Abstract
Molecular dynamics simulations are performed to characterize the nucleation behavior of organic compounds in the gas phase. Six basic molecular species are considered—ethylene, propylene, toluene, styrene, ethylbenzene, and para-xylene—in interaction with onion-like carbon nanostructures that model soot nanoparticles (NPs) at room temperature. We identify a shell-to-island aggregation process during the physisorption of aromatic molecules on the soot surface: The molecules tend to first cover the NP in a shell, on top of which additional adsorbates form island-shaped aggregates. We present results for the binding energy, suggesting that the NPs lead to the formation of more stable molecular aggregates in comparison with the pure gas phase. Our findings describe a plausible microscopic mechanism for the active role of soot in the formation and growth of organic particulate matter.
Introduction
Soot-based particulate matter (soot-PM) takes part in many environmental processes that impact climate and health. Its formation has been the focus of many experimental studies.1 Transmission electron microscopy observations revealed that soot-PM are frequently in the form of carbon nanoparticles (NPs) enveloped by organic coverings.2,3 Organic compounds from diverse environmental sources were reported to act as precursors in the condensation of soot-PM.4−6 New particle formation occurs in two distinct stages, nucleation to form a critical nucleus and subsequent growth of the critical nucleus to a larger size.7,8 However, the kinetic physicochemical processes at the root of the formation of soot-PM are unclear, particularly the molecular nucleation involving the physisorption of organic compounds on soot NPs.
Low-dimensional carbons have drawn considerable attention for molecular adsorption, thanks to their chemical inertness and peculiar structures. On the basis of density functional theory calculations, Lazar et al. reported that the static adsorption enthalpy of acetone, toluene, or hexane on graphene flakes ranges from 7.0 to 15.0 kcal/mol depending on the molecular species and the degree of the surface coverage.9 It was suggested that stable molecular aggregates of hydrocarbons could form on graphitic surfaces (such as the soot surface) via physisorption. On the basis of MD simulations, Veclani and Melchior studied the adsorption of neutral and zwitterionic forms of ciprofloxacin on single-walled carbon nanotubes in the gas phase and water. They found that the adsorbed molecules oriented parallel to the surface interacting via π–π interactions.10 Yang et al. systematically studied the adsorption of polycyclic aromatic hydrocarbons (PAHs) on various carbon nanomaterials. For different PAHs, adsorption seemed to relate with their molecular size, that is, the larger the molecular size, the lower the adsorbed volume capacity.11 In contrast, little is known about the kinetic adsorption process on soot. For example, about how the shape and the energetics of the molecular clusters change with different types of adsorbates during the growth of soot-PM.
There has been a great amount of experimental research on the adsorption of aliphatic and aromatic molecules on different adsorbents. For the alkenes, the adsorption capacity of C3H6 was higher than that of C2H4 for most of the adsorbents.12 The adsorption of BTEX on activated carbon was observed to proceed in the order xylene > ethylbenzene > toluene > benzene, as related to decreased solubility and increased molecular weight.13 It is also reported that carbon nanotubes have higher adsorption affinities for the organic compounds with a planar molecular configuration.14,15 The nucleation of organic molecules was often studied with MD simulations.16−18 We here used MD to simulate the physisorption of simple hydrocarbon molecules on the surface of carbon NPs in order to gain physicochemical insights into the formation of soot-PM. We selected six common carbohydrates due to their simple but diverse structures. The set comprises two aliphatic compounds, namely ethylene and propylene, and four aromatic ones, namely toluene, styrene, ethylbenzene, and para-xylene, as shown in the left panel of Figure 1. The nucleation of these molecules in their pure gas phase was also simulated to establish a baseline for comparison.
Figure 1.
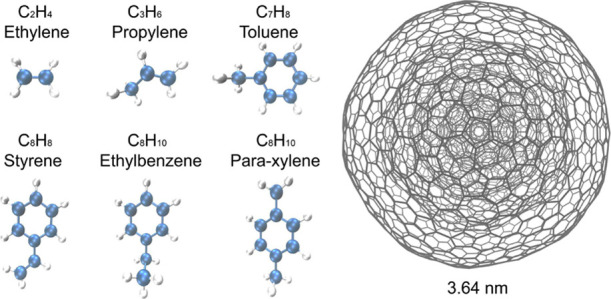
Structures and chemical formulas of the six organic compounds studied in this work. In the ball-and-stick model, carbon atoms are depicted in blue and hydrogen atoms in gray. The NP (of diameter 3.64 nm) used to model soot is also shown on the right-hand side of the figure.
Computational Methods
The geometry of our model soot NPs is based on experimental reports of onion-like carbon nanostructures characterized by means of spectrometry and electron microscopy.19−21 To mimic that kind of morphology, we built a bucky onion that contains a number of concentric fullerene layers, as shown in the right panel of Figure 1. We had to use relatively small soot NPs due to the limitation of our computational resources. We have therefore studied two systems of different sizes to see the size effect. Four and six concentric fullerene layers have been used to construct the soot particles of 3.64 and 4.58 nm, respectively. The quasi-spherical fullerenes are constructed by introducing Stone–Wales defects on the edges or medians of each triangle containing a pentagon at its vertices of the icosahedral or triacontahedral fullerenes using the method introduced in ref (22). We note that our model is a simplification because more complex morphologies of the soot monomer were reported. For example, a soot monomer was observed to exhibit an amorphous carbon core surrounded by a shell of crystalline graphite plates.23 The choice of the six organic compounds is based on their abundance that dominates the atmospheric pollution in modern cities.24,25 We have selected only hydrocarbons in this work for simplifying the computations.
We used the parallel MD package LAMMPS26 for our simulations. The interaction potential energies and forces were computed using the adaptive interatomic reactive empirical bond order (AIREBO) force field. The total energy is a collection of the contributions from individual bonds, which are functions of many-body interactions. The van der Waals interactions between the NP and the molecules, between the NP layers, and between the molecules are included in AIREBO using a parametrized long-range Lennard-Jones function with a cutoff radius of 1.0 nm. Details about the formulation, the parameters, and the benchmark calculation of this force field are provided elsewhere.27 We chose AIREBO because of its inclusion of bond rotation and torsion terms that are important for taking into account the deformation of the substrate caused by adsorbates and thermal fluctuation,28 which has been reported to influence gas adsorption.29 AIREBO is a widely used force field for hydrocarbon systems because it allows the breaking or the formation of chemical bonds that could occur between the molecules and the substrate. However, we did not observe any expected chemisorption in our simulations, probably because the studied temperature is relatively low.
At the beginning of the simulation, a specified number of organic molecules are placed at random sites near the surface of the NP in a periodic simulation cell of 10 × 10 × 10 nm3. In atmospheric conditions, the nucleation time is orders of magnitude longer than that can be treated by MD. It is a common MD approach to increase the molecular density for accelerating the process. Because of this simplification, we simulated different numbers of molecules and tried to understand the effect of molecular density on the adsorption behavior (Figure 2). Initial velocities are sampled from the Maxwell–Boltzmann distribution, and then, a Langevin thermostat is used with a friction coefficient in the Langevin equation corresponding to a coupling time of 0.05 ns for a period of 2.5 ns to let the NP progressively reach thermal equilibrium at 300 K. The simulation timestep is 0.0005 ps. The damping constant is 100 ps. The simulation duration is determined by a set of convergence tests, as shown in the Supporting Information. The gas phase (without NP) is also simulated under the same conditions for comparison, except that the thermostat is applied directly to the organic molecules instead of to the NP. We used different thermostats for the gas phase than in the case of NPs in order to reduce the simulation artifact. In the case of NPs, the molecules are set to be free, and their temperature is controlled by energy exchange with NPs. The NPT ensemble has to be applied for the pure gas phase. The simulations are still run for 0.5 ns after reaching thermal equilibrium for computing the binding energy. The total time of simulations is 3 ns for each system. The binding energy is computed at an interval of 0.5 ps where each coordination frame is saved and is averaged over 1000 points for a period of 0.5 ns after reaching thermal equilibrium. This work considers densities higher than those in real conditions because of the limitation of the simulation time. However, because the relaxation time is long enough to ensure that the system equilibrium is reached, the simulation can still provide microscopic insights into the adsorption process.
Figure 2.
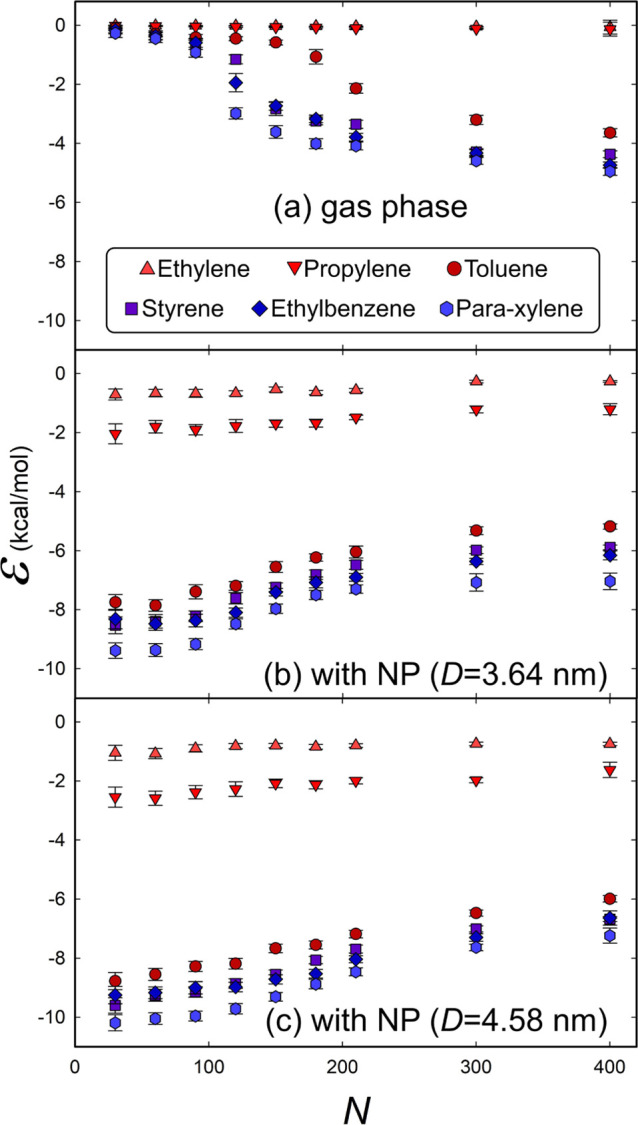
Binding energy per molecule vs the number of molecules for different molecular species in the gas phase without any NP (a) or on the surface of an NP with a diameter of 3.64 (b) or 4.58 nm (c). The data are averaged over the last 1000 frames of simulation for 0.5 ns. The error bars show the standard deviation.
Results and Discussion
We computed the binding energy per molecule ε, a key variable associated with the nucleation of gaseous compounds that indicates how easy or difficult it is for the molecules to form aggregates, as
| 1 |
where εtotal is the total interaction energy of the system, εa is the internal energy of the individual molecule, and εnp is the internal energy of the NP (or simply zero if no NP is present, i.e., in the gas phase). As shown in Figure 2, we plot ε versus the number of molecules N in the gas phase (a) and in the presence of NPs (b and c). ε is of the same order of magnitude as those reported in a previous study of organic molecules adsorbed on graphene.9 By comparing the vertical axis of the panels of Figure 2, it was observed that ε is, in general, lower for the two cases where NPs are present, suggesting that the clusters formed on the NP surface are more stable than the ones formed in the gas phase. These results could be correlated with the formation of the previously reported morphology of soot PM with organic coverings4−6 and indicate an active role of soot as an effective substrate for facilitating the initial growth of organic PM, a hypothesis also supported by the experimentally measured positive correlations between the concentrations of organic aerosols and soot (also called black carbon).30−35
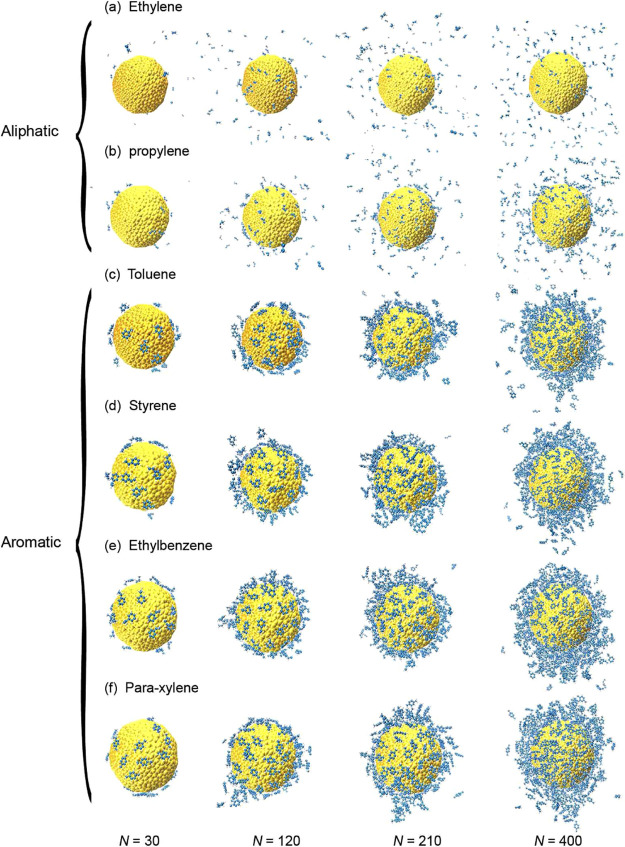
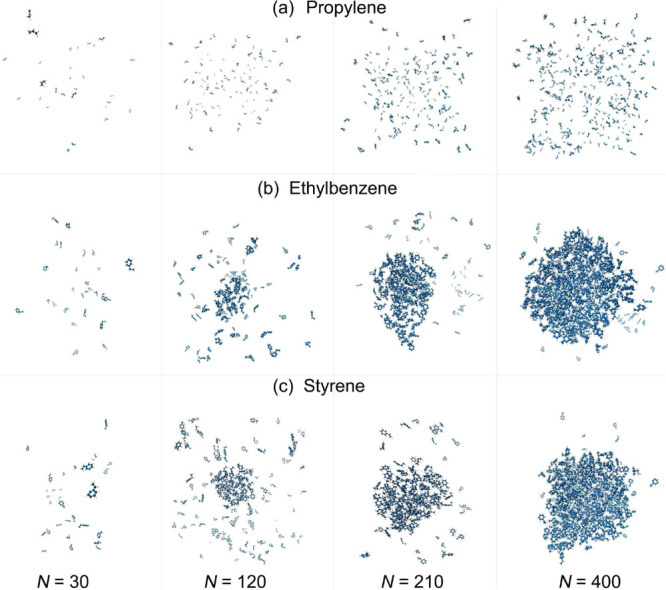
It is well known that the nucleation of aromatic compounds is different from that of aliphatic compounds;36 as expected, our calculated binding energy for the two aliphatic compounds (ethylene and propylene) exhibits clearly different magnitude and trend than those of the aromatic compounds (Figure 2). This contrast is caused not only by the different numbers of atoms in the molecules but also by a significant difference in the surface coverage. By examining the morphology of the formed clusters, we find that most of the aromatic molecules aggregate on the NPs more readily than the aliphatic ones, as shown in Figure 3, taking the cases of toluene and ethylene for comparison. This may stem from the characteristic stacking pattern of sp2-hybridized carbon,37,38 through which the planar molecular structure helps to form thermally stable aggregates not only on the NP surface but also in the gas phase. This hypothesis is consistent with the observation that the bigger the NP (hence the flatter its surface), the stronger the binding energy, as shown in Figure 2. It also agrees with previous experimental results that have shown that cyclic compounds contribute much more to PM formation than linear and branched compounds.39−41
Figure 3.
Atomistic configurations of different numbers of (a) ethylene, (b) propylene, (c) toluene, (d) styrene, (e) ethylbenzene, and (f) para-xylene molecules on an NP.
Our simulations reveal that, with an increasing number of adsorbates, the aromatic molecules first form a thin layer of molecules on the NP until its surface is saturated (e.g., N = 120, middle panel of Figure 3c). Island-shaped 3D aggregates are then observed for N = 400, as shown in the right panel of Figure 3c. In contrast, the ethylene molecules are found to be hard to adsorb on the NP as many molecules are isolated in the gas phase. A comprehensive characterization of this behavior is shown in the Supporting Information, which contains optimized molecular configurations in which the layer-to-island nucleation behavior can clearly be observed, at room temperature, for all the studied aromatic compounds.
To quantitatively characterize the layer-to-island stacking mechanism, as shown in Figure 4, we plot the distribution of the number of molecules at different distances d from the center of mass of the NP, taking toluene and para-xylene as examples, as well as the stacking angle θ, defined as the angle between the normals to the molecular plane and the NP surface. It can be observed that the stacking configurations of the molecules are strongly correlated with θ. For example, the first peak in the molecular distribution shown in the green zone of Figure 4a corresponds to the first layer of adsorbed molecules, which roughly orient parallel to the NP surface as exhibited by the relatively small value of the corresponding angle θ (Figure 4b). The second peak in the distribution curve might point to a transitional layer, as shown in the yellow region of Figure 4. However, a less ordered arrangement of the molecules was observed in the second transitional layer because the standard deviation of θ is larger than that obtained for the first layer. Above these two layers, the molecules stack randomly to form 3D islands, as evidenced by the relatively large values of θ and their standard deviations in the white area, as shown in Figure 4b. We also observe the desorption of molecules from the outermost layer. These molecules likely orient perpendicular rather than parallel to the surface before and after desorption and exhibit an average stacking angle larger than 45°.
Figure 4.
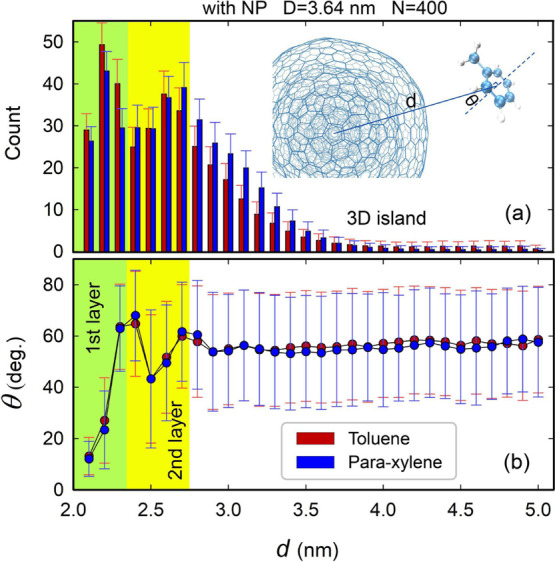
(a) Distribution of the numbers of adsorbed molecules at different distances d from the center of mass of the NP (D = 3.64 nm) for the cases of 400 toluene and para-xylene molecules. (b) Stacking angle θ of the 400 molecules as a function of d. The insets show how d and θ are defined. The data are averaged over the last 1000 frames of simulation for 500 ps. The error bars represent the standard deviations. Means and standard deviations are estimated over 1000 (a) and 1000 × count (b) samples, respectively.
Figure 5b,c shows that the molecules start to form aggregates approximately at a critical density of N = 120. This is consistent with the fact that the binding energy of the aromatic compounds (toluene, styrene, ethylbenzene, and para-xylene) evolves toward a different slope at this density, as shown in Figure 2a for the case of the gas phase where the energy is simply a function of the molecular density. It can also be observed that the energy curves change the slope again at N = 210, where only a very small portion of molecules remains disaggregated in the pure gas phase.
Figure 5.
Atomistic configurations of different numbers of (a) propylene, (b) ethylbenzene, and (c) styrene molecules in the gas phase.
Figure 6a shows the distribution of the number of molecules at different distances d from the center of mass of the aggregate formed by about 400 aromatic molecules in the gas phase. It is shown that the aggregations of styrene and toluene exhibit more homogeneous spatial distributions compared with para-xylene and ethylbenzene molecules. This difference in the nucleation behavior of the aromatic compounds is consistent with the calculations of binding energies, as shown in Figure 2a, which may stem from the various structures of their functional groups. The different spatial arrangements could be related to the different structures of the functional groups of the aromatic molecules. More specifically, toluene and styrene molecules exhibit smaller functional groups, and it is, therefore, easier for them to find a homogeneous spatial distribution than para-xylene and ethylbenzene molecules. Based on the corresponding distribution of the stacking angle (>45°) shown in Figure 6b, the benzene plane tends to have a shift from being perfectly perpendicular to the radial direction, which connects the mass center of the aggregate and that of the molecule. This behavior is particularly apparent for the outermost layer of the molecular aggregates, as shown in the insets of Figure 6b.
Figure 6.
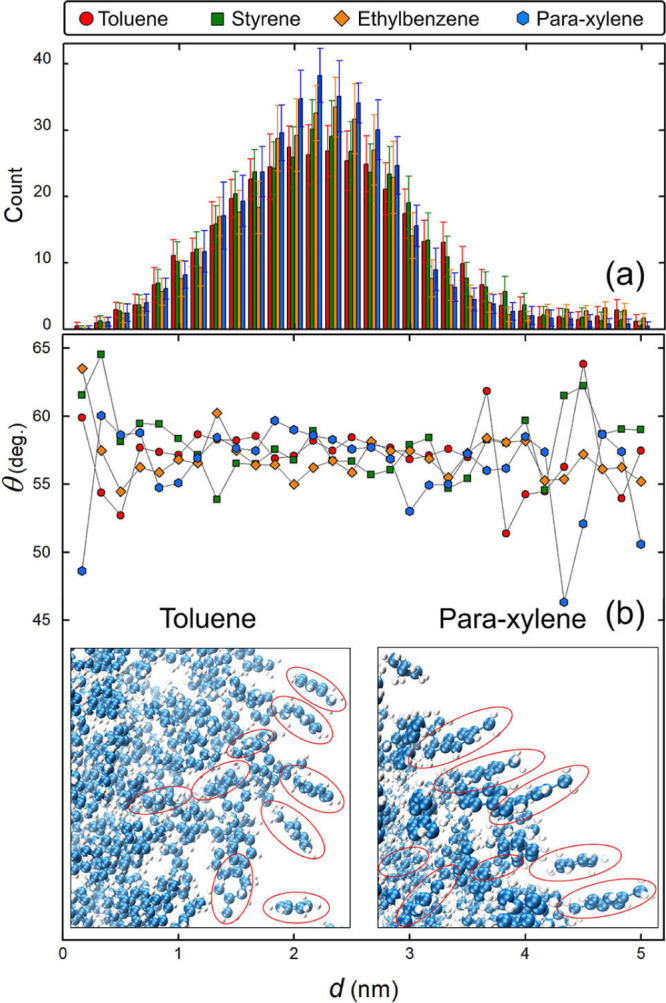
(a) Distribution of the numbers of molecules at different distances d from the center of mass of the aggregate of about 400 molecules formed in the gas phase. (b) Stacking angle θ of the 400 molecules as a function of d. θ is defined here in a similar fashion to what is shown in the inset of Figure 4, but the vector from the center of mass of the aggregate to that of the molecule is used instead of the surface normal. The insets show the positions of the molecules at the outermost layer of the aggregates. The data are averaged over the last 50 frames of simulation for 25 ps, considering that the translation and rotation of the clusters in the gas phase are more active than the case with the NP. The error bars represent the standard deviations. The data are estimated over 50 (a) and 50 × count (b) samples, respectively.
Conclusions
Using MD, we have simulated the physisorption of ethylene, propylene, toluene, styrene, ethylbenzene, and para-xylene molecules on soot NPs at room temperature. The calculation of the binding energy indicates that the soot NP could help to form thermally stable molecular clusters with respect to the case in the pure gas phase. This points to a possible active role of the soot in the formation of organic PM, in general agreement with previous experimental observations. As expected, a clear difference in the nucleation behaviors was observed between the studied aromatic and aliphatic compounds (the aromatic molecules aggregated more readily than the aliphatics did). Our simulations reveal a layer-to-island nucleation mechanism: With an increasing number of the adsorbed molecules, the adsorbates first formed a thin shell on the substrate until the surface was saturated. A second transitional layer then appears before the formation of aggregates in an island shape. These findings suggest a microscopic mechanism for the formation of soot-PM by the nucleation of organic compounds on soot NPs.
Acknowledgments
P. Blaha and K. Schwarz are acknowledged for helpful discussions. Support from the National Natural Science Foundation of China (11964002) is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03571.
Potential energy of the system versus simulation time; time-evolution of the distribution of the adsorbed molecules; snapshots of the simulated system; detailed explanation of the data files containing molecular configurations; and set of example LAMMPS input files for simulation (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- Zhang R.; Wang G.; Guo S.; Zamora M. L.; Ying Q.; Lin Y.; Wang W.; Hu M.; Wang Y. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- Baldelli A.; Trivanovic U.; Sipkens T. A.; Rogak S. N. On determining soot maturity: A review of the role of microscopy-and spectroscopy-based techniques. Chemosphere 2020, 252, 126532. 10.1016/j.chemosphere.2020.126532. [DOI] [PubMed] [Google Scholar]
- Nienow A. M.; Roberts J. T. Heterogeneous chemistry of carbon aerosols. Annu. Rev. Phys. Chem. 2006, 57, 105–128. 10.1146/annurev.physchem.57.032905.104525. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Khalizov A. F.; Pagels J.; Zhang D.; Xue H.; McMurry P. H. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 10291–10296. 10.1073/pnas.0804860105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin A.; Moffet R. C.; Gilles M. K. Chemical imaging of atmospheric particles. Acc. Chem. Res. 2019, 52, 3419–3431. 10.1021/acs.accounts.9b00396. [DOI] [PubMed] [Google Scholar]
- Adachi K.; Chung S. H.; Buseck P. R. Shapes of soot aerosol particles and implications for their effects on climate. J. Geophys. Res.: Space Phys. 2010, 115, D15206. 10.1029/2009jd012868. [DOI] [Google Scholar]
- Zhang R.; Khalizov A.; Wang L.; Hu M.; Xu W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2012, 112, 1957–2011. 10.1021/cr2001756. [DOI] [PubMed] [Google Scholar]
- Thanh N. T. K.; Maclean N.; Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. 10.1021/cr400544s. [DOI] [PubMed] [Google Scholar]
- Lazar P.; Karlický F.; Jurečka P.; Kocman M.; Otyepková E.; Šafářová K.; Otyepka M. Adsorption of small organic molecules on graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. 10.1021/ja403162r. [DOI] [PubMed] [Google Scholar]
- Veclani D.; Melchior A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. 10.1016/j.molliq.2019.111977. [DOI] [Google Scholar]
- Yang K.; Zhu L.; Xing B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. 10.1021/es052208w. [DOI] [PubMed] [Google Scholar]
- Su W.; Zhang A.; Sun Y.; Ran M.; Wang X. Adsorption properties of C2H4 and C3H6 on 11 adsorbents. J. Chem. Eng. Data 2017, 62, 417–421. 10.1021/acs.jced.6b00749. [DOI] [Google Scholar]
- Daifullah A. A. M.; Girgis B. S. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf., A 2003, 214, 181–193. 10.1016/s0927-7757(02)00392-8. [DOI] [Google Scholar]
- Liu C.-H.; Li J.-J.; Zhang H.-L.; Li B.-R.; Guo Y. Structure dependent interaction between organic dyes and carbon nanotubes. Colloids Surf., A 2008, 313–314, 9–12. 10.1016/j.colsurfa.2007.04.062. [DOI] [Google Scholar]
- Kragulj M.; Tričković J.; Dalmacija B.; Kukovecz Á.; Kónya Z.; Molnar J.; Rončević S. Molecular interactions between organic compounds and functionally modified multiwalled carbon nanotubes. Chem. Eng. J. 2013, 225, 144–152. 10.1016/j.cej.2013.03.086. [DOI] [Google Scholar]
- Li J.-R.; Kuppler R. J.; Zhou H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Wu H.; Gong Q.; Olson D. H.; Li J. Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks. Chem. Rev. 2012, 112, 836–868. 10.1021/cr200216x. [DOI] [PubMed] [Google Scholar]
- Düren T.; Bae Y.-S.; Snurr R. Q. Using molecular simulation to characterise metal–organic frameworks for adsorption applications. Chem. Soc. Rev. 2009, 38, 1237–1247. 10.1039/b803498m. [DOI] [PubMed] [Google Scholar]
- Li J.; Posfai M.; Hobbs P. V.; Buseck P. R. Individual aerosol particles from biomass burning in southern Africa: 2. Compositions and aging of inorganic particles. J. Geophys. Res.: Atmos. 2003, 108, 8484. 10.1029/2002jd002310. [DOI] [Google Scholar]
- Wentzel M.; Gorzawski H.; Naumann K.-H.; Saathoff H.; Weinbruch S. Transmission electron microscopical and aerosol dynamical characterization of soot aerosols. J. Aerosol Sci. 2003, 34, 1347–1370. 10.1016/s0021-8502(03)00360-4. [DOI] [Google Scholar]
- Müller J.-O.; Su D. S.; Wild U.; Schlögl R. Bulk and surface structural investigations of diesel engine soot and carbon black. Phys. Chem. Chem. Phys. 2007, 9, 4018–4025. 10.1039/b704850e. [DOI] [PubMed] [Google Scholar]
- Langlet R.; Mayer A.; Geuquet N.; Amara H.; Vandescuren M.; Henrard L.; Maksimenko S.; Lambin P. Study of the polarizability of fullerenes with a monopole-dipole interaction model. Diamond Relat. Mater. 2007, 16, 2145–2149. 10.1016/j.diamond.2007.10.019. [DOI] [Google Scholar]
- García Fernández C.; Picaud S.; Devel M. Calculations of the mass absorption cross sections for carbonaceous nanoparticles modeling soot. J. Quant. Spectrosc. Radiat. Transfer 2015, 164, 69–81. 10.1016/j.jqsrt.2015.05.011. [DOI] [Google Scholar]
- Hsieh C.-C.; Tsai J.-H. VOC concentration characteristics in Southern Taiwan. Chemosphere 2003, 50, 545–556. 10.1016/s0045-6535(02)00275-8. [DOI] [PubMed] [Google Scholar]
- Cao H.; Fu T.-M.; Zhang L.; Henze D. K.; Miller C. C.; Lerot C.; Abad G. G.; De Smedt I.; Zhang Q.; van Roozendael M.; et al. Adjoint inversion of Chinese non-methane volatile organic compound emissions using space-based observations of formaldehyde and glyoxal. Atmos. Chem. Phys. 2018, 18, 15017–15046. 10.5194/acp-18-15017-2018. [DOI] [Google Scholar]
- Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 1995, 117, 1–19. 10.1006/jcph.1995.1039. [DOI] [Google Scholar]
- Stuart S. J.; Tutein A. B.; Harrison J. A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. 10.1063/1.481208. [DOI] [Google Scholar]
- Wang Z.; Devel M. Periodic ripples in suspended graphene. Phys. Rev. B 2011, 83, 125422. 10.1103/physrevb.83.125422. [DOI] [Google Scholar]
- Vekeman J.; Sánchez-Marín J.; Sánchez de Merás A.; Garcia Cuesta I.; Faginas-Lago N. Flexibility in the graphene sheet: the influence on gas adsorption from molecular dynamics studies. J. Phys. Chem. C 2019, 123, 28035–28047. 10.1021/acs.jpcc.9b07503. [DOI] [Google Scholar]
- Ramanathan V.; Carmichael G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008, 1, 221–227. 10.1038/ngeo156. [DOI] [Google Scholar]
- Trompetter W. J.; Grange S. K.; Davy P. K.; Ancelet T. Vertical and temporal variations of black carbon in New Zealand urban areas during winter. Atmos. Environ. 2013, 75, 179–187. 10.1016/j.atmosenv.2013.04.036. [DOI] [Google Scholar]
- Shen L.; Li L.; Lü S.; Zhang X.; Liu J.; An J.; Zhang G.; Wu B.; Wang F. Characteristics of black carbon aerosol in Jiaxing, China during autumn 2013. Particuology 2015, 20, 10–15. 10.1016/j.partic.2014.08.002. [DOI] [Google Scholar]
- Chen Y.; Schleicher N.; Fricker M.; Cen K.; Liu X.-l.; Kaminski U.; Yu Y.; Wu X.-f.; Norra S. Long-term variation of black carbon and PM2.5 in Beijing, China with respect to meteorological conditions and governmental measures. Environ. Pollut. 2016, 212, 269–278. 10.1016/j.envpol.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Arif M.; Kumar R.; Kumar R.; Eric Z.; Gourav P. Ambient black carbon, PM2.5 and PM10 at Patna: Influence of anthropogenic emissions and brick kilns. Sci. Total Environ. 2018, 624, 1387–1400. 10.1016/j.scitotenv.2017.12.227. [DOI] [PubMed] [Google Scholar]
- Sarkar C.; Roy A.; Chatterjee A.; Ghosh S. K.; Raha S. Factors controlling the long-term (2009-2015) trend of PM2.5 and black carbon aerosols at eastern Himalaya, India. Sci. Total Environ. 2019, 656, 280–296. 10.1016/j.scitotenv.2018.11.367. [DOI] [PubMed] [Google Scholar]
- Wang Z. Selective conduction of organic molecules via free-standing graphene. J. Phys. Chem. C 2019, 123, 15166–15170. 10.1021/acs.jpcc.9b04131. [DOI] [Google Scholar]
- Björk J.; Hanke F.; Palma C.-A.; Samori P.; Cecchini M.; Persson M. Adsorption of aromatic and anti-aromatic systems on graphene through π-π stacking. J. Phys. Chem. Lett. 2010, 1, 3407–3412. 10.1021/jz101360k. [DOI] [Google Scholar]
- Roos M.; Künzel D.; Uhl B.; Huang H.-H.; Brandao Alves O.; Hoster H. E.; Gross A.; Behm R. J. Hierarchical interactions and their influence upon the adsorption of organic molecules on a graphene film. J. Am. Chem. Soc. 2011, 133, 9208–9211. 10.1021/ja2025855. [DOI] [PubMed] [Google Scholar]
- Jiang B.; Xia D.; Xie Y.; Liu X. Effect of the molecular structure of volatile organic compounds on atmospheric nucleation: A modeling study based on gas kinetic theory and graph theory. Atmos. Environ. 2019, 213, 215–222. 10.1016/j.atmosenv.2019.06.002. [DOI] [Google Scholar]
- Haritash A. K.; Kaushik C. P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Mu L.; Peng L.; Liu X.; He Q.; Bai H.; Yan Y.; Li Y. Emission characteristics and size distribution of polycyclic aromatic hydrocarbons from coke production in China. Atmos. Res. 2017, 197, 113–120. 10.1016/j.atmosres.2017.06.028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.