Abstract
Purpose:
Multiple Myeloma (MM), the second leading blood malignancy, has complex and costly disease management. We studied patterns of treatment disparities and unplanned interruptions among the MM patients after the Affordable Care Act to assess their prevalence and effect on survival.
Materials and Methods:
This retrospective study of 1002 MM patients at a tertiary referral center used standard guidelines as a reference to identify underuse of effective treatments. We used multivariate logistic regression and Cox proportionate hazard to study the prognostic effect on survival.
Results:
Median age in the cohort was 63.0 [IQR: 14] years. Non-Hispanic White (NHW) patients were older (p=0.007) and more likely to present with stage I disease (p=0.02). Underuse of maintenance therapy (aOR=1.98; 95%CI 1.12–3.48) and interruptions in treatment were associated with race/ethnicity and insurance (aOR=4.14; 95%CI: 1.78–9.74). Only underuse of induction therapy was associated with overall patient survival.
Conclusion:
Age, race, ethnicity and primary insurance contribute to the underuse of treatment and in unplanned interruptions in MM treatment. Addressing underuse causes in such patients is warranted.
Keywords: Multiple myeloma, induction therapy, sociodemographic disparities, patient outcome
Overview and Objectives
With more than 25,000 new cases diagnosed and 12,000 deaths per annum1, multiple myeloma (MM) is the second most common blood malignancy in the United States and its morbidity and mortality burden may vary by age, race and gender.1 The expansion of available therapeutic options in recent years has inspired optimism regarding the disease-related survival. Over 60% rates of very good partial response or better has been observed among younger and transplant eligible patients who had received pre-transplant induction therapy.2 However, disparities among racial groups are reported in several studies3–8 and the high cost of MM treatment can be a barrier to care.9 Given rising rates of MM particularly in New York City (NYC),10 improved therapies and national reports of disparities, we aimed to assess the extent of disparities in administration of induction therapy, Autologous Stem Cell Transplant (ASCT) and maintenance therapy in the years following the Affordable Care Act, in a state with generous Medicaid insurance at an inner city academic medical center. The key study aims are: 1. To assess disparities in treatment and unplanned care interruptions by sociodemographic factors and identify factors associated with underuse of treatment modalities by multivariate model; 2. To investigate if underuse of a specific type of treatment modality has an impact on patient survival and assess its relative significance compared with other covariates, including comorbidity and disease stage at diagnosis.
Materials and Methods
The study was approved by the Institutional Review Board.
Study population
We identified a retrospective cohort of 3101 patients at a NYC tertiary referral center between 2010 and 2014 using ICD −9 code 203.0. As this ICD did not distinguish between MM, smoldering myeloma and MGUS, we manually reviewed charts and excluded patients with smoldering myeloma and MGUS, leaving a final cohort of 1002 active MM patients.
Variables
We abstracted: demographic variables, primary insurance, Charlson Comorbidity Index (CCI), components of disease stage at diagnosis to calculate International Staging System (ISS), and treatment variables. If treatments were not given, we abstracted the reasons why as indicated in physicians’ progress notes. Patients’ self-reported race and ethnicity variables were merged to derive a race/ethnicity variable. Hispanic race/ethnicity included patients of Hispanic ethnicity irrespective of their racial background. Patients who either reported their background as Other or Unknown were categorized into Other. The CCI values were grouped into none (CCI=0), mild to moderate (CCI=1–4) and severe comorbidity (CCI >=5). Insurance was categorized as Medicaid for patients with Medicaid and dual eligible (Medicaid and Medicare), private for those with primary commercial insurance, Medicare for patients with Medicare only and with Medigap insurance, self-pay and unknown. Data were abstracted from progress notes to identify any unplanned interruptions in treatment. To define overall survival (OS), we obtained patients’ vital status, dates of last contact and death from the electronic medical record and from the American College of Surgeons certified tumor registry which must maintain 90% follow-up for ongoing certification.11
Treatment modalities and definition of underuse
Underuse was defined as lack of a particular treatment modality, not a specific regimen, that patient was eligible to receive for their primary treatment: Induction, ASCT, Maintenance- according to the NCCN 2014 guidelines (version 2.2014)12 (see Appendix). Primary treatment consists of completion of the first set of chemotherapy regimen for induction, followed by ASCT and maintenance.
All patients should undergo induction therapy, except those with poor prognosis due to other comorbid conditions. To be eligible for ASCT, patients had to: complete induction therapy with at least a partial response, be < 77 years of age, have albumin levels of >2.5, an ejection fraction (EF) of >20%, an ECOG of <4, and not have a poor prognosis from another comorbidity. We chose an EF of >20% based on expert opinion and data noting EFs <50% were permissible for patients treated at a center with strong cardiology specialty services.13, 14 Age cutoff was based on expert opinion and prior CMS restrictions. Performance status was taken at date of diagnosis or from initial visit closest to diagnosis. Patients who complete induction therapy, followed by ASCT, or transplant ineligible patients who obtained at least a stable disease are eligible for maintenance therapy.
Patients who were noted to receive treatment later, had known clinical contraindications to respective treatments according to the data obtained from progress notes or follow-up calls were excluded from underuse classification. We called patients assigned to underuse category to ascertain treatment and status.
Unplanned interruptions in treatment
When treatment was interrupted due to issues limiting access to care, such as loss or lapse of insurance coverage, lack of transportation, or inability to pay treatment-related out-of-pocket expenses as abstracted from progress notes, it was coded as unplanned treatment interruption.
Statistical analysis
Tests of association for underuse with categorical covariates were performed using the Chi-square test or Fisher’s exact test. One sample t-tests were performed to test the difference of means for continuous covariates. Multivariate logistic regression with generalized linear model was fitted to identify sociodemographic and other patient variables associated with the underuse of induction, transplant, maintenance and interrupted therapies. Univariate and multivariable Cox regression models with Breslow approximation to maintain backwards compatibility were applied to test the association of sociodemographic variables, treatment modalities, disease stage and comorbidities (CCI) as defined by the International Staging System (ISS) to the overall survival (OS). OS was defined as the time between primary diagnosis to the time of death (reported without specific cause of death) or the time of last contact (censored).
Results
Descriptive analysis
Median age in the study cohort was 63.0 [IQR: 14] years with 56.7% males; 22% of patients reported their race-ethnicity as Other (see Table 1). Patients with the Non-Hispanic White (NHW) and Other racial/ethnicity background (OTH) were older than NHB, Hispanic and Asian patients (NHW :63.8 ±11.5, Non-Hispanic Black (NHB): 61.2 ± 11.2, Hispanic: 60.3 ± 10.2, Asian :61.2 ± 9.4, Others (OTH): 63.5 ± 11.2; p=0.007). Statistically significant racial differences were observed among Asian, Hispanic White, NHB, NHW patients by their primary insurances (p<0.001). NHW patients had the lowest rate of Medicaid (3.9%) and highest rate of private insurance use (47.1%) (data not shown).
Table 1:
Patient characteristics and patterns of underuse and breaks in treatments.
| Overall | Underuse of induction therapy | Overall | Underuse of transplant | Overall | Underuse of maintenance therapy | Overall | Interruptions in treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P | No | Yes | P | No | Yes | P | No | Yes | P | |||||
| N | 1002 | 925 | 77 | 514 | 346 | 168 | 542 | 437 | 105 | 967 | 921 | 46 | ||||
| Age groups | <0.001 | <0.001 | 0.624 | 0.14 | ||||||||||||
| 18–45 y | 60 (6.0) | 57 (95.0) | 3 (5.0) | 35 (6.8) | 30 (85.7) | 5 (14.3) | 33 (6.1) | 27 (81.8) | 6 (18.2) | 59 (6.1) | 54 (91.5) | 5 (8.5) | ||||
| 45–65 y | 551 (55.0) | 525 (95.3) | 26 (4.7) | 328 (63.8) | 238 (72.6) | 90 (27.4) | 329 (60.7) | 262 (79.6) | 67 (20.4) | 540 (55.8) | 510 (94.4) | 30 (5.6) | ||||
| 65–80 y* | 329 (32.8) | 296 (90.0) | 33 (10.0) | 151 (29.4) | 78 (51.7) | 73 (48.3) | 170 (31.4) | 141 (82.9) | 29 (17.1) | 311 (32.2) | 302 (97.1) | 9 (2.9) | ||||
| >80 y | 62 (6.2) | 47 (75.8) | 15 (24.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (1.8) | 7 (70.0) | 3 (30.0) | 57 (5.9) | 55 (96.5) | 2 (3.5) | ||||
| Gender | 0.55 | 0.92 | 0.084 | 0.99 | ||||||||||||
| Female | 434 (43.3) | 36 (8.3) | 235 (45.7) | 76 (32.3) | 250 (46.1) | 210 (84.0) | 40 (16.0) | 422 (43.6) | 402 (95.3) | 20 (4.7) | ||||||
| Male | 568 (56.7) | 41 (7.2) | 279 (54.3) | 92 (33.0) | 292 (53.9) | 227 (77.7) | 65 (22.3) | 545 (56.4) | 519 (95.2) | 26 (4.8) | ||||||
| Race and ethnicity | 0.005 | 0.031 | 0.29 | <0.001 | ||||||||||||
| 458 (45.7) | 425 (92.8) | 33 (7.2) | 239 (46.5) | 162 (67.8) | 77 (32.2) | 250 (46.1) | 209 (83.6) | 41 (16.4) | 442 (45.7) | 432 (97.7) | 10 (2.3) | |||||
| Non-Hispanic Black | 199 (19.9) | 191 (96.0) | 8 (4.0) | 105 (20.4) | 75 (71.4) | 30 (28.6) | 111 (20.5) | 83 (74.8) | 28 (25.2) | 192 (19.9) | 176 (91.7) | 16 (8.3) | ||||
| Hispanic | 96 (9.6) | 91 (94.8) | 5 (5.2) | 58 (11.3) | 45 (77.6) | 13 (22.4) | 59 (10.9) | 45 (76.3) | 14 (23.7) | 94 (9.7) | 82 (87.2) | 12 (12.8) | ||||
| Asian | 27 (2.7) | 26 (96.3) | 1 (3.7) | 15 (2.9) | 11 (73.3) | 4 (26.7) | 16 (3.0) | 14 (87.5) | 2 (12.5) | 27 (2.8) | 25 (92.6) | 2 (7.4) | ||||
| Other | 222 (22.2) | 192 (86.5) | 30 (13.5) | 97 (18.9) | 53 (54.6) | 44 (45.4) | 106 (19.6) | 86 (81.1) | 20 (18.9) | 212 (21.9) | 206 (97.2) | 6 (2.8) | ||||
| Primary payor | <0.001 | 0.003 | 0.144 | <0.001 | ||||||||||||
| Private | 423 (42.2) | 399 (94.3) | 24 (5.7) | 245 (47.7) | 177 (72.2) | 68 (27.8) | 249 (45.9) | 195 (78.3) | 54 (21.7) | 414 (42.8) | 402 (97.1) | 12 (2.9) | ||||
| Medicaid | 96 (9.6) | 94 (97.9) | 2 (2.1) | 61 (11.9) | 48 (78.7) | 13 (21.3) | 61 (11.3) | 53 (86.9) | 8 (13.1) | 95 (9.8) | 81 (85.3) | 14 (14.7) | ||||
| Medicare | 431 (43.0) | 393 (91.2) | 38 (8.8) | 195 (37.9) | 113 (57.9) | 82 (42.1) | 214 (39.5) | 177 (82.7) | 37 (17.3) | 409 (42.3) | 393 (96.1) | 16 (3.9) | ||||
| Selfpay | 16 (1.6) | 13 (81.2) | 3 (18.8) | 6 (1.2) | 4 (66.7) | 2 (33.3) | 9 (1.7) | 5 (55.6) | 4 (44.4) | 16 (1.7) | 14 (87.5) | 2 (12.5) | ||||
| Unknown | 28 (2.8) | 21 (75.0) | 7 (25.0) | 4 (0.8) | 2 (50.0) | 2 (50.0) | 6 (1.1) | 5 (83.3) | 1 (16.7) | 26 (2.7) | 25 (96.2) | 1 (3.8) | ||||
| International Staging System | 0.04 | 0.084 | 0.264 | 0.49 | ||||||||||||
| Stage I | 298 (29.7) | 278 (93.3) | 20 (6.7) | 178 (34.6) | 128 (71.9) | 50 (28.1) | 183 (33.8) | 156 (85.2) | 27 (14.8) | 292 (30.2) | 280 (95.9) | 12 (4.1) | ||||
| Stage II | 178 (17.8) | 167 (93.8) | 11 (6.2) | 100 (19.5) | 63 (63.0) | 37 (37.0) | 106 (19.6) | 88 (83.0) | 18 (17.0) | 172 (17.8) | 166 (96.5) | 6 (3.5) | ||||
| Stage III | 226 (22.6) | 221 (97.8) | 5 (2.2) | 110 (21.4) | 66 (60.0) | 44 (40.0) | 123 (22.7) | 96 (78.0) | 27 (22.0) | 218 (22.5) | 205 (94.0) | 13 (6.0) | ||||
| Charlson Comorbidity Index | 0.92 | 0.008 | 0.177 | 0.937 | ||||||||||||
| None | 414 (41.3) | 384 (92.8) | 30 (7.2) | 223 (43.4) | 135 (60.5) | 88 (39.5) | 229 (42.3) | 177 (77.3) | 52 (22.7) | 397 (41.1) | 377 (95.0) | 20 (5.0) | ||||
| Mild to Moderate | 447 (44.6) | 411 (91.9) | 36 (8.1) | 222 (43.2) | 157 (70.7) | 65 (29.3) | 240 (44.3) | 197 (82.1) | 43 (17.9) | 433 (44.8) | 413 (95.4) | 20 (4.6) | ||||
| Severe | 141 (14.1) | 130 (92.2) | 11 (7.8) | 69 (13.4) | 54 (78.3) | 15 (21.7) | 73 (13.5) | 63 (86.3) | 10 (13.7) | 137 (14.2) | 131 (95.6) | 6 (4.4) | ||||
(bracketed values in overall columns are column percentages, whereas bracketed values in rest of the columns are row percentages; * 65–80 years age group in case of underuse of transplant represents 65–77 years)
Disease stage at diagnosis varied by race/ethnicity and primary insurance provider (p=0.5 and 0.84, respectively), with the highest rate of stage I at diagnosis among the OTH (49%) and NHWs (43%). Privately insured and self-paying patients had the highest rate of stage I presentation (46.8% and 50%, respectively). There was no significant association between missing ISS and underuse; 242 patients were unstaged.
Potential predictors of the underuse of treatment modalities (see Figure 1)
Figure 1: Multivariate model for potential predictors of underuse of treatment modalities and interruptions in treatment.
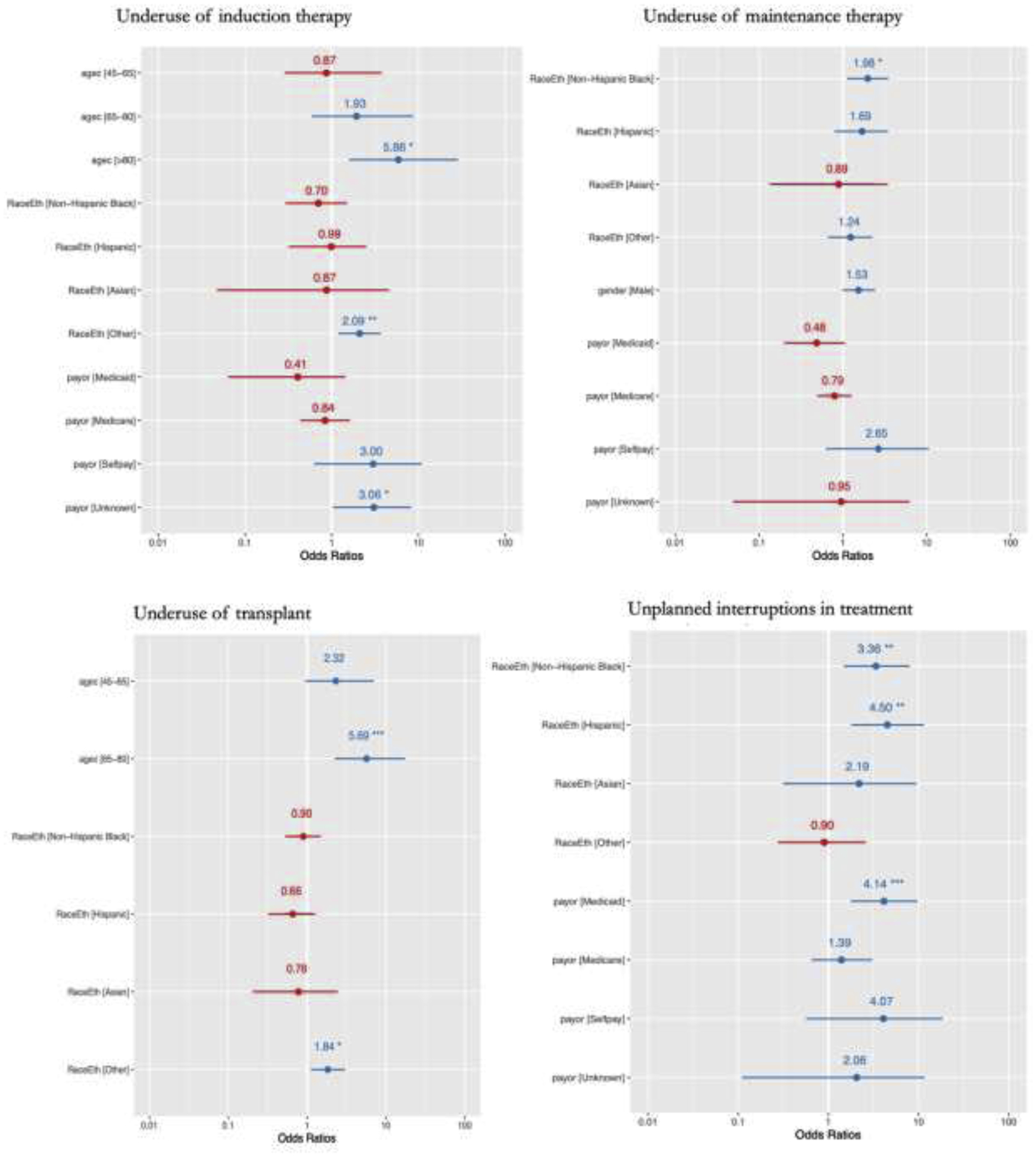
A: Underuse of therapeutic modalities
Underuse of induction
Overall, induction underuse was 7.7%. It was highest among patients with OTH and NHW backgrounds (7.2% and 13.5%, respectively), patients aged ≥65 years (>80 years: 24.2%; 65–80 years: 10.0%), and with ISS stage I (6.8%) and II (6.2%). The lowest underuse was observed among the Medicaid patients (Medicaid: 2.1%; Medicare: 8.8%; private insurance: 5.7%).
In a multivariate model, OTH race and ethnicity (adjusted odds ratio (aOR) = 2.1; 95% CI: 1.2–3.7, p=0.01), and age > 80 (aOR: 5.9, 95% CI: 1.6–28.6, p-value=0.01) and unknown primary payor (aOR: 3.1, 95% CI: 1.0–8.2, p-value=0.03) emerged as significant predictors.
Underuse of ASCT
Overall, 32.7% of eligible patients did not undergo ASCT. Differences in underuse of ASCT were significant by race and ethnicity, primary payor and comorbidity status. Underuse was more pronounced among the ≥65 year age group (48.3% vs. 14.3% among 18–45 years), NHWs (32.2%) and OTH (45.4%) backgrounds, patients with Medicare (42.1% vs. 21.3% among the Medicaid patients) and remarkably among the patients having no comorbidity (39.5% vs. 21.7% among the patients having severe comorbidity). In a multivariate model, OTH race and ethnicity (aOR = 1.8; 95% CI: 1.1–3.0, p=0.02) and age group 65–80 years (aOR: 5.7, 95% CI: 2.3–17.5, p-value<0.001) emerged as significant predictors.
Underuse of maintenance therapy
Overall, 10.4% of eligible patients did not receive maintenance therapy. Underuse of maintenance therapy was highest among the patients aged 45–65 years (20.4% vs. 17.1% among 65–80 years age group), NHB and Hispanic backgrounds (25.2% and 23.7%, respectively vs. 12.5% and 16.4% among Asian and NHW patients, respectively), and patients holding private insurance (21.7% vs. 13.1% and 17.3% among the Medicaid and Medicare, respectively vs. 44.4% and 21.7% among self-paying and patients having private insurance). Men had higher rates of maintenance underuse than women (22.3% v 15.0%).
In a multivariate model, the NHB background (aOR = 2.0; 95% CI: 1.1–3.5, p=0.02) and Medicaid insurance (aOR: 0.5; 95% CI: 0.2–1.1; p-value<0.09; compared to the private insurance) emerged as significant predictors. Moreover, we observed weaker significance for male gender (aOR = 1.5; 95% CI: 1.0–2.4, p=0.06).
Unplanned interruptions in treatment
Unplanned interruptions in treatment occurred in 4.8% of patients - most commonly among Medicaid beneficiaries (14.7% vs. 2.9% among patients with private insurance). Hispanic and NHBs had the highest rate of unplanned treatment interruptions (12.8% and 8.3%, respectively) vs 2.3% and 2.8% among NHW and OTH patients.
Multivariate modelling of unplanned interruptions in treatment found that patients with Medicaid (aOR: 4.1; 95% CI: 1.8–9.7; p-value=0.001), and those who were Hispanic (aOR: 4.5; 95% CI: 1.8–11.5; p-value=0.001) or Black (aOR: 3.4; 95% CI: 1.5–8.0; p-value=0.004) were most affected.
Patient survival
By November 2019, overall, 80% of patients were alive. The median follow-up period [IQR] was 57.1 [23.7–79.0] months, and it significantly differed by race and ethnicity (log rank test p=0.02). The longest median follow-up was observed among the Hispanics (68.1 months), and the shortest (38.2 months) among the patients with OTH background. Significant differences were observed in OS by race and ethnicity with shortest mean OS among OTH patients (40.0 months) and the longest among Asians (49.9 months).
Predictors of patient outcome
Univariate analysis testing the association of potential factors with the OS identified age (group) at primary diagnosis, disease stage by ISS, comorbidity (by CCI categories), underuse of induction therapy, primary insurance provider, race and ethnicity (Figure 2).
Figure 2: Differences in probability of survival by the ISS staging, Charlson Comorbidity Index and by underuse of induction therapy.
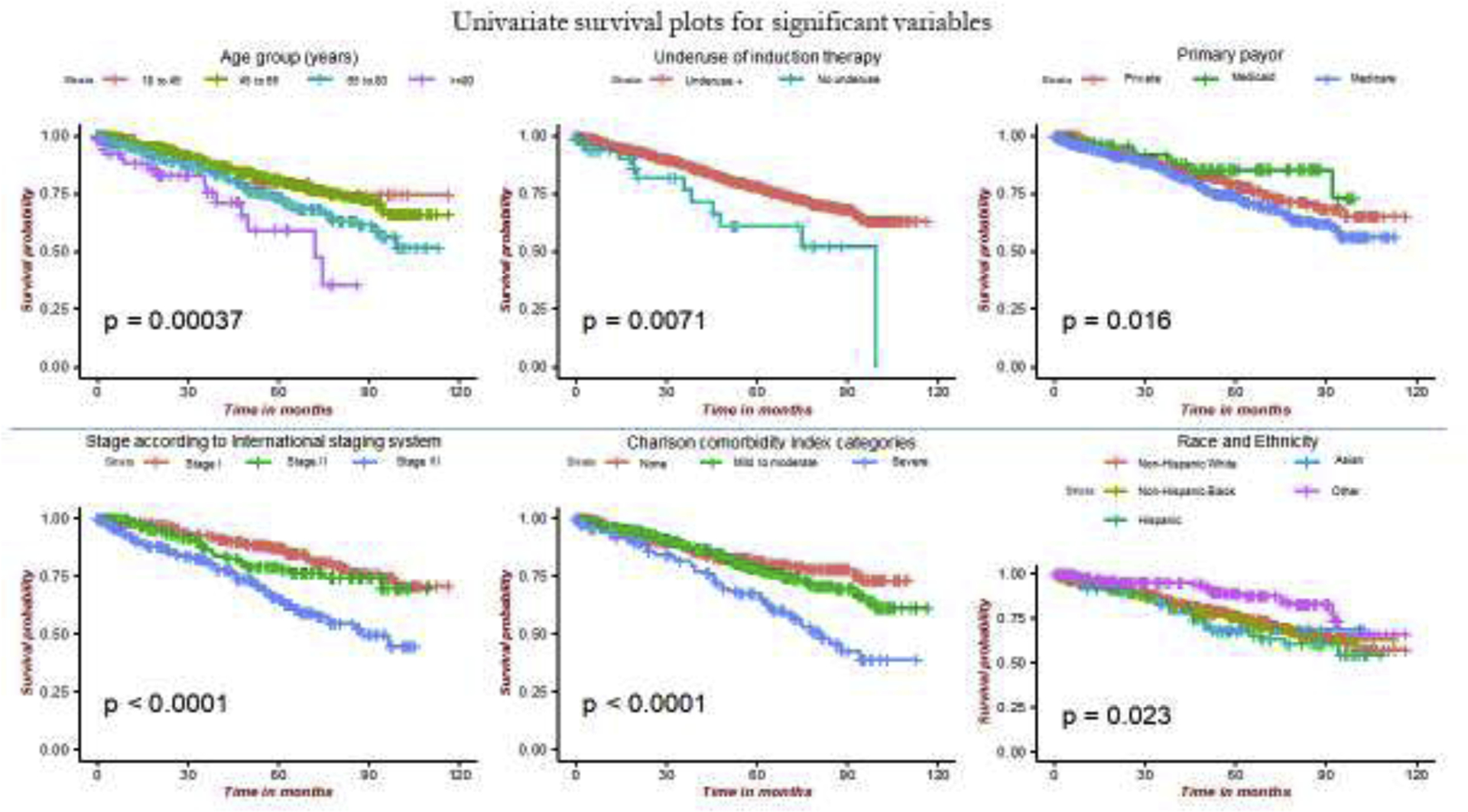
(ISS: international staging system; CCI: Charlson Comorbidity Index groups; under_induct: underuse of induction therapy; payor: Primary insurance payor; RaceEth: Race and ethnicity)
Among the treatment modalities, underuse of induction therapy was significantly associated with a lower OS in a univariate (p=0.007) with a median time (IQR) for follow-up of 6.10 months (0.67; 51.73) and in a multivariate Cox proportionate hazard model (aOR:5.0; 95% CI: 2.1–11.8; p<0.001) after controlling for disease stage, co-morbidity and gender (Figure 3).
Figure 3:
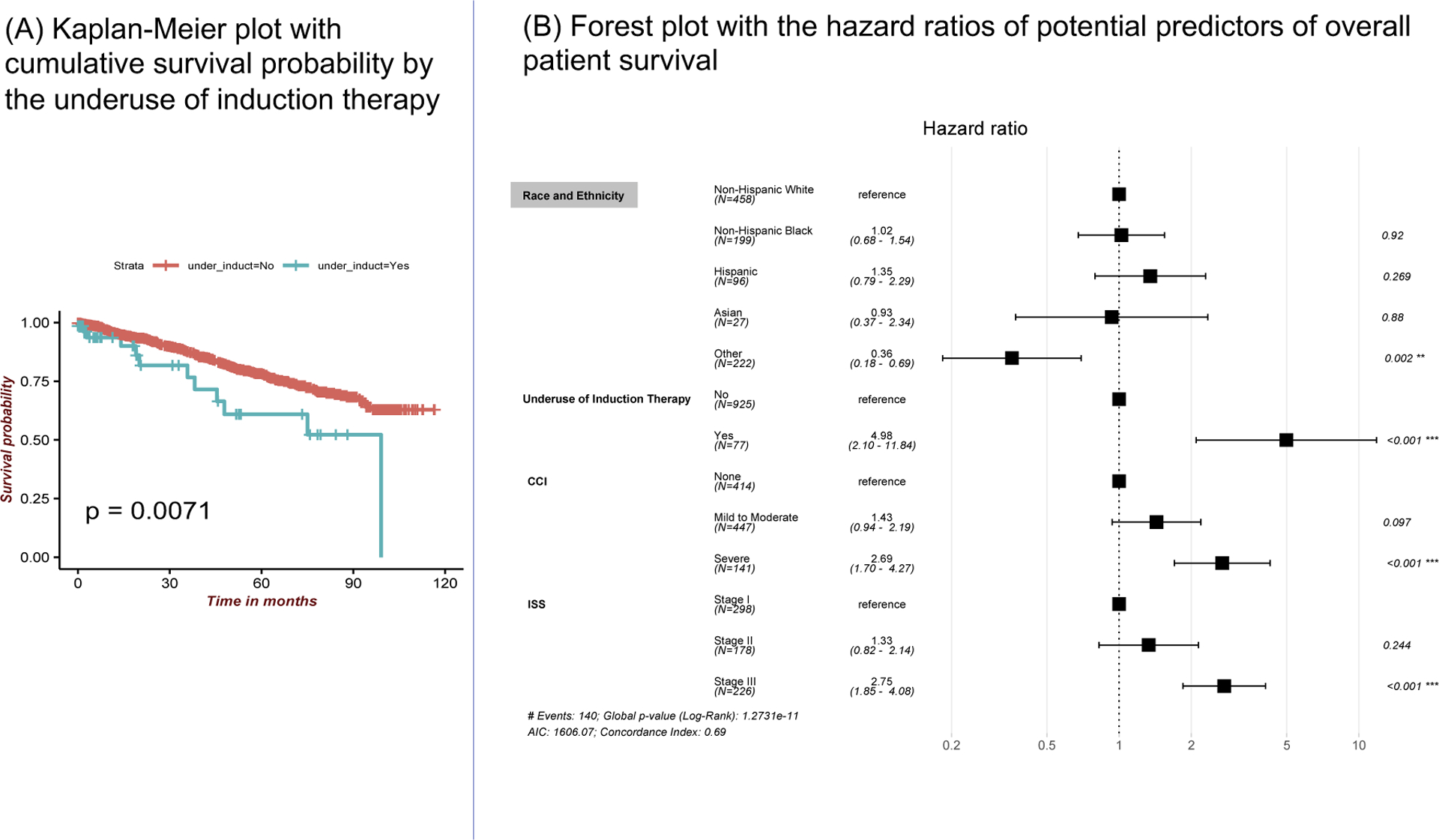
Results survival analysis: (A) Univariate Kaplan-Meier plot shows the cumulative probability of patient survival by the underuse of induction therapy. (B) Results of the multivariate Cox proportional hazards model of overall patient survival displayed as a forest plot with hazard ratios for underuse of induction therapy, race and ethnicity, ISS staging and Charlson comorbidity index. (ISS: international staging system; CCI: Charlson Comorbidity Index categories)
Among patients >65 years with Medicare, underuse of induction therapy was significantly associated with reduced OS (aOR: 4.5; 95% CI: 1.3–16.1) along with the comorbidity and disease stage indicating that the effect of underuse of induction therapy is independent of age or insurance. Further, in a subset of patients of 65–80 years age group with Medicare, Medicaid or commercial as their primary insurance, underuse of induction therapy was significantly associated with reduced survival (aOR:6.1; 95% CI: 1.2–31.1; p=0.03), as were comorbidities and the disease stage at diagnosis. Importantly, social determinants – race, ethnicity, primary insurance- did not affect overall survival.
Unplanned interruptions, underuse of transplant and underuse of maintenance therapy were not associated with the OS in our study.
Discussion
In this study, we found varying rates of underuse of effective treatments in the management of MM in a single institution serving an inner-city population. Not surprisingly, older age and sicker patients experienced higher rates of underuse.15 Importantly, minority race-ethnicity had varying effects– it played no role in underuse of induction or transplant but did affect use of maintenance therapy and interruptions in care as did those insured with Medicaid. The fact that race-ethnicity independently affected underuse suggests that patients and/or physicians’ beliefs and circumstances may exert an effect beyond access to care. Fortunately, these underuse episodes did not translate into worse survival.
More than one fifth of patients self-identified their race-ethnicity as “other.” We found greater underuse of induction and ASCT therapy for these patients. This group was most likely to present with early stage disease and to have commercial insurance. Because significant proportions of Hispanics self- identify their race as Hispanic,16 we classified Hispanic as its own racial-ethnic group and did not include them in the OTH category. We chose this approach as our primary hypothesis was that race-ethnicity would function as a social construct to affect treatment rates and thus, survival. Given the increasing proportions of individuals who self-identify as multi-racial/ethnic and as our nation moves towards greater granularity in collecting race-ethnicity data, we need to be clear about how these data will help stimulate change in care delivery and research.
Ensuring equity in care related decisions is vital to optimize MM related mortality and morbidity. Given known racial differences in incidence, progression and survival in MGUS, a premalignant MM condition, as well as in active MM,17, 18 age at diagnosis18 and heterogenous disease biology19 quantifying the impact of social determinants’ on quality of care and subsequent survival is paramount. Equal access to clinically recommended treatment modalities have been shown to mitigate specific disparities in patient outcomes.20 Therapy delivered is the measured outcome, not the offering of therapies. Thus, factors that may contribute to patient refusal or access to care are based on progress notes by physicians, physician assistants, or social workers and may not fully capture barriers resulting in underuse.
To date, racial disparities in MM care have been attributed to insurance coverage,21 delays in payer approvals,22 access and distance to cancer care facilities23 and patients’ ability to afford for uncovered expenses.24 The cost of cancer drugs is increasing exponentially26, 26 with the monthly cost of lenalidomide nearly $8,000,24, 27at a time when insurers are shifting more costs to patients. Given the mounting costs and the challenges posed by under-insurance, it is not surprising that we found underuse of maintenance among middle aged adults with private insurance.28
In our study, we were surprised to find even the small proportion of induction underuse we found. It is possible that this is an overestimate of actual underuse as some patients may have gone to outside institutions for their induction therapy. We tried to minimize the risk of potential underuse misclassification by calling patients with underuse to ascertain “outside” treatments that we may have missed as tracking patients and having them respond to survey is difficult in this age of telemarketing.29 Of note, the fact that induction underuse was significantly associated with poorer survival does provide face validity to this measure. Our findings do correlate with the studies that show significant impact on patient outcome by the induction therapy use,30 comorbidities,31, 32 disease stage,33 and contrasts from the studies that observed differences in patient survival by insurance status.34
The higher rate of ASCT underuse we found, as compared with induction and maintenance, is not surprising. Patients who responded to induction therapy would choose a more conservative treatment path, preferring to wait for progression prior to undergoing additional cycles of chemotherapy, is also not surprising. We are currently interviewing these underuse patients to get a clearer view of their treatment decision-making and get a better understanding of the role of culture and family in these decisions. Further complicating such decisions is the evolving debate of the role of ASCT as a frontline standard treatment, driven by the recent studies.35–38
Underuse of maintenance therapy was significantly associated with black race. Why race-ethnicity seems to have had this effect is uncertain. Both race and insurance were independently associated with underuse. Medicaid protected against underuse of this expensive therapy and black patients in our study were much less likely than Hispanics or Asians to have Medicaid (14% vs 22% & 33%, respectively). We did not find a survival advantage to maintenance therapy, a finding that corroborates the prevailing debate about the role of maintenance therapy in prolonging OS.39 We were unable to assess the impact of individual drug/combinations used in maintenance therapy on OS.
Limitations
Underuse was based on chart abstraction and may have misclassified patients treated elsewhere. We did reach out to patients who, based on chart abstraction experienced underuse of induction, transplant, or maintenance to ascertain their status, collecting updated information on treatment and survival. At the same time, limitations exist because we were only able to reach out to 60% of the patients due to challenges in finding and reaching patients. Duration of any unplanned interruptions were not collected; therefore, pattern of treatment could not be analyzed. Limited sample size for assessment of potential predictors underlying the underuse and OS is a key limitation of this study. Performance status was taken at date of diagnosis or from the initial visit close to diagnosis which may result in a bias as patients who complete induction and are referred for transplant or maintenance therapy will likely have improved performance status. However, patients referred for progressive disease likely have worse performance status. The study was unable to account for the biological heterogeneity of MM due to low sample size, and recent molecular advances in precision medicine have resulted in changes in treatment guidelines. Cause of death was not available in the data abstracted from the medical records and from subsequent follow-ups. Therefore, we were unable to study disease-specific survival. Our study population is younger than other population-based cohorts likely due to the many referrals to our institution for ASCT.
Conclusion
Racial-ethnic and insurance related disparities in underuse of MM treatment remain in a tertiary referral center that has standardized MM care delivery and pays special attention to social and access challenges. Underuse of induction therapy appears to affect patient survival. Although we did not find survival benefits with maintenance therapy, if this continues to be a standard of care, it is incumbent upon delivery systems to identify underinsured patients and encourage approaches to reduce associated financial toxicities. Appropriate policy measures to prevent the underuse of effective therapies are necessary in order to alleviate the morbidity and mortality burden from multiple myeloma. Moreover, this study suggests that encouraging accurate self-reporting of sociodemographic by patients can help to identify vulnerable groups, a keystone to design healthcare policies tailored to their needs.
Highlights:
Age, race, ethnicity, and insurance contribute to the underuse of myeloma treatment
Underuse of induction therapy was significantly associated with reduced survival
Minority race-ethnicity affected use of maintenance therapy and interruptions in care
Underuse of maintenance therapy was significantly associated with black race
Funding
Cancer Center Support Grant (CCSG P30CA196521) for HJ,KF,MM,NAB
APPENDIX
UNDERUSE DEFINITIONS*
Underuse is an episode in which effective MM treatment was not received, as per NCCN 2014 guidelines.1
A). Induction chemotherapy
All patients should undergo Induction therapy.
Exceptions: poor prognosis due to other comorbid condition (as documented in MD notes; excludes renal failure)
Underuse of induction= patient received no induction chemotherapy
Following Induction, patients were classified into two subgroups: 1) eligible to undergo autologous transplantation, and 2) ineligible to undergo an autologous transplantation.
B). Assessment of transplant eligibility
All candidates for high dose chemotherapy must have adequate liver, renal, pulmonary, and cardiac function, and frailty is a major determinant.2–4
Exceptions**:
>77years-old [CMS.gov – (CAG-00011N); expert opinion];
Albumin <2.5;
EF < 20%;
ECOG >4
Transplant eligible patients meet above criteria, received induction therapy and obtained at least a Partial Response (PR) to the induction therapy. A PR is defined as a 50% decrease either in measurable paraprotein (serum and/or urine) or in bone marrow infiltration by malignant plasma cells, and sustained for at least one month.
Underuse of Transplant = transplant eligible patient who does not undergo autologous hematopoietic cell transplantation (HCT).
C). Maintenance therapy
Patients who obtained a stable disease or a marginal, partial, or complete response in the first 100–110 days after undergoing an autologous HCT, or transplant ineligible patients who obtained a stable disease, marginal, partial or complete response in the first 100–110 days after completing induction therapy, are eligible for maintenance therapy [McCarthy PL. N Engl J Med 2012; 366:1770].
Exceptions:
Albumin <2.5;
EF < 20%;
ECOG >4
Underuse of Maintenance = patient eligible to receive maintenance therapy and does not receive maintenance therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Sundar Jagannath declares a conflict of interest with Bristol-Myers Squibb, Celgene Corporation, Janssen Pharmaceuticals, Karyopharm Therapeutics, Legend Biotech, Sanofi, and Takeda. All other authors declare that there is no conflict of interest.
Ethics approval and consent to participate
IRB# 16-00612
For each group, underuse for each suggested phase of treatment is based on NCCN 2014 MM guidelines. Note – no significant general treatment changes in guidelines from 2010–2014.
[As patients with poor renal function can undergo dialysis, renal function not included in eligibility criteria]
References
- 1.US Cancer Statistics Working Group: U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999–2016): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute [Internet]. 2019. Available from: www.cdc.gov/cancer/dataviz
- 2.Usmani SZ, Hoering A, Cavo M, et al. : Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma — an IMWG Research Project. Blood Cancer J 8:123, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ailawadhi S, Frank RD, Advani P, et al. : Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: a SEER-medicare analysis. Cancer Med 6:2876–2885, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ailawadhi S, Aldoss IT, Yang D, et al. : Outcome disparities in multiple myeloma: A SEER-based comparative analysis of ethnic subgroups. Br J Haematol, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Ailawadhi S, Jacobus S, Sexton R, et al. : Disease and outcome disparities in multiple myeloma: exploring the role of race/ethnicity in the Cooperative Group clinical trials [Internet]. Blood Cancer J 8:67, 2018[cited 2020 Feb 20] Available from: http://www.ncbi.nlm.nih.gov/pubmed/29980678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schriber JR, Hari PN, Ahn KW, et al. : Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatnagar V, Wu Y, Goloubeva OG, et al. : Disparities in black and white patients with multiple myeloma referred for autologous hematopoietic transplantation: A single center study [Internet]. Cancer 121:1064–1070, 2015[cited 2020 Feb 20] Available from: http://doi.wiley.com/10.1002/cncr.29160 [DOI] [PubMed] [Google Scholar]
- 8.Fiala MA, Wildes TM. Racial disparities in treatment use for multiple myeloma. Cancer. 2017;123(9):1590–1596. doi: 10.1002/cncr.30526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olszewski AJ, Dusetzina SB, Eaton CB, et al. : Subsidies for Oral Chemotherapy and Use of Immunomodulatory Drugs Among Medicare Beneficiaries With Myeloma. J Clin Oncol 35:3306–3314, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath GR, Renteria AS, Jagannath S, et al. : Where you live can impact your cancer risk: a look at multiple myeloma in New York City. Ann Epidemiol 48:43–50.e4, 2020 [DOI] [PubMed] [Google Scholar]
- 11.American College of Surgeons: Optimal Resources for Cancer Care:2020 Standards. 2020
- 12.NCCN panel: NCCN clinical practice guidelines in oncology : Multiple Myeloma. 2014 [DOI] [PubMed]
- 13.AymanSaad A, Mahindra A, Zhang MJ, Zhong X, Costa LJ, Drobyski WR, Freytes CO, Gale RP, Gasparetto CJ, Leona A.Holmberg LA, Kamble RT, Krishnan AY, Kyle RA, Marks D, Nishihori T, Pasquini MC, Ramanathan M, Lonia S, lSavani BN, Saber W, Sharma M, Sorror ML, Wirk BM, Hari PN. Hematopoietic Cell Transplant Comorbidity Index Is Predictive of Survival after Autologous Hematopoietic Cell Transplantation in Multiple Myeloma. Biology of Blood and Marrow Transplantation 2014; 20(3):402–408.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obiozor C, Subramaniam DP, Divine C, Shune L, Singh AK, Lin TL, Abhyankar S, Chen GJ, McGuirk J, Ganguly S. Evaluation of Performance Status and Hematopoietic Cell Transplantation Specific Comorbidity Index on Unplanned Admission Rates in Patients with Multiple Myeloma Undergoing Outpatient Autologous Stem Cell Transplantation. Biology of Blood and Marrow Transplantation 2017; 23(10):1641–1645. [DOI] [PubMed] [Google Scholar]
- 15.King AJ, Gooding S, Ramasamy K: Managing multiple myeloma in the over 70s: A review. Maturitas 80:148–154, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Parker K, Horowitz J, Morin R, et al. : Race and Multiracial Americans in the U.S. Census | Pew Research Center [Internet] 2015[cited 2020 Aug 25] Available from: https://www.pewsocialtrends.org/2015/06/11/chapter-1-race-and-multiracial-americans-in-the-u-s-census/
- 17.Greenberg AJ, Vachon CM, Rajkumar SV.: Disparities in the prevalence, pathogenesis and progression of monoclonal gammopathy of undetermined significance and multiple myeloma between blacks and whites. Leukemia 26:609–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waxman AJ, Mink PJ, Devesa SS, et al. : Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 116:5501–5506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss BM: Multiethnic myeloma. Blood 121:3062–3064, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Fillmore NR, Yellapragada SV., Ifeorah C, et al. : With equal access, African American patients have superior survival compared to white patients with multiple myeloma: a VA study [Internet]. Blood 133:2615–2618, 2019. Available from: https://ashpublications.org/blood/article/133/24/2615/261449/With-equal-access-African-American-patients-have [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmikla H, Ravi G, de Lima M, et al. : Stem Cell Transplant Minimizes Insurance Coverage-Driven Outcome Disparities for Multiple Myeloma Patients. Biol Blood Marrow Transplant 26:S46, 2020 [Google Scholar]
- 22.Bhatt VR, Loberiza FR, Schmit-Pokorny K, et al. : Time to Insurance Approval in Private and Public Payers Does Not Influence Survival in Patients Who Undergo Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 22:1117–1124, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Rao K, Darrington DL, Schumacher JJ, et al. : Disparity in Survival Outcome after Hematopoietic Stem Cell Transplantation for Hematologic Malignancies According to Area of Primary Residence. Biol Blood Marrow Transplant 13:1508–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Huntington SF, Weiss BM, Vogl DT, et al. : Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Haematol 2:e408–e416, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Yabroff KR, Bradley C, Shih Y-CT: Understanding Financial Hardship Among Cancer Survivors in the United States: Strategies for Prevention and Mitigation. J Clin Oncol 38:292–301, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang DM, Chan KKW, Jang RW, et al. : Anticancer drugs approved by the Food and Drug Administration for gastrointestinal malignancies: Clinical benefit and price considerations. Cancer Med 8:1584–1593, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntington SF: Cancer-related financial toxicity: beyond the realm of drug pricing and out-of-pocket costs. Ann Oncol 27:2143–2145, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Chino F, Peppercorn JM, Rushing C, et al. : Out-of-Pocket Costs, Financial Distress, and Underinsurance in Cancer Care. [Internet]. JAMA Oncol 3:1582–1584, 2017. Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2017.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy C, Hartig H: Phone survey response rates decline again | [Internet]. Pew Res Cent, 2019[cited 2021 Feb 9] Available from: https://www.pewresearch.org/fact-tank/2019/02/27/response-rates-in-telephone-surveys-have-resumed-their-decline/
- 30.Harousseau JL: Induction therapy in multiple myeloma. [Internet]. Hematology Am Soc Hematol Educ Program 306–312, 2008[cited 2020 Jun 26] Available from: https://pubmed.ncbi.nlm.nih.gov/19074101/ [DOI] [PubMed]
- 31.Fillmore N, DuMontier C, Cheng D, et al. : Multimorbidity patterns and their association with survival in a large national cohort of older veterans with multiple myeloma. J Clin Oncol 37:8033–8033, 2019 [Google Scholar]
- 32.Wildes T, Luo S, Colditz GA, et al. : Comorbidities Impact Survival in Multiple Myeloma: Analysis of the Veterans Health Administration National Database. Blood 120:760–760, 2012 [Google Scholar]
- 33.Greipp PR, Miguel JS, Dune BGM, et al. : International staging system for multiple myeloma. J Clin Oncol, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Chamoun K, De Lima MJG, Caimi PF, et al. : Insurance status and survival of multiple myeloma (MM) patients. J Clin Oncol 37:LBA107–LBA107, 2019 [Google Scholar]
- 35.Attal M, Lauwers-Cances V, Hulin C, et al. : Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma [Internet]. N Engl J Med 376:1311–1320, 2017. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1611750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palumbo A, Cavallo F, Gay F, et al. : Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg AS, Brunson A, Jonas BA, et al. : Association between autologous stem cell transplant and survival among californians with multiple myeloma. J Natl Cancer Inst, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gay F, Oliva S, Petrucci MT, et al. : Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 16:1617–1629, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Attal M, Roussel M: Maintenance Therapy for Myeloma: How Much, How Long, and at What Cost? Am Soc Clin Oncol Educ B, 2012 [DOI] [PubMed] [Google Scholar]
References:
- 1.National Comprehensive Cancer Network. (2014). NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma (Version 2.2014). Retrieved from http://williams.medicine.wisc.edu/myeloma.pdf. [DOI] [PubMed]
- 2.Gertz MA, dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 2014. August 7;124(6):882–90. doi: 10.1182/blood-2014-03-544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.cms.gov/medicare-coverage-database/(S(oydx2qf3ecue3wjb4itskjvw))/details/nca-decision-memo.aspx?NCAId=10&ver=7&NcaName=Autologous+Stem+Cell+Transplantation+(AuSCT)+for+Multiple+Myeloma&CALId=131&CalName=Prothrombin+Time+(PT)+and+Partial+Thromboplastin+Time+(PTT)+tests+(Removal+of+Unspecified+Joint+Replacements)&bc=BEAAAAAAABAA&.
- 4. https://www.bluecrossnc.com/sites/default/files/document/attachment/services/public/pdfs/bluemedicare/medicalpolicy/transplant_stem_cell.pdf.


