Abstract
The complement system plays a key role in opsonization and immune clearance of engineered nanoparticles. Understanding the efficiency, inter-subject, and inter-strain differences of complement opsonization in preclinical species can help with translational nanomedicine development and improve our ability to model complement response in humans. Dextran-coated superparamagnetic iron oxide (SPIO) nanoparticles and a wide range of non-magnetic iron oxide nanoparticle formulations are widely used in magnetic resonance imaging and as clinically approved iron supplements. Previously we found that opsonization of SPIO nanoworms (NW) with the third complement protein (C3) proceeds mostly via the alternative pathway in humans, and via the lectin pathway in mice. Here, we studied the pathway and efficiency of opsonization of 106 nm SPIO NW with C3 in different preclinical species and commonly used laboratory strains. In sera of healthy human donors (n=6), C3 opsonization proceeded exclusively through the alternative pathway. On the other hand, the C3 opsonization in dogs (6 breeds), rats (4 strains) and mice (5 strains) sera was either partially or completely dependent on the complement Ca2+-sensitive pathways (lectin and/or classical). Specifically, C3 opsonization in sera of Long Evans rat strain, and mouse strains widely used in nanomedicine research (BALB/c, C57BL/6J, and A/J) was only through the Ca2+-dependent pathways. Dogs and humans had the highest between-subject variability in C3 opsonization levels, while rat and mouse sera showed the lowest between-strain variability. Furthermore, using a panel of SPIO nanoparticles of different sizes and dextran coatings, we found that the level of C3 opsonization (C3 molecules per milligram Fe) in human sera was lower than in animal sera. At the same time, there was a strong predictive value of complement opsonization in dog and rat sera; nanoparticles with higher C3 deposition in animals showed higher deposition in humans, and vice versa. Notably, the opsonization decreased with decreasing size in all sera. The studies highlight the importance of the consideration of species and strains for predicting human complement responses (opsonization) towards nanomedicines.
Keywords: complement, nanomedicine, species, strains, mice, dogs, rats, serum, iron oxide
Graphical Abstract

INTRODUCTION
Depending on the intended application, nanomedicine performance depends on the ability to interact with, or to avoid the immune system. Complement is the critical part of innate immunity playing a key role in defense against pathogens and synthetic nanoparticles. [1] Accordingly, activation of complement presents a significant factor determining the in vivo performance of nanomedicines. [2] Knowledge of the molecular interactions between complement and nanoparticles is essential for predicting in vivo nanoparticle blood longevity, biodistribution, stability, and biological action. Complement is the evolutionary conserved system of innate immunity and in mammalian species it is triggered via the lectin pathway (LP), through recognition of surface sugars by mannose binding lectins, collectins, and ficolins; the classical pathway (CP), through binding of immunoglobulins and C1q; or the alternative pathway (AP), through initial deposition of C3b via spontaneous tick-over or other mechanisms, including immunoglobulin binding. [3] The third complement protein (C3) is the central factor required for immune recognition via surface opsonization and initiation of down-stream responses. Thus, the exposure of a nanosurface to serum or plasma results in a covalent attachment of C3b, release of extremely potent anaphylatoxins C3a and C5a, and immune recognition of C3 (iC3b/C3c/C3d)-opsonized particles by complement receptors on leukocytes, erythrocytes, and macrophages. [4-7] The potential consequences are phagocytic clearance, and in case of uncontrolled complement activation, the proinflammatory responses and anaphylaxis. 1
A wide range of nanoparticles trigger complement activation in sera of mice, dogs, rats and pigs as well as in vivo following intravenous injection. [2, 8-10] However, there is a limited understanding whether complement activation by nanomedicines in such preclinical species can predict human response. For instance, pigs have lower levels of complement factors and lower activity of all pathways than humans. [11, 12] Complement functionality is also different between guinea pigs and humans, [13] and between mice and humans. [14-16] While the murine model has long been used for assessing nanomedicine pharmacokinetics and clearance by macrophages of the reticuloendothelial system, there are differences in complement opsonization of nanomedicines between mouse and human sera. For example, nanoparticle coating with poly(2-methyl-2-oxazoline) overcomes C3 opsonization and confers “stealthing” properties in the murine model in vitro and in vivo, but in human sera C3 opsonization proceeds rapidly resulting in recognition of nanoparticles by blood leukocytes and monocyte-derived macrophages. [17] Notwithstanding these studies, and considering the limitation with murine models, the inter-subject/inter-strain differences in complement activation of nanoparticles have not been investigated and remain a grand challenge in relation to the standardization of assays and techniques used in nanomedicine. [18] Particularly, which pre-clinical species mimic the C3 opsonization pathway and efficiency in humans? Answering this question will help develop better predictive models of complement response towards nanoparticles in health and disease.
Multiple nanoparticle types have been used to study complement activation, including carbon nanotubes, [19, 20] micelles, [21] liposomes, [22] polymeric nanospheres, [23, 24] iron oxide, [25] and gold. [26] Of these, iron oxide-based nanoparticles have a number of advantages. First, 3 types of superparamagnetic iron oxide (SPIO) nanoparticles and at least 8 non-magnetic iron oxide nanoparticles are among the few nanomaterials FDA approved for patients. [27] The medical success of these materials results from their immediate applications in magnetic resonance imaging and to treat anemia, ease of manufacture, low cost of synthesis, and safety profiles. 24 Second, preclinical and clinical nanoparticles exist in a variety of sizes and coatings, which greatly expands the choice of materials for studying nanoparticle-complement activation. Previously we found that C3 opsonization of a promising class of preclinical SPIO nanoparticles, termed SPIO nanoworms (SPIO NW), is highly variable in humans (albeit gender- and age-independent). [28] We also found that in humans SPIO NW activates complement via the AP, but in BALB/c and C57/BL6 mice complement activation proceeds through the LP. [29-31] Due to differences in SPIO NW-triggered complement response in human and mouse sera, we hypothesized that these nanoparticles could be used to probe differences in C3 opsonization between humans and various preclinical animal models. Here we expanded these preliminary studies to multiple preclinical species and strains using several dextran-coated SPIO , either clinically available, or prepared in our laboratory. [3, 28, 31-33] We found significant subject-dependent and strain-dependent differences in the C3 opsonization and conclude that only some of the tested strains display complement response similar to humans. These studies lay a groundwork for understanding the biological implications of complement opsonization of nanomedicines for translational events and improving nanomedicine reproducibility issues, at least with respect to complement responses.
MATERIALS AND METHODS
Nanoparticles
Ferumoxytol (Feraheme®) was obtained as sterile leftovers from the University of Colorado Cancer Center pharmacy. SPIO NWs (large and small) were synthesized in presence of 20kDa native dextran, Fe (III) chloride and Fe (II) chloride, and ammonia (all from Sigma-Aldrich) in accordance with previously published procedures. [30, 34] Dextran/Fe ratio in the reaction was varied to obtain nanoparticles of different sizes. [30, 34] Ultrasmall SPIO and ferumoxtran-10 nanoparticles were synthesized using 10kDa reduced or 10kDa native (T-10) dextrans (Pharmacosmos), according to the procedure described in US Patent 6,599,498 example 29 and US Patent 6,599,498 examples 3 and 23, respectively. In examples 3 and 23 in the respective patents, 20kDa dextran was substituted for 10kDa dextran. The scale of each preparation presented in Patent 6,599,498 was adjusted to fit the equipment of a standard academic research laboratory. The difference in reported nanoparticle sizes between the patent and Table 1 likely represents the use of different instruments for sizing. Nanoparticles were dialyzed against double distilled water, filtered through 0.22 μm and stored at 4°C. Endotoxin level was measured with Pierce Limulus Amebocyte Lysate (LAL) assay (Thermo Fisher). The size and zeta potential of nanoparticles were determined at 25°C in 1:50 diluted PBS buffer in DDW using Zetasizer Nano ZS (Malvern Instruments).
Table 1.
Characterization of SPIO used in the study (average of 3 measurements). Nanoparticle concentration was either measured with NanoSight (large SPIO NW) or calculated theoretically, as described. [28] PDI, polydispersity index. All particle solutions were at 1 mg Fe/mL and had less than 0.2 EU/mL endotoxin as measured with Limulus amebocyte lysate assay.
| Particle name | Composition | Size (intensity weighted), nm | PDI | Zeta potential, mV |
|---|---|---|---|---|
| Large SPIO NW | 20kDa native dextran, multiple crystals per particle | 106 (Z-average), 126 ± 54.42 (peak) | 0.16 | −7.4 ± 6.8 |
| Small SPIO NW | 20kDa native dextran, multiple crystals per particle | 67 (Z-average), 81.36 ± 40.35 (peak) | 0.22 | −6.8 ± 8.8 |
| Ferumoxytol (Feraheme ®) | 10kDa reduced carboxymethyl dextran, 1 crystal per particle | 26 (Z-average), 26.86 ± 10.53 (peak) | 0.27 | −15.6 ± 7.4 |
| Ultrasmall SPIO (USPIO) | 10kDa reduced T-10 dextran SPIO, 1 crystal per particle | 26 (Z-average), 25.96 ± 7.64 (peak) | 0.23 | −5.0 ± 10.0 |
| Ferumoxtran-10 | 10kDa native T-10 dextran SPIO, 1-3 crystals per particle | 44 (Z-average), 51.19 ± 20.75 (peak) | 0.14 | −7.2 ± 9.5 |
Antibodies and ELISA kits
Dog complement C3 ELISA kit was from KAMIYA Biomedical Company (Seattle, WA, USA). Rat C3 ELISA Kit was from Crystal Chem (ELK Grove Village, IL, USA). Goat anti-dog C3 (Catalog No. A40-109A) was from Bethyl Laboratories (Montgomery, TX, UA), goat IgG fraction to mouse C3 (Catalog No. 55463) and goat IgG fraction to rat C3 (Catalog No. 55730) were from MP Biomedicals (Solon, OH, USA), goat anti-human C3 (Catalog No. A213) was from Complement Technology (Tyler, TX, USA), rabbit anti-pig polyclonal IgG (Catalog No. CAU26928) was from Biomatik Corporation (Kitchener, ON, Canada), and rabbit anti-pig polyclonal IgG (Catalog No. LS-C372631) was from LifeSpan Biosciences (Seattle, WA, USA). Secondary antibodies IRDye 800CW labeled donkey anti-goat, donkey anti-rabbit and goat anti-mouse were from LI-COR Biosciences (Lincoln, NE, USA).
Serum collection and preparation
Sera were collected by separation from clotted blood as described previously.[15, 35] Human whole blood were obtained in Vacutainer® Z (no anticoagulant) from consented healthy donors at the Children’s Hospital Colorado Blood Donor Center under the Center’s Institutional Review Board protocol for anonymous collection. Only age and gender were made available to the investigators. Sera from the canine patients were collected in Vacutainer® Z in accordance with the Colorado State University Clinical Review Board’s standard of care and with informed client consent. Canine patients were chosen randomly from dogs presenting to the Colorado State University Flint Animal Cancer Center. Patients were excluded if they were on any immunosuppressants, including steroids. The owners of the dog patients were consented. Breed and sex were the only data made available to investigators. Rat serum was collected in plastic Eppendorf tubes without anticoagulant from excess colonies at the University of Colorado Anschutz Medical Campus. The following rat strains were used: Brown Norway/Crl, Wistar, Fischer 344/NHsd, Long Evans (parent strain), and Sprague Dawley (parent strain). Mouse serum was collected according to the same protocol without anticoagulant from excess colonies at the University of Colorado Anschutz Medical Campus (BALB/c, C57BL/6J, DBA/2J) or purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The vendor used the same protocol for serum collection as in our laboratory.
Dot blot assay and C3 quantification
The assays were performed as described by us before. [3, 28, 36] Briefly, nanoparticles (1mg/ml Fe) were added to undiluted serum at 1:2 volume ratio (as opposed to 1:3 ratio in our previous studies due to serum availability), incubated for 30 min at 37°C, purified with ultracentrifugation and blotted on a nitrocellulose membrane at 1 μg Fe/dot. The C3 binding was detected with corresponding primary and secondary antibodies. For human C3 quantification, the purified human C3 standard was used. For dog and rat C3 quantification, the concentration of C3 in serum was first determined using corresponding dog and rat C3 ELISA. These sera were used for preparing standard dilutions. The known concentrations of C3 were dotted along with the samples and the number of C3 per mg Fe was calculated.
Statistical analysis
The variability between subjects/strains relative to the total variability in relative C3 levels after exposure to SPIO NW was calculated using the coefficient of determination (R2) from a one-way ANOVA. To examine differences in relative C3 levels in the presence of EDTA and EGTA, a linear mixed model (technical replicates in humans and dogs; biological replicates in mice and rats) was used to estimate treatment means and to compare between treatments. For comparison between species of absolute C3 levels, within species/nanoparticle medians were used to calculate correlation between species using a Spearman’s rank-order correlation. All statistical analyses and heatmaps were generating using R (version 4.0.4) and several other graphics and student’s t-tests were generated using GraphPad Prism (version 9.0.2, San Diego, CA, USA).
RESULTS
1. Pathway and variability of C3 opsonization of SPIO NW
Core-shell large SPIO NW (106 nm diameter, −7.4mV ζ-potential) were described by our group before (Table 1). [31, 37] These particles showed predominantly the LP activation in BALB/c and C57/BL6 mice, and the AP activation in humans. [29, 30] Unlike many other complement activation markers, anti-C3 antibodies for many species are commercially available. We used a quantitative dot-blot to determine the number of bound C3 and the pathway of C3 deposition on nanoparticles, [3, 28] and C3 cleavage products by Western blotting (Fig. 1A).
Fig. 1. Analysis of SPIO NW opsonization pathways.
A) Experimental workflow. Nanoparticles were incubated in serum, washed, and C3 deposition was analyzed by Western blot and quantitative dot blot; B) Three complement pathways converge into C3 cleavage and nanoparticle opsonization by C3 fragments (C3b/iC3b/C3c/C3d). AP is fully functional in the presence of 10mM EGTA/Mg2+, whereas Ca2+-sensitive pathways are inhibited. All pathways are fully inhibited by 10mM EDTA; C) Schematic diagram of C3 cleavage fragments. The dot blot assay does not distinguish between fragments; D) Western blot analysis of native C3 in sera and nanoparticle-deposited C3 in different species. C3 was detected by species-specific anti-C3 antibodies. Lane 1: serum 1:200 dilution (native C3 - control); Lane 2: SPIO NW after incubation in serum; Lane 3; SPIO NW after incubation in serum/EDTA; Lane 4: SPIO NW after incubation in serum/EGTA/Mg2+. All antibodies were able to clearly detect intact α-chain (115Da) and β-chain (75kDa) in serum, β-chain and α’2 (43kDa) on the particles. Some other α-chain fragments (e.g., α’1-chain) are likely to be in the high molecular weight fraction bound to other proteins via amide or ester bonds, and therefore could not be identified by their molecular weight.
A decrease or lack of C3 deposition in the presence of EGTA/Mg2+ usually suggests that the C3 deposition is due to the LP and/or CP, while no decrease suggests a predominant role for the AP. On the other hand, incomplete inhibition in the presence of EDTA suggests pathway-independent binding, likely due to a non-specific binding of the metastable “C3b-like” molecule C3(H2O) [38, 39] (Fig. 1B). First, we incubated SPIO NW in mouse (female, BALB/c), dog (male, mix breed), rat (female, Sprague Dawley) and human (male, 48 year old) sera, washed the particles and used Western blotting to detect intact C3 and cleaved C3 fragments (Fig. 1C). All anti-C3 antibodies detected intact α- and β-chains in native sera, and deposition of β-chain and cleaved α’2-chain on nanoparticles in human, dog, rat, and mouse sera (Fig. 1D). The presence of the α’2-chain and the absence of the intact 120kDa α-chain in all species suggest convertase-dependent cleavage of C3 into C3b and further proteolytic processing of surface-deposited C3b by Factor I/H to generate iC3b, C3dg and C3d (Fig. 1C). [40] Other fragments could not be identified by the molecular weight, since α’1-chain and its cleavage products are likely to be bound to other adsorbed proteins on nanoparticle surface via amide or ester bond. [35] While we observed either no effect or minimal inhibition by EGTA/Mg2+ in human, dog and rat sera, BALB/c serum showed complete inhibition by EGTA/Mg2+ (Fig. 1D). These data validate the anti-C3 antibodies for detection of C3 fragments on nanoparticles in all tested sera, and suggests some differences in C3 opsonization pathways between humans and animal species.
To investigate between-subject and between-strain variability in the activation pathway, and in the level of opsonization, we performed quantitative analysis of nanoparticle-bound C3 in sera collected from 6 healthy human donors (n=3 females, 3 males), 5 strains of mice (inbred BALB/c, n=4; inbred C57BL/6J, n=4; inbred DBA/2J, n=4; outbred J:ARC(S), n=4, inbred A/J, n=4), 4 strains of rats (outbred Sprague Dawley (SD), n=7; outbred Long Evans (LE), n=4; inbred Fischer 344 (F344), n=5; inbred Brown Norway (BN), n=4), and 6 patient dogs admitted to the Animal Cancer Center at Colorado State University (female Pitbull mix, male and female mix breed, female Golden Retriever, male English Springer Spaniel, and male Husky). According to Fig. 2A, pre-treatment of human sera with EGTA/Mg2+ did not inhibit C3 deposition, confirming the AP-driven complement activation. On the other hand, C3 opsonization in dogs’ sera was inhibited by 30–70% in the presence of EGTA/Mg2+, suggesting an important role for the involvement of Ca2+-dependent (LP and/or CP) pathways (Fig. 2A). The complement response in rats and mice, however, was more stochastic.
Fig. 2. C3 opsonization and variability of SPIO NWs in multiple species and strains.
A) From top to bottom: human, dog, rat and mouse sera. Left: bar graphs show levels of C3 (raw signal intensity). Right: heat maps of C3 levels (relative intensities within species) with statistical significance between means. Each heat map corresponds to the bar graph in the same row. In humans, letter and number denominate gender and age, respectively. Dogs: 1, female Pitbull mix; 2, male mix; 3, female Golden Retriever; 4, male English Springer Spaniel; 5, male Husky; and 6, female mix. Rats: SD, Sprague Dawley; LE, Long Evans; F344, Fischer 344; and BN, Brown Norway. The human and dog graphs and heat maps show means of 3 technical replicates per donors, and rat and mouse bar graphs and heat maps show means of multiple animals per strain. Details of statistical analysis in Methods; B) Western blot of C3 deposition in LE rat serum; C) Schematic representation of differences in C3 opsonization of SPIO NW between humans and preclinical species. Humans shows predominant AP activation by SPIO NW, whereas mice show predominant Ca2+-sensitive pathway activation. Dogs and rats have a mixed contribution of Ca2+-sensitive and AP.
While SD, BN and F344 rats sera showed 30-35% inhibition in C3 opsonization by EGTA/Mg2+, LE rats showed 93% inhibition (Fig. 2A) suggesting that the activation of Ca2+-sensitive pathways are predominantly responsible for C3 opsonization in this strain. This result was also confirmed with Western blotting (Fig. 2B). In mice, BALB/c, C57BL/6J and J ARC strains showed 80-90% inhibition, A/J showed 60-80% inhibition, and DBA/2J showed 15-30% inhibition by EGTA/Mg2+ (Fig. 2A) suggesting a highly variable role of the Ca2+-sensitive pathways in mice. The analysis of the mean effect of EGTA/Mg2+ in each species (heat maps in Fig. 2A) showed that in humans there was a significant increase in the C3 opsonization, while all other species showed a significant decrease in the C3 opsonization. This observation demonstrates the predominantly AP-dependent C3 opsonizations in humans, whereas the pathway of activation in other species is variable with only some strains and breeds exhibiting complement activation pathway similarity to humans with respect to SPIO NWs (Fig. 2C).
The analysis of between-subject and between-strain variability showed significant differences between strains/donors in C3 opsonization. Thus, dogs showed highest variability relative to total variability (R2 = 0.91, p<0.0001), followed by humans (R2 = 0.89, p<0.0001), rats (R2 = 0.69, p = 0.0004), and mice (R2=0.73, p = 0.0004).
2. C3 opsonization efficiency of different SPIO formulations in species/strains
The efficiency of C3 opsonization is one of the factors that determines the efficiency of immune clearance of nanomaterials. [31, 41-43] We previously found that the extent of C3 opsonization (C3 molecules per nanoparticle and C3 molecules per milligram) in sera of BALB/c mouse and humans correlates with nanoparticle diameter. [28, 34] To test whether this observation extends to multiple subjects, species and strains, we used a panel of SPIO nanoparticles of different sizes and surface dextran coatings [28, 34] (Fig. 3A, Table 1).
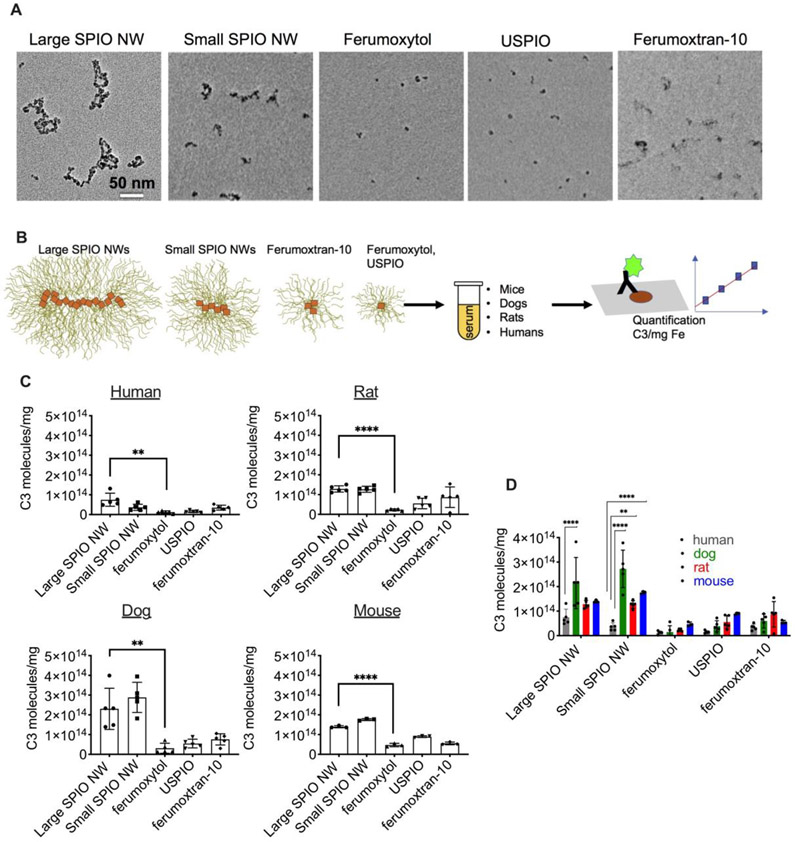
Fig. 3. Comparison of C3 opsonization (C3 molecules/mg Fe) of different SPIO formulations across species.
A) Transmission electron microscopy of the nanoparticles used in the study. Size bar 50nm for all images; B) Dot blot assay was performed along with the standard dilutions of C3 along with the samples to calculate number of deposited C3 molecules (described in Methods). The assay does not distinguish between different fragments; therefore all are referred as deposited C3; C) Mean values of C3 per mg Fe for designated particles, measured in humans (n=5 donors), dogs (n=5 breeds), rats (n=5 strains) and mice (n=3 strains). Large SPIO NW show significantly more C3 deposition than ferumoxytol (non-paired non-parametric t-test); D) Comparison of C3 deposition across species (2-way ANOVA with multiple comparisons).
These included: 1) 20kDa native dextran, 106 nm SPIO NW (large SPIO NW) that was used in the studies above; 2) 20kDa native dextran, 67 nm SPIO NW (small SPIO NW); 3) 10kDa native dextran, 44 nm small SPIO (ferumoxtran-10 (similar to Combidex® [44, 45]); 4) 10kDa reduced dextran, 26 nm monocrystalline ultrasmall SPIO (USPIO); and 5) 10kDa carboxymethyl reduced dextran, 26 nm monocrystalline USPIO (ferumoxytol (Feraheme®)). We used sera from 5 healthy human donors (3 females, 2 males), 5 rat strains (SD, BN, Wistar, LE, and F344), 5 dog donors (female Pitbull mix, male and female mix breed, female golden retriever, and male English springer spaniel) and 3 mouse strains (BALB/c, C57BL/6J and DBA/2J, all males). To compare C3 deposition on nanoparticles between humans and species, we calculated the number of deposited C3 molecules, using standard curves of C3 from each species, per mg Fe (Fig. 3B). The number of C3/mg Fe for ferumoxytol was significantly lower than for large SPIO NW in all sera, and was the lowest among all nanoparticles tested (Fig. 3C). The number of deposited C3/mg Fe for large SPIO NW was significantly lower in human sera than in dog sera, and for small SPIO NW it was significantly lower in human sera than in dog, rat, and mouse sera (Fig. 3D). The deposition of C3 on smaller nanoparticles (USPIO, ferumoxytol and ferumoxtran-10) was also lower in human than in animal sera, but this was not statistically significant (Fig. 3D). The correlation analysis of C3/mg for all particle types showed a significant linear correlation for rats vs humans (Fig. 4A, correlation coefficient 0.9; p=0.017). On the other hand, correlation for rats vs humans was linear but non-significant (Fig. 4B, correlation coefficient 0.9; p=0.083). Correlation for mice vs humans was also linear but non-significant (Fig. 4C, correlation coefficient 0.8; p=0.13). The analysis of C3 quantity on nanoparticles with non-charged dextran coating (excluding ferumoxytol) as a function of hydrodynamic diameter showed an increase in C3 deposition with the increase of nanoparticle diameter in all species (Fig. 4D). At the same time, human sera showed less steep slope (Fig. 4D) pointing to a somewhat less efficient complement activation as was observed in Fig. 3.
Fig. 4. Correlation of C3 deposition on nanoparticles between animals and humans.
Data presented in Figure 3 for different nanoparticles were used to plot correlations: A) Between rat vs. human serum; B) Between dog vs. human serum; C) between mouse vs. human serum. Only rats show significant positive correlation with humans in terms of the efficiency of opsonization; D) The number of C3/mg for nanoparticles (excluding ferumoxytol due to charged carboxymethyl dextran) increases with increasing diameter in all species. Each point is the mean of 5 donors/strains (except mice - 3 strains).
DISCUSSION
Considering recent concerns about reproducibility challenges in nanomedicine research and validation of animal models,15 the main objective for this study was to determine which preclinical species/strains display correlation with humans in terms of the activation pathway and variability of C3 opsonization. We chose preclinical and clinical dextran-coated iron oxide nanoparticles because of our expertise in their chemistry, biological behavior and toxicities, and the clinical and translational significance of these nanomaterials. For example, ferumoxtran-10 was used in clinical trials for metastatic cancer detection in lymph nodes, [46] whereas ferumoxytol is clinically approved for treatment of anemia and off label for MRI imaging in patients. [47] While the pig model has been extensively used for research on complement activation of nanomedicines, [48] we could not identify commercially available anti-pig antibody that reliably detects C3 fragments, therefore we did not include any specie of pigs in this study.
We found significant differences between human and animal sera in the pathways of complement opsonization/activation of large SPIO NW (Fig. 2C). In humans, the AP was the predominant pathway, whereas in dogs and rats, there was a significant dependency on the Ca2+-sensitive pathways. Among the tested rat strains, LE was an outlier as it showed almost no activation of the AP. Importantly, several mouse strains used in nanomedicine research (BALB/c, C57BL/6J, A/J) showed the critical dependency on Ca2+-sensitive pathways, and lack of robust AP activation. The strain-dependent difference in the activation pathways is intriguing and is something the nanomedicine community should pay close attention to. Our previous studies showed that large SPIO NW activated mouse complement in BALB/c and C57BL/6J sera via the LP. [29] It is not clear if SPIO NW incite the LP in other mouse strains, as well as in dogs and rats, but it is likely, given the polysaccharide coating of the nanoparticles. Some strains are known to be deficient or have decreased levels of complement factors (e.g., DBA/2J being deficient in C5 [49]). There is also a significant genetic drift occurring in inbred strains leading to differences from the original parent strains. Due to the genetic complexity and environmental factors modulating complement activation, [3, 28, 50-52] the mechanisms of the observed differences in activation pathways are not addressed in this study.
We also found higher between-subject variability in dogs and humans than between-strain variability in rats and mice. While we used both inbred and outbred mouse and rat strains, it is obvious that humans and dogs possess much higher genetic and environmental diversity, which could explain these differences. Also, the efficiency of activation (number of bound C3 molecules) was highest in dogs, and lowest in humans. There was a trend of decreasing in the number of bound C3 for particles with decreasing nanoparticle size, suggesting that particles that elicit higher response in animals are expected to trigger higher response in humans. Although the differences in surface properties such as dextran chain density, length, and orientation [45, 53] could determine the nanoparticle aggregation state and protein corona composition, [3, 54] and as a consequence modulate complement activation, the curvature remains an important constraint for the surface accommodation and functional operation of C3 convertases. [25, 28]
Given that all the tested animal species displayed similarities and differences with humans in C3 opsonization of SPIO nanoparticles, our conclusion is that for translational nanomedicine research, the choice of animal model needs to be carefully considered when studying complement activation and responses. While the pathway and opsonization efficiency in dog sera did not exactly match human sera with respect to iron oxide nanoparticles, dogs are a good preclinical model in the development of many therapeutics and diagnostics due to their relative similarity in immunocompetence and genetics to humans. [55] Complement activation by nanoparticles has been previously documented in canine patients. [56] Although it might not be possible to capture the variability inherent to humans using a single breed, the canine patients who present for medical care, and whose owners elect to have them as part of a clinical trial, has been previously successfully employed. [57-59] On the other hand, while rats and mice are more convenient, less expensive and readily available, caution should be exercised in the choice of a strain.
In conclusion, the observed differences in complement responses towards nanoparticles necessitate careful consideration of species and strains for nanomedicine studies that involve prediction of complement responses in humans.
Supplementary Material
ACKNOWLDEGMENTS
The study was supported by NIH grants R01EB022040 and R01AI154959 to D.S., R01AR51749 to NKB (Co-I), and R24 AA013162 to L.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ricklin D, Hajishengallis G, Yang K, Lambris JD, Complement: a key system for immune surveillance and homeostasis, Nature immunology, 11 (2010) 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Szebeni J, Simberg D, Gonzalez-Fernandez A, Barenholz Y, Dobrovolskaia MA, Roadmap and strategy for overcoming infusion reactions to nanomedicines, Nat Nanotechnol, 13 (2018) 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, Scheinman R, Saba L, Moghimi SM, Simberg D, Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles, Nat Nanotechnol, 14 (2019) 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Helmy KY, Katschke KJ Jr., Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M, CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens, Cell, 124 (2006) 915–927. [DOI] [PubMed] [Google Scholar]
- [5].Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S, Macrophage receptors and immune recognition, Annu. Rev. Immunol, 23 (2005) 901–944. [DOI] [PubMed] [Google Scholar]
- [6].Peng Q, Li K, Sacks SH, Zhou W, The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses, Inflammation & allergy drug targets, 8 (2009) 236–246. [DOI] [PubMed] [Google Scholar]
- [7].Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC, Material properties in complement activation, Adv Drug Deliver Rev, 63 (2011) 1000–1007. [DOI] [PubMed] [Google Scholar]
- [8].Moghimi SM, Simberg D, Translational gaps in animal models of human infusion reactions to nanomedicines, Nanomedicine (Lond), 13 (2018) 973–975. [DOI] [PubMed] [Google Scholar]
- [9].Moghimi SM, Simberg D, Papini E, Farhangrazi ZS, Complement activation by drug carriers and particulate pharmaceuticals: Principles, challenges and opportunities, Adv Drug Deliv Rev, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J, Methylation of the phosphate oxygen moiety of phospholipid-methoxy(polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production, Faseb J, 20 (2006) 2591–2593. [DOI] [PubMed] [Google Scholar]
- [11].Salvesen B, Mollnes TE, Pathway-specific complement activity in pigs evaluated with a human functional complement assay, Mol Immunol, 46 (2009) 1620–1625. [DOI] [PubMed] [Google Scholar]
- [12].Sakai R, Kitano E, Hatanaka M, Lo P, Matsuura R, Deguchi K, Eguchi H, Maeda A, Watanabe M, Matsunari H, Nagashima H, Okuyama H, Miyagawa S, Studies of Pig Complement: Measurement of Pig CH50, ACH50, and Components, Transplant Proc, 48 (2016) 1282–1284. [DOI] [PubMed] [Google Scholar]
- [13].Zhang Y, Suankratay C, Zhang X, Jones DR, Lint TF, Gewurz H, Calcium-independent haemolysis via the lectin pathway of complement activation in the guinea-pig and other species*, Immunology, 97 (1999) 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ratelade J, Verkman AS, Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica, Mol. Immunol, 62 (2014) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lachmann PJ, Preparing serum for functional complement assays, Journal of immunological methods, 352 (2010) 195–197. [DOI] [PubMed] [Google Scholar]
- [16].Ebanks RO, Isenman DE, Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity, Mol Immunol, 33 (1996) 297–309. [DOI] [PubMed] [Google Scholar]
- [17].Tavano R, Gabrielli L, Lubian E, Fedeli C, Visentin S, Polverino De Laureto P, Arrigoni G, Geffner-Smith A, Chen F, Simberg D, Morgese G, Benetti EM, Wu L, Moghimi SM, Mancin F, Papini E, C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes, ACS nano, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leong HS, Butler KS, Brinker CJ, Azzawi M, Conlan S, Dufes C, Owen A, Rannard S, Scott C, Chen C, Dobrovolskaia MA, Kozlov SV, Prina-Mello A, Schmid R, Wick P, Caputo F, Boisseau P, Crist RM, McNeil SE, Fadeel B, Tran L, Hansen SF, Hartmann NB, Clausen LPW, Skjolding LM, Baun A, Agerstrand M, Gu Z, Lamprou DA, Hoskins C, Huang L, Song W, Cao H, Liu X, Jandt KD, Jiang W, Kim BYS, Wheeler KE, Chetwynd AJ, Lynch I, Moghimi SM, Nel A, Xia T, Weiss PS, Sarmento B, das Neves J, Santos HA, Santos L, Mitragotri S, Little S, Peer D, Amiji MM, Alonso MJ, Petri-Fink A, Balog S, Lee A, Drasler B, Rothen-Rutishauser B, Wilhelm S, Acar H, Harrison RG, Mao C, Mukherjee P, Ramesh R, McNally LR, Busatto S, Wolfram J, Bergese P, Ferrari M, Fang RH, Zhang L, Zheng J, Peng C, Du B, Yu M, Charron DM, Zheng G, Pastore C, On the issue of transparency and reproducibility in nanomedicine, Nat Nanotechnol, 14 (2019) 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB, Complement activation and protein adsorption by carbon nanotubes, Mol Immunol, 43 (2006) 193–201. [DOI] [PubMed] [Google Scholar]
- [20].Andersen AJ, Robinson JT, Dai H, Hunter AC, Andresen TL, Moghimi SM, Single-walled carbon nanotube surface control of complement recognition and activation, ACS nano, 7 (2013)1108–1119. [DOI] [PubMed] [Google Scholar]
- [21].Hamad I, Hunter AC, Moghimi SM, Complement monitoring of Pluronic 127 gel and micelles: Suppression of copolymer-mediated complement activation by elevated serum levels of HDL, LDL, and apolipoproteins AI and B-100, J Control Release, 170 (2013) 167–174. [DOI] [PubMed] [Google Scholar]
- [22].Devine DV, Wong K, Serrano K, Chonn A, Cullis PR, Liposome-complement interactions in rat serum: implications for liposome survival studies, Biochim Biophys Acta, 1191 (1994) 43–51. [DOI] [PubMed] [Google Scholar]
- [23].Borchard G, Kreuter J, The role of serum complement on the organ distribution of intravenously administered poly (methyl methacrylate) nanoparticles: effects of pre-coating with plasma and with serum complement, Pharm Res, 13 (1996) 1055–1058. [DOI] [PubMed] [Google Scholar]
- [24].Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM, Distinct Polymer Architecture Mediates Switching of Complement Activation Pathways at the Nanosphere-Serum Interface: Implications for Stealth Nanoparticle Engineering, ACS nano, 4 (2010) 6629–6638. [DOI] [PubMed] [Google Scholar]
- [25].Pedersen MB, Zhou X, Larsen EK, Sorensen US, Kjems J, Nygaard JV, Nyengaard JR, Meyer RL, Boesen T, Vorup-Jensen T, Curvature of synthetic and natural surfaces is an important target feature in classical pathway complement activation, J Immunol, 184 (2010) 1931–1945. [DOI] [PubMed] [Google Scholar]
- [26].Dobrovolskaia MA, Patri AK, Zheng J, Clogston JD, Ayub N, Aggarwal P, Neun BW, Hall JB, McNeil SE, Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles, Nanomedicine, 5 (2009) 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anselmo AC, Mitragotri S, Nanoparticles in the clinic, Bioeng Transl Med, 1 (2016) 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Benasutti H, Wang G, Vu VP, Scheinman R, Groman E, Saba L, Simberg D, Variability of Complement Response toward Preclinical and Clinical Nanocarriers in the General Population, Bioconjug Chem, 28 (2017) 2747–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Banda NK, Mehta G, Chao Y, Wang G, Inturi S, Fossati-Jimack L, Botto M, Wu L, Moghimi S, Simberg D, Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum, Particle and fibre toxicology, 11 (2014) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang G, Chen F, Banda NK, Holers VM, Wu L, Moghimi SM, Simberg D, Activation of Human Complement System by Dextran-Coated Iron Oxide Nanoparticles Is Not Affected by Dextran/Fe Ratio, Hydroxyl Modifications, and Crosslinking, Front Immunol, 7 (2016) 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang G, Griffin JI, Inturi S, Brenneman B, Banda NK, Holers VM, Moghimi SM, Simberg D, In Vitro and In Vivo Differences in Murine Third Complement Component (C3) Opsonization and Macrophage/Leukocyte Responses to Antibody-Functionalized Iron Oxide Nanoworms, Front Immunol, 8 (2017) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Inturi S, Wang G, Chen F, Banda NK, Holers VM, Wu L, Moghimi SM, Simberg D, Modulatory Role of Surface Coating of Superparamagnetic Iron Oxide Nanoworms in Complement Opsonization and Leukocyte Uptake, ACS nano, 9 (2015) 10758–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chao Y, Makale M, Karmali PP, Sharikov Y, Tsigelny I, Merkulov S, Kesari S, Wrasidlo W, Ruoslahti E, Simberg D, Recognition of dextran-superparamagnetic iron oxide nanoparticle conjugates (Feridex) via macrophage scavenger receptor charged domains, Bioconjugate chemistry, 23 (2012) 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang G, Serkova NJ, Groman EV, Scheinman RI, Simberg D, Feraheme (Ferumoxytol) Is Recognized by Proinflammatory and Anti-inflammatory Macrophages via Scavenger Receptor Type AI/II, Molecular pharmaceutics, 16 (2019) 4274–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen F, Wang G, Griffin JI, Brenneman B, Banda NK, Holers VM, Backos DS, Wu L, Moghimi SM, Simberg D, Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo, Nat Nanotechnol, 12 (2017) 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gifford G, Vu VP, Banda NK, Holers VM, Wang G, Groman EV, Backos D, Scheinman R, Moghimi SM, Simberg D, Complement therapeutics meets nanomedicine: overcoming human complement activation and leukocyte uptake of nanomedicines with soluble domains of CD55, Journal of controlled release : official journal of the Controlled Release Society, 302 (2019) 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang G, Inturi S, Serkova NJ, Merkulov S, McCrae K, Russek SE, Banda NK, Simberg D, High-Relaxivity Superparamagnetic Iron Oxide Nanoworms with Decreased Immune Recognition and Long-Circulating Properties, ACS nano, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN, The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb), Mol Immunol, 45 (2008) 2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Klapper Y, Hamad OA, Teramura Y, Leneweit G, Nienhaus GU, Ricklin D, Lambris JD, Ekdahl KN, Nilsson B, Mediation of a non-proteolytic activation of complement component C3 by phospholipid vesicles, Biomaterials, 35 (2014) 3688–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Manning ML, Williams SA, Jelinek CA, Kostova MB, Denmeade SR, Proteolysis of complement factors iC3b and C5 by the serine protease prostate-specific antigen in prostatic fluid and seminal plasma, J Immunol, 190 (2013) 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gaikwad H, Li Y, Gifford G, Groman E, Banda NK, Saba L, Scheinman R, Wang G, Simberg D, Complement Inhibitors Block Complement C3 Opsonization and Improve Targeting Selectivity of Nanoparticles in Blood, Bioconjugate chemistry, 31 (2020) 1844–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kullberg M, Martinson H, Mann K, Anchordoquy TJ, Complement C3 mediated targeting of liposomes to granulocytic myeloid derived suppressor cells, Nanomedicine, 11 (2015)1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Francian A, Mann K, Kullberg M, Complement C3-dependent uptake of targeted liposomes into human macrophages, B cells, dendritic cells, neutrophils, and MDSCs, Int J Nanomedicine, 12 (2017) 5149–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, Oram DH, Jacobs IJ, Shepherd JH, Reznek RH, Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer, J. Clin. Oncol, 23 (2005) 2813–2821. [DOI] [PubMed] [Google Scholar]
- [45].Jung CW, Jacobs P, Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil, Magn. Reson. Imaging, 13 (1995) 661–674. [DOI] [PubMed] [Google Scholar]
- [46].Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R, Noninvasive detection of clinically occult lymph-node metastases in prostate cancer, N. Engl. J. Med, 348 (2003) 2491–2499. [DOI] [PubMed] [Google Scholar]
- [47].Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP, Gahramanov S, Harisinghani M, Macdougall I, Neuwelt A, Vasanawala SS, Ambady P, Barajas R, Cetas JS, Ciporen J, DeLoughery TJ, Doolittle ND, Fu R, Grinstead J, Guimaraes AR, Hamilton BE, Li X, McConnell HL, Muldoon LL, Nesbit G, Netto JP, Petterson D, Rooney WD, Schwartz D, Szidonya L, Neuwelt EA, Current and potential imaging applications of ferumoxytol for magnetic resonance imaging, Kidney Int., 92 (2017) 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Szebeni J, Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity, Toxicology, 216 (2005) 106–121. [DOI] [PubMed] [Google Scholar]
- [49].Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SW, Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice, J Neuroinflammation, 10 (2013) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Harris CL, Heurich M, Rodriguez de Cordoba S, Morgan BP, The complotype: dictating risk for inflammation and infection, Trends Immunol, 33 (2012) 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Heurich M, Martinez-Barricarte R, Francis NJ, Roberts DL, Rodriguez de Cordoba S, Morgan BP, Harris CL, Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk, Proc Natl Acad Sci U S A, 108 (2011) 8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gaya da Costa M, Poppelaars F, van Kooten C, Mollnes TE, Tedesco F, Wurzner R, Trouw LA, Truedsson L, Daha MR, Roos A, Seelen MA, Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population, Front Immunol, 9 (2018) 2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jung CW, Surface properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil, Magn. Reson. Imaging, 13 (1995) 675–691. [DOI] [PubMed] [Google Scholar]
- [54].Chao Y, Karmali PP, Mukthavaram R, Kesari S, Kouznetsova VL, Tsigelny IF, Simberg D, Direct recognition of superparamagnetic nanocrystals by macrophage scavenger receptor SR-AI, ACS nano, 7 (2013) 4289–4298. [DOI] [PubMed] [Google Scholar]
- [55].Tarone L, Barutello G, Iussich S, Giacobino D, Quaglino E, Buracco P, Cavallo F, Riccardo F, Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy, Cancer Immunol Immunother, 68 (2019) 1839–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Robinson K, Platt S, Bibi K, Banovic F, Barber R, Howerth EW, Madsen G, A Pilot Study on the Safety of a Novel Antioxidant Nanoparticle Delivery System and Its Indirect Effects on Cytokine Levels in Four Dogs, Front Vet Sci, 7 (2020) 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].De Clercq E, Tanovea (R) for the treatment of lymphoma in dogs, Biochem Pharmacol, 154 (2018) 265–269. [DOI] [PubMed] [Google Scholar]
- [58].Schlein LJ, Fadl-Alla B, Pondenis HC, Lezmi S, Eberhart CG, LeBlanc AK, Dickinson PJ, Hergenrother PJ, Fan TM, Immunohistochemical Characterization of Procaspase-3 Overexpression as a Druggable Target With PAC-1, a Procaspase-3 Activator, in Canine and Human Brain Cancers, Front Oncol, 9 (2019) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zinnen SP, Karpeisky A, Von Hoff DD, Plekhova L, Alexandrov A, First-in-Human Phase I Study of MBC-11, a Novel Bone-Targeted Cytarabine-Etidronate Conjugate in Patients with Cancer-Induced Bone Disease, Oncologist, 24 (2019) 303–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.