Abstract
Background
The ideal intravenous fluid for kidney transplantation has not been defined, despite the common use of normal saline during the peri‐operative period. The high chloride content of normal saline is associated with an increased risk of hyperchloraemic metabolic acidosis, which may in turn increase the risk of hyperkalaemia and delayed graft function. Balanced electrolyte solutions have a lower chloride content which may decrease this risk and avoid the need for dialysis due to hyperkalaemia in the immediate post‐transplant period. Randomised controlled trials (RCTs) addressing this issue have used biochemical outcomes to compare fluids and have been underpowered to address patient‐centred outcomes such as delayed graft function.
Objectives
To examine the effect of lower‐chloride solutions versus normal saline on delayed graft function, hyperkalaemia and acid‐base status in kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant's Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
RCTs of kidney transplant recipients that compared peri‐operative intravenous lower‐chloride solutions to normal saline were included.
Data collection and analysis
Two independent investigators assessed studies for eligibility and risk of bias. Data from individual studies were extracted using standardised forms and pooled according to a published protocol. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) and 95% CI for continuous outcomes.
Main results
Six studies (477 participants) were included in the review. All participants were adult kidney transplant recipients and 70% of participants underwent live‐donor kidney transplantation. The overall risk of bias was low for selection bias and unclear for remaining domains. There was no difference in the risk of delayed graft function (3 studies, 298 participants: RR 1.03, 95% CI 0.62 to 1.70) or hyperkalaemia (2 studies, 199 participants: RR 0.48, 95% CI 0.04 to 6.10) for participants who received balanced electrolyte solutions compared to normal saline. Intraoperative balanced electrolyte solutions compared to normal saline were associated with higher blood pH (3 studies, 193 participants: MD 0.07, 95% CI 0.05 to 0.09), higher serum bicarbonate (3 studies, 215 participants: MD 3.02 mEq/L, 95% CI 2.00 to 4.05) and lower serum chloride (3 studies, 215 participants: MD ‐9.93 mmol/L, 95% CI ‐19.96 to 0.11). There were four cases of graft loss in the normal saline group and one in the balanced electrolyte solution group, and four cases of acute rejection in the normal saline group compared to two cases in the balanced electrolyte solution group.
Authors' conclusions
Balanced electrolyte solutions are associated with less hyperchloraemic metabolic acidosis compared to normal saline, however it remains uncertain whether lower‐chloride solutions lead to improved graft outcomes compared to normal saline.
Plain language summary
Normal saline versus lower‐chloride solutions for kidney transplantation
What is the issue?
People with kidney failure may have a kidney transplant to replace the function of their own kidneys. During a kidney transplant operation, patients receive fluids through their veins to keep them hydrated. Maintaining good hydration helps the transplanted kidney to work after the operation. The choice of fluids that are given during and after the operation may have an effect on how the transplant kidney works after surgery and on the patient's acid‐base measures in the blood.
Normal saline is a type of fluid that is commonly given during an operation. It contains a high chloride level. Giving a kidney transplant patient normal saline might increase the acid level of the blood compared to giving the patient fluids that contain less chloride. High blood acid levels might be associated with high blood potassium levels, which is dangerous for the heart and often requires dialysis to correct.
What did we do?
We performed a systematic review to address the question of whether giving lower‐chloride fluids compared to normal saline during the kidney transplant operation alters the early function of the kidney, the number of patients with high blood potassium levels, and the acid level in the blood after the operation. We included studies that were published up to November 26, 2015.
What did we find?
We found six studies that included 477 kidney transplant patients. The majority of these patients had a kidney transplant from a living donor. The overall quality of the studies was low to average, and the main problem was the small number of studies and the small size of the studies. There was no information on funding source for most of the studies.
Compared to normal saline, giving kidney transplant patients solutions that contain less chloride during their transplant operation resulted in lower blood acid levels but did not affect how the transplant kidney worked after surgery, or the number of patients who had high blood potassium levels. Harmful effects were not reported in many studies. In the group of patients who were given lower‐chloride fluids, the transplant failed in one patient and one patient rejected the transplant. In the group of patients who were given normal saline, the transplant failed in four patients, and two patients rejected the transplant. However, this is probably an incomplete picture of harmful effects.
Background
Description of the condition
End‐stage kidney disease (ESKD) is a major cause of morbidity and mortality worldwide and was estimated to affect 1738 per million population in the USA in 2009, with 72% of these undergoing maintenance haemodialysis (Collins 2012). Australian registry data from 2010 estimated that 850 per million population receive renal replacement therapy, comprising of both dialysis patients and kidney transplant recipients (ANZDATA 2011). Kidney transplantation offers a significant survival benefit compared to dialysis and is the treatment of choice for the majority of patients. However, transplantation is limited by the number of available donor organs, and strategies that improve short‐ and long‐term graft function are important in the effective utilisation of this resource.
Delayed graft function has been variably defined as a failure of the serum creatinine to fall by 20% within the first 72 hours post‐transplantation, or a requirement for dialysis within seven days post‐transplant (Yarlagadda 2008). Delayed graft function has been associated with poorer short‐ and long‐term outcomes, including prolonged hospitalisation, higher transplantation costs, increased risk of acute rejection and decreased five‐year graft survival (Yarlagadda 2009). Delayed graft function is dependent on multiple factors, including donor type, comorbidities, and ischaemic time. In addition, the peri‐operative fluid status of the recipient and the regimen of fluid therapy are a possible consideration. While it is well accepted that maintaining adequate peri‐operative fluid volume facilitates early graft function (Schnuelle 2006), the type of fluid that is administered varies substantially between transplant units.
Description of the intervention
Normal saline, or 0.9% saline, contains 154 mmol/L of sodium chloride and is a widely accessible and commonly used fluid in kidney transplantation. A survey of United States transplant units demonstrated that normal saline is the most commonly used peri‐operative fluid for kidney transplantation (O'Malley 2002). However, there are concerns that the high chloride content of this solution leads to a hyperchloraemic metabolic acidosis (Handy 2008; Roche 2007; Scheingraber 1999). In response to this acidosis, potassium is released into the extracellular space in exchange for hydrogen ions as a compensatory mechanism (Halperin 1998), which may lead to the development of hyperkalaemia (O'Malley 2005). Hyperkalaemia is an indication for dialysis post‐transplant and may compromise the cardiovascular stability of the transplant recipient; in particular by increasing the risk of hyperkalaemia associated cardiac arrhythmias (Halperin 1998). Furthermore, the administration of normal saline has been associated with renal vasoconstriction and decreased kidney perfusion (Chowdhury 2012; Wilcox 1983), as well as an increased risk of acute kidney injury in critical care and general surgical populations (Yunos 2012; Shaw 2012). In the setting of kidney transplantation, these findings may support the hypothesis that normal saline could increase the risk of delayed graft function.
Lower‐chloride solutions refer to fluids with a lower chloride content compared to normal saline, and include both balanced electrolyte solutions and colloids. Balanced electrolyte solutions refer to crystalloid fluids that contain a more physiological level of chloride as well as bicarbonate precursors, and include fluids such as compound sodium lactate and Plasma‐lyte®. These fluids have been advocated in the setting of kidney transplantation as they are thought to limit the development of hyperchloraemic metabolic acidosis and subsequent hyperkalaemia, despite the fact that they contain potassium at a physiological concentration (Table 1). However, others caution the use of these fluids due to the risk of hyperkalaemia resulting from the potassium present in these fluids (Schnuelle 2006).
1. Electrolyte content of common fluids.
|
|
Crystalloids | ||||||
| Lower‐chloride solutions | |||||||
| Balanced electrolyte solutions | Colloids | ||||||
| Normal (0.9%) saline | Dextrose 5% | Plasma‐Lyte ® | Elo‐Mel isoton ® | CSL/Ringer's lactate | Albumin 4% | Gelatins | |
| Na+ (mmol/L) | 154 | 0 | 140 | 140 | 131 | 140 | 145 |
| K+ (mmol/L) | 0 | 0 | 5 | 5 | 4‐5 | 0 | 4‐5 |
| Cl‐ (mmol/L) | 154 | 0 | 98 | 108 | 112 | 128 | 120‐145 |
| HCO3‐ (mmol/L) | 0 | 0 | 50 (27 as acetate, 23 as gluconate) |
45 (as acetate) |
28 (as lactate) |
0 | 0 |
| Osmolarity (mOsm/L) | 310 | 252 | 297 | 302 | 255 | 250 | 284 |
Na+ ‐ sodium concentration; Cl‐ ‐ chloride concentration; CSL ‐ compound sodium lactate; HCO3‐ ‐ bicarbonate concentration; K+ ‐ potassium concentration
Why it is important to do this review
There have been a number of small randomised controlled trials (RCT) that have compared normal saline to certain balanced electrolyte solutions. However, the majority have assessed acid‐base measures as a primary outcome and were underpowered to address clinical endpoints such as delayed graft function or hyperkalaemia requiring dialysis (Hadimioglu 2008; Khajavi 2008; O'Malley 2005). In addition, none have addressed long‐term outcomes of graft or patient survival.
Objectives
The aims of this review were to compare normal saline to lower‐chloride containing solutions, in particular balanced electrolyte solutions and colloids, as fluid therapy in the acute peri‐transplant period.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that compared normal saline to lower‐chloride solutions without language restriction.
Types of participants
We included adults and children who received first or subsequent deceased‐donor or living‐donor kidney transplants.
Types of interventions
We included studies that compared intravenous normal saline to lower‐chloride solutions during the intraoperative and immediate postoperative period following kidney transplantation. There was no restriction on the volume or rate of fluid delivery.
Lower‐chloride solutions included:
Balanced electrolyte solutions: compound sodium lactate (also known as Hartmann’s solution or Ringer's lactate), Plasma‐lyte® and Elo‐Mel isoton®
Other crystalloids: dextrose 5%, dilutions of normal saline
Colloids: albumin 4%, gelatins (including Gelofusine® and Haemaccel®) and hydroxyethyl starches in balanced electrolytes (including Hextend®).
We excluded studies that compared fluids types to pharmacological agents (e.g. mannitol, dopamine, frusemide), or blood transfusion. In addition, we excluded colloid fluids that were made up in normal saline as they have the same chloride content as normal saline (e.g. hydroxyethyl starches in normal saline).
Specific comparisons were made between:
Normal saline and balanced electrolyte solutions
Normal saline and colloids
Normal saline and all lower‐chloride solutions.
Types of outcome measures
Primary outcomes
Delayed graft function, defined as the need for dialysis within seven days of kidney transplant surgery or failure of the serum creatinine to fall by 20% within 72 hours.
Clinically significant hyperkalaemia, defined as serum potassium > 5.5 mmol/L, or any hyperkalaemia requiring treatment (e.g. with dialysis, calcium gluconate, insulin, B₂ agonists, or ion‐exchange resins) within the first 72 hours post‐transplant.
Secondary outcomes
Acid‐base status, which was measured as the mean difference in blood pH, serum potassium concentration, serum chloride concentration, and serum bicarbonate concentration at the end of surgery compared to baseline (pre‐operative) and at day three compared to baseline (pre‐operative).
Adverse events, including death, graft loss, or cardiovascular events.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines, review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
We used the search strategy described to obtain titles and abstracts of studies relevant to the review. Two authors independently screened titles and abstracts, and discarded studies that were not applicable; however studies and reviews thought to include relevant data or information were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text of these studies, to determine which satisfied the inclusion criteria.
Data extraction and management
Two independent authors extracted the data using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of a study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any disagreement between authors regarding study selection or data extraction was resolved by discussion and referral to a third author where necessary.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes (e.g. delayed graft function and hyperkalaemia) were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. blood pH, serum potassium, chloride and bicarbonate concentrations), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. Adverse effects were assessed with descriptive techniques.
Dealing with missing data
Further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. Evaluation of data, including intention‐to‐treat, losses to follow‐up and withdrawals were investigated, and issues of missing data were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, as well as with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality). Heterogeneity among participants could be related to age, comorbidities, living versus deceased donor transplants, extended versus standard criteria donor kidneys, number of HLA mismatches, cold‐ischaemia time greater or less than 12 hours, and type of immunosuppression. Adverse effects were tabulated and assessed with descriptive techniques. Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Results
Description of studies
Results of the search
The process of study selection is outlined in Figure 1. We identified 20 potentially eligible citations through database searching and one through personal communication. Twenty‐one citations (19 studies) were retrieved for full‐text review. Cittanova 1996 and Pang 2011 each had two citations, one of which was a peer‐reviewed journal article, and the other was a conference abstract. We excluded 11 studies after full‐text review. One study (Nuraei 2010) is awaiting translation in order to be evaluated further, and one study is an RCT that is currently ongoing (ACTRN12612000023853). This left six studies for inclusion in the review. There were no disagreements between independent authors regarding any step of the review.
1.
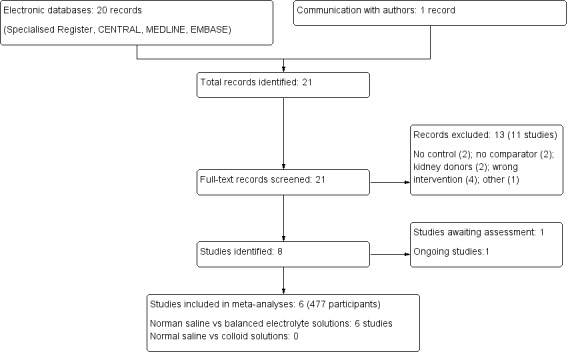
Study flow diagram
Included studies
Six studies (477 participants) were included in the review. Detailed descriptions of participant characteristics and study design are provided in the Characteristics of included studies table. O'Malley 2005 included both living and deceased‐donor kidney transplant recipients, Potura 2015 included deceased‐donor kidney transplant recipients only, and the remaining four studies included living‐donor kidney transplant recipients only. Hadimioglu 2008 compared normal saline with lactated ringers solution and Plasma‐lyte® in a three‐arm design, Potura 2015 compared normal saline with the balanced electrolyte solution Elo‐Mel isoton®, and the remainder compared normal saline with lactated ringers. Five out of six studies administered the study fluids during the intra‐operative period only, and the remaining study (Potura 2015) continued the study fluid until discharge from the anaesthetic recovery room. Two studies administered buffered crystalloid solution to all patients in the post‐operative period (Kim 2013; O'Malley 2005), and one study (Kim 2013) administered 5% albumin intra‐operatively to all patients. There were no RCTs comparing normal saline to colloids, dextrose or dilutions of normal saline. Therefore, the only comparisons that were made were between balanced electrolyte solutions (lactated ringers solution, Plasma‐lyte® and Elo‐Mel isoton®) and normal saline.
Excluded studies
Eleven studies were excluded from the review: three studies had no normal saline control arm (Dai 2011; Dawidson 1987; Wu 2010), two studies were of kidney donors (Cittanova 1996; Mertens zur Borg 2008), three studies investigated different rates or volumes of fluid administration (Hatch 1985; Magpantay 2011; Othman 2010), the intervention was a pharmacological agent in two studies (Pang 2011; Starke 2012), and one study had no comparator arm (Abdallah 2014). Further information is provided in the Characteristics of excluded studies table.
Studies awaiting classification
Nuraei 2010 is a quasi‐RCT comparing lactated ringers solution to normal saline in kidney transplant recipients. This study is awaiting translation in order to be classified.
Ongoing studies
ACTRN12612000023853 is an ongoing RCT comparing balanced electrolyte solutions to normal saline in adult kidney transplant recipients.
Risk of bias in included studies
A summary of the risk of bias assessment is provided in Figure 2 and Figure 3. The overall risk of bias was low for selection bias and unclear for the remaining domains.
2.
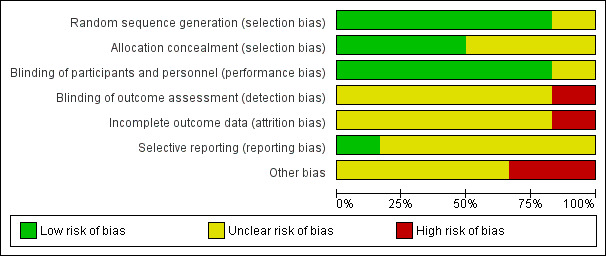
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
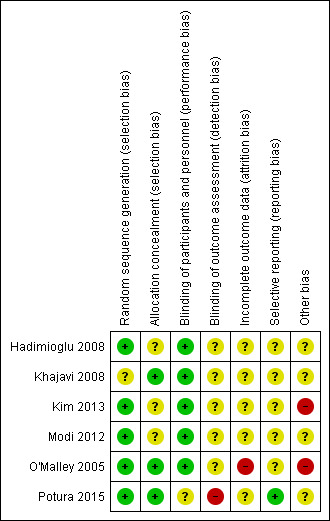
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five studies adequately performed and reported on random sequence generation (Hadimioglu 2008; Kim 2013; Modi 2012; O'Malley 2005; Potura 2015), with no information provided in the remaining study.
Three studies (Khajavi 2008; O'Malley 2005; Potura 2015) reported adequate allocation concealment techniques, with no information provided in the remaining three studies.
Blinding
Five studies reported on blinding for clinicians and participants, which was achieved by covering fluid bags with opaque tape (O'Malley 2005, Khajavi 2008, Modi 2012), or by the preparation of unlabelled fluid bags (Hadimioglu 2008; Kim 2013). Potura 2015 did not report on blinding of clinicians and participants, and no study reported on blinding for outcome or data assessors.
Incomplete outcome data
Only one study reported on loss to follow‐up (Potura 2015) and one study (Kim 2013) reported on dropout rate. The intention‐to‐treat principle was not adhered to in two studies; Potura 2015 excluded two patients from the balanced electrolyte solutions arm after randomisation due to "unsuitable vessels", and O'Malley 2005 excluded three patients after randomisation due to pre‐operative hyperkalaemia. No further information on these three patients was provided, including which groups they were randomised to. In the remaining four studies there was no information on whether or not intention‐to‐treat analysis was used.
Selective reporting
Selective reporting was unclear for the majority of studies. Potura 2015 was the only study registered with a clinical trials registry with a study protocol describing outcomes. The outcomes in the protocol were the same as the published study. The remaining studies did not have a protocol to assessment selective reporting.
Other potential sources of bias
Kim 2013 and O'Malley 2005 administered a buffered crystalloid solution to all patients in the post‐operative period, exposing the control group to a balanced electrolyte solution. Potura 2015 was the only study with a conflict of interest declaration and reported no industry sources of funding. The remaining studies did not provide information on funding sources.
Effects of interventions
Primary outcomes
Delayed graft function
Three studies reported on delayed graft function (Hadimioglu 2008; Kim 2013; Potura 2015). There was no difference in the risk of delayed graft function between participants receiving intra‐operative balanced electrolyte solutions compared with normal saline (Analysis 1.1 (3 studies, 298 participants): RR 1.03, 95% CI 0.62 to 1.70; I2 = 0%).
1.1. Analysis.
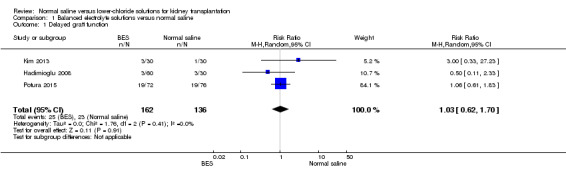
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 1 Delayed graft function.
Hyperkalaemia
Two studies reported on hyperkalaemia (O'Malley 2005; Potura 2015). There was no difference in the risk of hyperkalaemia in patients receiving balanced electrolyte solutions compared to normal saline (Analysis 1.2 (2 studies, 199 participants): RR 0.48, 95% CI 0.04 to 6.10). There was moderate heterogeneity for this outcome (I2 = 69%).
1.2. Analysis.
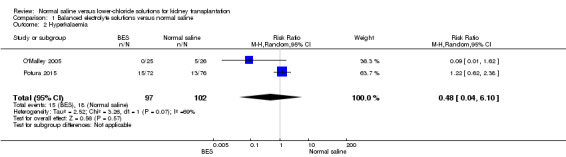
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 2 Hyperkalaemia.
Secondary outcomes
Acid‐base status
Three studies reported pH at end of surgery (Hadimioglu 2008; Khajavi 2008; O'Malley 2005). The mean blood pH was an average of 0.07 units higher in patients who received balanced electrolyte solutions compared to normal saline (Analysis 1.3 (3 studies, 193 participants): MD 0.07 units, 95% CI 0.05 to 0.09; I2 = 9%).
1.3. Analysis.
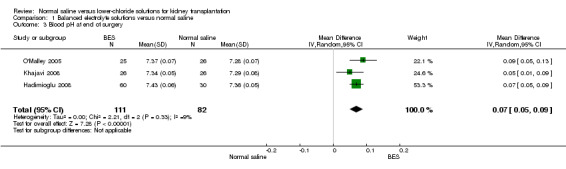
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 3 Blood pH at end of surgery.
Three studies reported serum bicarbonate at the end of surgery (Hadimioglu 2008; Modi 2012; O'Malley 2005). The mean serum bicarbonate was 3.02 mEq/L higher in the balanced electrolyte solutions group compared to the normal saline group (Analysis 1.4; (3 studies, 215 participants): MD 3.02 mEq/L, 95% CI 2.00 to 4.05; I2 = 21%).
1.4. Analysis.
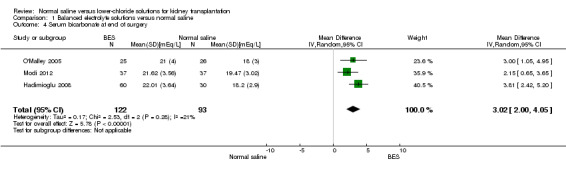
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 4 Serum bicarbonate at end of surgery.
Patients who received balanced electrolyte solutions had a mean chloride concentration 9.93 mmol/L lower at the end of surgery compared to the normal saline group (Analysis 1.5 (3 studies, 215 participants): MD ‐9.93 mmol/L, 95% CI ‐19.96 to 0.11) (Hadimioglu 2008; Modi 2012; O'Malley 2005). Heterogeneity was high (I2 = 99%)
1.5. Analysis.
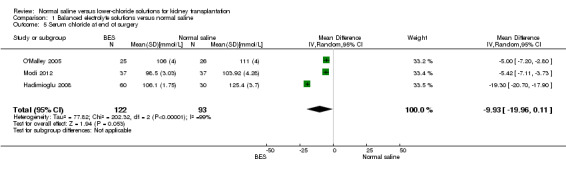
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 5 Serum chloride at end of surgery.
Four studies reported serum potassium at the end of surgery (Hadimioglu 2008; Khajavi 2008; Modi 2012; O'Malley 2005). There was no difference serum potassium in those who received balanced electrolyte solutions compared to normal saline (Analysis 1.6 (4 studies, 267 participants): MD ‐0.24 mmol/L, 95% CI ‐0.51 to 0.04). Heterogeneity was again high for this outcome (I2 = 70%).
1.6. Analysis.
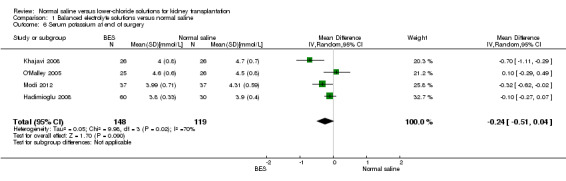
Comparison 1 Balanced electrolyte solutions versus normal saline, Outcome 6 Serum potassium at end of surgery.
Adverse events
Adverse events were reported by two studies. O'Malley 2005 reported graft loss in two patients from the normal saline group and one patient from the balanced electrolyte solutions group; however the timeframe for this loss was not reported. In addition, four episodes of biopsy‐proven acute rejection in the normal saline group and two in the balanced electrolyte solutions group were reported in this study. Khajavi 2008 reported two patients with acute renal artery thrombosis in the balanced electrolyte solutions group and none in the normal saline group.
Subgroup analysis
We performed subgroup analysis for living‐donor kidney transplant recipients for the outcome of delayed graft function; however this did not substantially alter the pooled result (data not shown). Subgroup analysis was not performed for the remaining pre‐specified groups or outcomes due to the small number of studies and events and the lack of data on these subgroups. In particular, there was only one study of deceased‐donor kidney transplant recipients.
Sensitivity analysis
Exclusion of studies that administered buffered normal saline solutions to all patients in the post‐operative period (Kim 2013; O'Malley 2005) resulted in a significantly lower mean serum potassium in the balanced electrolyte solutions group compared to the normal saline group (MD ‐0.33 mmol/L, 95% CI ‐0.65 to ‐0.01). We were unable to perform sensitivity analysis for the outcome of delayed graft function and hyperkalaemia due to the small study numbers, and further sensitivity analyses for the remaining outcomes did not change the magnitude or direction of effect.
Discussion
Summary of main results
Intra‐operative balanced electrolyte solutions were associated with a higher pH and bicarbonate level, and lower chloride concentration at the end of surgery compared to normal saline in patients undergoing kidney transplantation. There was no significant difference in the risk of delayed graft function or hyperkalaemia, and no difference in the mean serum potassium concentration between groups. These findings support the view that normal saline is associated with more hyperchloraemic metabolic acidosis than balanced electrolyte solutions; however the implications of this on clinical outcomes remain unclear.
Overall completeness and applicability of evidence
Nearly 70% of all participants included in this review underwent living‐donor kidney transplantation, and 98% of deceased‐donor transplant participants were included from one study (Potura 2015). Recipients of live‐donor kidneys are at low risk for delayed graft function because the main determinants of this are cold ischaemia time and donor age, which are usually optimised in the live‐donor setting. Since delayed graft function occurs less frequently in live‐ compared to deceased‐donor transplantation, there may be potential for a greater magnitude of effect to be seen in deceased‐donor transplant recipients, who represent a more high‐risk group. Nevertheless, even in this high risk group, no difference in the risk of delayed graft function was demonstrated in this review. This may be because other factors such as ischaemic time and donor age are stronger determinants of delayed graft function, and play a more important role than the type of intra‐operative fluid used. Subgroup analysis stratifying for delayed graft function risk would be useful to examine whether the choice of intra‐operative fluids might be more important in higher risk groups.
The available evidence assessed the impact of intravenous fluids in the intra‐operative setting only, a period that typically lasts two to four hours. However, during the immediate 48 to 72 hours post‐transplantation, large volumes of intravenous fluid are commonly administered to transplant recipients. Clinical outcomes of delayed graft function, hyperkalaemia and acid‐base status are therefore likely to be affected by the choice of post‐operative as well as intra‐operative fluids, and studies that assess post‐operative intravenous fluid choice during kidney transplant are required.
Individual studies described the administration of intra‐operative fluids to achieve a target central venous pressure, however, no study reported on the total volume of fluid delivered to study participants. The total volume of fluid is likely to influence the total chloride load and the development of hyperchloraemic metabolic acidosis, and may account for some of the heterogeneity seen. Future studies addressing this question should report on total fluid volume delivered.
Quality of the evidence
The overall quality of evidence included in this review was low to moderate. Randomisation and blinding of the included studies was adequate overall, however the reporting of important outcomes such as delayed graft function and hyperkalaemia was poor and could introduce reporting bias into this review. The low adverse event rates may reflect under‐reporting of these outcomes, although serious adverse events such as death or graft failure are uncommon in living‐donor transplant recipients, who were the majority of the review participants. The duration of follow‐up for all included studies was very short, and ranged from one day to six months post‐surgery. This did not allow for any assessment of long‐term clinical outcomes such as graft failure or patient survival.
Two of the studies in this review (Kim 2013; O'Malley 2005) administered buffered crystalloid solutions to all patients in the post‐operative period in the form of a dilution of normal saline with bicarbonate added. This may have diminished any effect of hyperchloraemic metabolic acidosis in the normal saline group, causing the pH, bicarbonate, chloride and potassium levels to be more similar between the two groups, and potentially leading to a bias towards the null. However, given that the time point for acid‐base measurements did not go beyond the end of surgery, it is unclear how these post‐operative buffered solutions might bias results. Nevertheless, when sensitivity analysis was performed excluding these studies, the magnitude of effect for mean difference in serum potassium between the two groups increased, and became significant in favour of balanced electrolyte solutions. This may also explain some of the heterogeneity seen in the analysis of serum potassium and chloride concentrations at the end of surgery.
Potential biases in the review process
Meta‐analysis remains retrospective research that is subject to the risks of bias of the included studies. The main limitation of this review is the low number of studies identified, and the resultant small sample size and low event rate. Significant publication bias cannot be excluded and is difficult to assess due to the small number of studies. In addition we were unable to translate one study (Nuraei 2010) in order to assess it for eligibility. However, we minimised the likelihood of bias by developing a detailed protocol prior to commencing this study, performing a meticulous and exhaustive search for published studies, and utilising explicit methodology for study selection, data extraction and data analysis.
Agreements and disagreements with other studies or reviews
This is the only systematic review to our knowledge that examines the effect of balanced electrolyte solutions compared to normal saline on clinically relevant outcomes in kidney transplant recipients. It agrees with recent reviews in the peri‐operative and critical care settings that report an association between normal saline and hyperchloraemic metabolic acidosis (Krajewski 2014; Myburgh 2013) without an increase in clinically important adverse effects (Krajewski 2014).
Authors' conclusions
Implications for practice.
Intra‐operative administration of balanced electrolyte solutions compared to normal saline is associated with less hyperchloraemic metabolic acidosis in kidney transplant recipients. Therefore, use of these solutions should be considered in patients at high risk of metabolic acidosis. However it remains uncertain whether lower‐chloride solutions lead to improved graft outcomes compared to normal saline.
Implications for research.
The current data mainly reflect live‐donor kidney transplant recipients, whereas the risk of delayed graft function is most relevant to the deceased‐donor kidney transplant population. High‐quality studies that assess deceased‐donor kidney transplant recipients are therefore required. In addition, further studies should evaluate intravenous fluids delivered during the post‐operative as well as intra‐operative period, and report on total volume of fluid delivered, while assessing clinically important outcomes such as delayed graft function and hyperkalaemia.
Acknowledgements
The authors wish to thank Dr Laurence Weinberg for reviewing and assisting with the manuscript. We also acknowledge the support of the Department of Nephrology at the Austin Hospital, Melbourne, as well as the assistance of the Cochrane Kidney and Transplant Editors and referees, and the Information Specialist.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID SP) |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Balanced electrolyte solutions versus normal saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Delayed graft function | 3 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.62, 1.70] |
| 2 Hyperkalaemia | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 6.10] |
| 3 Blood pH at end of surgery | 3 | 193 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.05, 0.09] |
| 4 Serum bicarbonate at end of surgery | 3 | 215 | Mean Difference (IV, Random, 95% CI) | 3.02 [2.00, 4.05] |
| 5 Serum chloride at end of surgery | 3 | 215 | Mean Difference (IV, Random, 95% CI) | ‐9.93 [‐19.96, 0.11] |
| 6 Serum potassium at end of surgery | 4 | 267 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.51, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hadimioglu 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Post‐operative fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer randomization program was used for patient group assignments." Page 264, paragraph 4 |
| Allocation concealment (selection bias) | Unclear risk | Information on allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study solutions were prepared in unlabeled bags by the hospital pharmacy. Patients and clinicians were blinded to group assignments." Page 264, paragraph 4 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific information on masking of outcome and data assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on intention‐to‐treat principle or missing outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol |
| Other bias | Unclear risk | No information on funding reported |
Khajavi 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Post‐operative fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was reported |
| Allocation concealment (selection bias) | Low risk | "Randomization was achieved using sealed envelopes." Page 536, paragraph 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The fluid bags were covered with tape so that the personnel and clinicians do not have any idea of the type of the fluid administered." Page 536, paragraph 6 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific information on masking of outcome and data assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on intention‐to‐treat principle or missing outcome date reported |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol |
| Other bias | Unclear risk | No information on funding reported |
Kim 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
Post‐operative fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "One day before transplant surgery, patients were assigned to either the NS group or the Plasmalyte group according to a random number sequence." Page 2192, paragraph 3 |
| Allocation concealment (selection bias) | Unclear risk | Information on allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study solutions were prepared in unlabeled bags by a staff nurse who was not involved in the study. Attending anesthesiologists and surgeons were blinded to group assignments." Page 2191, paragraph 3 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific information on masking of outcome and data assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on intention‐to‐treat principle or missing outcome date reported. However, there were no drop‐outs from the study |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol |
| Other bias | High risk | All patients received intra‐operative albumin 5% as well as postoperative 0.45% normal saline with 5 mmol/L KCl and 7 mmol/L HCO3. This could lead to non‐differential misclassification error as the control group was also exposed to a balanced electrolyte solution. No information on funding reported |
Modi 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Post‐operative fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer randomization program was used for patient group assignments." Page 135, paragraph 2 |
| Allocation concealment (selection bias) | Unclear risk | Information on allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The solutions were supplied by our hospital pharmacy after completely covering each bag with opaque tape to ensure blinding to study personnel and patients." Page 135, paragraph 2 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific information on masking of outcome and data assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on intention‐to‐treat principle or missing outcome date reported |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol |
| Other bias | Unclear risk | Published as a letter to the editor only. No information on funding reported |
O'Malley 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Post‐operative fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved by computer generation of random number lists..." Page 1519, paragraph 2 |
| Allocation concealment (selection bias) | Low risk | "Randomization was achieved by computer generation of random number lists, in blocks of four, and a closed envelope technique." Page 1519, paragraph 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The investigational pharmacy completely covered each bag of study fluid with opaque tape to ensure blinding of all study personnel and clinicians to the fluid type." Page 1520, paragraph 1 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Specific information on masking of outcome and data assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The intention‐to‐treat principle was not adhered to. 54 patients were randomised and data from 51 patients were analysed. 3 patients were excluded after randomisation due to preoperative hyperkalaemia. Page 1520, paragraph 7 |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol |
| Other bias | High risk | All patients received 5% dextrose/0.45% normal saline with 20 mEq/L HCO3. This could lead to non‐differential misclassification error as the control group was also exposed to a balanced electrolyte solution. No information on funding reported |
Potura 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Post‐operative (ward) fluid
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐based randomization was performed using sealed envelopes." Page 124, paragraph 4 |
| Allocation concealment (selection bias) | Low risk | "Computer‐based randomization was performed using sealed envelopes." Page 124, paragraph 4 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "We chose to perform an open label study...the patient is already sedated before start of surgery and then is totally anesthetized." Page128, paragraph 4 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "We chose to perform an open label study..." Page128, paragraph 4 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intention‐to‐treat principle was not adhered to. 74 patients randomised to the Balanced Solution group but only 72 patients analysed. 2 patients did not receive intervention due to "unsuitable vessel situation" (Page 126, Figure 1), however this is not discussed in the text further. It is unclear whether this infers that the transplant did not proceed for these 2 patients. There were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | The study was registered on clinicaltrials.gov with protocol outcomes the same as reported outcomes |
| Other bias | Unclear risk | Out of 397 eligible cadaveric transplants performed during the study period, only 150 recipients were included in the study and 247 excluded due to absence of study team personnel during those transplants. There was no information on any differences in characteristics between included and excluded transplant recipients making it difficult to assess selection bias due to this |
CVP ‐ central venous pressure; HCO3 ‐ bicarbonate; IV ‐ intravenous; M/F ‐ male/female; RCT ‐ randomised controlled trial; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdallah 2014 | No comparator arm (both groups received normal saline) |
| Cittanova 1996 | Intervention applied to kidney donors |
| Dai 2011 | No normal saline control arm |
| Dawidson 1987 | No comparator arm |
| Hatch 1985 | Intervention compared different rates of fluid administration |
| Magpantay 2011 | Intervention was not perioperative |
| Mertens zur Borg 2008 | Intervention applied to kidney donors |
| Othman 2010 | Intervention compared different rates of fluid administration |
| Pang 2011 | Intervention was parecoxib |
| Starke 2012 | Intervention was potassium citrate |
| Wu 2010 | Intervention compared 2 different types of colloids and both arms received lactated ringers solution |
Characteristics of studies awaiting assessment [ordered by study ID]
Nuraei 2010.
| Methods | Double‐blind quasi‐RCT |
| Participants | Adult live‐donor kidney transplant recipients |
| Interventions | Lactated ringers compared to normal saline |
| Outcomes | Acid‐base parameters at the end of surgery |
| Notes | This study is awaiting translation in order to be classified |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000023853.
| Trial name or title | Balanced fluid therapy and early kidney function in patients undergoing renal transplantation |
| Methods | RCT |
| Participants | Adult deceased‐donor kidney transplant recipients |
| Interventions | Plasma‐lyte® compared to normal saline |
| Outcomes | Hyperkalaemia and acid‐base balance |
| Starting date | 21‐11‐2012 |
| Contact information | |
| Notes |
RCT ‐ randomised controlled trial
Differences between protocol and review
-
Secondary outcomes
We intended to examine acid‐base status at the end of surgery compared to baseline and at day three compared to baseline. However, no studies reported on acid‐base status at day three, therefore only the comparison between end of surgery and baseline was made.
-
Assessment of reporting bias
We intended to use funnel plots to assess reporting bias, however this was not possible due to the small number of studies identified.
Contributions of authors
Draft the protocol: SW, PM, MR
Study selection: SW, PM
Extract data from studies: SW, PM
Enter data into RevMan: SW
Carry out the analysis: SW, PM, MR
Interpret the analysis: SW, PM, MR
Draft the final review: SW, PM, MR
Disagreement resolution: MR
Update the review: SW
Declarations of interest
Susan Wan: none known
Matthew A Roberts: I have received competitive research funding and speaker's honoraria for research in subjects not related to this current Cochrane Review
Peter Mount: is an investigator in an investigator initiated ongoing study entitled “Balanced fluid therapy and early kidney function in patients undergoing renal transplantation” (ACTRN12612000023853). The fluids used in this study are provided for and paid for by Baxter Healthcare.
New
References
References to studies included in this review
Hadimioglu 2008 {published data only}
- Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid‐base balance and early kidney function after kidney transplantation. Anesthesia & Analgesia 2008;107(1):264‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khajavi 2008 {published data only}
- Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysamie AP, Khashayar P, et al. Effects of normal saline vs. lactated ringer's during renal transplantation. Renal Failure 2008;30(5):535‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim SY, Huh KH, Lee JR, Kim SH, Jeong SH, Choi YS. Comparison of the effects of normal saline versus plasmalyte on acid‐base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplantation Proceedings 2013;45(6):2191‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Modi 2012 {published data only}
- Modi MP, Vora KS, Parikh GP, Shah VR. A comparative study of impact of infusion of Ringer's Lactate solution versus normal saline on acid‐base balance and serum electrolytes during live related renal transplantation. Saudi Journal of Kidney Diseases & Transplantation 2012;23(1):135‐7. [MEDLINE: ] [PubMed] [Google Scholar]
O'Malley 2005 {published data only}
- O'Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double‐blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesthesia & Analgesia 2005;100(5):1518‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Potura 2015 {published data only}
- Potura E, Lindner G, Biesenbach P, Funk GC, Reiterer C, Kabon B, et al. An acetate‐buffered balanced crystalloid versus 0.9% saline in patients with end‐stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesthesia & Analgesia 2015;120(1):123‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdallah 2014 {published data only}
- Abdallah E, El‐Shishtawy S, Mosbah O, Zeidan M. Comparison between the effects of intraoperative human albumin and normal saline on early graft function in renal transplantation. International Urology & Nephrology 2014;46(11):2221‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cittanova 1996 {published data only}
- Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P. Effect of hydroxyethylstarch in brain‐dead kidney donors on renal function in kidney‐transplant recipients. Lancet 1996;348(9042):1620‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cittanova ML, Leblanc I, Mouquet C, Riou B, Coriat P. Hydroxyethyl starch in brain‐dead kidney donors impairs renal function in kidney recipients [abstract]. British Journal of Anaesthesia 1996;76(Suppl 2):115. [CENTRAL: CN‐00269004] [Google Scholar]
Dai 2011 {published data only}
- Dai MH, Yan K, Lu C. Influence of different kinds of infusion solutions during renal transplantation on blood glucose levels in children. Zhongguo Dangdai Erke Zazhi [Chinese Journal of Contemporary Pediatrics] 2011;13(7):595‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Dawidson 1987 {published data only}
- Dawidson I, Berglin E, Brynger H, Reisch J. Intravascular volumes and colloid dynamics in relation to fluid management in living related kidney donors and recipients. Critical Care Medicine 1987;15(7):631‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hatch 1985 {published data only}
- Hatch DA, Barry JM, Norman DJ. A randomized study of intravenous fluid replacement following living‐donor renal transplantation. Transplantation 1985;40(6):648‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Magpantay 2011 {published data only}
- Magpantay L, Ziai F, Oberbauer R, Haas M. The effect of fluid intake on chronic kidney transplant failure: a pilot study. Journal of Renal Nutrition 2011;21(6):499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mertens zur Borg 2008 {published data only}
- Mertens zur Borg I, Biase M, Verbrugge S, Ijzermans JN, Gommers D. Comparison of three perioperative fluid regimes for laparoscopic donor nephrectomy: a prospective randomized dose‐finding study. Surgical Endoscopy 2008;22(1):146‐50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Othman 2010 {published data only}
- Othman MM, Ismael AZ, Hammouda GE. The impact of timing of maximal crystalloid hydration on early graft function during kidney transplantation. Anesthesia & Analgesia 2010;110(5):1440‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pang 2011 {published data only}
- Pang L, Lian X, Li Y, Nan L, Ma H. Efficacy and safety of parecoxib sodium after renal transplantation. African Journal of Pharmacy & Pharmacology 2011;5(22):2467‐73. [EMBASE: 2011702897] [Google Scholar]
- Yuan T, Pang L, Ma H. Efficacy and safety of parecoxib sodium after renal transplantation [abstract]. American Journal of Transplantation 2012;12:250. [EMBASE: 70746703] [Google Scholar]
Starke 2012 {published data only}
- Starke A, Corsenca A, Kohler T, Knubben J, Kraenzlin M, Uebelhart D, et al. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(9):1461‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu Y, Wu AS, Wang J, Tian M, Jia XY, Rui Y, et al. Effects of the novel 6% hydroxyethyl starch 130/0.4 on renal function of recipients in living‐related kidney transplantation. Chinese Medical Journal 2010;123(21):3079‐83. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Nuraei 2010 {published data only}
- Nuraei N, Khajenouri R, Soleimani M, Dabbagh A. The effects of intraoperative normal saline versus lactated ringer solution on clinical outcomes and laboratory findings in renal transplant patients. Tehran University Medical Journal 2010;68(4):243‐9. [EMBASE: 2010558117] [Google Scholar]
References to ongoing studies
ACTRN12612000023853 {published data only}
- Weinberg L. Balanced fluid therapy and early kidney function in patients undergoing renal transplantation. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347888 (accessed 27 June 2016).
Additional references
ANZDATA 2011
- McDonald S, Hurst K (editors). Thirty fourth annual report. 2011. Australia and New Zealand Dialysis & Transplant Registry. www.anzdata.org.au/anzdata/AnzdataReport/34thReport/2011c00_Front%20Pages_v1.6.pdf (accessed 27 June 2016).
Chowdhury 2012
- Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double‐blind crossover study on the effects of 2‐L infusions of 0.9% saline and plasma‐lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers.[Erratum appears in Ann Surg. 2013 Dec;258(6):1118]. Annals of Surgery 2012;256(1):18‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collins 2012
- Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end‐stage renal disease in the United States. American Journal of Kidney Diseases 2012;59(1 Suppl 1):A7, e1‐420. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halperin 1998
- Halperin ML, Kamel KS. Potassium. Lancet 1998;352(9122):135‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Handy 2008
- Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. British Journal of Anaesthesia 2008;101(2):141‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Krajewski 2014
- Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta‐analysis of high‐ versus low‐chloride content in perioperative and critical care fluid resuscitation. British Journal of Surgery 2015;102(1):24‐36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myburgh 2013
- Myburgh JA, Mythen MG. Resuscitation fluids. New England Journal of Medicine 2013;369(13):1243‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Malley 2002
- O’Malley CM, Frumento RJ, Bennet‐Guerrero E. Intravenous fluid therapy in renal transplant recipients: results of a US survey. Transplantation Proceedings 2002;34(8):3142‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roche 2007
- Roche AM, James MF. Fluid therapy in organ transplantation. Current Opinion in Organ Transplantation 2007;12(3):281‐6. [EMBASE: 2007250264] [DOI] [PubMed] [Google Scholar]
Scheingraber 1999
- Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999;90(5):1265‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schnuelle 2006
- Schnuelle P, Johannes van der Woude F. Perioperative fluid management in renal transplantation: a narrative review of the literature. Transplant International 2006;19(12):947‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaw 2012
- Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma‐Lyte. Annals of Surgery 2012;255(5):821‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilcox 1983
- Wilcox CS. Regulation of renal blood flow by plasma chloride. Journal of Clinical Investigation 1983;71(3):726‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarlagadda 2008
- Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrology Dialysis Transplantation 2008;23(9):2995‐3003. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarlagadda 2009
- Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta‐analysis. Nephrology Dialysis Transplantation 2009;24(3):1039‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yunos 2012
- Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride‐liberal vs chloride‐restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308(15):1566‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wan 2013
- Wan S, Roberts MA, Mount P. Normal saline versus lower‐chloride solutions for kidney transplantation. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010741] [DOI] [PMC free article] [PubMed] [Google Scholar]


