Abstract
Restriction fragment length polymorphism analysis after PCR amplification (RFLP-PCR) of the 16S rRNA gene has been previously proposed as a rapid method to identify Aeromonas species. In the present study, the precision of RFLP-PCR was evaluated with 62 Aeromonas reference strains. The analysis revealed that Aeromonas veronii biovar sobria strains produce various patterns, possibly leading to its misidentification as an environmental species. For most other Aeromonas species little variation was noted. This study supports the usefulness of RFLP-PCR analysis to separate three clinically important species but also reveals possible misidentifications that necessitate further biochemical tests to validate the preliminary identification.
For the identification of bacteria, the speed and precision of molecular approaches are attractive to many investigators, and such approaches can be used as either complements or alternatives to biochemical identification (5, 8, 23). Because the biochemical identification of Aeromonas species can be time-consuming and difficult (1, 2), there has been an interest in finding molecular methods. Promising approaches with species-specific PCR primers have been reported (7, 9, 19). Recently, a more powerful method has been published that relies on the use of a single pair of primers to amplify the DNA encoding a variable region of the 16S rRNA gene (4). The amplified DNA is subsequently subjected to restriction fragment length polymorphism (RFLP) analysis. Using this RFLP-PCR approach, the authors were able to separate most Aeromonas species and to identify unknown isolates (4). The validity of the method needed to be evaluated with multiple reference strains (8), because only one reference strain was tested for each species (4) and very small differences have been reported in the sequence of the amplified 16S rRNA gene (20).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study were identified to the species level by DNA-DNA hybridization by other groups (Table 1). The Aeromonas strains were grown on sheep blood agar at 30°C (11).
TABLE 1.
Aeromonas reference strains used in this study
| Species | Straina |
|---|---|
| A. hydrophila | A 82, A 162, AER 4, AER 19, ATCC 7966T |
| A. bestiarum | A 307, A 310, ATCC 14715, NCMB 1134 |
| A. salmonicida | A 8, A 14, A 63, A 99, A 132, CDC0434-84 |
| A. caviae | A 12, A 26, AER 5, AER 51, ATCC 15468T |
| A. media | A 6, A 81, A 117, A 225, A 284, A 912, CDC0862-83 (5a), CDC0435-84 (5b) |
| A. eucrenophila | A 1651, A 1653, ATCC 23309T |
| A. sobria | A 915, CIP 7433T |
| A. veronii biovar sobria | A 64, A 132, A 155, A 916, AER 28, AER 39, CDC0437-84, LMG 13694 (A 27), LMG 13695 (A 28) |
| A. jandaei | A 950, AER 14, ATCC 49568T, NMR1-6 |
| A. veronii biovar veronii | AER 397, ATCC 35624T, LMG 16334 (ATCC 35625) |
| A. encheleia | ATCC 35941, ATCC 43946, LMG 13076, LMG 16328, LMG 16329, LMG 16330T |
| A. schubertii | ATCC 43700T, LMG 12655, LMG 12668 |
| A. trota | AER 66, AER 370, ATCC 49657T |
| A. allosaccharophila | LMG 14021, LMG 14059T |
Strains were identified by DNA-DNA hybridization by other investigators. A, Institute for Medical Microbiology, Zürich, Switzerland (except isolates A 310 [originally from Sakazaki], A 132 [A. salmonicida], and A 81 [originally from Kuijper]); AER, Department of Health Services, Berkeley, Calif.; LMG, Culture collection of the Laboratorium voor Microbiologie Gent, University of Gent, Ghent, Belgium; and NCMB, National Collection of Marine Bacteria, Aberdeen, Scotland. If necessary the original strain designation is given in parentheses.
RFLP-PCR conditions.
The PCR for the RFLP-PCR was done as described previously (11). The sequence of the forward primer was 5′-TCA TGG CTC AGA TTG AAC GCT-3′, and the reverse primer was 5′-CGG GGC TTT CAC ATC TAA CTT ATC-3′ (MWG-Biotech, Ebersberg, Germany). After overnight growth on a blood agar plate, a single colony was resuspended in 20 μl of saline solution and the sample was placed into a boiling water bath for 10 min. Ten volumes of TE (Tris-EDTA, pH 7.6 [24]) was added, and the solution containing the DNA was stored at −20°C until use. One microliter of the DNA solution was added to the PCR mixture containing 1.5 mM MgCl2 (Boehringer Mannheim, Mannheim, Germany). The amplification conditions were as follows: (i) 1 cycle consisting of 94°C for 5 min; (ii) 30 cycles consisting of 94°C for 1 min, 57°C for 1 min, and 72°C for 1 min; and (iii) 1 cycle consisting of 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min. For the restriction digests, 3.5 μl of the amplified sample was digested with 10 U of either AluI, CfoI, or MnlI (Boehringer Mannheim and New England Biolabs, Beverly, Mass.). The digested samples were analyzed by agarose gel electrophoresis (3% ResoPhor [Eurobio, Les Ullis Cedex B, France]; 10 V/cm).
Biochemical identification.
Representative strains from different RFLP patterns were selected to verify their identities by biochemical testing. The identification scheme was the same as we used in a previous study (11). It is a biochemical test scheme that combines the results from several studies (1, 10, 12, 14, 16). Most of the test results were obtained with the test strips API 20 NE and API 20 E (BioMérieux, Marcy l’Etoile, France) according to the manufacturer’s instructions. For Aeromonas encheleia, the API 20 E tests were done at 28°C. Gas production from glucose was determined by using triple sugar iron agar. Resistance to cephalothin and to ampicillin (30 and 10 μg per Sensi-Disc, respectively; Becton Dickinson Europe, Meylan Cedex, France) was determined on blood agar plates at 30°C. Lactose fermentation was tested on MacConkey lactose agar (Oxoid, Columbia, Md.) after 18 h at 30°C.
RESULTS AND DISCUSSION
Various RFLP-PCR patterns for Aeromonas veronii biovar sobria.
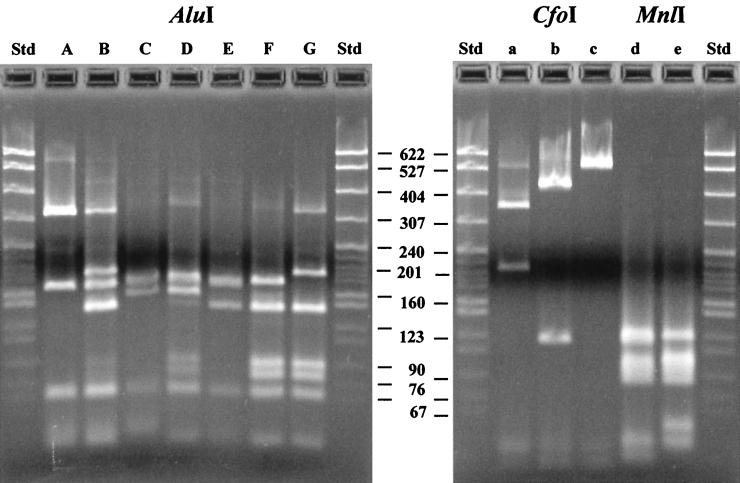
The ability to accurately identify Aeromonas species by RFLP-PCR analysis of the 16S rRNA gene was evaluated by comparing the RFLP patterns of 62 reference strains (Table 2 and Fig. 1). The digestion of the PCR product with the restriction endonuclease AluI revealed unexpected variation for A. veronii. A previous study reported no differences for the sequence of the 16S rRNA gene within A. veronii, and the same DNA sequence was reported for the two known biovars, A. veronii biovar sobria and A. veronii biovar veronii (20). While we observed the expected RFLP pattern (F) for both biovars, a second pattern (B/d) was detected for three A. veronii biovar sobria strains (CDC 0437-84, A 155, and A 916). This RFLP pattern corresponded to the pattern observed for Aeromonas media and Aeromonas allosaccharophila, possibly leading to a misidentification of A. veronii. Both of these species are considered to be environmental species, not human pathogens (15, 21).
TABLE 2.
Number of reference strains exhibiting the indicated RFLP patterns obtained from restriction digests of the amplified variable region of the 16S rRNA gene
| Patternb | No. of strains in speciesa
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ah | Ab | Asa | Ac | Am | Aeu | Aso | Avs | Aj | Avv | Aen | Asc | At | Aa | Totalc | |
| A/a | 5 | 1 | 6 | ||||||||||||
| A/b | 4 | 6 | 10 | ||||||||||||
| A/c | 4 | 4 | |||||||||||||
| B/c | 1 | 1 | |||||||||||||
| B/d | 6 | 3 | 2 | 11 | |||||||||||
| B/e | 3 | 3 | |||||||||||||
| C | 5 | 1 | 3 | 9 | |||||||||||
| D | 1 | 3 | 4 | ||||||||||||
| E/a | 1 | 1 | |||||||||||||
| E/c | 2 | 2 | |||||||||||||
| F | 4 | 3 | 1 | 8 | |||||||||||
| G | 3 | 3 | |||||||||||||
Ah, A. hydrophila; Ab, A. bestiarum; Asa, A. salmonicida; Ac, A. caviae; Am, A. media; Aeu, A. eucrenophila; Aso, A. sobria; Avs, A. veronii biovar sobria; Aj, A. jandaei; Avv, A. veronii biovar veronii; Aen, A. encheleia; Asc, A. schubertii; At, A. trota; Aa, A. allosaccharophila.
RFLP pattern. Capital letters refer to the pattern resulting from AluI digests; lowercase letters a, b, and c refer to CfoI digests; and d and e refer to MnlI digests. A strain with pattern combination A/a had pattern A when the PCR product was digested with AluI and pattern a when digested with CfoI.
Sum of the strains with a particular pattern.
FIG. 1.
RFLP patterns of the PCR-amplified gene encoding 16S rRNA from Aeromonas reference strains. Seven different RFLP patterns (A to G) were observed for AluI restriction digests. Three patterns (a to c) were observed for CfoI digests, and two patterns (d and e) were seen for MnlI. These patterns correspond to the patterns indicated in Table 2. The molecular weight standard (Std) was pBR322 DNA digested with MspI (New England Biolabs). The gels were photographed with Bio-print (version 6.21; Vilber Lourmat, Marne la Vallée, France), exported into Adobe Photoshop, and labeled in Macromedia Freehand 7.0.
Using different approaches, other studies have reported tremendous heterogeneity within A. veronii biovar sobria. The classic study by Popoff et al. (22) revealed that A. veronii biovar sobria was comprised of two distinct DNA-DNA hybridization groups (HG 8X and 8Y). Using a numerical classification approach, Kämpfer and Altwegg (18) described four biochemically distinct and very diverse clusters (clusters 1, 5, 6, and 8) of A. veronii strains. Similarly, Carnahan and Joseph (6) found that the type strain for HG 8X, CDC 0437-84 with RFLP pattern B/d, greatly differed phenotypically from other A. veronii biovar sobria strains. Multilocus enzyme analysis revealed four distinct phenons (groups of similar strains) for A. veronii biovar sobria (phenons 1, 3, 4, and 13), and the strains with RFLP pattern B/d belonged to phenon 3 (3). Studies analyzing the similarity of the genome from Aeromonas strains by comparing amplified fragment length polymorphism demonstrated that some A. veronii biovar sobria strains were more closely related to A. veronii biovar veronii while others (A 155 and A 916; RFLP pattern B/d) grouped with A. allosaccharophila (RFLP pattern Bld [13]). Finally, a mouse virulence model demonstrated a large variation in pathogenicity for A. veronii biovar sobria (17). Three of the A. veronii biovar sobria strains tested were included in this study. The least virulent A. veronii biovar sobria strain had pattern B/d (50% lethal dose, 9.0), while the other two strains had pattern F and were much more virulent (50% lethal dose, 6.6 and 7.2 [17]). When different criteria were analyzed, all of these studies revealed large variation within the species A. veronii, much larger than that observed for most other Aeromonas species. In several of these characteristics, ranging from biochemical tests to virulence, the A. veronii biovar sobria strains with RFLP pattern B/d differed from the strains with RFLP pattern F. These results suggest that variation in the 16S rRNA gene of A. veronii biovar sobria may reflect, and help to clarify, some of the phenotypic variation observed in this species.
RFLP-PCR patterns of other aeromonads.
In contrast to the different 16S rRNA patterns observed for A. veronii, most other Aeromonas species (10 species) exhibited a single RFLP pattern, reflecting the relatively conserved nature of the 16S rRNA gene in Aeromonas. In addition to A. veronii, only A. media (two of eight strains tested) and A. encheleia (two of six) isolates exhibited alternative RFLP patterns (Table 2). For A. media, these differences also reflected the phenotypic differences detected by Altwegg et al. (3), further suggesting that the variability in the sequence of the 16S rRNA gene may correlate with phenotypic differences.
Biochemical verification of species identification.
The additional RFLP patterns observed within a species could be due to actual variation of the 16S rRNA gene sequence or to false species identification. Thus, we attempted to verify the identities of strains with different RFLP patterns by biochemical tests. Of the five A. veronii biovar sobria strains verified, the test results for the two strains with RFLP patterns D and F were identical to those reported for A. veronii biovar sobria (1, 12, 14). For one strain (CDC 0437-84; RFLP pattern B/d) which had been previously reported to differ phenotypically from other Aeromonas veronii biovar sobria strains (e.g., negative for the Voges-Proskauer test [6]), we obtained the same test results that had been reported previously, thus confirming its identity. A second strain, also with RFLP pattern B/d, differed in the biochemical tests from CDC 0437-84 only by a very weak esculin reaction. In a previous study, we had isolated A. veronii biovar sobria strains from the medicinal leech, Hirudo medicinalis, that also had a positive esculin reaction (11). The fifth A. veronii biovar sobria strain, with RFLP pattern E/a, had unusual test results for A. veronii biovar sobria. It was esculin positive, Voges-Proskauer negative, and resistant to cephalothin. These results are in agreement with an identification as Aeromonas trota (RFLP pattern C). However, the strain was resistant to ampicillin, whereas A. trota is characteristically sensitive. The biochemical identification of this strain is uncertain, but the strain may be an unusual A. veronii. Thus four of the five strains tested are clearly A. veronii biovar sobria strains, suggesting that the variation in the RFLP-PCR patterns observed is due to differences in the sequences of the 16S rRNA genes.
The biochemical tests confirmed the identification of the three A. media strains (patterns A, B, and C [12, 16]). The two A. encheleia strains (patterns A and B) had test results identical to those reported previously except that they were negative for acid production from sucrose (14, 21). However in the original description one of the four strains examined was also negative for this test (21), suggesting that these strains are A. encheleia. One strain, Aeromonas jandaei (RFLP pattern A/a), was excluded from the study because it was biochemically similar to Aeromonas hydrophila (RFLP pattern A/a). The 10 strains that were verified included 7 strains with unexpected RFLP patterns. These results suggest that the variation in RFLP patterns observed in our study are unlikely to be due to strain switching or incorrect identification.
RFLP-PCR analysis of aeromonads.
The additional RFLP patterns within species and the lack of detectable differences between other species clearly limit the use of RFLP-PCR for species identification in Aeromonas. However, the three most frequently encountered clinical specimens (15), A. hydrophila (pattern A), Aeromonas caviae (C), and A. veronii biovar sobria (B and F) could be separated in all cases by using only the restriction enzyme AluI. Thus, RFLP-PCR of the 16S rRNA gene allows a rapid, presumptive species identification. If one excludes the RFLP patterns from infrequent isolates (single strains differing from the usual pattern of a species), one subsequent digest with a single additional restriction enzyme allows unambiguous species identification for seven species (Table 2). An additional four species could be grouped into pairs according to RFLP patterns (Aeromonas bestiarum-Aeromonas salmonicida and A. caviae-A. trota). The initial study by Borell et al. used a similar RFLP-PCR identification scheme to identify numerous clinical isolates (4). These results suggest that RFLP-PCR of the 16S rRNA gene can be used as a tool to aid in initial Aeromonas species identification. However, verification of species identification with biochemical tests is still appropriate for clinical diagnosis in light of the differences reported in our study.
The results presented in this study demonstrate variation in the 16S rRNA gene of A. veronii biovar sobria and reveal possible false identifications by RFLP-PCR analysis of the 16S rRNA. While molecular approaches provide fast and apparently straightforward results, problems can arise if not enough reference strains are used in establishing a broad database to validate the methodology (8). Future studies, such as sequencing the 16S rRNA gene from strains belonging to the different phenons, may help to explain the phenotypic variation observed in A. veronii and allow for the separation of biovars that do not present a clinical problem.
ACKNOWLEDGMENTS
I thank K. Schopfer and M. Täuber for their encouragement to pursue this study. I am especially thankful to K. Mühlemann for helpful discussion and A. Carnahan and E. S. Mirkin for commenting on the manuscript. I am grateful to M. Altwegg, J. M. Janda, and R. Powell for sending Aeromonas reference strains and R. Troller for excellent technical assistance with the biochemical identification.
I thank the Institute for Medical Microbiology of the University of Berne for financial support.
REFERENCES
- 1.Abbott S L, Cheung W K W, Kroske-Bystrom S, Malekzadeh T, Janda J M. Identification of Aeromonas strains to the genospecies level in the clinical laboratory. J Clin Microbiol. 1992;30:1262–1266. doi: 10.1128/jcm.30.5.1262-1266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott S L, Seli L S, Catino M, Jr, Hartley M A, Janda J M. Misidentification of unusual Aeromonas species as members of the genus Vibrio: a continuing problem. J Clin Microbiol. 1998;36:1103–1104. doi: 10.1128/jcm.36.4.1103-1104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altwegg M, Reeves M W, Altwegg-Bissig R, Brenner D J. Multilocus enzyme analysis of the genus Aeromonas and its use for species identification. Zentbl Bakteriol. 1991;275:28–45. doi: 10.1016/s0934-8840(11)80765-6. [DOI] [PubMed] [Google Scholar]
- 4.Borrell N, Acinas S G, Figueras M J, Martinez-Murcia A J. Identification of Aeromonas clinical isolates by restriction fragment length polymorphism of PCR-amplified 16S rRNA genes. J Clin Microbiol. 1997;35:1671–1674. doi: 10.1128/jcm.35.7.1671-1674.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardarelli-Leite P, Blom K, Patton C M, Nicholson M A, Steigerwalt A G, Hunter S B, Brenner D J, Barrett T J, Swaminathan B. Rapid identification of Campylobacter species by restriction fragment length polymorphism analysis of a PCR-amplified fragment of the gene coding for 16S rRNA. J Clin Microbiol. 1996;34:62–67. doi: 10.1128/jcm.34.1.62-67.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnahan A M, Joseph S W. Systematic assessment of geographically and clinically diverse Aeromonads. Syst Appl Microbiol. 1993;16:72–84. [Google Scholar]
- 7.Cascón A, Anguita J, Hernanz C, Sánchez M, Fernández M, Naharro G. Identification of Aeromonas hydrophila hybridization group 1 by PCR assays. Appl Environ Microbiol. 1996;62:1167–1170. doi: 10.1128/aem.62.4.1167-1170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton R A, Sutton G, Hinkle P S, Jr, Bult C, Fields C. Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa. Int J Syst Bacteriol. 1995;45:595–599. doi: 10.1099/00207713-45-3-595. [DOI] [PubMed] [Google Scholar]
- 9.Dorsch M, Ashbolt N J, Cox P T, Goodman A E. Rapid identification of Aeromonas species using 16S rDNA targeted oligonucleotide primers: a molecular approach based on screening of environmental isolates. J Appl Bacteriol. 1994;77:722–726. doi: 10.1111/j.1365-2672.1994.tb02825.x. [DOI] [PubMed] [Google Scholar]
- 10.Esteve C, Gutiérrez M C, Ventosa A. Aeromonas encheleia sp. nov., isolated from European eels. Int J Syst Bacteriol. 1995;45:462–466. doi: 10.1099/00207713-45-3-462. [DOI] [PubMed] [Google Scholar]
- 11.Graf J. The symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech: a novel model for digestive tract associations. Infect Immun. 1999;67:1–7. doi: 10.1128/iai.67.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hänninen M-L, Siitonen A. Distribution of Aeromonas phenospecies and genospecies among strains isolated from water, foods or from human clinical samples. Epidemiol Infect. 1995;115:39–50. doi: 10.1017/s0950268800058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 14.Huys G, Kämpfer P, Altwegg M, Coopman R, Janssen P, Gillis M, Kersters K. Inclusion of Aeromonas DNA hybridization group 11 in Aeromonas encheleia and extended descriptions of the species Aeromonas eucrenophila and A. encheleia. Int J Syst Bacteriol. 1997;47:1157–1164. doi: 10.1099/00207713-47-4-1157. [DOI] [PubMed] [Google Scholar]
- 15.Janda J M, Abbott S L. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 16.Janda J M, Abbott S L, Khashe S, Kellogg G H, Shimada T. Further studies on biochemical characteristics and serologic properties of the genus Aeromonas. J Clin Microbiol. 1996;34:1930–1933. doi: 10.1128/jcm.34.8.1930-1933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janda J M, Kokka R P. The pathogenicity of Aeromonas strains relative to genospecies and phenospecies identification. FEMS Microbiol Lett. 1991;90:29–34. doi: 10.1111/j.1574-6968.1991.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 18.Kämpfer P, Altwegg M. Numerical classification and identification of Aeromonas genospecies. J Appl Bacteriol. 1992;72:341–351. doi: 10.1111/j.1365-2672.1992.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 19.Karlyshev A V, MacIntyre S. Study of the intergenic exeF-exeG region and its application as a simple preliminary test for Aeromonas spp. FEMS Microbiol Lett. 1996;137:37–44. doi: 10.1111/j.1574-6968.1996.tb08079.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Murcia A J, Benlloch S, Collins M D. Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16S ribosomal DNA sequencing: lack of congruence with results of DNA-DNA hybridizations. Int J Syst Bacteriol. 1992;42:412–421. doi: 10.1099/00207713-42-3-412. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Murcia A J, Esteve C, Garay E, Collins M D. Aeromonas allosaccharophila sp. nov., a new mesophilic member of the genus Aeromonas. FEMS Microbiol Lett. 1992;91:199–206. doi: 10.1016/0378-1097(92)90698-n. [DOI] [PubMed] [Google Scholar]
- 22.Popoff M Y, Coynaults C, Kiredjian M, Lemelin M. Polynucleotide sequence relatedness among motile Aeromonas species. Curr Microbiol. 1981;5:109–114. [Google Scholar]
- 23.Riggio M P, Lennon A. Rapid identification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, and Haemophilus paraphrophilus by restriction enzyme analysis of PCR-amplified 16S rRNA genes. J Clin Microbiol. 1997;35:1630–1632. doi: 10.1128/jcm.35.6.1630-1632.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]