Abstract
Adolescent drinking is risky because neural circuits in the frontal lobes are undergoing maturational processes important for cognitive function and behavioral control in adulthood. Previous studies have shown that myelinated axons in the medial prefrontal cortex (mPFC) are particularly sensitive to alcohol drinking, especially in males. Pro-inflammatory mediators like toll-like receptor 4 (TLR4) and interleukin-1 beta (IL1b) have been implicated in alcohol induced-inflammation and demyelination; thus, herein we test the hypothesis that voluntary alcohol drinking early in adolescence elicits a pro-inflammatory state that is more pronounced in the brain of males compared to females. Adolescent male and female Wistar rats self-administered sweetened alcohol or sweetened water from postnatal days 28–42 and separate sets of brains were processed for 1) immunolabeling for ionized calcium-binding adapter molecule 1 to analyze microglial cell morphology, or 2) qPCR analysis of gene expression of pro-inflammatory mediators. Binge drinking of alcohol activated microglia in the mPFC and hippocampus of both males and females, suggesting that voluntary alcohol exposure initiates an inflammatory response. Il1b mRNA was upregulated in the mPFC of both sexes. Conversely, Tlr4 mRNA levels were elevated after drinking only in males, which could explain more robust effects of alcohol on myelin in this region in developing males compared to females. Il1b mRNA changes were not observed in the hippocampus, but alcohol elevated Tlr4 mRNA in both sexes, highlighting regional specificity in inflammatory responses to alcohol. Overall, these findings give insight into potential mechanisms by which low-to-moderate voluntary alcohol intake impacts the developing brain.
Keywords: adolescence; alcohol; operant self-administration; binge drinking; prefrontal cortex, mPFC; hippocampus; development; sex differences; male; female; rats; neuroinflammation; cytokines; Iba1; interleukin-1 beta; Il1b; toll-like receptor 4; Tlr4; microglia
1. Introduction
Adolescent alcohol drinking is highly prevalent in the US and it has been linked to an increased lifetime risk of alcohol use disorder (Chou and Pickering, 1992). There are sex differences in factors promoting early-onset drinking, as well as with the cortical structural changes that have been associated with heavy alcohol use (reviewed in (Flores-Bonilla and Richardson, 2020). Animal studies have tested the causality of these relationships and investigated underlying mechanisms. Adolescent drinking leads to heavy drinking later in adulthood in mice and rats (Alaux-Cantin et al., 2013; Alfonso-Loeches and Guerri, 2011; Wolstenholme et al., 2020). Likewise, we have found that a history of adolescent drinking caused higher levels of relapse-like drinking after short abstinent periods in adulthood in male rats (Gilpin et al., 2012). High alcohol exposure during development can also cause changes in brain structures important for learning and memory, cognition, and executive control of behaviors. For example, adolescent exposure to binge-like doses of alcohol elicits significant neuronal degeneration in the cortex and hippocampus, as indexed by high amino cupric silver staining in male rats (Crews et al., 2000; McClain et al., 2011). Intermittent alcohol exposure during early adolescence upregulates pro-inflammatory factors in the prefrontal cortex and this effect is also specific to that developmental period, as adults show no change in pro-inflammatory gene expression after the same alcohol treatment (Pascual et al., 2014). Moreover, voluntary binge drinking early in adolescence compromises axons that are undergoing de novo myelination at that time, and myelin deficits persist into adulthood in male rats (McDougall et al., 2018; Mengler et al., 2014; Vargas et al., 2014). Alcohol-induced inflammation and associated effects on myelin are accompanied by cognitive impairments later in adulthood, and blocking specific inflammatory pathways can reduce some of these negative consequences of alcohol on brain and behavior (Alfonso-Loeches et al., 2010; Montesinos et al., 2015; Pascual et al., 2007, 2014).
Inflammation and neural damage caused by high alcohol exposure is thought to be mediated by TLR4 because ablation of the gene encoding this receptor blocks upregulation of inducible nitric oxide synthase, as well as the COX2 enzyme, which is responsible for inflammation and pain, resulting in less cell death (Alfonso-Loeches et al., 2010). TLR4 agonists include damage-associated molecular patterns (DAMPs) and alarmins such as lipopolysaccharide (LPS). Thus, alcohol can activate these receptors indirectly through LPS release from the gut (Lu et al., 2008). In addition, in vitro studies using cultured astrocytes show that alcohol can also directly act on TLR4 to activate the pro-inflammatory cascade in the absence of LPS (Blanco et al., 2005). This receptor is also necessary for alcohol-induced microglia activation, as the absence of TLR4 prevents the activation of the pro-inflammatory cascade in microglia cell culture (Fernandez-Lizarbe et al., 2009). Microglia activation alone can lead to cognitive impairments, as stress-induced microglia activation results in worse performance in a working memory task and blocking microglia activation with minocycline eliminates this stress-induced behavioral effect (Hinwood et al., 2012). Finally, adolescent stress increases LPS-induced inflammatory responses in males, but not in females (Pyter et al., 2013). This, in turn, suggests that males might be more sensitive to inflammation during early adolescence as well.
We have previously shown that two weeks of voluntary alcohol drinking during adolescence had a more pronounced negative impact on myelin in male rats compared to females (Tavares et al., 2019). Thus, it is possible that males are more sensitive to the inflammatory effects of alcohol during this specific developmental time period. Alternatively, the differential sensitivity of myelinated axons may not be explained by a heightened neuroinflammatory response in adolescent males. To test this hypothesis, we exposed male and female rats to voluntary alcohol early in adolescence using an operant self-administration model of binge drinking and assessed microglial cell number and morphology in the anterior cingulate of the prefrontal cortex as well as in the different subregions of the hippocampus. We also measured inflammation-related gene expression in the prefrontal cortex, as well as the hippocampus, which develops earlier than the mPFC yet is highly sensitive to alcohol (Nickel and Gu, 2018). Alcohol drinking caused alterations in microglia structure and pro-inflammatory cytokine gene expression in the mPFC and hippocampus of both males and females. While the effects of alcohol were similar between males and females in the hippocampus, males seemed to have a more robust inflammatory response than females in the PFC, which could potentially be explained by a less mature PFC in males.
2. Materials and Methods
2.1. Animals
Male and female Wistar rats were shipped with mothers from Charles River at PD18. Rats were weaned at PD21 and housed in triads on a 12hr light cycle and ad libitum access to food and water. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
2.2. Operant self-administration
Adolescent rats were exposed to alcohol from PD28–42 using an operant model of binge self-administration (see (Gilpin et al., 2012; Karanikas et al., 2013) for details about this model and Fig 1 for the design of the current study). In brief, beginning at PD23–25, rats were first trained to lever press for sweetened water for 3–5 days. At PD28 they were randomly assigned to the alcohol or control groups and began overnight drinking sessions consisting of six 30-min bouts of access to sweetened alcohol or sweetened water, interweaved with 60-min breaks during which the lever was retracted. During these sessions, animals could press a lever that delivered 0.1 ml sweetened alcohol (3% glucose, 0.125% saccharin, 10% w/v alcohol) for alcohol animals and sweetened water (3% glucose, 0.125% saccharin) for control animals on a fixed ratio 1 schedule. A PC computer connected to the operant boxes was used to cap the maximum number of responses per 30-min bout to keep the amount of solution consumed by the control groups close to the level consumed by the alcohol groups. After the maximum number of responses was reached, the lever was retracted to avoid extinguishing self-administration responding in the control rats.
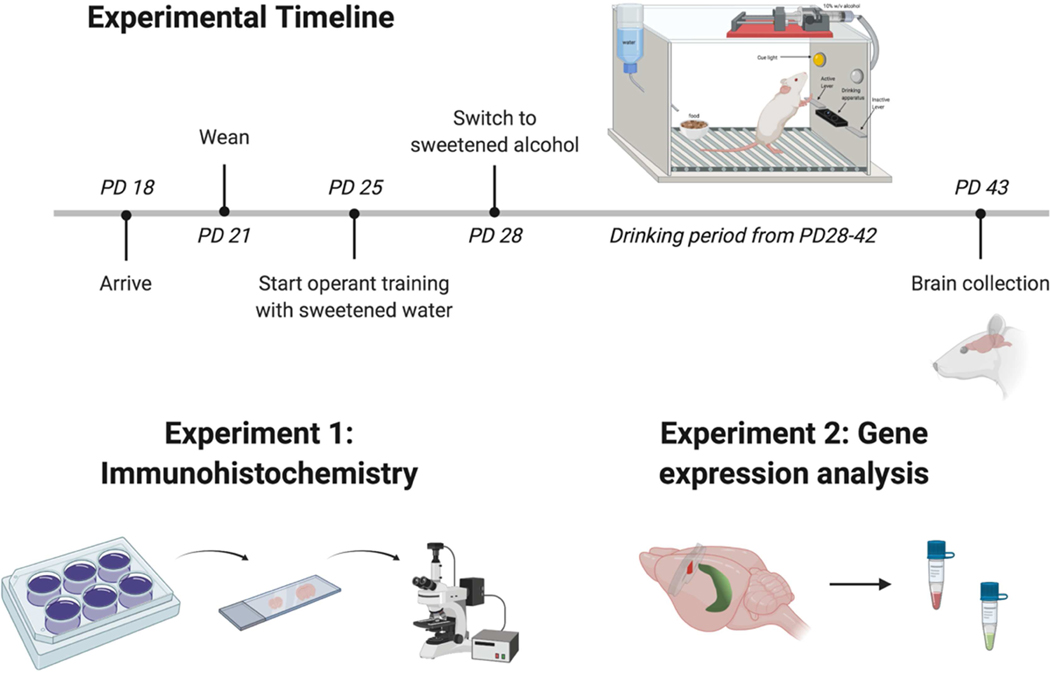
Figure 1. Timeline of operant self-administration and brain collection followed by gene expression and immunohistochemical analyses.
Rats were weaned at PD21 and started operant training at PD25, followed by self-administration of sweetened alcohol (alcohol groups) or water (control groups) from PD28–42. Brains were collected at PD43 and processed for immunohistochemical analysis of microglia cells (Experiment 1) or qPCR analysis of neuroimmune-related gene expression (Experiment 2). Created with BioRender.com
2.3. Experiment 1: Immunohistochemical analysis of microglia
2.3.1. Perfusions & Tissue Processing
Animals were deeply anesthetized with 50 mg/kg sodium pentobarbital 24 hours after their last drinking session and intracardially perfused with 0.9% saline for 5 minutes at room temperature, followed by cold 4% paraformaldehyde in 0.1M sodium tetraborate (pH 9.4) for 20 minutes as previously described (Gilpin et al., 2012; Karanikas et al., 2013; Tavares et al., 2019; Vargas et al., 2014). Brains were extracted and post-fixed in paraformaldehyde solution for 4 hours and then brains were dehydrated in 20% sucrose in PBS solution for ~48 hours and snap frozen and stored at −80°C until sectioning on a freezing sliding microtome. Brains were sectioned (coronal plane) at 35 μm thickness in strict anatomical order in a 1:10 series and stored in cryoprotectant at –20°C until immunohistochemistry experiments were conducted.
2.3.2. Immunohistochemistry
Free-floating sections from all treatment groups were rinsed in 0.05M Tris-buffered saline (TBS; pH 7.2). Sections were then rinsed in a solution containing 10% saline, 1% gelatin, 0.2% sodium azide, and 0.2% Triton-X in 0.05M TBS (gel TBS). Sections were then incubated in a blocking buffer containing 2% normal goat serum, 1% bovine serum albumin, and 1% hydrogen peroxide in gel TBS for 1 h. Sections were then incubated for 48h at 4°C with a rabbit polyclonal antibody raised against ionized calcium binding adapter molecule 1 (Iba1; Wako, Richmond, VA) at a dilution of 1:10,000 in 2% normal goat serum and 0.5% Triton X-100 in gel TBS. After primary incubation the sections were rinsed in TBS and incubated in biotinylated secondary antibody (goat anti-rabbit; Vector Laboratories, Burlingame, CA) in 2% normal goat serum in gel TBS for 90 min., followed by washes in gel TBS. Finally, the sections were incubated with an avidin-biotin horseradish peroxidase complex (Vecatstain ABC, Elite Kit; Vector Laboratories) for 90 min. at room temperature, washed in gel TBS and then washed in TBS. The sections were visualized with using nickel sulfate and 3,3’-diaminobenzidine tetrahydrochloride (DAB kit; Vector Laboratories). After this visualization step, the sections were rinsed in TBS and mounted serially onto gelatin-coated glass slides and coverslipped for microscopic analyses.
2.3.3. Microscopic analysis of labeled tissue
Whole brain sections containing mPFC (2.2 mm anterior to Bregma) and dorsal hippocampus (3.3 mm posterior to Bregma) sections were imaged using a Nikon microscope with high content analysis. The microscope was setup with Köhler illumination, the images were taken using an Andor Zyla 4.2 sCMOS camera, and NIS-Elements HC was used to carry out high content analysis. Slides were scanned at 4x to identify tissue location within the slide, along with general analysis to find the best plane of focus. Coordinates were determined from that and subsequently imaged at 20x magnification, as follows. In short, z-stacks were taken at 20x magnification, images were stitched, and extended depth of focus was used in order to find high contrast parts of each z-stack to assemble in a single plane, given the 3D nature of the brain sections. The regions of interest used for cell counts and morphological analyses of microglia in the mPFC and hippocampus are shown in Figs 3A and 4A, respectively.
2.3.4. Automatic cell counts
Cells were automatically counted using General Analysis within NIS-Elements Advance Research software. To highlight cell bodies only, images were preprocessed using a Gaussian convolution – size 5, and then thresholded using between 0 – 401 (darkest pixels). Regions were then smoothed and cleaned at 4x magnification. The regions of interest were filtered by area with a minimum of 5 μm2 to infinity. Each region was dilated by 1.3 μm. The objects were then automatically counted using NIS-Elements AR.
2.3.5. Microglia morphological analyses - ramifications
Microglia morphology was analyzed by quantifying the total branching points per cell using General Analysis 3 within NIS-Elements AR. The first step was to highlight the soma of the cells. The same steps were followed from the automatic cell counting protocol for this part to create a binary layer that would select cell bodies only (“Soma”, Fig 4B). Next, microglia ramifications were highlighted using the skeletonize feature. To do this, images were preprocessed to detect valleys with a Kernel count of 2x magnification and then were thresholded using pixels between 249–1253. Regions were smoothed and cleaned at 1x magnification and then skeletonized to create a second binary layer, “Skeletonized” (Fig 4B). The next step was to make sure that only the processes that belong to the cells in our sampling area were highlighted and counted, so a radius of 15 μm was chosen. To do this, we used the dilate function and selected 15 μm to create the third binary layer, “Expanded radius” (Fig 4B). The separate object function was then used so that the cells that overlapped with other cells were counted as individual objects. The “Expanded radius” and the “Skeletonized” binary layers were then put together using the logical operator AND, to get the final selection of ramifications per cell. Finally, using the object parenting function and the aggregate children function, the branch points per parent object were counted.
2.4. Experiment 2: Gene expression analysis of pro-inflammatory markers
Brain tissue processing.
One day after the last binge drinking session, rats were lightly anesthetized with CO2 and rapidly decapitated, brains were extracted and separated into two hemispheres. The medial prefrontal cortex (mPFC) and dorsal hippocampus were dissected from both hemispheres using a scalpel, and tissue was flash frozen with dry ice and stored in – 80* until further processing. Tissue from one hemisphere was used for qPCR analysis.
2.4.1. Real Time Polymerase Chain Reaction (qPCR) analysis.
RNA extraction from the dissected mPFC and hippocampus regions of one brain hemisphere was performed with QIAzol lysis reagent and RNeasy lipid tissue mini kit (Qiagen) according to the manufacturer’s instructions. RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher). cDNA was synthesized from RNA samples using “SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR” kit (Life Technologies, CA, USA). The amount of Tlr4, Il1b, Il33, and Mbp mRNA levels were measured and quantified by QuantiFast SYBR Green PCR (Qiagen, Hilden, Germany). Quantitaitve PCR reactions were carried out in duplicate in a 96-well plate RealPlex machine (Eppendorf). Primers were ordered from Integrated DNA Technologies (Coralville, IA, USA). Forward and reverse primer sequences for Tlr4 and Il1b, identical to those used in Nakata et al. (2006) and Tan et al. (2019), respectively, can be found in Table I. Beta-actin (Actb) and general transcription factor IIB (Gtf2b) were used as housekeeping genes. Finally, the ΔΔCt method (Livak and Schmittgen, 2001) was used to normalize the obtained Ct scores to the average of the two housekeeping genes and control group, and therefore, to calculate relative gene expression changes with a Grubbs correction for outliers.
Table I.
Primer sequences used for RT-PCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Tlr4 | GAGGACTGGGTGAGAAACGA | GAAACTGCCATGTCTGAGCA |
| Il1b | TCGTGCCTGACCCATGT | ACAAAGCTCATGGAGAATACCACTT |
| Il33 | TTCCTTTCCTGCACAATCAGGAG | TGATCTGGCGGAGAGACATCAC |
| Mbp | ACAGGAAACGGGGACTTAGG | TGGGCTCTGAGAGGAAACAG |
| Actb | AGGGAAATCGTGCGTGACAT | AAGGAAGGCTGGAAGAGAGC |
| Gtf2b | TGCGATAGCTTCTGCTTGTC | TCAGATCCACGCTCGTCTC |
2.5. Statistical analysis
Cumulative alcohol intake was analyzed using a mixed-model ANOVA, with sex as a between-subject variable and operant day as a within-subject variable. Cumulative glucose intake was analyzed using a mixed-model ANOVA, with sex and treatment as a between-subject variables and operant day as a within-subject variable. Microglia cell counts and fold-change were analyzed using two-way ANOVAs with sex and treatment as within-subject variables. Pearson correlation analyses were used to test for relationships between gene expression and cumulative intake, as well as relationship between Mbp and Il33 genes. A two-sample Kolmogorov-Smirnov test was used to compare distributions of total branching points between groups. Statistical significance was defined as p< 0.05 using two-tailed tests. Wherever appropriate, data are presented as mean ± SEM. Statistical analyses were performed using the R statistical software package (open source from https://www.r-project.org, R Core Team, 2017).
3. Results
3.1. Operant self-administration
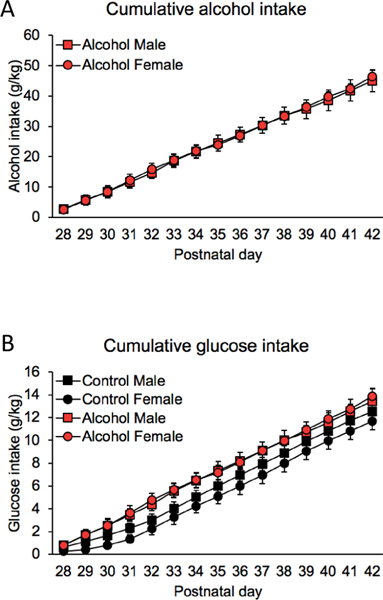
Fig 2 shows drinking data across the early adolescent period of exposure (PD28–42). Alcohol animals consumed between 38.5 and 59.5 g/kg of alcohol over the two-week period, and the total alcohol intake was comparable in males and females (p> 0.05, Fig 2A). Fig 2B shows consumption levels of glucose (g/kg), which was used to sweeten the alcohol and control solutions. Glucose intake was calculated based on the proportion of glucose (3%) in the sweetened alcohol consumed by binge animals or sweetened water consumed by control animals. Although there was a trend of slightly higher total glucose intake over the two-week period of alcohol rats exposure compared to control rats, this difference was not statistically significant (F(1,20)=3.19, p=0.09, Fig 2B). There were no significant sex differences or sex x treatment interactions in total glucose intake over the two-week period (all ps>0.05, Fig 2B). The average of total alcohol drinking was 45.59 ± 1.90 g/kg for animals in Experiment 1 and 48.22 ± 2.87 g/kg for animals in Experiment 2, which is comparable to the amount consumed by animals from our previous studies (Tavares et al., 2019; Vargas et al., 2014).
Figure 2. Cumulative alcohol and glucose intake throughout the drinking period.
(A) Cumulative alcohol intake (g/kg) for the alcohol groups, and (B) cumulative glucose intake (g/kg) for both control and alcohol groups over the binge period. Total alcohol and glucose intake were not significantly different across groups (all ps > 0.05).
3.2. Microglia activation
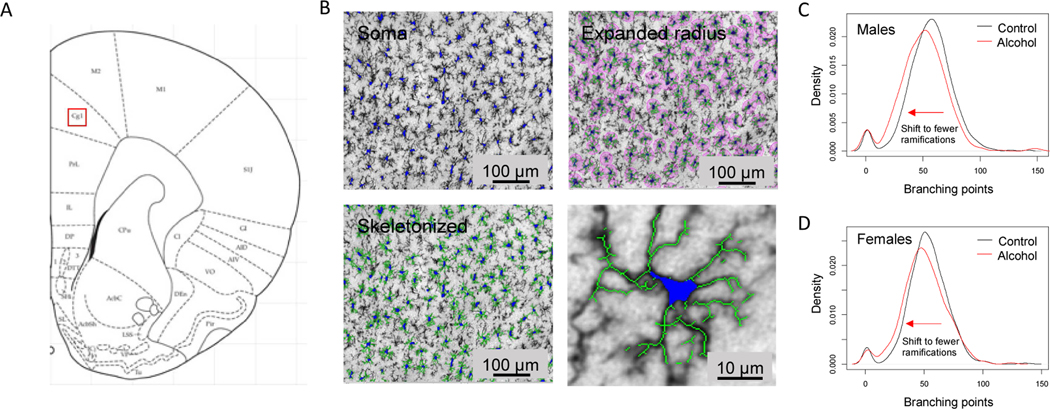
We first tested for evidence of microglial activation following voluntary drinking, as these are the resident immune cells of the brain (Kettenmann et al., 2011). To this end, we used immunohistochemistry to visualize cells and assessed changes in cellular morphology. To test for changes in microglia ramifications we measured the total number of branching points per cell and plotted the distribution. Alcohol drinking shifted the microglia cell population towards having fewer total branching points in both males and females (Fig 3; Two-sample Kolmogorov-Smirnov test, p < 0.001). Fewer branching points signifies that the cells are less ramified. This is a morphological change consistent with microglia activation, as processes retract when microglia are in a reactive or “alert” state (Marshall et al., 2013; Raivich et al., 1999). We did not detect sex differences or alcohol-induced changes in microglia cell number (data not shown).
Figure 3. Alcohol drinking produces a shift in microglial cell morphology to a more reactive state in the in the mPFC of adolescent male and female rats.
Automated morphological analysis of microglial cells was conducted using Nikon NIS-Elements Advanced Research software. We obtained a measurement of total branching points per microglial cell in the mPFC. Alcohol shifted the population distribution of microglial cell morphology to the left in male and female rats, indicating a more “alert” structure in which cellular branching is more retracted in these animals (C, D, n=6 rats for each sex and treatment group. Two-sample Kolmogorov-Smirnov test, both ps < 0.05).
Ramifications per microglia cell distribution in the hippocampus
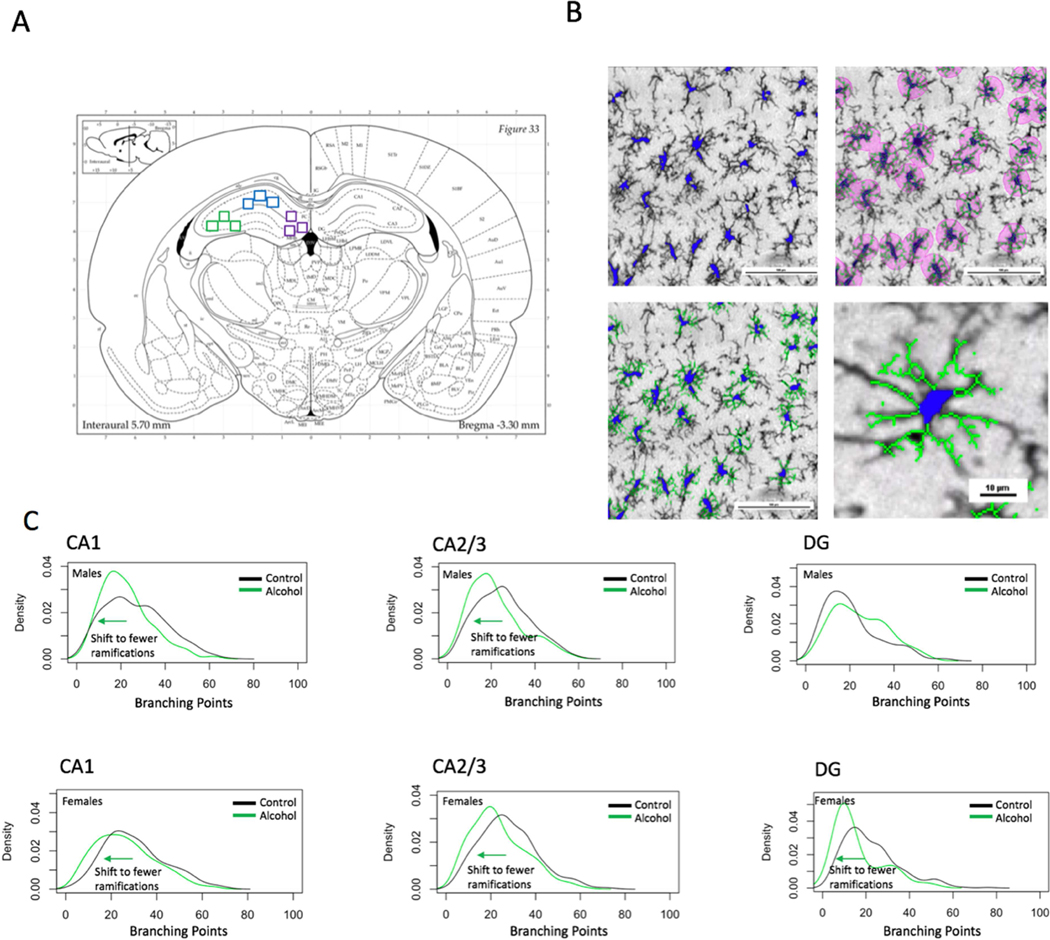
We also investigated the distribution of ramifications per microglial cell in the population in the dorsal hippocampus in the CA1, CA2/3, and DG regions (Fig 4). Using a density plot, we found a shift in the distribution to a state of cells having fewer ramifications in the alcohol group for both males and females in the CA2/3, in males in the CA1, and in females for the CA1 and DG. On the other hand, we found a shift to a state of more ramifications in the DG of alcohol-exposed males. Using Kolmogorov-Smirnov two-sample tests for each region, it was found that the alcohol and control groups’ distributions were significantly different for both sexes in every region (p < 0.05).
Figure 4. Alcohol drinking produces a shift in microglial cell morphology to a more reactive state in the hippocampus of adolescent males and females.
A) 3 cropped images were taken for each of the following regions for analysis: DG, CA1, CA2/3, shown by purple, blue, and green squares, respectively. B) Automated morphological analysis of microglial cells was conducted using Nikon NIS-Elements Advanced Research Software. C) The alcohol group in males and females for the CA1, CA2/3 and in females for the DG shifted the population of microglial cell morphology to the left, indicating a state of greater activation, characterized by retracted branchings. The DG of the male alcohol group had a shift to the right, indicating greater branching points, which supports an intermediate “hyper-ramified” activation state (n=3–6 rats for each sex and treatment group. Two-sample Kolmogorov-Smirnov test, p < 0.05).
3.3. Gene expression of pro-inflammatory factors
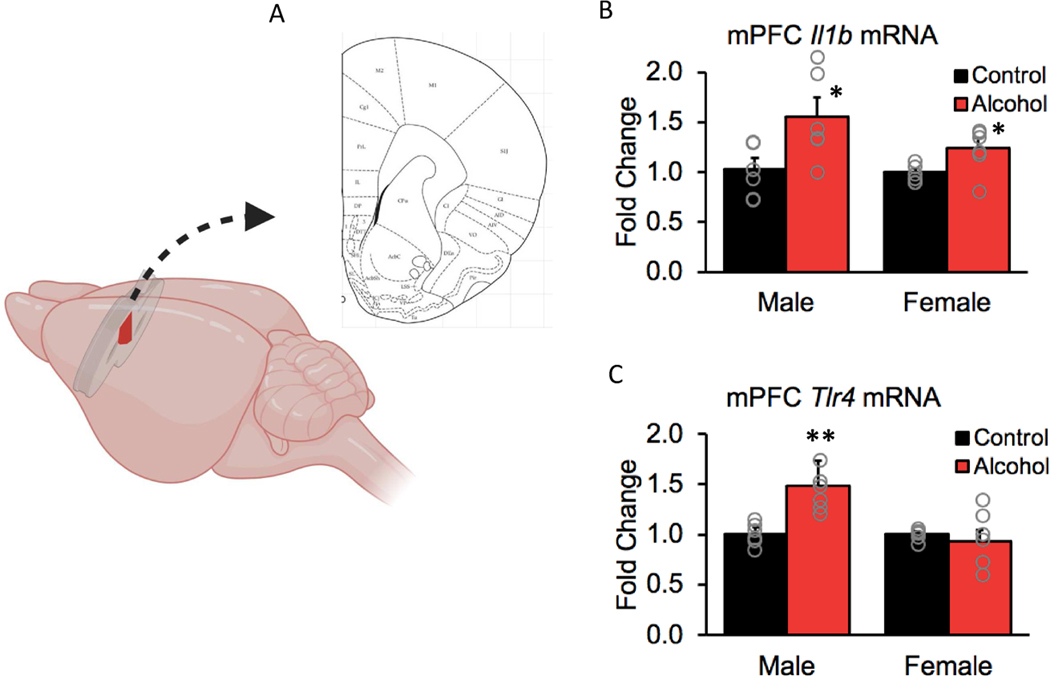
To investigate whether voluntary alcohol intake during adolescence leads to neuroinflammation in the mPFC, we examined gene expression of pro-inflammatory factors in this region (Fig 5A). Voluntary binge drinking increased expression of Il1b mRNA in both males and females (Fig 5B; F(1,19) = 15.46, p< 0.001). Il1b encodes for a cytokine involved in inflammatory responses and it has been shown to have a role in CNS repair, potentially through its effects on oligodendrocyte cell death and remyelination (Mason et al., 2001; Takahashi et al., 2003).
Figure 5. Voluntary binge drinking increased gene expression of pro-inflammatory factors more robustly in males than in females in the mPFC.
(A) Schematic showing the dissected portion of the mPFC used for qPCR analysis. (B) Alcohol drinking increased expression of the gene that encodes for IL1b, a pro-inflammatory cytokine, in both males and females (p < 0.001). (C) Adolescent drinking also increased expression of Tlr4 gene, which encodes for the receptor that activates the pro-inflammatory cascade but this effect was only observed in males (p < 0.01). Data are expressed as mean ± SEM (n = 5–6 rats for each sex and treatment group).
Binge drinking during adolescence also increased expression of the Tlr4 gene, which encodes for the receptor that activates the pro-inflammatory cascade (main effect of treatment, F(1,20) = 6.83, p < 0.05, Fig 5C). There was also a main effect of sex (F(1,20) = 9.85, p < 0.01) and a sex by treatment interaction (F(1,20) = 9.52, p < 0.01), with post-hoc analyses indicating that Tlr4 mRNA levels were increased by alcohol specifically in males (p = 0.003). TLR4 is one of the receptors that activates a pro-inflammatory cascade and it is also a key receptor in alcohol-induced myelin damage; consequently, upregulation of this receptor could render the system more vulnerable to subsequent insults (Alfonso-Loeches et al., 2012).
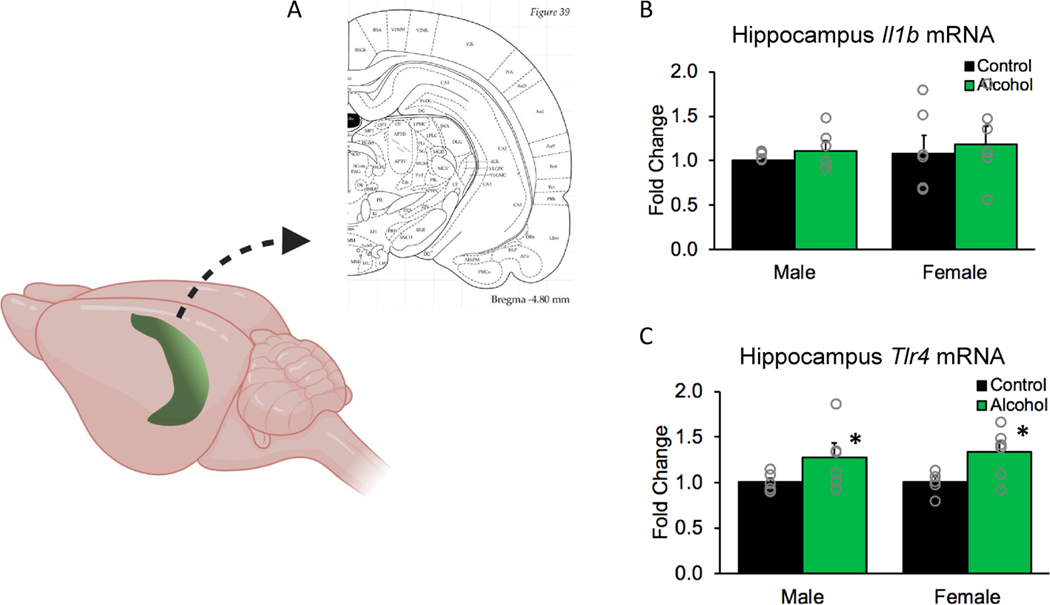
Given the high sensitivity of the hippocampus to alcohol-induced inflammation (Boschen et al., 2016; Drew et al., 2015; Vetreno et al., 2018), we also examined mRNA expression of these pro-inflammatory factors in this region (Fig 6A). Adolescent binge drinking during adolescence did not have an effect on Il1b gene expression in males or females (no effect of sex or treatment, all ps > 0.05, Fig 6B). However, Tlr4 was upregulated in the hippocampus of both sexes (main effect of treatment, F(1,19) = 9.32, p< 0.01, Fig 6C).
Figure 6. Binge drinking increased Tlr4 gene expression in the hippocampus of both males and females.
(A) Schematic showing the dissected portion of the hippocampus used for qPCR analysis. (B) Alcohol drinking did not change Il1b gene expression in the hippocampus of either males or females (p > 0.05). (C) Alcohol drinking increased expression of Tlr4 gene in both sexes (p < 0.01). Data are expressed as mean ± SEM (n = 5–6 rats for each sex and treatment group).
We next examined whether the variability in gene expression in some of our results could be explained by the amount of alcohol consumption. Table II shows the results of the Pearson correlation analyses between gene expression and cumulative alcohol drinking or cumulative glucose drinking for control animals. We found that Tlr4 was negatively correlated with alcohol drinking in the mPFC of females, suggesting alcohol might be having the opposite effect than in males (which had a significant increase in Tlr4 after alcohol exposure). In the hippocampus, Il1b was positively correlated with alcohol drinking in males. No significant correlations were found between other genes and alcohol or with cumulative glucose intake in any of the control groups.
Table II.
Results of regression analyses on gene expression vs. cumulative alcohol drinking
| Brain region | Sex | Treatment | Gene | R-squared values | p-values |
|---|---|---|---|---|---|
| mPFC | Males | Control | Tlr4 | 0.083 | 0.58 |
| Il1b | 0.217 | 0.35 | |||
| Il33 | 0.104 | 0.53 | |||
| Mbp | 0.206 | 0.37 | |||
| Alcohol | Tlr4 | 0.015 | 0.82 | ||
| Il1b | 0.123 | 0.50 | |||
| Il33 | 0.013 | 0.83 | |||
| Mbp | 0.098 | 0.55 | |||
| Females | Control | Tlr4 | 0.030 | 0.74 | |
| Il1b | 0.258 | 0.30 | |||
| Il33 | 0.039 | 0.75 | |||
| Mbp | 0.037 | 0.72 | |||
| Alcohol | Tlr4* (−) | 0.764 | 0.02 * | ||
| Il1b | 0.090 | 0.62 | |||
| Il33 | 0.037 | 0.71 | |||
| Mbp | 0.005 | 0.90 | |||
| Hippocampus | Male | Control | Tlr4 | 0.123 | 0.50 |
| Il1b | 0.039 | 0.71 | |||
| Il33 | 0.075 | 0.66 | |||
| Mbp | 0.103 | 0.53 | |||
| Alcohol | Tlr4 | 0.059 | 0.64 | ||
| Il1b* (+) | 0.878 | 0.02 * | |||
| Il33 | 0.106 | 0.53 | |||
| Mbp | 0.001 | 0.97 | |||
| Female | Control | Tlr4 | 0.020 | 0.82 | |
| Il1b | 0.209 | 0.36 | |||
| Il33 | 0.414 | 0.24 | |||
| Mbp | 0.222 | 0.35 | |||
| Alcohol | Tlr4 | 0.121 | 0.50 | ||
| Il1b | 0.026 | 0.76 | |||
| Il33 | 0.401 | 0.25 | |||
| Mbp | 0.188 | 0.39 |
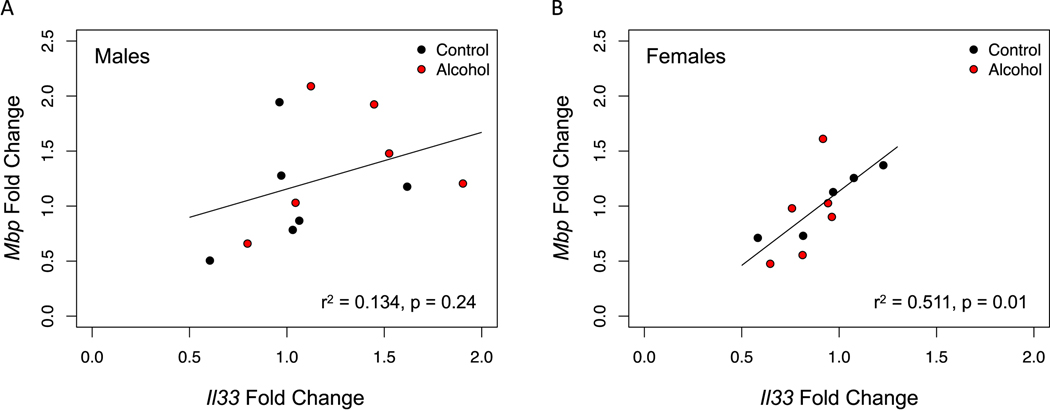
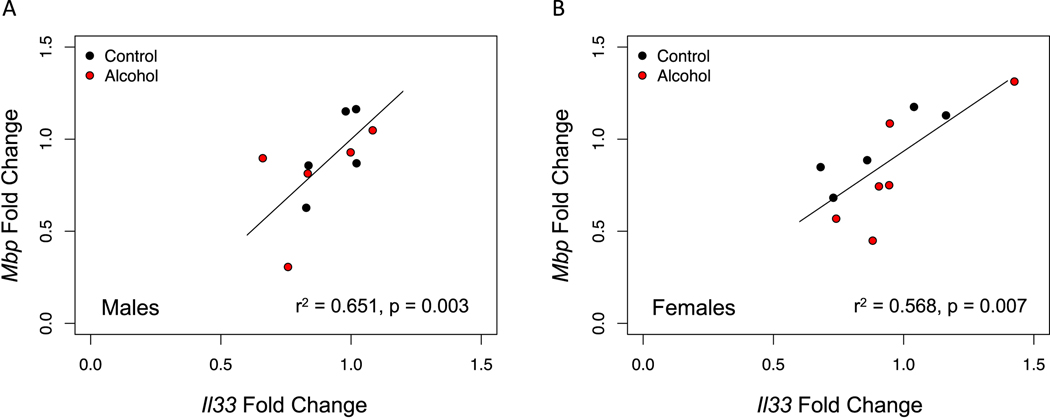
Previous studies indicate that alcohol drinking reduces myelin density in the mPFC (Tavares et al., 2019; Vargas et al., 2014), which could be due to the inflammatory response induced by alcohol. Herein, we tested whether alcohol drinking resulted in decreased expression of the myelin gene Mbp. Moreover, we measured gene expression of Il33, an alarmin that is expressed throughout development and has a role in the immune response and myelin repair after injury. While Il33 is not expressed in oligodendrocytes in early postnatal development, they have been shown to express it in adulthood (Gadani et al., 2015; Vainchtein et al., 2018). Thus, we examined the relationship between Il33 and Mbp, as this could be an indicator of oligodendrocyte maturation. While we did not observe an effect of alcohol in either one of these genes (all ps > 0.05, data not shown), we found that Il33 expression correlated positively with Mbp expression in the PFC of females (Fig 7B), as well as in the hippocampus of both males and females (Fig 8A, B). We did not observe the same correlation in the PFC of males, which is consistent with this region being less developed in males at this time (Fig 7A).
Figure 7. Mbp gene expression correlates with Il33 gene expression in the mPFC of females, regardless of alcohol drinking.
We examined the correlation between these two genes as a proxy for oligodendrocyte maturation. (A) There was no correlation between Mbp and Il33 in the mPFC of males (p=0.24). (B) In contrast, there was a strong correlation between Mbp and Il33 in the mPFC of females (p=0.01).
Figure 8. Mbp gene expression correlates with Il33 in the hippocampus of both males and females.
We examined the correlation between these genes in the hippocampus as a proxy of oligodendrocyte maturation and found that there was a strong correlation in both males (A, p=0.003) and females (B, p=0.007).
4. Discussion
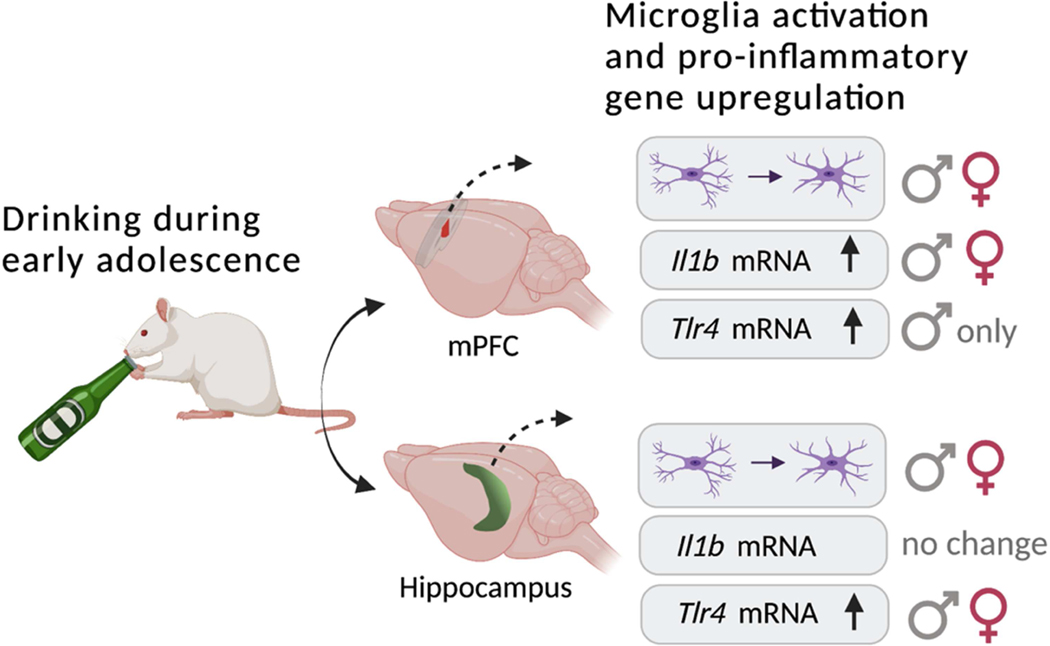
Neural circuits in the frontal lobes go through maturational processes such as axonal myelination during adolescence that are essential for cognitive function and behavioral control in adulthood (Giedd, 2004; Lebel et al., 2012; McDougall et al., 2018; Mengler et al., 2014). Thus, alcohol misuse at this young age may interfere with these neural developmental processes to have long-term effects on brain function and behavior. Previous research demonstrates prefrontal myelin deficits in rats following binge drinking early in adolescence, and these effects were more pronounced in males (Tavares et al., 2019; Vargas et al., 2014; Wolstenholme et al., 2017). The main objective of the present study was to examine whether low to moderate amounts of voluntary drinking during two weeks in adolescence were sufficient to induce an inflammatory response in in the mPFC, as well as in the hippocampus, as this region is extremely sensitive to alcohol even after adolescent maturation (Richardson et al., 2009; Vetreno et al., 2018). Our main findings are summarized in Fig 9. Alcohol caused a shift in the microglial cell population to an “alert” state in both the mPFC and hippocampus. Pro-inflammatory gene expression was upregulated in these structures as well, but the specific transcriptional changes varied with sex. Alcohol increased expression of Tlr4 mRNA in the mPFC and hippocampus in males, whereas in females Tlr4 mRNA was elevated only in the hippocampus. Alcohol intake was comparable in male and female rats during this early adolescent period, similar to our previous studies and expected based on the timing of when sex differences in drinking first emerge (Karanikas et al., 2013; Lancaster et al., 1996; Tavares et al., 2019). Thus, differences in alcohol exposure do not account for sex differences in gene expression. Patterns of change in Il1b mRNA were comparable in both sexes, but this upregulation in transcription was only evident in the mPFC. Overall, these results illustrate the impact even low-to-moderate alcohol drinking can have on the developing brain and how this differs with sex.
Figure 9. Summary of main findings.
Drinking alcohol early in adolescence (PD28–42) elicited a number of changes in frontolimbic structures in male and female rats. Alcohol caused a shift in the microglial cell population to an “alert” state in both the mPFC and hippocampus. Pro-inflammatory gene expression was upregulated in these structures as well, but the specific transcriptional changes varied with sex. In males, alcohol increased expression of Tlr4 mRNA in both the PFC and hippocampus, whereas in females, Tlr4 mRNA was upregulated only in the hippocampus. Patterns of change in Il1b mRNA were comparable in both sexes, but this upregulation in transcription was only evident in the mPFC. Overall, these results illustrate the impact even low-to-moderate alcohol drinking can have on the developing brain, especially in adolescent males.
Microglial cell populations are sensitive to low-to-moderate levels of alcohol exposure in the adolescent brain.
The current study replicates and extends previous reports showing that alcohol activates microglia in frontolimbic regions of the brain in adolescent male rodents (McClain et al., 2011; Walter et al., 2017). Four-days of intragastric exposure to high doses of alcohol elicits partial activation of microglia in the hippocampus of adolescent male rats, and this effect persists into adulthood (McClain et al., 2011). Intermittent alcohol exposure via intragastric administration for a longer period of time during adolescence activates microglia (Walter et al., 2017) and upregulates pro-inflammatory factors (Vetreno and Crews, 2012) in the PFC of males later in adulthood. The alcohol exposure method used in those studies was an intermittent dosing regimen in which 5 g/kg of alcohol is administered intragastrically on a two-day on/two-day off schedule from PD25 to PD55 (Vetreno and Crews, 2012). In the present study, we showed that lower doses of alcohol consumed during the first two weeks of adolescence activates microglia in the PFC as well as the hippocampus of both males and females.
Sex differences in Tlr4 gene expression after alcohol
Most of the previous investigations of alcohol-induced inflammation focused exclusively on males or exclusively female animals (Coleman et al., 2017; Crews et al., 2013, 2000; Fernandez-Lizarbe et al., 2009; Ibáñez et al., 2019; Pascual et al., 2007; Qin et al., 2008; Vetreno and Crews, 2012). One study that examined both sexes found that when adult female mice were exposed to chronic high alcohol for 5 months (10%v/v alcohol was the only source of liquid in the home cage), they exhibited a more robust neuroinflammatory and neurotoxic response compared to adult male mice (Alfonso-Loeches et al., 2013). This same alcohol treatment has also been shown to produce significant disarrangements of myelin sheaths and reductions in myelin proteins in the cortex of females (Alfonso-Loeches et al., 2012). Importantly, Tlr4 knock out female mice had minimal myelin damage after 5 months of drinking and their inflammatory responses were significantly lower compared to wildtype mice, indicating that both of these effects are mediated by these receptors.
Sensitivity of prefrontal myelin to alcohol drinking in adolescent males: potential role for TLR4
Previous studies from our lab have confirmed that adolescent binge drinking can alter myelinated axons at the microstructural level in the dorsal mPFC of both males and females. Other studies have shown that alcohol exposure during this time period results in decreased myelin proteins that extends to all of the prefrontal cortex, and this is accompanied by increased gene expression of pro-inflammatory cytokines and toll-like receptors in the same region (Pascual et al., 2014). In the present study, we found that alcohol upregulates Il1b gene in the mPFC of both males and females. This gene encodes for a pro-inflammatory cytokine that has been shown to augment the inflammatory response, which is important for subsequent repair (Mason et al., 2001). We also found that Tlr4 gene is upregulated in the mPFC of males, but not females. TLR4 plays an important role in alcohol-induced inflammation (Alfonso-Loeches et al., 2010) and its negative effects on myelin (Alfonso-Loeches et al., 2012), thus this specific sex difference in mPFC gene expression could be related to the heightened vulnerability of prefrontal myelin to alcohol in adolescent males (Tavares et al., 2019). However, given the dynamic nature of gene expression changes, it will be important to determine if this gene expression increase corresponds with increased protein levels. For example, we did not detect any changes in Mbp gene expression in the current study, however, we have unpublished data confirming that MBP protein levels are decreased in the mPFC in male rats after alcohol drinking. Something important to consider is that the PFC is undergoing myelination at that time and that the variability that is reflected on the data could be explained by the dynamic nature of myelination, which could make it difficult to detect effects of alcohol.
Maturational status of the oligodendrocyte lineage
IL-33 is an alarmin that has been shown to play a role in the immune response to promote recovery after CNS injury (Gadani et al). Aside from its immune role, IL-33 derived from astrocytes is also important for the maturation of circuits in early postnatal development (Vainchtein et al). While IL-33 is only expressed in astrocytes during this time period, somewhere in between early postnatal development and adulthood, oligodendrocytes also begin to express IL-33, and this protein has been shown to have a role in myelination and myelin repair after injury (Natarajan et al). We found that Il33 gene is correlated with myelin gene expression in the hippocampus in both sexes, and in the PFC of females only, suggesting that Il33 is expressed in oligodendrocytes in these regions. We did not observe the same relationship in the PFC of males, which could perhaps be explained by oligodendrocytes being in a less mature stage when compared to females and to other brain regions. Maturational status could contribute to differences in inflammatory cascade induced by alcohol in the PFC. Two weeks of binge-like intraperitoneal injections of alcohol increases pro-inflammatory cytokine levels and expression of toll-like receptors and decreases MBP protein in adolescent rats (Pascual et al., 2014). Adults are not affected by this same dosing regimen, suggesting that maturation provides protection against alcohol-induced neuroinflammation and myelin deficits (Pascual et al., 2014). Herein we show that two weeks alcohol self-administration is enough to upregulate Tlr4 gene expression in male, but not female adolescent rats in the mPFC and in both sexes in the hippocampus. Our results from this study together with our previous myelin studies suggest that the male PFC may be lagging behind developmentally when compared to the female PFC, which could be contributing to a higher sensitivity to alcohol-induced inflammation.
Limitations and future directions
Several limitations of the present study should be considered, especially in the context of sex differences in mechanisms of alcohol-induced inflammation. First, there are a number of other markers of inflammation that could be used to characterize microglia phenotype and determine which stage of the inflammatory process these cells are in (Marshall et al., 2013; Raivich et al., 1999). Additionally, quantifying gene expression of pro-inflammatory cytokines, like Il1b in this case, signifies that inflammatory cascades are being initiated; however, it does not provide information of actual cytokine production and release, which could directly impact myelin. The same can be said for the quantity of receptor, as here we show upregulation of the gene that encodes for TLR4. Thus, it will be important to examine expression of these pro-inflammatory factors at the protein level to determine the potential impact they can have. Second, other cell types might be contributing to alcohol-induced inflammation, which might be important to consider as a potential source of sex differences. Alcohol is known to initiate an inflammatory cascade in astrocytes directly via TLR4 and the receptor for IL1b (Blanco et al., 2005), contributing to the production and release of pro-inflammatory factors as well. Thus, assessing the involvement of astrocytes could provide additional insight into mechanisms of inflammation induced by our voluntary drinking model. It will also be important to consider pubertal maturation and the involvement of gonadal hormones in alcohol-induced inflammation, as estradiol has been shown to have anti-inflammatory properties (Vegeto et al., 2003). In our voluntary drinking model, females reach puberty towards the beginning of the drinking period, which means they already have high levels of circulating gonadal hormones when exposed to alcohol. In contrast, males do not reach puberty until the end of the drinking period (Tavares et al., 2019). It is therefore possible that higher levels of circulating gonadal hormones in females could be protecting them against the full effects of alcohol. Future studies could address the role of ovarian hormones in protecting females against alcohol-induced neuroinflammation during adolescence. One final consideration is that sex differences in alcohol metabolism and/or its clearance from the body could have contributed to differences in inflammatory gene response in the brain. Without assessing blood alcohol levels, we cannot rule this hypothesis out entirely. However, it should be noted that this is unlikely because while adult male and female rodents appear to have differences in alcohol clearance, this does not appear to be the case in adolescent animals (Macht et al., 2020; Varlinskaya and Spear, 2004).
Conclusions
The current study explored whether sex differences in alcohol-induced inflammatory mechanisms produced by a voluntary drinking model could help explain higher sensitivity to myelin deficits in adolescent males. Here we show that two weeks of voluntary drinking during early adolescence activated microglial cells and upregulated the expression of neuroinflammatory-related genes within the mPFC and hippocampus. However, within the mPFC, gene expression of TLR4 was only upregulated by alcohol in males. Given the role gonadal hormones play in attenuating neuroinflammation, the advanced pubertal status of females may have provided a level of protection for myelinated axons in the mPFC. This possibility remains to be tested. Nevertheless, the current findings highlight the vulnerability of frontolimbic brain regions to alcohol drinking and provide insight into a potential mechanism underlying sex differences in these effects.
Highlights.
Adolescent alcohol drinking activated microglia in male and female brains
Alcohol increased the gene expression of pro-inflammatory factors in both sexes
Toll-like receptor 4 gene increased in the medial prefrontal cortex of males only
Male prefrontal cortex may be more vulnerable to alcohol-induced inflammation
Sex differences in alcohol-induced inflammation may be due to maturational state
Acknowledgements
The authors thank Lynn Bengston for her assistance with the histological experiments, Aimee Lin for her help with the qPCR primer research and design, Dr. Samuel Hazen for lending his lab space and equipment for the qPCR experiments, and Dr. Said Akli and Annabelle Flores Bonilla for their feedback throughout the preparation of this manuscript. We performed the confocal analyses in the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, University of Massachusetts Amherst with support from the Massachusetts Life Science Center, and we thank the Director, Dr. James Chambers for his support and guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M, 2013. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 10.1016/j.neuropharm.2012.12.007 [DOI] [PubMed]
- Alfonso-Loeches S, Guerri C, 2011. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit. Rev. Clin. Lab. Sci. 48, 19–47. 10.3109/10408363.2011.580567 [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C, 2010. Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. J. Neurosci. 30, 8285–8295. 10.1523/jneurosci.0976-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gómez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C, 2012. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia 60, 948–964. 10.1002/glia.22327 [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C, 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311, 27–34. 10.1016/j.tox.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C, 2005. Involvement of TLR4/Type I IL-1 Receptor Signaling in the Induction of Inflammatory Mediators and Cell Death Induced by Ethanol in Cultured Astrocytes. J. Immunol. 175, 6893–6899. 10.4049/jimmunol.175.10.6893 [DOI] [PubMed] [Google Scholar]
- Boschen KE, Ruggiero MJ, Klintsova AY, 2016. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 324, 355–366. 10.1016/j.neuroscience.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SP, Pickering RP, 1992. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br. J. Addict. 87, 1199–1204. 10.1111/j.13600443.1992.tb02008.x [DOI] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Qin L, Crews FT, 2017. HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain. Behav. Immun. 1–17. 10.1016/j.bbi.2017.10.027 [DOI] [PMC free article] [PubMed]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ, 2000. Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats. Alcohol. Clin. Exp. Res. 24, 1712–1723. 10.1111/j.1530-0277.2000.tb01973.x [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J, 2013. High mobility group box 1/toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol. Psychiatry 73, 602–612. 10.1016/j.biopsych.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJM, 2015. Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 39, 445–454. 10.1111/acer.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C, 2009. Critical Role of TLR4 Response in the Activation of Microglia Induced by Ethanol. J. Immunol. 183, 4733–4744. 10.4049/jimmunol.0803590 [DOI] [PubMed] [Google Scholar]
- Flores-Bonilla A, Richardson HN, 2020. Sex Differences in the Neurobiology of Alcohol Use Disorder. Alcohol Res. 40, 04. 10.35946/arcr.v40.2.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J, 2015. The Glia-Derived Alarmin IL-33 Orchestrates the Immune Response and Promotes Recovery following CNS Injury. Neuron 85, 703–709. 10.1016/j.neuron.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, 2004. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 1021, 77–85. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN, 2012. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One 7. 10.1371/journal.pone.0031466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day T. a., Walker FR, 2012. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 22, 1442–1454. 10.1093/cercor/bhr229 [DOI] [PubMed] [Google Scholar]
- Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M, 2019. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J. Neuroinflammation 16, 1–14. 10.1186/s12974-019-1529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, Richardson HN, 2013. Adolescent drinking targets corticotropinreleasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience 249, 98–105. 10.1016/j.neuroscience.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A, 2011. Physiology of microglia. Physiol. Rev. 91, 461–553. 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB, 1996. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague-Dawley rats. Alcohol. Clin. Exp. Res. 20, 1043–1049. 10.1111/j.1530-0277.1996.tb01945.x [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS, 2008. LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151. 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Macht V, Elchert N, Crews F, 2020. Adolescent Alcohol Exposure Produces Protracted Cognitive-Behavioral Impairments in Adult Male and Female Rats. Brain Sci. 10, 785. 10.3390/brainsci10110785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain J. a., Kelso ML, Hopkins DM, Pauly JR, Nixon K, 2013. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 54, 239–251. 10.1016/j.nbd.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK, 2001. Interleukin-1β promotes repair of the CNS. J. Neurosci. 21, 7046–7052. 10.1523/jneurosci.21-1807046.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain J. a., Morris S. a., Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K, 2011. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain. Behav. Immun. 25, S120–S128. 10.1016/j.bbi.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S, Vargas Riad W, Silva-Gotay A, Tavares ER, Harpalani D, Li G-L, Richardson HN, 2018. Myelination of axons corresponds with faster transmission speed in the prefrontal cortex of developing male rats. Eneuro 5, ENEURO.0203-18.2018. 10.1523/ENEURO.0203-18.2018 [DOI] [PMC free article] [PubMed]
- Mengler L, Khmelinskii A, Diedenhofen M, Po C, Staring M, Lelieveldt BPF, Hoehn M, 2014. Brain maturation of the adolescent rat cortex and striatum: Changes in volume and myelination. Neuroimage 84, 35–44. 10.1016/j.neuroimage.2013.08.034 [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodríguez-Arias M, Miñarro J, Guerri C, 2015. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain. Behav. Immun. 45, 233–244. 10.1016/j.bbi.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Nickel M, Gu C, 2018. Regulation of Central Nervous System Myelination in Higher Brain Functions. Neural Plast. 2018, 1–12. 10.1155/2018/6436453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C, 2007. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 25, 541–550. 10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Miñarro J, Guerri C, 2014. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: A review with reference to human adolescent drinking. Alcohol Alcohol. 49, 187–192. 10.1093/alcalc/agt164 [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN, 2013. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain. Behav. Immun. 30, 88–94. 10.1016/j.bbi.2013.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT, 2008. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 1–17. 10.1186/1742-2094-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A language and environment for statistical computing. http://www.R-project.org/. [WWW Document]. R Found. Stat. Comput. Vienna, Austria. [Google Scholar]
- Raivich G, Bohatschek M, Kloss CUA, Werner A, Jones LL, Kreutzberg GW, 1999. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res. Rev. 10.1016/S0165-0173(99)00007-7 [DOI] [PubMed]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD, 2009. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol. Dis. 36, 1–10. 10.1016/j.nbd.2009.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW, 2003. Interleukin-1β promotes oligodendrocyte death through glutamate excitotoxicity. Ann. Neurol. 53, 588–595. 10.1002/ana.10519 [DOI] [PubMed] [Google Scholar]
- Tavares Silva-Gotay, Riad Bengston, Richardson, 2019. Sex differences in the effect of alcohol drinking on myelinated axons in the anterior cingulate cortex of adolescent rats. Brain Sci. 9, 167. 10.3390/brainsci9070167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV, 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science (80-. ). 359, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN, 2014. Alcohol Binge Drinking during Adolescence or Dependence during Adulthood Reduces Prefrontal Myelin in Male Rats. J. Neurosci. 34, 14777–14782. 10.1523/JNEUROSCI.3189-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, 2004. Acute Ethanol Withdrawal (Hangover) and Social Behavior in Adolescent and Adult Male and Female Sprague-Dawley Rats. Alcohol. Clin. Exp. Res. 28, 40–50. 10.1097/01.ALC.0000108655.51087.DF [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A, 2003. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. U. S. A. 100, 9614–9. 10.1073/pnas.1531957100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT, 2012. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience 226, 475–488. 10.1016/j.neuroscience.2012.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Lawrimore CJ, Rowsey PJ, Crews FT, 2018. Persistent adult neuroimmune activation and loss of hippocampal neurogenesis following adolescent ethanol exposure: Blockade by exercise and the anti-inflammatory drug indomethacin. Front. Neurosci. 12, 1–18. 10.3389/fnins.2018.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Vetreno RP, Crews FT, 2017. Alcohol and Stress Activation of Microglia and Neurons: Brain Regional Effects. Alcohol. Clin. Exp. Res. 41, 2066–2081. 10.1111/acer.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Mahmood T, Harris GM, Abbas S, Miles MF, 2017. Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC. Front. Mol. Neurosci. 10. 10.3389/fnmol.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Younis RM, Toma W, Damaj MI, 2020. Adolescent low-dose ethanol drinking in the dark increases ethanol intake later in life in C57BL/6J, but not DBA/2J mice. Alcohol 89, 85–91. 10.1016/j.alcohol.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]