Abstract
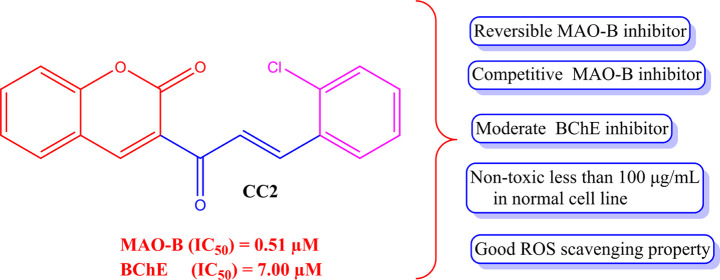
A series of halogenated coumarin–chalcones were synthesized, characterized, and their inhibitory activities against monoamine oxidases (MAOs), acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and β-site amyloid precursor protein cleaving enzyme 1 (BACE-1) were evaluated. Compound CC2 most potently inhibited MAO-B with an IC50 value of 0.51 μM, followed by CC1 (IC50 = 0.69 μM), with a selectivity index (SI) of >78.4 and >58.0, respectively, over MAO-A. However, none of the compounds effectively inhibited MAO-A, AChE, and BChE, except for CC2 and CC3 inhibiting BChE with IC50 values of 7.00 (SI > 5.73 over AChE) and 11.8 μM, respectively. CC1 and CC2 were found to be reversible and competitive inhibitors of MAO-B, with Ki values of 0.50 ± 0.06 and 0.53 ± 0.04 μM, respectively, and CC2 was also a reversible and competitive inhibitor of BChE, with a Ki value of 2.84 ± 0.09 μM. The parallel artificial membrane permeability assay (PAMPA) method showed that lead candidates can cross the blood–brain barrier (BBB). The in vitro toxicity analysis on the Vero cell line (Normal African green monkey kidney epithelial cells) by MTT confirmed that both CC1 and CC2 were nontoxic up to 100 μg/mL, which is almost equivalent to 100 times of their effective concentration used in biological studies. In addition, CC1 and CC2 attenuated H2O2-induced cellular damage via their reactive oxygen species (ROS) scavenging effect. These results suggest that CC1 and CC2 are selective and competitive inhibitors of MAO-B, and that CC2 is a selective and competitive inhibitor of BChE. Molecular docking studies of lead compounds provided the possible type of interactions in the targeted enzymes. Based on the findings, both compounds, CC1 and CC2, can be considered plausible drug candidates against neurodegenerative disorders.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that is genetically complex, irreversible, and slowly progressive in the older population.1 Due to the multifactorial pathogenesis of AD, researchers have focused on the development of multitarget-directed ligands (MTDL).2 Studies have been proposed for a novel paradigm shift to develop a therapeutic molecule that acts on different targets of the disease with different persuasion levels.3,4 Understanding the etiology and design of scaffolds by molecular hybridization induces potent MTDL development. The putative factors included in the pathology of AD are oxidative/nitrosative stress, altered apoptosis, mitochondrial damage, neuroinflammation of the immune system, genetics, Ca2+ and metal dyshomeostasis, autophagy, and enzymes such as cholinesterases (ChEs), β-site amyloid precursor protein cleaving enzyme 1 (β-secretase, BACE-1), and monoamine oxidases (MAOs).
MAOs have two types of enzymes (MAO-A and MAO-B), which are responsible for the degradation of neurotransmitters like epinephrine, norepinephrine, dopamine, and serotonin present in peripheral and central tissues.3,4 One of the major etiologies of neurodegenerative disease is oxidative damage of the brain, which is likely to be caused by the degradation of neurotransmitters. Reactive oxygen species (ROS) and hydrogen peroxide are produced by the degradation of amines, which are responsible for oxidative damage. Other than MAOs, ChEs, such as acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), also play important roles in the etiology of AD.5−9 Research studies have claimed that multitargeting drugs provide better efficacy than single-targeting drugs due to the multifactorial pathogenesis of AD.10−13
Drugs like donepezil (AChE inhibitor) increase the cholinergic system by inhibiting the enzyme acetylcholinesterase.14 On the other side, the N-methyl-d-aspartate (NMDA) receptor antagonist (memantine) acts by attenuating glutamate receptor activity.15 For AD therapy, the aforementioned drugs are insufficient. Therefore, the aim of the study is to develop a new class of drugs to reduce this impairment of their causative reason. Currently, the available drugs are single targets that help to improve cognition and memory loss. However, the single-target drugs are poor curative agents for AD. Due to the complex pathogenesis of AD, a multitarget drug could be more effective (synergistic effect) in leading to an improvement of cognition and memory.16,17 It has been suggested that chalcones are better pharmacophores for the development of multitarget ligands for targeting neurodegenerative disorders.18−20
Coumarin is a bicyclic heterocyclic compound comprising an aromatic ring fused to a 6-member lactone ring, which is chemically 1-benzopyran-2-one.21 Both the synthetic and natural coumarin compounds showed versatile pharmacological activities, such as antioxidant,12−24 antimicrobial,25 anticarcinogenic,26,27 MAO-B inhibiting,28 and anti-ChE activities.29,30 Chalcones are chemically olefinic (containing α,β-unsaturated ketones) compounds formed with a linkage between the two aryl/hetero aryl moieties. The presence of electron-withdrawing and electron-donating groups on ring A and ring B greatly influences MAO-B and AChE inhibitory activities, and the orientation of the electron-withdrawing and electron-donating groups greatly influences the electrophilic character of the Michael acceptor.31−48 Recent studies have reported that the presence of a halogen atom on ring B results in a compound with a highly selective MAO-B inhibitor.49,50
Due to the presence of olefinic linkage, chalcones exist both as cis and trans, where trans is more stable than cis.51 The presence of electron-withdrawing or electron-donating functional groups present in the benzene ring shows the fluorescent property of chalcones.52 Michael acceptors are electrophiles that are biologically and actively involved in the signaling pathway in cells. The α,β-unsaturated ketone present in the chalcone was perceived as a Michael acceptor. The electrophile present in the chalcone forms a covalent bond with the thiol or sulfhydryl group present in cysteine form, Michael adduct.53 Recently, Moya-Alvarado et al. synthesized and evaluated the MAO-B inhibitory profiles of some methoxy-bearing coumarin-based chalcones.54 Therefore, in this study, we synthesized a group of coumarin–chalcones and studied their ability to inhibit MAOs, ChEs, and BACE-1, which are responsible for neurodegenerative diseases. The most promising lead molecules were further evaluated for their biological safety profiles and ROS assay using the Vero cells in vitro system.
2. Results and Discussion
2.1. Synthesis
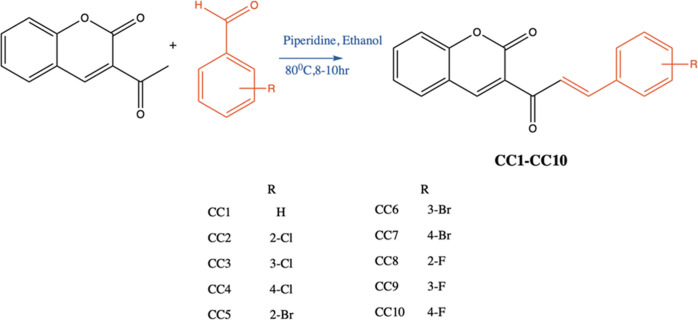
The synthesis of halogenated coumarin–chalcones was done by the condensation of 3-acetylcoumarin with various substituted halogenated benzaldehydes by Claisen–Schmidt condensation.55,561H NMR of CC2 showed the sharp doublet peak for Hα and Hβ at 8.29–8.26 and 7.95–7.92, respectively. A large coupling constant of 15–16 Hz of the double bond of chalcones confirmed its trans conformation.57 The presence of a sharp deshielded sp2 carbonyl carbon at 186.24 δ in the 13C-NMR of CC2 gave the confirmation of the α,β-unsaturated ketone system. The HRMS analysis showed the molecular weights of the targeted compounds (Supporting Information Figures S1–S21).
2.2. Inhibitory Activities Against MAOs, AChE, BChE, and BACE-1
Among the 10 derivatives tested, seven compounds showed residual activities of <50% for MAO-B at 10 μM. However, all compounds weakly inhibited MAO-A, AChE, BChE, and BACE-1, except that CC2 inhibited BChE with a residual activity of <50% at 10 μM (Table 1). Compound CC2 most potently inhibited MAO-B with an IC50 value of 0.51 μM, followed by CC1 (IC50 = 0.69 μM), with selectivity index (SI) values of >78.4 and >58.0, respectively, over MAO-A. Moreover, CC2 potently inhibited BChE (IC50 = 7.00 μM). All compounds weakly inhibited MAO-A, AChE, and BACE-1 with >50% of residual activity at 10 μM (Table 1). From the analysis of the synthesized compounds, the structure–activity relationship (SAR) indicates that the orientation of the halogen atoms at the various positions of phenyl ring B greatly influences MAO-B inhibition. Considering the halogen substitution on the phenyl B ring, chlorine at the ortho position leads to greater MAO-B inhibition compared to bromine and fluorine substitution.
Table 1. Inhibitions of Recombinant Human MAO-A, MAO-B, AChE, BChE, and BACE-1 by Compounds of the CC Series.
| residual
activity at 10 μM (%) |
IC50 (μM)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| compounds | MAO-A | MAO-B | AChEc | BChE | BACE-1 | MAO-A | MAO-B | AChE | BChE | SIb |
| CC1 | 94.8 ± 1.8 | 10.4 ± 4.4 | 86.0 ± 3.6 | 66.1 ± 1.5 | 74.6 ± 0.2 | >40 | 0.69 ± 0.09 | >40 | >40 | >58.0 |
| CC2 | 76.6 ± 1.8 | 19.8 ± 5.9 | 69.4 ± 3.6 | 43.9 ± 3.7 | 61.1 ± 1.0 | >40 | 0.51 ± 0.29 | 40.1 ± 0.12 | 7.00 ± 0.02 | >78.4 |
| CC3 | 95.5 ± 0.9 | 34.9 ± 0.7 | 77.1 ± 1.8 | 55.8 ± 4.1 | 91.0 ± 0.8 | >40 | 1.53 ± 0.39 | >40 | 11.80 ± 0.54 | >26.1 |
| CC4 | 86.8 ± 0.8 | 60.1 ± 4.5 | 76.9 ± 9.9 | 69.9 ± 3.9 | 78.1 ± 0.6 | >40 | 26.20 ± 0.22 | >40 | >40 | >1.53 |
| CC5 | 86.8 ± 5.7 | 69.6 ± 5.4 | 78.2 ± 0.0 | 62.1 ± 2.2 | 92.5 ± 0.8 | >40 | 23.60 ± 1.55 | >40 | >40 | >1.69 |
| CC6 | 86.2 ± 0.0 | 95.6 ± 4.5 | 73.7 ± 6.4 | 63.2 ± 6.4 | 92.8 ± 0.8 | >40 | 33.60 ± 1.21 | >40 | >40 | >1.19 |
| CC7 | 87.3 ± 7.7 | 31.6 ± 1.7 | 69.6 ± 0.5 | 85.4 ± 6.0 | 75.6 ± 0.2 | >40 | 2.50 ± 0.30 | >40 | >40 | >16.0 |
| CC8 | 83.1 ± 3.4 | 37.7 ± 1.2 | 69.6 ± 0.5 | 75.0 ± 3.0 | 89.1 ± 0.3 | >40 | 7.76 ± 0.81 | >40 | >40 | >5.15 |
| CC9 | 98.8 ± 3.4 | 39.3 ± 4.6 | 75.5 ± 0.5 | 55.7 ± 3.4 | 80.8 ± 0.4 | >40 | 3.61 ± 0.12 | >40 | 30.0 ± 2.00 | >11.1 |
| CC10 | 88.1 ± 5.1 | 30.8 ± 1.2 | 76.7 ± 3.8 | 75.0 ± 6.3 | 81.2 ± 0.2 | >40 | 3.36 ± 0.71 | >40 | >40 | >11.9 |
| toloxatone | 1.08 ± 0.03 | |||||||||
| lazabemide | 0.11 ± 0.02 | |||||||||
| clorgyline | 0.0070 ± 0.0007 | |||||||||
| pargyline | 0.14 ± 0.01 | |||||||||
| tacrine | 0.27 ± 0.02 | 0.060 ± 0.002 | ||||||||
| donepezil | 0.0095 ± 0.0019 | 0.180 ± 0.004 | ||||||||
Results are expressed as means ± standard errors of duplicate or triplicate experiments.
Selectivity index (SI) values are expressed for MAO-B as compared with MAO-A.
Values for reference compounds were determined after preincubation for 30 min with enzymes.
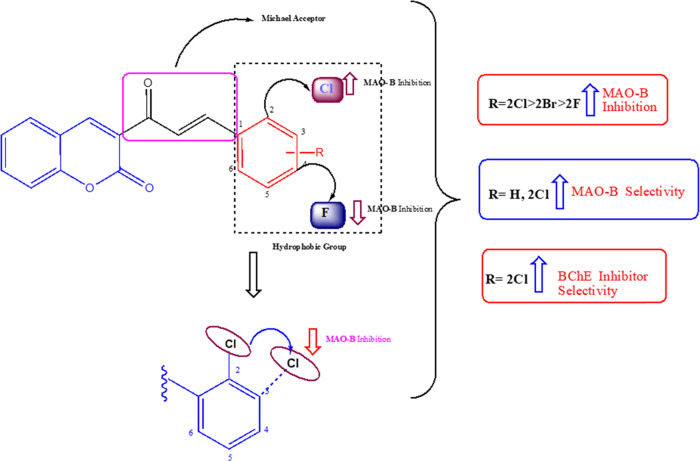
The rotatable bond in the α,β-unsaturated ketone was perceived as a Michael acceptor, which is responsible for binding with different enzyme targets.18 This depends on the bulkiness and nature of the groups attached on rings A and B of the chalcones, which greatly influence the binding interaction in the inhibitor cavity of the enzymes. It has been reported that in chalcones, the presence of electron-withdrawing groups (F, Cl, Br) and electron-donating groups (methyl, ethyl, methoxy, dimethylamino) at the para position of phenyl ring B lead to better MAO-B inhibition compared to MAO-A.19 For better AChE inhibition, a nitrogen-derived pharmacophore or methyl-containing group is essential in chalcones.18
This study shows that (i) the unsubstituted phenyl B ring shows better MAO-B inhibition with an IC50 value of 0.69 μM, having high residual activities for MAO-A, AChE, BChE, and BACE-1 (94.8, 86.8, 66.1, and 74.6%, respectively); (ii) the polarizability of the halogen atoms at the ortho position of the phenyl ring B shows MAO-B inhibition with IC50 values of 0.51, 7.76, and 23.6 μM for 2-Cl, 2-F, and 2-Br, respectively, i.e., Cl > F > Br in order, and with BACE-1 residual activities of 61.1, 89.1, and 92.5%, respectively, at 10 μM; (iii) moving the halogen atom Cl from ortho to meta position leads to less MAO-B inhibition (IC50 of 3-Cl = 1.50 μM); (iv) lipophilic atoms Br and F at the para position of the phenyl ring B show better MAO-B inhibition compared to the atoms at the meta position with IC50 values of 3.36 and 2.50 μM for 4-F and 4-Br, respectively; (v) the presence of the electron-withdrawing Cl atom at the ortho and meta positions leads to BChE inhibition with IC50 values of 7.00 and 11.8 μM for 2-Cl and 3-Cl, respectively. The fluorine atom at the meta position leads to BChE inhibition with an IC50 value of 30.0 μM. The SAR study concluded that all of the halogen-substituted compounds moderately inhibited MAO-B compared to MAO-A inhibition. Regarding AChE inhibition, the Cl atom at the ortho position of phenyl ring B shows an IC50 value of 40 μM.
For the development of dual-acting MAO-B and AChE inhibitors, researchers have used MTDL strategies. In AD, the production of neurotoxic free radicals, the production of β-amyloid, and the degradation of acetylcholine are caused by the enzymes MAO-B, AChE, and BACE-1, respectively; thus, studies have focused on the development of highly selective ligands to the target enzymes. BACE-1 inhibition from the coumarin–chalcones is not much promising. The lead molecule CC2 showed a moderate residual activity of 61.1% for BACE-1 at 10 μM. This study focused on the development of coumarin-based halogen-substituted chalcones on MAO-A, MAO-B, AChE, BChE, and BACE-1. Our results concluded that the lead molecules CC1 and CC2 selectively inhibit MAO-B at the submicromolar level, and CC2 moderately inhibits both AChE and BChE. The SAR is depicted in Figure 1.
Figure 1.
SAR analyses of halogenated coumarin–chalcones.
2.3. Kinetics of MAO-B and BChE Inhibitions
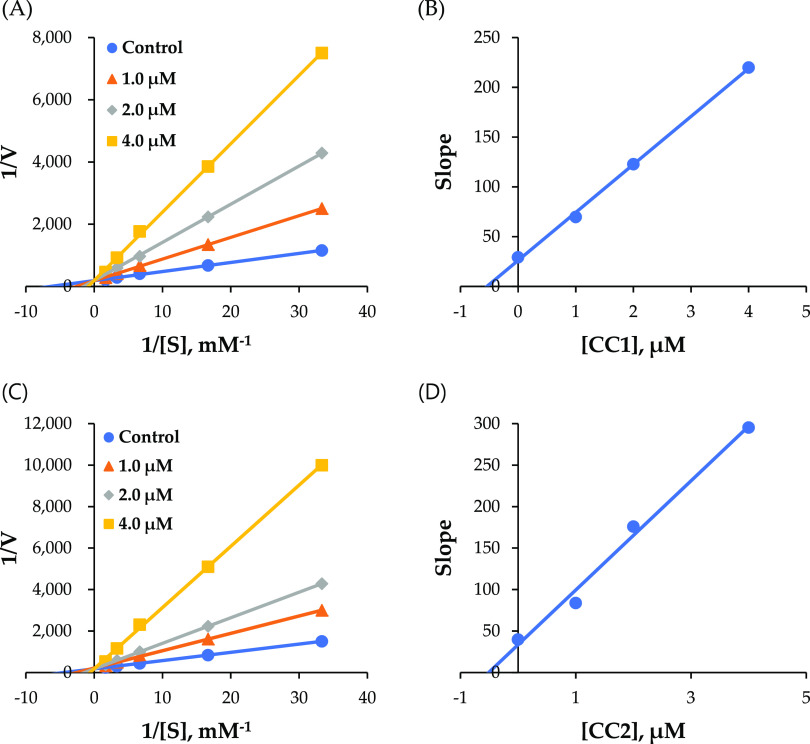
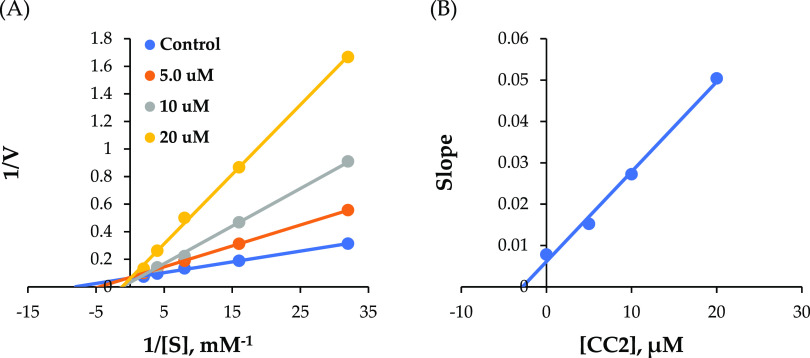
In kinetic studies of MAO-B by CC1 and CC2, Lineweaver–Burk and secondary plots showed that CC1 and CC2 were competitive inhibitors for MAO-B (Figure 2A,C), with Ki values of 0.50 ± 0.06 and 0.53 ± 0.04 μM, respectively (Figure 2B,D). In kinetic studies of BChE by CC2, Lineweaver–Burk and secondary plots showed that CC2 was a competitive inhibitor of BChE (Figure 3A), with Ki values of 2.84 ± 0.09 μM (Figure 3B).
Figure 2.
Lineweaver–Burk plots for MAO-B inhibition by CC1 and CC2 (A, C) and their respective secondary plots (B, D) of the slopes vs inhibitor concentrations.
Figure 3.
Lineweaver–Burk plots for BChE inhibition by CC2 (A) and their respective secondary plots (B) of the slopes vs inhibitor concentrations.
2.4. Reversibility Studies
In reversibility experiments using dialysis methods, the inhibition of MAO-B by CC1 was recovered from 37.6 (value of AU) to 87.9% (value of AD), and CC2 was also recovered from 37.7 (AU) to 90.2% (AD) (Figure 4). The recovery values were similar to those of the reversible reference lazabemide (from 36.8 to 85.5%). Inhibition of MAO-B by the irreversible inhibitor pargyline was not recovered (from 34.2 to 26.9%). In addition, inhibition of BChE by CC2 was recovered from 26.6 (value of AU) to 72.1% (value of AD) (Figure 5). The recovery value was similar to that of the reversible reference donepezil (from 26.8 to 85.4%). These experiments showed that inhibitions of MAO-B by CC1 and CC2 were recovered to the reversible reference level, and CC2 was also recovered to the reversible reference level by BChE, indicating that CC1 and CC2 are reversible inhibitors of MAO-B, and CC2 is a reversible inhibitor of BChE.
Figure 4.
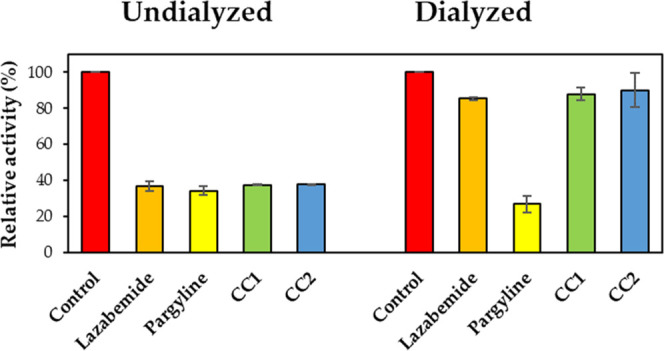
Recoveries of MAO-B inhibitions by CC1 and CC2 using dialysis experiments.
Figure 5.
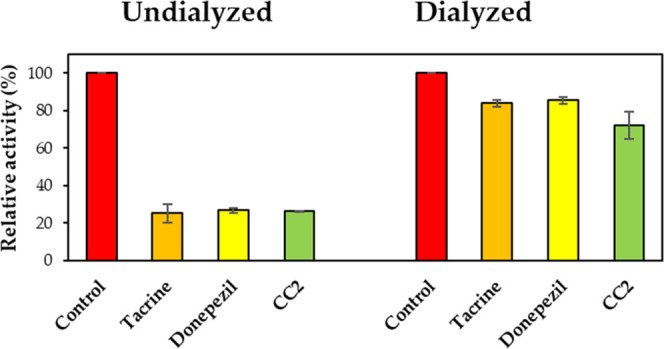
Recoveries of BChE inhibitions by CC2 using dialysis experiments.
Reversible inhibitors of monoamine oxidase selectively and reversibly inhibit MAO enzymes. Compared to irreversible MAO inhibitors, reversible ones are safe in single-drug overdoses due to their reversibility.58 Irreversible MAO inhibitors cause long-term enzyme inactivation, thus causing serious side effects that induce serotonin syndrome. For reversible MAO inhibitors, the formation of high levels of endogenous ligands can be decreased by displacing MAO inhibitors from the active site. Therefore, the discovery of selective and reversible MAO inhibitors is beneficial for the treatment of CNS disorders, particularly AD.59 Current studies on the reversible inhibition of MAO-B by CC1 and CC2 propose minimal disruption to the target.
2.5. In Vitro Toxicity Evaluation
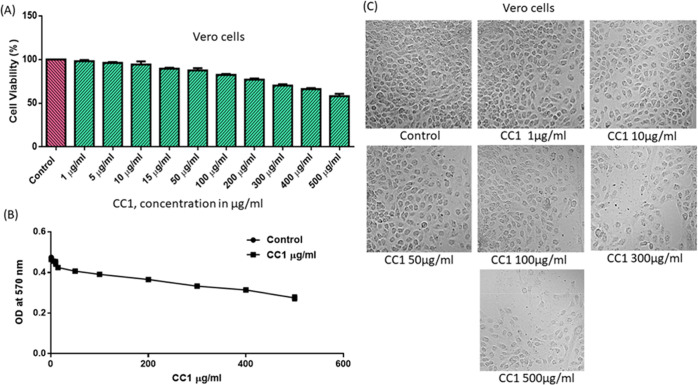
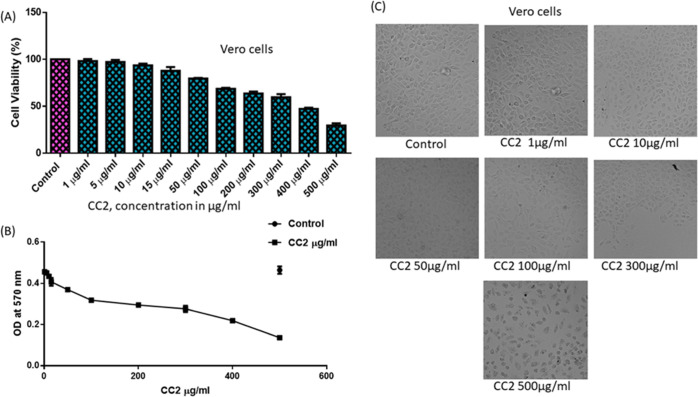
We further tested the in vitro toxicity of the compounds CC1 and CC2 on a normal epithelial cell line from the kidney of an African green monkey (Vero Cells) by MTT to prove the biological safety. The Vero cells were exposed to different concentrations (1–500 μg/mL) of the tested compounds for 48 h, and the relative cell viability was calculated as absorbance measured at λmax 540 nm using an ELISA microplate reader. The results indicated that the compounds induced concentration-dependent cell toxicity at the indicated time point of 48 h. It was found that more than 80% of the cells were even viable up to 100 μg/mL (363.4 μM) for CC1 (Figure 6A), whereas CC2 showed an approximate cell viability of 70% at the same concentration level (321.9 μM) (Figure 7A). The IC50 values of CC1 and CC2 were calculated from a dose–response curve plotted using GraphPad Prism 6.0 software. The IC50 values were 119.3 μg/mL (431.9 μM) for CC1 (Figure 6B) and 141.7 μg/mL (456.1 μM) for CC2 (Figure 7B), whereas the EC50 values of the respective compounds were below 1 μM. We also examined whether the addition of CC1 and CC2 to Vero cells had any effect on the cellular morphology using phase-contrast microscopy. The membrane integrity and reduction in cell numbers associated with cellular viability are also demonstrated morphologically on Vero cells. When CC1- and CC2-treated cells were compared with their respective controls, no distinct signs of toxicity were observed at 100 μg/mL (Figures 6C and 7C). The cells exposed to higher concentrations (300 and 500 μg/mL) showed marked morphological alterations typically associated with cytotoxicity, such as marked reduction in cellular density, cellular shrinkage, and blebbing. This study showed that CC1 and CC2 were not toxic for Vero cells up to a concentration of 100 μg/mL, which is almost equivalent to 100 times of their effective concentration in the biological study.
Figure 6.
Effect of CC1 on Vero cell viability. (A) Cell viability; a high viability (>80%) up to 100 μg/mL (363.4 μM). (B) Representation of a dose–response curve with IC50 119.3 μg/mL (431.9 μM). (C) Morphological observation of Vero cells with different concentrations under a phase-contrast microscope, exposed for 48 h. The control value was taken as 100%, and the data were presented as means ± SE from three independent experiments.
Figure 7.
Effect of CC2 on Vero cell viability. (A) Cell viability; a high viability (>70%) up to 100 μg/mL (321.9 μM). (B) Representation of a dose–response curve with IC50 141.7 μg/mL (456.1 μM). (C) Morphological observation of Vero cells with different concentrations under a phase-contrast microscope, exposed for 48 h. The control value was taken as 100%, and the data were presented as means ± SE from three independent experiments.
2.6. ROS Assay
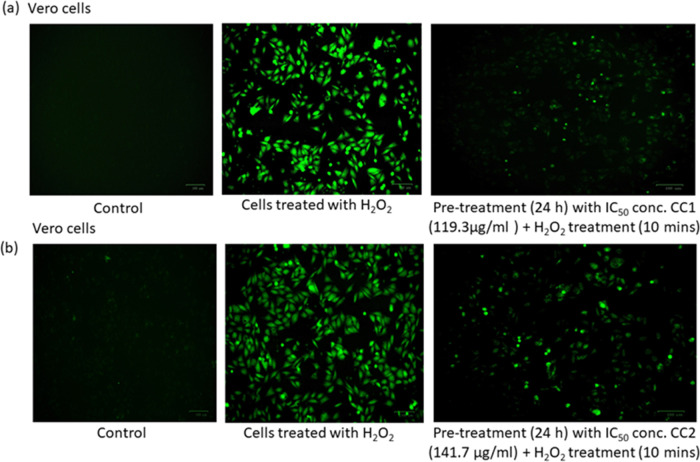
To further explore the intracellular ROS scavenging effect of CC1 and CC2, Vero cells were exposed to H2O2 for excess ROS generation for 10 min, and the images were taken under a fluorescence microscope.60 The IC50 concentrations of CC1 and CC2 (119.3 and 141.7 μg/mL, respectively) showed excellent ROS scavenging activity compared to that of the H2O2-treated control cells alone. These results indicate that CC1 and CC2 treatment inhibited intracellular ROS generation in H2O2-treated Vero cells. A decrease in ROS level was evaluated as a decrease in the intensity of fluorescence after 24 h of drug treatment, indicating the balance of prooxidant and antioxidant levels in the cell system (Figure 8A,B). Many studies have indicated that a high concentration of ROS leads to neural damage via oxidative stress in the brain during neurodegenerative disease.61 Both compounds can effectively regulate the ROS production induced by H2O2 treatment with its significant radical scavenging activity.
Figure 8.
Effects of CC1 (a) and CC2 (b) on Vero cells treated with H2O2 to generate ROS. Vero cells were treated with 100 μg/mL of 30% H2O2 for 10 min, and the production of ROS was evaluated immediately.
2.7. Computational Studies
To investigate the effect of interactions between compounds CC1 and CC2 toward MAOs, in silico analyses were performed, and the docking score and MM-GBSA values obtained from computational studies are provided in Table 2. For completeness, the reference values concerning cognate ligands within the targets are provided in Tables S1 and S2 of the Supporting Information.
Table 2. Docking Score and MM-GBSA Values of Compounds CC1 and CC2 toward MAOs.
| MAO-A |
MAO-B |
|||
|---|---|---|---|---|
| compound | docking score (kcal/mol) | MM-GBSA (kcal/mol) | docking score (kcal/mol) | MM-GBSA (kcal/mol) |
| CC1 | –8.70 | –32.11 | –10.47 | –49.54 |
| CC2 | –8.55 | –24.60 | –10.47 | –50.52 |
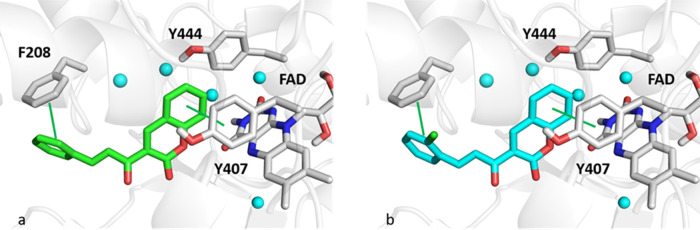
The main interactions engaged from compounds CC1 and CC2 toward MAO-A are shown in Figure 9. Interestingly, the coumarin ring can interact with Y407 through π–π contacts being placed within the hydrophobic region made up of FAD, Y407, and Y444, while the styrene moiety establishes π–π interaction with F208.
Figure 9.
Zoomed-in view of an MAO-A binding pocket. Panels (a) and (b) show the best pose returned from the docking analysis of CC1 (green sticks) and CC2 (cyan sticks), respectively. Cyan spheres indicate water molecules. Green lines show π–π contacts.
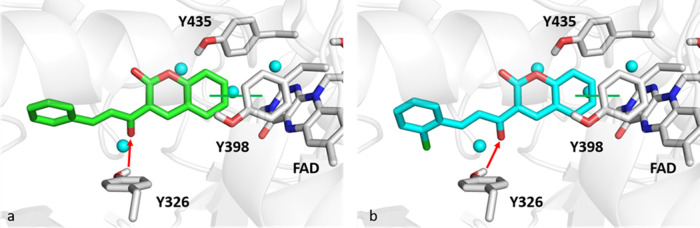
Given the binding modes toward MAO-B, the coumarin ring can make π–π interactions with Y398 that is trapped in the aromatic cage formed by FAD, Y398, and Y435 (Figure 10). Furthermore, the carbonyl group of the chalcone bridge can form a hydrogen bond with the hydroxyl group of the side chain of the Y326 MAO-B selective residue.
Figure 10.
Zoomed-in view of an MAO-B binding pocket. Panels (a) and (b) show the best pose returned from docking analysis of CC1 (green sticks) and CC2 (cyan sticks), respectively. Cyan spheres indicate water molecules. Green lines and red arrows show π–π contacts and hydrogen bonds, respectively.
The molecular docking and MM-GBSA analyses were helpful in explaining the binding toward MAO-A and MAO-B at a molecular level following the experimental IC50 inhibition data. The interaction of CC1 and CC2 with the selective MAO-B residue Y326 is crucial for binding toward MAO-B. Furthermore, both the docking score and MM-GBSA values confirmed the observed binding mode and the interactions toward MAO-B. In particular, with the MM-GBSA method, it was possible to select docking poses and perform a more accurate rescoring, considering some empirical corrections concerning both polar and hydrophobic interactions, thus providing more reliable results. For the sake of completeness, computational simulations have been performed also towards AChE and BChE targets: docking score and MM-GBSA values can be retrieved in Table S2 of the Supporting Information. Interestingly, compounds CC1 and CC2 did not reach the catalytic W82 in the AChE catalytic anionic site (CAS) and can interact with F295 and W286 (Figure S1). On the other hand, only compound CC2 can make π–π interactions with the catalytic W82 residue towards BChE (Figure S2), in agreement with the experimental data.
3. Conclusions
In summary, a series of 10 coumarin–chalcones substituted with halogens were synthesized and tested for MAOs, ChEs, and BACE-1 inhibitory activities. All of the compounds showed better MAO-B inhibitory activity compared to MAO-A. CC1 and CC2 in particular showed the best MAO-B inhibition. The nature and orientation of the halogen group at the phenyl B ring were responsible for the MAO-B and ChE inhibitory activities. From the series, the halogen atom substituted at the ortho position potentially inhibited MAO-B with IC50 values of 0.51, 7.76, and 23.6 μM for 2-Cl, 2-F = 7.76, and 2-Br, respectively, and CC2 moderately inhibited MAO-B. In addition, compound CC1 with an unsubstituted phenyl ring showed better MAO-B inhibition with an IC50 value of 0.51 μM. None of the compounds effectively inhibited MAO-A, AChE, and BChE, except for CC2 and CC3 inhibiting BChE with IC50 values of 7.00 (SI > 5.73 over AChE) and 11.8 μM, respectively. It is concluded that CC1 and CC2 are selective and competitive inhibitors of MAO-B, and CC2 is a selective and competitive inhibitor of BChE. Moreover, the biological safety of both CC1 and CC2 depicts the nontoxic behavior of the compounds at the EC50 level. We believe that this study provides the first evidence for the synthesized chalcone derivatives, CC1 and CC2, which have a free radical scavenging effect, thereby inhibiting oxidative stress in a disease condition. Therefore, this study may provide information for the design and development of a novel potent drug against neurodegenerative diseases.
4. Materials and Methods
4.1. Synthesis
The chemicals for the synthesis were purchased from Sigma-Aldrich (St. Louis, MO). Acetylcoumarin (0.01 mol) and substituted benzaldehyde (0.01 mol) were dissolved in 50 mL of ethanol in the presence of piperidine (0.001 mol) as a catalyst, and the mixture was stirred for 10 min at room temperature. It was refluxed for 8–10 h with a constant temperature at 80 °C and cooled, and the solution was then filtered, washed with water, and dried. The product was recrystallized with ethanol to obtain pure crystals.62,63 The purities of the compounds were analyzed by thin-layer chromatography [10:90 ethylacetate/hexane] (Scheme 1).
Scheme 1. Synthetic Route for CC Series.
4.2. Enzyme Assays
MAO activities were assayed using recombinant human MAO-A and MAO-B in the presence of kynuramine (0.06 mM) and benzylamine (0.3 mM) as substrates, respectively, as described previously.64 The substrate concentrations were 1.5× and 2.3× Km, respectively; the Km values of these substrates were 0.041 and 0.13 mM, respectively. AChE and BChE activities were measured using the enzymes from Electrophorus electricus Type VI-S and equine serum, respectively, in the presence of 0.5 mM acetylthiocholine iodide (ATCI) and butyrylthiocholine iodide (BTCI) as substrates, respectively, and 0.5 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) as a color-developing agent, as described previously.65,66 Inhibitory activities were measured after preincubating the substrates and inhibitors for 15 min. BACE-1 activity was measured using a BACE-1 activity detection kit at excitation 320 nm and emission 405 nm using a fluorescence spectrometer (FS-2, Scinco, Seoul, Korea), as described previously.67 The enzymes and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
4.3. Analysis of Enzyme Inhibitions and Kinetics
The inhibitory activities of 10 compounds against MAO-A, MAO-B, AChE, BChE, and BACE-1 were first observed at a concentration of 10 μM, and then IC50 values were determined for the compounds, of which the residual activities were <50%. Reversibility and kinetic studies were performed on the most potent inhibitors, i.e., CC1 and CC2 for MAO-B and CC2 for BChE, as previously described.68 Kinetic experiments were conducted at five concentrations of substrates and three inhibitor concentrations.
4.4. Analysis of Inhibitor Reversibility
The reversibility of compounds CC1 and CC2 was analyzed using a dialysis method after preincubating with MAO-B for 30 min, as previously described.69 In the reversibility of MAO-B, the concentrations used were 1.00 and 1.40 μM for CC1 and CC2, respectively, 0.20 μM for lazabemide (a reversible MAO-B reference inhibitor), and 0.28 μM for pargyline (an irreversible MAO-B reference inhibitor). In BChE experiments, the concentration used was 14.0 μM for CC2 and 0.36 μM for donepezil (a reversible BChE reference inhibitor).70 The relative activities for the undialyzed (AU) and dialyzed (AD) samples were compared to determine the reversibility patterns.71
4.5. Cytotoxicity and ROS Assay
The lead molecules CC1 and CC2 were evaluated for cytotoxicity and ROS scavenging potentiality, as previously described72,73 and as described shortly in the Supporting Information.
4.6. Computational Studies
The X-ray crystal structures of MAO-A and MAO-B were taken from the Protein Data Bank with entries 2Z5X and 2V5Z, respectively.74,75 These structures were treated with the Protein Preparation Wizard available in the Schrödinger Suite to predict the correct protonation states, remove nonfunctional water molecules, and minimize the total energy.76,77 Ligands were treated with the Ligprep Tool to generate all possible tautomers and the allowed conformations. The grid boxes were centered on the center of mass of the cognate ligands, and the SP docking protocol was set when using the GLIDE software. Finally, an estimation of the free energy of binding was done by employing the Molecular Mechanics Generalized Born Surface Area (MM-GBSA) approach.78,79
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant provided by the Korean Government (NRF-2019R1A2C1088967 to H.K.), Republic of Korea, and by a grant from Amrita Vishwa Vidyapeetham University (Seed Grant Number K-PHAR-20-628 to B.M.). The authors acknowledge Taif University Researchers Supporting Project number (TURSP-2020/68), Taif University, Taif, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04252.
Spectral data of 1H- and 13C-NMR and MS chromatograms for compounds (Figures S1–S21); zoomed-in views (Figures S22 and S23); and docking score and MMSBSA values (Tables S1 and S2) (PDF)
Author Contributions
◆ N.A.R., J.M.O., and B.M. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Hampel H.; Prvulovic D.; Teipel S.; Jessen F.; Luckhaus C.; Frolich L.; Riepe M. W.; Dodel R.; Leyhe T.; Bertram L.; Hoffmann W.; Faltraco F. The future of Alzheimer’s disease: the next 10 years. Prog. Neurobiol. 2011, 95, 718–728. 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Massoud F.; Gauthier S. Update on the pharmacological treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2010, 8, 69–80. 10.2174/157015910790909520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B.; Kim H. Inhibitors of monoamine oxidase and acetylcholinesterase as a front runner in CNS drug discovery. Comb. Chem. High Throughput Screening 2020, 23, 834–835. 10.2174/138620732309201127093123. [DOI] [PubMed] [Google Scholar]
- Sharma P.; Singh B.; Mathew B. An update of synthetic approaches and structure-activity relationships of various classes of human MAO-B inhibitors. ChemistrySelect 2021, 6, 1404–1429. 10.1002/slct.202004188. [DOI] [Google Scholar]
- Inestrosa N. C.; Alvarez A.; Perez C. A.; Moreno R. D.; Vicente M.; Linker C.; Casanueva O. I.; Soto C.; Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta- peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. 10.1016/S0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Alvarez A.; Alarcon R.; Opazo C.; Campos E. O.; Munoz F. J.; Calderon F. H.; Dajas F.; Gentry M. K.; Doctor B. P.; De Mello F. G.; Inestrosa N. C. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci. 1998, 18, 3213–3223. 10.1523/JNEUROSCI.18-09-03213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A.; Alafuzoff I.; Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging anddementia. J. Neurosci. Res. 1992, 31, 103–111. 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- Zheng W. H.; Bastianetto S.; Mennicken F.; Ma W.; Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211. 10.1016/S0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- Kar S.; Slowikowski S. P.; Westaway D.; Mount H. T. Inter- actions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004, 29, 427–441. [PMC free article] [PubMed] [Google Scholar]
- Cavalli A.; Bolognesi M. L.; Minarini A.; Rosini M.; Tumiatti V.; Recanatini M.; Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Bajda M.; Guzior N.; Ignasik M.; Malawska B. Multi- target-directed ligands in Alzheimer’s disease treatment. Curr. Med. Chem. 2011, 18, 4949–4975. 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- Leon R.; Marco-Contelles J. A step further towards multitarget drugs for Alzheimer and neuronal vascular diseases: targeting the cholinergic system, amyloid-beta aggregation and Ca(2+) dyshomeostasis. Curr. Med. Chem. 2011, 18, 552–576. 10.2174/092986711794480186. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010, 90, 465–494. 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- Francis P. T.; Palmer A. M.; Snape M.; Wilcock G. K. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol., Neurosurg. Psychiatry 1999, 66, 137–147. 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A. Pathologically-activated therapeutics for neuro- protection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr. Drug Targets 2007, 8, 621–632. 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- Csermely P.; Agoston V.; Pongor S. The efficiency of multi- target drugs: the network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Geldenhuys W. J.; Van der Schyf C. J. Designing drugs with multi- target activity: the next step in the treatment of neurodegenerative disorders. Expert Opin. Drug Discovery 2013, 8, 115–129. 10.1517/17460441.2013.744746. [DOI] [PubMed] [Google Scholar]
- Zhuang C.; Zhang W.; Sheng C.; Zhang W.; Xing C.; Miao Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi P.; Mathew B.; Secci D.; Carradori S. Chalcones: Unearthing their therapeutic possibility as monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2020, 205, 112650 10.1016/j.ejmech.2020.112650. [DOI] [PubMed] [Google Scholar]
- Irfan R.; Mousavi S.; Alazmi M.; Saleem R. S. Z. A Comprehensive review of aminochalcones. Molecules 2020, 25, 5381 10.3390/molecules25225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F.; Roleira F.; Milhazes N.; Santana L.; Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- Kostova I.; Bhatia S.; Grigorov P.; Balkansky S.; Parmar V. S.; Prasad A. K.; Saso L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- Fylaktakidou K. C.; Hadjipavlou-Litina D. J.; Litinas K. E.; Nico- laides D. N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- Atmaca M.; Bilgin H. M.; Obay B. D.; Diken H.; Kelle M.; Kale E. The hepatoprotective effect of coumarin and coumarin derivates on carbon tetrachloride-induced hepatic injury by antiox- idative activities in rats. J. Physiol. Biochem. 2011, 67, 569–576. 10.1007/s13105-011-0103-5. [DOI] [PubMed] [Google Scholar]
- Liaras K.; Geronikaki A.; Glamoclija J.; Ciric A.; Sokovic M. Thiazole-based chalcones as potent antimicrobial agents. Synthesis and biological evaluation. Bioorg. Med. Chem. 2011, 19, 3135–3140. 10.1016/j.bmc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Liu Z. J.; Wang Y.; Lin H. F.; Zuo D. Z.; Wang L. H.; Zhao Y. F.; Gong P. Design, synthesis and biological evaluation of novel thieno[3,2-d] pyrimidine derivatives containing diaryl urea moiety as potent antitumor agents. Eur. J. Med. Chem. 2014, 85, 215–227. 10.1016/j.ejmech.2014.07.099. [DOI] [PubMed] [Google Scholar]
- Emami S.; Dadashpour S. Current developments of coumarin- based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. 10.1016/j.ejmech.2015.08.033. [DOI] [PubMed] [Google Scholar]
- Chimenti F.; Secci D.; Bolasco A.; Chimenti P.; Bizzarri B.; Granese A.; Carradori S.; Yáñez M.; Orallo F.; Ortuso F.; Alcaro S. Synthesis, molecular modeling, and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J. Med. Chem. 2009, 52, 1935–1942. 10.1021/jm801496u. [DOI] [PubMed] [Google Scholar]
- Kurt B. Z.; Gazioglu I.; Dag A.; Salmas R. E.; Kayik G.; Durdagi S.; Sonmez F. Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thy- mol/carvacrol derivatives. Bioorg. Med. Chem. 2017, 25, 1352–1363. 10.1016/j.bmc.2016.12.037. [DOI] [PubMed] [Google Scholar]
- Hammuda A.; Shalaby R.; Rovida S.; Edmondson D. E.; Binda C.; Khalil A. Design and synthesis of novel chalcones as potent selective monoamine oxidase-B inhibitors. Eur. J. Med. Chem. 2016, 114, 162–169. 10.1016/j.ejmech.2016.02.038. [DOI] [PubMed] [Google Scholar]
- Hitge R.; Smit S.; Petzer A.; Petzer J. P. Evaluation of nitrocatechol chalcone and pyrazoline derivatives as inhibitors of catechol-O-methyltransferase and monoamine oxidase. Bioorg. Med. Chem. Lett. 2020, 30, 127188 10.1016/j.bmcl.2020.127188. [DOI] [PubMed] [Google Scholar]
- Kong Z.; Sun D.; Jiang Y.; Hu Y. Design, synthesis, and evaluation of 1, 4-benzodioxan-substituted chalcones as selective and reversible inhibitors of human monoamine oxidase B. J. Enzyme Inhib. Med. Chem. 2020, 35, 1513–1523. 10.1080/14756366.2020.1797711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti F.; Fioravanti R.; Bolasco A.; Chimenti P.; Secci D.; Rossi F.; Yáñez M.; Orallo F.; Ortuso F.; Alcaro S. Chalcones: a valid scaffold for monoamine oxidases inhibitors. J. Med. Chem. 2009, 52, 2818–2824. 10.1021/jm801590u. [DOI] [PubMed] [Google Scholar]
- Minders C.; Petzer J. P.; Petzer A.; Lourens A. C. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. 10.1016/j.bmcl.2015.09.049. [DOI] [PubMed] [Google Scholar]
- Sasidharan R.; Manju S. L.; Uçar G.; Baysal I.; Mathew B. Identification of indole-based chalcones: discovery of a potent, selective, and reversible class of MAO-B inhibitors. Arch. Pharm. 2016, 349, 627–637. 10.1002/ardp.201600088. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Haridas A.; Uçar G.; Baysal I.; Joy M.; Mathew G. E.; Lakshmanan B.; Jayaprakash V. Synthesis, biochemistry, and computational studies of brominated thienyl chalcones: A new class of reversible MAO-B Inhibitors. Chem. Med. Chem. 2016, 11, 1161–1171. 10.1002/cmdc.201600122. [DOI] [PubMed] [Google Scholar]
- Xiao G.; Li Y.; Qiang X.; Xu R.; Zheng Y.; Cao Z.; Luo L.; Yang X.; Sang Z.; Su F.; Deng Y. Design, synthesis and biological evaluation of 4′-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 1030–1041. 10.1016/j.bmc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Cao Z.; Yang J.; Xu R.; Song Q.; Zhang X.; Liu H.; Qiang X.; Li Y.; Tan Z.; Deng Y. Design, synthesis and evaluation of 4′-OH-flurbiprofen-chalcone hybrids as potential multifunctional agents for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2018, 26, 1102–1115. 10.1016/j.bmc.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Robinson S. J.; Petzer J. P.; Petzer A.; Bergh J. J.; Lourens A. C. Selected furanochalcones as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett. 2013, 23, 4985–4989. 10.1016/j.bmcl.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Jo G.; Ahn S.; Kim B. G.; Park H. R.; Kim Y. H.; Choo H. A.; Koh D.; Chong Y.; Ahn J. H.; Lim Y. Chromenylchalcones with inhibitory effects on monoamine oxidase B. Bioorg. Med. Chem. 2013, 21, 7890–7897. 10.1016/j.bmc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Mellado M.; González C.; Mella J.; Aguilar L. F.; Viña D.; Uriarte E.; Cuellar M.; Matos M. J. Combined 3D-QSAR and docking analysis for the design and synthesis of chalcones as potent and selective monoamine oxidase B inhibitors. Bioorg. Chem. 2021, 108, 104689 10.1016/j.bioorg.2021.104689. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Baek S. C.; Thomas Parambi D. G.; Lee J. P.; Mathew G. E.; Jayanthi S.; Vinod D.; Rapheal C.; Devikrishna V.; Kondarath S. S.; Uddin M. S.; Kim H. Potent and highly selective dual-targeting monoamine oxidase-B inhibitors: Fluorinated chalcones of morpholine versus imidazole. Arch. Pharm. 2019, 352, 1800309 10.1002/ardp.201800309. [DOI] [PubMed] [Google Scholar]
- Parambi D. G. T.; Oh J. M.; Baek S. C.; Lee J. P.; Tondo A. R.; Nicolotti O.; Kim H.; Mathew B. Design, synthesis and biological evaluation of oxygenated chalcones as potent and selective MAO-B inhibitors. Bioorg. Chem. 2019, 93, 103335 10.1016/j.bioorg.2019.103335. [DOI] [PubMed] [Google Scholar]
- Sang Z.; Wang K.; Zhang P.; Shi J.; Liu W.; Tan Z. Design, synthesis, in-silico and biological evaluation of novel chalcone derivatives as multi-function agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 238–252. 10.1016/j.ejmech.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Song Q.; Cao Z.; Li Y.; Tian C.; Yang Z.; Zhang H.; Deng Y. Design, synthesis and evaluation of chalcone Mannich base derivatives as multifunctional agents for the potential treatment of Alzheimer’s disease. Bioorg. Chem. 2019, 87, 395–408. 10.1016/j.bioorg.2019.03.043. [DOI] [PubMed] [Google Scholar]
- Shalaby R.; Petzer J. P.; Petzer A.; Ashraf U. M.; Atari E.; Alasmari F.; Kumarasamy S.; Sari Y.; Khalil A. SAR and molecular mechanism studies of monoamine oxidase inhibition by selected chalcone analogs. J. Enzyme Inhib. Med. Chem. 2019, 34, 863–876. 10.1080/14756366.2019.1593158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P.; Wang K.; Zhang P.; Shi J.; Cheng X.; Zhang Q.; Zheng C.; Cheng Y.; Yang J.; Lu X.; Sang Z. Development of chalcone-O-alkylamine derivatives as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2019, 183, 111737 10.1016/j.ejmech.2019.111737. [DOI] [PubMed] [Google Scholar]
- Mathew B. Privileged pharmacophore of FDA approved drugs in combination with chalcone framework: A new hope for Alzheimer’s treatment. Comb. Chem. High Throughput Screening 2020, 23, 842–846. 10.2174/1386207323999200728122627. [DOI] [PubMed] [Google Scholar]
- Nair A. S.; Oh J. M.; Koyiparambath V. P.; Kumar S.; Sudevan S. T.; Soremekun O.; Soliman M. E.; Khames A.; Abdelgawad M. A.; Pappachen L. K.; Mathew B.; Kim H. Development of halogenated pyrazolines as selective monoamine oxidase-B Inhibitors: Deciphering via molecular dynamics approach. Molecules 2021, 26, 3264 10.3390/molecules26113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B.; Carradori S.; Guglielmi P.; Uddin M. S.; Kim H. New aspects of monoamine oxidase b inhibitors: The key role of halogens to open the golden door. Curr. Med. Chem. 2020, 28, 266–283. 10.2174/0929867327666200121165931. [DOI] [PubMed] [Google Scholar]
- Wei H.; Ruan J. L.; Zhang X. J. Coumarin-chalcone hybrids: promising agents with diverse pharmacological properties. RSC Adv. 2016, 6, 10846–10860. 10.1039/C5RA26294A. [DOI] [Google Scholar]
- Serdiuk I. E.; Wera M.; Roshal A. D. Structural and spectral features of 4′-substituted 2′-hydroxychalcones in solutions and crystals: Spectroscopic and theoretical investigations. J. Phys. Chem. A 2018, 122, 2030–2038. 10.1021/acs.jpca.7b10361. [DOI] [PubMed] [Google Scholar]
- Sahu N. K.; Balbhadra S. S.; Choudhary J.; Kohli D. V. Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem. 2012, 19, 209–225. 10.2174/092986712803414132. [DOI] [PubMed] [Google Scholar]
- Moya-Alvarado G.; Yañez O.; Morales N.; González-González A.; Areche C.; Núñez M. T.; Fierro A.; García-Beltrán O. Coumarin-chalcone hybrids as inhibitors of MAO-B: Biological activity and in silico studies. Molecules 2021, 26, 2430 10.3390/molecules26092430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khode S.; Maddi V.; Aragade P.; Palkar M.; Ronad P. K.; Mamledesai S.; Thippeswamy A. H.; Satyanarayana D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. 10.1016/j.ejmech.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Ajani O. O.; Nwinyi O. C. Microwave-assisted synthesis and evaluation of antimicrobial activity of 3-{3-(s-aryl and s-heteroaromatic) acryloyl}-2H-chromen-2-one derivatives. J. Heterocycl. Chem. 2010, 47, 179–187. 10.1002/jhet.298. [DOI] [Google Scholar]
- Mathew B.; Adeniyi A. A.; Joy M.; Mathew G. E.; Ashona Singh-Pillay A. S.; Sudarsanakumar C.; Soliman M. E. S.; Suresh J. Anti-oxidant behavior of functionalized chalcone-a combined quantum chemical and crystallographic structural investigation. J. Mol. Struct. 2017, 1146, 301–308. 10.1016/j.molstruc.2017.05.100. [DOI] [Google Scholar]
- Carradori S.; Secci D.; Petzer J. P. MAO inhibitors and their wider applications: a patent review. Expert Opin. Ther. Pat. 2018, 28, 211–226. 10.1080/13543776.2018.1427735. [DOI] [PubMed] [Google Scholar]
- Tripathi A. C.; Upadhyay S.; Paliwal S.; Saraf S. K. Privileged scaffolds as MAO inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018, 145, 445–497. 10.1016/j.ejmech.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Kim S.; Kim M.; Kang M. C.; Lee H. H. L.; Cho C. H.; Choi I.; Park Y.; Lee S. H. Antioxidant effects of turmeric leaf extract against hydrogen peroxide-induced oxidative stress in vitro in vero cells and in vivo in zebrafish. Antioxidants 2021, 10, 112 10.3390/antiox10010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G.; Lloret A.; Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longevity 2019, 2105607 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G.; Siva Krishna V.; Sriram D.; Jachak S. M. Pyrazole-coumarin and pyrazole-quinoline chalcones as potential antitubercular agents. Arch. Pharm. 2020, 353, 2000077 10.1002/ardp.202000077. [DOI] [PubMed] [Google Scholar]
- Rodriguez S. V.; Guíñez R. F.; Matos M. J.; Azar C. O.; Maya J. D.; Santana L.; Borges F. Synthesis and trypanocidal properties of new coumarin-chalcone derivatives. Med. Chem. 2015, 5, 173–177. 10.4172/2161-0444.1000260. [DOI] [Google Scholar]
- Oh J. M.; Özdemir Z.; Büyüktuncel S. E.; Uysal M.; Mohamed A. A.; Musa A.; Gambacorta N.; Nicolotti O.; Mathew B.; Kim H.; et al. Design, synthesis, and biological evaluation of pyridazinones containing the (2-fluorophenyl) piperazine moiety as selective MAO-B inhibitors. Molecules 2020, 25, 5371 10.3390/molecules25225371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S. C.; Park M. H.; Ryu H. W.; Lee J. P.; Kang M. G.; Park D.; Park C. M.; Oh S. R.; Kim H. Rhamnocitrin isolated from Prunus padus var. seoulensis: A potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg. Chem. 2019, 83, 317–325. 10.1016/j.bioorg.2018.10.051. [DOI] [PubMed] [Google Scholar]
- Lee J. P.; Kang M. G.; Lee J. Y.; Oh J. M.; Baek S. C.; Leem H. H.; Park D.; Cho M. L.; Kim H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043 10.1016/j.bioorg.2019.103043. [DOI] [PubMed] [Google Scholar]
- Jeong G. S.; Kang M. G.; Han S. A.; Noh J. I.; Park J. E.; Nam S. J.; Park D.; Yee S. T.; Kim H. Selective inhibition of human monoamine oxidase B by 5-hydroxy-2-methyl-chroman-4-one isolated from an endogenous lichen fungus Daldinia fissa. J. Fungi 2021, 7, 84 10.3390/jof7020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. W.; Ryu H. W.; Kang M. G.; Park D.; Oh S. R.; Kim H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. 10.1016/j.bmcl.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Vishal P. K.; Oh J. M.; Khames A.; Abdelgawad M. A.; Nair A. S.; Nath L. R.; Gambacorta N.; Ciriaco F.; Nicolotti O.; Kim H.; Mathew B. Trimethoxylated halogenated chalcones as dual inhibitors of MAO-B and BACE-1 for the treatment of neurodegenerative disorders. Pharmaceutics 2021, 13, 850 10.3390/pharmaceutics13060850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew B.; Oh J. M.; Baty R. S.; Batiha G. E.; Parambi D.G.T.; Gambacorta N.; Nicolotti O.; Kim H. Piperazine-substituted chalcones: a new class of MAO-B, AChE, and BACE-1 inhibitors for the treatment of neurological disorders. Environ. Sci. Pollut. Res. 2021, 28, 38855–38866. 10.1007/s11356-021-13320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitepe H. E.; Oh J. M.; Doğan İ.S.; Yildirim S.; Aksel A. B.; Jeong G. S.; Khames A.; Abdelgawad M. A.; Gambacorta N.; Nicolotti O.; Mathew B.; Kim H. Synthesis of N′-(4-/3-/2-/Non-substituted benzylidene)-4-[(4-methylphenyl)sulfonyloxy] benzohydrazides and evaluation of their inhibitory activities against monoamine oxidases and β-secretase. Appl. Sci. 2021, 11, 5830 10.3390/app11135830. [DOI] [Google Scholar]
- Fotakis G.; Timbrell J. A. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Jambunathan N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 292–298. [DOI] [PubMed] [Google Scholar]
- Son S. Y.; Ma J.; Kondou Y.; Yoshimura M.; Yamashita E.; Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 5739–5744. 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C.; Wang J.; Pisani L.; Caccia C.; Carotti A.; Salvati P.; Edmondson D. E.; Mattevi A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J. Med. Chem. 2007, 50, 5848–5852. 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- Friesner R. A.; Banks J. L.; Murphy R. B.; Halgren T. A.; Klicic J. J.; Mainz D. T.; Repasky M. P.; Knoll E. H.; Shelley M.; Perry J. K.; Shaw D. E.; Francis P.; Shenkin P. S. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Schrödinger Release 2020-4: Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2016; Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC: New York, NY, 2020.
- Madhavi Sastry G.; Adzhigirey M.; Day T.; Annabhimoju R.; Sherman W. Protein and Ligand Preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Schrödinger Release 2020-4: LigPrep; Schrödinger, LLC: New York, NY, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.