Abstract
Sequential Knoevenagel condensation/cyclization leading to indene and benzofulvene derivatives has been developed. The reaction of 2-(1-phenylvinyl)benzaldehyde with malonates gave benzylidene malonates, cyclized indenes, and dehydrogenated benzofulvenes. The product selectivity depends on the reaction conditions. The reaction with piperidine, AcOH in benzene at 80 °C for 1.5 h gave a benzylidene malonate in 75% yield as a major product. The reactions with piperidine, AcOH in benzene at 80 °C for 17 h and with TiCl4-pyridine at room temperature gave an indene derivative in 56 and 79% yields, respectively, as a major product. The reaction with TiCl4-Et3N gave a benzofulvene in 40% yield selectively. Indene was transformed to a benzofulvene derivative using the reagents TiCl4-Et3N and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). The reaction of variously substituted aryl derivatives with dimethyl malonate gave indene and benzofulvene derivatives. The reactions of 2-(1-phenylvinyl)benzaldehyde with Meldrum’s acid or malononitrile also gave cyclized compounds in the suitable sequential or stepwise conditions. Furthermore, the reaction of 2-arylbenzaldehydes has been investigated. The limitation and scope have been described. The reaction mechanism of the cyclization steps has been examined by DFT calculations.
Introduction
Indenes and benzofulvenes are important core structures in organic chemistry due to their presence in many biologically active compounds1 and functional materials.2 Various methods to construct indene rings have been developed. For example, Lewis and Brønsted acid-catalyzed reactions such as Nazarov-type 4π-electrocyclization,3 Friedel–Crafts cyclization, and reaction of styrylmalonates with aromatic aldehydes4 have been reported recently. Among the methods developed, cyclization reactions of ortho-substitued arenes to provide functionalized indenes have been utilized efficiently. Transition-metal-catalyzed cyclization of ortho-substitued arenes has been investigated.5 Iodine-promoted6 and base-promoted7 cyclization reactions have been reported. Lewis and Brønsted acid-catalyzed reactions of alkene conjugate addition have been studied.8 It is desirable to find new efficient methods to construct variously functionalized indene derivatives.
Sequential reactions are considered to be efficient and favorable for the sustainable concepts.9 The Knoevenagel condensation is the reactions of aldehydes and ketones with active methylene compounds to give alkylidene- or benzylidene-dicarbonyls or analogous compounds, for example, in the presence of amines, ammonium salts, and Lewis acids with amines.10 Since Knoevenagel products are highly reactive compounds, several sequential reactions involving Knoevenagel condensation have been reported.11
For example, originally, Knoevenagel reported formation of bis-adducts.12 Various sequential reactions under the condensation conditions to give intermolecular Michael adducts including the reaction with two kinds of active methylene compounds and further transformation of the adducts and intermolecular hetero-Diels-Alder cycloadducts (Scheme 1A).13 Sequential intramolecular hetero-Diels-Alder14 and 1,5-hydride shift/cyclization15 reactions were also reported as efficient methods (Scheme 1B). The initial alkylidene or benzylidene compounds are directly transformed by the subsequent step under the reaction conditions. It is of interest to find new sequential reactions involving Knoevenagel condensation.
Scheme 1. (A) Sequential Intermolecular Reactions under Condensation Conditions to Give Michael Adducts and Hetero-Diels-Alder Adducts, (B) Sequential Intramolecular Hetero-Diels-Alder and 1,5-Hydride Shift/Cyclization Reactions, and (C) Sequential Knoevenagel Condensation/Cyclization.
In this work, a highly reactive diphenylethene moiety16 in ortho-substitution of arenealdehydes has been used to cause sequential Knoevenagel condensation/cyclization reactions (Scheme 1C). The reaction mechanism has been examined by DFT calculations.
Results and Discussion
The reaction of 2-(1-phenylvinyl)benzaldehyde 1a(5b) with methyl malonate 2a under the various Knoevenagel reaction conditions has been examined first.
The reaction of 1a and 2a with piperidine, AcOH in benzene at 80 °C for 1.5 h gave the benzylidene malonate 3a in 75% yield as a major product (Scheme 2). The same reaction conditions for 17 h gave an indene derivative 4a in 56% yield. The reaction with TiCl4-pyridine (1:4 equiv) in CH2Cl2 at room temperature gave an indene derivative 4a in 79% yield (Scheme 3, Table 1, entry 1). The reaction of variously substituted aryl derivatives 1 with dimethyl malonate 2a also gave indene derivatives 4 (Table 1).
Scheme 2. Reaction of 1a and 2a with Piperidine, AcOH in Benzene.
Scheme 3. Reaction of 1a–h and 2a,b with TiCl4-Pyridine (1:4 equiv) in CH2Cl2.
Table 1. Reaction of 1 and 2a,b with TiCl4-Pyridine (1:4).
| entry | 1 | R1 | R2 | R3 | 2 | R4 | 4 | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | H | H | H | 2a | Me | 4a | 79 |
| 2 | 1b | Me | H | H | 2a | Me | 4b | 46 |
| 3 | 1c | Cl | H | H | 2a | Me | 4c | 54 |
| 4 | 1d | H | Me | H | 2a | Me | 4d | 55 |
| 5 | 1e | H | Cl | H | 2a | Me | 4e | 57 |
| 6 | 1f | H | H | Cl | 2a | Me | 4f | 66 |
| 7 | 1g | H | F | H | 2a | Me | 4g | a |
| 8 | 1a | H | H | H | 2b | Et | 4h | 50 |
An inseparable mixture of possible 4g and 3g.
Transformation of 3a to 4a was also achieved by the reaction with catalytic amounts of Sc(OTf)3 in dichloromethane at 40 °C in 74% yield (Scheme 2).
Next, the reaction of 1a and 2a with TiCl4-Et3N (1:4 equiv ratio) was examined. The reaction gave a 1:1 mixture of 4a and 5a in 61% yield. After examining various ratios of TiCl4-Et3N, it was found that the reaction with TiCl4-Et3N (2:8 equiv) in CH2Cl2 at room temperature for 17 h gave a benzofulvene 5a in 40% yield selectively (Scheme 4, Table 2, entry 1). The reaction of variously substituted aryl derivatives 1 and 2a with TiCl4-Et3N also gave benzofulvene derivatives 5 as orange crystals (Table 2). The structure of 5e was determined by X-ray analysis (Figure S1 in the Supporting Information, CCDC 2105106). The reaction of 1a with diethyl malonate 2b gave the corresponding indene 4h and benzofulvene 5h17(Tables 1 and 2). The reaction of naphtyl derivative 1i and 2a with TiCl4-Et3N (2:8) also gave 5i as a major product in 41% yield.
Scheme 4. Reaction of 1a–g,i and 2a,b with TiCl4-Et3N (2:8 equiv) in CH2Cl2.
Table 2. Reaction of 1 and 2a,b with TiCl4-Et3N (2:8).
| entry | 1 | R1 | R2 | R3 | 2 | R4 | 5 | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | H | H | H | 2a | Me | 5a | 40 |
| 2 | 1b | Me | H | H | 2a | Me | 5b | 45 |
| 3 | 1c | Cl | H | H | 2a | Me | 5c | 57 |
| 4 | 1d | H | Me | H | 2a | Me | 5d | 61 |
| 5 | 1e | H | Cl | H | 2a | Me | 5e | 46 |
| 6 | 1f | H | H | Cl | 2a | Me | 5f | 52 |
| 7 | 1g | H | F | H | 2a | Me | 5g | 53 |
| 8 | 1a | H | H | H | 2b | Et | 5h | 41 |
| 9 | 1i | 2a | Me | 5i | 41 |
The indenes 4a,h were transformed to benzofulvene derivatives 5a,h using the reagents TiCl4-Et3N or DDQ in 67–98% yields (Scheme 5).
Scheme 5. 4a,h Transformation to Benzofulvene Derivatives 5a,h.
In order to examine the effect of phenyl on vinyl group of 1a, the reaction of 6 with 2a in the presence of TiCl4-pyridine/Et3N was carried out. The reaction under the examined conditions gave the Knoevenagel product 7 as an isolable product in 23–64% yield (Scheme 6).
Scheme 6. Reaction of 6 and 2a under the Examined Conditions Giving the Knoevenagel product 7.
The reactions of 2-(1-phenylvinyl)benzaldehydes 1 with other active methylene compounds were examined next. The reaction of 1a and Meldrum’s acid 8 in the presence of TiCl4-pyridine or TiCl4-Et3N gave a complex mixture. However, the reaction in the presence of piperidine (0.2 equiv) in benzene at room temperature gave cyclized product 9 in 80% yield (Scheme 7). The reaction with piperidine (0.2 equiv) and acetic acid (1 equiv) in benzene at room temperature gave 9 as an isolable product in lower yield (42%). The corresponding Knoevenagel adduct was not isolated under the examined conditions.
Scheme 7. Reaction in the Presence of Piperidine in Benzene Giving Cyclized Product 9 and Its Dehydrogenation with DDQ Giving Benzofulvene 10.
Since the properties of conjugated systems are of interest, dehydrogenation of indene products was examined.18,6b,6c Dehydrogenation of 9 proceeded by the reaction with DDQ (1 equiv) in benzene at room temperature to give benzofulvene 10(17) in 94% yield (Scheme 7).
The reaction of 1a and malononitrile 11 with TiCl4-pyridine or TiCl4-Et3N (1:4) gave Knoevenagel adduct 12a in 50–39% yields. The reaction with TiCl4-Et3N (2:8 ratio) gave a mixture of 12a and 13a in 17 and 11% yields, respectively. The reaction in the presence of piperidine (0.2 equiv) in benzene at room temperature gave adduct 12a in 82% yield (Scheme 8, Table 3). The reaction of 12a with catalytic amounts of Sc(OTf)3 in dichloromethane at room temperature gave 13a in 99% yield. Dehydrogenation of 13a with DDQ (1 equiv) in CH2Cl2 at room temperature provided 14a in 61% yield. Thus, the effective reaction conditions to give cyclized product sequentially could not be found. The reaction of the Me and Cl substituted derivatives 1b,c with malononitrile followed by cyclization and dehydration also proceeded stepwise to give 13b,c and 14b,c.
Scheme 8. Schematic of the Transformation of 1a–c to 14a–c.
Table 3. Stepwise Reaction of 1a–c and 11 to 12a–c, 13a–c, and 14a–c.
| entry | 1 | R1 | 12 | yield (%) | 13 | yield (%) | 14 | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | H | 12a | 82a | 13a | 99 | 14a | 61c |
| 2 | 1b | Me | 12b | 73a | 13b | 91 | 14b | 89c |
| 3 | 1c | Cl | 12c | 70b | 13c | 95 | 14c | 74d |
The reaction with piperidine (0.2 equiv) in benzene at r.t.
The reaction with piperidine (0.2 equiv) and acetic acid (1.0 equiv) in benzene at 80 °C.
The reaction with DDQ at r.t. in dichloromethane.
The reaction with DDQ at 80 °C in 1,2-dichloroethane.
The electrophilicity parameters are reported as ethyl benzylidenemalonate (−20.55) < benzylidenemalononitrile (−9.42) < benzylidene Meldrum’s acid (−9.15).19 However, these cyclization reactions may not be easy to compare, partly because the cyclization may be accelerated by Lewis acid or H+ coordination to O or N. In addition, some intermediates seem to be unstable under the Lewis acid conditions.
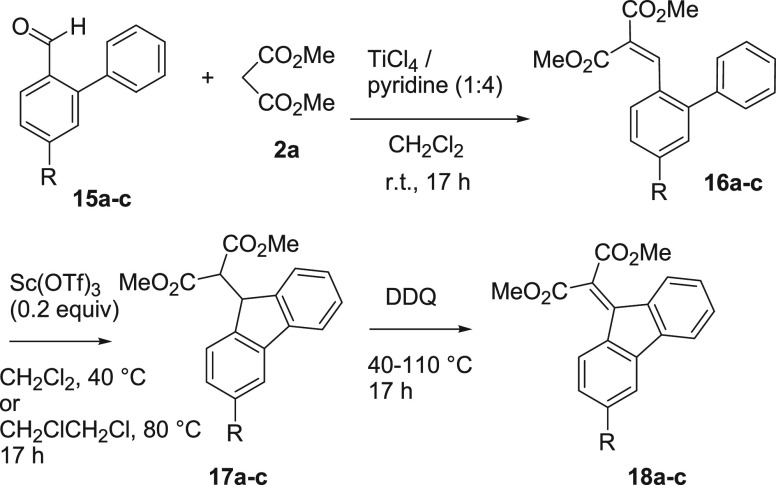
Furthermore, to extend the scope of sequential Knoevenagel condensation/cyclization reaction, the reactions of 2-arylbenzaldehydes 15a–c with an active methylene compound have been investigated (Scheme 9, Table 4). However, the reaction of 15a–c and methyl malonate 2a with TiCl4/Et3N or TiCl4/pyridine gave the normal Knoevenagel adduct 16a-c as major products. The stepwise reaction of 16a–c to fluorenes 17a–c with Sc(OTf)3 and subsequent treatment of 17a–c with DDQ gave 18a–c.
Scheme 9. Reactions of 2-Arylbenzaldehydes 15a–c to Form 18a–c.
Table 4. Stepwise Reaction of 15a–c and 2a to 16a–c, 17a-c, and 18a–c.
| entry | 15 | R | 16 | yield (%) | 17 | yield (%) | 18 | yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 15a | H | 16a | 78a | 17a | 94 | 18a | 69b |
| 2 | 15b | Me | 16b | 86 | 17b | 88 | 18b | 86c |
| 3 | 15c | Cl | 16c | 82 | 17c | 81 | 18c | 40d |
The reaction of 15a and 2a with TiCl4-Et3N (2:8) gave 16a in 81% yield.
At 40 °C in CH2Cl2.
At 80 °C in 1,2-dichloroethane.
At 110 °C in toluene.
On the other hand, the reaction of 15d with electron-donating 3,5-dimethoxyphenyl group and 2a in the presence of TiCl4/pyridine (1:4) gave cyclized product 17d in 63% yield in one pot (Scheme 10). However, the reaction with TiCl4/Et3N (2:8) gave a complex mixture including a small amount of 17d. The reaction of 17d with DDQ at room temperature gave 18d in 92% yield.
Scheme 10. Reaction of 15d to form 18d.
The reaction of 2-phenoxybenzaldehyde 19 and methyl malonate 2a with TiCl4/Et3N or TiCl4/pyridine gave only Knoevenagel adduct 20. The stepwise reaction of 20 to the xanthene derivative 21 with Sc(OTf)3 and subsequent treatment of 21 with DDQ gave 22 (Scheme 11).
Scheme 11. Reaction of 2-Phenoxybenzaldehyde 19 to Form 22.
The probable reaction mechanism to give products sequentially is shown in Scheme 12. First, Knoevenagel condensation of the active methylene compound such as 2a gives Lewis acid coordinated or protonated intermediate A, according to the reported mechanism.10,20 Intramolecular alkene addition affords the carbocation intermediate B, which is stabilized by two aryl groups. Intermediate B undergoes deprotonation to afford C. Protonation of the α-carbon of C may lead to indene 4a. Furthermore, dehydrogenation occurred to afford benzofulvene 5a in the presence of 2 equiv of TiCl4 and 8 equiv of Et3N in one pot.
Scheme 12. Probable Reaction Mechanism from 1a to Give Products 4a and 5a Sequentially.
The oxidative reactions using titanium tetrachloride and a tertiary amine have been reported previously.21 Based on the reports, the intermediate C can be dehydrogenated to give Ti-complex D, which leads to 5a.
The reaction mechanism of the cyclization step in Scheme 12 has been examined by the DFT calculations in order to compare the observed reactivities of various substrates.
The calculations were performed by the B3LYP/6-31G*22,23 level including the PCM24 solvent effect (solvent = CH2Cl2 or benzene). TS geometry was characterized by vibrational analysis, which checked whether the obtained geometry has single imaginary frequencies (ν‡). From TSs, reaction paths were traced by the intrinsic reaction coordinate (IRC) method25 to obtain the energy-minimum geometries. Relative Gibbs free energies in kcal/mol (T = 298.15 K, P = 1 atm) were refined by single-point calculations of RB3LYP/6-311+G(d,p) SCRF = (PCM, solvent = CH2Cl2 or benzene).
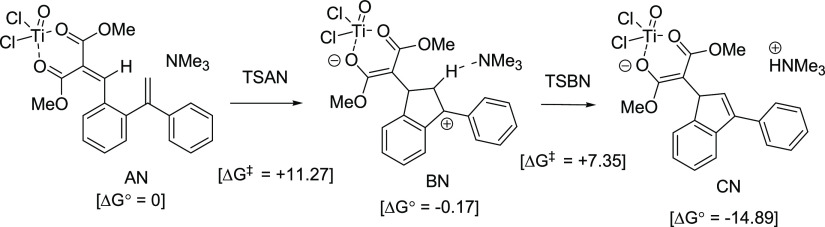
Based on the previous theoretical study by Marrone et al.,20a,20b the TiCl4-promoted Knoevenagel condensation of dimethyl malonate and aldehydes may give titanyl (TiOCl2) complex in situ. In this study, the reaction mechanism starting from the Knoevenagel adduct A (in Scheme 12) in situ was calculated by the use of the titanyl (TiOCl2) complex models.
Intramolecular addition of an alkene to the Knoevenagel adduct–TiOCl2 complex AN with Me3N, leading to the formation of intermediate BN, deprotonation of BN by Me3N (as a model for an amine) to form an alkene, and generation of the intermediate CN.
The steps AN → BN → CN were calculated (Scheme 13). The energy of transition state of cyclization, TSAN (ΔG‡ = +11.27 kcal/mol), is higher than that of deprotonation by Me3N, TSBN (ΔG‡ = +7.35 kcal/mol).
Scheme 13. Transition of AN to BN and then to CN.
Since TSAN is higher than TSBN, cyclization steps for various TiOCl2-coordinate substrate models without Me3N have been calculated and compared (Scheme 14). The activation energy of TSA1 is similar to that of TSAN of the model with Me3N. The transition state (TSA1) of cyclization for TiOCl2-coordinate 2-(1-phenylvinyl) derivative 3a, A1 to B1, is more stable than TSA2 for TiOCl2-coordinate 2-vinyl derivative 7, A2 to B2. The intermediate B1 is highly stabilized by two aryl groups. Furthermore, the reaction models A3 and A4 for Knowevenagel adducts 16a,d from 2-arybenzaldehydes 15a,d have been calculated. The activation energy of TS3A (+21.94 kcal/mol) is much higher than that of TSA1 due to destruction of the aromatic ring. However, the activation energy of TSA4 for the di-MeO derivative is +11.64 kcal/mol and comparable to the TSA1 because two electron-donating groups stabilize the cation intermediate. On the other hand, the activation energy of TSA5 for the oxygen-substituted derivative 20 is higher (+22.68 kcal/mol). This is probably because of both the electronic effect and the steric reason by the six-membered ring formation. Those calculations are in agreement with the experimental results.
Scheme 14. Cyclization Steps for Various TiOCl2-Coordinate Substrate Models.
Next, the reactivity between dimethyl malonate and Meldrum’s acid with dimethylammonium ion as a model of the piperidine-catalyzed reaction was compared (Scheme 15). TSA7 is more stable than TSA6. This is in agreement with the electrophilicity of benzylidenemalonate and benzylidene Meldrum’s acid, as described above.19
Scheme 15. Reactivity between Dimethyl Malonate and Meldrum’s Acid with the Dimethylammonium Ion.
Dehydrogenation step C to D in Scheme 16 was also examined. Hall et al. suggested formation of the iminium ion by the redox reaction between TiCl4 and Et3N.21e Therefore, hydride transfer of C1 with the iminium ion, formed in situ from TiCl4 and Me3N (as a model of Et3N), is considered. The removal of a hydride from the indene ring gives intermediate D1. Although the full mechanism of dehydrogenation by TiCl4-Et3N is not clear, the hydride transfer path by the iminium ion may be possible as shown by the model calculations. Dehydrogenation by DDQ may also involve the hydride transfer step.26
Scheme 16. Dehydrogenation Step C to D.
In summary, sequential Knoevenagel condensation/cyclization leading to indene and benzofulvene derivatives has been developed. The reaction of 2-(1-phenylvinyl)benzaldehyde with malonates gave benzylidene malonates, cyclized indenes, and dehydrogenated benzofulvenes. The product selectivity depends on the reaction conditions. Reaction of variously substituted aryl derivatives with dimethyl malonate gave indene and benzofulvene derivatives. The reactions of 2-(1-phenylvinyl)benzaldehyde with Meldrum’s acid or malononitrile also gave cyclized compounds in the suitable sequential or stepwise conditions. Furthermore, the reaction of 2-arylbenzaldehydes has been investigated. The limitation and scope have been described. The reaction mechanism of the cyclization steps has been examined by the DFT calculations.
Further study on the transformation and the utility of the products is under investigation.
Experimental Section
General Methods
1H chemical shifts are reported in ppm relative to Me4Si. 13C chemical shifts are reported in ppm relative to CDCl3 (77.1 ppm). 19F chemical shifts are reported in ppm relative to CFCl3. 13C mutiplicities were determined by DEPT and HSQC. Mass spectra were recorded at an ionizing voltage of 70 eV by EI or CI. The mass analyzer type used for EI and CI is double-focusing. All reactions were carried out under a nitrogen atmosphere. Column chromatography was performed on silica gel (75–150 μm).
1a–i and 6 were prepared according to the literature.5b15b,d were prepared according to the literature.2715c was prepared according to the literature method.27a
15c: (5 mmol scale, 853 mg, 75%); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 7.36–7.39 (m, 2H), 7.45–7.51 (m, 5H), 7.98 (dd, J = 8.1, 0.6 Hz, 1H), 9.92 (d, J = 0.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 128.24 (CH), 128.70 (CH), 128.74 (CH), 129.24 (CH), 130.00 (CH), 130.78 (CH), 132.12 (C), 136.44 (C), 139.91 (C), 147.44 (C), 191.26 (CH); IR (KBr) 2875, 1684, 1588, 1392, 1253, 1094 cm–1; MS (CI) m/z 219 ([M + H]+, 30), 217 ([M + H]+, 100%); HRMS (CI) m/z 217.0414, 219.0401 (calcd for C13H10ClO [M + H]+ 217.0420, 219.0391).
Procedure for Preparation of 3a
To a solution of 1a (491 mg, 2.3 mmol) in benzene (20 mL) were successively added dimethyl malonate 2a (0.32 g, 0.27 mL, 2.5 mmol), piperidine (0.20 g, 0.23 mL, 2.3 mmol), and AcOH (0.15 g, 0.14 mL, 2.5 mmol) at 0 °C and then heated at reflux. After heating for 1.5 h, the crude products were concentrated in vacuo, and the residue was purified by column chromatography over silica gel eluting with hexane-EtOAc to give 3a (586 mg, 75%).
3a: Rf = 0.1 (hexane-EtOAc = 10:1); pale yellow oil; 1H NMR (400 MHz, CDCl3). Compound 3a decomposes partially to 4a in CDCl3. δ (ppm) 3.738 (s, 3H), 3.742 (s, 3H), 5.23 (d, J = 1.0 Hz, 1H), 5.86 (d, J = 1.0 Hz, 1H), 7.23–7.46 (m, 9H), 7.81 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.46 (CH3), 118.17 (CH2), 126.13 (C), 127.30 (CH), 127.79 (CH), 127.99 (C), 128.01 (CH), 128.02 (CH), 128.40 (CH), 129.95 (CH), 130.24 (CH), 140.58 (C), 142.75 (C), 143.78 (CH), 147.33 (C), 164.25 (C), 166.91 (C); IR (neat) 2952, 1737, 1628, 1436, 1257, 1221, 1069 cm–1; MS (EI) m/z 322 (M+, 32), 290 (47), 262 (72), 202 (100%); HRMS (EI) m/z 322.1201 (calcd for C20H18O4 322.1205).
Procedure for Preparation of 4a
To a solution of 1a (1.05 g, 5.0 mmol) in CH2Cl2 (20 mL) was added dimethyl malonate 2a (660.6 mg, 0.55 mL, 5.0 mmol) and pyridine (1.57 g, 1.6 mL, 20 mmol). After cooling to 0 °C, TiCl4 (948.4 mg, 5.0 mmol) in CH2Cl2 (2.5 mL) was slowly added to the reaction mixture. The reaction mixture was allowed to warm to room temperature and stirred for 8 h. The mixture was quenched with 1 M HCl solution and extracted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography over silica gel eluting with hexane-EtOAc to give 4a (1.27 g, 79%).
4a: Rf = 0.4 (hexane-EtOAc = 10:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.57 (d, J = 8.6 Hz, 1H), 3.69 (s, 3H), 3.83 (s, 3H), 4.28 (dd, J = 8.6, 2.1 Hz, 1H), 6.53 (d, J = 2.1 Hz, 1H), 7.24 (ddd, J = 7.4, 7.4, 1.0 Hz, 1H), 7.33 (ddd, J = 7.4, 7.4, 0.7 Hz, 1H), 7.36–7.40 (m, 2H), 7.42–7.47 (m, 2H), 7.54 (d, J = 7.6 Hz, 1H), 7.56–7.59 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 47.66 (CH), 52.71 (CH3), 52.80 (CH3), 53.59 (CH), 120.83 (CH), 123.80 (CH), 125.61 (CH), 127.46 (CH), 127.78 (CH), 128.03 (CH), 128.66 (CH), 132.65 (CH), 135.33 (C), 143.59 (C), 144.67 (C), 145.70 (C), 168.36 (C), 169.02 (C); IR (neat) 2952, 1736, 1599, 1492, 1435, 1234, 1155, 1030 cm–1; MS (EI) m/z 322 (M+, 20), 262 (47), 207 (71), 202 (100%); HRMS (EI) m/z 322.1195 (calcd for C20H18O4 322.1205).
4b: 1 mmol scale, 154 mg, 46%; Rf = 0.2 (hexane-EtOAc = 10:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.40 (s, 3H), 3.55 (d, J = 8.8 Hz, 1H), 3.68 (s, 3H), 3.82 (s, 3H), 4.27 (dd, J = 8.8, 2.1 Hz, 1H), 6.49 (d, J = 2.1 Hz, 1H), 7.21–7.26 (m, 3H), 7.30–7.34 (m, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.47 (d-like, J = 8.0 Hz, 2H), 7.53 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.34 (CH3), 47.59 (CH), 52.66 (CH3), 52.75 (CH3), 53.61 (CH), 120.83 (CH), 123.74 (CH), 125.52 (CH), 127.40 (CH), 127.63 (CH), 129.33 (CH), 132.06 (CH), 132.39 (C), 137.82 (C), 143.71 (C), 144.69 (C), 145.53 (C), 168.36 (C), 169.01 (C); IR (neat) 2952, 1738, 1509, 1435, 1262, 1233, 1155 cm–1; MS (EI) m/z 336 (M+, 62), 276 (100%); HRMS (EI) m/z 336.1360 (calcd for C21H20O4 336.1362).
4c: 1 mmol scale, 174 mg, 46%; Rf = 0.9 (hexane-EtOAc = 1:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.59 (t, J = 8.6 Hz, 1H), 3.68 (s, 3H), 3.82 (s, 3H), 4.27 (dd, J = 8.6, 2.1 Hz, 1H), 6.54 (d, J = 2.1 Hz, 1H), 7.25 (ddd, J = 7.4, 7.4, 1.0 Hz, 1H), 7.33 (ddd, J = 7.5, 7.4, 0.7 Hz, 1H), 7.39–7.42 (m, 3H), 7.48 (d, J = 7.5 Hz, 1H), 7.50 (d-like, J = 8.6 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 47.65 (CH), 52.67 (CH3), 52.78 (CH3), 53.39 (CH), 120.57 (CH), 123.84 (CH), 125.76 (CH), 127.51 (CH), 128.83 (CH), 129.03 (CH), 133.09 (CH), 133.73 (C), 133.78 (C), 143.17 (C), 144.52 (C), 168.20 (C), 168.89 (C); IR (neat) 2953, 1735, 1488, 1434, 1234, 1155, 1089, 1014 cm–1; MS (EI) m/z 358 (M+, 11), 356 (M+, 32), 296 (70), 83 (100%); HRMS (EI) m/z 356.0812, 358.0781 (calcd for C20H17ClO4 356.0815, 358.0786).
4d: 0.92 mmol scale, 171 mg, 55%; Rf = 0.6 (hexane-ether = 1:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.37 (s, 3H), 3.53 (d, J = 8.8 Hz, 1H), 3.69 (s, 3H), 3.81 (s, 3H), 4.24 (dd, J = 8.8, 2.1 Hz, 1H), 6.51 (d, J = 2.1 Hz, 1H), 7.05 (d, J = 7.6 Hz, 1H), 7.26 (d, J = 7.6 Hz, 1H), 7.33 (s, 3H), 7.33–7.39 (m, 1H), 7.42–7.46 (m, 2H), 7.55–7.57 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.58 (CH3), 47.30 (CH), 52.60 (CH3), 52.67 (CH3), 53.68 (CH), 121.51 (CH), 123.45 (CH), 126.32 (CH), 127.75 (CH), 127.92 (CH), 128.59 (CH), 132.90 (CH), 135.40 (C), 137.15 (C), 141.69 (C), 143.75 (C), 145.60 (C), 168.34 (C), 168.98 (C); IR (neat) 2952, 1743, 1734, 1606, 1492, 1436, 1152, 1029 cm–1; MS (EI) m/z 336 (M+, 66), 276 (100%); HRMS (EI) m/z 336.1368 (calcd for C21H20O4 336.1362); anal. calcd for C21H20O4: C, 75.43; H, 5.43. Found: C, 75.81; H, 5.07.
4e: 1 mmol scale, 207 mg, 57%; Rf = 0.6 (hexane-Et2O = 1:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.60 (d, J = 8.2 Hz, 1H), 3.69 (s, 3H), 3.80 (s, 3H), 4.25 (dd, J = 8.2, 2.1 Hz, 1H), 6.58 (d, J = 2.1 Hz, 1H), 7.21 (dd, J = 8.0, 2.0 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.37–7.43 (m, 1H), 7.44–7.48 (m, 2H), 7.48 (d, J = 2.0 Hz, 1H), 7.51–7.54 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 47.34 (CH), 52.76 (CH3), 52.84 (CH3), 53.31 (CH), 121.14 (CH), 124.81 (CH), 125.53 (CH), 127.70 (CH), 128.31 (CH), 128.81 (CH), 133.64 (C), 134.25 (CH), 134.68 (C), 142.88 (C), 145.04 (C), 145.51 (C), 168.13 (C), 168.74 (C); IR (neat) 2953, 1754, 1730, 1598, 1565, 1491, 1435, 1156, 1072, 1029 cm–1; MS (EI) m/z 356 (M+, 47), 296 (100), 202 (64%); HRMS (EI) m/z 356.0810, 358.0793 (calcd for C20H17ClO4 356.0815, 358.0786).
4f: 1 mmol scale, 235 mg, 66%; Rf = 0.5 (hexane-Et2O = 1:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.58 (d, J = 8.6 Hz, 1H), 3.71 (s, 3H), 3.83 (s, 3H), 4.26 (dd, J = 8.6, 2.0 Hz, 1H), 6.52 (d, J = 2.0 Hz, 1H), 7.30 (dd, J = 8.0, 2.0 Hz, 1H), 7.37–7.41 (m, 2H), 7.43–7.47 (m, 3H), 7.51–7.55 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 47.51 (CH), 52.82 (CH3), 52.94 (CH3), 53.28 (CH), 121.60 (CH), 124.48 (CH), 127.64 (CH), 127.69 (CH), 128.26 (CH), 128.76 (CH), 131.74 (C), 132.84 (CH), 134.86 (C), 142.16 (C), 145.10 (C), 146.46 (C), 168.13 (C), 168.75 (C); IR (KBr) 2958, 1754, 1436, 1154, 1007 cm–1; MS (EI) m/z 356 (M+, 47), 296 (100), 202 (56%); HRMS (EI) m/z 356.0814, 358.0791 (calcd for C20H17ClO4 356.0815, 358.0786).
4h: 1 mmol scale, 174 mg, 50%; Rf = 0.5 (hexane-EtOAc = 10:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.12 (t, J = 7.2 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H), 3.64 (d, J = 7.8 Hz, 1H), 4.12 (q, J = 7.2 Hz, 2H), 4.25–4.30 (m, 3H), 6.57 (d, J = 2.1 Hz, 1H), 7.24 (ddd, J = 7.4, 7.4, 1.0 Hz, 1H), 7.30–7.34 (m, 1H), 7.35–7.39 (m, 1H), 7.41–7.46 (m, 3H), 7.53 (d, J = 7.6 Hz, 1H), 7.56–7.59 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 14.02 (CH3), 14.13 (CH3), 47.65 (CH), 53.72 (CH), 61.51 (CH2), 61.72 (CH2), 120.69 (CH), 123.94 (CH), 125.49 (CH), 127.31 (CH), 127.76 (CH), 127.95 (CH), 128.63 (CH), 132.90 (CH), 135.46 (C), 143.66 (C), 144.87 (C), 145.51 (C), 167.90 (C), 168.58 (C); IR (neat) 2981, 1749, 1732, 1598, 1446, 1369, 1153, 1035 cm–1; MS (EI) m/z 350 (M+, 51), 276 (100%); HRMS (EI) m/z 350.1519 (calcd for C22H22O4 350.1518).
Procedure for Preparation of 5a
To a solution of 1a (209.9 mg, 1.0 mmol) in CH2Cl2 (6 mL) were added dimethyl malonate 2a (132.1 mg, 0.11 mL, 1.0 mmol) and Et3N (809.5 mg, 1.12 mL, 8.0 mmol). After cooling to 0 °C, TiCl4 (379.4 mg, 2.0 mmol) in CH2Cl2 (1 mL) was slowly added to the reaction mixture. The reaction mixture was allowed to warm to room temperature and stirred for 17 h. The mixture was quenched with 1 M HCl solution and extracted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography over silica gel eluting with hexane-EtOAc to give 5a (135 mg, 40%).
5a: Rf = 0.4 (hexane-EtOAc = 15: 1); orange crystals; mp 108–109 °C (hexane); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.88 (s, 3H), 4.00 (s, 3H), 7.18 (ddd, J = 7.6, 7.6, 1.0 Hz, 1H), 7.31 (ddd, J = 7.6, 7.4, 1.0 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.42–7.50 (m, 5H), 7.65–7.68 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.76 (CH3), 53.18 (CH3), 121.06 (C), 121.56 (CH), 124.11 (CH), 124.76 (CH), 127.08 (CH), 127.76 (CH), 128.82 (CH), 129.34 (CH), 130.33 (CH), 134.27 (C), 135.22 (C), 143.14 (C), 149.24 (C), 151.93 (C), 164.30 (C), 166.95 (C); IR (KBr) 2949, 1723, 1617, 1437, 1252, 1223, 1116, 1050 cm–1; λmax (CH3CN) 250 (ε 22,700), 318 (10,000), 418 (2050) nm; MS (EI) m/z 320 (M+, 14), 202 (85), 201 (100%); HRMS (EI) m/z 320.1056 (calcd for C20H16O4 320.1049); anal. calcd for C20H16O4: C, 74.99; H, 5.03. Found: C, 75.08; H, 4.99.
5b: 1 mmol scale, 151 mg, 45%; Rf = 0.3 (hexane-EtOAc = 10:1); red-orange crystals; mp 108–110 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.42 (s, 3H), 3.87 (s, 3H), 3.99 (s, 3H), 7.18 (ddd, J = 7.6, 7.6, 1.2 Hz, 1H), 7.28 (d, J = 8.0 Hz, 2H), 7.30 (ddd, J = 7.6, 7.6, 1.0 Hz, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.45 (s, 1H), 7.46 (d, J = 7.6 Hz, 1H), 7.57 (d-like, J = 8.0 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.53 (CH3), 52.70 (CH3), 53.13 (CH3), 120.64 (C), 121.57 (CH), 124.01 (CH), 124.17 (CH), 126.99 (CH), 127.67 (CH), 129.50 (CH), 130.25 (CH), 131.36 (C), 135.34 (C), 139.53 (C), 143.20 (C), 149.38 (C), 151.93 (C), 164.35 (C), 167.01 (C); IR (KBr) 2951, 1729, 1725, 1605, 1251, 1218 cm–1; λmax (CH3CN) 251 (ε 19,400), 330 (10,000), 425 (2180) nm; MS (EI) m/z 334 (M+, 100), 303 (24%); HRMS (EI) m/z 334.1201 (calcd for C21H18O4 334.1205).
5c: 1 mmol scale, 184 mg, 57%; Rf = 0.3 (hexane-EtOAc = 10:1); yellow crystals; mp 115–117 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.87 (s, 3H), 4.00 (s, 3H), 7.19 (ddd, J = 7.6, 7.5, 1.0 Hz, 1H), 7.30 (ddd, J = 7.5, 7.4, 0.8 Hz, 1H), 7.39 (d, J = 7.4 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.43 (d-like, J = 8.5 Hz, 2H), 7.47 (s, 1H), 7.58 (d-like, J = 8.5 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.78 (CH3), 53.17 (CH3), 121.30 (CH), 121.38 (C), 124.17 (CH), 125.00 (CH), 127.21 (CH), 129.00 (CH), 129.05 (CH), 130.38 (CH), 132.64 (C), 135.06 (C), 135.15 (C), 142.70 (C), 148.90 (C), 150.55 (C), 164.16 (C), 166.78 (C); IR (KBr) 2958, 1746, 1730, 1617, 1251, 1210 cm–1; λmax (CH3CN) 252 (ε 32,700), 333 (11,200), 420 (3010) nm; MS (EI) m/z 356 (M+, 43), 354 (M+, 100%); HRMS (EI) m/z 354.0650, 356.0674 (calcd for C20H15ClO4 354.0659, 356.0629).
5d: 0.92 mmol scale, 188 mg, 61%; Rf = 0.5 (hexane-ether = 1:1); orange crystals; mp 112–113 °C (ether); 1H NMR (400 MHz, CDCl3) δ (ppm) 2.36 (s, 3H), 3.87 (s, 3H), 3.98 (s, 3H), 6.98 (d, J = 7.8 Hz, 1H), 7.25 (s, 1H), 7.28 (d, J = 7.8 Hz, 1H), 7.41–7.50 (m, 3H), 7.44 (s, 1H), 7.63–7.66 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.88 (CH3), 52.66 (CH3), 53.08 (CH3), 120.35 (C), 122.68 (CH), 124.02 (CH), 125.12 (CH), 127.41 (CH), 127.77 (CH), 128.79 (CH), 129.24 (CH), 132.43 (C), 134.37 (C), 140.87 (C), 143.46 (C), 149.32 (C), 151.86 (C), 164.40 (C), 167.03 (C); IR (KBr) 2949, 1735, 1728, 1604, 1430, 1250, 1218, 1048 cm–1; λmax (CH3CN) 253 (ε 25,800), 325 (9960), 419 (1640) nm; MS (EI) m/z 334 (M+, 100), 303 (23%); HRMS (EI) m/z 334.1201 (calcd for C21H18O4 334.1205).
5e: 1 mmol scale, 166 mg, 46%; Rf = 0.6 (hexane-ether = 1 1); orange crystals; mp 129–130 °C (ethanol); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.88 (s, 3H), 3.99 (s, 3H), 7.17 (dd, J = 8.2, 2.0 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.42 (d, J = 2.0 Hz, 1H), 7.45–7.51 (m, 3H), 7.48 (s, 1H), 7.60–7.63 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.85 (CH3), 53.21 (CH3), 121.87 (C), 122.06 (CH), 125.00 (CH), 126.09 (CH), 126.67 (CH), 127.69 (CH), 128.98 (CH), 129.59 (CH), 133.36 (C), 133.68 (C), 136.52 (C), 145.01 (C), 147.96 (C), 150.81 (C), 164.16 (C), 166.55 (C); IR (KBr) 2951, 1735, 1723, 1617, 1444, 1249, 1216, 1046 cm–1; MS (EI) m/z 356 (M+, 59), 354 (M+, 100), 296 (49%); λmax (CH3CN) 257 (ε 25,700), 334 (11,300), 413 (1930) nm; HRMS (EI) m/z 354.0659, 356.0648 (calcd for C20H15ClO4 354.0659, 356.0629); anal. calcd for C20H15ClO4: C, 67.71; H, 4.26. Found: C, 67.54; H, 4.42.
5f: 1 mmol scale, 186 mg, 52%; Rf = 0.5 (hexane-ether = 1:1); orange crystals; mp 99–100 °C (hexane-EtOAc); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.88 (s, 3H), 4.01 (s, 3H), 7.29 (dd, J = 8.0, 1.9 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.38 (d, J = 1.9 Hz, 1H), 7.42–7.50 (m, 3H), 7.44 (s, 1H), 7.61–7.64 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.90 (CH3), 53.26 (CH3), 122.12 (CH), 124.90 (CH), 124.94 (CH), 127.70 (CH), 128.92 (CH), 129.59 (CH), 129.84 (CH), 133.16 (C), 133.84 (C), 136.90 (C), 141.42 (C), 148.05 (C), 151.26 (C), 164.10 (C), 166.40 (C); IR (KBr) 2952, 1723, 1617, 1442, 1253, 1216, 1044 cm–1; λmax (CH3CN) 256 (ε 12,700), 316 (7400), 430 (1100) nm; MS (EI) m/z 356 (M+, 35), 354 (M+, 100), 323 (20), 189 (29%); HRMS (EI) m/z 354.0652, 356.0632 (calcd for C20H15ClO4 354.0659, 356.0629); anal. calcd for C20H15ClO4: C, 67.71; H, 4.26. Found: C, 67.70; H, 4.02.
5g: 1 mmol scale, 178 mg, 53%; Rf = 0.5 (hexane-ether = 1:1); orange crystals; mp 100–101 °C (hexane-EtOAc); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.88 (s, 3H), 3.99 (s, 3H), 6.86 (ddd, J = 8.6, 8.4, 2.3 Hz, 1H), 7.16 (dd, J = 8.8, 2.3 Hz, 1H), 7.39 (dd, J = 8.4, 4.9 Hz, 1H), 7.41–7.51 (m, 3H), 7.51 (s, 1H), 7.60–7.63 (m, 2H); 19F NMR (376 MHz, CDCl3) δ (ppm) −109.17 (ddd, JFH = 8.6, 8.6, 5.1 Hz); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.83 (CH3), 53.21 (CH3), 109.86 (d, JCF = 25 Hz, CH), 112.94 (d, JCF = 23 Hz, CH), 121.35 (C), 125.51 (d, JCF = 9.2 Hz, CH), 126.38 (CH), 127.61 (CH), 128.95 (CH), 129.56 (CH), 130.75 (d, JCF = 3.1 Hz, C), 133.71 (C), 145.91 (d, JCF = 8.4 Hz, C), 147.96 (C), 150.43 (d, JCF = 2.3 Hz, C), 164.21 (C), 164.33 (d, JCF = 251 Hz, C), 166.68 (C); IR (KBr) 2949, 1738, 1723, 1614, 1593, 1458, 1257, 1195, 1046 cm–1; λmax (CH3CN) 251 (ε 19,700), 321 (9370), 401 (1680) nm; MS (EI) m/z 338 (M+, 100), 220 (69), 207 (60%); HRMS (EI) m/z 338.0950 (calcd for C20H15FO4 338.0954); anal. calcd for C20H15FO4: C, 71.00; H, 4.47. Found: C, 71.13; H, 4.27.
5h:17 1.0 mmol scale, 143 mg, 41%; Rf = 0.4 (hexane-EtOAc = 10:1); orange crystals; mp 112.6–113.6 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.35 (t, J = 7.1 Hz, 3H), 1.41 (t, J = 7.1 Hz, 3H), 4.33 (q, J = 7.1 Hz, 2H), 4.47 (q, J = 7.1 Hz, 2H), 7.18 (ddd, J = 7.6, 7.6, 1.0 Hz, 1H), 7.30 (ddd, J = 7.6, 7.6, 1.0 Hz, 1H), 7.40–7.47 (m, 4H), 7.47 (s, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.64–7.67 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 14.03 (CH3), 14.18 (CH3), 61.71 (CH2), 62.08 (CH2), 121.42 (CH), 122.08 (C), 124.30 (CH), 124.90 (CH), 126.95 (CH), 127.75 (CH), 128.79 (CH), 129.23 (CH), 130.16 (CH), 134.40 (C), 135.33 (C), 143.14 (C), 148.54 (C), 151.56 (C), 163.97 (C), 166.40 (C); IR (KBr) 2981, 1724, 1708, 1451, 1250, 1217, 1047 cm–1; λmax (CH3CN) 250 (ε 22,100), 313 (11,800), 419 (1950) nm; MS (EI) m/z 348 (M+, 100), 303 (24), 202 (52%); HRMS (EI) m/z 348.1360 (calcd for C22H20O4 348.1362); anal. calcd for C22H20O4: C, 75.84; H, 5.79. Found: C, 75.49; H, 5.93.
5i: 0.89 mmol scale, 136 mg, 41%; Rf = 0.5 (hexane-ether = 1:1); dark red crystals; mp 124–125 °C (hexane-EtOAc); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.88 (s, 3H), 4.04 (s, 3H), 7.18 (ddd, J = 8.6, 6.7, 1.2 Hz, 1H), 7.26 (s, 1H), 7.63 (ddd, J = 8.1, 6.7, 1.2 Hz, 1H), 7.46–7.53 (m, 6H), 7.59 (d, J = 8.6 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.90 (CH3), 53.29 (CH3), 120.67 (CH), 123.08 (C), 125.15 (CH), 126.14 (CH), 126.54 (CH), 127.01 (CH), 127.57 (CH), 128.06 (C), 128.37 (CH), 128.45 (CH), 128.58 (CH), 128.69 (CH), 132.06 (C), 135.72 (C), 137.31 (C), 140.15 (C), 148.98 (C), 153.76 (C), 164.15 (C), 166.87 (C); IR (KBr) 2951, 1720, 1617, 1432, 1245, 1208, 1125, 1048 cm–1; λmax (CH3CN) 221 (ε 21,300), 291 (17,100), 334 (5410) nm; MS (EI) m/z 370 (M+, 100), 239 (27%); HRMS (EI) m/z 370.1198 (calcd for C24H18O4 370.1205).
5a was also obtained by treatment of 4a (182 mg, 0.56 mmol) with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) (127 mg, 0.56 mmol) in CH2Cl2 (2.0 mL) at room temperature for 17 h. Chromatography over silica gel eluting with hexane-EtOAc gave 5a (175 mg, 98%).
7: TiCl4/Et3N = 1:4 equiv, 1 mmol scale, 160.8 mg, 64%; Rf = 0.2 (hexane-AcOEt = 10:1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.72 (s, 3H), 3.87 (s, 3H), 5.44 (dd, J = 11.0, 1.1 Hz, 1H), 5.64 (dd, J = 17.3, 1.1 Hz, 1H), 6.90 (dd, J = 17.3, 11.0 Hz, 1H), 7.25 (ddd, J = 7.8, 7.8, 1.1 Hz, 1H), 7.31 (d-like, J = 7.8 Hz, 1H), 7.37 (ddd, J = 7.8, 7.8, 1.6 Hz, 1H), 7.50 (d-like, J = 7.8 Hz, 1H), 8.07 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.61 (CH3), 52.79 (CH3), 118.75 (CH2), 126.80 (CH), 127.43 (C), 127.80 (CH), 128.08 (CH), 130.23 (CH), 131.55 (C), 134.12 (CH), 137.89 (C), 143.04 (CH), 164.38 (C), 166.70 (C); IR (neat) 2952, 1735, 1625, 1437, 1260, 1215, 1070 cm–1; MS (EI) m/z 246 (M+, 1.4), 214 (100), 128 (59%); HRMS (EI) m/z 246.0884 (calcd for C14H14O4 246.0892).
Preparation of 9
To a solution of 1a (833 mg, 4.0 mmol) in benzene (6 mL) were added Meldrum’s acid 8 (576.5 mg, 4.0 mmol) and piperidine (68.1 mg, 0.08 mL, 0.8 mmol). The mixture was stirred at room temperature for 18 h. 1 M HCl was added to the mixture. The mixture was extracted with CH2Cl2. dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography over silica gel eluting with hexane-CH2Cl2 to give 9 (1.04 g, 80%).
9: Rf = 0.1 (hexane-CH2Cl2 = 1:2); colorless crystals; mp 129–130 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.75 (s, 3H), 1.82 (s, 3H), 4.27 (d, J = 3.7 Hz, 1H), 4.56 (dd, J = 3.7, 2.1 Hz, 1H), 6.44 (d, J = 2.1 Hz, 1H), 7.27 (ddd, J = 7.4, 7.4, 1.0 Hz, 1H), 7.33–7.47 (m, 5H), 7.57 (d, J = 7.6 Hz, 1H), 7.59–7.61 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 27.49 (CH3), 28.32 (CH3), 46.84 (CH), 47.06 (CH), 105.15 (C), 121.16 (CH), 122.34 (CH), 125.70 (CH), 127.43 (CH), 127.88 (CH), 128.05 (CH), 128.64 (CH), 130.65 (CH), 135.34 (C), 144.16 (C), 144.46 (C), 146.68 (C), 163.52 (C), 163.87 (C); IR (KBr) 3001, 2871, 1781, 1748, 1457, 1383, 1328, 1303, 1205, 1063 cm–1; MS (EI) m/z 334 (M+, 1.2), 276 (18), 248 (100), 232 (95%); HRMS (EI) m/z 334.1204 (calcd for C21H18O4 334.1205).
Preparation of 10
Reaction of 9 (32.8 mg, 0.10 mmol) with DDQ (22.7 mg, 0.10 mmol) in benzene (0.5 mL) at room temperature for 18 h. Chromatography over silica gel eluting with hexane-CH2Cl2 gave 10 (30.1 mg, 94%).
10:17 Rf = 0.2 (hexane-CH2Cl2 = 1: 1); red crystals; mp 130–131 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 1.81 (s, 6H), 7.23 (ddd, J = 7.6, 7.6, 1.2 Hz, 1H), 7.32 (ddd, J = 7.6, 7.3, 1.0 Hz, 1H), 7.39 (d, J = 7.3 Hz, 1H), 7.47–7.51 (m, 4H), 7.66–7.69 (m, 2H), 8.44 (d, J = 7.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 27.39 (CH3), 104.45 (C), 113.19 (C), 122.24 (CH), 126.26 (CH), 127.86 (CH), 128.76 (CH), 128.95 (CH), 129.33 (CH), 130.36 (CH), 132.13 (CH), 133.41 (C), 135.04 (C), 143.88 (C), 157.04 (C), 160.45 (C), 161.19 (C), 161.91 (C); IR (KBr) 2925, 1729, 1560, 1449, 1289, 1206 cm–1; λmax (CH3CN) 254 (ε 19,300), 344 (13,000), 480 (2340) nm; MS (EI) m/z 332 (M+, 9.9), 274 (100%); HRMS (EI) m/z 332.1048 (calcd for C21H16O4 332.1049).
12a: piperidine (0.2 equiv), 10.1 mmol scale, 2.14 g, 82%; Rf = 0.3 (hexane-CH2Cl2 = 2:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 5.20 (d, J = 0.7 Hz, 1H), 5.98 (d, J = 0.7 Hz, 1H), 7.19–7.22 (m, 2H), 7.32–7.36 (m, 3H), 7.39 (dd, J = 7.6, 1.2 Hz, 1H), 7.52 (ddd, J = 7.6, 7.6, 1.4 Hz, 1H), 7.60 (ddd, J = 7.6, 7.6, 1.4 Hz, 1H), 7.88 (s, 1H), 8.18 (d-like, J = 7.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 83.60 (C), 112.46 (C), 113.55 (C), 119.19 (CH2), 127.04 (CH), 128.39 (CH), 128.63 (CH), 128.79 (CH), 128.90 (CH), 129.81 (C), 130.97 (CH), 133.70 (CH), 139.86 (C), 144.84 (C), 146.47 (C), 159.68 (CH); IR (KBr) 3047, 2227, 1581 cm–1; MS (EI) m/z 256 (M+, 100), 255 (66), 191 (78%); HRMS (EI) m/z 256.0999 (calcd for C18H12N2 256.1000).
12b: piperidine (0.2 equiv), 5.5 mmol scale, 1.08 g, 73%; Rf = 0.3 (hexane-CH2Cl2 = 2:1); yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.35 (s, 3H), 5.13 (s, 1H), 5.94 (s, 1H), 7.09 (d-like, J = 8.2 Hz, 2H), 7.14 (d-like, J = 8.2 Hz, 2H), 7.38 (dd, J = 7.6, 1.2 Hz, 1H), 7.51 (ddd, J = 7.6, 7.4, 1.2 Hz, 1H), 7.59 (ddd, J = 7.6, 7.6, 1.3 Hz, 1H), 7.88 (s, 1H), 8.18 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.21 (CH3), 83.38 (C), 112.51 (C), 113.61 (C), 118.26 (CH2), 126.91 (CH), 128.30 (CH), 128.51 (CH), 129.55 (CH), 129.78 (C), 130.92 (CH), 133.66 (CH), 137.03 (C), 138.82 (C), 145.08 (C), 146.23 (C), 159.72 (CH); IR (neat) 3029, 2229, 1581, 1510, 1214 cm–1; MS (EI) m/z 270 (M+, 100), 255 (62), 205 (88%); HRMS (EI) m/z 270.1159 (calcd for C19H14N2 270.1157).
12c: piperidine (0.2 equiv), acetic acid (1.0 equiv) at 80 °C, 1.0 mmol scale, 204 mg, 70%; Rf = 0.3 (hexane-CH2Cl2 = 1:1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 5.21 (s, 1H), 5.98 (s, 1H), 7.14 (d-like, J = 8.6 Hz, 2H), 7.32 (d-like, J = 8.6 Hz, 2H), 7.36 (dd, J = 7.6, 1.4 Hz, 1H), 7.54 (ddd, J = 7.8, 7.6, 1.4 Hz, 1H), 7.61 (ddd, J = 7.6, 7.6, 1.4 Hz, 1H), 7.86 (s, 1H), 8.20 (dd, J = 7.8, 0.7 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 83.93 (C), 112.41 (C), 113.54 (C), 119.76 (CH2), 128.32 (CH), 128.54 (CH), 128.89 (CH), 129.11 (CH), 129.76 (C), 130.94 (CH), 133.84 (CH), 134.87 (C), 138.21 (C), 144.26 (C), 145.28 (C), 159.32 (CH); IR (KBr) 3037, 2236, 1588, 1487, 1089, 1010 cm–1; MS (EI) m/z 292 (M+, 22), 290 (M+, 64), 255 (100), 225 (90%); HRMS (EI) m/z 290.0615, 292.0595 (calcd for C18H11ClN2 290.0611, 292.0581).
Procedure for Preparation of 13a
To a solution of 12a (267 g, 1.0 mmol) in CH2Cl2 (3 mL) was added Sc(OTf)3 (104 mg, 0.2 mmol). The reaction mixture was stirred for 17 h. Saturated aqueous NaHCO3 was added to the mixture. The mixture was extracted with CH2Cl2, dried over Na2SO4, and concentrated in vacuo to give 13a (265 mg, 99%).
13a: yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 4.01 (d, J = 6.8 Hz, 1H), 4.06 (dd, J = 6.8, 2.1 Hz, 1H), 6.47 (d, J = 2.1 Hz, 1H), 7.37 (ddd, J = 7.4, 7.4, 1.1 Hz, 1H), 7.41–7.50 (m, 4H), 7.58–7.61 (m, 3H), 7.74 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 25.83 (CH), 47.44 (CH), 111.59 (C), 111.82 (C), 121.88 (CH), 123.91 (CH), 126.93 (CH), 127.48 (CH), 127.80 (CH), 128.89 (CH), 128.90 (CH), 129.19 (CH), 133.94 (C), 141.23 (C), 143.50 (C), 149.59 (C); IR (KBr) 3049, 2922, 2258, 1489, 1443, 1351, 1071, 1009 cm–1; MS (EI) m/z 256 (M+, 20), 191 (100%); HRMS (EI) m/z 256.0997 (calcd for C18H12N2 256.1000).
13b: 1 mmol scale, 247 mg, 91%; yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.41 (s, 3H), 3.97 (d, J = 6.8 Hz, 1H), 4.01 (dd, J = 6.8, 2.1 Hz, 1H), 6.41 (d, J = 2.1 Hz, 1H), 7.27 (d, J = 8.0 Hz, 2H), 7.34 (ddd, J = 7.4, 7.4, 1.0 Hz, 1H), 7.42 (ddd, J = 7.6, 7.4, 0.9 Hz, 1H), 7.48 (d-like, J = 8.0 Hz, 2H), 7.58 (d, J = 7.6 Hz, 1H), 7.71 (d-like, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.38 (CH3), 25.80 (CH), 47.38 (CH), 111.65 (C), 111.88 (C), 121.86 (CH), 123.85 (CH), 126.80 (CH), 126.93 (CH), 127.66 (CH), 129.11 (CH), 129.54 (CH), 131.04 (C), 138.86 (C), 141.30 (C), 143.62 (C), 149.39 (C); IR (KBr) 2901, 2259, 1508, 1457, 1115 cm–1; MS (EI) m/z 270 (M+, 18), 205 (100%); HRMS (EI) m/z 270.1154 (calcd for C19H14N2 270.1157).
13c: 0.94 mmol scale, 259 mg, 95%; pale yellow crystals; mp 141–142 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 4.04 (d, J = 6.6 Hz, 1H), 4.09 (dd, J = 6.6, 2.0 Hz, 1H), 6.48 (d, J = 2.0 Hz, 1H), 7.40 (ddd, J = 7.4, 7.4, 1.2 Hz, 1H), 7.42–7.49 (m, 3H), 7.52–7.55 (m, 3H), 7.75 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 25.83 (CH), 47.56 (CH), 111.44 (C), 111.72 (C), 121.73 (CH), 124.02 (CH), 127.19 (CH), 127.85 (CH), 129.14 (CH), 129.18 (CH), 129.35 (CH), 132.39 (C), 134.89 (C), 141.15 (C), 143.20 (C), 148.64 (C); IR (KBr) 2219, 1568, 1541, 1089 cm–1; MS (EI) m/z 292 (M+, 5.3), 290 (M+, 16), 227 (35), 225 (100%); HRMS (EI) m/z 290.0607, 292.0595 (calcd for C18H11ClN2 290.0611, 292.0581).
14a: r.t. in CH2Cl2, 17 h, 0.5 mmol scale, 77.1 mg, 61%; Rf = 0.5 (hexane-CH2Cl2 = 1:1); orange crystals; mp 153–154 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 6.66 (s, 1H), 7.33 (dd, J = 7.6, 7.6 Hz, 1H), 7.41 (ddd, J = 7.6, 7.4, 0.9 Hz, 1H), 7.48 (d, J = 7.4 Hz, 1H), 7.50–7.55 (m, 3H), 7.65–7.67 (m, 2H), 8.15 (d, J = 7.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 77.51 (C), 112.88 (C), 122.79 (CH), 123.16 (CH), 125.79 (CH), 127.83 (CH), 129.12 (CH), 129.18 (CH), 131.13 (CH), 132.38 (C), 132.95 (CH), 133.30 (C), 142.90 (C), 157.31 (C), 165.31 (C); IR (KBr) 3062, 2222, 1570, 1540, 1446, 1373, 1100 cm–1; MS (EI) m/z 254 (M+, 100%); HRMS (EI) m/z 254.0835 (calcd for C18H10N2 254.0844).
14b: r.t. in CH2Cl2, 18 h, 0.59 mmol scale, 140.4 mg, 89%; Rf = 0.5 (hexane-CH2Cl2 = 1:1); orange crystals; mp. 145 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.45 (s, 3H), 6.67 (s, 1H), 7.31–7.35 (m, 3H), 7.41 (ddd, J = 7.4, 7.4, 1.0, Hz, 1H), 7.50 (d, J = 7.4 Hz, 1H), 7.60 (d, J = 8.0 Hz, 2H), 8.17 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.74 (CH3), 77.00 (C), 113.08 (C), 122.23 (CH), 123.21 (CH), 125.76 (CH), 127.90 (CH), 129.11 (CH), 129.69 (C), 129.96 (CH), 132.86 (CH), 133.58 (C), 141.91 (C), 143.05 (C), 157.48 (C), 165.54 (C); IR (KBr) 2920, 2225, 1577, 1559, 1367, 1105 cm–1; MS (EI) m/z 268 (M+, 100%); HRMS (EI) m/z 268.1000 (calcd for C19H12N2 268.1000).
14c: 80 °C, in CH2ClCH2Cl, 20 h, 0.5 mmol scale, 105.8 mg, 74%; Rf = 0.5 (hexane-CH2Cl2 = 1:1); orange crystals; mp 208–210 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 6.70 (s, 1H), 7.36 (ddd, J = 7.4, 5.1, 3.7 Hz, 1H), 7.41–7.45 (m, 2H), 7.51 (d-like, J = 8.6 Hz, 2H), 7.63 (d-like, J = 8.6 Hz, 2H), 8.20 (ddd, J = 7.4, 0.9, 0.9 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 78.12 (C), 112.84 (C), 122.96 (CH), 123.14 (CH), 126.03 (CH), 129.15 (CH), 129.35 (CH), 129.59 (CH), 130.90 (C), 133.09 (CH), 133.27 (C), 137.25 (C), 142.71 (C), 156.01 (C), 165.12 (C); IR (KBr) 2921, 2259, 1490, 1401, 1096, 1012 cm–1; MS (EI) m/z 290 (M+, 34), 288 (M+, 100%); HRMS (EI) m/z 288.0453, 290.0439 (calcd for C18H9ClN2 288.0454, 290.0425).
Procedure for Preparation of 16a
To a solution of 15a (182.2 g, 1.0 mmol) in CH2Cl2 (5 mL) were added dimethyl malonate 2a (132.1 mg, 0.11 mL, 1.0 mmol) and pyridine (316 mg, 0.32 mL, 4 mmol). After cooling to 0 °C, TiCl4 (190 mg, 0.11 mL, 1.0 mmol) was slowly added to the reaction mixture. The reaction mixture was allowed to warm to room temperature and stirred for 17 h. The mixture was quenched with water and extracted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3, dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography over silica gel eluting with hexane-EtOAc to give 16a (230 mg, 78%).
16a: Rf = 0.4 (hexane-EtOAc = 10:1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.777 (s, 3H), 3.781 (s, 3H), 7.32–7.47 (m, 9H), 7.70 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.58 (CH3), 126.57 (C), 127.49 (CH), 127.92 (CH), 128.31 (CH), 128.41 (CH), 129.78 (CH), 130.17 (CH), 130.20 (CH), 131.94 (C), 139.68 (C), 142.70 (C), 144.33 (CH), 164.32 (C), 167.04 (C); IR (neat) 2976, 2868, 1737, 1626, 1437, 1116, 1069 cm–1; MS (EI) m/z 296 (M+, 9.2), 264 (94), 204 (100%); HRMS (EI) m/z 296.1042 (calcd for C18H16O4 296.1049).
16b: 2.7 mmol scale, 726 mg, 86%; Rf = 0.1 (hexane-EtOAc = 10:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.40 (s, 3H), 3.77 (s, 3H), 3.79 (s, 3H), 7.15 (d-like, J = 8.0 Hz, 1H), 7.24 (s, 1H), 7.31–7.44 (m, 6H), 7.68 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.47 (CH3), 52.53 (CH3), 52.60 (CH3), 125.59 (C), 127.84 (CH), 128.26 (CH), 128.32 (CH), 128.35 (CH), 129.01 (C), 129.77 (CH), 130.97 (CH), 139.75 (C), 140.62 (C), 142.88 (C), 144.22 (CH), 164.48 (C), 167.34 (C); IR (neat) 2952, 1735, 1626, 1607, 1436, 1256, 1070 cm–1; MS (EI) m/z 310 (M+, 11), 278 (80), 250 (52), 219 (100%); HRMS (EI) m/z 310.1204 (calcd for C19H18O4 310.1205).
16c: 0.5 mmol scale, 137 mg, 82%; Rf = 0.2 (hexane-EtOAc = 10:1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.79 (s, 3H), 3.80 (s, 3H), 7.30–7.34 (m, 3H), 7.37–7.47 (m, 5H), 7.60 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.71 (CH3), 52.76 (CH3), 127.02 (C), 127.64 (CH), 128.50 (CH), 128.60 (CH), 129.63 (CH), 130.18 (CH), 130.40 (C), 136.06 (C), 138.37 (C), 143.00 (CH), 144.28 (C), 164.14 (C), 166.82 (C); IR (neat) 2953, 1740, 1721, 1629, 1590, 1435, 1260, 1071 cm–1; MS (EI) m/z 332 (M+, 3.5), 330 (M+, 9.9), 298 (75), 263 (76), 239 (100%); HRMS (EI) m/z 330.0656, 332.0631 (calcd for C18H15ClO4 330.0659, 332.0629).
17d: 6.5 mmol scale, 1.46 g, 63%; Rf = 0.1 (hexane-CH2Cl2 = 1:1); colorless crystals; mp 96–98 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.24 (s, 3H), 3.85 (s, 3H), 3.885 (s, 3H), 3.890 (s, 3H), 4.69 (d, J = 3.5 Hz, 1H), 4.70 (d, J = 3.5 Hz, 1H), 6.42 (d, J = 2.1 Hz, 1H), 6.88 (d, J = 2.1 Hz, 1H), 7.27 (ddd, J = 7.6, 7.5, 1.1 Hz, 1H), 7.35 (dd, J = 7.6, 7.5 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 44.62 (CH), 51.81 (CH), 51.84 (CH3), 52.63 (CH3), 55.49 (CH3), 55.68 (CH3), 96.47 (CH), 97.80 (CH), 119.80 (CH), 123.12 (C), 125.55 (CH), 127.19 (CH), 127.53 (CH), 141.51 (C), 143.46 (C), 144.45 (C), 156.91 (C), 161.66 (C), 167.88 (C), 169.93 (C); IR (KBr) 2952, 1733, 1595, 1428, 1053 cm–1; MS (EI) m/z 356 (M+, 30), 296 (37), 225 (100%); HRMS (EI) m/z 356.1260 (calcd for C20H20O6 356.1260); anal. calcd for C20H20O6: C, 67.41; H, 5.66. Found: C, 67.39; H, 5.56.
Procedure for Preparation of 17a
To a solution of 16a (702 mg, 2.37 mmol) in CH2Cl2 (7.1 mL) was added Sc(OTf)3 (232 mg, 0.47 mmol). The reaction mixture was stirred at 40 °C for 17 h. After cooling to room temperature, saturated aqueous NaHCO3 was added to the mixture. The mixture was extracted with CH2Cl2, dried over Na2SO4, and concentrated in vacuo to give 17a (658 mg, 94%).
17a: colorless crystals; mp 129–131 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.66 (s, 6H), 3.88 (d, J = 6.4 Hz, 1H), 4.70 (d, J = 6.4 Hz, 1H), 7.28 (ddd, J = 7.6, 7.4, 1.2 Hz, 2H), 7.38 (dd, J = 7.6, 7.4 Hz, 2H), 7.46 (dd, J = 7.6, 0.8 Hz, 2H), 7.74 (d, J = 7.6 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 46.24 (CH), 52.56 (CH3), 55.34 (CH), 120.02 (CH), 124.76 (CH), 127.22 (CH), 127.93 (CH), 141.26 (C), 143.65 (C), 168.56 (C); IR (KBr) 2948, 1740, 1718, 1437, 1267, 1019 cm–1; MS (EI) m/z 296 (M+, 42), 236 (100%); HRMS (EI) m/z 296.1050 (calcd for C18H16O4 296.1049); anal. calcd for C18H16O4: C, 72.96; H, 5.44. Found: C, 72.97; H, 5.53.
17b: 0.39 mmol scale, 106.5 mg, 88%; Rf = 0.5 (hexane-EtOAc = 5: 1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.41 (s, 3H), 3.647 (s, 3H), 3.650 (s, 3H), 3.82 (d, J = 6.6 Hz, 1H), 4.65 (d, J = 6.6 Hz, 1H), 7.08 (dd, J = 7.8, 0.8 Hz, 1H), 7.25 (ddd, J = 7.5, 7.5, 1.2 Hz, 1H), 7.31–7.37 (m, 2H), 7.43 (dd, J = 7.5, 0.9 Hz, 1H), 7.54 (s, 1H), 7.69 (d, J = 7.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.53 (CH3), 45.86 (CH), 52.45 (CH3), 55.38 (CH), 119.82 (CH), 120.59 (CH), 124.36 (CH), 124.66 (CH), 126.99 (CH), 127.80 (CH), 128.10 (CH), 137.59 (C), 140.70 (C), 141.23 (C), 141.29 (C), 143.99 (C), 168.52 (C), 168.54 (C); IR (neat) 2952, 1740, 1435, 1256, 1157 cm–1; MS (EI) m/z 310 (M+, 43), 250 (100%); HRMS (EI) m/z 310.1202 (calcd for C19H18O4 310.1205).
17c: 2.0 mmol scale, 535 mg, 81%; colorless crystals; mp 117 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.63 (s, 3H), 3.68 (s, 3H), 3.90 (d, J = 6.1 Hz, 1H), 4.66 (d, J = 6.1 Hz, 1H), 7.25 (dd, J = 8.2, 2.1 Hz, 1H), 7.32 (ddd, J = 7.5, 7.5, 1.2 Hz, 1H), 7.38–7.42 (m, 2H), 7.47 (dd, J = 7.5, 0.8 Hz, 1H), 7.70–7.71 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 45.86 (CH), 52.64 (CH3), 52.70 (CH3), 55.02 (CH), 120.29 (CH), 124.81 (CH), 125.96 (CH), 127.14 (CH), 127.96 (CH), 128.15 (CH), 134.04 (C), 140.07 (C), 141.88 (C), 143.12 (C), 144.10 (C), 168.29 (C), 168.46 (C); IR (KBr) 2948, 1748, 1729, 1435, 1368, 1154 cm–1; MS (EI) m/z 332 (M+, 12), 330 (M+, 35), 270 (100%); HRMS (EI) m/z 330.0660, 332.0628 (calcd for C18H15ClO4 330.0659, 332.0629).
Preparation of 18a
To a solution of 17a (241 mg, 0.8 mmol) in CH2Cl2 (6.6 mL) was added DDQ (183 mg, 0.8 mmol). The reaction mixture was stirred at 40 °C for 17 h. After cooling to room temperature, the mixture was purified by chromatography over silica gel eluting with hexane-CH2Cl2 to give 18a (168 mg, 69%).
18a: Rf = 0.1 (hexane-CH2Cl2 = 1:1); yellow crystals; mp 79.2–80.0 °C (hexane-CH2Cl2); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.94 (s, 6H), 7.18 (ddd, J = 8.0, 7.6, 1.1 Hz, 2H), 7.34 (ddd, J = 7.6, 7.4, 0.9 Hz, 2H), 7.55 (d, J = 7.4 Hz, 2H), 7.74 (d, J = 8.0 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 53.07 (CH3), 119.75 (CH), 122.02 (C), 125.96 (CH), 127.76 (CH), 131.14 (C), 135.47 (C), 141.73 (C), 144.74 (C), 165.83 (C); IR (KBr) 2950, 1722, 1595, 1449, 1250, 1077 cm–1; MS (EI) m/z 294 (M+, 100), 263 (27), 195 (56%); HRMS (EI) m/z 294.0894 (calcd for C18H14O4 294.0892).
18b: 80 °C in 1,2-dichloroethane, 0.54 mmol scale, 144 mg, 86%; Rf = 0.4 (hexane-CH2Cl2 = 1:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 2.39 (s, 3H), 3.94 (s, 3H), 3.95 (s, 3H), 7.00 (dd, J = 8.0, 0.9 Hz, 1H), 7.18 (ddd, J = 7.8, 7.5, 1.2 Hz, 1H), 7.35 (ddd, J = 7.6, 7.5, 0.9 Hz, 1H), 7.38 (s, 1H), 7.54 (d, J = 7.6 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 21.78 (CH3), 53.05 (CH3), 119.63 (CH), 120.53 (CH), 121.18 (C), 126.02 (CH), 126.08 (CH), 127.73 (CH), 128.66 (CH), 131.07 (CH), 132.99 (C), 136.04 (C), 141.82 (C), 141.85 (C), 142.08 (C), 145.13 (C), 166.01 (C), 166.04 (C); IR (neat) 2950, 1732, 1716, 1615, 1456, 1244, 1076 cm–1; MS (EI) m/z 308 (M+, 100), 209 (67), 189 (64%); HRMS (EI) m/z 308.1044 (calcd for C19H16O4 308.1049).
18c: 110 °C in toluene, 0.53 mmol scale, 70 mg, 40%; Rf = 0.1 (hexane-CH2Cl2 = 2:1); mp 138.5–139.3 °C (hexane-CHCl3); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.95 (s, 3H), 3.97 (s, 3H), 7.17 (dd, J = 8.4, 2.0 Hz, 1H), 7.25 (ddd, J = 7.8, 7.7, 1.2 Hz, 1H), 7.39 (ddd, J = 7.7, 7.5, 0.9 Hz, 1H), 7.55 (d, J = 2.0 Hz, 1H), 7.56 (d, J = 7.5 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.78 (d, J = 8.4 Hz, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 53.21 (CH3), 120.09 (CH), 120.16 (CH), 122.56 (C), 126.05 (CH), 127.48 (CH), 127.72 (CH), 128.56 (CH), 131.36 (CH), 133.84 (C), 136.02 (C), 137.40 (C), 140.54 (C), 143.59 (C), 143.94 (C), 165.51 (C), 165.88 (C); IR (KBr) 2955, 1719, 1588, 1444, 1244, 1073 cm–1; MS (EI) m/z 330 (M+, 35), 328 (M+, 100), 297 (32), 229 (54%); HRMS (EI) m/z 328.0502, 330.0472 (calcd for C18H13ClO4 328.0502, 330.0473).
18d: r.t. in CH2Cl2, 0.5 mmol scale, 163 mg, 92%; Rf = 0.5 (hexane-CH2Cl2 = 1:2); yellow crystals; mp 159.5–161.0 °C (Et2O); 1H NMR (400 MHz, CDCl3) δ (ppm) 3.837 (s, 3H), 3.842 (s, 3H), 3.89 (s, 3H), 3.95 (s, 3H), 6.29 (d, J = 2.1 Hz, 1H), 6.78 (d, J = 2.1 Hz, 1H), 7.19 (ddd, J = 8.2, 7.4, 1.1 Hz, 1H), 7.32 (ddd, J = 7.4, 7.4, 1.2 Hz, 1H), 7.54–7.56 (m, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.45 (CH3), 53.02 (CH3), 55.66 (CH3), 55.77 (CH3), 97.88 (CH), 98.08 (CH), 116.70 (C), 119.93 (CH), 121.58 (C), 124.42 (CH), 128.06 (CH), 130.08 (CH), 138.09 (C), 140.82 (C), 142.69 (C), 144.81 (C), 158.57 (C), 164.41 (C), 166.86 (C), 168.97 (C); IR (KBr) 2954, 1734, 1715, 1591, 1430, 1249, 1146 cm–1; MS (EI) m/z 354 (M+, 100), 323 (52), 265 (58), 235 (79%); HRMS (EI) m/z 354.1096 (calcd for C20H18O6 354.1103).
20: TiCl4/pyridine = 1: 4 equiv, 1.0 mmol scale, 252 mg, 81%; Rf = 0.4 (hexane-AcOEt = 10:1); colorless oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.80 (s, 3H), 3.82 (s, 3H), 6.85 (dd, J = 8.4, 0.8 Hz, 1H), 7.01 (d-like, J = 7.6 Hz, 2H), 7.07 (dd, J = 7.8, 7.8 Hz, 1H), 7.15 (t-like, J = 7.3 Hz, 1H), 7.32 (dd, J = 8.4, 7.8 Hz, 1H), 7.33–7.38 (m, 2H), 7.44 (dd, J = 7.8, 1.6 Hz, 1H), 8.14 (s, 1H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.66 (CH3), 52.72 (CH3), 118.19 (CH), 119.43 (CH), 123.27 (CH), 124.04 (CH), 124.53 (C), 126.42 (C), 129.24 (CH), 129.97 (CH), 132.06 (CH), 138.44 (CH), 156.29 (C), 156.46 (C), 164.60 (C), 167.10 (C); IR (KBr) 2952, 1740, 1700, 1600, 1482, 1433, 1232, 1065 cm–1; MS (EI) m/z 312 (M+, 3.2). 280 (7.0), 248 (20), 219 (100%); HRMS (EI) m/z 312.1000 (calcd for C18H16O5 312.0998).
21: 1.0 mmol scale, 227 mg, 73%; Rf = 0.2 (hexane-AcOEt = 10:1); colorless crystals; mp 61.6–62.4 °C; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.56 (s, 6H), 3.60 (d, J = 9.2 Hz, 1H), 4.82 (d, J = 9.2 Hz, 1H), 7.06 (ddd, J = 7.4, 7.4, 1.2 Hz, 2H), 7.15 (d-like, J = 8.1 Hz, 2H), 7.26 (dd-like, J = 8.1, 7.4 Hz, 2H), 7.30 (d-like, J = 7.4 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 39.89 (CH), 52.58 (CH3), 59.94 (CH), 116.83 (CH), 122.68 (C), 123.45 (CH), 128.66 (CH), 128.88 (CH), 153.25 (C), 167.79 (C); IR (KBr) 2953, 1752, 1726, 1476, 1255, 1145 cm–1; MS (EI) m/z 312 (M+, 2.8), 181 (100%); HRMS (EI) m/z 312.1000 (calcd for C18H16O5 312.0998).
22: 0.5 mmol scale, 116.7 mg, 74%; Rf = 0.1 (hexane-AcOEt = 10:1); pale yellow oil; 1H NMR (400 MHz, CDCl3) δ (ppm) 3.75 (s, 6H), 7.16 (ddd, J = 8.0, 7.2, 1.2 Hz, 2H), 7.31 (dd, J = 8.0, 1.0 Hz, 2H), 7.43 (ddd, J = 8.0, 7.2, 1.6 Hz, 2H), 7.65 (dd, J = 8.0, 1.6 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ (ppm) 52.70 (CH3), 117.01 (CH), 119.97 (C), 120.89 (C), 123.25 (CH), 127.06 (CH), 131.01 (CH), 137.94 (C), 152.59 (C), 166.28 (C); IR (KBr) 2955, 1740, 1712, 1600, 1449, 1250, 1071 cm–1; MS (EI) m/z 310 (M+, 100), 279 (66%); HRMS (EI) m/z 310.0844 (calcd for C18H14O5 310.0841).
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan and JSPS KAKENHI Grant Number JP26410048. Part of this work was conducted in NAIST, supported by Nanotechnology Platform Program (Synthesis of Molecules and Materials) of MEXT.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05283.
The authors declare no competing financial interest.
Supplementary Material
References
- Recent examples,; a Watanabe N.; Nakagava H.; Ikeno A.; Minato H.; Kohayakawa C.; Tsuji J.-i. 4-(4-Alkylpiperazin-1-yl)phenyl group: A novel class of basic side chains for selective estrogen receptor modulators. Bioorg. Med. Chem. Lett. 2003, 13, 4317–4320. 10.1016/j.bmcl.2003.09.049. [DOI] [PubMed] [Google Scholar]; b Kolanos R.; Siripurapu U.; Pullagurla M.; Riaz M.; Setola V.; Roth B. L.; Dukat M.; Glennon R. A. Binding of isotryptamines and indenes at h5-HT6 serotonin receptors. Bioorg. Med. Chem. Lett. 2005, 15, 1987–1991. 10.1016/j.bmcl.2005.02.070. [DOI] [PubMed] [Google Scholar]; c Ahn J. H.; Shin M. S.; Jung S. H.; Kim J. A.; Kim H. M.; Kim S. H.; Kang S. K.; Kim K. R.; Rhee S. D.; Park S. D.; Lee J. M.; Lee J. H.; Cheona H. G.; Kim S. S. Synthesis and structure–activity relationship of novel indene N-oxide derivatives as potent peroxisome proliferator activated receptor c (PPARc) agonists. Bioorg. Med. Chem. Lett. 2007, 17, 5239–5244. 10.1016/j.bmcl.2007.06.073. [DOI] [PubMed] [Google Scholar]; d Alcalde E.; Mesquida N.; Lopez-Pérez S.; Frigola J.; Mercè R. Indene-Based Scaffolds. 2. An Indole–Indene Switch: Discovery of Novel Indenylsulfonamides as 5-HT6 Serotonin Receptor Agonists. J. Med. Chem. 2009, 52, 675–687. 10.1021/jm8009469. [DOI] [PubMed] [Google Scholar]; e Chanda D.; Saikia D.; Kumar J. K.; Thakur J. P.; Agarwal J.; Chanotiyy C. S.; Shanker K.; Negi A. S. 1- Chloro-2-formyl indenes and tetralenes as antitubercular agents. Bioorg. Med. Chem. Lett. 2011, 21, 3966–3969. 10.1016/j.bmcl.2011.05.016. [DOI] [PubMed] [Google Scholar]; f Iakovenko R. O.; Chicca A.; Daniela N.; Gertsch J.; Reynoso-Morenoc I.; Krasavin M.; Vasilyev A. Synthesis of various arylated trifluoromethyl substituted indanes and indenes, and study of their biological activity. Tetrahedron 2019, 75, 624–632. 10.1016/j.tet.2018.12.041. [DOI] [Google Scholar]
- Selected examples, (a)Yang J.; Lakshmikantham M. V.; Cava M. P.; Lorcy D.; Bethelot J. R. Synthesis and Characterization of 5,10-Bis(2-thienyl)indeno[2,1-a]indene Derivatives: The First Examples of Conducting Polymers Containing a Rigid Bis(thienyl)butadiene Core. J. Org. Chem. 2000, 65, 6739–6742. 10.1021/jo991140y. [DOI] [PubMed] [Google Scholar]; b Cappelli A.; Galeazzi S.; Giuliani G.; Anzini M.; Donati A.; Zetta L.; Mendichi R.; Aggravi M.; Giorgi G.; Paccagnini E.; Vomero S. Structural Manipulation of Benzofulvene Derivatives Showing Spontaneous Thermoreversible Polymerization. Role of the Substituents in the Modulation of Polymer Properties. Macromolecules 2007, 40, 3005–3014. 10.1021/ma0629236. [DOI] [Google Scholar]; c Basurto S.; García S.; Neo A. G.; Torroba T.; Marcos C. F.; Miguel D.; Barberá J.; Ros M. B.; de La Fuente M. R. Indene and Pseudoazulene Discotic Liquid Crystals: A Synthetic and Structural Study. Chem. – Eur. J. 2005, 11, 5362–5376. 10.1002/chem.200500155. [DOI] [PubMed] [Google Scholar]; d Nakano T.; Yade T.; Fukuda Y.; Yamaguchi T.; Okumura S. Macromolecules 2005, 38, 8140. 10.1021/ma0513564. [DOI] [Google Scholar]; e Londergan T. M.; Teng C. J.; Weber W. P. Free Radical Polymerization of 7-Methyl-1-methylene-3-phenylindene. Copoly(methylene/7-methyl-3-phenyl-1,1-indenylene)-Excimer Photoluminescence. Macromolecules 1999, 32, 1111–1114. 10.1021/ma9816113. [DOI] [Google Scholar]; f Kondo K.; Goda H.; Takemoto K.; Aso H.; Sasaki T.; Kawakami K.; Yoshida H.; Yoshida K. Micro- and macro-scopic second-order non-linear optical properties of fulvene compounds. J. Mater. Chem. 1992, 2, 1097. 10.1039/jm9920201097. [DOI] [Google Scholar]
- a Dillon A. S.; Kerr D. J.; Flynn B. L. Formation of Highly Substituted Indenes through Acid Promoted Cyclodehydration with Nucleophile Incorporation. J. Org. Chem. 2019, 84, 2756–2767. 10.1021/acs.joc.8b03042. [DOI] [PubMed] [Google Scholar]; b Smith C. D.; Rosocha G.; Mui L.; Batey R. A. Investigation of Substituent Effects on the Selectivity of 4π-Electrocyclization of 1,3-Diarylallylic Cations for the Formation of Highly Substituted Indenes. J. Org. Chem. 2010, 75, 4716–4727. 10.1021/jo100275q. [DOI] [PubMed] [Google Scholar]; c Mahesh S. K.; Nanubolu J. B.; Sudhakar G. Tandem Addition/Electrocyclization/Benzylation of Alkyl Aryl-1,3-dienes and Aromatic Aldehydes: Access to Highly Substituted Indenes. J. Org. Chem. 2019, 84, 7815–7828. 10.1021/acs.joc.9b00679. [DOI] [PubMed] [Google Scholar]
- a Novikov R. A.; Levina A. A.; Borisov D. D.; Volodin A. D.; Korlyukov A. A.; Tkachev Y. V.; Platonova Y. B.; Tomilova L. G.; Tomilov Y. V. Synthesis of the Cationic Gallium Phthalocyanines and Their Catalytic Application in Gallium(III)-Activated Processes for Donor–Acceptor Substrates. Organometallics 2020, 39, 2580–2593. 10.1021/acs.organomet.0c00113. [DOI] [Google Scholar]; b Borisov D. D.; Novikov R. A.; Tomilov Y. V. GaCl3-Mediated Reactions of Donor–Acceptor Cyclopropanes with Aromatic Aldehydes. Angew. Chem., Int. Ed. 2016, 55, 12233–12237. 10.1002/anie.201603927. [DOI] [PubMed] [Google Scholar]
- a Kundu K.; McCullagh J. V.; Morehead A. T. Hydroacylation of 2-Vinyl Benzaldehyde Systems: An Efficient Method for the Synthesis of Chiral 3-Substituted Indanones. J. Am. Chem. Soc. 2005, 127, 16042–16043. 10.1021/ja0564416. [DOI] [PubMed] [Google Scholar]; b Zhou Q.; Li S.; Zhang Y.; Wang J. Rhodium(II)- or Copper(I)-Catalyzed Formal Intramolecular Carbene Insertion into Vinylic C(sp2)–H Bonds: Access to Substituted 1H-Indenes. Angew. Chem., Int. Ed. 2017, 56, 16013–16017. 10.1002/anie.201709375. [DOI] [PubMed] [Google Scholar]; c Ye L.; Wang Y.; Aue D. H.; Zhang L. Experimental and Computational Evidence for Gold Vinylidenes: Generation from Terminal Alkynes via a Bifurcation Pathway and Facile C–H Insertions. J. Am. Chem. Soc. 2012, 134, 31–34. 10.1021/ja2091992. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jana A.; Misztal K.; Żak A.; Grela K. Synthesis of Selectively Substituted or Deuterated Indenes via Sequential Pd and Ru Catalysis. J. Org. Chem. 2017, 82, 4226–4234. 10.1021/acs.joc.7b00200. [DOI] [PubMed] [Google Scholar]; e Das B. G.; Chirila A.; Tromp M.; Reek J. N. H.; de Bruin B. CoIII–Carbene Radical Approach to Substituted 1H-Indenes. J. Am. Chem. Soc. 2016, 138, 8968–8975. 10.1021/jacs.6b05434. [DOI] [PubMed] [Google Scholar]
- a Martinelli C.; Cardone A.; Pinto V.; Talamo M. M.; D’arienzo M. L.; Mesto E.; Schingaro E.; Scordari F.; Naso F.; Musio R.; Farinola G. M. Synthesis and Structure of Conjugated Molecules with the Benzofulvene Core. Org. Lett. 2014, 16, 3424–3427. 10.1021/ol5015366. [DOI] [PubMed] [Google Scholar]; b Saunthwal R. K.; Danodia A. K.; Patel M.; Kumar S.; Verma A. K. Regioselective 5-endo-dig Electrophilic Iodocyclization of Enediynes: A Convenient Route to Iodo-substituted Indenes and Cyclopenta-Fused Arenes. Chem. – Asian J. 2016, 11, 3001–3007. 10.1002/asia.201601085. [DOI] [PubMed] [Google Scholar]; c Yagishita F.; Hoshi K.; Yoshida Y.; Ueta S.; Minagawa K.; Imada Y.; Kawamura Y. Facile Construction of Benzofulvene Scaffold from Tetraaryl[3]cumulene Through Electrophilic Iodocyclization. Eur. J. Org. Chem. 2021, 235–238. 10.1002/ejoc.202001200. [DOI] [Google Scholar]
- Qiu G.; Ding Q.; Gao K.; Peng Y.; Wu J. Efficient Assembly of 1-(1H-Imidazol-1-yl)-3-methylene-1H-indenes via Tandem Reaction of (2-(Alkynyl)benzylidene)malonates with Imidazoles. ACS Comb. Sci. 2011, 13, 13–18. 10.1021/co100006g. [DOI] [PubMed] [Google Scholar]
- a Qin Y.; Lv J.; Luo S.; Cheng J.-P. Direct Intramolecular Conjugate Addition of Simple Alkenes to α,β-Unsaturated Carbonyls Catalyzed by Cu(OTf)2. Org. Lett. 2014, 16, 5032–5035. 10.1021/ol502373u. [DOI] [PubMed] [Google Scholar]; b Dethe D. H.; Murhade G. M.; Ghosh S. FeCl3-Catalyzed Intramolecular Michael Reaction of Styrenes for the Synthesis of Highly Substituted Indenes. J. Org. Chem. 2015, 80, 8367–8376. 10.1021/acs.joc.5b01071. [DOI] [PubMed] [Google Scholar]; c Sarnpitak P.; Trongchit K.; Kostenko Y.; Sathalalai S.; Gleeson M. P.; Ruchirawat S.; Ploypradith P. Synthesis of Substituted 2-Arylindanes from E-(2-Stilbenyl)methanols via Lewis Acid-Mediated Cyclization and Nucleophililc Transfer from Trialkylsilyl Reagents. J. Org. Chem. 2013, 78, 8281–8296. 10.1021/jo4013755. [DOI] [PubMed] [Google Scholar]; d Bai J.-F.; Yasumoto K.; Kano T.; Maruoka K. Synthesis of 1-Aminoindenes through Aza-Prins-Type Cyclization. Chem. – Eur. J. 2018, 24, 10320–10323. 10.1002/chem.201802448. [DOI] [PubMed] [Google Scholar]; e Manojveer S.; Balamurugan R. In Situ Formed Acetal-Facilitated Synthesis of Substituted Indene Derivatives from o-Alkenylbenzaldehydes. Org. Lett. 2015, 17, 3600–3603. 10.1021/acs.orglett.5b01695. [DOI] [PubMed] [Google Scholar]
- a Tietze L. F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; b Tietze L. F.; Brasche G.; Gericke K. M.. Domino Reactions in Organic Synthesis. WILEY-VCH, 2006 10.1002/9783527609925. [DOI] [Google Scholar]
- a Tietze L.F.; Beifuss U.. The Knoevenagel Reaction. In: Trost B. M., Ed., Comprehensive Organic Synthesis, Pergamon Press, Oxford, 1991, 341–394. [Google Scholar]; b Ogata Y.; Tsuchida M. Kinetics of the Knoevenagel Condensation of Benzaldehydes with Diethyl Malonate. J. Am. Chem. Soc. 1959, 81, 2092–2094. 10.1016/B978-0-08-052349-1.00033-0. [DOI] [Google Scholar]; c van Beurden K.; de Koning S.; Molendijk D.; van Schijndel J. The Knoevenagel reaction: a review of the unfinished treasure map to forming carbon–carbon bonds. Green Chem. Lett. Rev. 2020, 13, 349–364. 10.1080/17518253.2020.1851398. [DOI] [Google Scholar]
- a Voskressensky L. G.; Festa A. A.; Varlamov A. V. Domino reactions based on Knoevenagel condensation in the synthesis of heterocyclic compounds. Recent advances. Tetrahedron 2014, 70, 551–572. 10.1016/j.tet.2013.11.011. [DOI] [Google Scholar]; b Sartori G.; Maggi R.; Bigi F.; Porta C.; Tao X.; Bernardi G. L.; Ianelli S.; Nardelli M. Selective synthesis of 1-indanones via tandem knoevenagel condensation-cycloalkylation of β-dicarbonyl compounds and aldehydes. Tetrahedron 1995, 51, 12179–12192. 10.1016/0040-4020(95)00772-Z. [DOI] [Google Scholar]; c Guo Y.-L.; Li Y.-H.; Chang H.-H.; Kuo T.-S.; Han J.-L. Molecular sieve mediated sequential Knoevenagel condensation/decarboxylative Michael addition reaction: efficient and mild conditions for the synthesis of 3,3-disubstituted oxindoles with an all carbon quaternary center. RSC Adv. 2016, 6, 74683–74690. 10.1039/C6RA16975A. [DOI] [Google Scholar]; d Pandey K.; Rangan K.; Kumar A. One-Pot Tandem Amidation, Knoevenagel Condensation, and Palladium-Catalyzed Wacker Type Oxidation/C-O Coupling: Synthesis of Chromeno-Annulated Imidazopyridines. J. Org. Chem. 2018, 83, 8026–8035. 10.1021/acs.joc.8b00884. [DOI] [PubMed] [Google Scholar]
- a Knoevenagel E. Ueber eine Darstellungsweise der Glutarsäure. Chem. Ber. 1894, 27, 2345–2346. 10.1002/cber.189402702229. [DOI] [Google Scholar]; b Knoevenagel E. Ueber eine Darstellungsweise des Benzylidenacetessigesters. Chem. Ber. 1896, 29, 172–174. 10.1002/cber.18960290133. [DOI] [Google Scholar]
- a Al-Majid A. M.; Islam M. S.; Barakat A.; Al-Qahtani N. J.; Yousuf S.; Choudhary M. I. Tandem Knoevenagel–Michael reactions in aqueous diethylamine medium: A greener and efficient approach toward bis-dimedone derivatives. Arabian J. Chem. 2017, 10, 185–193. 10.1016/j.arabjc.2014.04.008. [DOI] [Google Scholar]; b Sobhani S.; Hasaninejad A. R.; Maleki M. F.; Parizi Z. P. Tandem Knoevenagel–Michael Reaction of 1-Phenyl-3-methyl-5-pyrazolone with Aldehydes Using 3-Aminopropylated Silica Gel as an Efficient and Reusable Heterogeneous Catalyst. Synth. Commun. 2012, 42, 2245–2255. 10.1080/00397911.2011.555589. [DOI] [Google Scholar]; c Moosavi-Zare A. R.; Zolfigol M. A.; Khaledian O.; Khakyzadeh V.; Farahani M. D.; Kruger H. G. Tandem Knoevenagel–Michael-cyclocondensation reactions of malononitrile, various aldehydes and dimedone using acetic acid functionalized ionic liquid. New J. Chem. 2014, 38, 2342–2347. 10.1039/c3nj01509b. [DOI] [Google Scholar]; d Prakasha M. S.; Smitha G.; Somanatha T. M.; Suchetan P. A.; Swamy G. K.; Naveen S.; Lokanath N. K. Novel tandem Knoevenagel-Michael condensation product of naphtho[2,1-b]furan-2-carbaldehyde with dimedone: Synthesis, spectroscopic and crystal structure studies. Chem. Data Collect. 2020, 28, 100458. 10.1016/j.cdc.2020.100458. [DOI] [Google Scholar]
- a Tietze L.-F.; von Kiedrowski G.; Harms K.; Clegg W.; Sheldrick G. Stereocontrolled Intramolecular Diels-Alder Reaction of Heterodienes; Studies on the Synthesis of Cannabinoids. Angew. Chem., Int. Ed. Engl. 1980, 19, 134–135. 10.1002/anie.198001341. [DOI] [Google Scholar]; b Yadav J. S.; Reddy B. V. S.; Gopal A. V. H.; Rao R. N.; Somaiah R.; Reddy P. P.; Kunwar A. C. Domino Knoevenagel–hetero-Diels–Alder reactions: a stereoselective synthesis of sugar-annulated furo[3,2-b] pyrano[4,3-d]pyran derivatives. Tetrahedron Lett. 2010, 51, 2305–2308. 10.1016/j.tetlet.2010.02.132. [DOI] [Google Scholar]; c Bryhas A. O.; Matiychuk V. S.; Lis T.; Kinzhybalo V.; Smalius V. V.; Obushak M. D. A four-step domino Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2013, 54, 5667–5670. 10.1016/j.tetlet.2013.07.161. [DOI] [Google Scholar]
- Zhao T.; Zhang H.; Cui L.; Wang C.; Qu J.; Wang B. A ZnCl2-Catalyzed Knoevenagel Condensation/1,5-Hydride Shift/Cyclization Sequence: Synthesis of Novel Spiroisoxazol-5-one Tetrahydroquinolines. ChemistrySelect 2016, 1, 3713–3717. 10.1002/slct.201600823. [DOI] [Google Scholar]
- Wagner-Jauregg T. Thermische und photochemische Additionen von Dienophilen an Arene sowie deren Vinyloge und Hetero-Analoge; II. Synthesis 1980, 1980, 769–798. 10.1055/s-1980-29206. [DOI] [Google Scholar]
- Browne N. R.; Brown R. F. C.; Eastwood F. W.; Fallon G. D. The Chemistry of Ethyl 2-Ethoxycarbonyl-5,5-diphenylpenta-2,3,4-trienoate, a Potential Precursor of Ph2C=C=C=C=C=O. Aust. J. Chem. 1987, 40, 1675–1686. 10.1071/CH9871675. [DOI] [Google Scholar]
- a Basurto S.; Miguel D.; Moreno D.; Neo A. G.; Quesada R.; Torroba T. Simple 1-dicyanomethylene-2-chloro-3-aminoindene push–pull chromophores: applications in cation and anion sensing. Org. Biomol. Chem. 2010, 8, 552–558. 10.1039/B916700E. [DOI] [PubMed] [Google Scholar]; (b) Bonda C. A.; Hu S.; Zhang Q. J.; Zhang Z.. Compositions, apparatus, systems, and methods for resolving electronic excited states. US Patent 2014/0044654.
- Allgäuer D. S.; Jangra H.; Asahara H.; Li Z.; Chen Q.; Zipse H.; Ofial A. R.; Mayr H. Quantification and Theoretical Analysis of the Electrophilicities of Michael Acceptors. J. Am. Chem. Soc. 2017, 139, 13318–13329. 10.1021/jacs.7b05106. [DOI] [PubMed] [Google Scholar]
- a Marrone A.; Renzetti A.; De Maria P.; Gérard S.; Sapi J.; Fontana A.; Re N. Condensation of β-Diester Titanium Enolates with Carbonyl Substrates: A Combined DFT and Experimental Investigation. Chem. – Eur. J. 2009, 15, 11537–11550. 10.1002/chem.200901595. [DOI] [PubMed] [Google Scholar]; b Renzetti A.; Marrone A.; Gérard S.; Sapi J.; Nakazawa H.; Reb N.; Fontana A. TiCl4-promoted condensation of methyl acetoacetate. isobutyraldehyde, and indole: a theoretical and experimental study. Phys. Chem. Chem. Phys. 2015, 17, 8964–8972. 10.1039/C4CP05412A. [DOI] [PubMed] [Google Scholar]; c Dalessandro E. V.; Collin H. P.; Valle M. S.; Pliego J. R. Jr. Mechanism and free energy profile of base-catalyzed Knoevenagel condensation reaction. RSC Adv. 2016, 6, 57803–57810. 10.1039/C6RA10393F. [DOI] [Google Scholar]; d Dalessandro E. V.; Collin H. P.; Guimarães L. G. L.; Valle M. S.; Pliego J. R. Jr. Mechanism of the Piperidine-Catalyzed Knoevenagel Condensation Reaction in Methanol: The Role of Iminium and Enolate Ions. J. Phys. Chem. B 2017, 121, 5300–5307. 10.1021/acs.jpcb.7b03191. [DOI] [PubMed] [Google Scholar]
- a Matsumura Y.; Nishimura M.; Hiu H.; Watanabe M.; Kise N. Dependence of the Reactivities of Titanium Enolates on How They Are Generated: Diastereoselective Coupling of Phenylacetic Acid Esters Using Titanium Tetrachloride. J. Org. Chem. 1996, 61, 2809–2812. 10.1021/jo952204h. [DOI] [PubMed] [Google Scholar]; b Periasamy M.; Srinivas G.; Karunakar G. V.; Bharathi P. Reductive coupling of aromatic aldehydes and imines by the low valent titanium species generated in the reaction of TiCl4 with Et3N. Tetrahedron Lett. 1999, 40, 7577–7580. 10.1016/S0040-4039(99)01609-3. [DOI] [Google Scholar]; c Cież D.; Kalinowska-Tłuścik J. Titanium(IV) Enolates of 2-Nitrocarboxylic Esters and Their Oxidative Chlorination. A Convenient Route to α-Chloro-α-nitrocarboxylates. Synlett 2012, 2012, 267–271. 10.1055/s-0031-1290081. [DOI] [Google Scholar]; d Periasamy M.; Srinivas G.; Bharathi P. J. Org. Chem. 1999, 64, 4204–4205. 10.1021/jo982342h. [DOI] [Google Scholar]; e Hall H. K. Jr.; Padias A. B.; Williams P. A.; Gosau J.-M.; Boone H. W.; Park D.-K. Synthesis and Structure of Heterocyclic Quinone Arylimines as Model Compounds for Polyaromatic Quinone Imines. Macromolecules 1995, 28, 1–8. 10.1021/ma00105a001. [DOI] [Google Scholar]
- a Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. 10.1063/1.464913. [DOI] [Google Scholar]; b Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Gaussian 09, Revision D.01; Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian, Inc., Wallingford CT, 2009. [Google Scholar]
- a Cancès E.; Mennucci B.; Tomasi J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032. 10.1063/1.474659. [DOI] [Google Scholar]; b Cossi M.; Barone V.; Mennucci B.; Tomasi J. Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem. Phys. Lett. 1998, 286, 253–260. 10.1016/S0009-2614(98)00106-7. [DOI] [Google Scholar]; c Mennucci B.; Tomasi J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151. 10.1063/1.473558. [DOI] [Google Scholar]
- a Fukui K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. 10.1021/j100717a029. [DOI] [Google Scholar]; b Gonzalez C.; Schlegel H. B. An improved algorithm for reaction path following. J. Phys. Chem. 1989, 90, 2154. 10.1063/1.456010. [DOI] [Google Scholar]
- a Guo X.; Zipse H.; Mayr H. Mechanisms of Hydride Abstractions by Quinones. J. Am. Chem. Soc. 2014, 136, 13863–13873. 10.1021/ja507598y. [DOI] [PubMed] [Google Scholar]; b Trost B. M. Dehydrogenation Mechanisms. On the Mechanism of Dehydrogenation of Acenaphthene by Quinones. J. Am. Chem. Soc. 1967, 89, 1847–1851. 10.1021/ja00984a017. [DOI] [PubMed] [Google Scholar]; c Yamabe S.; Yamazaki S.; Sakaki S. A DFT study of hydride transfers to the carbonyl oxygen of DDQ. Int. J. Quantum Chem. 2015, 115, 1533–1542. 10.1002/qua.24967. [DOI] [Google Scholar]
- a Li Q.; Xu W.; Hu J.; Chen X.; Zhang F.; Zheng H. TfOH catalyzed synthesis of 9-arylfluorenes via tandem reaction under warm and efficient conditions. RSC Adv. 2014, 4, 27722–27725. 10.1039/C4RA03126A. [DOI] [Google Scholar]; b Zhao J.; Yue D.; Campo M. A.; Larock R. C. An Aryl to Imidoyl Palladium Migration Process Involving Intramolecular C–H Activation. J. Am. Chem. Soc. 2007, 129, 5288–5295. 10.1021/ja070657l. [DOI] [PubMed] [Google Scholar]; c Sun F.-L.; Zeng M.; Gu Q.; You S.-L. Enantioselective Synthesis of Fluorene Derivatives by Chiral Phosphoric Acid Catalyzed Tandem Double Friedel–Crafts Reaction. Chem. – Eur. J. 2009, 15, 8709–8712. 10.1002/chem.200901369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.