Abstract
Th17 cells have been implicated in the pathogenesis of numerous inflammatory and autoimmune conditions. At the ocular surface, Th17 cells have been identified as key effector cells in chronic ocular surface disease. Evidence from murine studies indicates that following differentiation and expansion, Th17 cells migrate from the lymphoid tissues to the eye, where they release inflammatory cytokines including, but not limited to, their hallmark cytokine IL-17A. As the acute phase subsides, a population of long-lived memory Th17 cells persist, which predispose hosts both to chronic inflammation and severe exacerbations of disease; of great interest is the small subset of Th17/1 cells that secrete both IL-17A and IFN-γ in acute-on-chronic disease exacerbation. Over the past decade, substantial progress has been made in deciphering how Th17 cells interact with the immune and neuroimmune pathways that mediate chronic ocular surface disease. Here, we review (i) the evidence for Th17 immunity in chronic ocular surface disease, (ii) regulatory mechanisms that constrain the Th17 immune response, and (iii) novel therapeutic strategies targeting Th17 cells.
I. Introduction
I.A. Socioeconomic burden of chronic ocular surface disorders
Anatomically, the ocular surface comprises the cornea, conjunctiva, lacrimal glands, accessory lacrimal glands, meibomian glands, lacrimal drainage apparatus and eyelids, and plays a fundamental role in protecting the refractive properties of the cornea [1]. The compromised function of any of these components may lead to tear film instability and ocular surface breakdown, which may in turn result in ocular discomfort, as well as impaired vision and decreased quality of life [2,3]. Ocular surface disorders include a broad spectrum of conditions, including but not limited to dry eye disease (DED), blepharitis, lid malposition, meibomian gland dysfunction, allergic conjunctivitis, contact lens wear, refractive surgery complications, exposure keratopathy, thyroid eye disease, graft-versus-host disease (GVHD) and irritation secondary to chronic use of eye drops containing preservatives like benzalkonium chloride [3,4]. Symptoms arising from ocular surface disorders include grittiness, burning, photophobia, dryness, foreign body sensation and visual disturbance [3].
Of all the optical interfaces of the eye, it is the tear film that makes the maximum contribution to the eye’s refractive power, as the passage of light from air to water is associated with a large shift in refractive index [5]. Tear film instability induced by ocular surface dysfunction may substantially impair the refractive function of the eye. Hence, the functional visual acuity (a simulation of daily gazing, measured after sustained eye opening for 10-20 seconds) is significantly impaired in DED patients [6]. DED has been shown to impose a substantial adverse impact on quality of life, not only due to visual quality change but also due to associated sleep and psychological disorders, in particular with major depressive disorder and generalized anxiety disorder [7,8].
Extrapolating from a study of approximately ten million US participants, sixteen million people are projected to be suffering from DED in the United States [9]. The average cost of managing DED is estimated to be ~$11,000 per patient, equivalent to more than $55 billion for the entire US population [10]. The anatomical and physiological changes that occur with aging predispose patients to ocular surface disease. Thus, a higher prevalence of DED is seen in patients aged greater than 50 years (11.7%), compared those aged 2-17 years (0.2%) [9]. As the aging population expands, the socioeconomic burden of DED is anticipated to grow further. Interestingly, a most recent study using a smartphone application survey (DryEyeRhythm) also suggests younger age as a risk factor for the development of DED, which may be attributed to increased digital device use since childhood [11]. Nevertheless, the DED in these younger groups is thought due to increased staring (from digital screen work) which is different from the more severe aqueous insufficient, immune mediated forms of DED we see more of in the elderly. It is important to note that the societal expenses of DED are not limited to direct costs (healthcare resources and medication expenditure), but also includes the indirect costs of reduced productivity, as DED results in an estimated 2-5 days off per worker per year [4,5,10,12].
I.B. T cell-mediated inflammation in ocular surface disease
The corneal and conjunctival epithelia serve as a physical barrier against environmental, microbial and inflammatory stimuli [13]. Both intrinsic and extrinsic insults may trigger immune cascades at the ocular surface, either by the activation of resident immune cells or by inducing corneal and conjunctival epithelial cells to express pro-inflammatory cytokines [13,14]. Hyperosmolarity of the tear film acts as an inflammatory stressor at the ocular surface, resulting in increased expression of pro-inflammatory cytokines (i.e., TNFα, IL-1β, IL-6), APC maturation and migration to the draining lymph nodes, T cell priming, and generation of effector T cells [13,15,16].
The paradigm of DED as a T cell-mediated disease is grounded in seminal experiments showing that adoptive transfer of T cells isolated from DED mice to naïve nude mice directly and specifically induce DED even if recipients were not exposed to desiccating stress [17]. Of the various T cell subsets, support for the involvement of Th1 cells in the pathogenesis of DED is provided in part by studies showing elevated levels of the Th1 signature cytokine IFN-γ at the ocular surface of murine and human DED [18,19]. However, our group has not observed a change in Th1 cell frequencies in the draining lymph nodes of DED mice compared to naïve [20]. In contrast, work by our group and others have shown a marked increase in Th17 cell frequencies in DED, and moreover have shown that these Th17 cells are relatively resistant to suppression by Tregs [20,21]. Interestingly, our group has further identified Th17-derived IFN-γ-secreting Th17/1 as a major source of increased IFN-γ in DED [22]. Following exposure to desiccating stress, ocular tissues express higher levels of the Th17-associated genes IL-6, TGF-β, IL-23 and IL-17A [23]. Th17 frequencies are increased not only at the ocular surface tissues, but also in the draining lymph nodes [21]. A significantly higher level of Th17-associated cytokines, including IL-17A and IL-22, are observed in tear samples of DED patients relative to healthy controls [24].
T cell-mediated immune responses have been implicated in a wide range of ocular surface disorders beyond ‘simple’ DED, including ocular graft-versus-host disease (oGVHD) and allergic eye disease [25,26]. oGVHD is a common and potentially severe complication of allogeneic hematopoietic stem cell transplantation (HSCT) [27,28]. Patients who receive allogeneic HSCT develop oGVHD in 40-60% of cases, which significantly impairs their quality of life [29,30]. Using a murine model of hematopoietic stem cell transplantation based on major histocompatibility match and minor histocompatibility mismatch, Heretes et al. have demonstrated the infiltration of donor CD4 (and also CD8) T cells in GVHD-associated corneal ulcerations [25]. The simultaneous observation of macrophages in the cornea suggests the interaction of infiltrating T cells with macrophages, driving a Th1 alloreactive response [25]. T cells have also been implicated in allergic eye disease, with polymorphonuclear neutrophils causing meibomian gland obstruction in Th17-mediated manner [26].
II. Pathogenic function of Th17 immunity in ocular surface disease
II.A. Autoreactive Th17 differentiation and activation
Th17 cells play a crucial role in host defense, yet these cells may become pathologic under certain conditions, and are known to promote inflammation in various autoimmune diseases [31–34]. During Th17 cell development, naïve T cells migrate from the thymus and differentiate into Th17 cells in lymphoid tissue, responding to antigens presented by activated antigen-presenting cells (APCs) and cues from the local cytokine milieu [35].
Increased levels of IL-6 and IL-23 have been reported in inflammatory ocular surface diseases, and these cytokines are produced by corneal epithelial cells as well as ocular surface-resident APCs [36,37]. Zheng et al. reported a significant upregulation of IL-6 and IL-23 production by corneal epithelial cells on exposure to hyperosmolar stress, leading to an increase in Th17 cell differentiation [19]. In homeostatic conditions, the cornea contains a heterogenous population of highly immature APCs with limited capacity to stimulate T cells. However, these cells acquire MHC class II, CD80 and CD86 expression upon exposure to inflammatory stress [38]. The upregulation of MHC class-II and costimulatory molecules expression has been attributed to IL-1, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, CD40L, and lipopolysaccharide [39–41] (Fig. 1). In the afferent arm of the Th17 response, the mature APCs migrate from the ocular surface to draining lymphoid tissue where they induce Th17 cell activation [42]. Kodati et al. demonstrated the functional importance of the chemokine receptor CCR7 in APC trafficking by treating dry eye mice with a neutralizing anti-CCR7 antibody, which yielded a decrease in APC migration to the lymph nodes, a reduced Th17 response and ameliorated clinical disease [43] (Figs. 1 & 2). Th17 cell differentiation is contingent upon exposure to certain cytokines, specifically IL-1β, IL-6, IL-21, IL-23, and TGF-β [35]. IL-6 serves to tilt the regulatory T cells (Tregs)/Th17 balance towards Th17 cells, and IL-23 induces the transcriptional regulators STAT3 and RORγt, which are crucial to Th17 cell maturation and function [44,45] (Fig. 2).
Figure 1: In the afferent arm of the Th17 response, activated APCs are poised to migrate from the ocular surface to the draining lymphoid tissue.
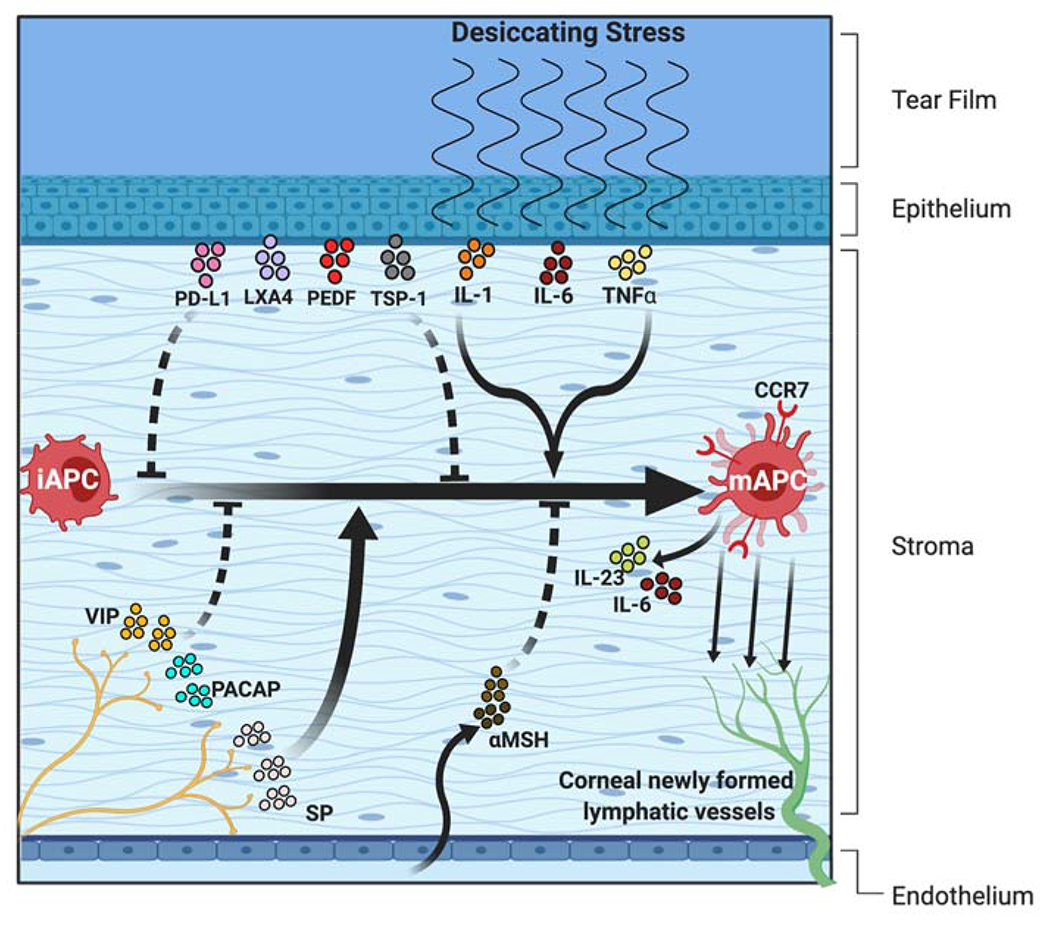
In homeostatic conditions, the cornea contains a heterogenous population of immature antigen presenting cells (iAPCs) with limited capacity to stimulate T cells. However, these cells acquire MHC class II, CD80 and CD86 expression on exposure to inflammatory stress to generate mature APCs (mAPCs). The upregulated expression of MHC class-II and costimulatory molecules has been attributed to IL-1, IL-6, and TNF-α from corneal epithelial cells, Substance P (SP) from the corneal nerves, and granulocyte-macrophage colony-stimulating factor (GM-CSF) from Th17 cells (not shown in the figure). Regarding immunoregulatory factors, various molecules including PD-L1, LXA4, PEDF, and TSP-1 derived from corneal epithelium, VIP and PACAP expressed by corneal nerves, and αMSH from the aqueous humor play an immunosuppressive role by limiting inflammatory cytokine-induced APC maturation. The chemokine receptor CCR7 expressed by mature APCs directs their trafficking to the lymph nodes via newly formed corneal lymphatic vessels.
Figure 2: Generation of autoreactive Th17 effector and memory cells.
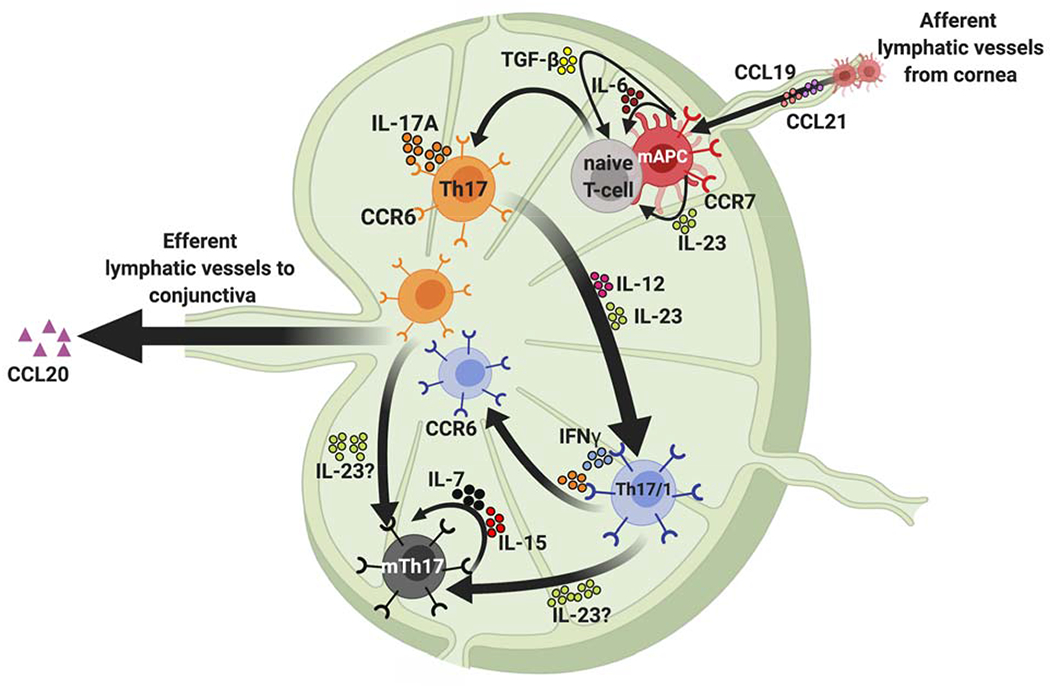
During Th17 cell development, naïve T cells migrate from the thymus and differentiate into Th17 cells in the draining lymphoid tissue on engagement with activated APCs and exposure to the local inflammatory cytokine milieu. TGF-β, IL-6 and IL-23 expressed by the APCs in the draining lymph node play a critical role in the development of pathogenic Th17 cells. Once generated, the migration of CCR6+ Th17 cells to the ocular surface is facilitated by the attractant chemokine CCL-20 at the ocular surface. In addition, activated Th17 cells produce IFN-γ under the influence of IL-12 and IL-23, and these Th17/1 cells contribute to disease exacerbation. In the chronic stage, effector Th17 cells develop into memory Th17 cells (mTh17), which are generated from both Th17 and Th17/1 subsets and maintained by IL-7 and IL-15. These mTh17 cells mediate the chronic inflammation in DED.
II.B. Migration and effector response of Th17 at the ocular surface
Following differentiation and expansion, Th17 cells migrate from the lymph nodes to the ocular surface. This migratory process is mediated by the CCR6/CCL20 chemokine axis [46]. Th17 cells express the chemokine receptor CCR6, and in DED the cornea and conjunctiva upregulate expression of the corresponding CCR6 ligand, CCL20. Previous work from our laboratory has demonstrated a decrease in Th17 cell migration to the ocular surface along with a corresponding reduction in disease severity upon disruption of CCR6/CCL20 binding using a topical CCL20 neutralizing antibody [46] (Figs. 2 & 3). These results are supported by a subsequent report in which it was shown that CCR6 knockout mice develop minimal signs of DED, and that adoptive transfer of CD4+ T cells from CCR6 knockouts to wild-type mice fails to induce disease [47].
Figure 3: Migration and effector response of Th17 cells at the ocular surface.
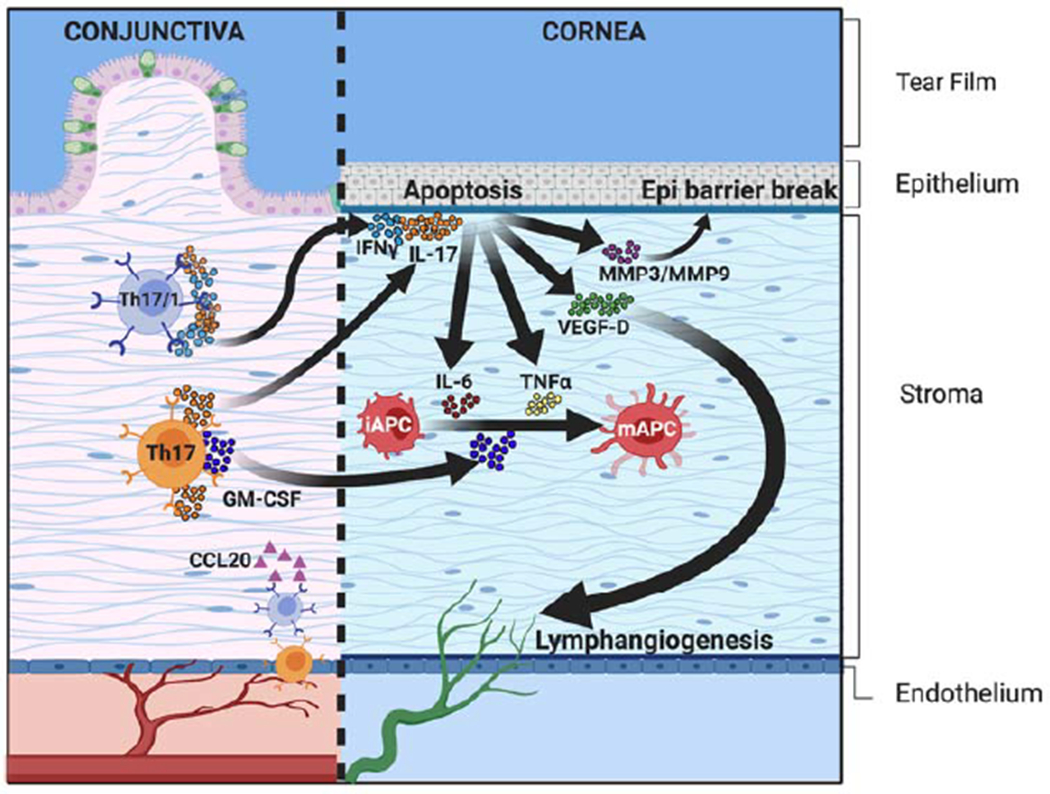
Th17 and Th17/1 cells express high levels of IL-17A (by Th17 and Th17/1) and IFN-γ (by Th17/1 cells). These inflammatory cytokines mediate the apoptosis of corneal epithelial cells both directly and indirectly (via the proteolytic matrix metalloproteinases [MMP-3 and MMP-9]). IL-17A also contributes to lymphangiogenesis by upregulating the expression of VEGF-D in the cornea, which facilitates the continuous migration of pro-inflammatory cells from the ocular surface to the draining lymphoid tissue. Th17 cells express GM-CSF, which promotes the maturation of iAPCs, and further increases ocular surface inflammation.
In 2009, Chauhan et al. were the first to demonstrate the role for Th17 cells in the immunopathogenesis of ocular surface disease [21]. Using a murine model of DED, the authors showed increased frequencies of Th17 cells and elevated levels of IL-17A, the signature effector cytokine of Th17 cells, in the draining lymph nodes and conjunctiva of DED mice. The functional relevance of Th17 cells was demonstrated by in vivo neutralization of IL-17A, which reduced Th17 cell expansion and clinical disease severity [21]. The pathogenic role of Th17 cells in ocular surface disease was supported by a report by De Paiva et al., in which the authors demonstrated elevated levels of Th17-associated cytokines at the ocular surface in humans with DED and in an animal model of DED, where they additionally observed increased levels of matrix metalloproteinases (MMPs) [23]. The authors showed that desiccating stress leads to induction of Th17 immunity and that blockade of IL-17A results in a significant reduction in expression of MMP-3 and MMP-9, with improvement in corneal epitheliopathy (Fig. 3). These findings implicate Th17 cells in the disruption of corneal epithelial barrier function, thereby contributing to the pathogenesis of ocular surface disease [23]. The De Paiva group has also demonstrated a Th17 cell response in an age-dependent Sjögren’s syndrome-like dry eye model developed in CD25 knockout mice, and interestingly, the level of clinical disease correlated with IL-17A expression [48].
As the signature effector cytokine of Th17 cells, IL-17A contributes to ocular surface disease pathogenesis in multiple ways. In addition to antagonizing Treg function and upregulating matrix metalloproteinases as described above, this cytokine also contributes to ocular surface disease by promoting lymphangiogenesis and B cell proliferation [49,50]. We have demonstrated lymphangiogenesis in a dry eye model, which contributes to the induction and maintenance of an immunoinflammatory response by facilitating the trafficking of APCs from the ocular surface to the draining lymphoid tissue [51]. Chauhan et al. have shown that IL-17A promotes corneal lymphangiogenesis by upregulating corneal epithelial cell production of VEGF-D, a key driver of lymphangiogenesis [52] (Fig. 3). IL-17A also promotes ocular surface inflammation through its effects on B cell proliferation. Subbarayal et al. have demonstrated that in a murine model of DED, IL-17A promotes germinal center formation, B cell proliferation and B cell differentiation into isotype-switched B cells and plasma cells. The authors showed that IL-17A promoted B cell proliferation by enhancing the effects of anti-IgM and anti-CD40 [50].
Along with IL-17A, Th17 cells produce additional effector cytokines including IL-17F, IL-21, IL-22 and GM-CSF [35]. Our group has demonstrated that Th17 cell-derived GM-CSF directly contributes to ocular surface disease pathogenesis in a murine model of DED [53]. We have shown that GM-CSF levels are significantly upregulated in dry eye and that GM-CSF promotes CD11b+ cell maturation and migration in vitro as well as in a murine model of DED. Topical neutralization of GM-CSF led to a reduction in CD11b+ cell maturation and migration to the ocular surface, along with a reduction in Th17 cell induction and a decrease in clinical signs of the disease [53]. This report elucidates the role of Th17 cell-derived GM-CSF in the pathogenesis of ocular surface disease via promotion of CD11b+ cell maturation and tissue infiltration.
Th17 cells are known to possess considerable heterogeneity and plasticity, and are capable of producing IFN-γ, the signature cytokine of Th1 cells, under certain conditions [54]. Our group recently identified a cellular source of IFN-γ in DED with the discovery of a major population of Th17 cell-derived Th17/1 cells at the ocular surface of mice exposed to desiccating stress [22] (Fig. 3). Through a series of adoptive transfer experiments, these cells were confirmed to be highly pathogenic. Furthermore, IL-12 and IL-23 signalling was shown to be necessary for the in vivo transition of pathogenic Th17 cells to IFN-γ producers [22] (Fig. 2). Identification of this cell population demonstrates the relevance of Th17 plasticity to DED, and details another mechanism by which Th17 cells contribute to ocular surface disease [22]. In addition, the plasticity and functional adaptability of Tregs are involved in the development of spontaneous lacrimal keratoconjunctivitis observed in a transgenic mouse strain of NOD.H-2b. It was reported that in aged NOD.H-2b mice, there was increased number but decreased suppressive function of Tregs, which was associated with their significant expressions of IL-17 and IFN-γ. Beyond losing the immune regulatory function, these inflammatory cytokine-producing Tregs further exerted effector T cell-like pathogenic function with the ability to transfer the disease to normal recipients, highlighting Tregs a potential cellular source contributing to Th17 pathogenicity in ocular surface inflammation [55].
II.C. Maintenance of chronic ocular surface inflammation by memory Th17 cells
Memory Th17 cells have been identified in multiple inflammatory conditions including inflammatory bowel disease and rheumatoid arthritis [56]. Although phenotypically similar to terminally differentiated memory T cells, they maintain a strong capacity for self-renewal [56]. These Th17 cells also maintain high IL-17A production and have robust proliferative and anti-apoptotic capabilities, making them a long-lived effector memory T cell population [57].
Like most other autoimmune and immune-based conditions, DED is a chronic condition that is characterized by intermittent disease exacerbations [49]. However, until recently, the vast majority of research on Th17 immunity in DED has utilized a model of disease characterized by acute exposure to desiccating stress with or without administration of anticholinergic drugs such as scopolamine [20]. In 2014 our group developed a model of chronic DED in which mice are exposed to desiccating stress for 14 days and then housed in a normal environment for four months [58]. Despite being in a normal humidity environment and having normal tear production, these mice exhibited long-lived low-grade corneal epitheliopathy, mimicking the chronic nature of dry eye seen in clinics [58]. Importantly, we observed that these mice possessed a significant population of memory Th17 cells at the ocular surface. Adoptive transfer of these memory Th17 cells induced disease in naïve mice, demonstrating a critical role for memory Th17 cells in disease pathogenesis of chronic dry eye [58]. Once formed, these memory Th17 cells are maintained by two key cytokines - IL-7 and IL-15 [59] (Fig. 2). Both IL-7 and IL-15 are present at the ocular surface of mice with chronic DED, and we have demonstrated their functional importance through a series of experiments in which blockade of either cytokine yielded a significant reduction in memory Th17 cells, while addition of IL-7 and IL-15 led to increased frequencies of memory Th17 cells [59].
Experiments conducted by our group have investigated the immune response when mice with chronic dry eye are re-exposed to desiccating stress [59]. Under these conditions, mice develop increased inflammation and corneal epitheliopathy, akin to an exacerbation or “flare” of disease. We have shown that during this period of re-exposure, memory Th17 cells differentiate into the previously described Th17/1 cells that produce both IL-17A and IFN-γ, and the frequency of Th17/1 cells corresponds to level of clinical disease [59]. Thus, while memory Th17 cells appear to be maintained by IL-7 and IL-15, we now recognize that acute-on-chronic disease exacerbation is due to Th17/1 cells which develop under the influence of IL-12 and IL-23 [22]. Currently, our group are actively investigating how mTh17 cells are generated from their effector precursors in DED. Our preliminary data suggest that both effector Th17 and effector Th17/1 cells give rise to memory cells driven by IL-23 during the resolution of acute inflammation.
II.D. Augmented Th17 response and higher susceptibility to ocular surface disease in aging
With aging, the immune system undergoes a variety of changes that lead to a state of chronic, low-level inflammation and increased risk of autoimmunity [60,61]. As DED is more prevalent and severe in middle-aged and elderly patients, it is important to consider the association between Th17 cell immunity and aging [9].
Several studies have demonstrated an augmented Th17 response with increasing age [62,63]. Ouyang et al. demonstrated significantly higher frequencies of Th17 cells and increased levels of the Th17-associated cytokines IL-17A, IL-17F, and IL-22 in both aged humans as well as mice. [62] The authors used a mouse model of inflammatory bowel disease to show that RAG knockout mice (deficient in both mature T and B cells) developed more severe colitis and increased colonic levels of Th17 cytokines upon adoptive transfer of T cells derived from aged as compared to young mice [62]. Additionally, in a separate investigation, Schmitt et al. evaluated Th17 cells in relation to Tregs as a function of age, and observed that the ratio of Th17 cells to Tregs increases with increasing age [63].
McClellan et al. investigated the effect of aging on the ocular surface and lacrimal glands, and reported spontaneous corneal epitheliopathy in elderly mice [64]. These clinical signs were associated with an increase in CD4+ T cells in both the conjunctiva and lacrimal glands. Importantly, the authors found increased IL-17A and IFN-γ in the conjunctiva and increased IL-17A and CCL20 in the cornea in the elderly mice [64]. These results support the epidemiological observation of increased prevalence of DED in the elderly, and the findings of increased Th17-associated cytokines at the ocular surface supports the role of Th17 cells in this process [64].
Recently, we have investigated the role of Th17 cells in aged mice exposed to desiccating stress. In these experiments, aged and young mice were exposed to 14 days of desiccating stress and showed no difference in disease severity. However, upon re-challenge with desiccating stress, the aged mice demonstrated a more rapid and severe clinical disease relative to young mice [65]. In addition, upon re-challenge, aged mice demonstrated an increase in effector Th17 cell frequencies in the draining lymph nodes that was substantially higher as compared to young mice. Treatment with anti-IL-15 antibody prior to rechallenge abrogated this increase in Th17 cell frequencies and reduced disease severity.
III. Regulation of Th17 immunity in ocular surface disease
III.A. Effect of corneal epithelial immunoregulatory factors on Th17 immunity
Ocular surface epithelial cells play an important role in maintaining immune homeostasis [42]. Various immunoregulatory factors including cell surface molecules and soluble proteins expressed at the epithelial cell surface act in concert to curb inflammation [42].
The protein thrombospondin 1 (TSP-1) is constitutively expressed by corneal and conjunctival epithelium, and plays an important role in activating latent TGF-β to upregulate its immunosuppressive and wound healing functions [66]. Conjunctival goblet cells have been shown to express and activate TGF- β2 in a TSP-1-dependent manner, thereby promoting the adoption of a tolerogenic dendritic cells phenotype [67,68]. In TSP-1 null mice, IL-17A protein has been observed to be significantly increased in tissue homogenates from lacrimal glands compared with those in wild-type mice, and decreased splenic Foxp3+ Treg frequencies have also been noted [69]. These findings suggest that TSP-1 plays a crucial role in maintaining homeostatic balance between Th17 cells and Tregs. Treatment with exogenous TSP-1 has been shown to inhibit Th17-induced IL-23 secretion by APCs, and attenuate DED severity by amplifying Tregs and inhibiting Th17 development [70]. In a recent study, we demonstrated the immunosuppressive effect of corneal epithelium derived TSP-1 on dendritic cell (DC) maturation in DED [71]. Topical treatment with recombinant TSP-1 resulted in decreased DC infiltration into corneal tissue, reduced generation of Th17 cells from naïve T-cells in the draining lymph nodes and decreased expression of Th17-associated cytokines in the conjunctiva. These immune changes following TSP-1 treatment resulted in an amelioration of DED.
Pigment epithelium-derived factor (PEDF) is a ubiquitous protein with known neurotrophic, anti-angiogenic and anti-inflammatory functions in the eye [70,72]. In unpublished data from our laboratory, we have observed increased PEDF expression by corneal epithelial cells on exposure to desiccating stress. We have also demonstrated the suppressive effect of corneal epithelium-derived PEDF on DC maturation in vitro. Our in vivo data indicates that topical application of PEDF reduces disease severity by suppressing DC maturation at the ocular surface, with reduced Th17 generation observed in the draining lymph nodes (Dana Lab unpublished data).
Lipoxin A4 (LXA4) is an anti-inflammatory molecule that is a metabolite of the arachidonic acid pathway [73]. Endogenous LXA4 at the ocular surface has been shown to be derived from polymorphonuclear neutrophils residing in the corneal limbus, lacrimal glands and cervical lymph nodes [74,75]. Abrogation of endogenous LXA4 by antibody depletion of tissue polymorphonuclear neutrophils leads to an increase of T effector cell activation [75]. Treatment of DED mice with LXA4 has been shown to suppress both Th1 and Th17 responses in the draining lymph nodes, increase Treg frequencies, and reduce dry eye pathogenesis [75]. The immunomodulatory effect of LXA4 may be mediated via modulation of DCs. In a model of microbial stimulation with an extract of Toxoplasma gondii, LXA4 has been down to decrease DC mobilization, CCR5 expression and IL-12 responses [76]. Collectively, these data underscore the anti-inflammatory role of LXA4 at the ocular surface and highlight its function in maintaining the balance between inflammatory and regulatory cell subsets.
PD-L1 derived from corneal epithelial cells plays a crucial role in limiting ocular surface inflammation [77,78]. There is evidence from experimental mouse models that DED induction downregulates corneal expression of PD-L1 [78]. Blockade of PD-L1 has been observed to substantially increase T cell corneal infiltration, with upregulated expression of chemokines and their receptors, as well as higher corneal fluorescein staining scores [78]. Among the upregulated chemokines in PD-L1-null DED mice, CXCL9 is notable for a 13-fold increase in the cornea [78]. CXCL9 has been shown to be an essential chemokine for the recruitment of CD4+ T cells into the cornea [78,79]. Similar findings have been observed in experimental autoimmune encephalomyelitis (EAE) [80]. Rui et al. demonstrated the development of EAE in PD-L1-null mice as a consequence of dysregulated macrophages and enhanced production of IL-6, leading to increased generation of Th17 cells [80]. Furthermore, the authors showed the direct suppressive action of PD-L1 on the activity of innate immune cells, thereby preventing autoreactive T-cell priming and differentiation into inflammatory effector Th17 cells [80]. In a murine model of acute GVHD (aGVHD), systemic overexpression of PD-L1 ameliorates aGVHD disease severity by inhibiting effector T cell function, including Th1 and Th17 responses [81]. In vitro experiments have shown that PD-L1 suppresses T cell proliferation, promotes T cell apoptosis and reduces the secretion of IL-2, IFN-γ and TNF-α by effector T cells [81]. These effects are independent of Tregs, with no effect of PD-L1 on Treg cell function observed [81].
III.B. Effect of regulatory T cells on Th17 immunity
Tregs play an essential role in limiting autoimmunity. Mutations in Foxp3 – the master transcriptional factor for the development and function of Tregs – results in fatal autoimmune diseases [82]. Tregs suppress the activity of effector T cells by 4 key mechanisms: by competing for metabolites required by effector T cells such as IL-2 (i.e. cytokine deprivation-mediated apoptosis), by Granzyme-B and perforin-mediated apoptosis of effector T cells, by releasing soluble immunosuppressive factors (e.g. IL-10, IL-35, TGF-β) and by suppressing APC maturation [83,84]. Tregs possess a TCR repertoire that is skewed toward the recognition of self-antigens [84]. When activated by TCR stimulation, Tregs downregulate the T-cell-priming capacity of APCs by trans-endocytosing the ligands CD80 and CD86 from the surface of APCs via its molecule CTLA-4, thereby inhibiting the expansion of potentially detrimental self-reactive T cells [84].
Dysfunction of the Treg compartment has been implicated in the pathogenesis of several autoimmune diseases; including DED, multiple sclerosis and rheumatoid arthritis [83,85–87]. Adoptive transfer of CD4+ T cells from DED mice into Treg deficient-mice results in increased disease severity, as compared to normal mice [17]. Previous work from our laboratory has shown that while Treg frequencies in DED mice remain unchanged, the capacity of these Tregs to suppress the proliferation of Th17 effector cells is impaired [21]. Unpublished data from our laboratory indicates that Treg dysfunction is persistent in chronic DED. Notably, the level of IL-6R expression on Tregs increases progressively during periods of desiccation, serving to transmit IL-6 signaling and suppress Treg differentiation and function [88]. Our group has previously shown that blockade of IL-17A restores Treg function in DED, implying that Th17-associated IL-17A is critically involved in causing Treg dysfunction [21]. Based on these studies, the augmentation of Treg function represents a potential strategy to ameliorate Th17-mediated autoimmune diseases, such as DED.
III.C. Cross regulation of nerve-derived factors and Th17 immunity of ocular surface
The ocular surface is extensively innervated by sensory and autonomic nerve fibers from the ophthalmic branch of the trigeminal nerve [89]. By releasing neuropeptides, these nerve fibers play a crucial role in the regulation of ocular surface homeostasis; including the maintenance of corneal epithelial integrity, the promotion of wound healing, and the control of inflammatory responses [89,90]. Substance P (SP) is an eleven-amino acid long neuropeptide that is markedly upregulated in chronic inflammation, and is associated with tissue infiltration of Th17 cells [90,91]. SP is a tachykinin neuropeptide that stimulates monocytes to secrete IL-1β, which in turn promotes the secretion of IL-17A by CD4+ memory T cells [90,92]; therefore, the finding of increased levels of IL-1β in the tears and ocular surface cells of DED patients suggests that SP may enhance the activation of infiltrating myeloid cells, thereby further augmenting the Th17 immune response in DED [90]. Relatedly, our group has reported that expression of SP is substantially upregulated in DED mice, and enhances expression of the MHC II maturation marker by BMDCs through acting on its preferred receptor (neurokinin-1 receptor, NK-1R) [93]. Blockade of SP with NK-1R inhibitors abrogates the effect of SP on BMDC maturation, and suppresses both Th17 activity and DED severity [93]. Collectively, these reports suggest that by modulating both innate and adaptive immune responses, and through the generation of inflammatory cytokines (such as IL-17A), SP upregulates autoimmune pathways [22,90].
Vasoactive intestinal polypeptide (VIP) is secreted by parasympathetic nerve fibers, and is well-known for its immunomodulatory effects [94,95]. VIP regulates inflammation by generating DCs with a tolerogenic phenotype, decreasing Th1 and Th17 frequencies, promoting differentiation into Th2 cells and by increasing both CD4+ and CD8+ Tregs [95–98]. VIP receptors (VPAC1 and VPAC2) are expressed in activated/expanded memory Th cells, and in the presence of VIP, memory Th cells shift toward a less pathogenic profile [99]. There is a growing body of evidence indicating that the application of exogenous VIP may regulate corneal inflammation [100]. In an experimental alkali burn murine model, exogenous VIP was observed to suppress the migration of polymorphonuclear leukocytes to the cornea [100]. VIP has also been shown to reduce corneal inflammation in bacterial keratitis, where in a model of Pseudomonas keratitis, VIP upregulates anti-inflammatory mediators (TGF-β and IL-10) and reduces pro-inflammatory molecules (IL-1β, TNF-α, MIP-2) [101]. In a separate study using a model of Pseudomonas keratitis, systemic treatment with VIP downregulated mRNA expression of proinflammatory TLRs and upregulated anti-inflammatory TLRs [102]. In studies using nonobese diabetic mice, Jimeno et al. have shown that VIP suppresses the functional phase of Th17 cells, and limits the increase in proportion of Th1 to Th17 cells [103]. The authors also demonstrated an increase in the Tregs/Th17 ratio in the spleen, indicative of an induction of immune tolerance [103].
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a sensory neuropeptide in the eye that demonstrates 68% sequence homology with VIP [104]. Like VIP, PACAP skews the immune response toward an anti-inflammatory profile. It has been reported that PACAP-null mice subjected to myelin oligodendrocyte glycoprotein develop more severe clinical and pathological manifestations of EAE compared to wild-type mice, due to a more pronounced Th17 and Th1 response, with downregulated Th2 and Treg pathways [105]. Mechanistically, macrophage and dendritic cells may be the targets of PACAP, that subsequently influence Th differentiation [105]. The capacity of both VIP and PACAP to ameliorate various autoimmune diseases lends support to their therapeutic potential at the ocular surface [104–107].
The melanocortin (MC) system plays an important role in regulating immune function, and encompasses multiple peptides including α-, β-, γ-melanocyte stimulating hormone (MSH), adrenocorticotropic hormone (ACTH), and five MC receptors (MCR1-5) [108]. Among these peptides, α-MSH (which is constitutively expressed in aqueous humor) exerts strong anti-inflammatory and immunosuppressive activity, and is crucial to the maintenance of ocular immune privilege [109], yet it remains unclear whether corneal nerves are one of the sources secreting α-MSH to the aqueous humor. α-MSH regulates the activity of T cells by suppressing the production of proinflammatory cytokines, increasing the expression of anti-inflammatory mediators, limiting the maturation of DCs, promoting cytolysis of effector T cells and converting effector T cells into Tregs [109,110]. Moreover, α-MSH has the ability to induce tolerogenic DCs, which are capable of expanding the Treg pool, and further suppress the proliferation and inflammatory cytokine secretion of pathogenic Th17 cells [109].
IV. Strategies targeting T cells for controlling ocular surface inflammation
IV.A. FDA-approved treatments targeting T cell response in ocular surface disease
In the US, the main FDA-approved treatments targeting T cell signaling for DED are cyclosporine-A (CsA) ophthalmic emulsion 0.05% (Restasis®; Allergan, Irvine, CA, USA), a nanomicellar formulation containing 0.09% CsA (Cequa® ; Sun Pharmaceutical Industries, Cranbury, NJ, USA) and lifitegrast ophthalmic solution 5.0% (Xiidra ®; Shire, Lexington, USA) [111].
CsA is a natural occurring fungal metabolite, widely used for the management of autoimmune diseases and prophylactically to prevent post-transplant organ rejection, due to its immunosuppressive properties [112]. CsA forms a complex with cyclophilin A to inhibit the action of calcineurin [112,113]. Blockade of calcineurin prevents translocation of NF-AT to the nucleus and decreases the production of IL-2, a pivotal factor in T cell replication and immune responses [112,113]. In addition, CsA protects mitochondrial function by binding to cyclophilin D, preventing Ca2+ influx into the mitochondria [113]. In a murine model of DED, topical CsA significantly reduced apoptosis of conjunctival epithelial cells and protected against goblet cell loss [114]. CsA ophthalmic emulsion 0.05% is approved by the FDA, having been launched in 2003. Based on data showing improvement of Schirmer scores after 6 months of treatment across 4 trials, CsA 0.05% is indicated to increase tear production in patients with ocular inflammation-mediated DED [111,115]. While several studies congruently reported that CsA 0.05% improved tear production and corneal staining scores, the study results regarding patient self-reported DED symptoms are inconsistent [116–120].
In order to overcome the hydrophobic property of CsA, Restasis is formulated in castor oil and water, yet this formulation has been reported to cause ocular adverse effects such as conjunctival hyperemia, ocular burning, stinging, instillation site pain, and blurred vision [111,113,116–119]. In 2018, the FDA approved a preservative-free CsA 0.09% nanomicellar topical formulation (OTX-101, 0.09%, Cequa®) for the treatment of DED [111,113]. The property of nanomicellar formulation improves CsA solubility and allows higher distribution of CsA into corneal and conjunctival cells [113]. Evaluation of the pharmacokinetics of namomicellar formulations demonstrate that 0.05% CsA nanomicellar (OTX-101 0.05%), with the same concentration as Restasis, generates 3-4 fold higher CsA concentrations in conjunctival and scleral tissues as compared to Restasis [113]. Two randomized, multicenter, double-masked clinical trials have validated the safety and efficacy of OTX-101 0.09% for DED patients, with the treatment group demonstrating increased tear production, improved corneal and conjunctival staining, and reduced symptom scores [115,121].
Lifitegrast is a tetrahydroisoquinoline derivative and is a lymphocyte function-associated antigen 1 (LFA-1) antagonist that blocks the interaction between LFA-1 and intercellular adhesion molecule (ICAM-1) [111,122]. The binding of LFA-1 on T cells to ICAM-1 on ocular surface epithelial cells and APCs stabilizes the immunological synapse, which in turn upregulates the production of downstream inflammatory mediators such as TNF-α and IL-1 [111,122–124]. Therefore, blocking the interaction between LFA-1 to ICAM-1 interrupts the ocular inflammatory cycle that characterizes DED [122,125]. The Lifitegrast ophthalmic solution 5.0% (Xiidra®), approved in the US in 2016, has shown promise in reducing corneal staining and improving DED symptoms across 4 clinical trials [126–129]. Notably, one study reported that patients with moderate-to-severe DED had significant improvement in their symptoms, but demonstrated no effect on the clinical signs of DED [130]. This contrasts with a recent study showing significant improvements in conjunctival and corneal staining as well as tear film breakup time (TBUT) in patients with moderate-to-severe DED [131]. No serious ocular adverse events have been reported, except for mild and transient discomfort at the instillation site [129,132].
IV.B. Novel therapeutic strategies suppressing Th17 immunity
Given the multi-faceted role of Th17 cells in the pathogenesis of ocular surface disease, these cells are an attractive target in the development of novel therapeutics (Fig. 4). Blockade of IL-17A successfully mitigates multiple aspects of the DED immune response including restoration of Treg function, inhibition of lymphangiogenesis and reduction of B cell formation [21,50,52]. Importantly, the neutralization of IL-17A in these studies additionally led to an improvement in clinical signs of disease. However, despite these successes in the laboratory setting, an effective therapeutic agent that specifically targets Th17 immunity in humans remains elusive. CsA is able to reduce Th17 cell frequencies in humans with primary Sjögren’s syndrome, but to date, there has only been one clinical trial performed in which IL-17A has been targeted [133]. In this trial, patients with DED received a single intravenous dose of Secukinumab, a monoclonal antibody that binds IL-17A, which is currently approved for the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. Secukinumab was compared to treatment with Canakinumab, a monoclonal antibody targeting IL-1β, and placebo [133]. The investigators found no significant difference in signs or symptoms of ocular surface disease with either treatment group. Possible explanations for the lack of efficacy in this study was the route of administration (a topical route of administration would be expected to be much more efficacious than systemic administration), dosing and pharmacokinetics, insufficient length of follow-up, the discrepancy between the symptoms and clinical signs, and the fact that patients were required to stop all other topical medications during the study.
Figure 4: Novel strategies suppressing Th17 to limit ocular surface inflammation.
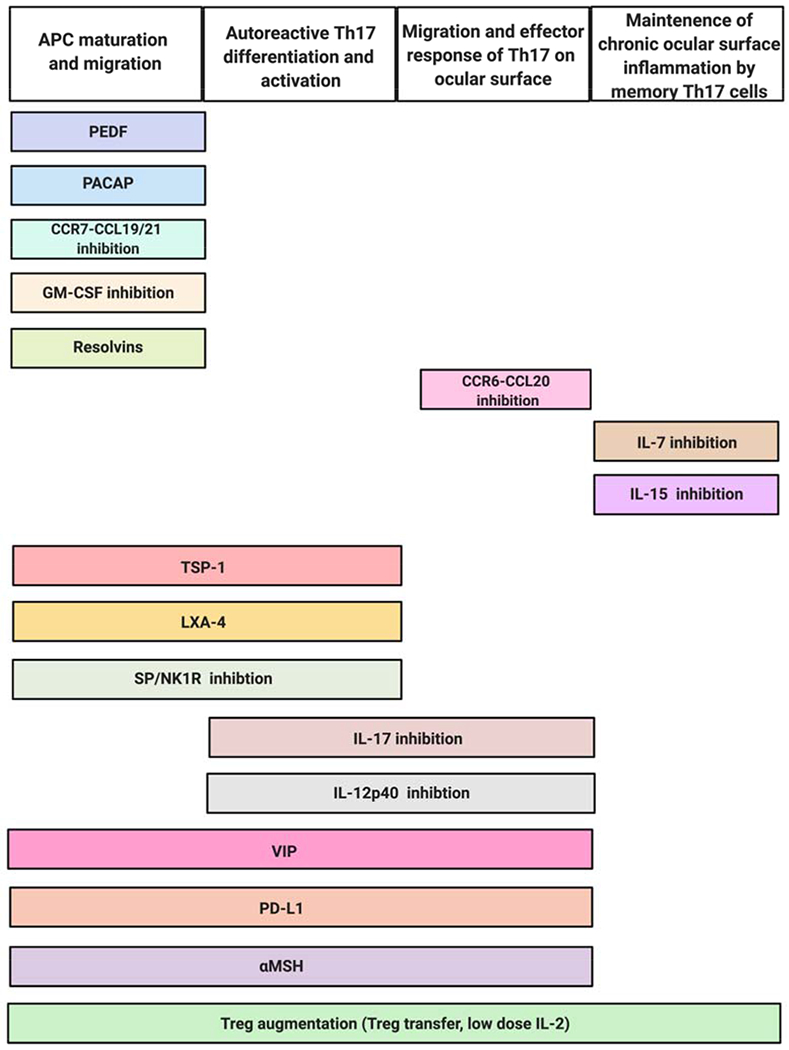
Multiple promising therapeutic strategies aim to ameliorate ocular surface inflammation by regulating the differentiation, activation, migration or effector responses of Th17 cells.
In addition to inhibiting IL-17A, other strategies for suppressing Th17 immunity have been employed in research settings [43,46]. One such strategy has been to target chemokine/chemokine receptor interactions to inhibit cell migration. In a murine model of disease, neutralization of CCL20 has successfully inhibited the migration of Th17 cells to the ocular surface, and neutralization of CCR7 has been used to inhibit APC migration to the draining lymphoid tissue, with subsequent downstream reduction in Th17 cell differentiation, as well as significant disease amelioration [43,46].
Recently, memory Th17 cells have been identified as important effector cells in chronic DED, as these cells are responsible for propagating long-term disease eye [58],[59]. IL-7 and IL-15 are recognized as critical mediators of memory Th17 cell formation and maintenance, and their inhibition can decrease memory Th17 cell formation along with clinical signs of disease [59]. Once reactivated, memory Th17 cells are capable of giving rise to effector Th17/1 cells, which are another recently identified effector cell in chronic dry eye that produce both IL-17A and IFN-γ and are responsible for disease exacerbations [22]. Th17/1 cells require IL-12 and IL-23 for their formation, and of note, IL-12p40 is a shared subunit between the receptors for IL-12 and IL-23, making this a potential novel target for suppressing Th17 immunity [134].
Resolvins are biosynthesized from the essential dietary omega-3 fatty acids, and both Resolvin D1 (RvD1) and E1 (RvE1) have anti-inflammatory activities including the early regulation of innate immune responses, and the suppression of CD4+ T cell recruitment [87,135]. Local delivery of RvE1 has been observed to promote corneal allograft survival by suppressing Th1-mediated inflammation [136]. The modulation of Th17 immunity by RvE1 is believed to be mediated via suppression of innate immunity, rather than by upregulating Tregs or IL-10 levels [136]. Recently, Asbell et al. investigated the effect of omega-3 fatty acids in the management of moderate to severe DED [137]. The authors found a significant reduction in Ocular Surface Disease Index scores in patients receiving a daily oral dose of 3000 mg of fish-derived omega-3 fatty acid derivatives - eicosapentaenoic and docosahexaenoic acids - compared to the placebo group over a follow-up period of one year [137].
Various other therapeutic strategies that have demonstrated efficacy in inhibiting Th17 cell function include administration of rituximab [138] and topical rebapimide [139], as well as inhibition of GMCSF [53], neurokinin-1 receptor [93], and phosphodiesterase-4 [140]. Additionally, depletion of natural killer cells has been shown to suppress Th17 cell immunity [141]. These studies have been conducted in animal models, and whether their promise can translate to the clinic remains to be seen.
Treg-based therapies are emerging as attractive therapeutic approaches in combatting autoimmune disorders, due to the capacity of Tregs to modulate the immune response [142]. Subconjunctival injection of Tregs into mice receiving corneal transplants has been shown to enhance the levels of IL-10 and TGF-β, decrease the frequencies of mature APCs and inhibit CD45+ cell infiltration in the graft, and has been shown to increase allograft survival [142]. These data suggest that exogenous Tregs may also have therapeutic potential in autoimmune disorders of the ocular surface.
In addition to the delivery of exogenous Tregs, another therapeutic strategy has been the expansion of endogenous Tregs using low-dose IL-2 [143]. IL-2 is required for Treg homeostasis and the survival of Tregs in the periphery [144]. Tregs have higher affinity to IL-2 than effector T cells due to high expression levels and trimeric configuration of the IL-2 receptor, and therefore can deprive effector T cells of IL-2 and inhibit their expansion [144,145]. The first experimental study to evaluate the effect of low dose IL-2 therapy was conducted in mice with type 1 diabetes, with the results demonstrating effective expansion of Tregs and reduced disease progression [146]. Human clinical trials of low dose IL-2 treatment (1,000,000 IU/day) for patients with various autoimmune diseases, including rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus and several forms of vasculitis, showed significant amelioration of disease severity with expansion and activation of Tregs [147]. The promising results of strategies to expand endogenous Tregs in these inflammatory diseases suggest their potential application in disorders of the ocular surface.
TGF-β in the absence of other pro-inflammatory cytokines induces Foxp3 expression, which limits the differentiation of Th17 cells by inhibiting the activity of RORγt – the master transcription factor for Th17 development [148]. Animal studies have shown that TSP-1-derived peptides, KRFYVVMWKK (4N1K) and Lys-Arg-Phe-Lys (KRFK), are able to facilitate TGF-β-mediated signaling [67,149]. 4N1K is derived from the C-terminal domain of TSP-1 (4N1K), that binds CD47 to suppress T cells [67,150]. KRFK activates latent TGF-β by competing for the binding of the Leu-Ser-Lys-leu sequence within the latent associated peptide [149,151]. Both of 4N1K and KRFK alleviate ocular surface inflammatory signs in TSP-1-deficient mice by inducing Tregs and suppressing Th17 development [67,149]. Other potential therapies employing specific cytokines include recombinant IL-10, IL-4 and IL-27, the application of which have been shown to suppress Th17 cells and inhibit IL-17A production [145,152,153]. Indeed, there is encouraging data demonstrating that the administration of recombinant proteins may control inflammatory responses in the setting of autoimmunity [152,153].
V. Conclusions
Th17 cells are recognized as important T effector cells mediating a range of ocular surface inflammatory disorders. The inflammatory cytokine IL-17A is known to jeopardize the integrity of the corneal epithelial barrier, to alter membrane-associated mucins and to abrogate the suppressive activity of Tregs. Our understanding of Th17 immunity at the ocular surface is expanding at a rapid rate, as novel studies (such as those employing knockout and lineage reporter mice, as well as new models of disease such as chronic DED) continue to be published. Data from these studies are suggestive of new therapeutic avenues to modulate Th17 activity; potential strategies that are made all the more compelling by our current lack of FDA-approved treatments that specifically target Th17 cells at the ocular surface. As discussed, the socioeconomic burden of ocular surface disease, as well as the personal cost to an affected individual’s quality of life, are substantial. Further basic and translational studies focused on Th17 cells are indicated so that we might treat debilitating inflammatory conditions of the ocular surface more effectively.
Acknowledgements
Dr. Nai-Wen Fan would like to thank the Yin Shu-Tien Foundation for their support through the Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program (No. 106-V-A-002).
Funding Source:
This work was supported by the National Eye Institute/National Institutes of Health grant R01EY020889 to R.D.
Footnotes
Conflicts of interest: Massachusetts Eye and Ear owns intellectual property related to anti-inflammation of targeting IL-17 and substance P in ocular surface diseases. R.D. is consultant to Dompé, Aldeyra, Santen, and holds equity in Claris Biotherapeutics and Aramis Biosciences.
References
- [1].Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2007;48:4390; 4391–8. 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khanna RC. Ocular surface disorders. Community Eye Heal 2017;30:S1–2. [PMC free article] [PubMed] [Google Scholar]
- [3].Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 2012;153:1–9.e2. 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- [4].Baudouin C Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol 2008;86:716–26. 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- [5].Benítez-Del-Castillo J, Labetoulle M, Baudouin C, Rolando M, Akova YA, Aragona P, et al. Visual acuity and quality of life in dry eye disease: Proceedings of the OCEAN group meeting. Ocul Surf 2017;15:169–78. 10.1016/j.jtos.2016.11.003. [DOI] [PubMed] [Google Scholar]
- [6].Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol 2002;133:181–6. 10.1016/S0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Y, Lin T, Jiang A, Zhao N, Gong L. Vision-related quality of life and psychological status in Chinese women with Sjogren’s syndrome dry eye: a case-control study. BMC Womens Health 2016;16:75. 10.1186/s12905-016-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ayaki M, Kawashima M, Negishi K, Tsubota K. High prevalence of sleep and mood disorders in dry eye patients: survey of 1,000 eye clinic visitors. Neuropsychiatr Dis Treat 2015;11:889–94. 10.2147/NDT.S81515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dana R, Bradley JL, Guerin A, Pivneva I, Stillman IO, Evans AM, et al. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am J Ophthalmol 2019. 10.1016/j.ajo.2019.01.026. [DOI] [PubMed] [Google Scholar]
- [10].Yu J, Asche CV, Fairchild CJ. The Economic Burden of Dry Eye Disease in the United States: A Decision Tree Analysis. Cornea 2011;30:379–87. 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- [11].Inomata T, Iwagami M, Nakamura M, Shiang T, Yoshimura Y, Fujimoto K, et al. Characteristics and Risk Factors Associated with Diagnosed and Undiagnosed Symptomatic Dry Eye Using a Smartphone Application. JAMA Ophthalmol 2020. 10.1001/jamaophthalmol.2019.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reddy P, Grad O, Rajagopalan K. The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea 2004;23:751–61. 10.1097/01.ico.0000134183.47687.75. [DOI] [PubMed] [Google Scholar]
- [13].Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease. Ophthalmology 2017;124:S4–13. 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental Dry Eye Stimulates Production of Inflammatory Cytokines and MMP-9 and Activates MAPK Signaling Pathways on the Ocular Surface. Investig Opthalmology Vis Sci 2004;45:4293. 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- [15].Li D-Q, Luo L, Chen Z, Kim H-S, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res 2006;82:588–96. 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luo L, Li D-Q, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005;31:186–93. [DOI] [PubMed] [Google Scholar]
- [17].Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, et al. Desiccating stress induces T cell-mediated Sjögren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol 2006;176:3950–7. [DOI] [PubMed] [Google Scholar]
- [18].Meadows JF, Dionne K, Nichols KK. Differential Profiling of T-Cell Cytokines as Measured by Protein Microarray Across Dry Eye Subgroups. Cornea 2016;35:329–35. 10.1097/ICO.0000000000000721. [DOI] [PubMed] [Google Scholar]
- [19].Zheng X, Bian F, Ma P, De Paiva CS, Stern M, Pflugfelder SC, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol 2010;222:95–102. 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, et al. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci 2013;54:2457–64. 10.1167/iovs.12-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009;182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-γ-Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol 2017:ji1602144. 10.4049/jimmunol.1602144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2009;2:243–53. 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tan X, Sun S, Liu Y, Zhu T, Wang K, Ren T, et al. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond) 2014;28:608–13. 10.1038/eye.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Herretes S, Ross DB, Duffort S, Barreras H, Yaohong T, Saeed AM, et al. Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants. Invest Ophthalmol Vis Sci 2015;56:2348–57. 10.1167/iovs.14-15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reyes NJ, Yu C, Mathew R, Kunnen CM, Kalnitsky J, Redfern RL, et al. Neutrophils cause obstruction of eyelid sebaceous glands in inflammatory eye disease in mice. Sci Transl Med 2018;10:eaas9164. 10.1126/scitranslmed.aas9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009;373:1550–61. 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bray LC, Carey PJ, Proctor SJ, Evans RG, Hamilton PJ. Ocular complications of bone marrow transplantation. Br J Ophthalmol 1991;75:611–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Y-C, Gau J-P, Lin P-Y, Liu CJ-L, Liu C-J, Liu J-H, et al. Conjunctival Acute Graft-versus-Host Disease in Adult Patients Receiving Allogeneic Hematopoietic Stem Cell Transplantation: A Cohort Study. PLoS One 2016;11:e0167129. 10.1371/journal.pone.0167129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saboo US, Amparo F, Abud TB, Schaumberg DA, Dana R. Vision-Related Quality of Life in Patients with Ocular Graft-versus-Host Disease. Ophthalmology 2015;122:1669–74. 10.1016/j.ophtha.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007. 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Van Langelaar J, Van Der Vuurst De Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, et al. T helper 17.1 cells associate with multiple sclerosis disease activity: Perspectives for early intervention. Brain 2018. 10.1093/brain/awy069. [DOI] [PubMed] [Google Scholar]
- [33].Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 2017. 10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van Hamburg JP, Tas SW. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J Autoimmun 2018. 10.1016/j.jaut.2017.12.006. [DOI] [PubMed] [Google Scholar]
- [35].Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol 2007;19:281–6. 10.1016/J.COI.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [36].Li M, Zhang X, Sun Z, Zhong L, Que L, Xia W, et al. Relationship Between Dynamic Changes in Expression of IL-17/IL-23 in Lacrimal Gland and Ocular Surface Lesions in Ovariectomized Mice. Eye Contact Lens Sci Clin Pract 2018;44:35–43. 10.1097/ICL.0000000000000289. [DOI] [PubMed] [Google Scholar]
- [37].Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjögren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci 1994;35:3493–504. [PubMed] [Google Scholar]
- [38].Dana R Corneal antigen presentation: Molecular regulation and functional implications. Ocul Surf 2005;3:169–72. 10.1016/s1542-0124(12)70248-3. [DOI] [PubMed] [Google Scholar]
- [39].Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- [40].Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor- α and interleukin-1β for migration. Immunology 1997;92:388–95. 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature 1992;360:258–61. 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- [42].Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends Immunol 2018;39:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kodati S, Chauhan SK, Chen Y, Dohlman TH, Karimian P, Saban D, et al. CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci 2014;55:5871–7. 10.1167/iovs.14-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015;74:5–17. 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol 2019;41:283–97. 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- [46].Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, et al. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci 2013;54:4081–91. 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine Receptors CCR6 and CXCR3 Are Necessary for CD4+ T Cell Mediated Ocular Surface Disease in Experimental Dry Eye Disease. PLoS One 2013;8:e78508. 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].De Paiva CS, Hwang CS, Pitcher JD, Pangelinan SB, Rahimy E, Chen W, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology 2010;49:246–58. 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stevenson W, Chauhan SK, Dana R. Dry Eye Disease. Arch Ophthalmol 2012;130:90. 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Subbarayal B, Chauhan SK, Di Zazzo A, Dana R. IL-17 Augments B Cell Activation in Ocular Surface Autoimmunity. J Immunol 2016;197:3464–70. 10.4049/jimmunol.1502641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of Corneal Lymphangiogenesis in Dry Eye Disease. Arch Ophthalmol 2010;128:819. 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, et al. A novel prolymphangiogenic function for Th17/1L-17. Blood 2011;118:4630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dohlman TH, Ding J, Dana R, Chauhan SK. T Cell-Derived Granulocyte-Macrophage Colony-Stimulating Factor Contributes to Dry Eye Disease Pathogenesis by Promoting CD11b+ Myeloid Cell Maturation and Migration. Invest Ophthalmol Vis Sci 2017;58:1330–6. 10.1167/iovs.16-20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Agalioti T, Villablanca EJ, Huber S, Gagliani N. T H 17 cell plasticity: The role of dendritic cells and molecular mechanisms. J Autoimmun 2018;87:50–60. 10.1016/j.jaut.2017.12.003. [DOI] [PubMed] [Google Scholar]
- [55].Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, De Paiva CS. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol 2017. 10.1038/mi.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McGeachy MJ. Th17 memory cells: live long and proliferate. J Leukoc Biol 2013;94:921–6. 10.1189/jlb.0313113. [DOI] [PubMed] [Google Scholar]
- [57].Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 Cells Are Long Lived and Retain a Stem Cell-like Molecular Signature. Immunity 2011;35:972–85. 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen Y, Chauhan SK, Soo Lee H, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol 2014;7:38–45. 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chen Y, Chauhan SK, Tan X, Dana R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun 2017;77:96–103. 10.1016/j.jaut.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- [61].Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci 2012;69:1615–23. 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, et al. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol 2011;266:208–17. 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol 2013;48:1379–86. 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- [64].McClellan AJ, Volpe EA, Zhang X, Darlington GJ, Li D-Q, Pflugfelder SC, et al. Ocular Surface Disease and Dacryoadenitis in Aging C57BL/6 Mice. Am J Pathol 2014;184:631–43. 10.1016/j.ajpath.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Foulsham W, Mittal SK, Taketani Y, Chen Y, Nakao T, Chauhan SK, et al. Aged mice exhibit severe exacerbations of dry eye disease with an amplified memory Th17 cell response. Am J Pathol n.d.;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Foulsham W, Dohlman TH, Mittal SK, Taketani Y, Singh RB, Masli S, et al. Thrombospondin-1 in ocular surface health and disease. Ocul Surf 2019;17:374–83. 10.1016/j.jtos.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Contreras Ruiz L, Mir FA, Turpie B, Masli S. Thrombospondin-derived peptide attenuates Sjögren’s syndrome-associated ocular surface inflammation in mice. Clin Exp Immunol 2017;188:86–95. 10.1111/cei.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 2015;10:e0120284. 10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjögren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol 2009;175:1136–47. 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].He J, Neumann D, Kakazu A, Pham TL, Musarrat F, Cortina MS, et al. PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection. Exp Eye Res 2017;161:153–62. 10.1016/j.exer.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tan X, Chen Y, Foulsham W, Amouzegar A, Inomata T, Liu Y, et al. The immunoregulatory role of corneal epithelium-derived thrombospondin-1 in dry eye disease. Ocul Surf 2018;16:470–7. 10.1016/j.jtos.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Matsui T, Nishino Y, Maeda S, Yamagishi S. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc Res 2012;84:105–8. 10.1016/j.mvr.2012.02.006. [DOI] [PubMed] [Google Scholar]
- [73].Chandrasekharan JA, Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res 2015;8:181–92. 10.2147/JIR.S90380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kenchegowda S, Bazan NG, Bazan HEP. EGF Stimulates Lipoxin A4 Synthesis and Modulates Repair in Corneal Epithelial Cells through ERK and p38 Activation. Investig Opthalmology Vis Sci 2011;52:2240. 10.1167/iovs.10-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol 2015;195:3086–99. 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol 2002;3:76–82. 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- [77].Yang W, Li H, Chen PW, Alizadeh H, He Y, Hogan RN, et al. PD-L1 Expression on Human Ocular Cells and Its Possible Role in Regulating Immune-Mediated Ocular Inflammation. Investig Opthalmology Vis Sci 2009;50:273. 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- [78].El Annan J, Goyal S, Zhang Q, Freeman GJ, Sharpe AH, Dana R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Invest Ophthalmol Vis Sci 2010;51:3418–23. 10.1167/iovs.09-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wuest T, Farber J, Luster A, Carr DJJ. CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol 2006;243:v. 10.1016/j.cellimm.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rui Y, Honjo T, Chikuma S. Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response. Proc Natl Acad Sci U S A 2013;110:16073–8. 10.1073/pnas.1315828110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tang L, Ma S, Gong H, Wang J, Xu Y, Wu D, et al. PD-L1 Ameliorates Murine Acute Graft-Versus-Host Disease by Suppressing Effector But Not Regulatory T Cells Function. Arch Immunol Ther Exp (Warsz) 2019;67:179–87. 10.1007/s00005-019-00539-4. [DOI] [PubMed] [Google Scholar]
- [82].Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001;182:18–32. 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- [83].Foulsham W, Marmalidou A, Amouzegar A, Coco G, Chen Y, Dana R. Review: The function of regulatory T cells at the ocular surface. Ocul Surf 2017;15:652–9. 10.1016/j.jtos.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006;212:8–27. 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- [85].Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of Functional Suppression by CD4+CD25+ Regulatory T Cells in Patients with Multiple Sclerosis. J Exp Med 2004;199:971–9. 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised Function of Regulatory T Cells in Rheumatoid Arthritis and Reversal by Anti-TNFα Therapy. J Exp Med 2004;200:277–85. 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci 2009;50:4743–52. 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–5. 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- [89].Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol 2019;97:137–45. 10.1111/aos.13844. [DOI] [PubMed] [Google Scholar]
- [90].Cunin P, Caillon A, Corvaisier M, Garo E, Scotet M, Blanchard S, et al. The Tachykinins Substance P and Hemokinin-1 Favor the Generation of Human Memory Th17 Cells by Inducing IL-1β, IL-23, and TNF-Like 1A Expression by Monocytes. J Immunol 2011;186:4175–82. 10.4049/jimmunol.1002535. [DOI] [PubMed] [Google Scholar]
- [91].Remröd C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res 2007;299:85–91. 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- [92].Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 2009;183:4432–9. 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yu M, Lee S-M, Lee H, Amouzegar A, Nakao T, Chen Y, et al. Neurokinin-1 Receptor Antagonism Ameliorates Dry Eye Disease by Inhibiting Antigen-Presenting Cell Maturation and Th17 Cell Activation. Am J Pathol 2019. 10.1016/j.ajpath.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 2004;56:249–90. 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- [95].Villanueva-Romero R, Gutiérrez-Cañas I, Carrión M, Pérez-García S, Seoane IV, Martínez C, et al. The Anti-Inflammatory Mediator, Vasoactive Intestinal Peptide, Modulates the Differentiation and Function of Th Subsets in Rheumatoid Arthritis. J Immunol Res 2018;2018:6043710. 10.1155/2018/6043710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med 2007;13:241–51. 10.1016/j.molmed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- [97].Anderson P, Delgado M. Endogenous anti-inflammatory neuropeptides and pro-resolving lipid mediators: a new therapeutic approach for immune disorders. J Cell Mol Med 2008;12:1830–47. 10.1111/j.1582-4934.2008.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci U S A 2005;102:13562–7. 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jimeno R, Gomariz RP, Garín M, Gutiérrez-Cañas I, González-Álvaro I, Carrión M, et al. The pathogenic Th profile of human activated memory Th cells in early rheumatoid arthritis can be modulated by VIP. J Mol Med 2015;93:457–67. 10.1007/S00109-014-1232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tuncel N, Yildirim N, Gurer F, Basmak H, Uzuner K, Sahinturk V, et al. Effect of vasoactive intestinal peptide on the wound healing of alkali-burned corneas. Int J Ophthalmol 2016;9:204–10. 10.18240/ijo.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Carion TW, McWhirter CR, Grewal DK, Berger EA. Efficacy of VIP as Treatment for Bacteria-Induced Keratitis Against Multiple Pseudomonas aeruginosa Strains. Invest Ophthalmol Vis Sci 2015;56:6932–10. 10.1167/iovs.15-17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jiang X, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Vasoactive intestinal peptide downregulates proinflammatory TLRs while upregulating anti-inflammatory TLRs in the infected cornea. J Immunol 2012;189:269–78. 10.4049/jimmunol.1200365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jimeno R, Gomariz RP, Gutiérrez-Cañas I, Martínez C, Juarranz Y, Leceta J. New insights into the role of VIP on the ratio of T-cell subsets during the development of autoimmune diabetes. Immunol Cell Biol 2010;88:734–45. 10.1038/icb.2010.29. [DOI] [PubMed] [Google Scholar]
- [104].Nakamachi T, Ohtaki H, Seki T, Yofu S, Kagami N, Hashimoto H, et al. PACAP suppresses dry eye signs by stimulating tear secretion. Nat Commun 2016;7:12034. 10.1038/ncomms12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tan Y-V, Abad C, Lopez R, Dong H, Liu S, Lee A, et al. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 2009;106:2012–7. 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 2001;7:563–8. 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- [107].Azuma Y-T, Hagi K, Shintani N, Kuwamura M, Nakajima H, Hashimoto H, et al. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J Cell Physiol 2008;216:111–9. 10.1002/jcp.21381. [DOI] [PubMed] [Google Scholar]
- [108].Wang W, Guo D-Y, Lin Y-J, Tao Y-X. Melanocortin Regulation of Inflammation. Front Endocrinol (Lausanne) 2019;10:683. 10.3389/fendo.2019.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Auriemma M, Brzoska T, Klenner L, Kupas V, Goerge T, Voskort M, et al. α-MSH-stimulated tolerogenic dendritic cells induce functional regulatory T cells and ameliorate ongoing skin inflammation. J Invest Dermatol 2012;132:1814–24. 10.1038/jid.2012.59. [DOI] [PubMed] [Google Scholar]
- [110].Taylor AW, Lee DJ. The Alpha-Melanocyte Stimulating Hormone Induces Conversion of Effector T cells into Treg cells. J Transplant 2011;2011:246856. 10.1155/2011/246856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul Surf 2019;17:412–23. 10.1016/j.jtos.2019.02.012. [DOI] [PubMed] [Google Scholar]
- [112].Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 2012;31:271–85. 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm Res 2019;36:36. 10.1007/s11095-018-2556-5. [DOI] [PubMed] [Google Scholar]
- [114].Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea 2005;24:80–5. [DOI] [PubMed] [Google Scholar]
- [115].Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology 2019;126:1230–7. 10.1016/j.ophtha.2019.03.050. [DOI] [PubMed] [Google Scholar]
- [116].Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease11Reprint requests to: Linda Lewis, 575 Anton Blvd, Suite 900, Costa Mesa, CA 92626. Ophthalmology 2000;107:631–9. 10.1016/S0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- [117].Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. J Ocul Pharmacol Ther 2010;26:157–64. 10.1089/jop.2009.0091. [DOI] [PubMed] [Google Scholar]
- [118].Chen M, Gong L, Sun X, Xie H, Zhang Y, Zou L, et al. A comparison of cyclosporine 0.05% ophthalmic emulsion versus vehicle in Chinese patients with moderate to severe dry eye disease: an eight-week, multicenter, randomized, double-blind, parallel-group trial. J Ocul Pharmacol Ther 2010;26:361–6. 10.1089/jop.2009.0145. [DOI] [PubMed] [Google Scholar]
- [119].Prabhasawat P, Tesavibul N, Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 2012;31:1386–93. 10.1097/ICO.0b013e31823cc098. [DOI] [PubMed] [Google Scholar]
- [120].Baiza-Durán L, Medrano-Palafox J, Hernández-Quintela E, Lozano-Alcazar J, Alaníz-de la O JF. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol 2010;94:1312–5. 10.1136/bjo.2008.150011. [DOI] [PubMed] [Google Scholar]
- [121].Tauber J, Schechter BA, Bacharach J, Toyos MM, Smyth-Medina R, Weiss SL, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol 2018;12:1921–9. 10.2147/OPTH.S175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Abidi A, Shukla P, Ahmad A. Lifitegrast: A novel drug for treatment of dry eye disease. J Pharmacol Pharmacother 2016;7:194. 10.4103/0976-500X.195920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 1987;51:813–9. 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- [124].Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol 2016;10:1083–94. 10.2147/OPTH.S110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a Novel Integrin Antagonist for Treatment of Dry Eye Disease. Ocul Surf 2016;14:207–15. 10.1016/j.jtos.2016.01.001. [DOI] [PubMed] [Google Scholar]
- [126].Tauber J, Davitt WF, Bokosky JE, Nichols KK, Yerxa BR, Schaberg AE, et al. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 2004;23:784–92. 10.1097/01.ico.0000133993.14768.a9. [DOI] [PubMed] [Google Scholar]
- [127].Holland EJ, Luchs J, Karpecki PM, Nichols KK, Jackson MA, Sall K, et al. Lifitegrast for the Treatment of Dry Eye Disease: Results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3). Ophthalmology 2017;124:53–60. 10.1016/j.ophtha.2016.09.025. [DOI] [PubMed] [Google Scholar]
- [128].Sheppard JD, Torkildsen GL, Lonsdale JD, D’Ambrosio FA, McLaurin EB, Eiferman RA, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology 2014;121:475–83. 10.1016/j.ophtha.2013.09.015. [DOI] [PubMed] [Google Scholar]
- [129].Semba CP, Torkildsen GL, Lonsdale JD, McLaurin EB, Geffin JA, Mundorf TK, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol 2012;153:1050–60.e1. 10.1016/j.ajo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [130].Pepose JS, Qazi MA, Devries DK. Longitudinal changes in dry eye symptoms and signs following lifitegrast therapy and relationship to tear osmolarity. Clin Ophthalmol 2019;13:571–9. 10.2147/OPTH.S196593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tong AY, Passi SF, Gupta PK. Clinical Outcomes of Lifitegrast 5% Ophthalmic Solution in the Treatment of Dry Eye Disease. Eye Contact Lens 2019:1. 10.1097/ICL.0000000000000601. [DOI] [PubMed] [Google Scholar]
- [132].Murphy CJ, Bentley E, Miller PE, McIntyre K, Leatherberry G, Dubielzig R, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci 2011;52:3174–80. 10.1167/iovs.09-5078. [DOI] [PubMed] [Google Scholar]
- [133].Grosskreutz CL, Hockey H-U, Serra D, Dryja TP. Dry Eye Signs and Symptoms Persist During Systemic Neutralization of IL-1β by Canakinumab or IL-17A by Secukinumab. Cornea 2015;34:1551–6. 10.1097/ICO.0000000000000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015;75:249–55. 10.1016/j.cyto.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lee J-E, Sun Y, Gjorstrup P, Pearlman E. Inhibition of Corneal Inflammation by the Resolvin E1. Invest Ophthalmol Vis Sci 2015;56:2728–36. 10.1167/iovs.14-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang H, Zhao Q, Luo D, Yin Y, Li T, Zhao M. Resolvin E1 Inhibits Corneal Allograft Rejection in High-Risk Corneal Transplantation. Investig Opthalmology Vis Sci 2018;59:3911. 10.1167/iovs.18-24562. [DOI] [PubMed] [Google Scholar]
- [137].Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, Pistilli M, Ying G, Szczotka-Flynn LB, et al. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med 2018;378:1681–90. 10.1056/NEJMoa1709691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Di Benedetto P, et al. Interleukin (IL)-17-producing pathogenic T lymphocytes co-express CD20 and are depleted by rituximab in primary Sjögren’s syndrome: a pilot study. Clin Exp Immunol 2016;184:284–92. 10.1111/cei.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fu R, Jiang Y, Zhou J, Zhang J. Rebamipide ophthalmic solution modulates the ratio of T helper cell 17/regulatory T cells in dry eye disease mice. Mol Med Rep 2019;19:4011–8. 10.3892/mmr.2019.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Sadrai Z, Stevenson W, Okanobo A, Chen Y, Dohlman TH, Hua J, et al. PDE4 inhibition suppresses IL-17-associated immunity in dry eye disease. Invest Ophthalmol Vis Sci 2012;53:3584–91. 10.1167/iovs.11-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhang X, Volpe EA, Gandhi NB, Schaumburg CCS, Siemasko KKF, Pangelinan SSB, et al. NK Cells Promote Th-17 Mediated Corneal Barrier Disruption in Dry Eye. PLoS One 2012;7:e36822. 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Shao C, Chen Y, Nakao T, Amouzegar A, Yin J, Tahvildari M, et al. Local Delivery of Regulatory T Cells Promotes Corneal Allograft Survival. Transplantation 2018. 10.1097/TP.0000000000002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tahvildari M, Omoto M, Chen Y, Emami-Naeini P, Inomata T, Dohlman TH, et al. In Vivo Expansion of Regulatory T Cells by Low-Dose Interleukin-2 Treatment Increases Allograft Survival in Corneal Transplantation. Transplantation 2016;100. 10.1097/TP.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Trinath J, Bayry J. Current trends with FOXP3+ regulatory T cell immunotherapy to contest autoimmunity and inflammation. Immunotherapy 2019;11:755–8. 10.2217/imt-2019-0069. [DOI] [PubMed] [Google Scholar]
- [145].Astry B, Venkatesha SH, Moudgil KD. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine 2015;74:54–61. 10.1016/j.cyto.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 2009;30:204–17. 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Collison J Low-dose IL-2 therapy for autoimmune diseases. Nat Rev Rheumatol 2019;15:2. 10.1038/s41584-018-0142-1. [DOI] [PubMed] [Google Scholar]
- [148].Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008;453:236–40. 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Soriano-Romaní L, Contreras-Ruiz L, López-García A, Diebold Y, Masli S. Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation. Int J Mol Sci 2018;20:9. 10.3390/ijms20010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis DS, et al. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol 2002;157:509–19. 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol 2012;31:178–86. 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Dai Q, Li Y, Yu H, Wang X. Suppression of Th1 and Th17 Responses and Induction of Treg Responses by IL-18-Expressing Plasmid Gene Combined with IL-4 on Collagen-Induced Arthritis. Biomed Res Int 2018;2018:5164715. 10.1155/2018/5164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J Biol Chem 2011;286:2817–25. 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]


