Abstract
While only four globally important rotavirus G serotypes (1 to 4) have been documented, many studies suggest that serotype G9 viruses may be widely distributed and more important than previously recognized. We have evaluated 10 serotype G9 rotavirus-neutralizing monoclonal antibodies (MAbs) directed to VP7, which bound by direct enzyme immunoassay (EIA) to P1A[8], G9 rotaviruses F45, WI61, and AU32, for their ability to recognize the New Delhi G9 rotavirus 116E. Only one MAb (MAb F45:1) bound to P[11], G9 virus 116E to a high titer by EIA. This MAb was incorporated into an indirect EIA for G serotyping, which was validated with prototype cultivable human rotaviruses of G types 1 to 4 and 9. The EIA was compared with genotyping by reverse transcriptase PCR (RT-PCR) under code for the determination of the G types of rotaviruses obtained from neonates in New Delhi, India. The sensitivities of RT-PCR and EIA (after two additional freeze-thaw cycles) for the typing of G9 rotaviruses were 91 and 86%, respectively, for 24 culture-adapted rotavirus strains. The untypeable culture-adapted rotavirus samples also were unreactive with VP7 group antigen-reactive MAb 60. After two additional freeze-thaw cycles, only 26 of 42 (62%) of stools containing rotavirus typed as G9 by RT-PCR were positive for G9 rotavirus by EIA. Stools containing rotavirus untypeable by EIA contained significantly less MAb 60-reactive VP7 antigen (P = 0.0001) than the stools containing typeable rotavirus. Thus, RT-PCR genotyping was the more sensitive method for determination of G9 type, but a serotype was readily determined in rotavirus samples containing MAb 60-reactive VP7 antigen by an EIA that incorporates MAb F45:1.
Group A rotaviruses are the major etiologic agents of severe acute diarrhea in infants and young children worldwide (33). Infectious virions comprise six structural proteins in three protein layers enclosing 11 segments of double-stranded RNA (dsRNA). Rotavirus serotype classification is based on differences in antigenic determinants that elicit neutralizing antibodies on the major component of the outer capsid, VP7 (G serotypes), and the spike protein, VP4 (P serotypes), whose proteolytic cleavage activates rotavirus infectivity. VP7 is a glycoprotein encoded by gene segment 7, 8, or 9, whereas VP4 is encoded by gene segment 4, so that VP7 (G) and VP4 (P) serotypes can segregate independently (30). Nucleotide sequence analysis of rotavirus variants selected for resistance to neutralization by VP7-specific monoclonal antibodies (MAbs) has allowed the definition of six antigenic regions, regions A to F, on VP7 (8, 16, 17, 34, 35). Apart from region D (amino acid [aa] 291), all these regions correspond to areas of the VP7 protein that are divergent between serotypes (23, 28). All regions may participate in conformation-dependent neutralization.
Rotavirus serotypes were originally defined by using cross-neutralization assays with hyperimmune serum, and it was shown subsequently that serotypes so defined relate primarily to VP7 and correspond to G serotypes (6). P serotypes were defined in neutralization assays by using hyperimmune antisera raised to baculovirus-expressed VP4 (24) or to reassortant rotaviruses (29). At least 10 G serotypes (serotypes G1 to G6, G8 to G10, and G12) and 7 P serotypes (serotypes P1A, P1B, P2A, P3 to P5, and P8) of human rotaviruses have been found to date. Both G and P serotypes can now be identified by enzyme immunoassay (EIA) that incorporates VP7- and VP4-reactive, serotype-specific MAbs (4, 6, 11, 42, 45, 47). However, P serotypes show cross-reactivity more frequently than G serotypes, making P serotyping by EIA difficult. Alternative P-typing methods have been developed on the basis of the degree of amino acid sequence variation in VP4 of rotavirus strains of different P serotypes. These include hybridization (38), restriction fragment length polymorphism assay (31), and reverse transcriptase PCR (RT-PCR) with seminested primers (21). These techniques are also applicable to G-genotype determination (12, 19, 25, 26). Among human rotaviruses, eight genomic P types (genotypes) which correspond to some of the described P serotypes have been defined. As the correlation between VP4 (P) serotypes and genotypes is not completely established, both are used to describe rotaviruses. P genotypes are included within brackets, whereas P serotypes are open numbers, with letters used to designate current subtypes. For example, the prototype human rotavirus strain RV-4 is designated P1A[8], G1 (18). In this paper, the G types of rotaviruses for which only the G genotype has been determined also will be indicated with brackets.
Numerous epidemiological studies have shown that G1 rotaviruses predominate worldwide as a cause severe rotavirus gastroenteritis, with G2, G3, and G4 strains being responsible for the majority of the residual disease (22). Most P-genotyping studies have shown that the rotaviruses of G1, G3, and G4 are P[8] and that the G2 strains are associated with P[4]. When the P serotypes of these G1 to G4 rotaviruses have been determined, they generally correspond to the genotype determined or to the P type predicted (4, 6, 42), so that, in descending order, the predominant rotaviruses that cause disease are P1A[8] G1, P1A[8] G4, P1B[4] G2, and P1A[8] G3 (22).
Although rotaviruses of the G9 serotype have been found less often than serotypes G1 to G4, they have been important causes of diarrhea in India (43), Bangladesh (50), and the United States (44). P1A[8], G9 rotavirus WI61 was isolated in Philadelphia, Pa., in 1983 and 1984, and viruses of this RNA electropherotype caused 9% of rotavirus disease at that time (3). In Japan in 1985 and 1986, 12% of cases of rotavirus disease in Yamagata (39) and 52% of cases of rotavirus disease in Osaka (32) were attributed to G9 rotaviruses, of which F45 (P1A[8], G9) (27) and AU32 (P1A[8], G9) (40), respectively, are representative. In India in 1993, P[6], G[9] rotaviruses were the most commonly detected type in children with diarrhea (43). P[11], G[9] rotaviruses, represented by culture-adapted strain 116E (20), and P[6], G[9] rotaviruses were the predominant strains isolated from neonates in New Delhi, India, between 1986 and 1993 (12). The G serotype of 116E was determined to be 9 by cross-neutralization with hyperimmune antisera, but the G serotypes of the stool viruses were not determined.
The results of these studies raise the question of whether G9 rotaviruses have been underdiagnosed and suggest that inclusion of G9-specific MAbs in G-serotyping EIA protocols is warranted. MAbs directed to VP7 of G9 rotaviruses have been derived (34, 37). One panel of 10 MAbs all bound by EIA and neutralized G9 rotavirus strains F45, WI61, and AU32, and the MAbs were mapped to antigenic regions A, B, C, and F (17, 34, 35). However, an evaluation of existing MAbs for their utility in EIA for stool rotaviruses has not been performed (34, 37). We therefore tested the panel of 10 G9 MAbs for their ability to react with the G9 rotavirus strain 116E by EIA and neutralization assay and then further evaluated 3 MAbs for EIA detection of G[9] rotaviruses in culture and in stools using the New Delhi P[11], G[9] and P[6], G[9] rotaviruses.
MATERIALS AND METHODS
Viruses.
The following prototype cultivable rotaviruses whose origins have been described previously (4, 8, 10) were used for the evaluation of the G9-reactive MAbs for serotyping: human viruses RV-4 (P1A[8], G1), RV-5 (P1B[4], G2), ST-3 (P2A[6], G4), Hosokawa (P1A[8], G4), and F45, WI61, and AU32 (P1A[8], G9); simian virus SA11 (P[2], G3); and porcine virus TFR41 (P2B[7], G4). The P[11], G9 rotavirus 116E was isolated from a New Delhi neonate infected asymptomatically (13). Rotaviruses were propagated in MA 104 cells in the presence of 1 μg of porcine trypsin (Sigma) per ml following activation with 10 μg of porcine trypsin per ml as described previously (10). Rotaviruses and mock-infected MA 104 cells (for use as a negative control) were partially purified as described previously for EIA antigen (4) for use in the direct and serotyping EIA formats.
A panel of 50 stool samples and 24 culture-adapted rotaviruses isolated from a separate set of stools from newborn infants at six government hospitals in New Delhi between 1986 and 1988 and between 1992 and 1993, as well as strains from a longitudinal study (1, 12), were evaluated. Since the serotype of the viruses in almost all of the stools and culture-adapted rotaviruses from which the 74 samples were selected were G[9] by RT-PCR (12), the selection of the samples for study was random. A few samples with non-G[9] rotavirus were chosen to serve as controls, and the viruses in these samples were representative of the small number of non-G[9] rotaviruses present in this population. G9 rotavirus strain 116E was serotyped by cross-neutralization with hyperimmune antisera (13) and was representative of the majority of the New Delhi G9 rotaviruses, which also were P[11]. A minority of these G9 rotaviruses were P[6] (12).
MAbs.
The derivation and characterization of the VP7-specific, rotavirus-neutralizing MAbs and selection of rotavirus escape mutants with these MAbs have been the subjects of previous reports (5–11, 17, 34–36). Antibody designation, immunoglobulin class, and G-serotype specificity are summarized in Table 1. Antibodies were titrated against prototype cultivable rotaviruses by using a direct EIA, in which partially purified virus or cell control antigen was adsorbed to the solid phase and then serial twofold dilutions of MAbs were added and allowed to bind to immobilized virus. Bound antibody was detected with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulins (Silenus, Melbourne, Victoria, Australia) and then with substrate containing 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma Chemical Co., St. Louis, Mo.) (4, 10, 11). MAb F45:1 was also titrated by the serotyping EIA format (see below). Neutralization titers of MAbs against prototype cultivable rotaviruses were determined by fluorescent focus reduction neutralization (FFN) assay, as described previously (7).
TABLE 1.
Reciprocal FFN and EIA titers of G9 rotavirus-neutralizing MAbs with G9 rotaviruses
| MAb | G serotype reactivity in decreasing order | Immunoglobulin classa | Reciprocal FFN titer with 116E | Fold change in FFN titer to 116E over other that to G9 virusesb | Reciprocal direct EIA titer with 116E | Fold change in EIA titer to 116E over other that to G9 virusesb |
|---|---|---|---|---|---|---|
| F45:6 | 9, 5 | M | 1 × 103 | <−1,000 to −200 | 1 × 103 | −300 to −30 |
| F45:5 | 9 | G3 | 5 × 103 | −16 to −6.0 | 3 × 104 | −66 to −3.0 |
| WI61:1 | 9, 8, 5 | M | 5 × 104 | <−20 to −4.0 | 6 × 104 | −18 to 1.0 |
| F45:7 | 9, 5 | M | 2 × 103 | −150 to −50 | 2 × 103 | −30 to −15 |
| F45:9 | 9 | M | 1 × 105 | −10 to −0.5 | 4 × 103 | −100 to −75 |
| F45:8 | 9 | M | <1 × 102 | <−104 to <−100 | 8 × 102 | −5,000 to −125 |
| RV-3:2 | 3, 9, 5 | G2bc | 3 × 102 | −170 to −67 | 3 × 103 | −660 to 1.5 |
| RV-3:4 | 3, 9 | G1 | 4 × 102 | −50 to >4.0 | 2 × 104 | 1.0 to 10 |
| F45:1 | 9, 4 | G2a | >1 × 106 | 1.0 to >5.0 | >1 × 106 | ≥−4.0 to >1.0 |
| F45:2 | 9, 3, 4 | A | 4 × 103 | −2.5 to 4.0 | 8 × 103 | −125 to −4.0 |
Determined with an EIA kit with subclass- and isotype-specific antisera (Commonwealth Serum Laboratories, Parkville, Victoria, Australia).
The other G9 rotaviruses tested were F45, WI61, and AU32. FFN and EIA titers of MAbs F45:1, F45:2, F45:5, F45:6, F45:7, F45:8, F45:9, and WI61:1 with these rotaviruses were determined previously (34).
Described previously (7).
Serotyping EIA.
The serotyping EIA method for G types 1 to 4 has been described previously (6, 11, 49). It was adapted to include G9 specificity by the inclusion of rabbit hyperimmune antiserum raised to F45 rotavirus (11) diluted 1 in 4,000 in phosphate-buffered saline (PBS; pH 7.4) to coat the solid phase and MAb F45:1 to detect bound virus. Otherwise, the EIA was performed as described previously. In brief, for each sample to be tested, wells of a microtiter plate were coated separately with rabbit hyperimmune antisera to each of rotavirus G types 1 to 4 and 9. Test samples diluted in PBS containing 0.05% (vol/vol) Tween 20 and 2.5% (wt/vol) skim milk powder (PBS-T-SMP) were added, followed by the addition of purified, serotype-specific MAbs diluted in PBS-T-SMP relative to ascitic fluid as indicated: RV-4:2 (G1-specific), 1 in 1,000; RV-5:3 (G2-specific), 1 in 4,300; RV-3:1 (G3-specific), 1 in 20,000; ST-3:1 (G4-specific), 1 in 4,500; F45:1, 1 in 10,000; F45:9, 1 in 2,000; and WI61:1, 1 in 10,000. A VP7-cross-reactive, nonneutralizing MAb, MAb 60 (41, 46), was also included. It was diluted 1 in 2,000 in PBS-T-SMP and was used to detect the presence of VP7 antigen in wells coated with antiserum to F45 rotavirus. Bound MAbs were detected by addition of HRP-conjugated antimouse immunoglobulins and TMB substrate as described above. Optimal dilutions of reagents were determined by checkerboard titration.
G typing by RT-PCR.
Genomic dsRNA was extracted from fecal samples containing rotavirus by the glass powder method (21). Stocks of rotavirus grown in MA 104 cells were frozen and thawed three times and were then clarified by low-speed centrifugation to remove cell debris. The dsRNA in the supernatant was extracted by the phenol-chloroform method, followed by ethanol precipitation and glass powder extraction. Rotavirus genotypes were determined by a one-step RT-PCR amplification method with type-specific primers, agarose gel electrophoresis, and ethidium bromide staining as described previously (12). Markers (123-bp ladder; Gibco BRL, Gaithersburg, Md.) and products amplified from prototype human rotaviruses possessing G types 1 to 4 and 9 were included for genotype determination, as described previously (43).
Statistical analysis.
The correlation between MAb EIA reactivities was examined by the nonparametric two-tailed Spearman test. The significance of differences in MAb 60 reactivity between groups of rotavirus samples either typeable or nontypeable by EIA was assessed by the nonparametric two-tailed Mann-Whitney test. Significance was set at the 99% level.
RESULTS
Reactivity of G9 rotavirus-neutralizing MAbs with cultivable prototype rotaviruses by FFN, direct EIA, and serotyping EIA.
A panel of 10 MAbs was evaluated for the ability to bind to and neutralize P[11], G9 virus 116E (Table 1). All MAbs except F45:8 neutralized 116E, and all MAbs bound to 116E, albeit some (F45:6 and F45:8) bound very weakly. All of the MAbs that mapped to the A-antigenic region (F45:5, F45:6, F45:7, F45:8, F45:9, and WI61:1) and one (RV-3:2) of two directed to the B-antigenic region showed lower FFN and EIA titers with 116E rotavirus than with the other G9 rotaviruses. MAb F45:2, which mapped to the antigenic F region, showed somewhat reduced levels of binding by EIA. Only one MAb, MAb F45:1, which mapped to the C-antigenic region of VP7, neutralized and bound to 116E virus to a high titer, at levels similar to those obtained with the other G9 rotaviruses tested.
MAb F45:1 was assessed by titration in the G-serotyping EIA format with 10 prototype cultivable rotavirus strains representing G types 1 to 5 and 9 (Table 2). This MAb reacted at the highest titer with all four G9 rotaviruses, at a medium titer with the G4 strains Hoso and ST-3, and at a very low titer with G1 to G3 and G5 rotaviruses. For use in the serotyping EIA, a single dilution of the MAb was chosen; the dilution chosen was the highest dilution that gave the maximum optical density at 450 nm (OD450) with the four G9 rotaviruses (strains WI61, F45, 116E, and AU32). For MAb F45:1, this was 1 in 10,000.
TABLE 2.
Reactivities of MAbs used to distinguish G types 1 to 4 and 9 by serotyping EIA with a panel of cell culture-adapted rotaviruses
| Rotavirus strain | G serotype | Reciprocal titer of MAb F45:1 in serotyping EIA formata | OD450 with given MAb (MAb G serotype specificity)b:
|
|||||
|---|---|---|---|---|---|---|---|---|
| F45:1 (9) | RV-4:2 (1) | RV-5:3 (2) | RV-3:1 (3) | ST-3:1 (4) | 60 (VP7) | |||
| 116E | 9 | >1 × 107 | 1.283 | 0.184 | 0.136 | 0.173 | 0.116 | 1.450 |
| F45 | 9 | >1 × 107 | 2.768 | 0.200 | 0.153 | 0.199 | 0.243 | 2.657 |
| WI61 | 9 | >1 × 107 | 0.970 | 0.179 | 0.127 | 0.172 | 0.215 | 1.462 |
| AU32 | 9 | >1 × 107 | 0.463 | 0.187 | 0.124 | 0.168 | 0.210 | 0.747 |
| Hoso | 4 | 2 × 106 | 2.586 | 0.040 | 0.039 | 0.040 | 2.495 | 2.868 |
| ST-3 | 4 | 2 × 104 | 0.130 | 0.038 | 0.036 | 0.043 | 1.654 | 2.839 |
| RV-4 | 1 | 5 × 103 | 0.127 | 2.491 | 0.037 | 0.039 | 0.039 | 2.791 |
| RV-5 | 2 | 7 × 103 | 0.133 | 0.040 | 0.362 | 0.035 | 0.036 | 0.955 |
| SA11 | 3 | 5 × 103 | 0.110 | 0.041 | 0.038 | 2.680 | 0.045 | 2.977 |
| TFR41 | 5 | 5 × 103 | 0.104 | 0.038 | 0.037 | 0.041 | 0.220 | 0.879 |
All rotavirus strains tested reacted with MAb F45:1. However, the dilution of MAb F45:1 used to screen samples in the serotyping EIA was higher than its titers with RV-4, RV-5, SA11, and TFR41, so these rotaviruses were not detected in the serotyping EIA with the single optimal dilution of MAb F45:1.
Positive reactions in the serotyping EIA, in which MAbs were used at a single optimal dilution, are shown in boldface.
In the serotyping EIA (Table 2), all viruses tested reacted with MAb 60, and their OD450s with this MAb showed a significant correlation with their OD450s with the G-typing MAb(s) that gave a positive result(s) (r = 0.7781, P = 0.01). The G1 to G5 rotaviruses all reacted with the G1- to G4-typing MAbs in the expected pattern. These results showed that the virus samples contained native VP7 in sufficient quantity to be serotypeable. MAb F45:1 reacted strongly with all four G9 rotaviruses and the G4 virus Hosokawa, consistent with its ability to neutralize (34) and bind to this rotavirus. MAb F45:1 did not detect the other G4 rotavirus tested, ST-3, probably because the dilution of the purified MAb used (1 in 10,000 relative to ascitic fluid) was only twofold lower than the endpoint titer of this MAb with ST-3 (1 in 20,000). No reaction of MAb F45:1 with G1, G2, G3, or G5 rotavirus was detected, consistent with its use at a dilution of 1 in 10,000 in the serotyping EIA.
Comparison of MAb EIA and RT-PCR for determination of G types of culture-adapted and stool rotaviruses.
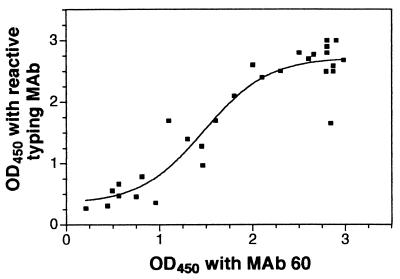
The G types of 24 rotavirus strains that were adapted to culture and that were obtained from stools from New Delhi neonates were determined by RT-PCR and EIA with MAb F45:1 for the detection of G9 rotaviruses (Table 3). Since MAb F45:1 binds to some G4 rotaviruses at the dilution used in the serotyping EIA, we used a correlation of reactivity with MAb F45:1 and failure to react with the G4-specific MAb ST-3:1 as the criterion for assignment of type G9. All the strains that were G[9] were also P[11] by RT-PCR. Identical results were obtained by the two VP7 typing methods for 14 (58%) of strains (13 G9 strains and 1 G3 strain). When either method detected a mixture of G9 virus with another type (n = 3), the non-G9 type was not detected in the alternative system. Neither of the viruses typed as G[2] by RT-PCR was typeable by EIA, and neither reacted with MAb 60. Three strains that were typed as G[9] by RT-PCR were untypeable by EIA. Conversely, two viruses that were typed as G9 by EIA were untypeable by RT-PCR. Thus, of the 21 strains typed as G9 by either method, 19 (91%) were typed by RT-PCR and 18 (86%) were typed by EIA, so the two methods had similar sensitivities. The relation between the levels of reaction of MAb 60 and the appropriate type-specific MAb with the prototype cultivable rotaviruses listed in Table 2 (n = 10) and the culture-adapted New Delhi viruses which were typeable by EIA (n = 19; Table 3) was examined (Fig. 1). These MAb reactivities showed a significant correlation (r = 0.85; P < 0.0001), suggesting that the reactivity of a sample of cultivable virus with MAb 60 shows that it contains sufficient native VP7 for successful serotyping by EIA.
TABLE 3.
Comparison of MAb serotyping EIA and RT-PCR for determination of G9 types of cell culture-adapted human rotaviruses
| G genotype determined by RT-PCR | No. of strains with the following G serotype determined by MAb EIAa:
|
Total | ||||
|---|---|---|---|---|---|---|
| 9 | 9+3 | 9+4 | 3 | Nontypeable | ||
| 9 | 13 | 1 | 1 | 0 | 3 | 18 |
| 9+3 | 1 | 0 | 0 | 0 | 0 | 1 |
| 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| 3 | 0 | 0 | 0 | 1 | 0 | 1 |
| Nonreactiveb | 2 | 0 | 0 | 0 | 0 | 2 |
| Total | 16 | 1 | 1 | 1 | 5 | 24 |
All samples that contained viral antigen typeable by EIA also reacted with MAb 60, whereas none of the samples that contained nontypeable antigen reacted with MAb 60.
No RT-PCR product was visible, even though a product of the expected size from control prototype rotaviruses was obtained.
FIG. 1.
Relation between levels of typing MAb and MAb 60 reactivities with 25 cultivable human rotaviruses of G serotypes 1 to 4 and 9. The OD450 was obtained by the serotyping EIA. The sigmoidal curve of best fit shown was determined by regression analysis (r2 = 0.89).
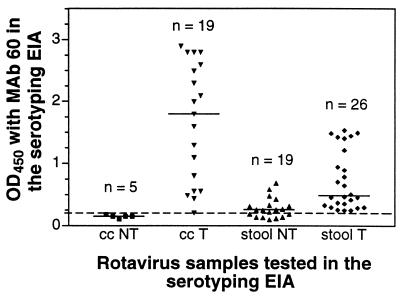
The G types of rotavirus detectable in 50 stool specimens collected from New Delhi neonates were determined by RT-PCR and EIA with MAb F45:1 (Table 4). As for the cultivable viruses, when either method detected a mixture of G9 virus with another type (n = 2), the non-G9 type was not detected in the alternative system. The single G3 rotavirus was typed by both methods, and four stool samples contained virus untypeable by either method. Of the 42 stool samples in which G9 rotavirus was detected by either method, all were positive by RT-PCR, but only 26 (62%) were positive by EIA. All the stool extracts containing viral antigen typeable by EIA also reacted with MAb 60. However, only 12 (63%) of the stool extracts, which contained rotavirus that was typeable or not, that were tested by RT-PCR and whose viruses were not typeable by EIA (n = 19) contained VP7 antigen detectable with MAb 60. The relation between the level of virus reactivity with MAb 60 and success in obtaining a G serotype by EIA (Fig. 2) suggests that MAb 60 reactivity is a marker for the ability to type VP7 by EIA in stools and virus stocks. Virus reactive with this antibody was G typeable by EIA significantly more often both in stools (P = 0.0001) and in culture (P = 0.008).
TABLE 4.
Comparison of MAb serotyping EIA and RT-PCR for determination of G9 type in stool extracts containing human rotaviruses
| G genotype determined by RT-PCR | No. of strains with the following G serotype determined by MAb EIA:
|
Total | |||
|---|---|---|---|---|---|
| 9 | 9+4 | 3 | Nontypeablea | ||
| 9 | 23 | 1 | 0 | 16 | 40 |
| 9+1 | 0 | 0 | 0 | 1 | 1 |
| 9+2 | 1 | 0 | 0 | 0 | 1 |
| 3 | 0 | 0 | 1 | 0 | 1 |
| Nonreactiveb | 0 | 0 | 0 | 4 | 4 |
| Not tested | 1 | 0 | 0 | 2 | 3 |
| Total | 25 | 1 | 1 | 23 | 50 |
Only 12 (63%) of the stools containing rotavirus typeable or not tested by RT-PCR and not typeable by EIA (n = 19) contained VP7 antigen detectable with MAb 60, whereas all stools that contained viral antigen typeable by EIA also reacted with MAb 60.
See footnote b of Table 3.
FIG. 2.
Relation of level of MAb 60 reactivity to serotyping MAb reactivity in the EIA. cc, cell culture-adapted rotavirus; stool, rotavirus-positive stool sample; NT, not typeable by G-serotyping EIA; T, typeable by G-serotyping EIA. Horizontal bars indicate median values for each group. The positive-negative cutoff for MAb 60 reactivity by EIA is shown with a dashed line.
Since MAb F45:1 detected only 62% of stool rotaviruses genotyped as 9 by RT-PCR, two additional G9-reactive MAbs, MAbs WI61:1 and F45:9, were evaluated. These were chosen on the basis of their consistently high EIA titers to cultivable G9 rotaviruses, including strain 116E (Table 1). MAb F45:5 was not evaluated because of its low affinity in the EIA. As shown in Table 5, MAb WI61:1 typed 14 (61%) of the 23 stool G9 rotaviruses typed by MAb F45:1, whereas MAb F45:9 typed only 2 (9%) of these stool viruses. MAb WI61:1 also typed as G9 5 of 16 (31%) of stool viruses that contained G9 virus by RT-PCR but that did not react with MAb F45:1. Used individually, MAb WI61:1 and MAb F45:9 were not as suitable as MAb F45:1 for G9 rotavirus typing for these stool samples. However, inclusion of MAb WI61:1 as well as MAb F45:1 for G9 typing increased the overall typing rate by EIA from 62 to 74%, although two (6%) of the reactions with MAb WI61:1 (n = 32) may have been false positive since no MAb 60-reactive antigen was detected in these samples. Interpretation of results obtained with MAb WI61:1 may be complicated by the cross-reactivity of this MAb with G5 and G8 rotaviruses (Table 1).
TABLE 5.
Comparison of G9-reactive MAbs F45:1, WI61:1, and F45:9 for determination of G9 type in stool extracts containing human rotaviruses
| G type by RT-PCR/EIA | No. of stool samples | % stool samples reactive with the following MAb in serotyping EIA:
|
|||
|---|---|---|---|---|---|
| F45:1 | WI61:1 | F45:9 | 60 | ||
| 9/9 | 23 | 100 | 61 | 9 | 100 |
| 9+2/9 | 1 | 100 | 100 | 0 | 100 |
| 9/9+4 | 1 | 100 | 100 | 0 | 100 |
| 9/NTa | 16 | 0 | 31b | 0 | 44 |
| 9+1/NT | 1 | 0 | 0 | 0 | 0 |
| NRc/NT | 4 | 0 | 0 | 0 | 0 |
NT, nontypeable.
The total included two stool specimens not reactive with MAb 60 in the serotyping EIA.
NR, nonreactive.
DISCUSSION
We have demonstrated that MAb F45:1 can be used to serotype G9 rotaviruses by EIA. It was found that both culture-adapted and fecal rotaviruses assigned to serotype G9 by RT-PCR genotyping were also G9 by EIA, thus confirming that serotype G9 rotaviruses were a common cause of rotavirus infections of neonates in India in 1993. This study is especially timely because of the recent detection of G9 rotavirus in multiple cities of the United States and in Bangladesh, suggesting with earlier studies that G9 strains probably have a global distribution and may be much more prevalent than was previously believed (13, 33, 39, 43, 44, 50). In view of the recent introduction of the tetravalent rhesus-human reassortant rotavirus vaccine (which contains the VP7 antigens of serotypes G1 to G4 only) in the United States, it will be crucial to conduct large-scale surveillance studies for G9 rotavirus to help determine the effectiveness of the vaccine against these novel strains. Such studies would be facilitated by the availability of EIA-based methods for the serotyping of rotaviruses directly from fecal specimens, as genotyping by RT-PCR is an indirect measure of virus serotype.
It was interesting that of 10 G9-neutralizing MAbs, only one proved to be suitable for detection of all four of the prototype cultivable G9 human rotaviruses by EIA. This was primarily because this was the only MAb that did not show reduced neutralization and binding titers with 116E compared with those with the three other viruses. These results suggest that 116E differs antigenically from the three other G9 rotaviruses at positions that affect VP7 at antigenic regions A, B, and F but not region C. It is possible that 116E is a G9 monotype or subtype (5, 9) different from those of the other G9 strains. Comparison of the amino acid sequences of VP7 of 116E, WI61, F45, and AU32 shows that 116E differs from the other G9 viruses at aa 87 (D to G) and aa 100 (A to I) in antigenic region A and at aa 220 (A to T) and aa 221 (S to N) in antigenic region C. Although MAb-resistant variants with mutations at these positions have not been selected in any rotavirus strain studied, these changes may affect antigenicity. Rotavirus 116E also differs from WI61 and F45 in region B at aa 145 (D to T). This produces a new potential glycosylation site, which, if used, could explain the reduced binding of the B-region MAbs to 116E. A change in region F of 116E at aa 242 (T to N) may also explain the reduced binding of MAb F45:2 to 116E.
It is also possible that differences in other structural proteins, particularly VP4, affect the antigenicity of strain 116E VP7. Interactions between VP4 and VP7 of heterologous parent origin affected the presentation of a VP4 epitope (2), and the position of amino acid mutations in the VP7 of antigenic variants was altered in reassortants heterologous for the remaining 10 genes (35). The interaction between VP4 and VP7 of murine P10[16], G3 rotavirus EW (18) was likely to have blocked or altered antigenic A- and C-region epitopes of VP7 (15). The conformation of the A and C regions of herpes simplex virus type 1-expressed VP7 appeared to be dependent on interaction with other rotaviral proteins (14). Since strain 116E has a P serotype (P8[11]) different from that for strains WI61, F45, and AU32 (P1A[8]), this difference represents another possible explanation for its altered reactivity to VP7 MAbs (18, 20).
The sensitivities of EIA and RT-PCR for determination of the G9 serotype were similar for cultivable rotaviruses, but EIA detected only 62% of stool rotaviruses genotyped as 9 by RT-PCR. RT-PCR has shown greater sensitivity than EIA for determination of human rotavirus G types 1 to 4 in previous studies (48, 51). Reaction of the VP7 group antigen-specific MAb 60 with virus-containing samples correlated with the ability to serotype the virus by EIA with MAb F45:1 and the MAbs specific for G types 1 to 4 and with the level of typing MAb binding. It therefore appears that degradation of VP7 antigen was a major factor in the loss of typing ability by EIA. This has been reported previously by use of this assay for typing of G1 to G4 rotaviruses (6, 11, 49). Both cultivable and stool rotavirus samples were frozen and thawed two extra times after RT-PCR analysis before EIA typing was performed. It is likely that the exposure of stool (but not cultivable) rotavirus to proteolytic enzymes during these freeze-thaw cycles was responsible for the higher levels of VP7 degradation for virus in stools compared with those for culture-adapted rotaviruses. Inclusion of MAb 60 or a similar antibody in rotavirus G-serotyping EIA protocols will help in the differentiation between the inability to type the strain because of VP7 degradation and a lack of a reaction because a novel serotype or monotype is present (9).
One New Delhi culture-adapted rotavirus and one rotavirus-positive stool sample reacted strongly and several other samples had slightly elevated OD450 readings with G4-typing MAb ST-3:1 by EIA. By RT-PCR, these samples contained only G9 rotavirus. The presence of a mixture of rotaviruses in these samples could not be confirmed by RNA electropherotyping or subgroup analysis (data not shown). It is thus possible that MAb ST-3:1 may cross-react with some G9 rotaviruses, although no significant cross-reaction was found with prototype G9 strains 116E, F45, WI61, and AU32. By EIA with this MAb these viruses showed slightly elevated OD450 readings which were well below the positive-negative cutoff. A significant G-serotype cross-reaction with this MAb has not been observed previously (4, 6, 11, 49). It will be important to evaluate this possible G4-G9 cross-reactivity in further studies of G9 rotavirus strains, particularly with culture-adapted rotaviruses that bind to both MAb ST-3:1 and MAb F45:1. Neutralization-resistant variants of ST-3 virus selected with MAb ST-3:1 showed an amino acid mutation in the A-antigenic region at position 94 (Ser-Asn) (9). If MAb ST3:1 is cross-reactive with type G9 strains, it may be due to the close homology in the A-antigenic region of VP7 between G4 and G9 strains. Rotaviruses 116E, F45, WI61, and AU32 differ from ST-3 in the A region only at position 94 (Ser-Gly), and since neutralization-resistant variants of ST-3 selected with MAb ST-3:1 also have a single mutation in VP7 at the same position, then G4-G9 cross-reactivity would be the most likely one for MAb ST-3:1 to exhibit.
The importance of the epitope specificity of MAbs used for rotavirus serotyping by EIA is also highlighted by our comparison of MAbs F45:1, WI61:1, and MAb F45:9 for the typing of G9 rotaviruses in stools. All three MAbs showed different reactivity patterns. Of the two A-antigenic region MAbs, WI61:1 and F45:9, only WI61:1 reacted specifically with a significant number of stool specimens. The EIA reactivity of this MAb overlapped that of MAb F45:1, but it also detected G9 virus in some additional stool samples, including two that were not reactive with MAb 60 by EIA. Should this reactivity prove to be specific for G9 rotaviruses, the use of a combination of MAbs F45:1 and WI61:1 may improve the sensitivity of EIA for the detection of G9 rotavirus in stool samples. In addition, other G9 MAbs, such as F45:9, bound to strain 116E at a low titer and barely detected this virus (or G9 rotaviruses in stools) when it was used as a detector antibody in the standard EIA format. Evaluation of these MAbs or hyperimmune antiserum to 116E as capture antibodies might also improve the sensitivity of this assay.
Our findings with Indian neonates need to be extended by evaluation of this panel of MAbs for EIA serotyping of G9 rotavirus-containing fecal samples collected in other locations and at other times from both neonates and older children. It will be of special interest to analyze the G9 strains which have been detected recently in the United States, as the introduction of a universal vaccination campaign against rotavirus with the tetravalent rhesus-human reassortant rotavirus vaccine makes it imperative that the effectiveness of this vaccine against type G9 be understood.
The G serotypes of rotaviruses in stools are most easily and inexpensively determined by an EIA with MAbs. However, RT-PCR is particularly useful for obtaining a rotavirus G genotype in the smaller number of stool samples that contain virus that cannot be typed by EIA. As shown in this study, use of a combination of these methods is advisable to combine maximum sensitivity (RT-PCR) and direct serotype determination (MAb EIA) for the typing of G9 rotaviruses in stools.
ACKNOWLEDGMENTS
We are grateful to John Tam and Harry Greenberg for provision of MAb 60.
This project was supported by project grants 940315 and 980635 from the National Health and Medical Research Council of Australia and by grants from the Indo-U.S. Vaccine Action Program and the National Vaccine Program.
REFERENCES
- 1.Bhan M K, Lew J F, Sazawal S, Das B K, Gentsch J R, Glass R I. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–287. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 2.Chen D Y, Estes M K, Ramig R F. Specific interactions between rotavirus outer capsid proteins VP4 and VP7 determine expression of a cross-reactive, neutralizing VP4-specific epitope. J Virol. 1992;66:432–439. doi: 10.1128/jvi.66.1.432-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark H F, Hoshino Y, Bell L M, Groff J, Hess G, Bachman P, Offit P A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987;25:1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulson B S. Typing of human rotavirus VP4 by an enzyme immunoassay using monoclonal antibodies. J Clin Microbiol. 1993;31:1–8. doi: 10.1128/jcm.31.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulson B S. Variation in neutralization epitopes of human rotaviruses in relation to genomic RNA polymorphism. Virology. 1987;159:209–216. doi: 10.1016/0042-6822(87)90457-0. [DOI] [PubMed] [Google Scholar]
- 6.Coulson B S. VP4 and VP7 typing using monoclonal antibodies. Arch Virol Suppl. 1996;12:113–118. doi: 10.1007/978-3-7091-6553-9_13. [DOI] [PubMed] [Google Scholar]
- 7.Coulson B S, Fowler K J, Bishop R F, Cotton R G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985;54:14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulson B S, Kirkwood C. Relation of VP7 amino acid sequence to monoclonal antibody neutralization of rotavirus and rotavirus monotype. J Virol. 1991;65:5968–5974. doi: 10.1128/jvi.65.11.5968-5974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulson B S, Kirkwood C D, Masendycz P J, Bishop R F, Gerna G. Amino acids involved in distinguishing between monotypes of rotavirus G serotypes 2 and 4. J Gen Virol. 1996;77:239–245. doi: 10.1099/0022-1317-77-2-239. [DOI] [PubMed] [Google Scholar]
- 10.Coulson B S, Tursi J M, McAdam W J, Bishop R F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986;154:302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- 11.Coulson B S, Unicomb L E, Pitson G A, Bishop R F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987;25:509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das B K, Gentsch J R, Cicirello H G, Woods P A, Gupta A, Ramachandran M, Kumar R, Bhan M K, Glass R I. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das B K, Gentsch J R, Hoshino Y, Ishida S, Nakagomi O, Bhan M K, Kumar R, Glass R I. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197:99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- 14.Dormitzer P R, Ho D Y, Mackow E R, Mocarski E S, Greenberg H B. Neutralizing epitopes on herpes simplex virus-1-expressed rotavirus VP7 are dependent on coexpression of other rotavirus proteins. Virology. 1992;187:18–32. doi: 10.1016/0042-6822(92)90291-v. [DOI] [PubMed] [Google Scholar]
- 15.Dunn S J, Burns J W, Cross T L, Vo P T, Ward R L, Bremont M, Greenberg H B. Comparison of VP4 and VP7 of five murine rotavirus strains. Virology. 1994;203:250–259. doi: 10.1006/viro.1994.1482. [DOI] [PubMed] [Google Scholar]
- 16.Dunn S J, Ward R L, McNeal M M, Cross T L, Greenberg H B. Identification of a new neutralization epitope on VP7 of human serotype 2 rotavirus and evidence for electropherotype differences caused by single nucleotide substitutions. Virology. 1993;197:397–404. doi: 10.1006/viro.1993.1601. [DOI] [PubMed] [Google Scholar]
- 17.Dyall Smith M L, Lazdins I, Tregear G W, Holmes I H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci USA. 1986;83:3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1625–1655. [Google Scholar]
- 19.Flores J, Sears J, Perez-Schael I, White L, Garcia D, Lanata C, Kapikian A Z. Identification of human rotavirus serotype by hybridization to polymerase chain reaction-generated probes derived from a hyperdivergent region of the gene encoding outer capsid protein VP7. J Virol. 1990;64:4021–4024. doi: 10.1128/jvi.64.8.4021-4024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentsch J R, Das B K, Jiang B, Bhan M K, Glass R I. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology. 1993;194:424–430. doi: 10.1006/viro.1993.1280. [DOI] [PubMed] [Google Scholar]
- 21.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentsch J R, Woods P A, Ramachandran M, Das B K, Leite J P, Alfieri A, Kumar R, Bhan M K, Glass R I. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174:S30–S36. doi: 10.1093/infdis/174.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 23.Glass R I, Keith J, Nakagomi O, Nakagomi T, Askaa J, Kapikian A Z, Chanock R M, Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985;141:292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- 24.Gorziglia M, Larralde G, Kapikian A Z, Chanock R M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci USA. 1990;87:7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouvea V, Ramirez C, Li B, Santos N, Saif L, Clark H F, Hoshino Y. Restriction endonuclease analysis of the vp7 genes of human and animal rotaviruses. J Clin Microbiol. 1993;31:917–923. doi: 10.1128/jcm.31.4.917-923.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green K Y, Hoshino Y, Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989;168:429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 28.Green K Y, Midthun K, Gorziglia M, Hoshino Y, Kapikian A Z, Chanock R M, Flores J. Comparison of the amino acid sequences of the major neutralization protein of four human rotavirus serotypes. Virology. 1987;161:153–159. doi: 10.1016/0042-6822(87)90181-4. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino Y, Kapikian A Z. Rotavirus antigens. Curr Top Microbiol Immunol. 1994;185:179–227. doi: 10.1007/978-3-642-78256-5_7. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino Y, Sereno M M, Midthun K, Flores J, Kapikian A Z, Chanock R M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci USA. 1985;82:8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iizuka M, Chiba M, Masamune O, Gerna G, Nakagomi O. Molecular characterization of human rotavirus VP4 genes by polymerase chain reaction and restriction fragment length polymorphism assay. Microbiol Immunol. 1993;37:729–735. doi: 10.1111/j.1348-0421.1993.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 32.Ikegami N, Akatani K, Hosaka T, Ushijima H. Program and abstracts of the VII International Congress of Virology. Ottawa, Ontario, Canada: National Research Council of Canada; 1987. Prevalence of a new serotype of group A human rotaviruses in Japan, abstr. R11.29; p. 113. [Google Scholar]
- 33.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1657–1708. [Google Scholar]
- 34.Kirkwood C, Masendycz P J, Coulson B S. Characteristics and location of cross-reactive and serotype-specific neutralization sites on VP7 of human G type 9 rotaviruses. Virology. 1993;196:79–88. doi: 10.1006/viro.1993.1456. [DOI] [PubMed] [Google Scholar]
- 35.Lazdins I, Coulson B S, Kirkwood C, Dyall Smith M, Masendycz P J, Sonza S, Holmes I H. Rotavirus antigenicity is affected by the genetic context and glycosylation of VP7. Virology. 1995;209:80–89. doi: 10.1006/viro.1995.1232. [DOI] [PubMed] [Google Scholar]
- 36.Lazdins I, Sonza S, Dyall Smith M L, Coulson B S, Holmes I H. Demonstration of an immunodominant neutralization site by analysis of antigenic variants of SA11 rotavirus. J Virol. 1985;56:317–319. doi: 10.1128/jvi.56.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Midthun K, Valdesuso J, Kapikian A Z, Hoshino Y, Green K Y. Identification of serotype 9 human rotavirus by enzyme-linked immunosorbent assay with monoclonal antibodies. J Clin Microbiol. 1989;27:2112–2114. doi: 10.1128/jcm.27.9.2112-2114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagomi O, Oyamada H, Nakagomi T. Use of alkaline Northern blot hybridization for the identification of genetic relatedness of the fourth gene of rotaviruses. Mol Cell Probes. 1989;3:263–271. doi: 10.1016/0890-8508(89)90007-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakagomi T, Akatani K, Ikegami N, Katsushima N, Nakagomi O. Occurrence of changes in human rotavirus serotypes with concurrent changes in genomic RNA electropherotypes. J Clin Microbiol. 1988;26:2586–2592. doi: 10.1128/jcm.26.12.2586-2592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagomi T, Ohshima A, Akatani K, Ikegami N, Katsushima N, Nakagomi O. Isolation and molecular characterization of a serotype 9 human rotavirus strain. Microbiol Immunol. 1990;34:77–82. doi: 10.1111/j.1348-0421.1990.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 41.Noel J S, Beards G M, Cubitt W D. Epidemiological survey of human rotavirus serotypes and electropherotypes in young children admitted to two children’s hospitals in northeast London from 1984 to 1990. J Clin Microbiol. 1991;29:2213–2219. doi: 10.1128/jcm.29.10.2213-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padilla-Noriega L, Mendez-Toss M, Menchaca G, Contreras J F, Romero-Guido P, Puerto F I, Guiscarfe H, Mota F, Herrera I, Cedillo R, Munoz O, Calva J, De Lourdes Guerrero M, Coulson B S, Greenberg H B, Lopez S, Arias C F. Antigenic and genomic diversity of human rotavirus VP4 in two consecutive epidemic seasons in Mexico. J Clin Microbiol. 1998;36:1688–1692. doi: 10.1128/jcm.36.6.1688-1692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramachandran M, Das B K, Vij A, Kumar R, Bhambal S S, Kesari N, Rawat H, Bahl L, Thakur S, Woods P A, Glass R I, Bhan M K, Gentsch J R. Unusual diversity of human rotavirus G and P genotypes in India. J Clin Microbiol. 1996;34:436–439. doi: 10.1128/jcm.34.2.436-439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandran M, Gentsch J R, Parashar U D, Jin S, Woods P A, Holmes J L, Kirkwood C D, Bishop R F, Greenberg H B, Urasawa S, Gerna G, Coulson B S, Taniguchi K, Bresee J S, Glass R I the National Rotavirus Strain Surveillance System Collaborating Laboratories. Detection and characterization of novel rotavirus strains in the United States. J Clin Microbiol. 1998;36:3223–3229. doi: 10.1128/jcm.36.11.3223-3229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw R D, Stoner-Ma D L, Estes M K, Greenberg H B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985;22:286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw R D, Vo P T, Offit P A, Coulson B S, Greenberg H B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986;155:434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi K, Urasawa T, Morita Y, Greenberg H B, Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987;155:1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi K, Wakasugi F, Pongsuwanna Y, Urasawa T, Ukae S, Chiba S, Urasawa S. Identification of human and bovine rotavirus serotypes by polymerase chain reaction. Epidemiol Infect. 1992;109:303–312. doi: 10.1017/s0950268800050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unicomb L E, Coulson B S, Bishop R F. Experience with an enzyme immunoassay for serotyping human group A rotaviruses. J Clin Microbiol. 1989;27:586–588. doi: 10.1128/jcm.27.3.586-588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unicomb L E, Podder G, Gentsch J R, Hasan K Z, Faruque A S G, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ushijima H, Koike H, Mukoyama A, Hasegawa A, Nishimura S, Gentsch J. Detection and serotyping of rotaviruses in stool specimens by using reverse transcription and polymerase chain reaction amplification. J Med Virol. 1992;38:292–297. doi: 10.1002/jmv.1890380412. . (Erratum, 44:165, 1994.) [DOI] [PubMed] [Google Scholar]