Abstract
Background & Aims:
Acute pouchitis is the most common non-surgical complication after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC). We used validated case-finding definitions for pouchitis to search administrative claims data and determine the incidence of pouchitis in the first 2 years after IPAA.
Methods:
We identified all patients who underwent proctocolectomy with IPAA for UC in the IQVIA Legacy PharMetrics Adjudicated Claims Database, from January 1, 2007 through June 1, 2016. The primary outcome was the development of pouchitis within 2 years after IPAA. Secondary outcomes included isolated acute vs recurrent pouchitis, immunosuppressive therapy, further surgery, and admission to the hospital.
Results:
Among 594 patients, the cumulative incidence of pouchitis within 2 years of IPAA was 48% (95% CI, 44%–52%). The cumulative incidence of isolated acute pouchitis was 29% (95% CI, 26%–33%). Compared to patients with isolated acute pouchitis, patients who received a diagnosis of recurrent pouchitis (cumulative incidence, 19%: 95% CI, 16%–22%) demonstrated increased outpatient visits, emergency department visits, and inpatient admissions (all P<0.001). Patients who developed pouchitis were more likely to have a history of primary sclerosing cholangitis (adjusted odds ratio [aOR], 3.94; 95% CI, 1.05–14.8) and anti-tumor necrosis factor alpha therapy prior to colectomy (aOR 1.63; 95% CI, 1.09–2.45). Among patients with pouchitis, the cumulative frequency of new immunosuppressive therapy was 40% (95% CI, 35%–46%) and the cumulative incidence of pouch excision was 1.0% (95% CI, 0.4%–3.0%). The cumulative incidence of a new diagnosis of Crohn’s disease after IPAA for UC was 9.0% (95% CI, 7.2%–11%).
Conclusions:
In a geographically diverse population, 48% of patients with UC developed pouchitis within the first 2 years after IPAA. Patients with pouchitis had greater use of healthcare resources, indicating a significant burden of disease.
Keywords: hospitalization, antibiotics, utilization, inflammation
INTRODUCTION
Acute antibiotic responsive pouchitis is the most common complication after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA). Among patients undergoing IPAA as a treatment for medically refractory ulcerative colitis (UC) or UC-related neoplasia, prior studies have estimated that 40% of patients will develop acute pouchitis within the first year after surgery.1 The lifetime symptom burden may be greater, as up to 80% of patients can experience pouchitis symptoms.2, 3
The majority of our understanding about the natural history of the pouch after IPAA for UC, including the incidence of pouchitis, risk factors for development, and the disease course after diagnosis has been generated from studies of select patient populations.3–6 Large administrative claims databases have proven to be a valuable resource for understanding associations and identifying high-risk populations in other epidemiologic studies of inflammatory bowel disease (IBD).7, 8 Administrative claims data have been used to perform studies of comparative effectiveness,9 to investigate adverse effects of therapy,8 and to evaluate epidemiologic trends among patients with Crohn’s disease (CD) and UC.7, 10 The ability to study patients after IPAA, and to evaluate patients with pouchitis in particular, has previously been limited by a lack of a valid and reliable case-finding definition.11 Establishing the true incidence of pouchitis is important to improving our understanding of the disease course. This may allow for an improved understanding of the epidemiology of pouch-related disorders and identification of risk factors for both pouchitis and other inflammatory pouch conditions after IPAA.
In this study, we aimed to establish the incidence of pouchitis in the first 2 years after the final stage of an IPAA for UC in the current treatment era, using a large administrative claims database. Additionally, we aimed to evaluate patterns of healthcare resource utilization among patients after IPAA, including antibiotic prescriptions, Emergency Department (ED) visits, and inpatient hospitalizations.
METHODS
Data Source
The IQVIA Legacy PharMetrics Adjudicated Claims Data includes longitudinal pharmacy, hospital, and medical claims in a de-identified manner. Between January 1, 2007 and June 1, 2016, approximately 100 health plans and 78 million de-identified individuals were represented. Prior studies have reported the IQVIA Legacy PharMetrics Adjudicated Claims Data to be representative of the commercially insured population in the United States (US).7 Given the de-identified nature of the data, this study was determined to be exempt by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Patient Selection
All patients age ≥ 18 and <64 years with at least 6 months of continuous health plan enrollment between January 1, 2007 and July 1, 2016 were eligible for inclusion. We first identified all patients who underwent a colectomy followed by an IPAA for UC. Patients with UC were identified using previously published criteria.7, 12 All patients undergoing proctocolectomy with IPAA were identified using Current Procedural Terminology (CPT) coding (Supplementary Table 1). The final stage of surgery was defined as a CPT code for an IPAA or a CPT code for an ileostomy takedown following a CPT code for a total abdominal colectomy or total proctocolectomy (with or without IPAA). The final stage of surgery represented the index date for the study. Patients were required to have at least 6 months of continuous enrollment prior to the final stage of surgery.
Outcome Measures
We identified patients with pouchitis using a previously validated case-finding definition for patients with pouchitis within administrative claims data.11 Patients were required to have 1) a preoperative diagnosis of UC, 2) CPT coding for an IPAA, 3) an International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) or ICD, 10th revision, (ICD-10-CM) diagnosis code for pouchitis (569.71 for ICD-9-CM and K91.850 for ICD-10-CM) or a prescription for ciprofloxacin or metronidazole during the first 2 years after IPAA.
The primary outcome was the incidence of pouchitis within the first 2 years after IPAA. Secondary outcomes included the incidence of isolated acute pouchitis, defined as a single/isolated pouchitis episode, and the incidence of recurrent pouchitis, defined as one or more recurrent episodes of pouchitis during the follow-up period. In additional exploratory analyses, we used an alternative cohort definition requiring only 12 months of follow-up after IPAA with no 2-year limit on follow-up duration. We also evaluated the frequency of a change in diagnosis to CD, identified by an ICD-9-CM or ICD-10-CM code for CD on at least 3 separate occasions. Finally, we evaluated measures of utilization including antibiotic use, ED and outpatient clinic visits, hospitalizations, pouchoscopy, and further IBD-related surgeries.
Covariates
Available patient demographics included age at index date, sex, insurance plan type, year of surgery, and US census region. We analyzed the time to development of pouchitis and clinical variables that have previously been associated with an increased risk for pouchitis (Supplementary Methods).
Follow-up
For each patient who underwent a proctocolectomy with IPAA, the follow-up period began on the date of the final surgery (the index date for the study). Follow-up continued until whichever of the following occurred first: discontinuation of insurance coverage, age >65, 2 years from the time of IPAA, or death.
Statistical analysis
We used descriptive statistics to summarize baseline demographic and clinical characteristics when comparing patients with pouchitis and those who did not develop pouchitis. Continuous variables are reported as means with accompanying standard deviation (SD) while categorical variables are reported as raw values with percentages. Continuous variables were compared using T-tests and Wilcoxon Rank Sum testing as appropriate, while categorical variables were compared using Fisher Exact and Chi-Square testing. Multivariable logistic regression was performed to analyze the relationship between clinical and demographic factors and the development of pouchitis, adjusting for potential confounders. Among patients who developed pouchitis, Kaplan-Meier analysis and Cox-Proportional Hazard modeling were utilized in the evaluation of the time to development of a second episode of pouchitis, where patients were stratified based on whether the first episode of pouchitis occurred in the first 6 months after IPAA. For all analyses, 2-sided p-values of 0.05 or less were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
A total of 2,476 patients with UC and proctocolectomy with IPAA were identified between January 1, 2007 and July 1, 2016. Of these, 594 patients had at least 6 months enrollment prior to the index date and at least 24 months of enrollment after the index date (Figure 1). The median age of the cohort was 44 years (interquartile range 32–53) with 269 (45%) women.
Figure 1.
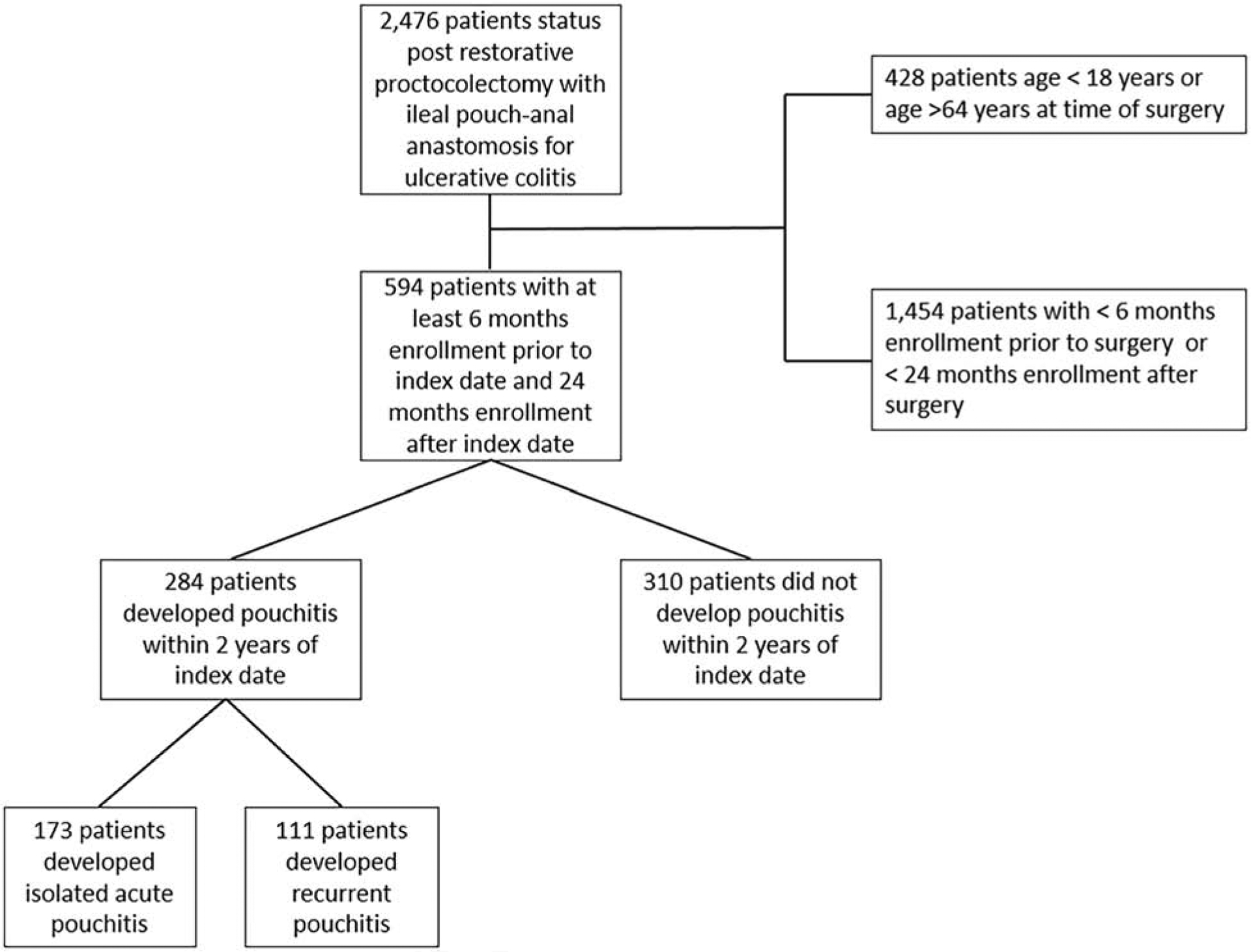
Flow diagram for evaluation of patients after restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis between January 1, 2007 and June 1, 2016
During the 2-year follow-up period after IPAA, the cumulative incidence of pouchitis was 48% (95% CI 44% – 52%). Patients who developed pouchitis were significantly more likely to have a diagnosis of PSC prior to IPAA when compared to those patients who did not develop pouchitis (5% vs 1%, P=0.007, Table 1). When evaluating IBD-specific medication use in the 6 months prior to the first stage of surgery in bivariate analysis, patients who developed an episode of pouchitis were more likely to be treated with an anti-TNF therapy (35% vs 24%, P=0.003) when compared to patients who did not develop pouchitis (Table 2).
Table 1.
Demographic and clinical characteristics of patients with and without pouchitis in the two years following an ileal pouch-anal anastomosis
| Patients without pouchitis (n=310) | Patients with pouchitis (n=284) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Age in years (Mean, SD) | 42.6 | 12.8 | 41.8 | 12.5 |
| Female Sex | 127 | 41 | 141 | 50 |
| Clostridioides difficile infection in 6 months prior to colectomy with IPAA | 10 | 3 | 10 | 4 |
| Tobacco use/smoking | 23 | 7 | 21 | 8 |
| Primary Sclerosing Cholangitis | 3 | 1 | 13 | 5 |
| Diagnosis of colon cancer or dysplasia in 6 months prior to colectomy with IPAA | 26 | 8 | 23 | 8 |
| Residence Region | ||||
| Northeast | 75 | 24 | 61 | 21 |
| Midwest | 111 | 36 | 113 | 40 |
| South | 97 | 31 | 84 | 30 |
| West | 27 | 9 | 26 | 9 |
| Pay type | ||||
| Commercial Plan | 261 | 84 | 242 | 85 |
| Medicaid | 14 | 5 | 8 | 3 |
| Self-Insured | 28 | 9 | 27 | 10 |
| Unknown/Missing | 7 | 2 | 7 | 2 |
| Year of index date | ||||
| 2007 | 11 | 3 | 12 | 4 |
| 2008 | 57 | 18 | 51 | 18 |
| 2009 | 61 | 20 | 65 | 23 |
| 2010 | 57 | 18 | 55 | 19 |
| 2011 | 44 | 14 | 44 | 15 |
| 2012 | 36 | 12 | 31 | 11 |
| 2013 | 25 | 8 | 17 | 6 |
| 2014 | 19 | 6 | 9 | 3 |
Ileal pouch-anal anastomosis (IPAA); standard deviation (SD)
Table 2.
Inflammatory bowel disease-specific medication use in the six months prior to initial stage of surgery
| Patients without pouchitis n=287 | Patients with pouchitis n=260 | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Mesalamine | 107 | 37 | 141 | 54 | <0.001 |
| Sulfasalazine | 11 | 4 | 9 | 3 | 0.817 |
| Balsalazide | 27 | 9 | 25 | 10 | 0.934 |
| Immunomodulator (azathiopurine, mercaptopurine, or methotrexate) | 58 | 20 | 66 | 25 | 0.149 |
| Tacrolimus | 4 | 1 | 3 | 1 | 0.803 |
| Cyclosporine | 2 | 1 | 1 | 0 | 0.664 |
| Anti-TNF therapy | 68 | 24 | 92 | 35 | 0.003 |
| Prednisone | 133 | 46 | 144 | 55 | 0.035 |
| Budesonide (MMX and enteral release) | 13 | 5 | 21 | 8 | 0.086 |
Anti-tumor necrosis factor alpha (anti-TNF); multimatrix (MMX)
In a multivariable analysis, patients with a history of PSC prior to IPAA demonstrated a significant increase in odds of developing pouchitis (adjusted Odds Ratio [aOR] 3.94, 95% CI 1.05 – 14.8, Table 3). Additionally, use of anti-TNF prior to colectomy was associated with increased risk of pouchitis (aOR 1.63, 95% CI 1.09 – 2.45). There was no significant difference in the odds of developing pouchitis when comparing patients who underwent an emergent colectomy or a colectomy for dysplasia/cancer compared to those patients who underwent colectomy for medical refractory colitis.
Table 3.
Odds of developing pouchitis after ileal pouch-anal anastomosis for ulcerative colitis
| Odds Ratio | 95% Confidence Interval | |
|---|---|---|
| Indication for Colectomy | ||
| Medically-refractory ulcerative colitis | Reference | Reference |
| Dysplasia or cancer | 0.80 | 0.49 – 1.31 |
| Emergent colectomy | 0.75 | 0.35 – 1.62 |
| Female sex | 1.46 | 1.04 – 2.07 |
| Age | ||
| 18–34 years | Reference | Reference |
| 35–44 years | 1.61 | 0.99 – 2.62 |
| 45–54 years | 1.41 | 0.90 – 2.23 |
| 55–64 years | 1.10 | 0.66 – 1.83 |
| Primary sclerosing cholangitis | 3.94 | 1.05 – 14.8 |
| Smoker/tobacco abose | 1.24 | 0.64 – 2.39 |
| Deyo Modification of Charlson Comorbidity Index Score | ||
| 0 | Reference | Reference |
| 1 | 1.05 | 0.68 – 1.62 |
| 2 | 0.97 | 0.53 – 1.78 |
| 3 or more | 1.19 | 0.66 – 2.17 |
| Residence Region | ||
| Northeast | Reference | Reference |
| Midwest | 1.06 | 0.67 – 1.68 |
| South | 0.94 | 0.56 – 1.56 |
| West | 1.11 | 0.55 – 2.22 |
| Plan type | ||
| Preferred Provider Organization | Reference | Reference |
| Health Maintenance Organization | 0.29 | 0.07 −1.15 |
| Consumer Directed Health Care | 1.27 | 0.78 – 2.05 |
| Other | 0.83 | 0.49 – 1.42 |
| Year of index date | ||
| 2007 | Reference | Reference |
| 2008 | 0.84 | 1.32 – 2.18 |
| 2009 | 0.86 | 0.33 – 2.56 |
| 2010 | 0.83 | 0.32 – 2.17 |
| 2011 | 0.88 | 0.33 – 2.36 |
| 2012 | 0.77 | 0.28 – 2.12 |
| 2013 | 0.72 | 0.24 – 2.12 |
| 2014 | 0.39 | 0.12 – 1.30 |
| Use of mesalamine prior to colectomy | 1.72 | 1.19 – 2.48 |
| Use of immunomodulator prior to colectomy | 1.04 | 0.68 – 1.59 |
| Use of anti-TNF prior to colectomy | 1.63 | 1.09 – 2.45 |
| Use of corticosteroids prior to colectomy | 1.09 | 0.75 – 1.59 |
Anti-tumor necrosis factor alpha (anti-TNF); ileal pouch-anal anastomosis (IPAA)
In an evaluation of the treatments for pouchitis, ciprofloxacin (70%), metronidazole (60%), and amoxicillin-clavulanate (20%) were the most common therapies. Ciprofloxacin and metronidazole were also the most commonly prescribed antibiotics as a first treatment regimen for pouchitis (Supplementary Table 2). Among all patients, the cumulative incidence of isolated acute pouchitis was 29% (95% CI 26% – 33%) while the cumulative incidence of recurrent pouchitis was 19% (95% CI 16% – 22%). Those patients with recurrent pouchitis demonstrated a greater use of several different antibiotic regimens as compared to patients with acute pouchitis, including ciprofloxacin (83% vs 62%, P<0.001), metronidazole (71% vs 52%, P<0.001), and amoxicillin-clavulanate (41% vs 6%, P<0.001, Table 4). Patients with recurrent pouchitis also demonstrated a greater mean number of antibiotic prescription fills in the 2 years after IPAA (8.9 vs 3.6, P<0.001). There was no increased risk of developing Clostridioides difficile infection in the 2 years following IPAA among patients who developed pouchitis compared to patients without pouchitis (3% vs 2%, P=0.212) or when comparing patients with recurrent pouchitis to patients with acute pouchitis (3% vs 3%, P=0.719). The mean time to the first episode of pouchitis after IPAA was significantly shorter in patients with recurrent pouchitis when compared to patients with isolated acute pouchitis (mean 169.3 days vs 298.6 days, P<0.001).
Table 4.
Use of antibiotics after ileal pouch-anal anastomosis in the first two years after ileal pouch-anal anastomosis
| Patients with acute pouchitis n=173 | Patients with recurrent pouchitis n=111 | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Ciprofloxacin | 108 | 62 | 92 | 83 | <0.001 |
| Metronidazole | 90 | 52 | 79 | 71 | <0.001 |
| Amoxicillin | 7 | 4 | 42 | 38 | <0.001 |
| Amoxcillin-clavulanate | 10 | 6 | 46 | 41 | <0.001 |
| Vancomycin | 1 | 1 | 1 | 1 | 0.783 |
| Rifaximin | 2 | 1 | 27 | 24 | <0.001 |
| Sulfamethoxazole-trimethoprim | 10 | 6 | 39 | 35 | <0.001 |
Note: Patients could use combinations of antibiotics and sequences of antibiotics during the two-year follow-up period
The cumulative frequency of immunosuppressive therapy utilization after IPAA among patients with pouchitis was 40% (95% CI 35% – 46%). Among patients with pouchitis, 20 (7%) went on to require anti-TNF therapy after IPAA while 19 (7%) were treated with an immunomodulator and 59 (21%) were treated with mesalamine. Patients with recurrent pouchitis were more likely to utilize an anti-TNF when compared to patients with acute pouchitis (11% vs 5%, P=0.047, Supplementary Table 3).
Patients with pouchitis were more likely to have one or more evaluations in the ED as compared to patients without pouchitis, (35% vs 25%, P=0.006) and were more likely to have a documented inpatient admission (50% vs 32%, P<0.001). The three most common diagnoses in the ED or inpatient admission in the 2 years after IPAA were 1) abdominal pain, 2) intestinal obstruction, and 3) UC. Patients with recurrent pouchitis were significantly more likely to have one or more evaluations in the ED when compared to patients with isolated acute pouchitis (44% vs 29%, P<0.001) and were more likely to require an inpatient admission during the follow-up period (63% vs 41%, P<0.001).
Patients with pouchitis also demonstrated a significantly greater number of outpatient visits in the 2-year follow-up when compared to patients without pouchitis (mean 24.9 vs 17.9, P<0.001). Patients with recurrent pouchitis required more outpatient visits compared to patients with isolated acute pouchitis (mean 29.5 vs 21.8, P<0.001). Patients diagnosed with pouchitis were significantly more likely to undergo pouchoscopy than those patients without pouchitis (45% vs 17%, P<0.001). The cumulative incidence of a new diagnosis of CD after IPAA for UC was 9.0% (95% CI, 7.2%–11%). During the study period, 33 (12%) patients with pouchitis had at least 3 ICD codes for CD. There was no difference when comparing the frequency of CD codes among patients with acute and recurrent pouchitis. Additionally, 20 (6%) patients without pouchitis had at least 3 ICD codes for CD. In the 2 years after IPAA, the cumulative incidence of pouch excision among patients with pouchitis was 1.0% (95% CI 0.4% – 3.0%).
In an exploratory analysis requiring only a minimum of 12 months of follow-up after IPAA, 950 patients were identified. In this population, the cumulative incidence of pouchitis was 57% (95% CI 53% - 60%), with 25% developing recurrent pouchitis (95% CI 22% – 28%). The demographics and clinical characteristics of this exploratory cohort were similar to the original cohort (Supplementary Table 4).
Among patients with pouchitis in this larger cohort, we analyzed the time to a second episode of pouchitis in a Kaplan-Meier analysis (Figure 2). There was no significant difference when patients were categorized based on time from surgery to the first episode of pouchitis (log-rank P=0.107). Similarly, in a Cox-Proportional Hazards model adjusting for other factors, there was no significant difference in the risk of developing a second episode of pouchitis with early versus late-onset pouchitis (Supplementary Table 5).
Figure 2.
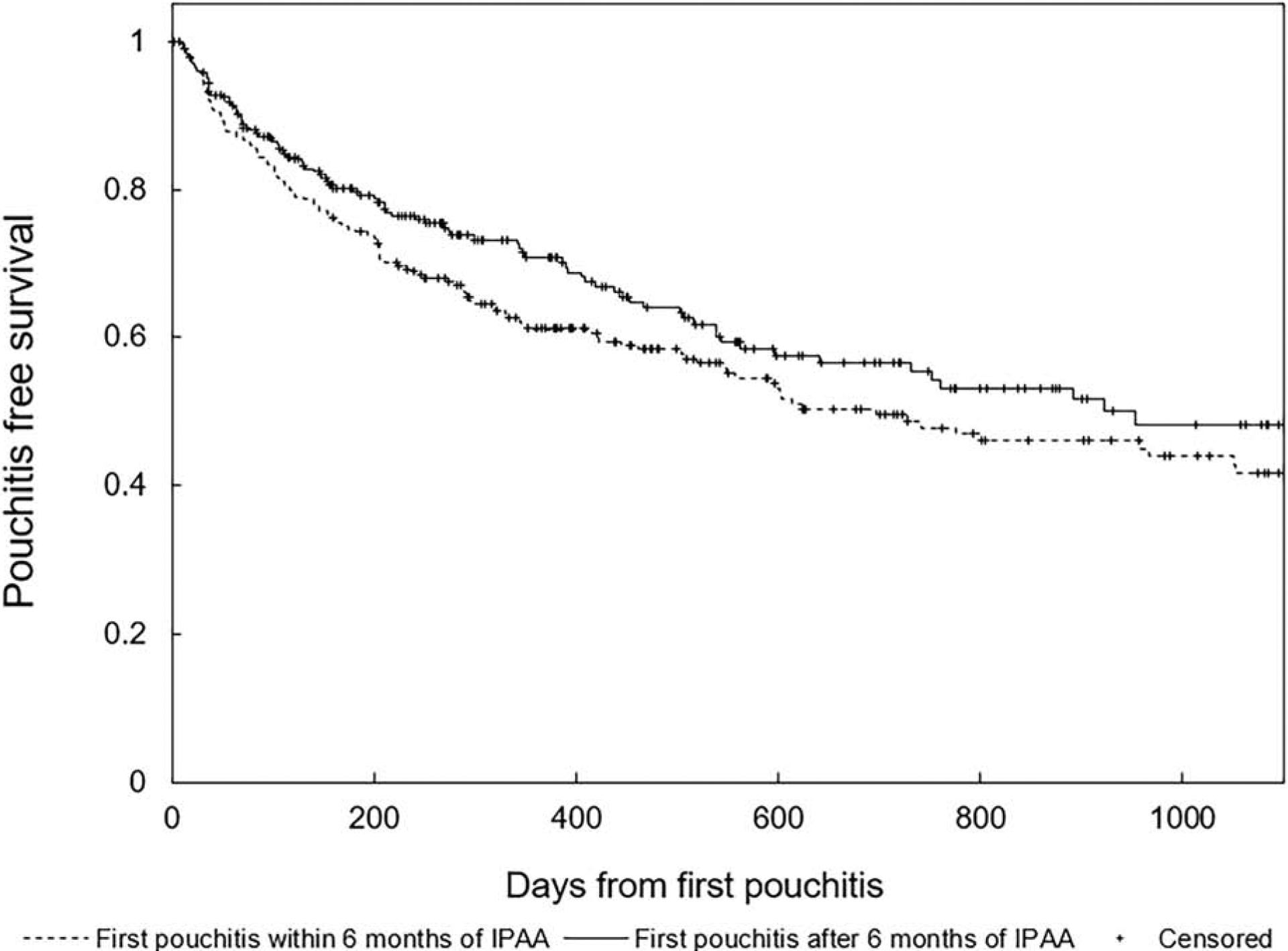
Pouchitis free survival after an initial episode of pouchitis comparing patients who developed pouchitis within the first six months of ileal pouch-anal anastomosis for ulcerative colitis to those who developed pouchitis greater than six months after surgery
Legend: log-rank p=0.107
DISCUSSION
This study utilized a validated case-finding method for the identification of patients with pouchitis, allowing for an evaluation of pouch pathology following IPAA for UC across a geographically diverse population. This approach offers several advantages including the ability to evaluate treatment patterns, resource utilization, and time to development of pouchitis among patients treated in a variety of healthcare settings across the US. In using these strategies to establish the cumulative incidence of pouchitis within the first 2 years after IPAA and evaluate patterns of antibiotic use and healthcare utilization, we are able to provide new information regarding the impact of inflammatory complications following an IPAA for UC. In this evaluation where all regions of the US were represented, almost one-half of patients developed pouchitis within the first 2 years after IPAA. Therefore, inflammatory pouch conditions can occur quickly following IPAA, resulting in an elevated burden of healthcare utilization. This incidence rate can further inform gastroenterologists and surgeons regarding the expectation of the development of pouchitis after IPAA, allowing for the establishment of treatment algorithms or protocols in individual practices.
Traditionally, the incidence of pouchitis in the first year after IPAA has been estimated at 40%, based in large part on the placebo arm of a single-center clinical trial.4 In this study, we demonstrated a 2-year incidence rate of 48%. Although our case-finding definitions were not designed to identify patients with chronic pouchitis, the incidence rate of recurrent pouchitis is similar to previously published incidence rates.13, 14 Additionally, patients with recurrent pouchitis averaged 8.9 antibiotic fills during the follow-up period, which aligns with previously published algorithms for the definition of chronic pouchitis where patients that experience episodes that are not resolved or experience ≥ 3 episodes per year are diagnosed with chronic pouchitis.15
Administrative claims data are a valuable resource in understanding the epidemiology of many disease states, including CD and UC.7, 10 Although prior studies have described the natural history after IPAA in single centers,3, 14, 16–18 the analysis of all four US census regions allows for a more generalizable estimate of the incidence rate of pouchitis in the first 2 years after IPAA. These data are nationally representative of the commercially insured population in the US and allowed us to longitudinally evaluate the disease course of individual patients from the time of IPAA. In demonstrating incidence rates of pouchitis that were similar to established estimates from single-center populations,4, 13, 14 we both confirm the high burden of inflammatory complications after IPAA and further validate administrative data as a new resource for studying patients after IPAA.
The earlier time to pouchitis diagnosis and the greater utilization of antibiotics in the recurrent pouchitis group seems to indicate a more aggressive phenotype as compared to those patients with isolated acute pouchitis. Whether the earlier development of pouchitis demonstrated among patients with recurrent pouchitis was due to underlying differences in the microbiota, unmeasured clinical or surgical factors, or another etiology remains unknown and may require follow-up analyses. Patients who developed pouchitis were more likely to have been exposed to an anti-TNF therapy in the 6 months prior to colectomy for UC, a finding that has also been suggested in a recent evaluation from the University of Chicago.19 Whether exposure to anti-TNF therapy prior to colectomy leads to underlying changes in the ileum and ultimate development of pouchitis is unknown, as the use of anti-TNF therapy may also be a surrogate for more severe colitis.
Ciprofloxacin and metronidazole are the most commonly recommended therapies for the treatment of acute pouchitis.20, 21 These treatment patterns were reflected in our results. Relatively high utilization of ciprofloxacin or metronidazole is potentially expected given the validated case-finding definition for pouchitis used in this study. However, the subsequent antibiotic patterns utilized by patients with pouchitis, particularly the high rate of alternative antibiotics among patients with recurrent pouchitis point towards the need for alternation of antibiotics due to loss of response over time. Although patients with pouchitis demonstrated a high rate of antibiotic utilization, there were no significant differences in the development of Clostridium difficile infection after IPAA when comparing those patients with pouchitis to those with no pouchitis. A similarly low rate of Clostridium difficile infection was noted in a prior evaluation of long term use of ciprofloxacin and metronidazole for pouchitis.22
Given that approximately one-half of patients will develop pouchitis in the first 2 years after IPAA, gastroenterologists should strongly consider standardized protocols for the assessment and management of patients after IPAA for UC. These may include the use of standard antibiotic regimens, including rotating antibiotics for recurrent pouchitis patients.23 For patients who develop early pouchitis symptoms after IPAA, future research efforts may focus on new innovative protocols after an episode of pouchitis or prophylaxis for the prevention of recurrent pouchitis. Further efforts to risk stratify patients at the time of IPAA, utilizing factors such as a diagnosis of PSC, may allow for individualized treatment algorithms.
Our study has limitations worth mentioning. As with other epidemiologic studies utilizing administrative data, the possibility of misclassification of the exposure and the outcome is possible. We attempted to minimize this using previously validated strategies for the identification of patients with UC in the preoperative setting,7 and pouchitis after IPAA.11 A 2-year follow-up after IPAA was utilized based on the case-finding definition for pouchitis, and in an attempt to maximize follow-up time recognizing the high turnover of commercial insurance coverage in the US.24 Although the use of administrative claims data allowed for a more generalizable evaluation of the disease course after IPAA, elderly patients and the uninsured were not represented in this study. Older age at the time of IPAA has been suggested as a risk factor for development of chronic antibiotic dependent pouchitis,25 however any patient >65 years of age was excluded from this study given the potential for dual insurance coverage with Medicare. We attempted to evaluate any differences in risk factors for the development of pouchitis and particularly recurrent pouchitis, however we could not evaluate previously identified serologic and stool biomarkers for pouch-related conditions.26 Although we attempted to evaluate utilization patterns after IPAA, our case-finding definitions may also be more likely to identify the patients at an increased risk to develop refractory pouchitis, thus leading to increased estimates of utilization after an IPAA. Additionally, these case-finding definitions and administrative claims data in particular are not designed to evaluate chronic conditions where variability in the diagnostic criteria may exist such as Crohn’s-like disease of the pouch,27 as evidenced by the frequency of patients with ICD codes for CD after an IPAA for UC.
In summary, among commercially insured patients in the US, the incidence rate of pouchitis within the first 2 years after IPAA for UC was 48%. Those patients with recurrent pouchitis required a variety of antibiotic regimens during the 2-year follow-up period after IPAA. This study establishes the feasibility of evaluating the disease course after IPAA for UC using administrative claims, and provides a foundation for future evaluations of pouch outcomes after IPAA for UC.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
Acute pouchitis is the most common non-surgical complication after restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis (UC).
Findings:
In a geographically diverse population, 48% of patients with UC developed pouchitis within the first 2 years after IPAA. Patients with pouchitis had greater use of healthcare resources.
Implications for patient care:
Acute pouchitis occurs in almost half of patients with UC who undergo restorative proctocolectomy and poses a significant burden of disease.
Acknowledgements:
The statements, findings, conclusions and opinions contained and expressed in this manuscript are based in part on data obtained under license from the IQVIA Legacy PharMetrics Adjudicated Claims Data, All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities.
Grant Support:
This research was supported by grants from the Crohn’s & Colitis Foundation [567497, (ELB)] and the National Institutes of Health [P30DK034987, (RSS)]
Conflicts of Interest:
Edward L. Barnes has served as a consultant for AbbVie, Takeda, and Target Pharmasolutions.
Hans H. Herfarth has served as a consultant for Alivio, AMAG, Finch, Gilead, Lycera, Merck, Otsuka, Pfizer, PureTech, Seres and research support from Pfizer and Artizan Biosciences
Michael D. Kappelman has served as a consultant for Abbvie, Takeda, Janssen, and Eli Lilly and has received research support from Abbvie and Janssen.
Amy Lightner has served as a consultant for Takeda.
Millie D. Long has served as a consultant for AbbVie, UCB, Takeda, Janssen, Pfizer, Salix, Valeant, Target Pharmasolutions and has received research support from Pfizer and Takeda.
Robert S. Sandler have no relevant disclosures or conflicts of interest.
Abbreviations:
- aOR
adjusted odds ratio
- CD
Crohn’s disease
- CPT
Current Procedural Terminology
- ED
emergency department
- IPAA
ileal pouch-anal anastomosis
- IBD
inflammatory bowel disease
- ICD
International Classification of Diseases
- ICD-9
ICD-9th Clinical Modification
- ICD-10
ICD-10th Clinical Modification
- PSC
primary sclerosing cholangitis
- SD
standard deviation
- UC
ulcerative colitis
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shen B Acute and chronic pouchitis--pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2012;9:323–33. [DOI] [PubMed] [Google Scholar]
- 2.Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-Related Symptoms and Quality of Life in Patients with Ileal Pouch-Anal Anastomosis. Inflamm Bowel Dis 2017;23:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lightner AL, Mathis KL, Dozois EJ, et al. Results at Up to 30 Years After Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis 2017;23:781–790. [DOI] [PubMed] [Google Scholar]
- 4.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 2003;124:1202–9. [DOI] [PubMed] [Google Scholar]
- 5.Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2008;6:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen B, Fazio VW, Remzi FH, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:81–9; quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 7.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- 8.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143:390–399 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of infliximab and adalimumab in patients with ulcerative colitis. Aliment Pharmacol Ther 2016;43:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coward S, Clement F, Benchimol EI, et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019;156:1345–1353 e4. [DOI] [PubMed] [Google Scholar]
- 11.Barnes EL, Kochar B, Herfarth HH, et al. Creation of a Case-Finding Definition for Identifying Patients with Acute Pouchitis in Administrative Claims Data. Clin Gastroenterol Hepatol 2020. epublished March 5. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol 1999;149:916–924. [DOI] [PubMed] [Google Scholar]
- 13.Madiba T, Bartolo D. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. J R Coll Surg Edinb 2001;46:334–7. [PubMed] [Google Scholar]
- 14.Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013;257:679–85. [DOI] [PubMed] [Google Scholar]
- 15.Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther 2017;45:581–592. [DOI] [PubMed] [Google Scholar]
- 16.Kayal M, Plietz M, Rizvi A, et al. Inflammatory Pouch Conditions Are Common After Ileal Pouch Anal Anastomosis in Ulcerative Colitis Patients. Inflamm Bowel Dis 2019. epublished October 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2007;5:952–8; quiz 887. [DOI] [PubMed] [Google Scholar]
- 18.Yanai H, Ben-Shachar S, Mlynarsky L, et al. The outcome of ulcerative colitis patients undergoing pouch surgery is determined by pre-surgical factors. Aliment Pharmacol Ther 2017;46:508–515. [DOI] [PubMed] [Google Scholar]
- 19.Bertucci Zoccali M, Hyman NH, Skowron KB, et al. Exposure to Anti-tumor Necrosis Factor Medications Increases the Incidence of Pouchitis After Restorative Proctocolectomy in Patients With Ulcerative Colitis. Dis Colon Rectum 2019;62:1344–1351. [DOI] [PubMed] [Google Scholar]
- 20.Barnes EL, Lightner AL, Regueiro M. Peri-operative and Post-operative Management of Patients with Crohn’s Disease and Ulcerative Colitis. Clin Gastroenterol Hepatol 2020;18:1356–1366. [DOI] [PubMed] [Google Scholar]
- 21.Shen B Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol 2013;11:1538–49. [DOI] [PubMed] [Google Scholar]
- 22.Bar N, Dubinsky V, Avraham Y, et al. Long Term Use of Ciprofloxacin and Metronidazole for Pouchitis The Low Rate of Adverse Events and the High Association with Antibiotic Resistance. Gastroenterology 2018;154:S–28. [Google Scholar]
- 23.Dubinsky V, Reshef L, Bar N, et al. Predominantly Antibiotic-resistant Intestinal Microbiome Persists in Patients With Pouchitis Who Respond to Antibiotic Therapy. Gastroenterology 2020;158:610–624.e13. [DOI] [PubMed] [Google Scholar]
- 24.Long MD, Hutfless S, Kappelman MD, et al. Challenges in designing a national surveillance program for inflammatory bowel disease in the United States. Inflamm Bowel Dis 2014;20:398–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver KN, Kochar B, Hansen JJ, et al. Chronic Antibiotic Dependent Pouchitis Is Associated With Older Age at the Time of Ileal Pouch Anal Anastomosis (J-pouch) Surgery. Crohns Colitis 360 2019;1:otz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Sharma PK, Loftus EV Jr., et al. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther 2013;37:867–75. [DOI] [PubMed] [Google Scholar]
- 27.Barnes EL, Kochar B, Jessup HR, et al. The Incidence and Definition of Crohn’s Disease of the Pouch: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2019;25:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


