Abstract
Hemostasis and thrombosis are believed to be so intricately linked that any strategies that reduce thrombosis will have an inevitable impact on hemostasis. Consequently, bleeding is viewed as an unavoidable side effect of anticoagulant therapy. Emerging evidence suggests that factor XI is important for thrombosis but has a minor role in hemostasis. This information raises the possibility that anticoagulants that target factor XI will be safer than currently available agents. The authors provide a visual representation of the coagulation pathways that distinguishes between the steps involved in thrombosis and hemostasis to explain why factor XI inhibitors may serve as hemostasis-sparing anticoagulants. A safer class of anticoagulants would provide opportunities for treatment of a wider range of patients, including those at high risk for bleeding. Ongoing clinical studies will determine the extent to which factor XI inhibitors attenuate thrombosis without disruption of hemostasis.
Keywords: anticoagulation, factor XI, hemostasis, thrombosis
Thromboembolic disorders are estimated to cause 1 in 4 deaths worldwide. As the common underlying mechanism of venous thromboembolism (VTE) and most cases of myocardial infarction and ischemic stroke, thrombosis is a leading cause of morbidity and mortality (1).
Anticoagulation therapy is a cornerstone for the prevention and treatment of thrombosis. For many years, vitamin K antagonists (VKAs) such as warfarin were the only available oral anticoagulants. Although effective, VKAs are burdensome to administer because the dose varies from person to person. This variability reflects drug-drug and drug-food interactions as well as common genetic polymorphisms that affect the pharmacokinetic and pharmacodynamic properties of VKAs. Consequently, patients taking VKAs need regular coagulation monitoring and dose adjustment to ensure that the international normalized ratio (INR), the measure of the anticoagulant effect of VKAs, remains within the therapeutic range. If the INR is too low, the risk of thrombosis is increased, and if the INR is too high, the risk of bleeding is increased. Although INR monitoring reduces the risk of bleeding with VKAs, the risk of intracranial hemorrhage, the most feared complication of VKA treatment, is up to 10-fold higher in patients taking VKAs than in those not taking an anticoagulant (2,3). This is despite the INR being within the therapeutic range in at least two-thirds of patients with VKA-associated intracranial bleeding (4).
VKAs exert their antithrombotic effects by lowering the functional levels of the vitamin K-dependent clotting proteins, factor (F) II (prothrombin), FVII, FIX, and FX. Reduction in the levels of prothrombin and FX appears to be most important for achieving a therapeutic effect. Consistent with this, parenteral agents that specifically inhibit the activated forms of these proteins, thrombin (eg, argatroban or bivalirudin) or FXa (eg, fondaparinux), have proven to be effective anticoagulants. Over the past 15 years, oral inhibitors targeting thrombin (ie, dabigatran) or FXa (ie, rivaroxaban, apixaban, and edoxaban) have become available. These drugs are collectively referred to as direct oral anticoagulants (DOACs). A meta-analysis of pivotal trials of patients with nonvalvular atrial fibrillation showed that, compared with warfarin, the high-dose DOAC regimens were associated with a 19% reduction in the risk of stroke/systemic embolism and a 10% reduction in all-cause mortality. This was accompanied by a 50% reduction in intracranial hemorrhage and a 50% reduction in hemorrhagic stroke (5). Post-marketing observational data are consistent with the randomized trial results for all marketed DOACs, revealing a 40% reduction in major bleeding (6-11).
Despite the improved safety profile of the DOACs compared with VKAs, their use is still associated with a significant risk of bleeding. The fear of bleeding likely contributes to undertreatment of certain types of patients who may benefit from anticoagulation. A recent registry-based study revealed that 13% of patients with atrial fibrillation admitted with stroke had been deemed unsuitable for anticoagulation due to a high bleeding risk. Furthermore, an additional 42% of patients with known atrial fibrillation had not been given an anticoagulant despite being eligible for thromboprophylaxis, indicating that fear of bleeding is an obstacle to anticoagulation (12,13).
Prior to the introduction of the DOACs, VTE treatment started with heparin or low-molecular-weight heparin as patients were bridged to a VKA. Randomized trials comparing this approach with treatment with DOACs revealed a similar risk of recurrent VTE, but a 40% reduction in major bleeding with DOACs (14). Consequently, DOACs rapidly replaced VKAs for VTE treatment. Low-molecular-weight heparins such as dalteparin were the standard of care for treatment of cancer-associated VTE because they were shown to be superior to VKAs for prevention of recurrent VTE (15). Randomized trials comparing DOACs with dalteparin in such patients reveal a lower risk of recurrent VTE with the DOACs, but more major bleeding, at least with some of the drugs (16). The excess bleeding with the DOACs in cancer patients mainly reflects gastrointestinal bleeding in those with unresected tumors of the upper gastrointestinal tract. However, even in patients with atrial fibrillation, the rates of gastrointestinal bleeding are higher with some of the DOACs than with warfarin (5). This emphasizes the need for new anticoagulants that spare hemostasis (17).
FXI inhibitors have emerged as a particularly promising class of anticoagulants. With the publication of early- and mid-stage trial data for drugs directed against FXI and its activated form FXIa, it is a good time to take a fresh look at how targeting this protein prevents thrombosis while leaving hemostasis largely intact. In this paper, we propose a new visual framework for coagulation based on the latest science that explains the potential of inhibitors of FXI and FXIa to uncouple thrombosis from hemostasis (Central Illustration).
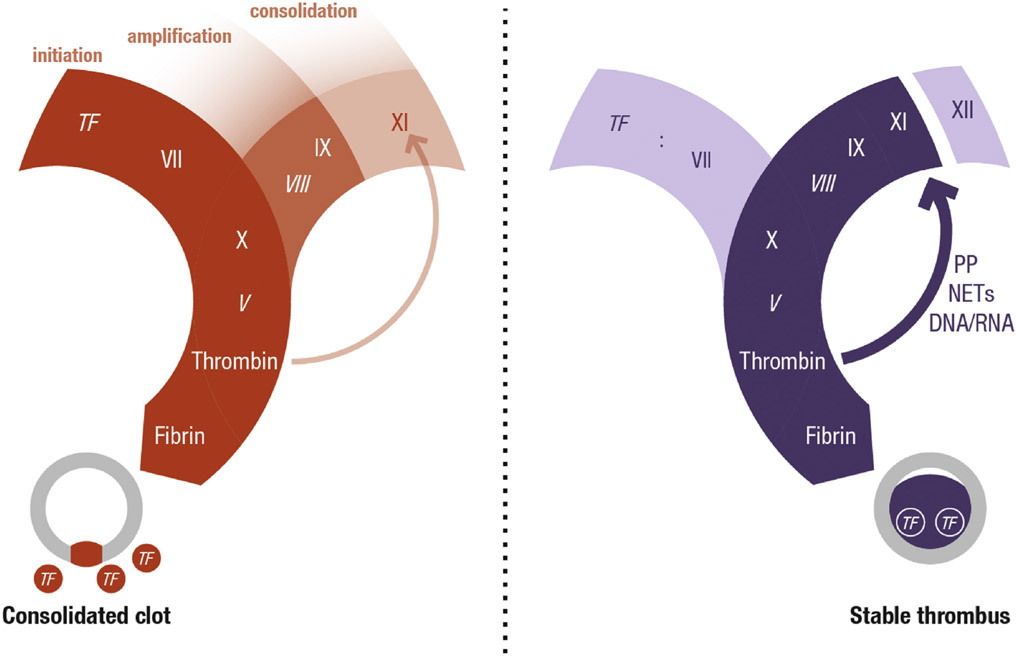
CENTRAL ILLUSTRATION. Hemostasis and Thrombosis.
Schematic representation of the key differences between hemostasis and thrombosis showing coagulation factor activation pathways and cross sections of a blood vessel. Two related but distinct processes of coagulation are represented, one characterized by clot formation to seal off a vessel wall injury (hemostasis) and the other by intraluminal clot formation resulting in obstruction of blood flow (thrombosis). Tissue factor initiates both processes but, whereas FXI plays a subsidiary role in hemostasis, in thrombosis FXI is required for thrombus growth within the vessel lumen. The predicted relative importance of the pathway components is denoted by the intensity of shading. Italics denote cofactors. NETs = neutrophil extracellular traps; PP = polyphosphate; TF = tissue factor.
UNCOUPLING HEMOSTASIS AND THROMBOSIS
Hemostasis and thrombosis involve many of the same enzymatic reactions but produce different results, as set out in the Central Illustration. In hemostasis, bleeding from injured vessels is stopped by formation of a hemostatic plug within the vessel wall and the immediate extravascular environment. In thrombosis, a thrombus forming within the blood vessel lumen partially or completely blocks blood flow, thereby leading to organ damage (Central Illustration). Understanding the similarities and differences in these processes is required for rational design of drugs that uncouple antithrombotic and antihemostatic effects.
HEMOSTASIS.
The goal of hemostasis, a physiological response to injury to a blood vessel wall, is to seal the leak and to stop bleeding, with minimal disruption of normal blood flow within vessels. The hemostatic process is initiated when FVII or its activated form FVIIa in blood binds to extravascular tissue factor (TF) exposed at the site of blood vessel injury (Figure 1). The FVIIa:TF complex activates FX to FXa, which then converts a limited amount of prothrombin into the key coagulation enzyme thrombin. This early thrombin generation initiates fibrin formation and, along with FXa, provides positive feedback to activate the key cofactor proteins, FV and FVIII.
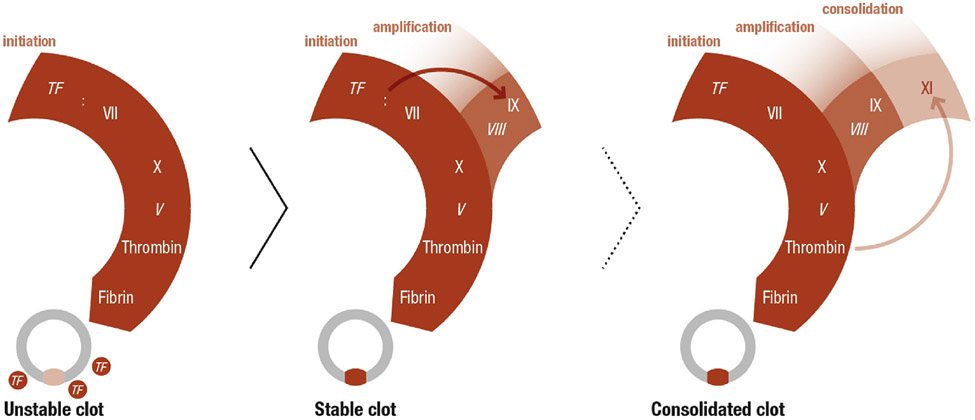
FIGURE 1. Schematic Representation of Processes Required for Hemostasis.
Hemostasis is initiated when FVII or FVIIa in blood binds to extravascular TF exposed at the site of injury (left). The FVIIa/TF complex converts FX to FXa leading to formation of sufficient thrombin to convert fibrinogen to fibrin and to form an unstable hemostatic plug. The FVIIa/TF complex also activates FIX (center), which amplifies and sustains FXa and thrombin generation. This additional thrombin strengthens the clot and maintains its stability over time. In some patients and in certain situations, conversion of FXI to FXIa is required to consolidate the hemostatic plug (right). FXI is thought to be activated by thrombin generated early in hemostasis, and contributes to sustained thrombin generation by activating FIX in addition to the FIX activated by the FVIIa/TF complex. Italics indicate cofactors. TF = tissue factor.
The limited amount of thrombin generated early in hemostasis is only sufficient to initiate the response to injury. In addition to activating FX, the FVIIa/TF complex converts FIX to FIXa (Figure 1). FIXa, with its cofactor factor VIIIa, maintains thrombin generation over hours to days post-injury through sustained activation of FX. The importance of this pathway in hemostasis is reflected by the severe bleeding disorders associated with inherited FVIII or FIX deficiency (hemophilia A and hemophilia B, respectively) (18).
FXI plays a relatively limited role in hemostasis. In some individuals, it is required to stem bleeding with certain types of injuries. During hemostasis, FXI is thought to be converted to FXIa by thrombin, and this process consolidates coagulation through activation of FIX (Figure 1). FXI appears to be most important with injury to the oropharynx and urinary tract. Bleeding in patients with congenital FXI deficiency is relatively mild compared with that in hemophilia A or hemophilia B, and the spontaneous bleeds into muscles or joints and the life-threatening intracranial or gastrointestinal bleeding seen with hemophilia A or B are not features of FXI deficiency (19).
THROMBOSIS.
A pathologic process in which clots form within the blood vessel lumen, thrombosis compromises blood flow. In most cases, thrombus formation appears to be triggered by TF exposed on disrupted vascular endothelium (eg, disrupted atherosclerotic plaques) or expressed on leukocytes, microvesicles, or diseased endothelial cells (Figure 2). However, the capacity of the FVIIa/TF complex to propagate intraluminal thrombus growth may be limited once the thrombus enlarges and extends beyond the TF source at the site of vessel wall damage. Here, FXI may drive thrombus growth (Figure 2). Epidemiological data and studies in animals support the importance of FXI in thrombosis. FXI-deficient individuals have reduced incidences of VTE and ischemic stroke compared with the general population, whereas those with high FXI levels carry more than twice the risk of VTE (20,21). Thrombosis in response to injury is attenuated in mice deficient in FXI, and FXI knockdown or inhibition reduces thrombosis in a variety of animal models (22-25).
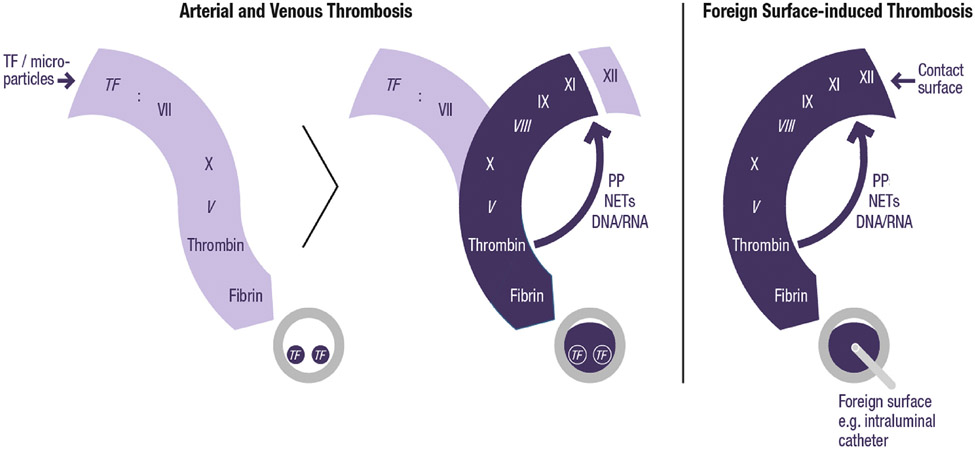
FIGURE 2. Schematic Representation of Processes That Contribute to Thrombosis.
Tissue factor (TF) on the luminal surface of blood vessels initiates thrombin generation by forming a complex with FVII/FVIIa (left). However, thrombus growth away from the vessel wall depends on activation of FXI to amplify the process (center). In most cases, FXI is likely to be activated by thrombin, but in some situations, particularly those in which blood is exposed to artificial surfaces (right), FXIIa may be the major activator of FXI. Italics indicate cofactors. NETs = neutrophil extracellular traps; PP = polyphosphate.
Thrombin is likely the predominant activator of FXI in thrombosis. FXIIa appears to be less important because epidemiologic studies fail to show an association between FXII levels and the risk of VTE, ischemic stroke, or myocardial infarction. Nonetheless, naturally occurring polyanions that are likely to be present in a growing thrombus, including chromatin extruded from activated neutrophils (neutrophil extracellular traps) or inorganic phosphate polymers (polyphosphate) released from activated platelets, can activate FXII and support FXI activation by FXIIa. Furthermore, in situations where thrombosis is triggered by exposure of blood to artificial surfaces (eg, cardiopulmonary bypass, hemodialysis, central venous catheters), thrombin generation may be primarily initiated by FXIIa activation of FXI (Figure 2), rather than by the FVIIa/TF complex. Regardless of the mechanism of FXI activation, on balance, FXIa appears to be more important for thrombosis than for hemostasis.
Therapies directed at FXI or FXIa have demonstrated efficacy in preventing VTE in patients undergoing total knee arthroplasty in two phase 2 studies (26,27). During knee arthroplasty, a number of factors may increase VTE risk including exposure of blood to TF, collagen, or products of cell breakdown such as nucleic acids and polyphosphate. FXI reduction achieved by administering an antisense oligonucleotide starting 35 days before surgery, or FXIa inhibition with osocimab, an inhibitory antibody given immediately before or after surgery, was at least as effective as standard therapy in preventing postoperative VTE in patients undergoing total knee arthroplasty. Importantly, therapy directed at FXI/FXIa did not appear to compromise hemostasis. Thus, many of the patients who received the antisense oligonucleotide or osocimab preoperatively had the full effect of FXI inhibition therapy during surgery and did not have excessive bleeding (26,27). An ideal treatment would have the same efficacy and bleeding profile as the antisense oligonucleotide but would have a rapid onset of action, which would enable administration after surgery.
The models for hemostasis and thrombosis in Figures 1 and 2 provide insight into the effects of different classes of anticoagulants on hemostasis (Figure 3). Current anticoagulants either reduce plasma levels of prothrombin and FX (VKAs), or inhibit their activated forms (heparins and DOACs). Because thrombin and FXa are as important in the host response to injury as they are in thrombosis, their inhibition must compromise hemostasis to some extent if they are to be effective antithrombotic agents. However, this puts a limitation on the intensity of therapy that can be administered, and means that some patients with high bleeding risk will not be eligible for therapy. FXI, by contrast, serves an ancillary role in hemostasis, and its relative contributions to thrombosis appear to be disproportionately large. Because patients with severe FXI deficiency rarely have spontaneous bleeding and are not at increased risk for bleeding into the central nervous system or gastrointestinal tract, therapies targeting FXI or FXIa should be associated with a relatively low risk for serious bleeding.
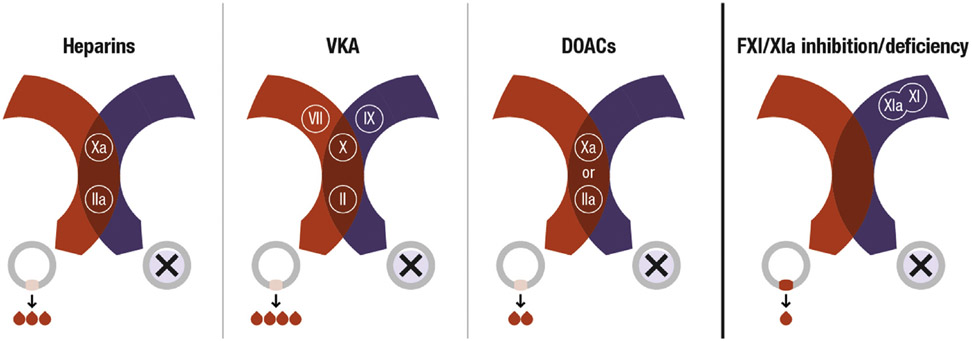
FIGURE 3. Impact of Various Anticoagulants on Hemostasis Versus Thrombosis.
Current anticoagulants inhibit either FXa or thrombin (IIa) (DOACs), or both (heparins), or reduce synthesis of their precursors (VKAs). This will attenuate thrombosis but also compromise hemostasis. The greater role of FXI in thrombosis relative to hemostasis provides a potential target for hemostasis-sparing anticoagulation. Cross-sectional view of vessels shown with hemostasis in red and thrombosis in purple. DOACs = direct oral anticoagulants; VKA = vitamin K antagonist.
EFFECT OF FXI OR FXIa INHIBITORS ON COAGULATION TESTS
Despite the observation of a minimal bleeding propensity in patients with congenital FXI deficiency, laboratory tests may, on the surface, appear to suggest otherwise. FXI is part of the traditional intrinsic pathway of coagulation that, along with FVIII, FIX, and FXII, initiates thrombin generation and clot formation in the activated partial thromboplastin time (aPTT) assay. Consequently, deficiency or inhibition of FXI/FXIa prolongs the aPTT, but does not affect the prothrombin time assay, which is used to assess coagulation initiated by the FVIIa/TF complex via the extrinsic pathway. Indeed, if FXI-directed therapy inhibits most of the protein’s plasma activity, the aPTT may be prolonged to a greater extent than that observed in patients with severe hemophilia A or B. Although this may seem to indicate a significant defect in hemostasis, in practice, there is poor correlation between plasma FXI levels as determined by the aPTT and bleeding propensity. This apparent paradox reflects the fact that the aPTT requires FXI to initiate clot formation (the assay readout for the aPTT), a role which FXI does not perform in vivo. As shown in Figure 1, initiation of clotting in vivo is triggered by the FVIIa/TF complex, which is not measured by the aPTT assay. When required, FXI serves as a consolidator of coagulation in vivo, and not as an initiator. This consolidating activity is not captured by the aPTT assay. In fact, no readily available coagulation assay accurately reflects the role of FXI in hemostasis. Therefore, when studying FXI or FXIa inhibitors, it is important to understand the limitations of the aPTT as an indicator of bleeding propensity even though it may be a good biomarker for presence of the drug.
CONCLUSIONS AND FUTURE DIRECTIONS
Current anticoagulation strategies are based on the premise that the enzymatic processes involved in hemostasis and thrombosis are similar. Following this approach, prevention of thrombosis requires inhibition of the hemostatic system, which increases the risk for bleeding. A substantial body of work over the past 20 years indicates that this notion, while intuitive, is an oversimplification. Plasma components such as FXI that have relatively small impacts on hemostasis make important contributions to thrombosis in humans and are, therefore, attractive targets for hemostasis-sparing therapies. Inhibition of FXI or FXIa has been effective at preventing VTE in phase 2 studies of patients undergoing total knee arthroplasty. Ongoing trials are assessing these agents for prevention of major adverse vascular events in patients with end-stage kidney disease undergoing hemodialysis or as adjuncts to antiplatelet therapy for prevention of recurrent ischemic events in patients with acute myocardial infarction or noncardioembolic stroke. Other studies are comparing FXI or FXIa inhibitors head to head with DOACs for stroke prevention in patients with atrial fibrillation. The results of these trials will not only establish the efficacy of FXI or FXIa inhibition, but will determine whether such an approach truly attenuates thrombosis with little or no disruption of hemostasis.
HIGHLIGHTS.
Although currently available anticoagulants are effective, bleeding is the most frequent side effect.
Factor XI is important for thrombosis but less so for hemostasis.
Factor XI inhibitors may serve as hemostasis-sparing anticoagulants with better safety profiles than currently available anticoagulants.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The development of this paper was funded by Anthos Therapeutics. Drs Gailani and Weitz had complete editorial control of the manuscript with Anthos providing project management support. Dr Hsu was a contractor to Anthos Therapeutics during the development of this paper. Dr Hutt has received consulting fees from Anthos Therapeutics. Dr Bloomfield is an employee of Anthos Therapeutics. Drs Gailani and Weitz have received consulting fees and honoraria from Anthos Therapeutics, Bayer, Bristol Myers Squibb, Ionis, and Janssen. Dr. Weitz holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J. F. Mustard Chair in Cardiovascular Research at McMaster University.
ABBREVIATIONS AND ACRONYMS
- aPTT
activated partial thromboplastin time
- DOAC
direct oral anticoagulant
- INR
international normalized ratio
- TF
tissue factor
- VKA
vitamin K antagonist
- VTE
venous thromboembolism
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapata-Wainberg G, Ximenez-Carrillo Rico A, Benavente Fernandez L, et al. Epidemiology of intracranial haemorrhages associated with vitamin K antagonist oral anticoagulants in Spain: TAC registry. Interv Neurol. 2015;4:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson D, Charidimou A, Shakeshaft C, et al. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology. 2016;86:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–262. [DOI] [PubMed] [Google Scholar]
- 5.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 6.Carmo J, Moscoso Costa F, Ferreira J, Mendes M. Dabigatran in real-world atrial fibrillation. Meta-analysis of observational comparison studies with vitamin K antagonists. Thromb Haemost. 2016;116:754–763. [DOI] [PubMed] [Google Scholar]
- 7.Huisman MV, Rothman KJ, Paquette M, et al. Two-year follow-up of patients treated with dabigatran for stroke prevention in atrial fibrillation: Global Registry on Long-Term Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) registry. Am Heart J. 2018;198:55–63. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez CAA, Lanas F, Radaideh G, et al. XANTUS-EL: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation in Eastern Europe, Middle East, Africa and Latin America. Egypt Heart J. 2018;70:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XS, Deitelzweig S, Keshishian A, et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. A propensity-matched analysis of 76,940 patients. Thromb Haemost. 2017;117:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in Asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol. 2018;72:838–853. [DOI] [PubMed] [Google Scholar]
- 12.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42(5):373–498. 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 13.Han TS, Fry CH, Fluck D, et al. Anticoagulation therapy in patients with stroke and atrial fibrillation: a registry-based study of acute stroke care in Surrey, UK. BMJ Open. 2018;8, e022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968–1975. [DOI] [PubMed] [Google Scholar]
- 15.Carrier M, Prandoni P. Controversies in the management of cancer-associated thrombosis. Expert Rev Hematol. 2017;10:15–22. [DOI] [PubMed] [Google Scholar]
- 16.Mulder FI, Bosch FTM, Young AM, et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood. 2020;136:1433–1441. [DOI] [PubMed] [Google Scholar]
- 17.Plow EF, Wang Y, Simon DI. The search for new antithrombotic mechanisms and therapies that may spare hemostasis. Blood. 2018;131:1899–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brummel-Ziedins KE, Branda RF, Butenas S, Mann KG. Discordant fibrin formation in hemophilia. J Thromb Haemost. 2009;7:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton-Maggs PH. Factor XI deficiency and its management. Haemophilia. 2000;6(Suppl 1):100–109. [DOI] [PubMed] [Google Scholar]
- 20.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. [DOI] [PubMed] [Google Scholar]
- 21.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Cheng Q, Xu L, et al. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. [DOI] [PubMed] [Google Scholar]
- 23.Tucker EI, Marzec UM, White TC, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosby JR, Marzec U, Revenko AS, et al. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013;33:1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau JW, Liao P, Fredenburgh JC, et al. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–2107. [DOI] [PubMed] [Google Scholar]
- 26.Buller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]