Abstract
5-Hydroxymethylfurfural (5-HMF) exists in a wide range of sugar-rich foods and traditional Chinese medicines. The role of 5-HMF in antiviral innate immunity and its mechanism have not been reported previously. In this study, we reveal for the first time that 5-HMF upregulates the production of retinoic acid-inducible gene I (RIG-I)-mediated type I interferon (IFN) as a response to viral infection. IFN-β and IFN-stimulated chemokine gene expressions induced by the vesicular stomatitis virus (VSV) are upregulated in RAW264.7 cells and primary peritoneal macrophages after treatment with 5-HMF, a natural product that appears to inhibit the efficiency of viral replication. Meanwhile, 5-HMF-pretreated mice show enhanced innate antiviral immunity, increased serum levels of IFN-β, and reduced morbidity and viral loads upon infection with VSV. Thus, 5-HMF can be seen to have a positive effect on enhancing type I IFN production. Mechanistically, 5-HMF upregulates the expression of RIG-I in macrophages, resulting in an acceleration of the RIG-I signaling pathway activation. Additionally, STAT1 and STAT2 phosphorylations, along with the expression of IFN-stimulated chemokine genes induced by IFN-α/β, were also enhanced in macrophages cotreated with 5-HMF. In summary, these findings indicate that 5-HMF not only can induce type I IFN production but also can enhance IFN-JAK/STAT signaling, leading to a novel immunomodulatory mechanism against viral infection. In conclusion, our study reveals a previously unrecognized effect of 5-HMF in the antiviral innate immune response and suggests new potential of utilizing 5-HMF for controlling viral infection.
Introduction
Viral infection activates the host’s innate immune response, which is the first line of defense in protecting the host against infection.1 Retinoic acid-inducible gene I (RIG-I)-like receptors, such as melanoma differentiation-associated gene 5 (MDA5) and RIG-I, are important pattern recognition receptors (PRRs) that initiate the innate immune response in response to RNA virus invasion.2 After recognization of viral infection, the PRR pathway such as the RIG-I signaling pathway can activate immune cells to produce type I interferons (IFNs)3 in response to viral infections. Secreted type I IFNs, such as IFN-α and IFN-β, bind to the IFN-α/β receptor (IFNAR) and activate a range of antiviral gene expressions, including IFN-stimulated genes (ISGs),4 which suppress viral replication and result in the clearance of the virus from host cells.5
RIG-I-like receptors, such as MDA5 and RIG-I, recognize viral pathogen-associated molecular patterns, including double-stranded RNA (dsRNA) and polyinosinic/polycytidylic acid [poly(I/C)] in the cytoplasm.6 These PRRs then use N-terminal caspase activation and recruitment domains to recruit mitochondrial antiviral signaling protein (MAVS; also known as IPS-1, Cardif, or VISA) to initiate IFN-β signaling.3,7 Afterward, MAVS recruits TNF receptor-associated factor 3 (TRAF3) and activates TANK-binding kinase 1 (TBK1). The activated TBK1 then phosphorylates the transcription factor interferon regulatory factor 3 (IRF3) and initiates the dimerization of IRF3, resulting in its nuclear translocation. IRF3 subsequently binds to IFN stimulation response elements (ISREs), which triggers type I IFN transcription.3,8 Moreover, this induction of type I IFN production largely determines the host cells’ antiviral immune activity.9 Type I IFNs exert their biological act via the activation of the Janus kinase signal transducer and activator of transcription (JAK/STAT) signaling pathway, which in turn triggers the expression of ISGs.10 Briefly, IFN-α/β binds to the IFNAR in an autocrine or paracrine fashion, activating receptor-associated JAKs and leading to STAT1 and STAT2 phosphorylation. Once phosphorylated, STAT1 and STAT2 heterodimerize and form the transcription factor complex ISGF3 with IFN regulatory factor 9 (IRF9).11 The resulting complex is translocated to the nucleus and, in binding the ISREs, initiates the transcription of hundreds of ISGs. These processes trigger an antiviral state within the cells, further resulting in the inhibition of viral replication. Although type I IFNs are used clinically against numerous human viral pathogens, this clinical use is limited due to the ability of viruses to acquire and evade IFN-induced innate antiviral responses.12,13 In this sense, identifying natural compounds to modulate type I IFN-related antiviral immune response is of great significance for developing novel effective antiviral agents.
5-Hydroxymethylfurfural (5-HMF, Figure 1) is a heterocyclic furan-containing aldehyde generated by the Maillard reaction (a common reaction involving sugars at high temperatures).14 5-HMF is present in various sugar-containing foods which undergo thermal processing and long-term storage, including honey, coffee, fruit juices, and dried fruits.15 5-HMF is also detected in numerous heat-processed traditional Chinese medicines (TCMs) such as black garlic extract16 and Codonopsis pilosula.17 Recently, some studies have shown that 5-HMF exhibits various biological functions, such as the antioxidant activity,18 antiproliferative activity,18 cardioprotective effects,19 and protection against alcohol-associated oxidative liver injury.20 Regarding the immunomodulatory functions of 5-HMF, it has been shown that 5-HMF exerted the anti-inflammatory effect on human umbilical vein endothelial cells (HUVECs)16 and lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.21 However, the complete role of 5-HMF in the antiviral immune response remains unknown.
Figure 1.
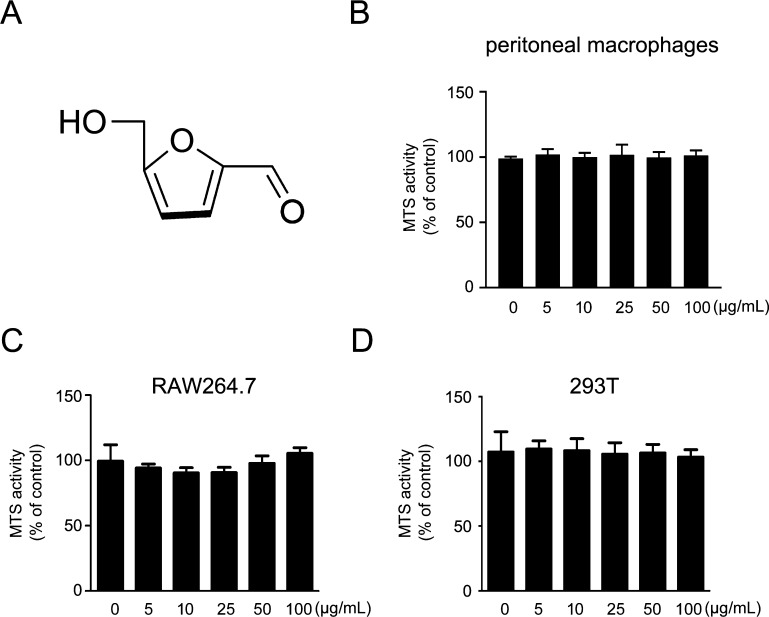
5-HMF’s chemical structure and effects on the survival of various cell lines. (A) Chemical structure of 5-HMF; (B-D) cell viability via the MTS assay of mouse peritoneal macrophages (B), RAW264.7 cells (C), and 293T cells (D) treated with various concentrations of 5-HMF for 48 h. Data denote the mean ± SEM of three independent experiments. * and ** mean p < 0.05 and p < 0.01, respectively.
In this paper, we elucidate the novel role of 5-HMF as an antiviral immune response regulator for the first time. At present, we have identified 5-HMF as a novel antivirus molecule by inhibiting vesicular stomatitis virus (VSV) replication, both in vivo and in vitro. Moreover, we have demonstrated that 5-HMF enhanced RIG-I expression to increase the activation of IRF-3 and production of type I IFNs. We have also demonstrated that 5-HMF upregulates IFN-α/β-triggered STAT1 and STAT2 phosphorylation and ISGs expression. These results provide an understanding of 5-HMF’s role in the context of the host’s innate immunity, suggesting that 5-HMF improves the RIG-I-induced type I IFN production and IFN-induced JAK/STAT signaling pathway and 5-HMF has the potency to be a promising agent for the treatment of viral infections.
Materials and Methods
Chemicals, Reagents, and Antibodies
5-HMF (purity > 99.5%) and LPS (055:B5) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Poly(I/C) and poly(deoxyadenylic/deoxythymidylic) acid [poly(dA/dT); B-DNA] were purchased from InvivoGen (San Diego, CA) and used at a concentration of 1 μg/mL for transfection. Recombinant mouse IFN-α and IFN-β proteins were purchased from R&D Systems (Minneapolis, MN). Anti-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-MAVS and anti-MDA5 antibodies were purchased from Abcam (Cambridge, UK). Cell Signaling Technology (Beverly, MA, USA) provided anti-RIG-I, anti-TBK1, anti-IRF3, anti-phosphorylated IRF3 (Ser396), anti-STAT1, anti-phosphorylated STAT1 (Tyr701), anti-STAT2, and anti-phosphorylated STAT2 (Tyr690) antibodies. Santa Cruz Biotechnology’s (Santa Cruz, CA, USA) HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG secondary antibodies were procured for use. VSV was purchased from the China Center for Type Culture Collection (Wuhan, China).
Cell Culture
The following protocol was used to prepare primary mouse peritoneal macrophages from C57BL/6J mice (6–8 weeks old): mice were injected with 3% thioglycolate [intraperitoneally (i.p.)], the peritoneal cells of the mice were harvested after 72 h, and macrophages enriched by rapid adhesion were isolated. HEK293T and RAW264.7 cell lines, which were obtained from the American Type Culture Collection, were cultured with 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen-Life Technologies) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 mg/mL streptomycin (Sigma-Aldrich) and 100 U/mL penicillin (Sigma-Aldrich) at 37 °C and 5% CO2. For the transfection of plasmids, the X-tremeGENE HP DNA transfection reagent (Roche) was used according to the manufacturer’s protocol.
Cell Viability Assay
A 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was utilized to investigate 5-HMF’s effect on cell viability. In brief, mouse primary peritoneal macrophages, RAW 264.7 cells, and 293T cells were harvested, and the cell suspensions were prepared in DMEM. The cells were seeded at a density of 1 × 103 to 1 × 104 cells/well into 96-well plates, with the wells at the edge of each plate filled with sterile phosphate-buffered saline (PBS). The concentrations of 5-HMF treatment were 0, 5, 10, 25, 50, and 100 μg/mL, and the medium was removed after 48 h incubation. To each well, 20 μL of MTS (Sigma-Aldrich) and 100 μL of the medium were added. The culture was terminated subsequently at 4 h and agitated until crystals were fully dissolved (10 min). The absorbance at 490 nm was measured, and the inhibition rate was calculated for each well.
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
RNA was isolated using TRIzol (Invitrogen) as per the manufacturer’s protocol. The results were analyzed using the ABI Prism 7500 7500 Real-Time PCR System after quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was conducted in three dependent trials using the SYBR Green PCR Master Mix (Applied Biosystems). The ΔΔCt method was used to determine relative expression levels, with Gapdh serving as the endogenous control. Several primers were utilized, including Ifnb, forward, 5′-AGCTCCAAGAAAGGACGAACA-3′, reverse, 5′-GCCCTGTAGGTGAGGTTGAT-3′; Ifit1, forward, 5′-CTGAGATGTCACTTCACATGGAA-3′, reverse, 5′-GTGCATCCCCAATGGGTTCT-3′; Ifit2, forward, 5′- GGAGAGCAATCTGCGACAG-3′, reverse, 5′- GCTGCCTCATTTAGACCTCTG-3′; Isg15, forward, 5′-GGTGTCCGTGACTAACTCCAT-3′, reverse, 5′-TGGAAAGGGTAAGACCGTCCT-3′; Ccl5, forward, 5′- GCTGCTTTGCCTACCTCTCC-3′, reverse, 5′-TCGAGTGACAAACACGACTGC-3′; Mx1, forward, 5′-GACCATAGGGGTCTTGACCAA-3′, reverse, 5′- AGACTTGCTCTTTCTGAAAAGCC-3′; Ifitm1, forward, 5′- GACAGCCACCACAATCAACAT-3′, reverse, 5′- CCCAGGCAGCAGAAGTTCAT-3′; Isg20, forward, 5′- TGGGCCTCAAAGGGTGAGT-3′, reverse, 5′- CGGGTCGGATGTACTTGTCATA-3′; Gapdh, forward, 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse, 5′-TGTAGACCATGTAGTTGAGGTCA-3′; and VSV mRNA, forward, 5′- ACGGCGTACTTCCAGATGG-3′, reverse, 5′-CTCGGTTCAAGATCCAGGT-3.
Luciferase Reporter Assays
Professor Chengjiang Gao (Shandong University, Jinan, China) generously donated IFN and ISRE reporter plasmids. The pGL3-Ddx58 reporter plasmid was generated by the PCR amplification of 1.6 kb Ddx58 promoter DNA sequences. Following this, the Ddx58 promoter DNA fragment was inserted into the pGL3 vector (Promega), and DNA sequencing was used to confirm all constructs.
In order to investigate the luciferase activity, transfection of 293T cells by the plasmids was carried out using the X-tremeGENE HP DNA transfection reagent (Roche). After 24 h, the cells were treated with either 5-HMF or DMSO and then stimulated with different reagents for different times, as indicated. According to the manufacturer’s protocol, the cells were harvested and lysed for dual-luciferase assays (Promega) to investigate the cellular luciferase activity.22 Then, to normalize the data for transfection efficiency, the firefly luciferase activity was divided by the Renilla luciferase activity.
ELISA and Western Blot
Using an ELISA kit purchased from R&D Systems, the IFN-β levels in the sera of C57BL/6J mice were determined by measuring the culture supernatant of mouse peritoneal macrophages or RAW264.7 cells. For western blot analysis, whole-cell lysates were obtained, and equivalent quantities of extracts were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes for analysis, as mentioned previously.22
Animal Experiments
From SLAC Laboratory Experimental Animal Co. (Shanghai, China), we procured 6–8 weeks old, 16–22 g, male C57BL/6J mice. All mice were housed in a facility free of pathogens. The ethical committee for Animal Care and the Use of Laboratory Animals at Hangzhou Normal University authorized the experimental procedures, which were strictly followed according to the university guidelines. For the investigation of the effect of 5-HMF on a virus-infected mouse model, the mice were randomly divided into four groups (n = 5), including the PBS control group, 5-HMF treatment group, VSV infection group, and VSV infection treated with the 5-HMF (5-HMF + VSV) group. The PBS control group mice were instilled by 200 μL of PBS per mouse i.p. The mice in the 5-HMF treatment group and the mice in the 5-HMF + VSV group were injected with 5-HMF at a dose of 12 mg/kg i.p. for a total volume of 100 μL per mouse, and after 1 h, the 5-HMF group mice were instilled by 100 μL of PBS i.p., and the 5-HMF + VSV group mice were instilled by a total volume of 100 μL of VSV (5 × 107 pfu/g) i.p. The mice in the VSV infection group were injected with a total volume of 100 μL of VSV (5 × 107 pfu/g) i.p. Mice were sacrificed 18 h after infection, and the samples were taken from the sera of the mice for ELISA cytokine analysis.
VSV replication in the tissues was measured using real-time qPCR on lung, liver, and spleen samples. The group dosed with PBS was selected as the negative control. Lung samples from the mice of the control group or infected with VSV were dissected for the histological study. The samples were prepared following a multistep procedure involving fixation with 10% phosphate-buffered formalin, embedding in paraffin, sectioning, staining with eosin and hematoxylin (H&E), and assessing via light microscopy.
To investigate the effects of the compound on the body weight, lung index, and pulmonary fibrosis of mice, the mice were randomly divided into four groups (five mice per group): the PBS control group, 5-HMF treatment group, VSV group, and VSV + 5-HMF group. Mice were treated according to the same procedure as that described in the previous paragraph, except that the dose of VSV was adjusted to 3 × 107 pfu/g in this experiment. After 48 h of infection, mice were sacrificed. The body weights of the animals were recorded. The lung tissues were harvested and weighed. The lung index was calculated using the following formula: lung index = lung weight (g)/body weight (g) × 100. The lung tissues were fixed, paraffin-embedded, and cut into 4 μm sections, which were subjected to Masson’s trichrome staining according to the manufacturer’s instructions (Servicebio, Wuhan, China) to identify collagen fibers. In order to detect the expression of α-SMA (smooth muscle actin), collagen I, and collagen III in mouse lung tissues, the sections were also subjected to immunohistochemical staining by using specific antibodies such as the anti-α-SMA antibody (1:200), anti-collagen I antibody (1:150), and anti-collagen III antibody (1:150) (Servicebio, Wuhan, China).
In mortality studies, the C57BL/6 mice were randomly divided into two groups (n = 10). For each group, the mice were injected with 5-HMF (12 mg/kg i.p.) or PBS. One hour later, the VSV-infected mouse model was established through VSV injection (1 × 108 pfu/g, i.p.), and the experiment was performed in the VSV group and VSV + 5-HMF (12 mg/kg) group. Each group’s status of survival was noted at various intervals as previously described.23
Statistical Analysis
Unless otherwise indicated, all data are presented as the mean ± SEM of each experiment. In order to determine the statistical significance, Student’s t-test was used, with a P-value of 0.05 or less suggesting statistical significance. All calculations were executed via the Prism software (GraphPad Software).
Results
5-HMF Enhances IFN-β Production in Macrophages
5-HMF is a ubiquitous natural product that exists in a variety of different foods and numerous TCMs. To determine the most appropriate concentrations of 5-HMF treatment for the subsequent experiment, the effect of 5-HMF on cell viability was evaluated by using the MTS colorimetric assay. At the highest tested concentration of 100 μg/mL, 5-HMF did not show cytotoxicity against the primary peritoneal macrophages and the cell lines RAW264.7 and 293T (Figure 1).
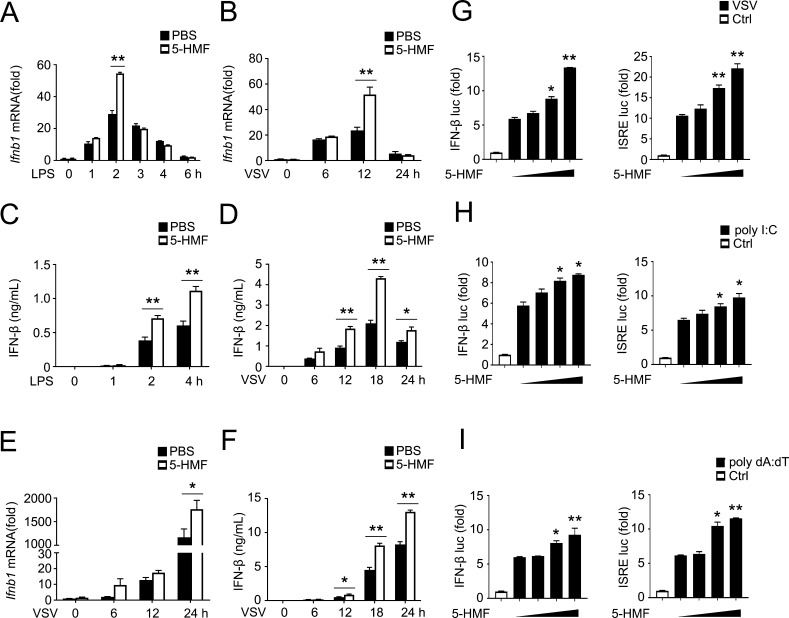
During the early stages of viral infection, IFN-β is the most crucial effector as part of the first-line defense for virus clearance. We then investigated 5-HMF’s possible effect on in vitro IFN-β production in macrophages following viral infection or stimulation with the toll-like receptor (TLR) ligand. In this study, mouse peritoneal macrophages were pretreated with 5-HMF and then underwent LPS stimulation (TLR4 ligand) or infection with VSV (a typical model ssRNA virus recognized by RIG-I) for varying time intervals. As shown in Figure 2, peritoneal macrophages treated by 5-HMF had higher IFN-β mRNA expression levels than nontreated cells after 2 h of stimulation with LPS or 12 h infection by VSV (Figure 2A,B). Consistently, LPS- and VSV-induced IFN-β secretion in the supernatant was also significantly increased in the presence of 5-HMF (Figure 2C,D). Similarly, the 5-HMF treatment also enhanced VSV-induced IFN-β expression and secretion in RAW264.7 cells (Figure 2E,F). According to these results, 5-HMF can promote the production of IFN-β triggered by the TLR4 signal or viral infection in macrophages.
Figure 2.
5-HMF enhances the production of IFN-β in macrophages. (A–D) qPCR (A,B) and ELISA (C,D) analyses of the production of IFN-β in PBS-pretreated peritoneal macrophages or 5-HMF-pretreated peritoneal macrophages (50 μg/mL) after subsequent stimulation with LPS (100 ng/mL, A) or infection with VSV (MOI = 1, B) for the indicated times. (E and F) Real-time PCR (E) and ELISA (F) analyses of IFN-β production in RAW 264.7 cells pretreated with PBS or 5-HMF and then infected with VSV (MOI = 1) at each specified duration. (G to I) In HEK293T cells, transfected with a plasmid-encoding luciferase reporter for the IFN-β promoter (IFN-β–luc) or ISRE (ISRE-luc; 100 ng each), followed by pretreatment with PBS or 5-HMF (0, 10, 25 and 50 μg/mL; wedge), subsequently undergoing no infection (Ctrl) or infection with VSV (MOI = 1, G), and transfected with poly(I/C) (1 μg/mL) [poly(I/C), H] or transfected with poly(dA/dT) (1 μg/mL) [poly(dA/dT), I], luciferase activity was measured. The results are presented relative to the Renilla luciferase activity. * and ** indicate p < 0.05 and p < 0.01, respectively.
To further investigate 5-HMF’s potential effects on antiviral immunity, we performed human embryonic kidney cell (HEK293T cells) transfections with the IFN-β-promoter luciferase reporter and the ISRE-luciferase reporter, which contains an ISRE sequence and only needs activation with IRF3. Because the coordination of IRF3 activation is necessary for IFN activation, this luciferase reporter assay was used to determine whether 5-HMF’s modulatory effect on IRF-3 was responsible for the increase in type I IFN performance. HEK293T cells underwent treatment with 5-HMF for 30 min after transfection with the luciferase reporter plasmids and were then infected with VSV for 12 h or transfected with the synthetic RNA duplex poly(I/C) or poly(dA/dT) for 6 h. The IFN-β and ISRE luciferase reporter assays showed that the treatment of 5-HMF significantly enhanced the IFN-β promoter and ISRE luciferase reporter activation triggered by the infection of VSV (Figure 2G) in HEK293T cells. Similar findings were observed in HEK293T cells transfected with poly(I/C) (Figure 2H), which is recognized by RIG-I and activates downstream signaling, or poly(dA/dT) (Figure 2I), which is known to trigger RIG-I signaling through RNA polymerase III-mediated transcription of poly(dA/dT) into poly(I/C). In combination, these findings support the hypothesis that 5-HMF enhances the virus-induced type I IFN signaling pathway.
5-HMF Enhances Cellular Antiviral Responses
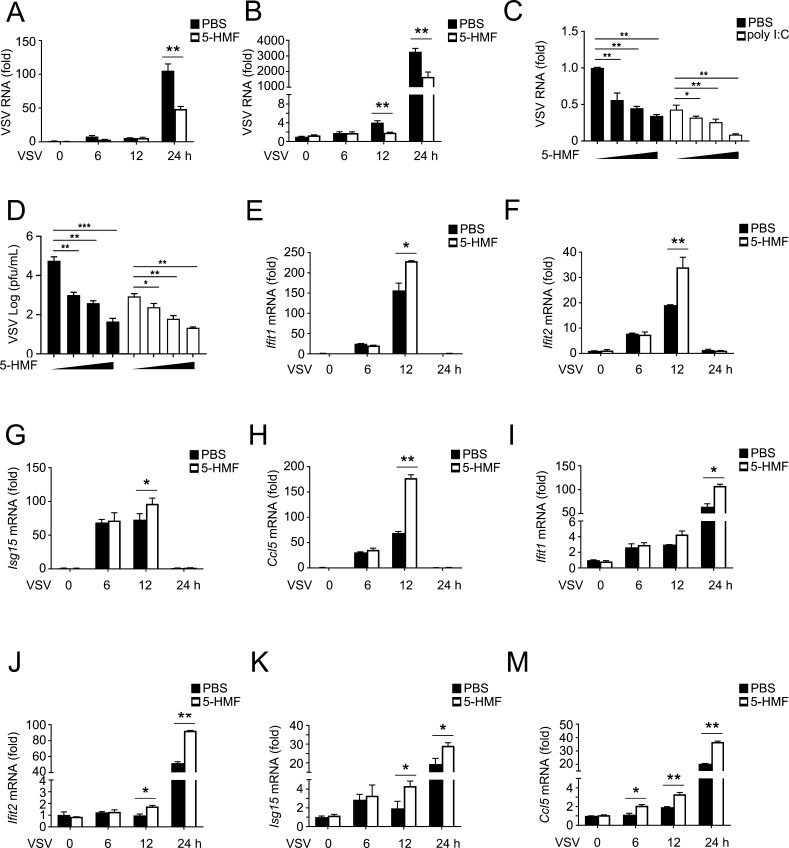
It has long been established that type I IFNs play a critical role in the immune system’s response to viral infection.24 Therefore, we examined the function of 5-HMF in antiviral immunity. As measured by qPCR, VSV RNA replicates were greatly reduced in 5-HMF-treated mouse peritoneal macrophages (Figure 3A) 24 h after infection or in RAW264.7 cells 12 and 24 h after infection (Figure 3B). Similarly, the qPCR experiment to detect VSV titers (Figure 3C) and the plaque assays of mouse peritoneal macrophages (Figure 3D) showed that 5-HMF substantially inhibited viral replication in a dose-dependent manner, independent of poly(I/C)’s absence or presence. These data suggest that 5-HMF significantly increased cellular antiviral responses.
Figure 3.
5-HMF enhances cellular antiviral responses. (A,B) Mouse peritoneal macrophages (A) or RAW264.7 cells (B) pretreated with PBS or 5-HMF (50 μg/mL), followed by infection with VSV (MOI = 1) for the indicated times. qPCR was used to determine the number of intracellular VSV RNA replicates. (C and D) After being pretreated with increasing concentrations of 5-HMF, mouse peritoneal macrophages were infected with VSV for 24 h, followed by poly(I/C) (20 μg/mL) or PBS treatment, as indicated. qPCR was used to determine the number of intracellular VSV RNA replicates (C), and standard plaque assays were used to assess VSV titers (D). (E–M) mRNA expression levels of Ifit1 (E,I), Ifit2 (F,J), Isg15 (G,K), and Ccl5 (H,M) were determined by qPCR in mouse peritoneal macrophages (E–H) and RAW 264.7 cells (I–M) pretreated with PBS or 5-HMF (50 μg/mL) and then infected with VSV for the indicated times, similar to that in (A,B). * and ** indicate p < 0.05 and p < 0.01, respectively.
To better understand the effect of 5-HMF on the expression of IFN-responsive genes, we detected the mRNA expression levels of several ISGs, such as Ifit1, Ifit2, Isg15, and Ccl5 in 5-HMF-pretreated or untreated macrophages after the infection of VSV for different times. We found that in both the mouse peritoneal macrophages (Figure 3E–H) and RAW 264.7 cells (Figure 3I–M), infection with VSV in 5-HMF-pretreated cells resulted in a greater increase in the expression of ISGs than that in untreated cells. These results suggest that the 5-HMF treatment enhanced the ISG expression triggered by viral infection.
5-HMF Protects Mice from Viral Infection by Increasing IFN-β Production
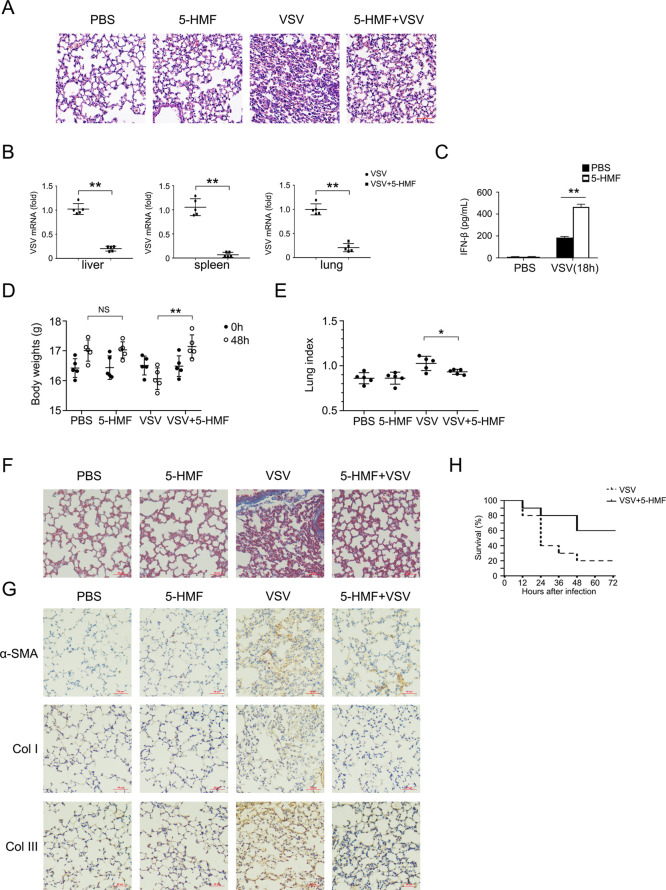
Next, we investigated the in vivo physiological and pathological relevance of these regulatory 5-HMF effects in the context of VSV infection. In mice infected with VSV, the 5-HMF-pretreated group showed a lower degree of monocyte infiltration and lung tissue damage than the control group (Figure 4A). Accordingly, fewer VSV viral loads in the organs such as the liver, lung, and spleen were observed in the 5-HMF-treated group (Figure 4B). Furthermore, the IFN-β production in the serum increased significantly compared to that in the control group (Figure 4C). After virus infection for 48 h, a loss of weight was observed in mice with VSV infection. In contrast, animals pretreated with 5-HMF showed a significantly higher body weight (Figure 4D) and a significantly lower lung index (Figure 4E) 48 h after VSV infection. Masson’s trichrome staining of lung sections showed that in mice infected with VSV, an abundance of collagen fibers was induced in lung tissues compared with that in the normal control group, and 5-HMF treatment significantly reduced collagen deposition (Figure 4F). The increased expression levels of α-SMA, collagen I, and collagen III after 48 h of VSV infection were detected by immunohistochemical staining, while 5-HMF pretreatment suppressed the increase in α-SMA, collagen I, and collagen III expression levels in lung tissues of the mice infected with VSV (Figure 4G). Finally, we evaluated the mortality effect of VSV-infected mice upon the administration of 5-HMF. Furthermore, the mice were dosed with 5-HMF (6 and 12 mg/kg, i.p.) or PBS before being challenged with VSV. As shown in Figure 4H, the overall survival was prolonged in the 5-HMF-treated group compared to that in the control group (Figure 4H). Consequently, taking all of this into consideration, these data demonstrate that 5-HMF protects mice against RNA virus infection by augmenting IFN-β production and facilitating the cellular antiviral response.
Figure 4.
5-HMF-treated mice are more resistant to viral infection due to the increased IFN-β production. (A–C) C57BL/6 mice (n = 5 mice/group) pretreated with 5-HMF (12 mg/kg, i.p.) or PBS were injected with VSV (5 × 107 pfu/g, i.p.) for 18 h. (A) Representative H&E stain of the lung tissue from the indicated group (×200); scale bar, 50 μm. (B) VSV loads in organs were determined by real-time PCR. (C) IFN-β levels in the sera were examined by ELISA. All results are expressed as the mean ± SEM: * and ** indicate p < 0.05 and p < 0.01, respectively, by Student’s t-test. (D–G) C57BL/6 mice (n = 5 mice/group) pretreated with 5-HMF (12 mg/kg, i.p.) or PBS were injected with VSV (3 × 107 pfu/g, i.p.) for 48 h. (D) Body weights of the mice before and after VSV infection. (E) Lung index (lung/body weight ratio) of the mice. (F) Masson’s trichrome staining of the lung tissue from the indicated group (×200). (G) Immunohistochemical staining of α-SMA, collagen I. and collagen III among different groups of mouse lung tissues. (H) C57BL/6 mice (n = 10 mice/group) pretreated with 5-HMF (6 and 12 mg/kg, i.p.) or PBS were injected with VSV (1 × 108 pfu/g, i.p.), and the survival rates of mice from each group were analyzed (**p < 0.01).
5-HMF Upregulates RIG-I Expression to Augment Antiviral Signaling
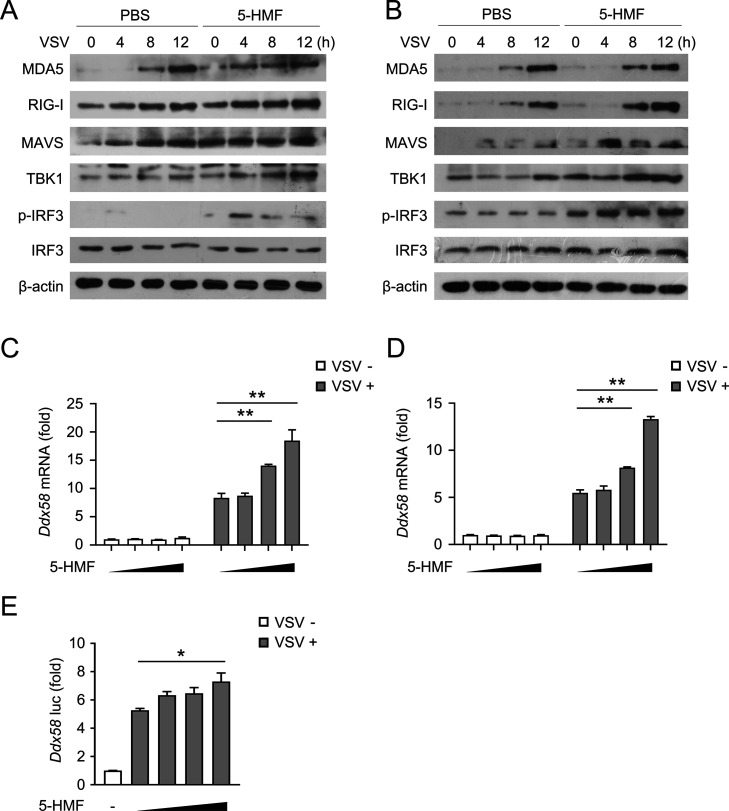
The results mentioned above indicate that 5-HMF can regulate type I IFN signaling following infection with VSV. Since the significant role of RIG-I-mediated antiviral response has been established in the development of VSV-induced IFNs,7 we hypothesized that 5-HMF may influence IFN production through the regulation of RIG-I-mediated signaling. Therefore, we used VSV to stimulate mouse peritoneal macrophages (Figure 5A) and RAW264.7 cells (Figure 5B) for different times combined with or without 5-HMF treatments. As shown in Figure 5A,B, the VSV-induced expression of RIG-I, MAVS, and TBK1 and phosphorylation of IRF3 was elevated in 5-HMF-pretreated cells.
Figure 5.
5-HMF regulates the type I IFN signaling pathway by inducing RIG-I expression. (A,B) Western blot analysis of RIG-I, MDA5, MAVS, TBK1, total and phosphorylated (p-) IRF3 in peritoneal macrophage cells (A) and RAW264.7 cells (B) pretreated with PBS or 5-HMF (50 μg/mL), followed by infection with VSV (MOI = 1) for different times. (C,D) mRNA expression levels of Ddx58 (which encodes RIG-I) were determined by qPCR in mouse peritoneal macrophages (C) and RAW 264.7 cells (D) pretreated with PBS or 5-HMF (0, 10, 25, and 50 μg/mL; wedge), followed by no infection (Ctrl) or infection with VSV (MOI = 1), respectively. (E) Luciferase activity in 293T cells transfected with a plasmid encoding a luciferase reporter for the Ddx58 promoter (Ddx58–luc, 100 ng each), pretreated with PBS or 5-HMF (0, 10, 25, and 50 μg/mL; wedge), followed by no infection (Ctrl) or infection with VSV (MOI = 1); results are presented relative to the Renilla luciferase activity. * and ** indicate p < 0.05 and p < 0.01, respectively.
Furthermore, we surveyed the VSV-induced Ddx58 gene expression (which encodes RIG-I protein) in mouse peritoneal macrophages and RAW264.7 cells, which had been pretreated with different dose concentrations of 5-HMF. mRNA levels of Ddx58, as measured by qPCR, are shown in Figure 5C,D. The increased expression of Ddx58 mRNA was initiated in the cells pretreated with 5-HMF (P < 0.05). Similarly, 5-HMF treatment increased the Ddx58 luciferase reporter gene activity in HEK293T cells challenged with VSV in a dose-dependent fashion (Figure 5E). Collectively, these results demonstrate that 5-HMF has a regulatory effect on the RNA virus-induced type I IFN signaling pathway through the upregulation of RIG-I expression.
5-HMF Enhances IFN-Induced STAT1 and STAT2 Activation and ISG Expression
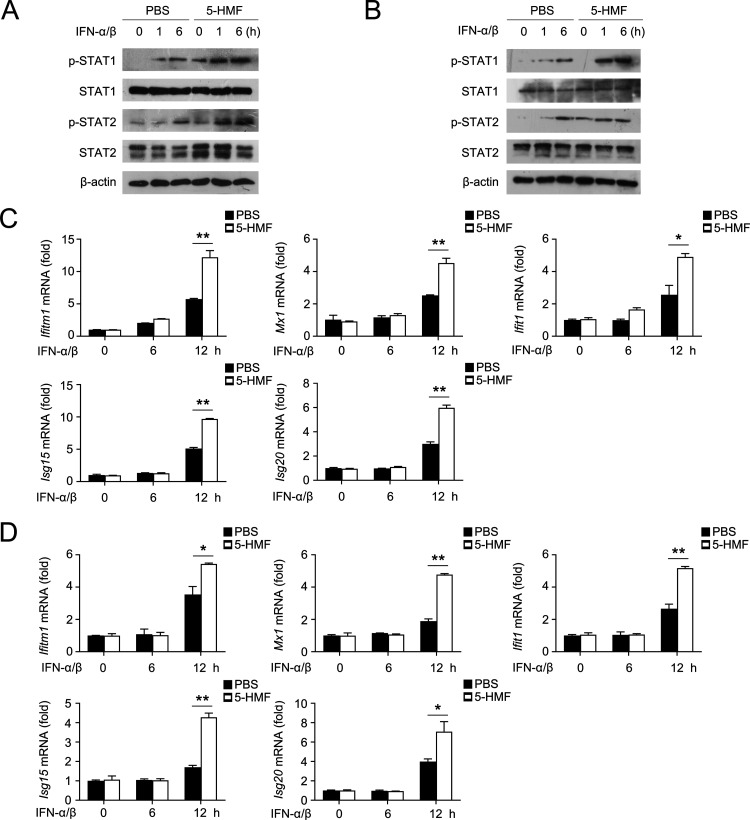
Type I IFNs, such as IFN-α and IFN-β, induce the JAK-STAT signaling pathway by upregulating the phosphorylation of STAT1 and STAT2, subsequently initiating the transcription of a set of ISGs which contribute to antiviral immunity.10 To determine whether 5-HMF regulates Type I IFN-triggered STAT1 and STAT2 activation; we investigated the phosphorylation state of STAT1 and STAT2 in RAW264.7 cells and primary peritoneal macrophages under IFN-α/β stimulation. As shown in Figure 6A,B, both STAT1 and STAT2 phosphorylation levels were significantly enhanced in 5-HMF-pretreated peritoneal primary macrophages (Figure 6A) and RAW264.7 cells (Figure 6B) compared to that in control cells.
Figure 6.
5-HMF facilitates IFN-induced STAT1 and STAT2 phosphorylation and ISG expression. (A,B) Western blot analysis of STAT1, phosphorylated (p-) STAT1, STAT2, and phosphorylated (p-) STAT2 in peritoneal macrophages (A) and RAW264.7 cells (B) pretreated with PBS or 5-HMF (50 μg/mL), followed by treatment with IFN-α/β (20 ng/mL) for 0, 1, and 6 h. (C,D) mRNA expression levels of Ifitm1, Mx2, Ifit1, Isg15, and Isg20 were determined by qPCR in mouse peritoneal macrophages (C) and RAW 264.7 cells (D) pretreated with PBS or 5-HMF and treated with IFN-α/β (20 ng/mL) for the specified periods.
Increased signaling through STAT1 and STAT2 leads to the enhancement of ISG expression. We then investigated the effect of 5-HMF on mRNA expression of the ISG genes in combination with IFN-α/β. As is evident in Figure 6C,D, compared with that of the cells under IFN-α/β treatment alone, the mRNA expression of the ISG genes, such as Ifitm1, Mx2, Ifit1, Isg15, and Isg50, significantly increased in cells treated with a combination of 5-HMF and IFN-α/β for 12 h in both primary peritoneal macrophages (Figure 6C) and RAW264.7 cells (Figure 6D). Therefore, 5-HMF significantly promotes type I IFN-triggered STAT1 and STAT2 signaling and ISG expression in macrophages.
Discussion
5-HMF is an organic compound generated by the Maillard reaction (sugar reduction);25 therefore, it is commonly found in daily heat-processed food products such as honey, coffee, dried fruit, and fruit juices.26 Recently, scientists uncovered 5-HMF’s capability to modulate the innate immune responses through anti-inflammatory or antiallergic effects.18,27 For example, it has been reported that 5-HMF shows antioxidant and anti-inflammatory effects in interferon-γ and phorbol ester-induced Caco-2 cells.28 In addition, 5-HMF was found to inhibit the production of ROS and inflammatory cytokines such as NO, PGE2, IL-1β, IL-6, and TNF-α in LPS-stimulated RAW264.7 macrophages.18 However, the biological properties of 5-HMF in the antiviral immune response in macrophages are less documented.
Considering the important role of innate immunity in various physiological conditions and the broad application of immune modulation in clinical settings, we determined the effects of 5-HMF on primary macrophages and cell line RAW264.7 after VSV infection. To better understand the molecular mechanisms of 5-HMF modulating the antiviral immune response, we investigated mRNA and protein expression of IFN-β (and their relevant ISGs) in VSV-infected primary macrophages or RAW264.7 cells. Regarding cell viability following 5-HMF treatment, 5-HMF was nontoxic to cells in our experiments at concentrations below 100 μg/mL. Thus, 5-HMF concentrations of 0, 10, 25, and 50 μg/mL were used to evaluate the effect on antiviral immune response in macrophages (Figure 1).
Type I IFNs are released rapidly by host cells to induce an antiviral state in response to most forms of viral infection and are a crucial component of innate defense. Optimum levels of type I IFN production are crucial to eliminate the invading viruses.4,29 Unfortunately, in order to survive in the host cells, viruses can develop several evasive mechanisms that inhibit the type I IFN expression and protect them from immune-mediated clearance. Since the secretion of type I IFNs requires tight control, the development of novel natural compounds that modulate type I IFN production is urgently needed.
Many compounds isolated or extracted from medicinal herbs and plants have been shown to have measurable antiviral effects. For example, certain Epimedium koreanum Nakai, with components such as quercetin, can trigger type I IFNs and proinflammatory cytokine secretion, which displayed a striking in vitro and in vivo antiviral effect.30 Lyu et al. investigated the antiherpetic activity of natural flavonoids in vitro against both herpes simplex virus types 1 and 2 (HSV-1 and HSV-2).31 Di et al. reported that the natural compound cirsitakaoside enhances the antiviral activity by inducing IFN-β production in host cells.32 Moreover, Jie et al. reported that the natural bisindole alkaloid from Isatis indigotica, indirubin, promotes IFN-β generation after infection with influenza in stressed mice.33 Our study identifies 5-HMF as a novel positive regulator of IFN-β induction downstream of LPS stimulation or VSV infection (Figure 2). Meanwhile, 5-HMF treatment boosts IFN-β and ISRE activation, which is triggered by poly(I/C), poly(dA/dT), and VSV infection (Figure 2).
VSV infection not only directly infects respiratory epithelial cells, stromal cells, and macrophages, causing cell necrosis and damage, but also induces the body to produce a large number of inflammatory factors through various antigenic components of the virus, resulting in lung inflammation. In the present study, we evaluated the protective effect of 5-HMF by examining the pathological changes of lung tissues of VSV-infected mice under treatment with 5-HMF. As shown in Figure 4A, 18 h after VSV infection, lung histopathology examination showed pulmonary interstitial lesions, including interstitial congestion, edema, thicken alveolar septum, and lymphocyte infiltration. 5-HMF can significantly reduce the congestion and thickening of pulmonary stroma and the infiltration of inflammatory cells (Figure 4A). These results showed that 5-HMF can significantly alleviate the pathological changes of the lung tissue of mice under viral infection. Furthermore, the viral loads in the lung, liver, and spleen of the VSV-infected mice were significantly reduced by 5-HMF treatment (Figure 4B). Additionally, we revealed that 5-HMF-pretreated mice were shielded from VSV infection in vivo probably due to the increased IFN-β production. In the intermediate and later stages of many virus infection-related lung diseases, acute lung injury eventually would lead to pulmonary fibrosis. Herein, we investigated the ability of 5-HMF to alleviate pulmonary fibrosis after virus infection. Mice treated with 5-HMF were found to have reduction in the expression of various fibrosis marker genes such as α-SMA, collagen I, and collagen III, further demonstrating 5-HMF’s ability to enhance lung repair through limiting fibroproliferation. Meanwhile, 5-HMF significantly reduced the decline in the survival rate caused by VSV infection in mice (Figure 4H). These in vivo and in vitro observations show a positive impact of 5-HMF on the reduction of VSV susceptibility.
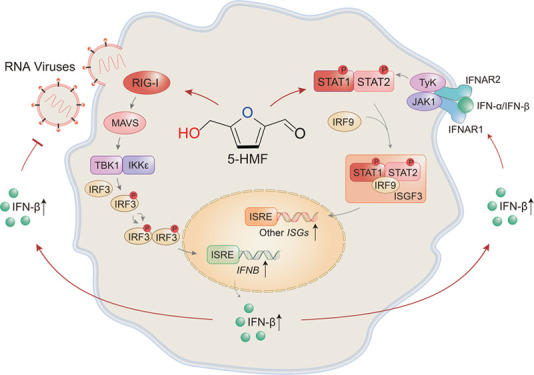
RIG-I is an important PRR that recognizes double-stranded viral RNA in the cytoplasm. RIG-I can activate the innate immune response and also regulate adaptive immunity. Macrophages and dendritic cells are host innate immune cells that express high levels of RIG-I and play a vital role in protection against viral infection.34 Several natural compounds have been reported for their bioactivity to modulate the RIG-I signaling pathway. For example, ribavirin and 4(3H)-quinazolone have been seen to inhibit virus-induced RIG-I expression signaling via the downregulation of the RIG-I expression. In our present study, VSV activation of the RIG-I signaling pathway is initiated through the upregulation of the key signaling molecule RIG-I. This change activates the nuclear transcription factor IRF3 through signaling transduction, which triggers IFN-β production. 5-HMF amplifies the RIG-I activation signaling pathway and also upregulates the expression of other key signaling molecules (such as MAVS and TBK1) and the phosphorylation of IRF-3, finally promoting IFN-β expression. We also confirmed that 5-HMF could augment the VSV-induced RIG-I expression at the mRNA level (Figure 5). In summary, we discovered a novel role of 5-HMF in promoting the antiviral response. The mechanism of this action of 5-HMF is partially due to the increasing production of type I IFN via upregulating the RIG-I signaling pathway in macrophages.
ISGs have been studied extensively due to their important role in innate immune defense. The synthesized type I IFNs during virus infection initiate JAK/STAT signaling, which shows a major contribution to ISG transcription.35 The canonical JAK/STAT signaling pathway is mediated by STAT1 and STAT2 phosphorylation.36 Therefore, the JAK/STAT pathway, which plays a central role in the antiviral defense system, is a potential target for antiviral drug development. However, despite the discovery of many natural JAK/STAT pathway inhibitors which control immune responses, natural compounds which act as activators of the JAK/STAT pathway are less reported.
He et al. described that emodin (1,3,8-trihydroxy-6-methyl anthraquinone) activated JAK/STAT signaling and improved the antiproliferative effect of IFN-α.37 Emodin is a naturally occurring anthraquinone derivative found in certain plant roots and bark. Wang et al. reported that luteolin, a common bioflavonoid found in fruits and vegetables, increased the production of ISGs by enhancing STAT1 phosphorylation, resulting in an inhibition of RSV replication.38 Kawaguchi et al. reported that the bioactive constituent tryptanthrin, found in the indigo plants Polygonum tinctrorium and Isatis tinctoria, altered STAT1 phosphorylation and nuclear translocation in HUVECs.39 In the present study, we found that compared to that of the untreated group, the expression levels of Ifit1, Ifit2, Isg15, and Ccl5 mRNA in the 5-HMF-treated macrophages infected with VSV were significantly upregulated (Figure 3). These results illustrate that during the period of VSV infection, 5-HMF may improve the antiviral immune function by evaluating the synthesis and release of ISGs, accounting for one aspect of its antiviral protective effects.
Moreover, we also found that 5-HMF significantly upregulated the phosphorylation levels of STAT1 and STAT2 in macrophages triggered by IFN-α/β. This upregulation of phosphorylated STAT1 and STAT2 was synergistically associated with the expression levels of Ifitm1, Mx1, Ifit1, Isg15, and Isg20 mRNA in our study (Figure 6), accounting for another aspect of the antiviral protective effects of 5-HMF. These results reveal that 5-HMF can enhance the antiviral effects of IFN-α/β during viral infection, thus paving the way for further investigation into the mechanisms by which 5-HMF regulates the immune system.
Collectively, in our present study, 5-HMF has been uncovered to show the antivirus activity both in vitro and in vivo. 5-HMF modulates the innate antiviral response by promoting IFN-β production, leading to the augmentation of the immune response to VSV infection. Mechanistically, we demonstrated that 5-HMF upregulates the RIG-I expression and RIG-I downstream signaling pathway. Meanwhile, 5-HMF upregulates the JAK/STAT signaling pathway triggered by type I IFNs in macrophages, which leads to the increased expression levels of ISGs. In conclusion, our results indicate that the natural compound 5-HMF shows promising antiviral effects and the potential for development as a novel antivirus drug. Additionally, 5-HMF’s potential impact on various subtypes of viruses warrants further investigation.
Acknowledgments
We thank Prof. Chengjiang Gao for providing the plasmids used in this work.
Author Contributions
H.Zou and T.W. contributed equally to this work. H.Zhang, X.T., and Y.X.-Y. conceived and supervised the project. H.Zhang and Y.X.-Y. wrote the manuscript. H.Zhang and Y.K. designed the experiments and analyzed the data. H.Zou, W.T., S.Q., W.Y., X.L., J.Z., and L.X. performed the experiments.
This work was funded by the National Natural Science Foundation of China (81730108, 81973635, 81900011, and 82073686), the Zhejiang Provincial Natural Science Foundation of China (LQ19C080001), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021KY962), the Key Project of Hangzhou Ministry of Science and Technology (20212013B03), the Hangzhou Normal University startup fund (4125C5021920419), the Hangzhou Normal University School of Medicine Teaching Reform Fund (4125b30100112), the Project of National Students’ Platform for Innovation and Entrepreneurship Training Program (201910346035), and the Program of “Xinmiao” Talents in Zhejiang Province of China (2020R427042).
The authors declare no competing financial interest.
References
- Schneider W. M.; Chevillotte M. D.; Rice C. M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S.; Thomsen A. R. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012, 86, 2900–2910. 10.1128/jvi.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M.; Onomoto K.; Jogi M.; Akaboshi T.; Fujita T. Viral RNA detection by RIG-I-like receptors. Curr. Opin. Immunol. 2015, 32, 48–53. 10.1016/j.coi.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Wang B. X.; Fish E. N. The yin and yang of viruses and interferons. Trends Immunol. 2012, 33, 190–197. 10.1016/j.it.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Yoneyama M.; Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009, 227, 54–65. 10.1111/j.1600-065x.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- Loo Y.-M.; Gale M. Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L. A. J.; Golenbock D.; Bowie A. G. The history of Toll-like receptors - redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Hu Y. W.; Zhang J.; Wu X. M.; Cao L.; Nie P.; Chang M. X. TANK-Binding Kinase 1 (TBK1) Isoforms Negatively Regulate Type I Interferon Induction by Inhibiting TBK1-IRF3 Interaction and IRF3 Phosphorylation. Front. Immunol. 2018, 9, 84. 10.3389/fimmu.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson D. S.; Horvath C. M. A road map for those who don’t know JAK-STAT. Science 2002, 296, 1653–1655. 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Der S. D.; Zhou A.; Williams B. R. G.; Silverman R. H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 15623–15628. 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Liu Q.; Zhou J.; Xie W.; Chen C.; Wang Z.; Yang H.; Cui J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discovery 2017, 3, 17006. 10.1038/celldisc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.; Vijayan M.; Pritzl C. J.; Fuchs S. Y.; McDermott A. B.; Hahm B. Hemagglutinin of Influenza A Virus Antagonizes Type I Interferon (IFN) Responses by Inducing Degradation of Type I IFN Receptor 1. J. Virol. 2015, 90, 2403–2417. 10.1128/JVI.02749-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirbes L.; Nguyen B. K.; de Graaf D. C.; De Meulenaer B.; Reybroeck W.; Haubruge E.; Saegerman C. Hydroxymethylfurfural: a possible emergent cause of honey bee mortality?. J. Agric. Food Chem. 2013, 61, 11865–11870. 10.1021/jf403280n. [DOI] [PubMed] [Google Scholar]
- Anese M.; Manzocco L.; Calligaris S.; Nicoli M. C. Industrially applicable strategies for mitigating acrylamide, furan, and 5-hydroxymethylfurfural in food. J. Agric. Food Chem. 2013, 61, 10209–10214. 10.1021/jf305085r. [DOI] [PubMed] [Google Scholar]
- Kim H. K.; Choi Y.-W.; Lee E. N.; Park J. K.; Kim S.-G.; Park D.-J.; Kim B.-S.; Lim Y.-T.; Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFalpha-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-kappaB activation. Phytother. Res. 2011, 25, 965–974. 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- Feng Y. J.; Wang X. X.; Zhuang P. Y.; Zhang D. Y.; Gao L.; Chen J. M.; Han G. [Study on chemical constituents of Codonopsis pilosula]. Zhongguo Zhongyao Zazhi 2017, 42, 135–139. 10.19540/j.cnki.cjcmm.20161222.046. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Chen J.; Su J.; Li L.; Hu S.; Li B.; Zhang X.; Xu Z.; Chen T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- Wölkart G.; Schrammel A.; Koyani C. N.; Scherübel S.; Zorn-Pauly K.; Malle E.; Pelzmann B.; Andrä M.; Ortner A.; Mayer B. Cardioprotective effects of 5-hydroxymethylfurfural mediated by inhibition of L-type Ca(2+) currents. Br. J. Pharmacol. 2017, 174, 3640–3653. 10.1111/bph.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Qu X.-N.; Han Y.; Zheng S.-W.; Wang J.; Wang Y.-P. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int. J. Mol. Sci. 2015, 16, 2446–2457. 10.3390/ijms16022446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F.; Lee B. H.; Wei K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-kappaB and mTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275. 10.3390/molecules24020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.; Kang Y.; Zou H.; Cheng X.; Xie T.; Shi L.; Zhang H. beta-elemene attenuates macrophage activation and proinflammatory factor production via crosstalk with Wnt/beta-catenin signaling pathway. Fitoterapia 2018, 124, 92–102. 10.1016/j.fitote.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Kang Y.; Zhang H.; Zhao Y.; Wang Y.; Wang W.; He Y.; Zhang W.; Zhang W.; Zhu X.; Zhou Y.; Zhang L.; Ju Z.; Shi L. Telomere Dysfunction Disturbs Macrophage Mitochondrial Metabolism and the NLRP3 Inflammasome through the PGC-1alpha/TNFAIP3 Axis. Cell Rep. 2018, 22, 3493–3506. 10.1016/j.celrep.2018.02.071. [DOI] [PubMed] [Google Scholar]
- Stetson D. B.; Medzhitov R. Type I interferons in host defense. Immunity 2006, 25, 373–381. 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Janzowski C.; Glaab V.; Samimi E.; Schlatter J.; Eisenbrand G. 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. 10.1016/s0278-6915(00)00070-3. [DOI] [PubMed] [Google Scholar]
- Shapla U. M.; Solayman M.; Alam N.; Khalil M. I.; Gan S. H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem. Cent. J. 2018, 12, 35. 10.1186/s13065-018-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M.; Khodaei H.; Mesgari Abbasi M.; Saleh-Ghadimi S. Assessing the effect of 5-hydroxymethylfurfural on selected components of immune responses in mice immunised with ovalbumin. J. Sci. Food Agric. 2017, 97, 3979–3984. 10.1002/jsfa.8261. [DOI] [PubMed] [Google Scholar]
- Kitts D. D.; Chen X.-M.; Jing H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar-amino acid maillard reaction products. J. Agric. Food Chem. 2012, 60, 6718–6727. 10.1021/jf2044636. [DOI] [PubMed] [Google Scholar]
- Porritt R. A.; Hertzog P. J. Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol. 2015, 36, 150–160. 10.1016/j.it.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Cho W.-K.; Weeratunga P.; Lee B.-H.; Park J.-S.; Kim C.-J.; Ma J.; Lee J.-S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses 2015, 7, 352–377. 10.3390/v7010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu S.-Y.; Rhim J.-Y.; Park W.-B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharmacal Res. 2005, 28, 1293–1301. 10.1007/bf02978215. [DOI] [PubMed] [Google Scholar]
- Di Q.; Zhu H.; Pu D.; Zhao X.; Li X.; Ma X.; Xiao W.; Chen W. The natural compound Cirsitakaoside enhances antiviral innate responses against vesicular stomatitis virus in vitro and in vivo. Int. Immunopharmacol. 2020, 86, 106783. 10.1016/j.intimp.2020.106783. [DOI] [PubMed] [Google Scholar]
- Jie C.; Luo Z.; Chen H.; Wang M.; Yan C.; Mao Z.-F.; Xiao G.-K.; Kurihara H.; Li Y.-F.; He R.-R. Indirubin, a bisindole alkaloid from Isatis indigotica, reduces H1N1 susceptibility in stressed mice by regulating MAVS signaling. Oncotarget 2017, 8, 105615–105629. 10.18632/oncotarget.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H.; Flavell R. A. Innate recognition of non-self nucleic acids. Genome Biol. 2008, 9, 211. 10.1186/gb-2008-9-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C. E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. table of contents 10.1128/cmr.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J. W.; Rice C. M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Huang J.; Wang P.; Shen X.; Li S.; Yang L.; Liu W.; Suksamrarn A.; Zhang G.; Wang F. Emodin potentiates the antiproliferative effect of interferon alpha/beta by activation of JAK/STAT pathway signaling through inhibition of the 26S proteasome. Oncotarget 2016, 7, 4664–4679. 10.18632/oncotarget.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Ling Y.; Yao Y.; Zheng G.; Chen W. Luteolin inhibits respiratory syncytial virus replication by regulating the MiR-155/SOCS1/STAT1 signaling pathway. Virol. J. 2020, 17, 187. 10.1186/s12985-020-01451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S.; Sakuraba H.; Kikuchi H.; Numao N.; Asari T.; Hiraga H.; Ding J.; Matsumiya T.; Seya K.; Fukuda S.; Imaizumi T. Tryptanthrin suppresses double-stranded RNA-induced CXCL10 expression via inhibiting the phosphorylation of STAT1 in human umbilical vein endothelial cells. Mol. Immunol. 2021, 129, 32–38. 10.1016/j.molimm.2020.11.003. [DOI] [PubMed] [Google Scholar]