Abstract
Background:
The long-term prognosis of relapsing-remitting multiple sclerosis (RRMS) is usually unfavorable as most patients transition to secondary progressive multiple sclerosis (SPMS) with accumulative disability. A rare form of non-progressive multiple sclerosis (MS) also exists, known as benign MS (BMS or NPMS), which lacks disease progression defined as Expanded Disability Status Scale (EDSS) ≤3 after 15 years of disease onset without treatment.
Purpose:
Our study aims to identify soluble plasma factors predicting disease progression in multiple sclerosis (MS).
Research Design and Study Sample:
We utilized Luminex multiplex to analyze plasma levels of 33 soluble factors, comparing 32 SPMS patients to age-, sex-, and disease duration-matched non-progressive BMS patients, as well as to RRMS patients and healthy controls.
Results:
Plasma levels of EGF, sCD40L, MCP1/CCL2, fractalkine/CX3CL1, IL-13, Eotaxin, TNFβ/LTα, and IL-12p40 were significantly different between the various types of MS. Plasma sCD40L was significantly elevated in SPMS compared to BMS and RRMS. The combination of MCP1/CCL2 and sCD40L discriminated between RRMS and SPMS. MCP1/CCL2 was found to be the most effective classifier between BMS and RRMS, while BMS was most effectively distinguished from SPMS by the combination of sCD40L and IFNγ levels.
Conclusions:
These differences may facilitate personalized precision medicine and aid in the discovery of new therapeutic targets for disease progression through the improvement of patient stratification.
Keywords: Benign MS, multiple sclerosis, relapsing remitting MS, secondary progressive multiple sclerosis, soluble CD40 ligand
Introduction
Multiple sclerosis (MS) is an autoimmune disease associated with the central nervous system (CNS). The pathology of MS is characterized by T- and B-lymphocyte and innate immune cell infiltration into the CNS, myelin sheath breakdown, oligodendrocyte damage, and axonal degeneration.1,2 The causes of MS have not been fully elucidated, although certain genes and environmental factors increase susceptibility.
MS typically presents between the ages of 20 and 40 years. The disease phenotype is heterogeneous and varies from person to person in terms of disease trajectory and progression. In most cases, relapsing-remitting MS (RRMS) progresses to secondary progressive MS (SPMS) indicated by a steady increase in long-term disability and disease severity. In less than 5–10% of cases, relapses are mild, and disease progression does not occur. This is known as non-progressive benign MS (NPMS; BMS), where individuals are functionally unaffected in terms of disability progression. 3 As non-progressive BMS is typically diagnosed with 15–20 years of disease duration by the lack of progression without the use of disease-modifying therapies (DMTs), those diagnosed with BMS and SPMS tend to be naturally matched for age and disease duration, and the only major discernable difference is the rate of progression. Most prior studies examining MS-associated factors have focused primarily on RRMS, and those that studied SPMS usually compared them to RRMS. However, individuals with RRMS and SPMS typically differ on a variety of features including age, disease duration, disease trajectory, and the use of DMTs. Thus, differentially expressed factors may be unrelated to disease progression. Aging affects the levels of many cytokines4,5 and influences the levels of soluble factors previously associated with disease progression, including neurofilament light. 6
While cerebrospinal fluid (CSF) derived factors are expected to most closely tie to disease pathology for CNS diseases, MS involves a dysregulation of immunity in both central and peripheral compartments. In comparison to CSF samples, plasma samples are more accessible and practical to obtain, and thus more suitable for routine clinical practice. Therefore, we analyzed plasma levels of cytokine, chemokine, and growth factor in different forms of MS, including RRMS, SPMS, and BMS. Specifically, we took a novel approach by using non-progressive BMS, age- and disease duration-matched to progressive SPMS, to dissect progression-specific mechanisms. There is currently a poor understanding of the mechanisms underlying disease progression, which has made it difficult to develop effective therapies for progressive MS. Successful identification of specific markers to predict MS disease progression has the potential to uncover biological drivers of progression and targets for therapeutic intervention. Plasma markers could also help to stratify individuals according to the rate of progression, allowing for better tailoring of clinical treatment plans. We found a set of plasma biomarkers that can help differentiate between various forms of MS, which may be especially useful for progressive MS and potentially lead to therapeutic interventions.
Materials and Methods
Study Approval and Participating Subjects Recruitment
Our study consisted of a total of 52 MS participants (32 SPMS, 8 RRMS, and 12 BMS) and 5 healthy control (HC) participants. HC and MS participants were recruited from the University of Michigan Multiple Sclerosis Clinic and the Autoimmunity Center of Excellence. RRMS and SPMS were defined by the 2010 Revised McDonald criteria and were not treated with disease-modifying therapy (DMT) at the time of the study. Non-progressive BMS patients were not treated with disease-modifying therapy and were defined as having an Expanded Disability Status Scale (EDSS) score ≤3 with more than 15 years of disease duration. The SPMS group included baseline patients in the AMS04 study prior to randomization. The AMS04 is a multicentered SPMS mechanistic study of siponimod. Detailed inclusion and exclusion criteria can be found in clinicaltrials.gov identifier NCT02330965. 7 SPMS patients had a unified and documented recent progression as defined by a progressive increase in disability (of at least 6 months duration) in the absence of relapses or independent of relapses: EDSS progression in the 2 years prior to the study of ≥1 point for patients with EDSS <6.0 at baseline, and ≥.5 point for patients with EDSS ≥6.0 at baseline. Written informed consent was obtained from all patients prior to participation in this study, which was approved by the University of Michigan Institutional Review Board (Backspaceunder biomarker study HUM00066792 and AMS04 study HUM00084719). The demographic and disease-associated characteristics of the participants are shown in Table 1.
Table 1.
Demographics of Participants of this Study.
| Groups | Number of patients | Age (SD) | Female/male | Race (white/other) | MS diagnosis duration (SD) | EDSS (SD) |
| HC | 5 | 56.3 (7.8) | 4/1 | 5/0 | — | — |
| RRMS | 8 | 40.7 (13.5) | 7/1 | 7/1 | 3.2 (3.0) | 1.2 (.9) |
| BMS | 12 | 57.0 (7.3) | 11/1 | 12/0 | 24.9 (9.8) | 1.2 (.7) |
| SPMS | 32 | 53.0 (7.1) | 24/8 | 29/3 | 20.4 (10.5) | 5.7 (1.3) |
Age, average age of the group; BMS, non-progressive benign MS; EDSS, The Expanded Disability Status Scale; HC, healthy control; MS diagnosis duration, average MS disease duration after MS diagnosis; RRMS, relapsing-remitting MS; SD, standard deviation; SPMS, secondary progressive MS.
Peripheral Blood Mononuclear Cells (PBMC) and Plasma Isolation
About 60 mL of heparinized peripheral blood was collected from each participant in Sodium HeparinVacutainerTM (BD Biosciences). Plasma was collected from a clear top layer after a spin of 400g for 10 min, followed by centrifugation at 1800g for 15 min without a break. The clear plasma was aliquoted and stored at −80oC before use.
Luminex Assay
The frozen plasma collected from HC and participants with RRMS, BMS, and SPMS was used to measure chemokine and cytokine concentrations. Luminex assays of the plasma cytokine profile were measured using the HCYTMAG-60K-PX33 kit purchased from Sigma-Millipore (Burlington, MA, USA), according to the manufacturer’s recommended protocol. The kit is designed to detect these inflammation-related cytokines and chemokines: EGF, FGF-2, Eotaxin, G-CSF, Flt3L, GM-CSF, Fractalkine (CX3CL1), IFN-α2, IFNγ, Groα (CXCL1), IL-10, IL-12p40 MDC (CCL22), IL-12p70, IL-13, IL-15, sCD40L, IL-17A, IL-9, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL8), IP-10 (CXCL10), MCP-1(CCL2), MIP-1α (CCL3), MIP-1β (CCL4), TNFα, TNFβ (LTα), and VEGF. All plasma samples were spun for 10 s at 16000g, and the clear supernatants were added to the wells of a Luminex assay plate. Each sample was assayed in duplicate.
Statistical Analysis
The plasma derived from HC, RRMS, BMS, and SPMS were analyzed using the Luminex kit from Millipore. Kruskal–Wallis test and multiple comparison with Dunn’s modification between different groups were performed. Median of plasma concentration (pg/mL) are presented with 25-75% range. Non-parametric Mann–Whitney U tests were used when data derived from 2 groups were analyzed. Receiver operator characteristic (ROC) analysis, multiple logistic regression analysis and Spearman correlation coefficient, and 95% CI and two-tail P values were calculated using Prism GraphPad software (version 8.4.3). P < .05 and r > .3 for correlation are considered statistically significant. Z-scores used in the heatmap were calculated according to the formula: z-score is z = (x−μ)/σ, where x is the individual raw soluble factor concentration, μ is the population mean of the soluble factor of all sample analyzed, and σ is the population standard deviation.
Results
Overview
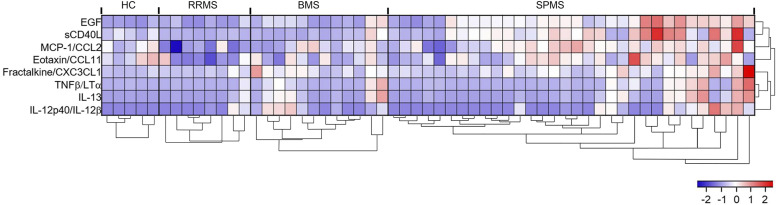
To identify cytokine, chemokine, or soluble factor in plasma that differentiate between HC and MS participants, or among different MS types, we measured the plasma concentration of 33 cytokine/chemokines with Luminex using plasma samples derived from 4 groups of individuals including HC, RRMS, BMS, and SPMS. The resulting concentrations were analyzed using the Kruskal–Wallis method comparing all 4 groups. Differences in EGF (P ≤ .0001), soluble CD40L (sCD40L) (P = .0009), MCP-1/CCL2 (P = .0029), Fractalkine/CX3CL1 (P = .0128), IL-13 (P = .0358), Eotaxin (P = .0360), IL-12 (p40) (P = .0420), and TNFβ/LTα (P = .0482) (Figure 1, Table 2) all showed statistical significance (P < .05). IL-8/CXCL8 (P = .0748), IFNγ (P = .0812), IL-4 (P = .0848), and IL-15 (P = .0905) showed a trend toward significance (Supplemental Table 1). No statistically significant differences were detected for the following cytokines and chemokines: MIP-1α/CCL3, IL-10, IL-6, VEGF, IFN-α2, TNFβ, IL-7, MDC/CCL22, IP-10/CXCL10, IL-9, IL-17A, FGF2, GM-CSF, G-CSF, MIP-1β/CCL4, IL-5, IL-2, IL-3, GROα/CXCL1, IL-12 (p70), and Flt-3L (Supplemental Table 1).
Figure 1.
Z-score heatmap comparing relative expression levels of different soluble factors detected in plasma among HC and 3 types of MS. Expression levels of 8 significantly different (P < .05) plasma soluble factors detected using Luminex and analyzed with Kruskal–Wallis test comparing HC, RRMS, BMS, and SPMS are displayed after Z-transformation. HC (n = 5), RRMS (n = 8), BMS (n = 12), and SPMS (n = 33). Hierarchical clustering of soluble factors and patient groups was completed in R using divisive clustering methodology (DIANA) from the cluster package.
Table 2.
Significantly Different Plasma Cytokine/Chemokine/Growth Factor Levels Among Different Types of Multiple Sclerosis and Healthy Controls.
| Analyte | Kruskal–Wallis test | Median (25%–75% range) | Dunn’s multiple × comparison (P value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HC vs | RRMS vs | BMS | |||||||||
| P value | HC | RRMS | BMS | SPMS | RRMS | BMS | SPMS | BMS | SPMS | SPMS | |
| EGF | <.0001 | 18.30 (6.965–29.50) | 20.63 (6.528–43.17) | 44.10 (17.78–73.24) | 113.5 (80.51–209.7) | >.9999 | .8213 | .007 | .8429 | .0016 | .1371 |
| sCD40L | .0009 | 925.4 (471.1–1468) | 324.32 (199.4–421.8) | 384.6 (125.2–586.14) | 2416 (587.4–5107) | >.9999 | >.9999 | >.9999 | >.9999 | .0053 | .014 |
| MCP1/CCL2 | .0029 | 501.5 (392.1–583.1) | 311.4 (263.1–359.8) | 440.3 (359.1–598.8) | 554.7 (442.5–700.3) | .3841 | >.9999 | >.9999 | .2976 | .0016 | .6333 |
| Fractalkine/CX3CL1 | .0128 | 34.62 (18.31–84.08) | 65.85 (39.40–105.0) | 183.2 (80.28–337.9) | 147.4 (72.97–263.3) | >.9999 | .0666 | .046 | .3523 | .2689 | >.9999 |
| IL-13 | .0358 | .525 (.525–4.075) | .13 (.13–47.66) | 54.98 (.525–253.7) | 15.75 (.525–168.0) | >.9999 | .4602 | .841 | .0776 | .1354 | >.9999 |
| Eotaxin | .0360 | 476.8 (394.7–1067) | 201.9 (165.6–481.6) | 423.5 (291.6–778.9) | 598.8 (334.9–888.2) | .1676 | >.9999 | >.9999 | .9355 | .0427 | >.9999 |
| IL-12p40/IL12B | .0420 | .95 (.95–2.14) | .3550 (.19–60.21) | 36.22 (4.76–152.70) | .95 (.95–87.14) | >.9999 | .3291 | >.9999 | .0616 | .3292 | >.9999 |
| TNFβ/LTα | .0482 | .475 (.475–18.08) | .115 (.115–14.10) | 54.23 (.475–400.9) | 8.70 (.475–298.5) | >.9999 | >.9999 | >.9999 | .0583 | .1182 | >.9999 |
Plasma derived from HC (n = 5), patients with RRMS (n = 8), BMS (n = 12), and SPMS (n = 32) were analyzed using Luminex kit from Millipore. Kruskal–Wallis test and multiple comparison with Dunn’s modification between different groups were performed. Median of plasma concentration (pg/mL) is presented followed by 25–75% range in the brackets.
Several circulating cytokines/chemokines were found to be significantly different between the different types of MS (Figure 1, Table 2). Using Dunn’s multiple comparison, significant differences between HC and SPMS were found in circulating EGF (P = .007) and Fractalkine/CX3CL1 (P = .046) (Table 2). Several cytokines were found to be significantly different comparing RRMS with SPMS, including EGF (P = .0016), MCP1/CCL2 (P = .0016), sCD40L (P = .0053), and Eotaxin (P = .0427). No soluble biomarkers were significantly different between RRMS and BMS, while TNFβ/LTα (P = .0583), IL-12p40/IL-12B (P = .0616), and IL-13 (P = .0776) showed a trend of difference. sCD40L was the only soluble factor that was found to be significantly different between BMS and SPMS (P = .014) (Table 2).
Circulating Soluble Factors are Influenced by Age and Disease Duration to Varying Degrees
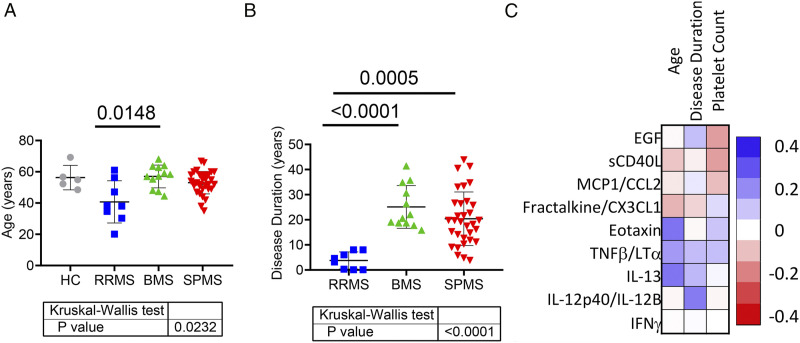
Like past research, when compared SPMS to RRMS, RRMS subjects were significantly younger (Figure 2A) and had a shorter disease duration (Figure 2B). Therefore, our study took a unique approach by comparing non-progressive benign MS (BMS), naturally age- and disease duration-matched, to progressive SPMS to dissect progression-specific mechanisms (Figures 2A and 2B). Spearman correlation analysis less than 0.3 is considered statistically negligible 8 (Figure 2C). The analysis indicated that Eotaxin (r = .3398, 95% CI: .0791-.5570, P = .0097), TNFβ/LTα (r = .3133, 95% CI: .0495-.5362, P = .0177), and IL-13 (r = .3383, 95% CI: .0774-.5559, P = .0101) were moderately associated with age, while IL-12p40/IL-12 was moderately associated with disease duration (r = .3339, 95% CI: .0589-.5618, P = .0156. Other factors are not significantly associated with age and disease duration. EGF and sCD40L could potentially be affected by platelet count, 9 our analysis of plasma levels of EGF and sCD40L did not show a noticeable association with platelet count. EGF can be derived from multiple cellular sources, which may have disparate effects, and levels may be influenced by comorbidities, suggesting a lack of specificity for MS. 10
Figure 2.
Correlation analysis of plasma cytokine levels against age, disease duration, and platelet count. Ages of all participants (A) and disease duration (B) of all MS patients in this study were analyzed using the Kruskal–Wallis test. Statistically significant P values between groups calculated using Dunn’s adjusted multiple comparison are shown above horizontal lines. Spearman rank correlation analysis was performed using the plasma levels of 8 most significantly different cytokines among different groups against age, disease duration, and circulating platelet count of corresponding individuals. Their correlation coefficient values are shown in scale of color as matrix (C). HC (n = 5), RRMS (n = 8), BMS (n = 12), and SPMS (n = 32).
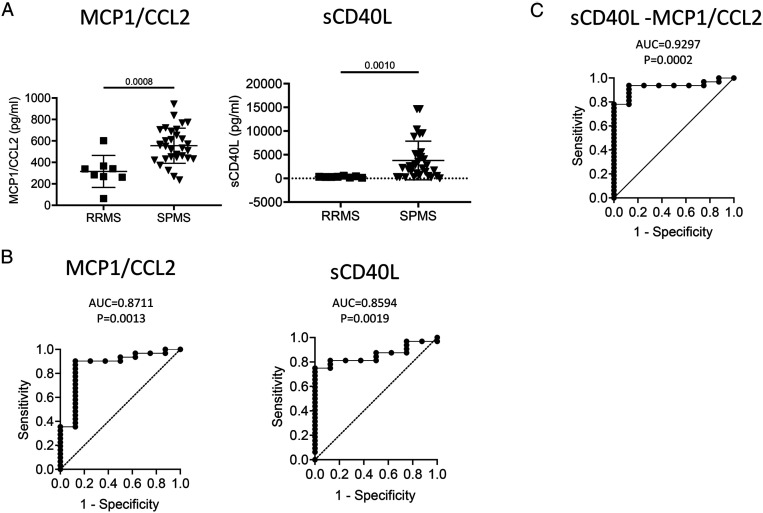
Plasma Levels of MCP1/CCL2 and sCD40L are Elevated in SPMS as Compared to RRMS
To evaluate whether any of the soluble factors can be used to discriminate SPMS from RRMS, receiver-operating characteristic (ROC) curve analyses were performed (Supplemental Table 2). Plasma levels of MCP1/CCL2 and sCD40L were significantly increased in SPMS patients compared with RRMS patients (Figure 3A). ROC analysis revealed that MCP1/CCL2 (AUC = .8711 ± .0762, 95% CI: .7218–1.000, P = .0013) and sCD40L (AUC = .8594 ± .0577, 95% CI: .7462–.9725, P = .0019) were the best biomarkers to stratify SPMS from RRMS (Figure 3B). Multiple logistic regression analysis combining plasma levels of MCP/CCL2 and sCD40L gave a higher predictive power (AUC = .9297 ± .04193, 95% CI: .8475–1.000, P = .0002, Figure 3C), which gave a negative predictive power of 77.78% and a positive predictive power of 96.77% to discriminate SPMS from RRMS.
Figure 3.
Plasma biomarkers discriminate SPMS from RRMS. (A) Column scatter graph of plasma concentrations of MCP1/CCL2 and sCD40L of RRMS and SPMS patients. P value using Mann–Whitney test are shown above the line. (B) ROC analysis using plasma concentrations of MCP1/CCL2 and sCD40L derived from RRMS and SPMS groups. (C) Multiple logistic regression analysis using plasma concentration of sCD40L and MCP1/CCL2 derived from patients of both RRMS and SPMS groups. RRMS (n = 8), SPMS (n = 30). ROC, receiver-operating characteristic; AUC, area under curve.
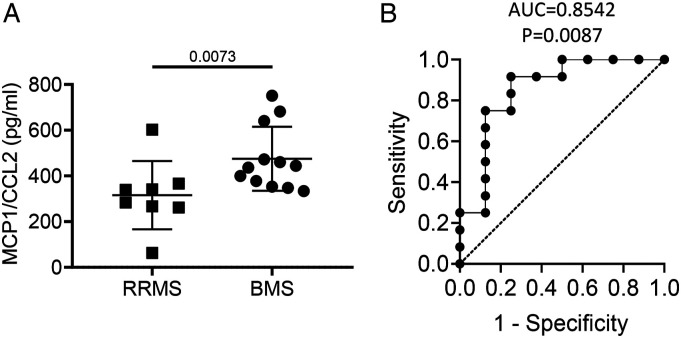
Plasma Levels of MCP1/CCL2 are Elevated in BMS as Compared to RRMS
Plasma levels of MCP1/CCL2 were significantly increased in BMS compared with RRMS (P = .0073, Figure 4A). ROC curve analysis indicated that MCP1/CCL2 was the best classifier to discriminate BMS from RRMS (AUC = .8542 ± .0965, 95% CI: .6651–1.000, P = .0087, Figure 4), which gave a sensitivity of 75% and a specificity of 87.5% (Supplemental Table 2).
Figure 4.
Plasma levels of MCP1/CCL2 discriminate BMS from RRMS. Plasma concentration of MCP1/CCL2 derived from RRMS and BMS patients were compared along with their ROC plots. (A) Column scatter graph comparing plasma level of MCP1/CCL2 of RRMS group (n = 8) with BMS group (n = 12). P value using Mann–Whitney test are shown above the line. (B) ROC analysis using MCP/CCL2 to discriminate BMS from RRMS. ROC, receiver-operating characteristic; AUC, area under curve.
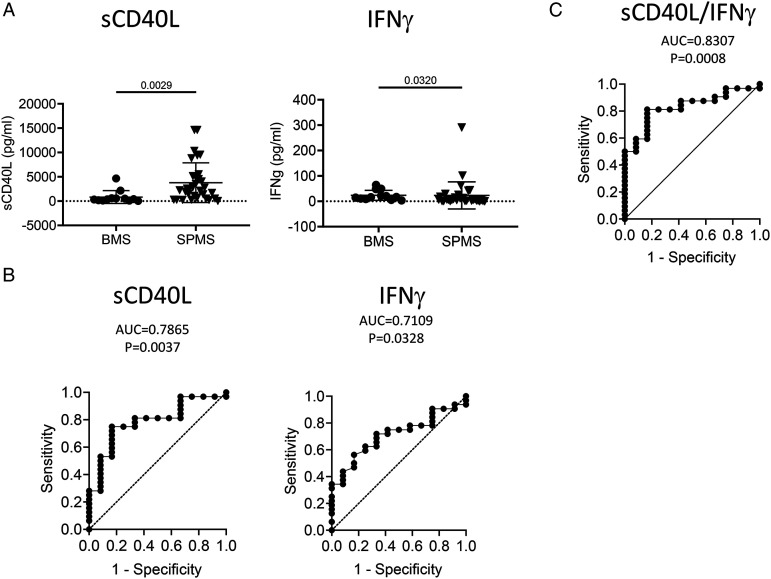
Plasma Levels of sCD40L and IFN-γ Differentiate Non-progressive BMS from SPMS
Comparison of non-progressive BMS and SPMS is the most informative analysis to reveal underlying mechanisms of MS disease progression given that these groups typically have similar age and disease duration but different disease progression rates. Plasma levels of sCD40L detected by Luminex assay revealed significant elevation in patients with SPMS compared to those with BMS (P = .0029; Figure 5A). Mann–Whitney analysis comparing the BMS group and the SPMS group also revealed IFNγ significantly discriminates between non-progressive BMS and SPMS (P = .0320, Figure 5A). ROC analysis revealed that sCD40L (AUC = .7865 ± .0743, 95% CI: .6408–.9321, P = .0037) and IFNγ (AUC = .7109 ± .07829, 95% CI: .5575–.8644, P = .0328) could discriminate SPMS from BMS (Figure 5B). Multiple logistic regression analysis showed that combining IFNγ with sCD40L improved the stratification (AUC = .8307 ± .0627, 95% CI: .7078–.9536, P = .0008, Figure 5C), which gave a negative predictive power of 60.00 and a positive predictive power of 82.35% to discriminate BMS and SPMS.
Figure 5.
Plasma levels of sCD40L and IFNγ discriminate SPMS from BMS. Plasma concentration of sCD40L and IFNγ in BMS and SPMS patients were compared along with their ROC plots. (A) Column scatter graph of plasma concentrations of sCD40L and IFNγ derived from BMS and SPMS patients. P value using Mann–Whitney test are shown above the line. (B) ROC analysis using plasma concentrations of sCD40L and IFNγ derived from BMS and SPMS groups. (C) Multiple logistic regression analysis combining plasma concentration of sCD40L and IFNγ derived from patients of both BMS and SPMS groups. n: BMS = 12, SPMS = 32. ROC, receiver-operating characteristic; AUC, area under curve.
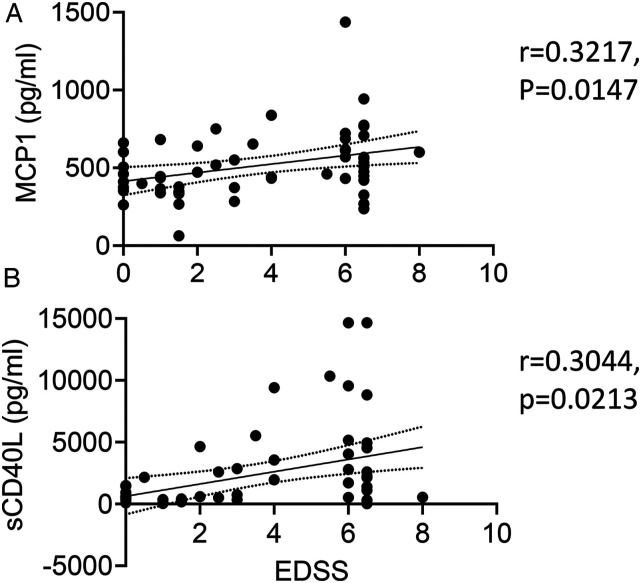
MCP1/CCL2 and sCD40L are Positively Correlated MS Disease Progression
Spearman correlation analysis was used to determine whether any of the plasma soluble factors are linked to MS progression across all cohorts using EDSS as a disease progression marker. Only MCP1/CCL2 (r = .3217, 95% CI: .05890–.5428, P = .0147, Figure 6A) and sCD40L (r = .3044, 95% CI: .03975–.5292, P = .0213, Figure 6B) showed positive correlation with EDSS, suggesting that their upregulation is associated with MS disease progression. It is unclear whether these factors play a causative role in MS progression or reflect the body’s physiological response directly or indirectly to MS progression.
Figure 6.
MCP1/CCL2 and sCD40L are significantly correlated with MS disease progression. Spearman correlation analysis was performed using plasma levels of MCP1/CCL2 (A) and sCD40L (B) against corresponding EDSS scores in all participants. r is the Spearman correlation coefficient. The solid line is the best fit line of linear regression with 95% CI (dotted line).
Correlations of Inflammatory Cytokines are SPMS-specific
To find out whether any of the plasma soluble factors are correlated with each other specifically in SPMS, we did a comparative correlation analysis using all of the factors analyzed in this study to compare the SPMS group with the non-SPMS group including BMS/RRMS (Supplemental Figure 1). Significant positive correlations were found among the majority of cytokines for SPMS groups, suggesting a shift to a global pro-inflammatory innate immune environment in SPMS patients. Notably, there was a significant correlation of collectively elevated cluster of soluble factors only with SPMS, which includes sCD40L, Eotaxin, EGF, and MCP1/CCL2. sCD40L showed a significant positive correlation with EGF (r = .6087, P = .0002), Eotaxin (r = .5943, P = .0003), as well as MCP1/CCL2, although less significantly (r = .4713, P = .0065) in the SPMS group of patients. However, their correlations in HC, RRMS, and BMS were limited and insignificant (Supplemental Figure 1). EGF seemed to show a significant correlation with many other cytokines in non-SPMS. Our study suggests that sCD40L, along with Eotaxin/CCL11 and MCP/CCL2, appears to have a close association with MS progression into the SPMS stage and could play an important role in the pathogenic process of MS progression. Therefore, they could be potential biomarkers and therapeutic targets for SPMS.
Discussion
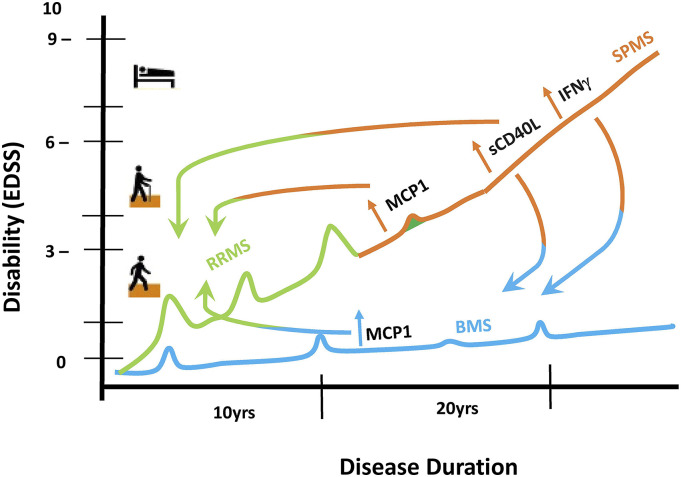
The lack of effective therapies for progressive MS makes the identification of progression-related factors crucial. Our study aims to identify soluble plasma factors predicting disease progression in MS. Previous studies often omit non-progressive BMS. 11 Our study took a novel approach by comparing age- and disease duration-matched non-progressive BMS to SPMS. We found notable plasma marker differences between non-progressive BMS and SPMS that may be used as potential biomarkers specific for MS disease progression (Figure 7).
Figure 7.
Summary. RRMS (green) becomes SPMS (orange) over time with increased disability; BMS (blue) does not accumulate disability despite long duration of disease. Progressing to SPMS from RRMS (indicated with a half orange/half green arrow), sCD40L and MCP1 are increased. Comparing BMS to RRMS (indicated with a half blue/half green arrow), only MCP1 is increased. Although both BMS and SPMS have long disease duration, SPMS exhibits increased sCD40L and IFNγ compared to non-progressive BMS (indicated with a half orange/half blue arrow). Both MCP1 and sCD40L were found to be correlated with EDSS.
Role of sCD40L in MS Progression
The combined sCD40L and IFNγ levels distinguish between non-progressive BMS and SPMS with AUC = .8307 (P = .0008) in the multiple logistic regression analysis (Figures 5 and 6). IFNγ is known for worsening demyelination and axonal injury in MS.12,13 Many earlier studies also suggested that CD40L may play an important role in MS pathogenesis. Membrane-bound CD40L is a co-stimulatory molecule on T cells; it interacts with CD40 on the surface of antigen-presenting cells and has an integral function in the T-B cell interaction that occurs during the development of a successful humoral immune response by promoting B cell proliferation, suppressing B cell apoptosis and initiating antibody class-switching. 14 In experimental autoimmune encephalomyelitis (EAE) models of MS, the loss of CD40-CD40L signaling have been shown to decrease the severity of the disease. 15 Soluble CD40L, which is a secreted form that originates from platelets and activated T cells, has been shown to increase disease severity in EAE and induce inflammation in the CNS by disrupting the BBB. 16 Serum sCD40L concentrations are positively correlated with the CSF/serum albumin ratio, which affects BBB permeability. 17 These mechanisms are likely clinically relevant due to the fact that sCD40L has been shown to be elevated in the CSF and serum of MS patients and was correlated with BBB permeability in this population.18-20 Interestingly, an allele of the receptor for sCD40L, the CD40 SNP rs4810485*T allele, increases the risk of MS and is associated with a lower serum level of IL-10, suggesting that genetic differences affecting the CD40-CD40L pathway may also contribute to MS predisposition. 21 Additionally, the DMTs glatiramer acetate, 22 IFN-β, 23 and natalizumab 24 were shown to reduce serum concentrations of sCD40L in MS, suggesting sCD40L levels may be a useful biomarker to monitor treatment efficacies. Further, our proof-of-concept phase I study using an anti-CD40L monoclonal antibody (mAb) (Toralizumab) was shown to be safe and feasible in an MS cohort. 25
Role of EGF in MS Remains Controversial
The role of epidermal growth factor (EGF) in MS remains controversial because it is associated with not only the growth and proliferation of CNS cells (i.e., neurons, astrocytes, and oligodendrocytes) but also neurotoxic immune responses. 26 Although we identified EGF as the top differential cytokine across cohorts, the secretion of EGF by various cell types complicates the interpretation of these differences. Circulating EGF levels have been implicated as a prognostic marker for other neurodegenerative diseases, 27 and EGF dysregulation is also associated with kidney disease, 28 suggesting that changes in EGF are not specific for MS and that levels may be influenced by the presence of comorbidities. Consequently, circulating EGF levels may be highly influenced by patient characteristics as well as heterogeneity within and across cohorts. This heterogeneity may explain the discrepancy concerning EGF levels with respect to our actively progressive SPMS cohort compared to those of a previous study that used a less defined progressive population combining PPMS with SPMS. 11
Role of MCP1/CCL2, Fractalkine/CX3CL1, and Eotaxin-1/CCL11 in MS Disease Progression
Chemokines mediate the migration of immune cells into the CNS 29 MCP1/CCL2 is a monocyte chemoattractant that has an important role in the migration of monocytes, activated T cells, and macrophages across the endothelium of blood vessels. 30 The up-regulation of MCP1/CCL2 during inflammation has been linked to the disruption of the BBB, 31 thereby inducing disease and relapse in EAE models.29,32,33 Consistent with our results, a previous comparison between RRMS and a progressive MS group showed a significant increase (P < .05) in serum levels of MCP1/CCL2 in the progressive disease group, suggesting that it may play a role in MS disease progression. 11
Both Fractalkine/CX3CL1 and Eotaxin-1/CCL11 showed significant age effects in our analysis. Fractalkine/CX3CL1 is a chemokine exhibiting chemoattractant effects on effector cells with cytotoxic function in the membrane-bound form, including natural killer (NK) cells and cytotoxic T cells. 34 In the CNS, the interaction between fractalkine/CX3CL1 and its corresponding receptor (CX3CR1) modulates microglial activation, and loss of fractalkine signaling is associated with neuroprotection in animal models. 35 However, it has been hypothesized that the soluble form of fractalkine may decrease the ability for leukocyte migration into the CNS to occur due to competitive binding with the corresponding receptor. 36 This could be consistent with our finding that soluble fractalkine/CX3CL1 levels tend to be higher in BMS than SPMS or RRMS. Furthermore, the decrease in soluble fractalkine levels with age may enhance neuroinflammatory processes that promote neurodegeneration in the CNS.
Eotaxin-1/CCL11, an eosinophil recruiting chemokine in allergic diseases, has recently garnered attention for its potential role in neurodegenerative diseases. 37 This chemokine has been shown to cross the BBB without disruption, where it could inhibit neurogenesis, causing reduction of cognitive function in mice.38,39 In the CNS, it has been shown that activated astrocytes could produce Eotaxin-1/CCL11, which increased microglial migration and production of reactive oxygen species, causing neuronal cell death. 40 Serum and plasma levels of Eotaxin-1/CCL11 have been previously described as being increased in SPMS compared to RRMS, which is consistent with our study. 41 The progression from RRMS to SPMS typically occurs in older adults as circulating levels of Eotaxin-1/CCL11 increase. 42 The increase in Eotaxin-1/CCL11 levels with age may then contribute to the neurodegenerative mechanisms of progression and promote the transition from RRMS to SPMS.
Summary
Our study shows that sCD40L can be used as a potential biomarker associated with disease progression in MS. This corroborates earlier studies23-25 which proposed that sCD40L along with IL-31 are important prognostic markers when analyzing MS progression. A limitation of our study is the small sample size. Despite this, some significant differences were seen. The results support the idea that CD40/CD40L can be used as a therapeutic target to control MS progression. In addition to sCD40L, our study also recognizes the important role that MCP1/CCL2 and IFNγ may play in different stages of MS progression (Figure 7). Future studies with a larger number of participants ought to confirm our findings. With better stratification of MS patient populations, clinical trials focused on actively progressing MS may be more effectively carried out, leading to improved treatments for these patients.
Supplemental Material
Supplemental Material, sj-pdf-1-cns-10.1177_11795735211050712 for Elevated sCD40L in Secondary Progressive Multiple Sclerosis in Comparison to Non-progressive Benign and Relapsing Remitting Multiple Sclerosis by Qi Wu, Qin Wang, Jennifer Yang, Jacob WS Martens, Elizabeth A Mills, Aiya Saad, Pavani Chilukuri, Catherine A Dowling and Yang Mao-Draayer in Journal of Central Nervous System Disease
Acknowledgments
We sincerely thank all our patients and family for participating in the study.
Author Contributions: Conducted experiments: QWu and QWang
Contributed to study design and analyzed data: QWu, QWang, JY, and YM-D
Served as clinical coordinators: AS and CDWrote the manuscript: QWu, QWang, EAM, JY, JWSM, PC, and YM-D
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: QWu, QWang, JY, JWSM, EAM, AS, PC, and CD have nothing to disclose. YM-D has served as a consultant and/or received grant support from Acorda, Bayer Pharmaceutical, Biogen Idec, Celgene/Bristol Myers Squibb, EMD Serono, Sanofi-Genzyme, Roche-Genentech, Janssen, Novartis, Questor, and Teva Neuroscience.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: YM-D is currently supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557-05, UM1 AI144298-01, Chugai, PCORI, Novartis, Sanofi-Genzyme, and Genentech.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
References
- 1.Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: A comprehensive review. J Autoimmun. 2015;64:13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019;26(1):27-40. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015;11(3):134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson A, Carlsson L, Gordh T, Lind A-L, Thulin M, Kamali-Moghaddam M. The effects of age and gender on plasma levels of 63 cytokines. J Immunol Methods. 2015;425:58-61. [DOI] [PubMed] [Google Scholar]
- 6.Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Mills EA, Wang Q, et al. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight. 2020;5(3):e134251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69-71. [PMC free article] [PubMed] [Google Scholar]
- 9.Biancotto A, Feng X, Langweiler M, Young NS, Philip McCoy J. Effect of anticoagulants on multiplexed measurement of cytokine/chemokines in healthy subjects. Cytokine. 2012;60(2):438-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng F, Harris RC. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev Biol. 2014;28:2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tejera-Alhambra M, Casrouge A, de Andrés C, et al. Plasma biomarkers discriminate clinical forms of multiple sclerosis. PLoS One. 2015;10(6):e0128952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bever CT, Panitch HS, Levy HB, McFarlin DE, Johnson KP. Gamma-interferon induction in patients with chronic progressive MS. Neurology. 1991;41(7):1124-1127. [DOI] [PubMed] [Google Scholar]
- 13.Maña P, Liñares D, Fordham S, Staykova M, Willenborg D. Deleterious role of IFNγ in a toxic model of central nervous system demyelination. Am J Pathol. 2006;168(5):1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarts SABM, Seijkens TTP, van Dorst KJF, Dijkstra CD, Kooij G, Lutgens E. The CD40-CD40L dyad in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol. 2017;8:1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda H, Mori M, Umehara K, et al. Soluble CD40 ligand disrupts the blood-brain barrier and exacerbates inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2018;316:117-120. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Mori M, Uchida T, Uzawa A, Ohtani R, Kuwabara S. Soluble CD40 ligand contributes to blood-brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J Neuroimmunol. 2017;305:102-107. [DOI] [PubMed] [Google Scholar]
- 18.Sosvorova L, Kanceva R, Vcelak J, et al. The comparison of selected cerebrospinal fluid and serum cytokine levels in patients with multiple sclerosis and normal pressure hydrocephalus. Neuroendocrinol Lett. 2015;36(6):564-571. PMID: 26812299. [PubMed] [Google Scholar]
- 19.Burman J, Svensson E, Fransson M, et al. The cerebrospinal fluid cytokine signature of multiple sclerosis: A homogenous response that does not conform to the Th1/Th2/Th17 convention. J Neuroimmunol. 2014;277(1-2):153-159. DOI: 10.1016/j.jneuroim.2014.10.005. Epub 2014 Oct 18. PMID: 25457841. [DOI] [PubMed] [Google Scholar]
- 20.Du L, Chang H, Wei Y, Zhang X, Yin L. Different roles of soluble CD40 ligand in central nervous system damage. Neurol Res. 2020;42(5):372-378. [DOI] [PubMed] [Google Scholar]
- 21.Smets I, Fiddes B, Garcia-Perez JE, et al. Multiple sclerosis risk variants alter expression of co-stimulatory genes in B cells. Brain. 2018;141(3):786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrieri PB, Carbone F, Perna F, et al. Longitudinal assessment of immuno-metabolic parameters in multiple sclerosis patients during treatment with glatiramer acetate. Metabolism. 2015;64(9):1112-1121. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero-García JdJ, Rojas-Mayorquín AE, Valle Y, et al. Decreased serum levels of sCD40L and IL-31 correlate in treated patients with relapsing-remitting multiple sclerosis. Immunobiology. 2018;223(1):135-141. [DOI] [PubMed] [Google Scholar]
- 24.Balasa RI, Simu M, Voidazan S, et al. Natalizumab changes the peripheral profile of the Th17 panel in MS patients: New mechanisms of action. CNS Neurol Disord Drug Targets. 2017;16(9):1018-1026. [DOI] [PubMed] [Google Scholar]
- 25.Fadul CE, Mao-Draayer Y, et al. Safety and immune effects of blocking CD40-ligand in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2021. Nov;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy YA, Fainberg KM, Amidror T, Regev K, Auriel E, Karni A. High and dysregulated secretion of epidermal growth factor from immune cells of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2013;257(1-2):82-89. [DOI] [PubMed] [Google Scholar]
- 27.Lim NS, Swanson CR, Cherng H-R, et al. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3(5):346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaka Y. Epidermal growth factor as a prognostic biomarker in chronic kidney diseases. Ann Transl Med. 2016;4(suppl 1):S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczuciński A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115(3):137-146. [DOI] [PubMed] [Google Scholar]
- 30.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29(6):313-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71(4):683-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192(6):899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the Cc chemokine receptor (Ccr2). J Exp Med. 2000;192(7):1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: A potential new target for inflammatory diseases. Mol Interv. 2010;10(5):263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917-924. [DOI] [PubMed] [Google Scholar]
- 36.Kastenbauer S, Koedel U, Wick M, Kieseier BC, Hartung HP, Pfister HW. CSF and serum levels of soluble fractalkine (CX3CL1) in inflammatory diseases of the nervous system. J Neuroimmunol. 2003;137(1-2):210-217. [DOI] [PubMed] [Google Scholar]
- 37.Huber AK, Giles DA, Segal BM, Irani DN. An emerging role for eotaxins in neurodegenerative disease. Clin Immunol. 2018;189:29-33. [DOI] [PubMed] [Google Scholar]
- 38.Erickson MA, Morofuji Y, Owen JB, Banks WA. Rapid transport of CCL11 across the blood-brain barrier: Regional variation and importance of blood cells. J Pharmacol Exp Therapeut. 2014;349(3):497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parajuli B, Horiuchi H, Mizuno T, Takeuchi H, Suzumura A. CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia. 2015;63(12):2274-2284. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci USA. 2020;117(23):12952-12960. DOI: 10.1073/pnas.1912839117. Epub 2020 May 26. PMID: 32457139; PMCID: PMC7293699.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoefer J, Luger M, Dal-Pont C, Culig Z, Schennach H, Jochberger S. The “aging factor” eotaxin-1 (CCL11) is detectable in transfusion blood products and increases with the donor’s age. Front Aging Neurosci. 2017;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cns-10.1177_11795735211050712 for Elevated sCD40L in Secondary Progressive Multiple Sclerosis in Comparison to Non-progressive Benign and Relapsing Remitting Multiple Sclerosis by Qi Wu, Qin Wang, Jennifer Yang, Jacob WS Martens, Elizabeth A Mills, Aiya Saad, Pavani Chilukuri, Catherine A Dowling and Yang Mao-Draayer in Journal of Central Nervous System Disease