Abstract
Background
The hepatitis C virus (HCV) infection affects about 2% of the world's population and can cause chronic liver infection and persistent long‐term sequelae such as cirrhosis and liver cancer.
The prevalence of HCV infection among people on haemodialysis is often higher than the general population. The virus is easily transmitted parenterally, and blood transfusions have previously played a significant role in transmission; however, erythropoietin therapy has reduced the need for transfusions, and coupled with improved screening of donated blood, has significantly decreased transmission by transfusion. Although control of hospital‐acquired infection has improved with the advent of biosafety measures, stopping HCV transmission in haemodialysis units remains challenging.
Isolating people infected with HCV involves physical separation from others to limit direct or indirect transmission and includes a number of strategies during dialysis. The evidence for isolating people infected with HCV during haemodialysis is sparse with some inconsistencies.
Objectives
To evaluate the benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review. We also searched the Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 2015), Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S, 1990 to 2015), ProQuest Dissertations & Theses Database (1990 to 2015), and Open Grey (1990 to 2015).
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster RCTs evaluating the clinical benefits and harms of isolating HCV‐infected patients during haemodialysis on the transmission of HCV to other patients. We considered incidence of dialysis‐acquired HCV infection, all‐cause mortality, and adverse effects associated with isolation as the primary outcomes.
Data collection and analysis
Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes.
Main results
Only one study, which included 12 centres was identified: four centres used dedicated haemodialysis machines for HCV‐infected patients and eight centres used non‐dedicated machines. The total number of patients enrolled was 593. One centre was excluded after randomisation. Random sequence generation was not described and allocation concealment was not performed. Participants and personnel were not blinded and blinding of outcome assessors was not reported. Only 74.5% of the patients were followed for 9 months; and 47.3% were followed for an additional 9 months. The authors only reported one outcome, measuring the difference in the incidence of HCV in both groups. The authors did not consider the exposure time, to determine the adjusted rate of seroconversion risk/patient‐year. The study reported that the incidence of HCV infection during the first follow‐up period (9 months) was 1.6% in the dedicated group, and 4.7% in the non‐dedicated one (446 patients analysed out of 593 randomised; RR 0.34, 95% CI 0.11 to 1.07). During the second follow‐up period (18 months) the incidence was 1.3% in the dedicated group and 5.8% in the control (281 patients analysed out of 593 randomised; RR 0.22, 95% CI 0.05 to 1.02). Therefore, we found no differences in terms of the number of participants developing HCV infection when comparing the dedicated group with the usual care. Moreover, the evidence was of very low quality, which means that we have very little confidence in the effect estimate.
Authors' conclusions
The benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients are uncertain. Evidence from one short‐duration cluster‐randomised study with a high risk of bias did not find differences in terms of the number of participants developing HCV infection when comparing the use of dedicated haemodialysis machines for HCV infected patients with the use of non‐dedicated machines.
Plain language summary
Isolation as a strategy for controlling the transmission of hepatitis C virus (HCV) infection in haemodialysis units
What is the issue?
The hepatitis C virus (HCV) is easily transmitted intravenously, such as blood transfusions and the use of haemodialysis. It can cause a persistent infection and chronic liver disease. The frequency of HCV is higher among people on haemodialysis than the general population; and is associated with increased risk of death from heart disease and liver. We wanted to find out if the isolation of people with HCV during haemodialysis (using a different room, machines or dedicated staff, a specific shift) was effective in limiting the direct or indirect transmission of the virus to non‐infected patients.
What did we do?
We conducted an extensive literature search to November 26, 2015, but only found one study looking at isolation as a strategy for controlling the transmission of HCV infection.
What did we find?
This one study included 12 centres (593 patients). Four centres assigned HCV‐infected patients to a dedicated haemodialysis machine and eight centres did not. This study reported the incidence of HCV in haemodialysis patients decreased with the use of dedicated machines; however it was not possible to determine the benefits and harms associated with isolation, cost, or mortality from the disease.
Conclusions
There is insufficient evidence, but additional studies would help clarify the role of isolation to reduce the transmission of HCV in haemodialysis patients.
Summary of findings
Summary of findings for the main comparison. Dialysis machine separation versus usual care.
| Should patients with HCV be isolated in haemodialysis units for controlling the transmission of HCV? | ||||||
|
Patient or population: patients in haemodialysis
Setting: ambulatory Intervention: isolation Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with isolation | |||||
| Incidence of HCV infection (9 months) | Study population | RR 0.34 (0.11 to 1.07) | 446 (1) | ⊕⊝⊝⊝ VERY LOW | Very low quality of evidence due to high risk of bias and imprecision | |
| 47 per 1.000 | 16 per 1.000 (5 to 50) | |||||
| Incidence of HCV infection (18 months) | Study population | RR 0.22 (0.05 to 1.02) | 281 (1) | ⊕⊝⊝⊝ VERY LOW | Very low quality of evidence due to high risk of bias and imprecision | |
| 58 per 1.000 | 13 per 1.000 (3 to 59) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
Hepatitis C virus (HCV) infection affects approximately 3% of the global population, or about 170 million people (Lee 2014). HCV causes persistent infection and chronic liver disease; long‐term sequelae include cirrhosis (30%) and liver cancer (1% to 5%) (Hsu 2015; Webster 2015).
Extrahepatic manifestations of chronic HCV infection are considered to be of immunologic origin and include cryoglobulinaemia, membranoproliferative glomerulonephritis, (Morales 2012) and porphyria cutanea tarda.
Prevalence of HCV infection in haemodialysis patients is usually higher than in the general population (Fabrizi 2012). The overall incidence rate of HCV infection is 1.47/100 patient‐years; 4.44/100 patient‐years in low‐ to middle‐income countries, and 0.99/100 patient‐years in high‐income countries (Su 2013). Prevalence ranges from less than 5% in most Northern European countries (Fissell 2004; Schneeberger 2000) to over 70% in many parts of the world, including countries in Asia, Latin America and North Africa (Sun 2009; Vladutiu 2000). However, the prevalence of HCV in European dialysis centres declined sharply in the early 2000s. This was attributed to reduced risk of hospital‐acquired infection and occupational HCV infection (Jadoul 2004), increased mortality rates, and stabilisation of the incidence of acute HCV infection (Espinosa 2004). Falling prevalence rates emphasise the importance of adhering to recommended infection control precautions and virological follow‐up to detect anti‐HCV antibodies using sensitive, specific new‐generation serological tests (Saune 2011).
HCV‐infected haemodialysis patients are at increased risk of liver or cardiovascular disease‐related death compared with non‐infected patients (Fabrizi 2012). HCV infection is associated with increased morbidity and mortality in kidney transplant recipients (Batty 2001; Butt 2007; Mahmoud 2004). Anti‐HCV‐positive patients on dialysis are at increased risk compared with anti‐HCV‐negative patients.
Description of the intervention
Isolating HCV‐infected patients (or patients waiting HCV screening results) during haemodialysis is defined as physical segregation from others for the express purpose of limiting direct or indirect transmission of HCV. Isolation policies may include strategies for HCV‐infected patients with different grades of intensity, such as use of a dedicated dialysis machine, personnel, room, or shift, or other barrier precautions (such as aprons, gowns, or gloves), by healthcare professionals attending these patients. These strategies may be implemented in combination.
How the intervention might work
HCV is easily transmitted parenterally and its control has therefore been a challenge in dialysis settings (Natov 2005; Su 2013). More recently erythropoietin therapy, which reduces requirements for transfusions, together with more sensitive tests to detect HCV in donated blood, have significantly reduced transmission via transfusion (Marwaha 2014). The post‐transfusion risk has been calculated at around 0.0001% in the USA (i.e. 1 blood transfusion in 1 million units of blood), with similar dramatic improvements in the viral safety of blood in other western countries (Selvarajah 2012).
The WHO has estimated an overall prevalence of 3% for HCV infection in the global population, but there is a wide geographic variability: fewer than 5% of people in most northern European countries are infected, close to 10% in southern Europe and the USA, and estimates range from 10% to 50% and up to 70% in many low‐ to middle‐income countries. HCV infection incidence has however decreased to less than 1.2% of people in high‐income countries (Espinosa 2004; Finelli 2005; Jadoul 2012).
This reduction was initially attributed to decreased rates of post‐transfusion infection (Djordjevic 2000; Valtuille 2002), but it was later ascribed to other infection control measures used to prevent hospital‐acquired infection rates in dialysis units. Prevalence of HCV infection among people on haemodialysis is generally below 10% in most countries, but may be higher (> 20%) where social crisis, war, or economic downturn exist (Ali 2011; Selm 2010; Voiculescu 2010). In these situations, maintenance of chronic haemodialysis programs is highly challenging, and infection control programs are difficult to maintain.
In spite of reduced rates of infection, HCV transmission in haemodialysis units remains an unsolved problem. Despite advances in screening blood products for HCV people on haemodialysis it remains at a higher risk of infection than in the general population (Ozer Etik 2015)
HCV seroconversion (change from anti‐HCV negative to anti‐HCV positive) has been detected in patients who were never transfused (Agarwal 2011) therefore other mechanisms of transmission occur in dialysis units. Shared haemodialysis machines (Elamin 2011; Sartor 2004) and reprocessing of dialysers from people with HCV have been linked to HCV transmission (Bashiri 2013). Other factors include physical proximity to an infected person and sharing personal items (Al‐Ghamdi 2004; Fabrizi 2008); breakdown in standard infection control practices, including improper handling and preparation of medications (Alter 2008; CDC 2009; Samandari 2005; Thompson 2009; Williams 2004); poor environmental cleaning (CDC 2009; Girou 2008; Kamili 2007; Patel 2010; Thompson 2009) and basic hygiene practices (Alfurayh 2000; Patel 2010); staff numbers and workload (Arenas 2005; CDC 2009; KDIGO 2008; Patel 2010; Shimokura 2011).
Why it is important to do this review
The evidence for or against the use of isolation of HCV‐infected patients during haemodialysis is weak and certain inconsistencies exist regarding the recommendations for its use among different guidelines. The centres for Disease Control and Prevention (CDC 2001; Mbaeyi 2013) published guidelines to prevent the transmission of HCV and other infections among haemodialysis patients, but did not recommend the isolation of HCV‐infected patients. KDIGO 2008 stated that haemodialysis units should ensure implementation of and adherence to strict infection‐control procedures designed to prevent transmission of blood‐borne pathogens, including HCV, but isolation of HCV‐infected patients was not recommended as an alternative to strict infection‐control procedures (unless in cases of continued hospital‐acquired transmission, where a local isolation policy may be deemed necessary). The UK Renal association stated that patients with HCV patients do not need to be dialysed in a segregated area, however more experienced staff should be assigned. If nosocomial transmission continues to occur, despite reinforcement and audit of the precautions, a local segregation policy may be deemed necessary (Geddes 2011)
The European Best Practice (ERBP) Work Group considers that implementation of universal hygienic measures should be the standard of care. Isolation of positive patients could be considered, but only if this practice does not have a negative impact on the implementation and reinforcement of basic hygienic measures in the unit as a whole (Covic 2009). Some investigators support isolating patients with HCV infection in a specific haemodialysis room (Fabrizi 2008), or suggest that the no isolation policy should not be generalised. Whether or not HCV‐positive patients should be isolated is still debated, particularly since isolation policies to control HCV infection transmission in haemodialysis units involves significant logistic problems that should be considered.
Objectives
This review aimed to evaluate the benefits and harms of isolation of HCV‐infected patients during haemodialysis on the transmission of HCV to other patients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), and cluster RCTs and cluster quasi‐RCTs (where centres rather than individual patients were randomised), looking at isolation of HCV‐infected patients during haemodialysis were eligible for inclusion.
Types of participants
All patients (adults and children) undergoing maintenance haemodialysis and dialysed in a haemodialysis centre (e.g. hospital unit, outpatient clinic) were eligible for inclusion.
Types of interventions
Intervention group
Any strategy targeting the isolation during haemodialysis of HCV‐infected patients or patients waiting HCV screening results was eligible. Isolation was defined as the physical segregation of these patients from others with the express purpose of limiting direct or indirect transmission of HCV to other patients. Isolation policies could include a number of strategies with different grades of intensity, such as the use of a dedicated dialysis machine, personnel, room or dialysis shift.
Control group
We considered any control group that enabled comparison to determine the relative effect of the isolation strategy as eligible for inclusion. Studies comparing two types of isolation strategies were also eligible.
Types of outcome measures
Primary outcomes
Incidence of dialysis‐acquired HCV infection
All‐cause mortality
Adverse effects associated to the isolation strategy (such as negative effects on patient mental well‐being, or adverse effects related to supportive care failures).
Secondary outcomes
Incidence of dialysis‐acquired non‐HCV infections
Patient satisfaction with treatment, measured with a validated tool
Isolation costs.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 26 November 2015 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on Cochrane Kidney and Transplant scope. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
We searched the following electronic databases.
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 2015)
Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S, 1990 to 2015) (accessed via ISI Web of Science)
ProQuest Dissertations & Theses Database (1990 to 2015)
Open Grey (1990 to 2015).
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles and relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews thought to include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts, and if necessary the full text, of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was to be carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were to be grouped together and the publication with the most complete data used in analyses. Where relevant outcomes were only published in earlier versions these data were to be used. Any discrepancy between published versions was to be highlighted.
Assessment of risk of bias in included studies
The following items were to be independently assessed by two authors using the risk of bias assessment tool (Higgins 2011a) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
Was recruitment bias adequately prevented?
Were baseline imbalances (in terms of either the clusters or the individuals) adequately addressed?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Was the study analysed by correct statistical methods (i.e. taking the clustering into account)?
Measures of treatment effect
For dichotomous data and for counts of rare events and rates (dialysis‐acquired HCV infections, dialysis‐acquired non‐HCV infections, mortality due to dialysis‐acquired infections, all‐cause mortality, adverse effects) we planned to report risk ratios (RR); for continuous data (patient satisfaction with treatment) we planned to use the mean difference (MD) with 95% CI. Where different scales were used to measure continuous outcomes, we planned to calculate a standardised mean difference (SMD).
Unit of analysis issues
We planned to include cluster RCTs, and when possible, extract effect measures and standard error rates from an analysis taking clustering into account. If that was not possible, we planned to extract the number of clusters and estimate the intra‐cluster correlation coefficient to inform a reliable analysis. If this was not possible, we planned to disregard the clustering and investigate the effect of this in a sensitivity analysis (Deeks 2011).
Dealing with missing data
We planned to extract data for intention‐to‐treat analyses (ITT) and contact authors if required information was missing. Where ITT analysis was not possible, we planned to extract data from an available case analysis and assess the risk of bias from attrition.
Assessment of heterogeneity
We planned to analyse heterogeneity using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and the I² statistic.
Assessment of reporting biases
We did not expect that a sufficient number of studies would be identified to create a useful funnel plot. Assessing reporting bias is difficult, but we planned to note whether outcomes that we considered important were reported. We planned to contact authors about possible unpublished outcomes.
Data synthesis
We planned to use a random‐effects model and to express the results as both relative risks and number‐needed‐to‐screen to achieve the relevant outcomes, both beneficial and harmful.
Subgroup analysis and investigation of heterogeneity
We planned to perform the subgroup analyses
Mean duration of the haemodialysis treatment
-
The degree of missing primary outcome data
High prevalence of HCV in the haemodialysis unit: studies implemented in a context of high HCV prevalence (< 5%) versus non‐high HCV prevalence (≥ 5%)
Outbreak situation: studies implemented when healthcare associated infection (any pathogen) were noted to be increasing or to exceed a recognised benchmark versus non outbreak situation
Types of isolation.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors on effect size.
Repeating the meta‐analysis to assess the effect of excluding studies with high risk of bias
Exploring the impact of the assumptions taken in the available case analysis by performing a sensitivity analysis with imputation of missing data
Assessing the effect of the statistical model chosen for meta‐analysis (fixed‐effect model versus random‐effects model)
Repeating the meta‐analysis to assess the effect of including only studies with allocation to interventions at the group level (cluster designs) (Ukoumunne 1999).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
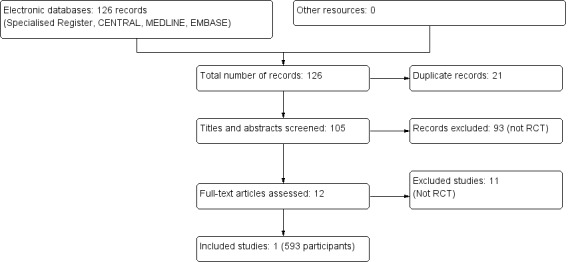
The combined search of MEDLINE, EMBASE and CENTRAL identified a total of 126 articles, of which 125 were excluded.
Twenty one records were duplicates
After reviewing the abstracts, 93 records were excluded because they did not meet our inclusion criteria
After full text review an additional 11 records, which were not RCTs, were excluded.
We therefore included one study enrolling 593 patients (Shamshirsaz 2004). A flow chart of the study selection process is shown in Figure 1
1.
Included studies
Shamshirsaz 2004 was a prospective RCT evaluating the effect of dialysis machine separation in reducing HCV transmission to haemodialysis patients. Selected centres were randomly divided into dedicated and non‐dedicated haemodialysis machine groups. Positive cases were confirmed by RT‐PCR. Information regarding age, sex, occupation (health care personnel, surgeons and dentists), HCV‐infected relatives, previous peritoneal dialysis, surgery during last 2 months, duration of haemodialysis, number of blood product transfusions, history of organ transplantation, and the causes of ESRD was collected. Outcomes included incidence of HCV infection in both groups.
Patients were dialyzed for 4 to 4.5 hours, 2 or 3 times/week, using standard haemodialysis techniques. All included haemodialysis patients were HIV and hepatitis B surface antigen (HBsAg) negative. Dialysis membranes were low pressure and used only once and haemodialysis machines were bleached and rinsed between dialysis sessions according to the manufacturers' instruction. All machines were located in dialysis wards and not in separate rooms for both groups. Patient to staff ratio in the dedicated and non‐dedicated groups was not statistically different (3.1 and 3.4 respectively) and all staff members were negative for anti‐HCV. Education courses hygiene guidelines the CDC were conducted for all personnel involved in patient care, a checklist of the practice was used. In all centres the patients had specific dialysis place. It was specified how often the tests were performed HCV patients during the study or whether this was routinely carried out.
The patients were followed for 9 months (first follow‐up population) and 281 patients who remained within the study were followed for an additional 9 months (second follow‐up population). See Characteristics of included studies.
Excluded studies
Eleven studies were not RCTs (Agarwal 2009; Barril 2003; Gallego 2006; Garcia‐Valdecasas 1994; Huang 1995; Mohamed 2010; Ross 2009; Saxena 2003; Shebeb 2006; Valtuille 1998; Yang 2003)
Risk of bias in included studies
See risk of bias in Figure 2 and Figure 3.
2.
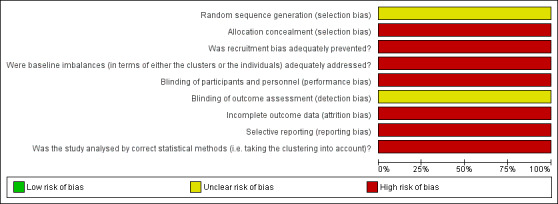
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across one included study.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for one included study.
Allocation
This study did not describe the use of a random number table to generate the allocation sequence. Allocation concealment was not performed
Randomisation was performed by dialysis centre. It was a cluster RCT, which included four centres (dedicated) compared with eight centres (non‐dedicated).
Blinding
Participants (patients and investigators) were not blinded. Blinding of outcome assessors was not reported.
Incomplete outcome data
Only 74.5% (446/593) of the patients were followed for 9 months, and 47.3% (281/593) were followed for an additional 9 months.
One centre was excluded after randomisation causing a deviation from protocol.
Selective reporting
The authors only reported one outcome, measuring the difference in the incidence of HCV in both groups. No secondary outcomes were reported.
The authors did not consider the exposure time, to determine the adjusted rate of seroconversion risk/patient‐year.
Other potential sources of bias
The assessments of the risk of bias domains relative to cluster designs are detailed in the risk of bias table (see Characteristics of included studies).
Effects of interventions
See: Table 1
Incidence of dialysis‐acquired HCV infection
Shamshirsaz 2004 reported the incidence of HCV infection was 1.6% in the dedicated group and 4.7% in the non‐dedicated one (Analysis 1.1 (1 study 446 participants): RR 0.34, 95% CI 0.11 to 1.07) during the first follow‐up period (9 months). During the second follow‐up period (18 months), the incidence was 1.3% in the dedicated group and 5.8% in the non‐dedicated group (Analysis 1.2 (1 study, 281 participants): RR 0.22, 95% CI 0.05 to 1.02). Therefore, we found no differences in terms of the number of participants developing HCV infection when comparing the dedicated group with the usual care. Moreover, the evidence was of very low quality, which means that we have very little confidence in the effect estimate and that the true effect is likely to be substantially different from the estimate of effect.
1.1. Analysis.
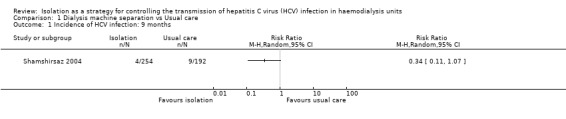
Comparison 1 Dialysis machine separation vs Usual care, Outcome 1 Incidence of HCV infection: 9 months.
1.2. Analysis.
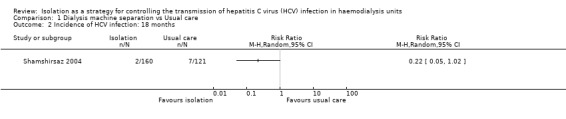
Comparison 1 Dialysis machine separation vs Usual care, Outcome 2 Incidence of HCV infection: 18 months.
All‐cause mortality
Mortality was not reported.
Adverse effects
Adverse effects associated with the isolation strategy were not reported.
Incidence of dialysis‐acquired non‐HCV infections
Incidence of dialysis‐acquired non‐HCV infections were not reported.
Patient satisfaction with treatment
Patient satisfaction with treatment was not reported.
Isolation costs
Isolation costs were not reported.
Discussion
Summary of main results
Only one study meeting our inclusion criteria was identified (Shamshirsaz 2004). This study selected haemodialysis centres, one by one to reach a total number of 593 patients (12 centres). Selected centres were randomly divided in to dedicated and non‐dedicated haemodialysis machine groups, including 297 patients in the dedicated group (4 centres) and 296 patients in the non‐dedicated group (8 centres). This study found that the use of dialysis machines dedicated for HCV infected individuals, as compared with the use of non‐dedicated dialysis machines, made no difference in terms of reducing the incidence incidence of HCV infection during the first (9 months) or second (18 months) follow‐up periods. The quality of the evidence was very low (see Table 1).
This study did not report any of our other primary outcomes of interest (all‐cause mortality, adverse effects associated with the strategy of isolation).
Overall completeness and applicability of evidence
We planned to include patients (adults and children) undergoing maintenance haemodialysis and dialysed in a haemodialysis centre and assess whether strategies isolation machine, room, staff or dialysis shift influence the transmission of hepatitis C. Our search just found one study where the separation of machines is evaluated as a form of isolation.
Consideration should also be given to both the prevalence of hepatitis C and the geographical region as this may influence the possibility of seroconversion. Regions with high prevalence may require isolation combined strategies: room, machine, and personnel. No costs are included for the isolation strategy.
Methods of randomisation were unclear, participants and patients were not blinded, so that the results of this study must be considered with some caution. Confirmatory research is required. Any further studies conducted in this area must be well designed RCTs assessing these primary outcomes.
Quality of the evidence
This review is based on the evidence of one RCT (Shamshirsaz 2004) that included a total of 12 haemodialysis centres (593 patients) divided into a group of dedicated dialysis machines involving four centres: 297 patients, 267 negative and 30 positive for HCV and a group of centres with non‐dedicated machines: 8 centres: 296 patients, 275 negative and 21 positive for HCV.
The authors did not disclose the details of the method of randomisation, participants and patients were not blinded, and blinding of outcome assessors was not reported. The authors decided to exclude, after randomisation, one of dialysis centres in the non‐dedicated group due to non‐adherence to CDC hygienic guidelines early in the study. There was a high risk of bias due to incomplete outcome data: only 74.5% of patients were followed for 9 months, and 47.3% were followed for an additional 9 months. In addition, the estimation of the effect of the intervention was imprecise. This makes the quality of evidence very low (see Table 1).
Potential biases in the review process
We followed the Cochrane Collaboration guidelines for conducting this systematic review and meta‐analysis. Strengths of our review include the searching of several databases. Study selection, assessment of risk of bias, and data extraction were performed by two authors, which reduced the risk of error and bias. Although efforts were made to collect relevant data, the possibility of missing data cannot be excluded. Publication bias remains a possible source of important bias. Meanwhile, interpretation of the result should be done with extreme caution.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic review on this topic; many authors have reported some reduction (but not full prevention) of HCV transmission in haemodialysis, and after the adoption of an isolation policy, most of the studies have compared their results with their own historical control (before‐and‐after). Thus, it is unclear whether the reported improvement resulted from the adoption of an isolation policy or rather from the simultaneous raising of awareness and reinforcement of the application of hygienic precautions. These isolation policies include: implementing the isolation of a room (Agarwal 2009; Barril 2003); using exclusive machines (Garcia‐Valdecasas 1994); and isolating machines, room and staff (Gallego 2006; Huang 1995; Saxena 2003); and Mohamed 2010, Ross 2009 and Shebeb 2006, compared isolation versus universal precautions. In all cases isolation was found to decrease the rate of seroconversion. In addition, Yang 2003 conducted a study with three sets of patients: one set without isolation, a second set with a dedicated area and a dedicated machine in the same room and a third set of patients isolated in a separate room, and showed that isolation in a different room was better than dedicated machines.
In contrast, a DOPPS multicentre study concluded that isolation does not protect against transmission of HCV in haemodialysis patients (Fissell 2004). A prospective observational study by Jadoul 1998 showed a reduction from 1.4% to 0% of the annual incidence of HCV seroconversion. They have reported a reduction of HCV transmission after the reinforcement of basic hygienic precautions, without any isolation measures.
Authors' conclusions
Implications for practice.
Data from robust RCTs are not available to allow conclusions to be drawn about the relative effectiveness of isolation as a strategy to control HCV transmission in haemodialysis units. Therefore, the benefits and harms of isolation remain unknown.
Implications for research.
Large, multicentre long‐term RCTs of good quality are required to answer the questions concerning the benefits and harms of isolation of HCV‐infected patients during haemodialysis. These studies should evaluate mortality, costs and complications associated with isolation. These studies should ensure the physical separation of either the centre or room; these programs should have strict isolation strategies in place that include staff as well as machines dedicated to HCV‐infected haemodialysis patients.
Acknowledgements
We wish to acknowledge the Cochrane Kidney and Transplant editorial team and the referees for the comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement |
Appendix 3. Risk of bias assessment tool for cluster randomised studies
| Potential source of bias | Assessment criteria |
| Was recruitment bias adequately prevented? | Low risk of bias: Individuals were not recruited to the trial after the clusters had been randomised. |
| High risk of bias: Individuals were recruited to the trial after the clusters had been randomised (the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited). | |
| Unclear risk of bias: Insufficient information to permit judgement. | |
| Were baseline imbalances (in terms of either the clusters or the individuals) adequately addressed? | Low risk of bias: The randomised groups were similar at baseline; or the randomised groups were imbalanced at baseline but finally controlled for at the design (such as using stratified or pair matched randomisation of clusters) or analysis stage of the study. |
| High risk of bias: There were baseline imbalances between the randomised groups, but finally they were not controlled for at the design or analysis stage of the study. | |
| Unclear risk of bias: Insufficient information to permit judgement. | |
| Were loss of clusters and participants adequately addressed? | See Appendix 2: "Incomplete outcome data" for criteria of how we will assess this domain. |
| Was the study analysed by correct statistical methods (i.e. taking the clustering into account)? | Low risk of bias: The cluster‐randomised trial was analysed by correct statistical methods, taking the clustering into account. Ways to avoid unit‐of‐analysis errors in cluster‐randomised trials are (see Cochrane Handbook 16.3.3, Higgins 2011b): to conduct the analysis at the same level as the allocation; to conduct the analysis at the level of the individual while accounting for the clustering in the data. Such an analysis might be based on a ‘multilevel model’, a ‘variance components analysis’ or a ‘generalized estimating equations (GEEs)’, among other techniques. |
| High risk of bias: The cluster‐randomised trial was analysed by incorrect statistical methods, not taking the clustering into account. Such analyses tend to create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. Although they do not lead to biased estimates of effect, if they remain uncorrected, they will receive too much weight in a meta‐analysis. | |
| Unclear risk of bias: insufficient information to permit judgement. |
Data and analyses
Comparison 1. Dialysis machine separation vs Usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of HCV infection: 9 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of HCV infection: 18 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Shamshirsaz 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process Randomisation was performed by dialysis centre, it was a cluster randomised trial which included four centres (intervention) compared with eight centres (control) |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not performed |
| Was recruitment bias adequately prevented? | High risk | Individuals were recruited to the trial after the clusters had been randomised (the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited). |
| Were baseline imbalances (in terms of either the clusters or the individuals) adequately addressed? | High risk | There were baseline imbalances between the randomised groups, but finally they were not controlled for at the design or analysis stage of the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was not performed. There was an "available case analysis" done with substantial departure of the intervention received from that assigned at randomisation. Only 74.5% of the patients were followed for 9 months and 47.3% were followed for an additional 9 months They lost > 10% of their population One centre was excluded after randomisation, causing a deviation from protocol |
| Selective reporting (reporting bias) | High risk | The authors only reported one result, measuring the difference in the incidence of HCV in both groups, based on that issued its findings, did not have secondary outcomes. The authors did not consider the exposure time (i.e. years patients at risk) in determining the rate of seroconversion |
| Was the study analysed by correct statistical methods (i.e. taking the clustering into account)? | High risk | "Comparisons between groups were made by the chi square test method for categorical variables and by the t test for quantitative variables." The cluster‐randomised trial was analysed by incorrect statistical methods, not taking the clustering into account |
CDC ‐ Centers for Disease Control and Prevention; HCV ‐ hepatitis C virus; HD ‐ haemodialysis; SE ‐ standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2009 | Cohort study 2003 to 2006, room isolation, HCV seroconversion from 42% in 1995 to 1998 (without isolation) to 4% in 2003 to 2006 (with isolation) |
| Barril 2003 | Cohort, multicentre study: 44 centres, room isolation, evaluate prevalence of hepatitis C and time, it divide its population in 4 quartiles according prevalence of hepatitis C, after isolation they observe decrease of seroconversion in all quartiles |
| Gallego 2006 | Analytic study, comparing two periods of 1993 to 1995 in which isolation of machines for patients with hepatitis C is applied, and where are 2 seroconversions and second period 1996 to 2003 applied isolation room and staff, which do not show any seroconversion. Progressive decline observed prevalence of hepatitis C in its population 30.5% vs 6.8% |
| Garcia‐Valdecasas 1994 | Analytic study, three rooms of dialysis: Room A: 27 patients and 6 HCV (+), Room B: 28 patients; 7 HCV (+), Room C: 25 patients, 14 with HCV (+); No isolation: 1990, 1991 to 1992 isolation of machine only in rooms A and B, isolation |
| Huang 1995 | Prospective study, from April 1992, 32 patients negative for HCV, isolated machine and room, after 14.2 + 3 months, a patient seroconverted, apparently associated with blood transfusion, seroconversion rate 14.6% versus 3.1% prior to isolation (historical data) |
| Mohamed 2010 | Prospective study was conducted on 36 seronegative HD patients. All patients were managed with strict application of infection control guidelines as well as isolation of HCV‐positive patients. After five years of follow‐up, They found that the incidence of HCV seroconversion was zero. |
| Ross 2009 | Large prospective multicentre study was conducted. In the first and second rounds, 150 (5.2%) of 2909 and 114 (5.4%) of 2100 patients were anti‐HCV positive, respectively, and 4% of individuals were viraemic. 20% of these patients no nosocomial Hepatitis C transmission occurred during the observational period suggests that the lack of HCV seroconversions was not only attributable to the isolation of HCV‐infected patients but also to the strict adherence universal hygienic precautions |
| Saxena 2003 | Retrospective study, In the first phase, 189 patients who were receiving maintenance HD from 1995 to 2000 were studied about prevalence of HCV. Phase II involved stringent isolation of anti‐HCV positive patients detected during phase, with dedicated space, dialysis equipment, and nursing staff from December 2000 to December 2001. Prevalence rate of 43.9% (83/189) and an annual HCV seroconversion rate of 6.8% were identified in this cohort. Only 2 new HCV seroconversions (1.01% (2/198)) were identified after isolation. |
| Shebeb 2006 | This study compared strict adherence to the universal precautions and anti HCV seropositive patient isolation. Three units: A: education intervention program, 30 patients and 12 staff personal, B: none of the preventive measures were applied, 66 patients nursed by 16 staff C: had 67 patients nursed by 27 staff. The incidence rate of anti HCV seroconversion decreased in unit A from (10% to 0%), and in unit C from (24.4% to 10%), 6 months of the follow. It increased in unit B, where no measure was taken, from 10.5% to 16.7%. |
| Valtuille 1998 | Study applying extreme conditions of permeability to the dialysis membrane and avoiding the use of heparin and dialysis bath. They obtained samples from the ultrafiltrate at the beginning of 18 HD sessions carried out in 6 HCV RNA‐positive patients. HCV RNA was detected by PCR in 3 (16.7%) ultrafiltrate samples belonging to 1 of the patients. HCV genotype was the same as that found in positive ultrafiltrate samples and in the serum corresponding to this patient. |
| Yang 2003 | Retrospective study, 325 HD patients from1993 to 2000 were included, isolation started after 1997. Patients positive for either hepatitis B or C were clustered in 1 area. Anti‐HCV‐negative and HBsAg ‐ negative patients were assigned either to a segregated zone (Area 2) in the same room or to a separate independent room (Area 3). Forty months after the implementation of the isolation policy, there was significant reduction in the total prevalence (49.7 vs 31.7%) and incidence (9.1 vs 2.9 % patient‐years) of HCV infection. |
HBsAg ‐ hepatitis B surface antigen; HCV ‐ hepatitis C virus; HD ‐ haemodialysis
Differences between protocol and review
Additional risk of bias assessments have been added due to the inclusion of cluster RCTs.
Contributions of authors
Writing of protocol and review: JB, CLM, JLA Screening of titles and abstracts: JB, CLM, JLA Assessment for inclusion: JB, CLM, JLA Quality assessment: JB, CLM, JLA Data extraction: JB, CLM Data entry into RevMan: JB, CLM Data analysis: JB, CLM, JLA
Sources of support
Internal sources
None known, Peru.
External sources
No sources of support supplied
Declarations of interest
Jessica I Bravo Zuñiga: none known
César Loza Munárriz: none known
Jesús López‐Alcalde: none known
New
References
References to studies included in this review
Shamshirsaz 2004 {published data only}
- Shamshirsaz AA, Kamgar M, Bekheirnia MR, Ayazi F, Hashemi SR, Bouzari N, et al. The role of hemodialysis machines dedication in reducing hepatitis C transmission in the dialysis setting in Iran: a multicenter prospective interventional study. BMC Nephrology 2004;5:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2009 {published data only}
- Agarwal SK, Dash SC, Gupta S, Pandey RM. Hepatitis C virus infection in haemodialysis: the 'no‐isolation' policy should not be generalized. Nephron 2009;111(2):c133‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barril 2003 {published data only}
- Barril G, Traver JA. Decrease in the hepatitis C virus (HCV) prevalence in hemodialysis patients in Spain: effect of time, initiating HCV prevalence studies and adoption of isolation measures. Antiviral Research 2003;60(2):129‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gallego 2006 {published data only}
- Gallego E, Lopez A, Perez J, Llamas F, Lorenzo I, Lopez E, et al. Effect of isolation measures on the incidence and prevalence of hepatitis C virus infection in hemodialysis. Nephron 2006;104(1):c1‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Valdecasas 1994 {published data only}
- Garcia‐Valdecasas J, Bernal MC, et al. Efficacy of preventive measures to avoid the transmission of the hepatitis C virus in dialysis units [abstract]. Nephrology Dialysis Transplantation 1994;9(10):1506. [CENTRAL: CN‐00261074] [Google Scholar]
Huang 1995 {published data only}
- Huang CC, Liaw JF, Fang JT, Lee CC, Chien HC. Prevention of hepatitis C viral infection in the dialysis unit: to reuse dialyzer or not? [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:522. [CENTRAL: CN‐00509241]
Mohamed 2010 {published data only}
- Mohamed, Waleed Z. Prevention of hepatitis C virus in hemodialysis patients: five years experience from a single center. Saudi Journal of Kidney Diseases & Transplantation 2010;21(3):548‐54. [MEDLINE: ] [PubMed] [Google Scholar]
Ross 2009 {published data only}
- Ross RS, Viazov S, Clauberg R, Wolters B, Fengler I, Eveld K, et al. Lack of de novo hepatitis C virus infections and absence of nosocomial transmissions of GB virus C in a large cohort of German haemodialysis patients. Journal of Viral Hepatitis 2009;16(4):230‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saxena 2003 {published data only}
- Saxena AK, Panhotra BR, Sundaram DS, Naguib M, Venkateshappa CK, Uzzaman W, et al. Impact of dedicated space, dialysis equipment, and nursing staff on the transmission of hepatitis C virus in a hemodialysis unit of the Middle East. American Journal of Infection Control 2003;31(1):26‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shebeb 2006 {published data only}
- Shebeb AM, Kotkat AM, Abd El Reheim SM, Farghaly AG, Fetohy EM. An intervention study for prevention of HCV infection in some hemodialysis units in Alexandria. Journal of the Egyptian Public Health Association 2006;81(1‐2):119‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Valtuille 1998 {published data only}
- Valtuille R, Fernandez JL, Berridi J, Moretto H, Pino N, Rendo P, et al. Evidence of hepatitis C virus passage across dialysis membrane. Nephron 1998;80(2):194‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2003 {published data only}
- Yang CS, Chang HH, Chou CC, Peng SJ. Isolation effectively prevents the transmission of hepatitis C virus in the hemodialysis unit. Journal of the Formosan Medical Association 2003;102(2):79‐85. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Agarwal 2011
- Agarwal SK. Hemodialysis of patients with HCV infection: isolation has a definite role. Nephron 2011;117(4):c328‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Al‐Ghamdi 2004
- Al‐Ghamdi SM. Nurses' knowledge and practice in hemodialysis units: Comparison between nurses in units with high and low prevalence of hepatitis C virus infection. Saudi Journal of Kidney Diseases & Transplantation 2004;15(1):34‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Alfurayh 2000
- Alfurayh O, Sabeel A, Al Ahdal MN, Almeshari K, Kessie G, Hamid M, et al. Hand contamination with hepatitis C virus in staff looking after hepatitis C positive hemodialysis patients. American Journal of Nephrology 2000;20(2):103‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ali 2011
- Ali I, Siddique L, Rehman LU, Khan NU, Iqbal A, Munir I, et al. Prevalence of HCV among the high risk groups in Khyber Pakhtunkhwa. Virology Journal 2011;8:296. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alter 2008
Arenas 2005
- Arenas MD, Sanchez‐Paya J, Barril G, Garcia‐Valdecasas J, Gorriz JL, Soriano A, et al. A multicentric survey of the practice of hand hygiene in haemodialysis units: factors affecting compliance. Nephrology Dialysis Transplantation 2005;20(6):1164‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bashiri 2013
- Bashiri H, Omrani H, Mami M, Rezaee M. Comparison of contamination passing through Iranian and non‐ Iranian filters of ultra filtration dialysis machines in patients with hepatitis C. Hepatitis Monthly 2013;13(1):e5912. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batty 2001
- Batty DS Jr, Swanson SJ, Kirk AD, Ko CW, Agodoa LY, Abbott KC. Hepatitis C virus seropositivity at the time of renal transplantation in the United States: associated factors and patient survival. American Journal of Transplantation 2001;1(2):179‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Butt 2007
- Butt AA, Skanderson M, McGinnis KA, Ahuja T, Bryce CL, Barnato AE, et al. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. Journal of Viral Hepatitis 2007;14(10):688‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CDC 2001
- Anonymous. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR ‐ Morbidity & Mortality Weekly Report. Recommendations & Reports 2001;50(RR‐5):1‐43. [MEDLINE: ] [PubMed] [Google Scholar]
CDC 2009
- Centers for Disease Control and Prevention (CDC). Hepatitis C virus transmission at an outpatient hemodialysis unit‐‐New York, 2001‐2008. MMWR ‐ Morbidity & Mortality Weekly Report 2009;58(8):189‐94. [MEDLINE: ] [PubMed] [Google Scholar]
Covic 2009
- Covic A, Abramowicz D, Bruchfeld A, Leroux‐Roels G, Samuel D, Biesen W, et al. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) hepatitis C guidelines: a European Renal Best Practice (ERBP) position statement. Nephrology Dialysis Transplantation 2009;24(3):719‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Djordjevic 2000
- Djordjevic V, Stojanovic K, Stojanovic M, Stefanovic V. Prevention of nosocomial transmission of hepatitis C infection in a hemodialysis unit. A prospective study. International Journal of Artificial Organs 2000;23(3):181‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Elamin 2011
- Elamin S, Abu‐Aisha H. Prevention of hepatitis B virus and hepatitis C virus transmission in hemodialysis centers: review of current international recommendations. Arab Journal of Nephrology and Transplantation 2011;4(1):35‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Espinosa 2004
- Espinosa M, Martín‐Malo A, Ojeda R, Santamara R, Soriano S, Aguera M, et al. Marked reduction in the prevalence of hepatitis C virus infection in hemodialysis patients: causes and consequences. American Journal of Kidney Diseases 2004;43(4):685‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fabrizi 2008
- Fabrizi F, Messa P, Martin P. Transmission of hepatitis C virus infection in hemodialysis: current concepts. International Journal of Artificial Organs 2008;31(12):1004‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fabrizi 2012
- Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality?. Journal of Viral Hepatitis 2012;19(9):601‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Finelli 2005
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis‐associated diseases in the United States, 2002. Seminars in Dialysis 2005;18(1):52‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fissell 2004
- Fissell RB, Bragg‐Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney International 2004;65(6):2335‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geddes 2011
- Geddes C, Lindley E, Duncan N. Renal Association Clinical Practice Guideline on prevention of blood borne virus infection in the renal unit. Nephron 2011;118 Suppl 1:c165‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girou 2008
- Girou E, Chevaliez S, Challine D, Thiessart M, Morice Y, Lesprit P, et al. Determinant roles of environmental contamination and noncompliance with standard precautions in the risk of hepatitis C virus transmission in a hemodialysis unit. Clinical Infectious Diseases 2008;47(5):627‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hsu 2015
Jadoul 1998
- Jadoul M, Cornu C, Ypersele de Strihou C. Universal precautions prevent hepatitis C virus transmission: a 54 month follow‐up of the Belgian Multicenter Study. The Universitaires Cliniques St‐Luc (UCL) Collaborative Group. Kidney international 1998;53(4):1022‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jadoul 2004
- Jadoul M, Poignet JL, Geddes C, Locatelli F, Medin C, Krajewska M, et al. The changing epidemiology of hepatitis C virus (HCV) infection in haemodialysis: European multicentre study. Nephrology Dialysis Transplantation 2004;19(4):904‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jadoul 2012
- Jadoul M, Barril G. Hepatitis C in hemodialysis: epidemiology and prevention of hepatitis C virus transmission. Contributions to Nephrology 2012;176:35‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kamili 2007
- Kamili S, Krawczynski K, McCaustland K, Li X, Alter MJ. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infection Control & Hospital Epidemiology 2007;28(5):519‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2008
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney International ‐ Supplement 2008;73(109):S1‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2014
- Lee MH, Yang HI, Yuan Y, L'Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World Journal of Gastroenterology 2014;20(28):9270‐80. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmoud 2004
- Mahmoud IM, Elhabashi AF, Elsawy E, El‐Husseini AA, Sheha GE, Sobh MA. The impact of hepatitis C virus viremia on renal graft and patient survival: a 9‐year prospective study. American Journal of Kidney Diseases 2004;43(1):131‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marwaha 2014
- Marwaha N, Sachdev S. Current testing strategies for hepatitis C virus infection in blood donors and the way forward. World Journal of Gastroenterology 2014;20(11):2948‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbaeyi 2013
- Mbaeyi C, Thompson ND. Hepatitis C virus screening and management of seroconversions in hemodialysis facilities. Seminars in Dialysis 2013;26(4):439‐46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales 2012
- Morales JM, Kamar N, Rostaing L. Hepatitis C and renal disease: epidemiology, diagnosis, pathogenesis and therapy. Contributions to Nephrology 2012;176:10‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Natov 2005
- Natov SN, Pereira BJ. Hepatitis C virus in chronic dialysis patients. Minerva Urologica e Nefrologica 2005;57(3):175‐97. [MEDLINE: ] [PubMed] [Google Scholar]
Ozer Etik 2015
- Ozer Etik D, Ocal S, Boyacioglu AS. Hepatitis C infection in hemodialysis patients: a review. World Journal of Hepatology 2015;7(6):885‐95. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2010
- Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. American Journal of Kidney Diseases 2010;56(2):371‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Samandari 2005
- Samandari T, Malakmadze N, Balter S, Perz JF, Khristova M, Swetnam L, et al. A large outbreak of hepatitis B virus infections associated with frequent injections at a physician's office. Infection Control & Hospital Epidemiology 2005;26(9):745‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sartor 2004
- Sartor C, Brunet P, Simon S, Tamalet C, Berland Y, Drancourt M. Transmission of hepatitis C virus between hemodialysis patients sharing the same machine. Infection Control & Hospital Epidemiology 2004;25(7):609‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saune 2011
- Saune K, Kamar N, Miedouge M, Weclawiak H, Dubois M, Izopet J, et al. Decreased prevalence and incidence of HCV markers in haemodialysis units: a multicentric French survey. Nephrology Dialysis Transplantation 2011;26(7):2309‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schneeberger 2000
- Schneeberger PM, Keur I, Loon AM, Mortier D, Coul KO, Haperen AV, et al. The prevalence and incidence of hepatitis C virus infections among dialysis patients in The Netherlands: a nationwide prospective study. Journal of Infectious Diseases 2000;182(5):1291‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selm 2010
- Selm SB. Prevalence of hepatitis C virus infection among hemodialysis patients in a single center in Yemen. Saudi Journal of Kidney Diseases & Transplantation 2010;21(6):1165‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Selvarajah 2012
- Selvarajah S, Busch MP. Transfusion transmission of HCV, a long but successful road map to safety. Antiviral Therapy 2012;17(7 Pt B):1423‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimokura 2011
- Shimokura G, Chai F, Weber DJ, Samsa GP, Xia GL, Nainan OV, et al. Patient‐care practices associated with an increased prevalence of hepatitis C virus infection among chronic hemodialysis patients. Infection Control & Hospital Epidemiology 2011;32(5):415‐24. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2013
- Su Y, Norris JL, Zang C, Peng Z, Wang N. Incidence of hepatitis C virus infection in patients on hemodialysis: a systematic review and meta‐analysis. Hemodialysis International 2013;17(4):532‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sun 2009
- Sun J, Yu R, Zhu B, Wu J, Larsen S, Zhao W. Hepatitis C infection and related factors in hemodialysis patients in China: systematic review and meta‐analysis. Renal Failure 2009;31(7):610‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thompson 2009
- Thompson ND, Novak RT, Datta D, Cotter S, Arduino MJ, Patel PR, et al. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infection Control & Hospital Epidemiology 2009;30(9):900‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment (Winchester, England) 1999;3(5):iii‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Valtuille 2002
- Valtuille R, Moretto H, Lef L, Rendo P, Fernandez JL. Decline of high hepatitis C virus prevalence in a hemodialysis unit with no isolation measures during a 6‐year follow‐up. Clinical Nephrology 2002;57(5):371‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vladutiu 2000
- Vladutiu DS, Cosa A, Neamtu A, State D, Braila M, Gherman M, et al. Infections with hepatitis B and C viruses in patients on maintenance dialysis in Romania and in former communist countries: yellow spots on a blank map?. Journal of Viral Hepatitis 2000;7(4):313‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Voiculescu 2010
- Voiculescu M, Iliescu L, Ionescu C, Micu L, Ismail G, Zilisteanu D, et al. A cross‐sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South‐Eastern regions of Romania. Journal of Gastrointestinal & Liver Diseases 2010;19(1):43‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Webster 2015
- Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015;385(9973):1124‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2004
- Williams J. Guidelines to determine who should be tested for hepatitis C. Nursing Times 2004;100(28):28‐9. [MEDLINE: ] [PubMed] [Google Scholar]
References to other published versions of this review
Bravo Zuniga 2007
- Bravo Zuñiga JI, Loza Munarriz C, López‐Alcalde J. Isolation as a strategy for controlling the transmission of hepatitis C virus (HCV) infection in haemodialysis units. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006420] [DOI] [PMC free article] [PubMed] [Google Scholar]


