gabbay V, Coffey BJ, Babb JS, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcus: comparison of diagnosis and treatment in the community and at a specialty clinic. Pediatrics. 2008;122(2):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pupillo J PANDAS/PANS treatments, awareness evolve, but some experts skeptical. AAP News. American Academy of Pediatrics; 2017. [Google Scholar]
- 36.Bejerot S, Hesselmark E. The Cunningham Panel is an unreliable biological measure. Transl Psychiatry. 2019;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powers SW, Coffey CS, Chamberlin LA, et al. Trial of amitripty-line, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376(2):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362(9):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallee F, Kohegyi E, Zhao J, et al. Randomized, double-blind, placebo-controlled trial demonstrates the efficacy and safety of oral aripiprazole for the treatment of Tourette’s disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017; 27(9):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280(20):1752–1756. [DOI] [PubMed] [Google Scholar]
- 41.International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23(5):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto MA. Antibiotics have a role in PANS even with no infection. Clinical Psychiatry News. 2017. [Google Scholar]
- 43.Berende A, Ter Hofstede HJM, Vos FJ, et al. Effect of prolonged antibiotic treatment on cognition in patients with Lyme borreliosis. Neurology. 2019;92(13):e1447–e1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurlan R, Johnson D, Kaplan EL; Tourette Syndrome Study Group. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. 2008;121(6):1188–1197. [DOI] [PubMed] [Google Scholar]
- 45.Leckman JF, King RA, Gilbert DL, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2011;50(2): 108–118.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooperstock MS, Swedo SE, Pasternack MS, Murphy TK; for the PANS/PANDAS Consortium. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part III—treatment and prevention of infections. J Child Adolesc Psychopharmacol. 2017;27(7):594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer HS, Giuliano JD, Zimmerman AM, Walkup JT. Infection: a stimulus for tic disorders. Pediatr Neurol. 2000;22(5):380–383. [DOI] [PubMed] [Google Scholar]
- 48.Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R. Moving from PANDAS to CANS. J Pediatr. 2012;160(5):725–731. [DOI] [PubMed] [Google Scholar]
- 49.Shulman ST. Pediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS): update. Curr Opin Pediatr. 2009;21(1):127–130. [DOI] [PubMed] [Google Scholar]
- 50.Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME. Association of streptococcal throat infection with mental disorders: testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry. 2017;74(7): 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dileepan T, Smith ED, Knowland D, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J Clin Invest. 2016;126(1):303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy TK, Parker-Athill EC, Lewin AB, Storch EA, Mutch PJ. Cefdinir for recent-onset pediatric neuropsychiatric disorders: a pilot randomized trial. J Child Adolesc Psychopharmacol. 2015; 25(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy TK, Lewin AB, Parker-Athill EC, Storch EA, Mutch PJ. Tonsillectomies and adenoidectomies do not prevent the onset of pediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Pediatr Infect Dis J. 2013;32(8):834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavone P, Rapisarda V, Serra A, et al. Pediatric autoimmune neuropsychiatric disorder associated with group a streptococcal infection: the role of surgical treatment. Int J Immunopathol Pharmacol. 2014;27(3):371–378. [DOI] [PubMed] [Google Scholar]
- 55.Williams KA, Swedo SE, Farmer CA, et al. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Am Acad Child Adolesc Psychiatry. 2016;55(10): 860–867.e2. [DOI] [PubMed] [Google Scholar]
- 56.Frankovich J, Swedo S, Murphy T, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part II—use of immunomodulatory therapies. J Child Adolesc Psychopharmacol. 2017;27(7):574–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang K, Frankovich J, Cooperstock M, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015;25(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol. 2015;25(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calaprice D, Tona J, Parker-Athill EC, Murphy TK. A survey of pediatric acute-onset neuropsychiatric syndrome characteristics and course. J Child Adolesc Psychopharmacol. 2017;27(7): 607–618. [DOI] [PubMed] [Google Scholar]
- 60.Murphy TK, Brennan EM, Johnco C, et al. A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2017;27(7):640–651. [DOI] [PubMed] [Google Scholar]
- 61.Advisory Commission on Childhood Vaccines. Rockville, MD: Health Resources and Services Administration, Department of Health and Human Services; 2017. [Google Scholar]
- 62.Decision on Entitlement, No. 15–1146 V. United States Court of Federal Claims OoSM. 2017. [Google Scholar]
- 63.Hornig M The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013; 25(4):488–795. [DOI] [PubMed] [Google Scholar]
- 64.Bos-Veneman NG, Olieman R, Tobiasova Z, et al. Altered immunoglobulin profiles in children with Tourette syndrome. Brain Behav Immun. 2011;25(3):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. [DOI] [PubMed] [Google Scholar]
- 67.Kissinger B, Dwyer M. Illinois becomes 1st state requiring insurers to cover treatment for kids with PANDAS. WGN TV. 2017. [Google Scholar]
- 68.Taylor RB, Mason W, Kong K, Wennberg R. Reversible paraneoplastic encephalomyelitis associated with a benign ovarian teratoma. Can J Neurol Sci. 1999;26(4):317–320. [DOI] [PubMed] [Google Scholar]
- 69.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armangue T, Titulaer MJ, Malaga I, et al. Pediatric anti-N-methyl-d-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. 2013;162(4): 850–856.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groselj U, Tansek MZ, Battelino T. Fifty years of phenylketonuria newborn screening—a great success for many, but what about the rest? Mol Genet Metab. 2014;113(1–2):8–10. [DOI] [PubMed] [Google Scholar]
- 74.la Marca G Mass spectrometry in clinical chemistry: the case of newborn screening. J Pharm Biomed Anal. 2014;101:174–182. [DOI] [PubMed] [Google Scholar]
- 75.Hesselmark E, Bejerot S. Biomarkers for diagnosis of Pediatric Acute Neuropsychiatric Syndrome (PANS)—sensitivity and specificity of the Cunningham Panel. J Neuroimmunol. 2017;312: 31–37. [DOI] [PubMed] [Google Scholar]
- 76.Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med. 2002;156(4):356–361. [DOI] [PubMed] [Google Scholar]
- 77.Kopeloff N, Kirby GH. Focal infection and mental disease. Am J Psychiatry. 1923;80(2):149–197. [Google Scholar]
- 78.Pearl J, Mackenzie D. The Book of Why: The New Science of Cause and Effect. New York, NY: Basic Books; 2018. [Google Scholar]
- 79.Swedo SE, Frankovich J, Murphy TK. Overview of treatment of pediatric acute-onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2017;27(7):562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wakeford R. Association and causation in epidemiology—half a century since the publication of Bradford Hill’s interpretational guidance. J R Soc Med. 2015;108(1):4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ioannidis JPA. The proposal to lower P value thresholds to .005. JAMA. 2018;319(14):1429–1430. [DOI] [PubMed] [Google Scholar]
- 82.Hellmuth J, Rabinovici GD, Miller BL. The rise of pseudomedicine for dementia and brain health. JAMA. 2019;321(6):543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789–800. [DOI] [PubMed] [Google Scholar]
- 84.Thienemann M, Murphy T, Leckman J, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part I—psychiatric and behavioral interventions. J Child Adolesc Psychopharmacol. 2017;27(7):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown K, Farmer C, Farhadian B, Hernandez J, Thienemann M, Frankovich J. Pediatric acute-onset neuropsychiatric syndrome response to oral corticosteroid bursts: an observational study of patients in an academic community-based PANS clinic. J Child Adolesc Psychopharmacol. 2017. ’27(7):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown KD, Farmer C, Freeman GM Jr, et al. Effect of early and prophylactic nonsteroidal anti-inflammatory drugs on flare duration in pediatric acute-onset neuropsychiatric syndrome: an observational study of patients followed by an academic community-based pediatric acute-onset neuropsychiatric syndrome clinic. J Child Adolesc Psychopharmacol. 2017;27(7):619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paul KC, Ling C, Lee A, et al. Cognitive decline, mortality, and organophosphorus exposure in aging Mexican Americans. Environ Res. 2018;160:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pezzini A, Grassi M, Del Zotto E, et al. Influence of acute blood pressure on short- and mid-term outcome of ischemic and hemorrhagic stroke. J Neurol. 2011;258(4):634–640. [DOI] [PubMed] [Google Scholar]
- 89.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. [DOI] [PubMed] [Google Scholar]
- 90.Feynman RP, Robbins J, Sturman H, Löhnberg A. The Pleasure of Finding Things Out. Amsterdam: Nieuw Amsterdam; 2005. [Google Scholar]
- 91.Wingerter M Oklahoma state-funded program sends out claim donkey milk treats disease. The Oklahoman. 2018. [Google Scholar]
- 92.Yvon S, Olier M, Leveque M, et al. Donkey milk consumption exerts anti-inflammatory properties by normalizing antimicrobial peptides levels in Paneth’s cells in a model of ileitis in mice. Eur J Nutr. 2018;57(1):155–166. [DOI] [PubMed] [Google Scholar]
- 93.Lewis A PANS/PANDAS. https://sanctafamiliacenter.com/specialty/panspandas/.
- 94.Kirsch I Response expectancy and the placebo effect. Int Rev Neurobiol. 2018;138:81–93. [DOI] [PubMed] [Google Scholar]
- 95.Dodd S, Dean OM, Vian J, Berk M. A review of the theoretical and biological understanding of the nocebo and placebo phenomena. Clin Ther. 2017;39(3):469–476. [DOI] [PubMed] [Google Scholar]
- 96.Shahar E, Shahar DJ. Causal diagrams, the placebo effect, and the expectation effect. Int J Gen Med. 2013;6:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]


