Abstract
Microwave-assisted organic reaction enhancement (MORE) has become more important in synthetic organic chemistry for efficient resource utilization. In this study, we synthesized bioactive compounds using both traditional and microwave methods. Microwave-assisted synthesis takes less time and produces higher yields and quality than conventional approaches. We reported the synthesis of N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) substituted hydrazides (4a–t). We also tested them against two strains: M. tuberculosis H37Ra and M. bovis BCG. Against M. tuberculosis H37Ra, the compounds 4e, 4h, 4k, 4p, and 4s were the most effective. Compounds 4f, 4g, and 4s showed significant activity against M. bovis BCG. The structures of newly synthesized molecules were determined using spectral methods. Furthermore, molecular docking investigations into the active site of mycobacterial InhA yielded well-clustered solutions for these compounds’ binding modalities producing a binding affinity in the range of −10.366 to −8.037. Theoretical results were in good accord with the observed experimental values. The docking score of compound 4e was −10.366, and the Glide energy was −66.459 kcal/mol.
Introduction
In the recent past, 10 million new cases of tuberculosis were reported worldwide.1 Despite the fact that medications to treat tuberculosis are available in the market, bacteria started developing resistance toward drugs like isoniazid and rifampicin. Due to this, there is a continuous need to search for improved medicines. Pyrazole is an interesting heterocycle to investigate. It has also gotten a lot of interest because it is being studied for a broad-spectrum biological property.2−14 The 1,3,4-oxadiazole is being studied because of its potentially relevant pharmacological activity.15−26
To improve antitubercular activity, an efficient synthetic protocol for combining the pyrazole moiety with oxadiazole has been established. Figure 1 depicts commercially accessible pharmaceuticals with pyrazole and 1,3,4-oxadiazole motifs. We present the synthesis of certain novel structural hybrids of pyrazole and 1,3,4-oxadiazole pharmacophores in a single molecular framework to investigate their antitubercular efficacy in vitro.Scheme 1 depicts the traditional and microwave-assisted synthesis pathways for named compounds.
Figure 1.
Design concept based on commercially available drugs comprising the heterocycles pyrazole and oxadiazole.
Scheme 1. Synthetic Pathway of Titled Compounds 4a-t.
Microwave-assisted organic reaction enhancement (MORE) has gained popularity because microwave-assisted procedures offer the advantage of maximizing resource utilization and providing a pure product with fewer undesirable side products.27 Microwave-assisted synthesis takes less time and produces higher yields of derivatives.
Strategically constructed innovative antitubercular drugs can exemplify two or more active scaffolds to create a single molecule with improved biological potency in the hybrid strategy to integrate physiologically active heterocycles. The novel pyrazole and oxadiazole hybrids described in this paper could be a valuable addition for the development of new antitubercular agents with a novel mechanism of action. We created scaffolds 4a–t by incorporating the pharmacophore feature of thioacetazone (antitubercular antibiotic), furamizole (antibiotic), and lonazolac (anti-inflammatory) in pursuit of novel heterocycles with potency to deal with tuberculosis resistance. The structure–activity relationship (SAR) of the various pharmacophores was taken into account when designing the molecule (Figure 1). By changing the side chain of thioacetazone and altering the dihydrooxadiazole ring of furamizole, the novel scaffolds 4a–t were created. The inclusion of an active pharmacophore was linked to the introduction of a 1,3,4-substituted pyrazole ring from lonazolac. The phenyl ring of thioacetazone was replaced by a five-membered heterocyclic ring (dihydro-1,3,4-oxadiazole), and the side chain of thioacetazone was terminated by substituted phenyl rings containing distinct electron-donating and electron-withdrawing groups.
We have reported several 1-(2-(1H-indol-3-yl)-5-(pyridin-4-yl)-1,3,4-oxadiazol-3(2H)-yl)-3-(aryl)prop-2-en-1-ones28 and investigated their antitubercular efficacy in vitro. The results revealed that the compounds tested have antitubercular activity. The antitubercular potency of 1-(2-(1H-indol-3-yl)-5-(pyridin-4-yl)-1,3,4-oxadiazol-3(2H)-yl)-3-(2-nitrophenyl)prop-2-en-1-one with MIC and IC50 values against M. bovis BCG in the active state was 1.03 and 0.48 μg/mL, respectively, among the studied substances. The active molecule had a docking score of −8.267 and a Glide energy of −54.856 kcal/mol when it docked into the active site of the mycobacterial enoyl reductase (InhA) enzyme. These findings piqued our interest in developing new hybrids based on the oxadiazole pharmacophore. Herein, we reported the synthesis of N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) substituted hydrazides (4a–t) and in vitro antitubercular efficacy. The structural changes, such as replacing the indole moiety with a substituted pyrazole ring, increased the pharmacological potency. The Schiff base linker was used to replace the chalcone linker between the nitrophenyl ring and the oxadiazole ring. At three different concentrations, the synthesized compounds showed substantial inhibition. In the active state, compound 4e had excellent antitubercular activity against M. tuberculosis H37Ra, with MIC and IC50 values of 0.92 and 0.52 μg/mL, respectively. The docking score of compound 4e was −10.366, and the Glide energy was −66.459 kcal/mol. Figure 2 shows the structural alterations, as well as a comparative study of activity and molecular docking research.
Figure 2.
Comparison and structural modifications in our previous study based on oxadiazole hybrids with the reported work.
Results and Discussion
Chemistry
The traditional and microwave-assisted synthesis pathways for novel N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) substituted hydrazides (4a–t) are shown in Scheme 1. The chalcone derivative, N′-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazole–4-carbaldehyde)benzohydrazide (2), was prepared by dissolving 3-(4-chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1) and benzohydrazide in 1,4-dioxane and refluxing for 4 h. The generation of chalcone derivative (2) is explained by the nucleophilic attack of nitrogen on the carbonyl carbon of aldehyde followed by proton transfer, protonation, removal of water molecule, and deprotonation. The intermediate 1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethenone (3) is produced by refluxing the chalcone derivative (2) in acetic anhydride at 140 °C for 5 h. A well-dissolved mixture of intermediate (3), appropriate benzohydrazides, and a pinch of anhydrous ZnCl2 in ethanol is refluxed for 7–9 h to yield N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) substituted hydrazides (4a–t). Microwave-assisted synthesis of the identical compounds reduces reaction time from 7–9 h to 9–10 min, resulting in a 79–92% improvement in product yield (see Table 1). In the experimental part, the details of microwave synthesis are described.
Table 1. Optimization of Reaction Conditions for the Conventional and Microwave Methods.
| conventional method |
microwave method |
||||
|---|---|---|---|---|---|
| entry | -R | yield (%) | reaction time (min) | yield (%) | reaction time (min) |
| 4a | -C6H5 | 77 | 420 | 92 | 10 |
| 4b | -2-OH-C6H4 | 65 | 450 | 80 | 9 |
| 4c | -3-OH-C6H4 | 69 | 450 | 83 | 8 |
| 4d | -4-OH-C6H4 | 61 | 450 | 80 | 9 |
| 4e | -4-NO2-C6H4 | 58 | 420 | 79 | 8 |
| 4f | -3-CH3-C6H4 | 73 | 450 | 90 | 9 |
| 4g | -4-CH3-C6H4 | 71 | 450 | 90 | 8 |
| 4h | -4-OCH3-C6H4 | 62 | 450 | 85 | 9 |
| 4i | -3-Cl-C6H4 | 68 | 480 | 88 | 10 |
| 4j | -4-Cl-C6H4 | 74 | 480 | 92 | 10 |
| 4k | -CH2-C6H5 | 57 | 450 | 79 | 9 |
| 4l | -CH2NHCO-C6H5 | 60 | 540 | 81 | 10 |
| 4m | -CH2-O-C6H4-2-CH3 | 64 | 540 | 85 | 10 |
| 4n | -CH2-O-C6H4-3-CH3 | 63 | 540 | 85 | 9 |
| 4o | -CH2-O-C6H4-4-CH3 | 70 | 540 | 90 | 9 |
| 4p | -CH2-O-C6H4-2-NO2 | 61 | 480 | 83 | 10 |
| 4q | -CH2-O-C6H4-3-NO2 | 55 | 480 | 80 | 9 |
| 4r | -CH2-O-C6H4-4-NO2 | 66 | 480 | 85 | 9 |
| 4s | -CH2-O-C6H4-2-Cl | 73 | 450 | 91 | 8 |
| 4t | -CH2-O-C6H4-4-Cl | 69 | 450 | 87 | 8 |
The final structures of N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) substituted hydrazides (4a–t) were confirmed by different spectroscopic analysis data. Compound 4c exhibited a distinct absorption band at 1691 cm–1, which corresponded to the presence of a carbonyl group in the structure. The Schiff base’s N–H stretching (secondary amide stretching) and O–H stretching of the −OH group linked to the phenyl ring caused different vibrations at 3367 and 3447 cm–1. The aromatic ring’s C–H stretching and the −CH=CH– group produced vibrations at 3066 and 3024 cm–1, respectively. The C–O–C stretching of the oxadiazole nucleus and C–Cl stretching caused absorptions at 1232 and 747 cm–1. A singlet signal at 5.69 ppm in the 1H NMR spectra of compound 4c was attributable to the oxadiazole ring’s C2–H, while peaks at 2.17 and 5.30 ppm were three −CH3 group protons and one hydroxyl proton, respectively. The presence of the −CONH– group in the molecule’s arrangement was demonstrated by a singlet peak at 12.30 ppm.
The methyl carbon of the methyl group produced a signal at 12.9 ppm in 13C NMR, while the carbon connected to the −CH3 group produced a signal at 147.3 ppm. Two signals appeared due to carbons of the oxadiazole ring at 157.6 and 78.4 ppm, proving the presence of the oxadiazole ring, while three signals appeared due to carbons of the pyrazole ring at 117.9, 123.1, and 149.4 ppm, proving the presence of the pyrazole ring in the structure of compound 4c. At 163.7 ppm, the carbonyl carbon of the amide group produced a distinct signal. In mass spectra, m/z = 577.17 corresponds to the molecular weight suggested. The details of spectral information are given in the Supplementary Information.
Biological Evaluation
Antimycobacterial Activity
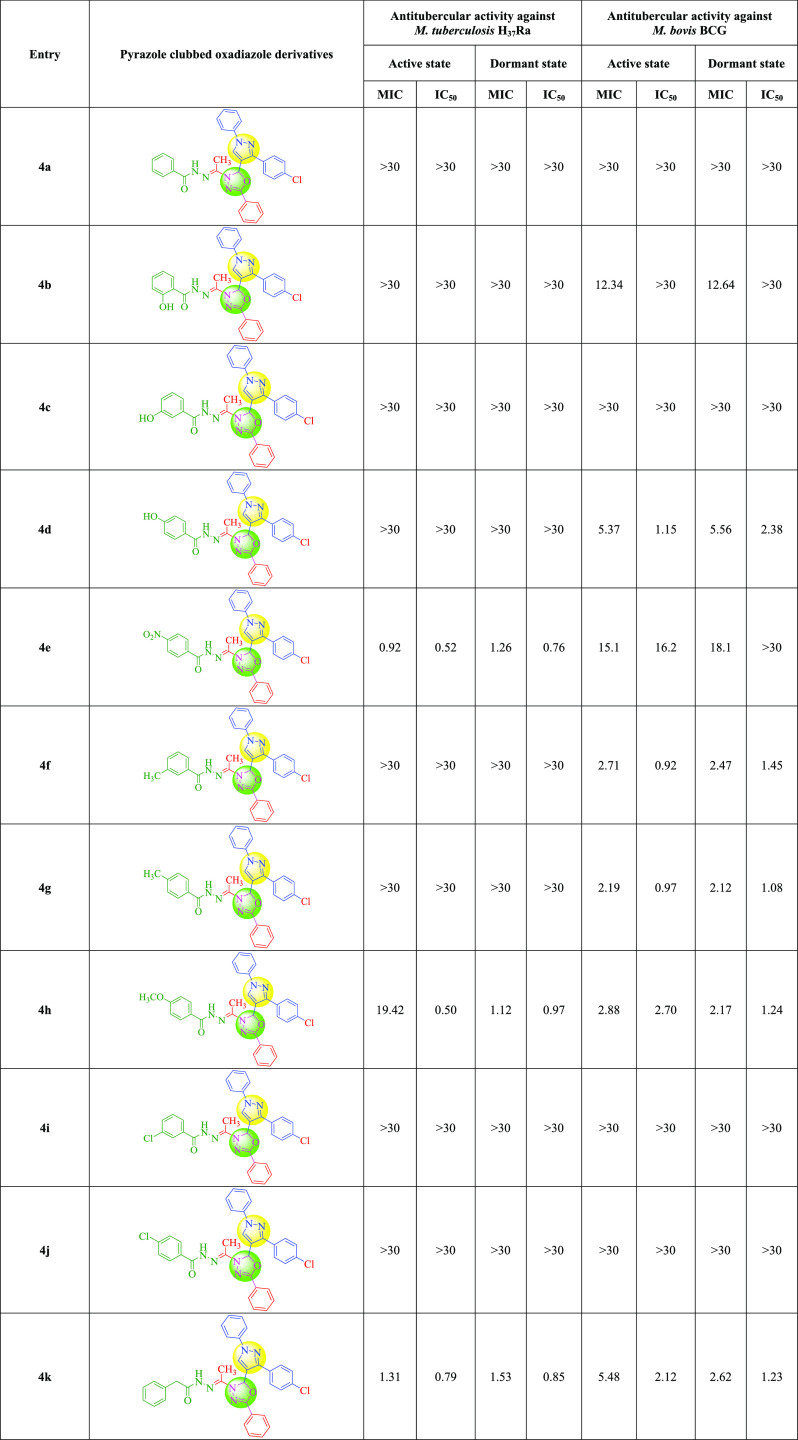
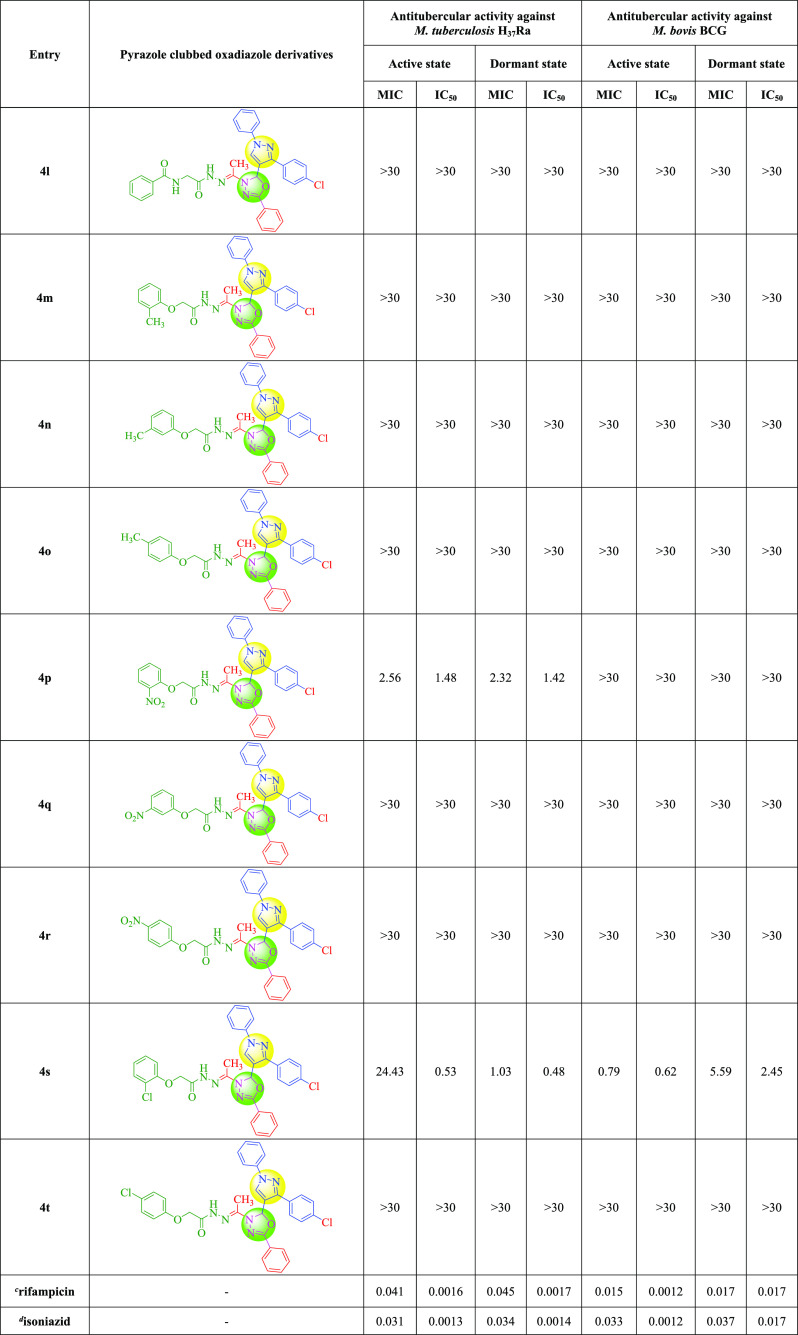
In both active and dormant modes, the newly synthesized compounds demonstrated good effectiveness against M. bovis BCG and M. tuberculosis H37Ra. The antitubercular action of the pyrazole and oxadiazole hybrids 4e, 4h, 4k, 4p, and 4s against M. tuberculosis H37Ra was described. The compounds 4e, 4k, and 4p had excellent MICs against M. tuberculosis H37Ra in an active state, ranging from 0.92 to 2.56 μg/mL. Compound 4e was shown to be the most effective against active M. tuberculosis H37Ra, with an active MIC of 0.92 μg/mL and a dormant MIC of 1.26 μg/mL. In active and dormant stages, compound 4k had MICs of 1.31 and 1.53 μg/mL, respectively, against M. tuberculosis H37Ra. Compound 4p was around 2 to 3 times less active against M. tuberculosis H37Ra than the most active compound 4e (active state MIC: 2.56 μg/mL, dormant state MIC: 2.32 μg/mL). In a dormant condition, compound 4s had an excellent MIC value of 1.03 μg/mL against M. tuberculosis H37Ra. Compounds 4e, 4h, 4k, 4p, and 4s had MICs ranging from 1.03 to 2.32 μg/mL against M. tuberculosis H37Ra in a dormant condition. Compounds 4f (active state MIC: 2.71 μg/mL, dormant state MIC: 2.47 μg/mL) and 4g (active state MIC: 2.19 μg/mL, dormant state MIC: 2.12 μg/mL) were found to have excellent MIC values for M. bovis BCG. In the active state of M. bovis BCG, compound 4s had an excellent MIC value of 0.79 μg/mL. The antitubercular action was greatly impacted by the substitution pattern at the phenyl ring, as shown in Tables 2 and 3. The antitubercular activity against M. tuberculosis H37Ra was affected by electron-withdrawing groups like −NO2 and −Cl, but the antitubercular activity against M. bovis BCG was affected by electron-donating groups like −CH3 and −OCH3.
Table 2. Results of Primary Screening against M. tuberculosis H37Ra and M. bovis BCG of N′-(1-(2-(3-(4-Bromophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-(aryl)-benzohydrazides.
| at dormant M. tuberculosis H37Ra |
at dormant M. bovis BCG |
||||||
|---|---|---|---|---|---|---|---|
| entry | -R | 30 μg/mL | 10 μg/mL | 3 μg/mL | 30 μg/mL | 10 μg/mL | 3 μg/mL |
| 4a | -C6H5 | 61.72 | 51.64 | 32.34 | 40.34 | 34.70 | 20.43 |
| 4b | -2-OH-C6H4 | 54.14 | 60.21 | 21.99 | 100.53 | 100.80 | 99.85 |
| 4c | -3-OH-C6H4 | 37.94 | 46.17 | 14.61 | 0.29 | 6.92 | 7.51 |
| 4d | -4-OH-C6H4 | 98.45 | 94.86 | 14.86 | 99.55 | 100.57 | 99.63 |
| 4e | -4-NO2-C6H4 | 95.09 | 92.48 | 11.22 | 99.55 | 100.38 | 38.49 |
| 4f | -3-CH3-C6H4 | 98.47 | 85.31 | 25.24 | 99.24 | 100.48 | 98.79 |
| 4g | -4-CH3-C6H4 | 7.98 | 19.22 | 3.22 | 96.14 | 98.65 | 99.47 |
| 4h | -4-OCH3-C6H4 | 95.44 | 91.47 | 86.22 | 97.25 | 99.82 | 100.25 |
| 4i | -3-Cl-C6H4 | 46.72 | 26.60 | 18.61 | 99.03 | 81.67 | 15.34 |
| 4j | -4-Cl-C6H4 | 28.24 | 3.92 | 35.08 | 75.22 | 19.21 | 20.53 |
| 4k | -CH2-C6H5 | 98.74 | 93.71 | 96.13 | 97.77 | 89.29 | 56.75 |
| 4l | -CH2NHCO-C6H5 | 3.09 | –17.07 | 35.30 | 58.41 | 21.15 | 8.60 |
| 4m | -CH2-O-C6H4-2-CH3 | 38.95 | 7.09 | 33.03 | 37.25 | 37.51 | 33.01 |
| 4n | -CH2-O-C6H4-3-CH3 | –22.83 | –35.88 | 3.56 | 71.56 | 4.17 | –18.98 |
| 4o | -CH2-O-C6H4-4-CH3 | 85.53 | 78.95 | 0.75 | 81.96 | 48.21 | –1.06 |
| 4p | -CH2-O-C6H4-2-NO2 | 95.19 | 90.23 | 94.58 | 98.81 | 94.62 | 91.41 |
| 4q | -H2-O-C6H4-3-NO2 | 34.13 | 31.12 | 13.95 | 7.79 | 0.04 | –7.87 |
| 4r | -CH2-O-C6H4-4-NO2 | 54.11 | 40.25 | 7.88 | 33.24 | 26.42 | 20.26 |
| 4s | -CH2-O-C6H4-2-Cl | 93.68 | 98.02 | 93.52 | 82.60 | 86.27 | 83.22 |
| 4t | -CH2-O-C6H4-4-Cl | 11.41 | –1.79 | –4.38 | 9.62 | 25.12 | 13.69 |
Table 3. Antitubercular Screening Results of the Tested Compounds against Mycobacterium tuberculosis H37Ra and Mycobacterium bovis BCG Strainsa,b.
MIC = minimum inhibitory concentration in μg/mL.
IC50 = 50% inhibitory concentration in μg/mL.
Rifampicin = reference for Mycobacterium tuberculosis H37Ra and Mycobacterium bovis BCG strains.
Isoniazid = reference for Mycobacterium tuberculosis H37Ra and Mycobacterium bovis BCG strains.
The antitubercular activity of titled compounds 4a–t was determined using a Nitrate reductase assay and an XTT Reduction Menadione assay, respectively, against M. tuberculosis H37Ra and M. bovis BCG. The primary screening was done at doses of 30, 10, and 3 μg/mL at first. For the current study, we chose drugs that showed 90% bacilli inhibition at or below 30 μg/mL for the dose–response curve. We chose the drugs rifampicin and isoniazid as a reference, both of which are commercially accessible for clinical use.29Tables 2 and 3 summarize the findings.
Structure–Activity Relationship
We created some novel pyrazole–oxadiazole hybrids as possible antitubercular agents in this study. The current study’s findings revealed some encouraging results in terms of the structure–activity relationship. To investigate the impact of functional groups on antitubercular assessment, we used a variety of electron-withdrawing and electron-donating functional groups. Functional groups have played a key role in improving antitubercular activity in both primary and secondary screening. In primary and secondary screening, the electron-withdrawing −NO2 group at the fourth position in benzohydrazide derivatives exhibited significant antitubercular action. In initial screening, it displayed 95.09% inhibition against the M. tb. strain, with MIC values of 0.92 and 1.26 μg/mL in active and dormant states, respectively. In primary and secondary screening, the electron-donating −OCH3 group, which was placed at the fourth position, showed significant action against the M. tb. strain. It inhibited the M. tb. strain by 95.44% in the primary screening, with MIC values of 19.42 and 1.12 μg/mL in active and dormant states, respectively.
The antitubercular activity of both the screening, the electron-donating −CH3 group at the third and fourth positions had showed excellent activity against the M. bovis strain. In primary screening, a 3-CH3 derivative of the benzohydrazide motif showed 99.24% inhibition and a MIC of 2.71 μg/mL in the active state and 2.47 μg/mL in the dormant state against the M. bovis strain. In the active and dormant states, the 4-CH3 derivative of the benzohydrazide scaffold inhibited M. bovis with a MIC of 2.19 and 2.12 μg/mL, respectively. In both screenings, the SAR study of benzohydrazide derivatives revealed that electron-donating tendencies were more prevalent than electron-withdrawing tendencies. The functional groups like −CH3 and −OCH3 at various positions showed increased effectiveness against M. tb. and M. bovis strains. Electron-withdrawing functional groups in acetohydrazide derivatives exhibited good efficacy against the M. tb. strain. In primary screening, the −NO2 group at the second position showed 95.19% inhibition against the M. tb. strain, with MIC values of 2.56 μg/mL in the active state and 2.32 μg/mL in the dormant state. The electron-withdrawing −Cl group in the second position showed 93.68% inhibition in primary and secondary screening against the M. tb. strain, having MIC values of 24.43 and 1.03 μg/mL in active and dormant states, respectively. The electron-withdrawing −NO2 and −Cl groups at the second position are more prominent than electron-donating groups at various positions, according to an evaluation of acetohydrazide derivatives. The cumulative impacts of active motifs of pyrazole–oxadiazole hybrids (4a–t) on the structure–activity relationship are demonstrated in Figure 3.
Figure 3.

Primary and secondary antitubercular screening of pyrazole–oxadiazole hybrids (4a–t) revealed a structure–activity relationship.
Molecular Docking Study
Molecular docking is now a well-established computational technique for predicting the predominant binding mode(s) of a ligand within a protein (biological target) of a given three-dimensional structure, and it has become a vital aspect of drug development research. It gives researchers information on binding affinities, binding mechanisms, and types of interactions that guide protein–ligand complexation, allowing them to develop and examine the potential of existing lead compounds and their equivalents. In the absence of resources to perform enzymatic assays, in silico computational chemistry techniques such as molecular docking have become critical for identifying the targets for various molecules and their associated thermodynamic interactions with the target enzyme that govern pathogen inhibition. Mycobacterial enoyl reductase is said to be inhibited by the oxadiazole scaffold (InhA).30−32 As a result, the 3D structure of M. tuberculosis mycobacterial enoyl reductase (InhA) complexed with its inhibitor was chosen as the target protein for the molecular docking study for the active compounds (4e, 4f, 4g, 4p, and 4s) in the series to rationalize the promising antitubercular results and investigate the possible interactions of the studied compounds. The enoyl-ACP (CoA) reductase (FabI/ENR/InhA) is a key enzyme in the type II fatty acid biosynthesis pathway in mycobacteria, and its inhibition stunts the growth of a fast-growing model organism. Mycobacterium smegmatis has been demonstrated to block mycolic acid formation and promote cell lysis,33 making it a suitable target for medication development against tuberculosis.
The outcome of the molecular docking study revealed that all the studied compounds (4e, 4f, 4g, 4p, and 4s) were successfully docked, making a snug fit into the active site of InhA at coordinates very close to that of the native ligand in the crystal structure with varying degrees of affinities. Their complexes with protein structure were stabilized by several bonded and nonbonded interactions with the amino acids surrounding the active site. The findings of the molecular docking investigation are described in Table 4, which shows that there is a substantial association between docking scores and biological activity, with active molecules producing higher scores and those with low activity producing lower docking scores. Their binding energies were likewise found to be negative (−66.459 to −55.157 kcal/mol), indicating that they could be used to rationally design compounds that target InhA.
Table 4. Results of the Per-Residue Interaction Analysis for the Pyrazole Clubbed Oxadiazole Hybrid Heterocycles.
| per-residue interaction energy analysis |
||||||
|---|---|---|---|---|---|---|
| entry | docking score | binding energy | van der Waals (kcal/mol) | electrostatic (kcal/mol) | π–π stacking | H-bonding |
| 4e | –10.366 | –66.459 | Ile215 (−2.438), Leu207 (−1.711), Ile202 (−1.788), Met199 (−2.915), Ala198 (−2.376), Thr196 (−3.184), Ile194 (−2.484), Pro193 (−1.343), Ala191 (−1.968), Lys165 (−1.685), Met161 (−2.058), Tyr158 (−7.209), Ala157 (−1.567), Met155 (−1.093), Phe149 (−4.211), Met147 (−2.55), Met103 (−4.69), Met98 (−1.443), Met97 (−1.903), Gly96 (−1.844), Ile95 (−0. 956), Ser94 (−2.361), Ile21 (−2.832), Ser20 (−1.992), Gly14 (−1.127) | Ile210 (−1.968), Ser209 (−1.711), Lys165 (−2.343), 115 (−1.527), Ala22 (−1.008), Ser20 (−1.905) | Phe149 (2.135) | Ser94 (2.41), Ala22 (2.57), Ser20 (2.77) |
| 4f | –8.037 | –55.157 | Ile215 (−1.248), Leu207 (−1.001), Ile202 (−1.748), Met199 (−1.831), Ala198 (−2.032), 197 (−0.967), Thr196 (−1.522), Met161 (−1.928), Tyr158 (−1.92), Phe149 (−2.145), Met147 (−1.768), Met103 (−2.843), 99 (−1.067), Met98 (−1.018), Met97 (−1.186), Gly96 (−2.182), Ile95 (−1.439), Ser94 (−1.943), Ile21 (−1.881), Ser20 (−1.187), Ile16 (−1.326) | Ile219 (−1.587), Lys165 (−1.019) | Lys165 (1.98) | |
| 4g | –8.159 | –58.133 | Ile215 (−1.36), Leu207 (−1.033), Ile202 (−2.438), Met199 (−2.054), Ala198 (−2.077), Thr196 (−1.48), Lys165 (−1.782), Met161 (−1.636), Tyr158 (−2.63), Ala157 (−0.931), Phe149 (−3.592), 148 (−1.487), Met147 (−2.197), Met103 (−3.31), Met98 (−1.465), Met97 (−1.211), Gly96 (−2.566), Ile95 (−1.145), Ser94 (−1.875), Ile21 (−1.679), Ser20 (−1.193), Ile16 (−0.987) | 261 (−0.972), Met199 (−1.122), Thr196 (−1.626), 169 (−1.101), 148 (−2.797), Ser20 (−1.246) | Thr196 (2.30) | |
| 4p | –8.909 | –62.547 | Ala198 (−2.206), 197 (−1.785), Thr196 (−2.607), Lys165 (−1.098), Met161 (−2.168), Phe149 (−0.964), 148 (−1.325), Met147 (−2.952), 122 (−1.526), Met103 (−1.463), 100 (−1.971), 99 (−1.184), Met98 (−1.394), Met97 (−1.587), Gly96 (−2.034), Ile95 (−2.61), Ser94 (−2.111), 41 (−2.473), Ile21 (−2.046), Ser20 (−1.798), Ser19 (−1.078), Thr17 (−1.89), Ile16 (−2.208) | Ile219 (−1.134), Ser209 (−1.199), Lys165 (−2.278), 115 (−1.104), Gly96 (−1.514) | Phe97 (2.362) | Lys165 (2.14) |
| 4s | –9.972 | –64.465 | Ile215 (−2.153), Ile202 (−2.073), Met199 (−2.55), Ala198 (−2.256), Thr196 (−3.008), Ile194 (−2.197), Pro193 (−1.352), 192 (−0.964), Ala191 (−1.812), Met161 (−3.095), Tyr158 (−4.064), Phe149 (−4.414), 148 (−1.567), Met147 (−3.112), Met103 (−3.963), Met98 (−1.447), Met97 (−1.818), Gly96 (−2.095), Ile21 (−2.087), Ser20 (−1.446), Ile16 (−1.076) | Ile219 (−1.949), Ile210 (−1.879), Ser209 (−1.298), Lys165 (−2.62), Met147 (−0.907) | Phe149 (2.644) | Lys165 (1.99) |
Furthermore, the thermodynamic elements influencing the binding of these compounds to InhA were investigated in-depth by analyzing per-residue interactions between the enzyme and these ligands. However, for the sake of brevity, we have discussed these details for compound 4e, which is the most active, while we summarized for other molecules in Table 4.
The best docked configuration of the most active oxadiazole derivative 4e into the active site of mycobacterial enoyl reductase (InhA) (Figure 4) revealed that the inhibitor snugly fits the active site, making multiple intimate contacts through bonded and nonbonded interactions. This molecule has the highest affinity in the series (docking score of −10.366 and binding energy of −66.459 kcal/mol), which can be explained in terms of unique bonded and nonbonded per-residue interactions with the active site residues. The visual inspection of the bound pose of 4e revealed that the compound is stabilized within the active site through an extensive network of favorable van der Waals contacts with Ile215 (−2.438 kcal/mol), Ile202 (−1.788 kcal/mol), Met199 (−2.915 kcal/mol), Ala198 (−2.376 kcal/mol), Thr196 (−3.184 kcal/mol), Ile194 (−2.484 kcal/mol), Pro193 (−1.343 kcal/mol), Ala191 (−1.968 kcal/mol), Tyr158 (−7.209 kcal/mol), Ala157 (−1.567 kcal/mol), and Phe149 (−4.211 kcal/mol) through the N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-4-nitrobenzohydrazidebenzohydrazide component of 4e got engaged in a similar network of van der Waals interactions with Leu207 (−1.711 kcal/mol), Lys165 (−1.685 kcal/mol), Met161 (−2.058 kcal/mol), Met155 (−1.093 kcal/mol), Met147 (−2.55 kcal/mol), Met103 (−4.69 kcal/mol), Met98 (−1.443 kcal/mol), Met97 (−1.903 kcal/mol), Gly96 (−1.844 kcal/mol), Ile95 (−0.956 kcal/mol), Ser94 (−2.361 kcal/mol), Ile21 (−2.832 kcal/mol), Ser20 (−1.992 kcal/mol), and Gly14 (−1.127 kcal/mol) residues lining the active site of InhA. Significant electrostatic interactions with Ile210 (−1.968 kcal/mol), Ser209 (−1.711 kcal/mol), Lys165 (−2.343 kcal/mol), 115 (−1.527 kcal/mol), Ala22 (−1.008 kcal/mol), and Ser20 (−1.905 kcal/mol) residues in the active site are also responsible for 4e’s increased binding affinity. Furthermore, at bonding distances of 2.41, 2.25, and 2.77, respectively, a strong hydrogen bonding interaction was detected between the nitro group and the Ser94, Ala22, and Ser20 residues. A significant π–π stacking was also observed between the Phe149 (2.135) and the p-chlorophenyl ring of 4e. Such hydrogen bonding interactions and the pi–pi stacking serve as an ″anchor″ guiding the 3D space position of the ligand in the active site and facilitate the steric and electrostatic interactions adding to the stability of the enzyme-inhibitor complex. The other oxadiazole analogues (4f, 4g, 4p, and 4s) were also seen to possess an optimum binding affinity for the enzyme engaging in a similar network of interactions with the active side residues but quantitatively decreasing gradually with their observed antitubercular activity. Overall, the per-residue interaction analysis revealed that the primary driving forces for mechanical interlocking of these ligands are the steric complementarity between these ligands and the active site of InhA that is reflected in the relatively higher number of favorable van der Waals interactions compared to other components contributing to the overall binding affinity.
Figure 4.
Binding mode of compound 4e into the active site of mycobacterial enoyl reductase (InhA) (on the right side: the π–π stacking interaction is represented using green lines, while pink lines represent the hydrogen bonding interactions).
Conclusions
The findings of the investigation suggested that the titled compounds will be important in the development of antitubercular drugs in the future. Microwave-assisted synthesis and the hybrid molecule idea were used to produce and develop the active chemicals. Our findings showed that the chemical transformation took place in a relatively short period, the purity of the compounds was higher, and the yields were higher than the traditional approach. As a result, the pharmaceutical industry will rely heavily on this technique. Our previously published work was used to describe the compounds (Figure 2). The fact that the molecule with the nitro group at the para position has outstanding activity and a high docking score motivated us to develop new hybrids based on the oxadiazole core structure and explore their antitubercular activity. The study found that compounds with electron-withdrawing functional groups had excellent MICs against M. tuberculosis H37Ra, whereas compounds with electron-donating functional groups have antitubercular action against M. bovis BCG but no activity against M. tuberculosis H37Ra. The pyrazole–oxadiazole hybrids 4e, 4h, 4p, and 4s were found to be most effective against M. tuberculosis H37Ra, while 4f and 4g have substantial activity against M. bovis BCG. Molecular docking studies were used to gain an insight into the mechanism of action, which suggested that these molecules possess significant binding affinity toward the critical target mycobacterium InhA. These findings revealed important information about possible binding interactions, implying that this class of compounds can be further selected to build InhA specific inhibitors. Iterative synthesis and computer modeling are being used to make a variety of structural changes, and the results will be shared as soon as possible.
Experimental Section
Materials and Methods
Melting points were determined on an electrothermal melting point apparatus and are reported uncorrected. The completion of the reaction and purity of all compounds were checked on aluminum-coated TLC plates 60 F254 (E. Merck) using chloroform/methanol (9.5:0.5 v/v) as the mobile phase and visualized under ultraviolet (UV) light or iodine vapor. Elemental analysis (% C, H, N) was carried out by a Perkin-Elmer 2400 CHN analyzer. IR spectra of all compounds were taken on a Perkin-Elmer FT-IR spectrophotometer in KBr. 1H NMR and 13C NMR spectra were recorded on Bruker (400 MHz) and (100 MHz) spectrometers, respectively, using DMSO-d6 as a solvent and TMS as an internal standard. Chemical shifts are reported in parts per million (ppm). A mass spectrum was scanned on a Shimadzu LC–MS 2010 spectrophotometer. Anhydrous reactions were carried out in oven-dried glassware in a nitrogen atmosphere. Microwave-assisted synthesis was carried out using Synthos 3000.34
General Procedure for the Preparation of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) Substituted Hydrazides (4a-t) by the Conventional Method
Preparation of N′-((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) Benzohydrazide (2)
3-(4-Chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1, 0.01 mol) and benzohydrazide (0.01 mol) were dissolved in 1,4-dioxane (20 mL) and refluxed for 4 h. The crystals developed as soon as the product was cooled, and the product was crystallized from methanol to obtain compound 2. Yield 63%, mp 187–189 °C.
Preparation of 1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethanone (3)
Acetic anhydride (0.03 mol) was added to compound N′-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)benzohydrazide (2, 0.01 mol) and refluxed at 140 °C for 5 h. The resulting mixture was transferred to crushed ice once the reaction mixture reached the room temperature. The precipitates were filtered and crystallized with methanol after being oven-dried, yielding compound 3. Yield 73%, mp 180–182 °C. Anal. calcd for C25H19ClN4O2: C-67.79, H-4.32, N-12.62; found: C-67.86, H-4.4, N-12.6%.
Preparation of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) Substituted Hydrazides (4a-t)
An equimolar mixture of intermediate compound 1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethanone (3, 0.01 mol) and appropriate benzohydrazides (0.01 mol) was dissolved in ethanol (95%) (20 mL), and a pinch of anhydrous ZnCl2 was added to the mixture. The reaction mixture was then refluxed for 7–9 h at 80 °C. The crystals formed after cooling the reaction mixture were filtered and recrystallized from methanol to give compound 4a–t.
General Procedure for the Preparation of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) Substituted Hydrazides (4a–t) by Microwave-Assisted Synthesis
Preparation of N′-((3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) Benzohydrazide (2)
Compounds 3-(4-chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (1, 0.01 mol), benzohydrazide (0.01 mol), and 1,4-dioxane (5 mL) were swirled together and microwave irradiated for 30 s. This reaction mixture was heated for a further 5 min. When the reaction was finished, the contents were placed in crushed ice with water and recrystallized in methanol to form compound 2, resulting in an 82% yield.
Preparation of 1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethanone (3)
This silica gel (1 g) was added to a combination of compound N′-((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)benzohydrazide (2, 0.01 mol) and acetic anhydride (3 mL) at room temperature. Under microwave irradiation, the absorbed material was air-dried and magnetically agitated for 5 min at a maximum power of 250 W at 30 s intervals. The product was extracted from methanol when the reaction mixture was cooled. A crude product was obtained by diluting methanolic extract with ice-cold water, which was then filtered, washed, and recrystallized from methanol to yield compound 3, resulting in an 88% yield.
Preparation of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene) Substituted Hydrazides (4a–t)
1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethanone (3, 0.01 mol) and appropriate benzohydrazides (0.01 mol) were dissolved in pure alcohol (99.9%), and a pinch of anhydrous ZnCl2 was added. Under microwave irradiation, the reaction mixture was magnetically agitated for 8–10 min with a maximum power of 300 W intermittently at 30 s intervals. The reaction mixture was then quenched, and the resulting precipitates were filtered, washed, and recrystallized from methanol to yield compounds 4a–t.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)benzohydrazide (4a)
Yield 77%, mp 229–232 °C; IR (KBr) λmax: 3365 (N–H stretching, secondary amide), 3066, 3024 (C–H stretching, aromatic ring), 1691 (C=O stretching), 1548, 1488 (C=C, C=N stretching, aromatic ring), 1232 (C–O–C stretching, oxadiazole ring), 747 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.16 (s, 3H, −CH3), 5.65 (s, 1H, C2–H, oxadiazole ring), 6.88–8.04 (m, 20H, Ar–H and C5–H pyrazole), 12.36 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.5, 78.2, 117.6, 119.4 (2), 123.4, 125.3 (2), 126.1, 127.3 (2), 128.7 (4), 128.8 (2), 129.3 (4), 131.2, 131.7, 132.1, 132.5, 132.6, 134.8, 139.4, 147.3, 149.2, 157.6, 163.8; LCMS: m/z = 561.2 (M+); anal. calcd for C32H25ClN6O2: C, 68.51; H, 4.49; N, 14.98; found: C, 68.62; H, 4.55; N, 15.00%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-hydroxybenzohydrazide (4b)
Yield 65%, mp 227–230 °C; IR (KBr) λmax: 3465 (O–H stretching, Ar–OH), 3370 (N–H stretching, secondary amide), 3068, 3028 (C–H stretching, aromatic ring), 1696 (C=O stretching), 1550, 1492 (C=C, C=N stretching, aromatic ring), 1200 (C–O–C stretching, oxadiazole ring), 740 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.19 (s, 3H, −CH3), 5.35 (s, 1H, −OH), 5.69 (s, 1H, C2–H oxadiazole ring), 6.92–8.09 (m, 19H, Ar–H and C5–H pyrazole), 12.35 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.8, 78.6, 117.2, 117.3 (2), 119.3 (2), 119.4, 121.5, 123.4, 126.4 (2), 128.6, 128.9 (2), 129.1, 129.4 (4), 129.5, 129.7, 131.0, 131.2, 133.6, 134.8, 139.2, 147.2, 149.3, 150.2, 159.3, 163.7; LCMS: m/z = 577.6 (M+); anal. calcd for C32H25ClN6O3: C, 66.61; H, 4.37; N, 14.56; found: C, 66.63; H, 4.50; N, 14.70%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-3-hydroxybenzohydrazide (4c)
Yield 69%, mp 209–211 °C; IR (KBr) λmax: 3447 (O–H stretching, Ar–OH), 3367 (N–H stretching, secondary amide), 3066, 3024 (C–H stretching, aromatic ring), 1691 (C=O stretching), 1548, 1488 (C=C, C=N stretching, aromatic ring), 1232 (C–O–C stretching, oxadiazole ring), 747 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.17 (s, 3H, −CH3), 5.30 (s, 1H, −OH), 5.69 (s, 1H, C2–H oxadiazole ring), 6.90–8.05 (m, 19H, Ar–H and C5–H pyrazole), 12.30 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.9, 78.4, 115.3, 117.9, 119.4, 119.6 (2), 120.4, 123.1, 125.4 (2), 126.3, 128.4 (2), 128.8 (2), 129.3 (4), 130.5, 131.5, 131.9, 134.1, 135.9, 139.2, 147.3, 149.4, 157.6, 158.2, 158.3, 163.7; LCMS: m/z = 577.1 (M+); anal. calcd for C32H25ClN6O3: C, 66.61; H, 4.37; N, 14.56; found: C, 66.70; H, 4.50; N, 14.65%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-4-hydroxybenzohydrazide (4d)
Yield 65%, mp 241–244 °C; IR (KBr) λmax: 3434 (O–H stretching, Ar–OH), 3380 (N–H stretching, secondary amide), 3070, 3012 (C–H stretching, aromatic ring), 1612 (C=O stretching), 1509, 1412 (C=C, C=N stretching, aromatic ring), 1232 (C–O–C stretching, oxadiazole ring), 730 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.15 (s, 3H, −CH3), 5.32 (s, 1H, −OH), 5.66 (s, 1H, C2–H oxadiazole ring), 6.90–8.09 (m, 19H, Ar–H and C5–H pyrazole), 12.36 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.3, 78.1, 116.3 (2), 117.6, 119.4 (2), 123.7, 125.1, 125.6 (2), 126.4, 128.0, 128.1 (2), 128.4 (2), 128.6, 129.3 (4), 131.0, 131.7, 132.4, 134.8, 139.4, 147.1, 149.4, 157.4, 161.7, 163.7; LCMS: m/z = 577.2 (M+); anal. calcd for C32H25ClN6O3: C, 66.61; H, 4.37; N, 14.56; found: C, 66.62; H, 4.39; N, 14.60%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-4-nitrobenzohydrazide (4e)
Yield 58%, mp 271–273 °C; IR (KBr) λmax: 3388 (N–H stretching, secondary amide), 3077, 3013 (C–H stretching, aromatic ring), 1530, 1610 (C=O stretching), 1530 (N=O stretching, Ar–NO2), 1500, 1429 (C=C, C=N stretching, aromatic ring), 1200 (C–O–C stretching, oxadiazole ring), 700 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.12 (s, 3H, −CH3), 5.60 (s, 1H, C2–H oxadiazole ring), 6.85–8.04 (m, 19H, Ar–H and C5–H pyrazole), 12.32 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.0, 78.7, 117.4, 119.7 (2), 123.5, 124.4 (2), 125.3 (2), 126.7, 128.3 (2), 128.4 (2), 129.3 (4), 129.4 (2), 131.4, 134.4, 138.3, 138.4, 139.4, 147.2, 149.5, 150.7, 151.0, 157.5, 163.7; LCMS: m/z = 605.2 (M+); anal. calcd for C32H24ClN7O4: C, 63.42; H, 3.99; N, 16.18; found: C, 63.55; H, 4.10; N, 16.20%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-3-methylbenzohydrazide (4f)
Yield 73%, mp 245–247 °C; IR (KBr) λmax: 3344 (N–H stretching, secondary amide), 3066, 3002 (C–H stretching, aromatic ring), 2930 (C–H stretching, −CH3 group), 1619 (C=O stretching), 1503, 1412 (C=C, C=N stretching, aromatic ring), 1214 (C–O–C stretching, oxadiazole ring), 705 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.11 (s, 3H, −CH3), 2.34 (s, 3H, −CH3), 5.61 (s, 1H, C2–H oxadiazole ring), 6.82–8.02 (m, 19H, Ar–H and C5–H pyrazole), 12.31 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.1, 21.7, 78.1, 117.5, 119.5 (2), 123.7, 124.7, 125.0, 125.1 (2), 126.7, 128.1 (2), 128.4 (2), 129.4 (4), 131.5, 131.8, 131.9, 132.2, 132.4, 134.4, 134.7, 138.3, 139.2, 147.3, 149.4, 157.9, 163.5; LCMS: m/z = 575.7 (M+); anal. calcd for C33H27ClN6O2: C, 68.92; H, 4.73; N, 14.61; found: C, 68.96; H, 4.80; N, 14.69%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-4-methylbenzohydrazide (4g)
Yield 71%, mp 233–236 °C; IR (KBr) λmax: 3312 (N–H stretching, secondary amide), 3016, 3020 (C–H stretching, aromatic ring), 2924 (C–H stretching, −CH3 group), 1600 (C=O stretching), 1546, 1440 (C=C, C=N stretching, aromatic ring), 1240 (C–O–C stretching, oxadiazole ring), 701 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.08 (s, 3H, −CH3), 2.34 (s, 3H, −CH3), 5.68 (s, 1H, C2–H oxadiazole ring), 6.80–7.99 (m, 19H, Ar–H and C5–H pyrazole), 12.35 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.4, 21.8, 78.1, 117.3, 119.3 (2), 123.7, 125.3 (2), 126.8, 127.4 (2), 128.6 (2), 128.9 (2), 129.3 (4), 129.4 (2), 129.8, 131.5, 131.8, 132.8, 134.8, 139.4, 147.2, 149.7, 151.1, 157.3, 163.7. LCMS: m/z = 575.3 (M+); anal. calcd for C33H27ClN6O2: C, 68.92; H, 4.73; N, 14.61; found: C, 68.96; H, 4.63; N, 14.70%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-4-methoxybenzohydrazide (4h)
Yield 62%, mp 248–251 °C; IR (KBr) λmax: 3320 (N–H stretching, secondary amide), 3014, 3020 (C–H stretching, aromatic ring), 2850 (C–H stretching, −OCH3),2824, 1605(C=O stretching), 1554, 1428 (C=C, C=N stretching, aromatic ring), 1212 (C–O–C stretching, oxadiazole ring), 758 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.05 (s, 3H, −CH3), 3.80 (s, 3H, −OCH3), 5.72 (s, 1H, C2–H oxadiazole ring), 6.80–7.80 (m, 19H, Ar–H and C5–H pyrazole), 12.33 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.8, 55.6, 78.4, 114.7 (2), 117.7, 119.4 (2), 123.0, 125.4 (2), 126.7, 128.1, 128.4 (2), 128.7 (2), 128.8 (2), 129.4 (4), 131.2, 131.7, 132.0, 134.7, 139.4, 147.4, 149.4, 157.1, 163.7, 164.0; LCMS: m/z = 591.2 (M+); anal. calcd for C33H27ClN6O3: C, 67.06; H, 4.60; N, 14.22; found: C, 67.09; H, 4.70; N, 14.26%.
Characterization of 3-Chloro-N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)benzohydrazide (4i)
Yield 68%, mp 183–185 °C; IR (KBr) λmax: 3338 (N–H stretching, secondary amide), 3023, 3020 (C–H stretching, aromatic ring), 1609 (C=O stretching), 1562, 1430 (C=C, C=N stretching, aromatic ring), 1219 (C–O–C stretching, oxadiazole ring), 775, 758 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.06 (s, 3H, −CH3), 5.71 (s, 1H, C2–H oxadiazole ring), 6.82–7.99 (m, 19H, Ar–H and C5–H pyrazole), 12.34 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.4, 78.1, 117.4, 119.7 (2), 120.1, 123.1, 125.4 (2), 125.7, 126.7, 127.4, 128.1 (2), 128.4 (2), 129.1 (4), 130.7, 131.5, 132.4, 132.7, 134.7, 134.8, 135.7, 139.4, 147.1, 149.2, 157.5, 163.4; LCMS: m/z = 594.1 (M+); anal. calcd for C32H24Cl2N6O2: C, 64.54; H, 4.06; N, 14.11; found: C, 64.70; H, 4.10; N, 14.20%.
Characterization of 4-Chloro-N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)benzohydrazide (4j)
Yield 74%, mp 191–193 °C; IR (KBr) λmax: 3336 (N–H stretching, secondary amide), 3014, 3001 (C–H stretching, aromatic ring), 1606 (C=O stretching), 1534, 1429 (C=C, C=N stretching, aromatic ring), 1212 (C–O–C stretching, oxadiazole ring), 770, 754 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.06 (s, 3H, −CH3), 5.73 (s, 1H, C2–H oxadiazole ring), 6.82–7.96 (m, 19H, Ar–H and C5–H pyrazole), 12.33 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.0, 78.3, 117.3, 119.2 (2), 120.3, 123.7, 125.4 (2), 126.1, 128.0 (2), 128.1 (4), 129.6 (4), 130.3 (2), 130.8, 131.7, 132.7, 134.4, 137.1, 139.2, 147.1, 149.3, 157.3, 163.9; LCMS: m/z = 595.2 (M+); anal. calcd for C32H24Cl2N6O2: C, 64.54; H, 4.06; N, 14.11; found: C, 64.60; H, 4.10; N, 14.15%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-phenylacetohydrazide (4k)
Yield 57%, mp 244–246 °C; IR (KBr) λmax: 3333 (N–H stretching, secondary amide), 3014, 3020 (C–H stretching, aromatic ring), 2855 (C–H stretching, −CH2−), 1595 (C=O stretching), 1554, 1428 (C=C, C=N stretching, aromatic ring), 1218 (C–O–C stretching, oxadiazole ring), 758 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.00 (s, 3H, −CH3), 3.81 (s, 2H, −CH2), 5.70 (s, 1H, C2–H oxadiazole ring), 7.26–7.62 (m, 20H, Ar–H and C5–H pyrazole), 12.36 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.9, 16.5, 42.7, 79.2, 117.3, 119.7 (2), 123.8, 125.7 (2), 126.7, 127.4, 128.2 (2), 128.4 (2), 129.1 (2), 129.3 (4), 129.6 (2), 130.7, 131.7, 134.7, 135.7, 139.8, 147.0, 149.4, 150.4, 171.9; LCMS: m/z = 575.1 (M+); anal. calcd for C33H27ClN6O2: C, 68.52; H, 4.73; N, 14.61; found: C, 68.60; H, 4.75; N, 14.70%.
Characterization of N-(2-(2-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)hydrazinyl)-2-oxoethyl)benzamide (4l)
Yield 60%, mp 275–278 °C; IR (KBr) λmax: 3012, 3020, 3030 (C–H stretching, aromatic ring), 2867 (C–H stretching, −CH2−), 1700, 1632 (C=O stretching), 1550, 1430 (C=C, C=N stretching, aromatic ring), 1210 (C–O–C stretching, oxadiazole ring), 715 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.02 (s, 3H, −CH3), 2.23 (s, 2H, −CH2), 5.69 (s, 1H, C2–H oxadiazole ring), 6.99–7.99 (m, 19H, Ar–H and C5–H pyrazole), 7.51 (s, 1H, −NHCH2), 7.56 (s, 1H, NH), 12.33 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.4, 45.7, 78.1, 117.5, 119.2 (2), 123.7, 125.7 (2), 126.3, 127.4 (2), 128.5 (4), 128.7 (2), 129.4 (4), 131.5, 131.7, 132.4, 132.7, 134.7, 134.8, 139.4, 147.4, 149.1, 157.4, 167.2, 171.2; LCMS: m/z = 618.2 (M+); anal. calcd for C34H28ClN7O3: C, 66.07; H, 4.57; N, 15.86; found: C, 66.10; H, 4.75; N, 15.96%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(o-tolyloxy)acetohydrazide (4m)
Yield 64%, mp 226–228 °C; IR (KBr) λmax: 3009, 3035, 3025 (C–H stretching, aromatic ring), 2930 (C–H stretching, −CH3 group), 2860 (C–H stretching, −CH2−), 1735, 1635 (C=O stretching), 1555, 1435 (C=C, C=N stretching, aromatic ring), 1325, 1200 (C–O–C stretching, oxadiazole ring), 720 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.01 (s, 3H, −CH3), 2.16 (s, 3H, CH3), 4.66 (s, 2H, −OCH2), 5.69 (s, 1H, C2–H oxadiazole ring), 6.84–7.99 (m, 19H, Ar–H and C5–H pyrazole), 12.33 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.0, 15.2, 69.4, 78.1, 112.4, 117.3, 119.4 (2), 120.7, 123.1, 125.1 (2), 126.2, 126.4, 126.6, 128.3 (2), 128.4 (2), 129.3 (4), 131.4, 131.6, 131.7, 132.4, 134.0, 139.1, 147.2, 149.5, 157.1, 158.4, 171.3; LCMS: m/z = 605.3 (M+); anal. calcd for C34H29ClN6O3: C, 67.49; H, 4.57; N, 15.86; found: C, 67.56; H, 4.63; N, 15.90%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(m-tolyloxy)acetohydrazide (4n)
Yield 63%, mp 212–215 °C; IR (KBr) λmax: 3012, 3040, 3030 (C–H stretching, aromatic ring), 2925 (C–H stretching, −CH3 group), 2800 (C–H stretching, −CH2−), 1695, 1640 (C=O stretching), 1528, 1418 (C=C, C=N stretching, aromatic ring), 1302, 1215 (C–O–C stretching, oxadiazole ring), 730 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.03 (s, 3H, −CH3), 2.19 (s, 3H, CH3), 4.73 (s, 2H, −OCH2), 5.71 (s, 1H, C2–H oxadiazole ring), 6.78–7.93 (m, 19H, Ar–H and C5–H pyrazole), 12.39 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.1, 21.1, 69.3, 78.2, 111.6 (2), 113.4, 117.3, 119.4 (2), 121.6, 123.5, 125.2 (2), 126.8, 128.3 (2), 128.7 (2), 129.2, 129.4 (4), 131.5, 132.2, 134.7, 139.4, 139.5, 147.2, 149.3, 157.2, 160.1, 171.1; LCMS: m/z = 605.4 (M+); anal. calcd for C34H29ClN6O3: C, 67.49; H, 4.57; N, 15.86; found: C, 67.58; H, 4.63; N, 15.96%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(p-tolyloxy)acetohydrazide (4o)
Yield 70%, mp 239–241 °C; IR (KBr) λmax: 3014, 3045, 3035 (C–H stretching, aromatic ring), 2920 (C–H stretching, −CH3 group), 2813 (C–H stretching, −CH2−), 1697, 1647 (C=O stretching), 1530, 1416 (C=C, C=N stretching, aromatic ring), 1336, 1216 (C–O–C stretching, oxadiazole ring), 763 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.09 (s, 3H, −CH3), 2.20 (s, 3H, CH3), 4.79 (s, 2H, −OCH2), 5.75 (s, 1H, C2–H oxadiazole ring), 6.87–7.95 (m, 19H, Ar–H and C5–H pyrazole),.42 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.8, 21.7, 69.6, 78.1, 114.3 (2), 117.5, 119.1 (2), 123.8, 125.9 (2), 126.3, 128.6 (2), 128.8 (2), 129.4 (4), 130.5 (2), 130.8, 131.4, 131.8, 132.5, 134.7, 139.4, 147.3, 149.3, 155.3, 157.3, 171.3; LCMS: m/z = 605.4 (M+); anal. calcd for C34H29ClN6O3: C, 67.49; H, 4.83; N, 13.89; found: C, 67.53; H, 4.90; N, 13.96%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(2-nitrophenoxy)acetohydrazide (4p)
Yield 61%, mp 272–275 °C; IR (KBr) λmax: 3036, 3012, 3060 (C–H stretching, aromatic ring), 2860 (C–H stretching, −CH2−), 1612, 1623 (C=O stretching), 1533 (N=O stretching, Ar–NO2), 1545, 1420 (C=C, C=N stretching, aromatic ring), 1220 (C–O–C stretching, oxadiazole ring), 723 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.12 (s, 3H, −CH3), 4.72 (s, 2H, −OCH2), 5.75 (s, 1H, C2–H oxadiazole ring), 6.94–7.98 (m, 19H, Ar–H and C5–H pyrazole), 12.42 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.4, 68.1, 78.0, 115.0, 117.6, 119.4 (2), 121.9, 123.4, 125.9 (2), 126.7, 127.1, 128.3 (2), 128.7 (2), 129.4 (4), 131.2, 131.4, 131.9, 132.2, 134.0, 139.5, 140.7, 147.1, 149.3, 152.4, 157.2, 171.6; LCMS: m/z = 636.2 (M+); anal. calcd for C33H26ClN7O5: C, 62.31; H, 4.12; N, 15.41; found: C, 62.36; H, 4.05; N, 15.60%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(3-nitrophenoxy)acetohydrazide (4q)
Yield 55%, mp 183–185 °C; IR (KBr) λmax: 3066, 3085, 3012 (C–H stretching, aromatic ring), 2865 (C–H stretching, −CH2−), 1620, 1612 (C=O stretching), 1520 (N=O stretching, Ar–NO2), 1515, 1425 (C=C, C=N stretching, aromatic ring), 1225 (C–O–C stretching, oxadiazole ring), 712 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.18 (s, 3H, −CH3), 4.65 (s, 2H, −OCH2), 5.70 (s, 1H, C2–H oxadiazole ring), 7.38–7.99 (m, 19H, Ar–H and C5–H pyrazole), 12.44 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.3, 69.2, 78.1, 111.0, 116.3, 117.1, 119.8 (2), 120.1, 123.8, 125.6 (2), 126.3, 128.3 (2), 128.8 (2), 129.6 (4), 130.7, 131.4, 131.9, 132.4, 134.6, 139.1, 147.1, 149.3, 151.1, 157.9, 161.9, 171.3; LCMS: m/z = 635.6 (M+); anal. calcd for C33H26ClN7O5: C, 62.31; H, 4.12; N, 15.41; found: C, 62.36; H, 4.20; N, 15.50%.
Characterization of N′-(1-(2-(3-(4-Chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)-2-(4-nitrophenoxy)acetohydrazide (4r)
Yield 66%, mp 207–210 °C; IR (KBr) λmax: 3061, 3080, 3014 (C–H stretching, aromatic ring), 2800 (C–H stretching, −CH2−), 1610, 1623 (C=O stretching), 1525 (N=O stretching, Ar–NO2), 1517, 1410 (C=C, C=N stretching, aromatic ring), 1220 (C–O–C stretching, oxadiazole ring), 725 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.14 (s, 3H, −CH3), 4.63 (s, 2H, −OCH2), 5.72 (s, 1H, C2–H oxadiazole ring), 7.25–8.15 (m, 19H, Ar–H and C5–H pyrazole), 12.47 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.7, 69.3, 78.4, 115.0 (2), 117.9, 119.3 (2), 123.0, 125.1 (2), 125.6 (2), 126.7, 128.0 (2), 128.6 (2), 129.4 (4), 131.0, 131.5, 132.7, 134.7, 139.5, 140.6, 147.8, 149.7, 157.6, 164.6, 171.9; LCMS: m/z = 636.1 (M+); anal. calcd for C33H26ClN7O5: C, 62.41; H, 4.12; N, 15.41; found: C, 62.50; H, 4.23; N, 15.50%.
Characterization of 2-(2-Chlorophenoxy)-N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)acetohydrazide (4s)
Yield 73%, mp 269–272 °C; IR (KBr) λmax: 3061, 3080, 3014 (C–H stretching, aromatic ring), 2800 (C–H stretching, −CH2−), 1610, 1623 (C=O stretching), 1525, 1410 (C=C, C=N stretching, aromatic ring), 1220 (C–O–C stretching, oxadiazole ring), 740, 725 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.18 (s, 3H, −CH3), 4.69 (s, 2H, −OCH2), 5.71 (s, 1H, C2–H oxadiazole ring), 6.95–7.98 (m, 19H, Ar–H and C5–H pyrazole), 12.52 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.0, 68.9, 78.0, 112.7, 117.3, 119.2 (2), 122.8, 122.9, 123.0, 125.3 (2), 126.7, 127.3, 128.6 (2), 128.8 (2), 129.5 (4), 131.0, 131.8, 132.0, 132.7, 134.9, 139.3, 147.9, 149.4, 154.3, 157.6, 171.6; LCMS: m/z = 625.3 (M+); anal. calcd for C33H26Cl2N6O3: C, 63.37; H, 4.19; N, 13.44; found: C, 63.40; H, 4.20; N, 13.55%.
Characterization of 2-(4-Chlorophenoxy)-N′-(1-(2-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-phenyl-1,3,4-oxadiazol-3(2H)-yl)ethylidene)acetohydrazide (4t)
Yield 69%, mp 280–282 °C; IR (KBr) λmax: 3051, 3088, 3012 (C–H stretching, aromatic ring), 2850 (C–H stretching, −CH2−), 1612, 1623 (C=O stretching), 1512, 1413 (C=C, C=N stretching, aromatic ring), 1224 (C–O–C stretching, oxadiazole ring), 750, 725 (C–Cl stretching) cm–1; 1H NMR (DMSO-d6, 400 MHz): 2.23 (s, 3H, −CH3), 4.72 (s, 2H, −OCH2), 5.70 (s, 1H, C2–H oxadiazole ring), 6.90–7.98 (m, 19H, Ar–H and C5–H pyrazole), 12.58 (s, 1H, −CONH); 13C NMR (DMSO-d6, 100 MHz): 12.8, 69.5, 78.4, 117.0, 117.6 (2), 119.7 (2), 123.0, 125.8 (2), 126.0, 126.9, 128.7 (2), 128.9 (2), 129.3 (4), 130.7 (2), 131.7, 131.9, 132.7, 134.9, 139.6, 147.8, 149.0, 156.9, 157.6, 171.4; LCMS: m/z = 625.4 (M+); anal. calcd for C33H26Cl2N6O3: C, 63.37; H, 4.19; N, 13.44; found: C, 63.50; H, 4.30; N, 13.55%.
Methodology for Molecular Docking
The molecular docking study was performed with the Glide (Grid-Based Ligand Docking with Energetics)35−37 module incorporated in the Schrödinger molecular modeling software (Schrödinger, Inc., USA, 2016) to gain an insight into the binding modes of the oxadiazole derivatives (4a–t) into the active site of the mycobacterial enoyl reductase (InhA) enzyme. With this purpose, the crystal structure of the mycobacterial enoyl reductase (InhA) complexed with its inhibitor was retrieved from the protein data bank (PDB) (PDB code: 4TZK).38 Since the protein structure file obtained from the PDB was not suitable for use in molecular docking studies, first, the optimization and minimization of the enzyme-inhibitor complex were performed using the protein preparation wizard tool applying the OPLS-2005 force field. This involved eliminating the crystallographically observed water molecules (water without H bonds) since no water molecule was found to be conserved in the ligand–protein interaction, adding hydrogen atoms corresponding to pH 7.0 considering the appropriate ionization states for the acidic as well as basic amino acid residues assigning the correct bond orders. Following the assignment of charge and protonation state, finally, energy minimization was performed using the OPLS-2005 force field until the average root-mean-square deviation (RMSD) of the nonhydrogen atoms reached 0.3 Å to relieve the steric clashes among the residues due to the addition of hydrogen atoms.
On the other hand, the initial 3D structures of the oxadiazole derivatives were sketched using the build panel in Maestro and subsequently optimized through the LigPrep module that performs addition of hydrogen atoms; adjustment of realistic bond lengths as well as angles; and correction of chiralities, ionization states, tautomers, stereochemistries, and ring conformations. Partial atomic charges were ascribed using the OPLS-2005 force field, and possible ionization states were generated for each ligand at a pH of 7.0. Finally, the geometries of these compounds were optimized by energy minimization until a gradient of 0.01 kcal/mol/Å was reached.
After ensuring that the ligand and protein structures were in the correct form, the shape and properties of the binding site of the InhA enzyme were defined and set up for docking using the receptor grid generation panel. Since the inhibitor’s coordinates in the complex are known, the active site was defined by a 10 × 10 × 10 Å box centered on the native ligand in the crystal complex. The receptor grid generation program uses two cubical boxes with a common centroid for calculations: a larger enclosing and a smaller binding box. With the non-covalently bound cocrystallized ligand, the grid file was created using this native ligand at the center of the two boxes which were kept sufficiently large in order to allow the ligands to explore a large portion of the enzyme structure in search of the best binding conformation.
These optimized protein and ligand geometries were used as input for the binding affinity study. Glide is an interactive molecular graphics program for studying ligand–receptor interactions and identifying the probable binding site for the biomolecules. The final scoring and ranking of the docked poses were carried out using Schrödinger’s proprietary extra precision (i.e., with GlideXP) scoring function. The scoring function is equipped with force-field-based parameters that account for contributions from steric and electrostatic interaction energies, solvation and repulsive interactions, hydrophobic interactions, hydrogen bonding, and metal–ligand interactions, all incorporated in the empirical energy functions. Maestro’s Pose Viewer utility was used to visualize and analyze the output file generated in terms of the docking poses of the ligands for the key elements of interaction with the enzyme.
Acknowledgments
Prof. Nisheeth C. Desai is thankful to the University Grant Commission, New Delhi, for the financial support under BSR Faculty-Fellowship 2019 (F18-1/2011 (BSR)). Jahnvi D. Monapara is grateful to the Department of Science and Technology, INSPIRE PROGRAM, for the award of INSPIRE Fellowship (IF180817). The authors are thankful to the National Repository of Small Molecules (NRM) facility, CSIR-National Chemical Laboratory (CSIR-NCL), Pune, for performing the antitubercular activity. The authors are also thankful to Schrödinger Inc. for providing the demo license of Schrödinger Suite that has tremendously helped in the computational study. The authors are also thankful to Priyanka Desai, founder of iScribblers, for the linguistic editing of the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04411.
Additional figures illustrating the characterization of synthetics by 1H NMR and 13C NMR, mass, and IR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization. Tuberculosis Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Accessed 13 April 2020.
- Rao H. S. P.; Adigopula L. N.; Ramadas K. One-Pot Synthesis of Densely Substituted Pyrazolo[3,4-b]-4,7-dihydropyridines. ACS Comb. Sci. 2017, 19, 279–285. 10.1021/acscombsci.6b00156. [DOI] [PubMed] [Google Scholar]
- El-Borai M. A.; Rizk H. F.; Sadek M. R.; El-Keiy M. M. An Eco-Friendly Synthesis of Heterocyclic Moieties Condensed with Pyrazole System under Green Conditions and Their Biological Activity. Green Sustainable Chem. 2016, 06, 88–100. 10.4236/gsc.2016.62008. [DOI] [Google Scholar]
- Nitulescu G. M.; Matei L.; Aldea I. M.; Draghici C.; Olaru O. T.; Bleotu C. Ultrasound-Assisted Synthesis and Anticancer Evaluation of New Pyrazole Derivatives as Cell Cycle Inhibitors. Arab. J. Chem. 2019, 12, 816–824. 10.1016/j.arabjc.2015.12.006. [DOI] [Google Scholar]
- Mehranpour A. M.; Hashemnia S.; Abbasi S. Synthesis of New Pyrazole Derivatives Using Vinamidinium Salts. Org. Chem. Res. 2017, 3, 187–190. 10.22036/ORG.CHEM.2017.83427.1089. [DOI] [Google Scholar]
- Naim M. J.; Alam O.; Nawaz F.; Alam M. J.; Alam P. Current Status of Pyrazole and its Biological Activities. J. Pharm. Bioallied. Sci. 2016, 8, 2–17. 10.4103/0975-7406.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudeva M. G.; Vivek H. K.; Kumar K. A. Synthesis of Novel Pyrazole Carboxamides using Reusable Catalyst as Antimicrobial Agents and Molecular Docking Studies. Chem. Data Collect. 2019, 20, 100193. 10.1016/j.cdc.2019.100193. [DOI] [Google Scholar]
- Takate S. J.; Shinde A. D.; Karale B. K.; Akolkar H.; Nawale L.; Sarkar D.; Mhaske P. C. Thiazolyl-Pyrazole Derivatives as Potential Antimycobacterial Agents. Bioorg. Med. Chem. Lett. 2019, 29, 1199–1202. 10.1016/j.bmcl.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Gedawy E. M.; Kassab A. E.; El Kerdawy A. M. Design, Synthesis and Biological Evaluation of Novel Pyrazole Sulfonamide Derivatives as Dual COX-2/5-LOX Inhibitors. Eur. J. Med. Chem. 2020, 189, 112066. 10.1016/j.ejmech.2020.112066. [DOI] [PubMed] [Google Scholar]
- Yamali C.; Gul H. I.; Ece A.; Taslimi P.; Gulcin I. Synthesis, Molecular Modeling, and Biological Evaluation of 4-[5-aryl-3-(thiophen-2-yl)-4, 5-dihydro-1h-pyrazol-1-yl] benzenesulfonamides Toward Acetylcholinesterase, Carbonic Anhydrase I and II Enzymes. Chem. Biol. Drug Des. 2017, 91, 854–866. 10.1111/cbdd.13149. [DOI] [PubMed] [Google Scholar]
- Abadi A. H.; Eissa A. A. H.; Hassan G. S. Synthesis of Novel 1, 3, 4-Trisubstituted Pyrazole Derivatives and Their Evaluation as Antitumor and Antiangiogenic Agents. Chem. Pharm. Bull. 2003, 51, 838–844. 10.1248/cpb.51.838. [DOI] [PubMed] [Google Scholar]
- Ohno R.; Watanabe A.; Matsukawa T.; Ueda T.; Sakurai H.; Hori M.; Hirai K. Synthesis and Herbicidal Activity of New Pyrazole-4-Carboxamide Derivatives. J. Pestic. Sci. 2004, 29, 15–26. 10.1584/jpestics.29.15. [DOI] [Google Scholar]
- Meng F.-J.; Sun T.; Dong W.-Z.; Li M.-H.; Tuo Z.-Z. Discovery of Novel Pyrazole Derivatives as Potent Neuraminidase Inhibitors against Influenza H1N1 Virus. Arch. Pharm. 2016, 349, 168–174. 10.1002/ardp.201500342. [DOI] [PubMed] [Google Scholar]
- Zhao P. L.; Wang L.; Zhu X. L.; Huang X.; Zhan C. G.; Wu J. W.; Yang G. F. Subnanomolar Inhibitor of Cytochrome Bc1 Complex Designed by Optimizing Interaction with Conformationally Flexible Residues. J. Am. Chem. Soc. 2010, 132, 185–194. 10.1021/ja905756c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh A.; Meshram J. Novel 1,3,4-Oxadiazole Derivatives of Dihydropyrimidinones: Synthesis, Anti-Inflammatory, Anthelmintic, and Antibacterial Activity Evaluation. J. Heterocycl. Chem. 2016, 53, 1176–1182. 10.1002/jhet.2377. [DOI] [Google Scholar]
- Modi V.; Modi P. Oxadiazole: Synthesis, Characterization and Biological Activities. J. Saudi Chem. Soc. 2012, 16, 327–332. 10.1016/j.jscs.2011.12.017. [DOI] [Google Scholar]
- Bhat K.; Sufeera K.; Chaitanya S. Synthesis, Characterization and Biological Activity Studies of 1,3,4-Oxadiazole Analogs. J. Young Pharm. 2011, 3, 310–314. 10.4103/0975-1483.90243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. G.; Das N.; Shengule S. A.; Srivastava R. S.; Shrivastava S. K. Synthesis, Characterization, Evaluation and Molecular Dynamics Studies of 5, 6-Diphenyl-1,2,4-triazin-3(2H)-one Derivatives Bearing 5-Substituted 1,3,4-Oxadiazole as Potential Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2015, 101, 81–95. 10.1016/j.ejmech.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Sreenivasulu R.; Tej M. B.; Jadav S. S.; Sujitha P.; Kumar C. G.; Raju R. R. Synthesis, Anticancer Evaluation and Molecular Docking Studies of 2,5-Bis(indolyl)-1,3,4-oxadiazoles. J. Mol. Struct. 2020, 1208, 127875. 10.1016/j.molstruc.2020.127875. [DOI] [Google Scholar]
- Rathore A.; Sudhakar R.; Ahsan M. J.; Ali A.; Subbarao N.; Jadav S. S.; Umar S.; Yar M. S. In vivo Anti-Inflammatory Activity and Docking Study of Newly Synthesized Benzimidazole Derivatives Bearing Oxadiazole and Morpholine Rings. Bioorg. Chem. 2017, 70, 107–117. 10.1016/j.bioorg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Shaykoon M. S.; Marzouk A. A.; Soltan O. M.; Wanas A. S.; Radwan M. M.; Gouda A. M.; Youssif B. G. M.; Abdel-Aziz M. Design, Synthesis and Antitrypanosomal Activity of Heteroaryl-Based 1,2,4-Triazole and 1,3,4-Oxadiazole Derivatives. Bioorg. Chem. 2020, 100, 103933. 10.1016/j.bioorg.2020.103933. [DOI] [PubMed] [Google Scholar]
- Ambhore A. N.; Kamble S. S.; Kadam S. N.; Kamble R. D.; Hebade M. J.; Hese S. V.; Gaikwad M. V.; Meshram R. J.; Gacche R. N.; Dawane B. S. Design, Synthesis and in silico Study of Pyridine Based 1,3,4-Oxadiazole Embedded Hydrazinecarbothioamide Derivatives as Potent Anti-Tubercular Agent. Comput. Biol. Chem. 2019, 80, 54–65. 10.1016/j.compbiolchem.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Desai N. C.; Trivedi A.; Somani H.; Jadeja K. A.; Vaja D.; Nawale L.; Khedkar V. M.; Sarkar D. Synthesis, Biological Evaluation, and Molecular Docking Study of Pyridine Clubbed 1,3,4-Oxadiazoles as Potential Antituberculars. Synth. Comm. 2018, 48, 524–540. 10.1080/00397911.2017.1410892. [DOI] [Google Scholar]
- Khan A.; Sarkar D. A Simple Whole Cell Based High Throughput Screening Protocol Using Mycobacterium Bovis BCG for Inhibitors Against Dormant and Active Tubercle bacilli. J. Microbiol. Methods 2008, 73, 62–68. 10.1016/j.mimet.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Zhu X. L.; Jiang L. L.; Liu Z. M.; Yang G. F. Synthesis, Antifungal Activity and CoMFA Analysis of Novel 1,2,4-Triazolo[1,5-a]Pyrimidine Derivatives. Eur. J. Med. Chem. 2008, 43, 595–603. 10.1016/j.ejmech.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Zhang M. Z.; Mulholland N.; Beattie D.; Irwin D.; Gu Y. C.; Chen Q.; Yang G. F.; Clough J. Synthesis and Antifungal Activity of 3-(1,3,4-Oxadiazol-5-Yl)-Indoles and 3-(1,3,4-Oxadiazol-5-Yl)Methyl-Indoles. Eur. J. Med. Chem. 2013, 63, 22–32. 10.1016/j.ejmech.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Pathak G.; Das D.; Rokhum S. L. A Microwave-Assisted Highly Practical Chemoselective Esterification and Amidation of Carboxylic Acids. RSC Adv. 2016, 6, 93729–93740. 10.1039/C6RA22558F. [DOI] [Google Scholar]
- Desai N. C.; Somani H.; Trivedi A.; Bhatt K.; Nawale L.; Khedkar V. M.; Jha P. C.; Sarkar D. Synthesis, Biological Evaluation and Molecular Docking Study of Some Novel Indole and Pyridine Based 1, 3, 4-Oxadiazole Derivatives as Potential Antitubercular Agents. Bioorg. Med. Chem. Lett. 2016, 26, 1776–1783. 10.1016/j.bmcl.2016.02.043. [DOI] [PubMed] [Google Scholar]
- Leung A. K.; White E. L.; Ross L. J.; Reynolds R. C.; DeVito J. A.; Borhani D. W. Structure of Mycobacterium tuberculosis FtsZ Reveals Unexpected, G Protein-Like Conformational Switches. J. Mol. Biol. 2004, 342, 953–970. 10.1016/j.jmb.2004.07.061. [DOI] [PubMed] [Google Scholar]
- Asgaonkar K. D.; Mote G. D.; Chitre T. S. QSAR and Molecular Docking Studies of Oxadiazole-Ligated Pyrrole Derivatives as Enoyl-ACP (CoA) Reductase Inhibitors. Sci. Pharm. 2014, 82, 71–85. 10.3797/scipharm.1310-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini S. G.; Bhat A. R.; Bryant B.; Williamson J. S.; Dayan F. E. Synthesis, Antitubercular Activity and Docking Study of Novel Cyclic Azole Substituted Diphenyl Ether Derivatives. Eur. J. Med. Chem. 2009, 44, 492–500. 10.1016/j.ejmech.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Mathew B.; Suresh J.; Mathew G. E.; Sonia G.; Krishnan G. K. Design, Synthesis, Toxicity Estimation and Molecular Docking Studies of N-(furan-2-yl)-1-(5-substituted) phenyl-1,3,4-oxadiazol-2-yl methanimine as Antitubercular Agents. Indian J. Pharm. Sci. 2014, 76, 401–406. [PMC free article] [PubMed] [Google Scholar]
- Vilchèze C.; Morbidoni H. R.; Weisbrod T. R.; Iwamoto H.; Kuo M.; Sacchettini J. C.; Jacobs W. R. Inactivation of the inhA-Encoded Fatty Acid Synthase II (FASII) Enoyl-Acyl Carrier Protein Reductase Induces Accumulation of the FASI End Products and Cell Lysis of Mycobacterium smegmatis. J. Bacteriol. 2000, 182, 4059–4067. 10.1128/JB.182.14.4059-4067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. C.; Kotadiya G. M. Microwave-Assisted Synthesis of Benzimidazole bearing 1, 3, 4-Oxadiazole Derivatives: Screening for Their in vitro Antimicrobial Activity. Med. Chem. Res. 2014, 23, 4021–4033. 10.1007/s00044-014-0978-0. [DOI] [Google Scholar]
- Friesner R. A.; Banks J. L.; Murphy R. B.; Halgren T. A.; Klicic J. J.; Mainz D. T.; Repasky M. P.; Knoll E. H.; Shelley M.; Perry J. K.; Shaw D. E.; Francis P.; Shenkin P. S. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Halgren T. A.; Murphy R. B.; Friesner R. A.; Beard H. S.; Frye L. L.; Pollard W. T.; Banks J. L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- Jadhav C. K.; Nipate A. S.; Chate A. V.; Dofe V. S.; Sangshetti J. N.; Khedkar V. M.; Gill C. H. Rapid Construction of Substituted Dihydrothiophene Ureidoformamides at Room Temperature Using Diisopropyl Ethyl Ammonium Acetate: A Green Perspective. ACS Omega 2020, 5, 29055–29067. 10.1021/acsomega.0c03575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.rcsb.org/pdb/. (Accessed on August 1st, 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.