Abstract
Aims
In response to the COVID-19 pandemic, guidelines on reduced fractionation for patients treated with curative-intent radiotherapy were published, aimed at reducing the number of hospital attendances and potential exposure of vulnerable patients to minimise the risk of COVID-19 infection. We describe the changes that took place in the management of patients with stage I–III lung cancer from April to October 2020.
Materials and methods
Lung Radiotherapy during the COVID-19 Pandemic (COVID-RT Lung) is a prospective multicentre UK cohort study. The inclusion criteria were: patients with stage I–III lung cancer referred for and/or treated with radical radiotherapy between 2nd April and 2nd October 2020. Patients who had had a change in their management and those who continued with standard management were included. Data on demographics, COVID-19 diagnosis, diagnostic work-up, radiotherapy and systemic treatment were collected and reported as counts and percentages. Patient characteristics associated with a change in treatment were analysed using multivariable binary logistic regression.
Results
In total, 1553 patients were included (median age 72 years, 49% female); 93 (12%) had a change to their diagnostic investigation and 528 (34%) had a change to their treatment from their centre's standard of care as a result of the COVID-19 pandemic. Age ≥70 years, male gender and stage III disease were associated with a change in treatment on multivariable analysis. Patients who had their treatment changed had a median of 15 fractions of radiotherapy compared with a median of 20 fractions in those who did not have their treatment changed. Low rates of COVID-19 infection were seen during or after radiotherapy, with only 21 patients (1.4%) developing the disease.
Conclusions
The COVID-19 pandemic resulted in changes to patient treatment in line with national recommendations. The main change was an increase in hypofractionation. Further work is ongoing to analyse the impact of these changes on patient outcomes.
Key words: COVID-19, lung cancer, radiotherapy, reduced fractionation
Introduction
At the outset of the coronavirus pandemic in early 2020, there was paucity of data about the risk of COVID-19 in patients with lung cancer. Initial reports suggested that patients with cancer had both a higher risk of COVID-19 and an increased incidence of intensive care unit admissions and death [1]. There were concerns that immunosuppression due to chemotherapy and thoracic radiotherapy [2] would increase the likelihood of severe COVID-19 infection in patients with lung cancer. Moreover, cigarette smoking and comorbidities, such as hypertension and chronic obstructive pulmonary disease, that are common in patients with lung cancer, increased the risk of hospital admission or death from COVID-19 [3]. Consequently, in the UK, patients undergoing radical radiotherapy for lung cancer were identified as clinically extremely vulnerable and advised to shield [4].
Radiotherapy is used in the primary treatment of 20–60% of patients with lung cancer [5]. It was therefore crucial to find ways to balance continued access of cancer patients to radiotherapy while minimising the risk of COVID-19 infection. To this end, at the start of the first wave of the COVID-19 pandemic in the UK, a group of clinical oncologists published guidelines based on current literature and practice, on how to safely reduce the number of fractions (and therefore hospital visits) when delivering curative-intent radiotherapy in patients with lung cancer [6].
Following the publication of the UK recommendations [6], it is now important to assess their impact on practice and on the clinical outcome of lung cancer patients considered for radiotherapy in the UK during the COVID-19 pandemic. Lung Radiotherapy during the COVID-19 Pandemic (COVID-RT Lung) is a UK-wide cohort study established to record and analyse changes in lung cancer management and outcomes, with a specific focus on radical radiotherapy treatment. The study aims to assess the effect of the COVID-19 pandemic on the diagnostic and treatment pathways for patients with lung cancer. Here we outline the initial results from COVID-RT Lung.
Materials and Methods
COVID-RT Lung is a national, multicentre prospective registry cohort study. Radiotherapy centres in the UK were sent a letter of invitation to participate in COVID-RT Lung. Interested centres then registered the project locally as a clinical audit. Local approval to enter anonymous patient data was obtained by each participating centre from their Caldicott Guardian.
Patient Cohort
Patients were eligible for inclusion in COVID-RT Lung if they had stage I–III lung cancer and were referred for curative-intent radiotherapy (biologically equivalent dose >50 Gy) at a participating centre between 2nd April and 2nd October 2020. Participating radiotherapy centres prospectively collected data from the patient records on all patients referred for and treated with radical radiotherapy during the inclusion period, whether or not their treatment was changed due to the COVID-19 pandemic. Radiotherapy centres were divided by UK region.
A patient was defined as having a change to their diagnostic investigations if standard investigations that would usually be carried out prior to radiotherapy at their treating centre were not undertaken as a result of the COVID-19 pandemic. A patient was defined as having a change to treatment if they had a different treatment to their centre's standard of care treatment, taking into account individual patient characteristics, such as performance status, and tumour characteristics, such as stage.
The following information was collected for each patient: age at the time of treatment; gender; histology; stage; baseline Eastern Cooperative Oncology Group Performance Status (PS) and comorbidities; radiotherapy dose and fractionation; dates of radiotherapy; chemotherapy or immunotherapy delivery. Stereotactic ablative body radiotherapy (SABR) was defined as radiotherapy delivered in ≤8 fractions of more than 6 Gy per fraction. Specific details on the chemotherapy drugs used were not collected. A systemic therapy dose reduction was defined as a reduction in the number of planned cycles of therapy and/or a reduction in the dose for any single cycle.
The presence of the following comorbidities was recorded: ischaemic heart disease; congestive heart failure; cardiac arrhythmia, hypertension; chronic obstructive pulmonary disease; chronic kidney disease; diabetes; stroke; dementia and any previous malignancy prior to the current lung cancer diagnosis. Other comorbidities were recorded as free text.
Data were collected on COVID-19 diagnosis. Patients were classified as having COVID-19 if they had a positive reverse transcriptase polymerase chain reaction (RT-PCR) nasopharyngeal swab or if they had a clinical diagnosis of COVID-19 in the absence of an RT-PCR swab.
In addition, the following data were collected if available: Rockwood clinical frailty scale; smoking history; administration of granulocyte colony stimulating factor (G-CSF) during treatment; neutrophil and lymphocyte count in the final week of radiotherapy.
Study data were collected and managed using the Research Electronic Data Capture (REDCap) cloud platform (nPhase Inc, CA, USA) [7] administered by the University of Manchester Clinical Trials Unit.
Statistical Analysis
Key baseline characteristics were summarised as categorical variables and reported as counts and percentages. Adjusted odds ratios and 95% confidence intervals for a change to diagnostic investigations and treatment from the centre's standard of care were estimated by multivariable binary logistic regression. Age was dichotomised at 70 years in line with the UK Government's shielding advice. The Rockwood frailty score was excluded from multivariable analysis as more than 50% of the data were missing. North West England was chosen as the base factor in regional analysis as it had the largest number of patients. The median number of radiotherapy fractions were compared using the Wilcoxon signed rank test. The statistical analysis was carried out in Rstudio version 3.6.3.
Results
Data on 1553 patients were available for analysis on 17 March 2021. The median age was 72 years (37–93 years), 762 (49%) were female. There were 906 patients (58.3%) with non-small cell lung cancer (NSCLC) and 482 (31%) had a radiological diagnosis of cancer. Only 167 patients (10.8%) had no comorbidities prior to their diagnosis of lung cancer and 624 patients (40.2%) had three or more comorbidities. The most common comorbidity was chronic obstructive pulmonary disease, recorded in 667 patients (42.9%). A list of participating radiotherapy centres and their region is given in Supplementary Table S1. Baseline characteristics are summarised in Table 1 .
Table 1.
Baseline characteristics stratified by change to treatment [n (%)]
| No change | Changed | Total | ||
|---|---|---|---|---|
| Total n (%) | 1025 (66.0) | 528 (34.0) | 1553 | |
| Age (years) | <70 | 405 (39.5) | 203 (38.4) | 608 (39.2) |
| ≥70 | 610 (59.5) | 325 (61.6) | 935 (60.2) | |
| Missing | 10 (1.0) | 0 (0.0) | 10 (0.6) | |
| Gender | Female | 524 (51.1) | 238 (45.1) | 762 (49.1) |
| Male | 495 (48.3) | 290 (54.9) | 785 (50.5) | |
| Missing | 6 (0.6) | 0 (0.0) | 6 (0.4) | |
| Performance status | 0 | 118 (11.5) | 96 (18.2) | 214 (13.8) |
| 1 | 509 (49.7) | 309 (58.5) | 818 (52.7) | |
| 2–3 | 390 (38.0) | 123 (23.3) | 513 (33.0) | |
| Missing | 8 (0.8) | 0 (0.0) | 8 (0.5) | |
| Clinical frailty scale | 1 | 18 (1.8) | 14 (2.7) | 32 (2.1) |
| 2 | 72 (7.0) | 59 (11.2) | 131 (8.4) | |
| 3 | 143 (14.0) | 115 (21.8) | 258 (16.6) | |
| 4 | 101 (9.9) | 62 (11.7) | 163 (10.5) | |
| 5 | 56 (5.5) | 22 (4.2) | 78 (5.0) | |
| 6 | 27 (2.6) | 10 (1.9) | 37 (2.4) | |
| 7 | 7 (0.7) | 1 (0.2) | 8 (0.5) | |
| Missing | 601 (58.6) | 245 (46.4) | 846 (54.5) | |
| Smoking status | Current smoker | 298 (29.1) | 148 (28.0) | 446 (28.7) |
| Ex-smoker | 594 (58.0) | 317 (60.0) | 911 (58.7) | |
| Never smoker | 29 (2.8) | 22 (4.2) | 51 (3.3) | |
| Missing | 104 (10.1) | 41 (7.8) | 145 (9.3) | |
| Histology | NSCLC | 576 (56.2) | 330 (62.5) | 906 (58.3) |
| SCLC | 87 (8.5) | 70 (13.3) | 157 (10.1) | |
| Radiological diagnosis | 354 (34.5) | 128 (24.2) | 482 (31.0) | |
| Missing | 8 (0.8) | 0 (0.0) | 8 (0.5) | |
| Stage | I | 473 (46.1) | 189 (35.8) | 662 (42.6) |
| II | 164 (16.0) | 71 (13.4) | 235 (15.1) | |
| III | 380 (37.1) | 265 (50.2) | 645 (41.5) | |
| Missing | 8 (0.8) | 3 (0.6) | 11 (0.7) | |
| Region | North West England | 166 (16.2) | 159 (30.1) | 325 (20.9) |
| Yorkshire & North East England | 161 (15.7) | 106 (20.1) | 267 (17.2) | |
| South East England | 151 (14.7) | 77 (14.6) | 228 (14.7) | |
| London | 46 (4.5) | 17 (3.2) | 63 (4.1) | |
| South West England | 50 (4.9) | 28 (5.3) | 78 (5.0) | |
| Midlands | 123 (12.0) | 49 (9.3) | 172 (11.1) | |
| Northern Ireland | 88 (8.6) | 30 (5.7) | 118 (7.6) | |
| Wales | 63 (6.1) | 42 (8.0) | 105 (6.8) | |
| Scotland | 177 (17.3) | 20 (3.8) | 197 (12.7) | |
| IHD | No IHD | 819 (79.9) | 439 (83.1) | 1258 (81.0) |
| IHD | 206 (20.1) | 89 (16.9) | 295 (19.0) | |
| CHF | No CHF | 964 (94.0) | 506 (95.8) | 1470 (94.7) |
| CHF | 61 (6.0) | 22 (4.2) | 83 (5.3) | |
| Cardiac arrhythmia | No arrhythmia | 912 (89.0) | 471 (89.2) | 1383 (89.1) |
| Arrhythmia | 113 (11.0) | 57 (10.8) | 170 (10.9) | |
| Hypertension | No hypertension | 660 (64.4) | 354 (67.0) | 1014 (65.3) |
| Hypertension | 365 (35.6) | 174 (33.0) | 539 (34.7) | |
| COPD | No COPD | 574 (56.0) | 312 (59.1) | 886 (57.1) |
| COPD | 451 (44.0) | 216 (40.9) | 667 (42.9) | |
| CKD | No CKD | 961 (93.8) | 507 (96.0) | 1468 (94.5) |
| CKD | 64 (6.2) | 21 (4.0) | 85 (5.5) | |
| Diabetes | No diabetes | 859 (83.8) | 449 (85.0) | 1308 (84.2) |
| Diabetes | 166 (16.2) | 79 (15.0) | 245 (15.8) | |
| Stroke/TIA | No stroke | 930 (90.7) | 493 (93.4) | 1423 (91.6) |
| Stroke | 95 (9.3) | 35 (6.6) | 130 (8.4) | |
| Dementia | No dementia | 1011 (98.6) | 522 (98.9) | 1533 (98.7) |
| Dementia | 14 (1.4) | 6 (1.1) | 20 (1.3) | |
| Previous malignancy | No previous malignancy | 772 (75.3) | 423 (80.1) | 1195 (76.9) |
| Previous malignancy | 253 (24.7) | 105 (19.9) | 358 (23.1) |
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; IHD, ischaemic heart disease; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; TIA, transient ischaemic attack.
One hundred and ninety-three patients (12%) had their diagnostic investigations affected by the pandemic (Table 2 ). The characteristics of patients who had their diagnostic investigations changed are listed in Supplementary Table S2. The most common change was not obtaining histology prior to treatment in 66 patients.
Table 2.
Changes to diagnostic investigations
| Change to diagnostic investigations | Patients n = 1553 |
|---|---|
| Histology not obtained | 66 (4.3%) |
| No nodal sampling | 38 (2.5%) |
| No pulmonary function tests | 29 (1.9%) |
| No brain imaging | 32 (2.1%) |
| No PET-CT or PET-CT out of date∗ | 50 (3.2%) |
| Delays in diagnosis | 11 (0.7%) |
PET-CT, positron emission tomography-computed tomography.
Defined by the local clinical teams.
Radiotherapy details were not recorded for 11 patients. In 33 patients (2.1%), a watch and wait approach was adopted, 26 of whom went on to have radiotherapy at a later date. Three patients (0.2%) had best supportive care instead of radical treatment and 26 patients referred for radical treatment had a palliative radiotherapy schedule. In patients with stage I–II lung cancer, 579 (64.5%) had SABR, 296 (33%) had fractionated curative-intent radiotherapy. Eight patients had single fraction SABR with 34 Gy. In patients with stage III lung cancer, 356 patients (55%) had sequential or concurrent chemoradiotherapy and 264 patients (40.9%) had curative-intent radiotherapy alone.
Changes to treatment due to the pandemic are shown in Table 3 . The most common change was to the centre's standard radiotherapy dose or fractionation. The median number of fractions of radiotherapy received by patients who had their treatment changed was significantly lower than those without a change to their standard radiotherapy (15 fractions versus 20 fractions, P < 0.001).
Table 3.
Changes made to patients' treatment according to lung cancer stage (information on stage was missing for four patients)
| Change to treatment | Stage I–II (n = 897) | Stage III (n = 645) |
|---|---|---|
| Any change | 260 (29%) | 265 (41.1%) |
| Change to radiotherapy dose/fractionation | 144 (16.1%) | 126 (19.5%) |
| Radiotherapy given instead of surgery | 85 (9.5%) | 18 (2.8%) |
| Chemotherapy dose reduced | 12 (1.3%) | 59 (6.8%) |
| Chemotherapy omitted | 9 (1%) | 69 (10.7%) |
| Immunotherapy dose reduced or omitted | 0 | 8 (1.2%) |
| Watch and wait | 31 (3.5%) | 2 (0.3%) |
| Best supportive care | 1 (0.1%) | 2 (0.3%) |
| Other | 2 (0.2%) | 4 (0.6%) |
A higher proportion of patients with small cell lung cancer (SCLC) had their treatment changed compared with patients with NSCLC (44.6% versus 36.4%). The median radiotherapy dose per fraction for patients with SCLC was 2.67 Gy to a total of 40 Gy, delivered once daily. This schedule was used with concurrent chemotherapy in 18 patients, with sequential chemotherapy in 65 patients and without chemotherapy in 10 patients. The schedule of 45 Gy in 30 fractions bi-daily with concurrent chemotherapy was delivered in 25 patients with SCLC.
The median radiotherapy dose per fraction for patients with NSCLC was 2.75 Gy to a total of 55 Gy, delivered with sequential chemotherapy in 142 patients, concurrent chemotherapy in 146 patients and without chemotherapy in 616 patients. In patients with a radiological diagnosis of lung cancer (assumed to be NSCLC by the multidisciplinary team), the median radiotherapy dose per fraction was 11 Gy to a total of 55 Gy; five patients with a radiological diagnosis had chemotherapy.
Five hundred and twenty-eight patients (34%) had their treatment changed from their centre's standard of care treatment due to the COVID-19 pandemic. The North West and Yorkshire/North East of England had the highest proportion of patients who had their treatment changed from their centre's standard of care. Multivariable analysis revealed that male gender, age ≥ 70 years and stage III lung cancer were associated with a change in treatment (Table 4 ). Patients of performance status 2–3 were less likely to have their treatment changed. A change in diagnostic investigations was associated with a radiological diagnosis of lung cancer, chronic kidney disease and treatment in Northern Ireland.
Table 4.
Adjusted odds ratio (aOR) of baseline factors with change to treatment and change to diagnostic investigations
| Change to treatment aOR (95% CI) | Change to investigations aOR (95% CI) | |
|---|---|---|
| Age (years) <70 versus ≥70 | 1.34 (1.03–1.74) | 0.95 (0.64–1.42) |
| Gender, female versus male | 1.36 (1.07–1.73) | 0.90 (0.63–1.29) |
| Performance status, versus 0 | ||
| 1 | 0.78 (0.55–1.12) | 1.00 (0.57–1.82) |
| 2–3 | 0.37 (0.25–0.56) | 0.72 (0.38–1.39) |
| Smoking status, versus current smoker | ||
| Ex-smoker | 0.96 (0.73–1.25) | 0.74 (0.50–1.10) |
| Never smoker | 1.51 (0.78–2.86) | 0.65 (0.20–1.77) |
| Histology, versus NSCLC | ||
| SCLC | 1.27 (0.86–1.89) | 0.97 (0.46–1.90) |
| Radiological diagnosis | 0.88 (0.64–1.20) | 3.83 (2.44–6.09) |
| Stage, versus stage I | ||
| Stage II | 0.95 (0.65–1.38) | 1.25 (0.73–2.10) |
| Stage III | 1.56 (1.15–2.13) | 1.42 (0.88–2.30) |
| Region, versus North West England | ||
| Yorkshire & North East England | 0.62 (0.43–0.90) | 0.81 (0.43–1.46) |
| South East England | 0.41 (0.27–0.61) | 0.34 (0.13–0.75) |
| London | 0.34 (0.18–0.63) | 0.29 (0.05–1.02) |
| South West England | 0.41 (0.23–0.71) | 1.62 (0.75–3.36) |
| Midlands | 0.41 (0.26–0.65) | 0.64 (0.29–1.31) |
| Northern Ireland | 0.33 (0.20–0.54) | 13.87 (8.05–24.47) |
| Wales | 0.62 (0.39–1.01) | 2.36 (1.22–4.48) |
| Scotland | 0.09 (0.05–0.14) | 0.44 (0.21–0.88) |
| Ischaemic heart disease, no versus yes | 0.94 (0.68–1.28) | 1.22 (0.78–1.89) |
| Congestive heart failure, no versus yes | 0.98 (0.54–1.74) | 0.54 (0.20–1.27) |
| Arrhythmia, no versus yes | 1.07 (0.71–1.60) | 1.04 (0.56–1.86) |
| Hypertension, no versus yes | 0.80 (0.62–1.03) | 1.15 (0.79–1.68) |
| COPD, no versus yes | 1.24 (0.96–1.60) | 1.29 (0.88–1.88) |
| Chronic kidney disease, no versus yes | 0.78 (0.43–1.36) | 2.13 (1.03–4.19) |
| Diabetes, no versus yes | 0.99 (0.71–1.39) | 1.08 (0.65–1.75) |
| Stroke, no versus yes | 0.83 (0.52–1.30) | 1.03 (0.54–1.87) |
| Dementia, no versus yes | 1.19 (0.39–3.27) | 0.91 (0.18–3.36) |
| Previous malignancy, no versus yes | 0.86 (0.64–1.15) | 1.12 (0.72–1.70) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
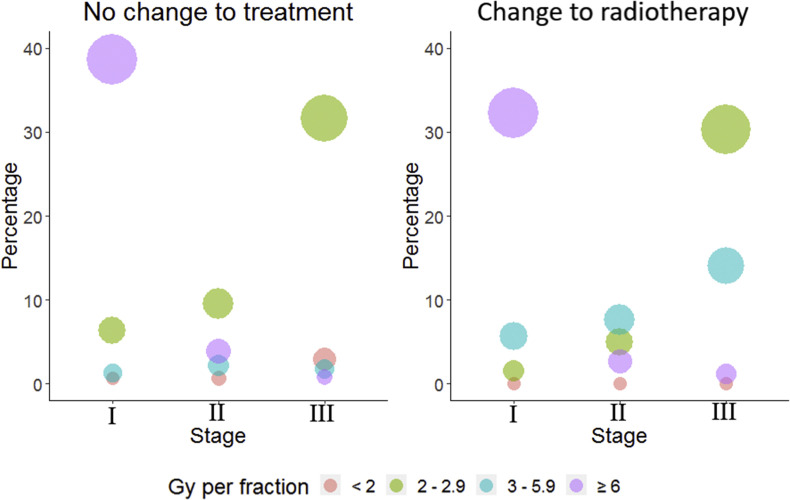
Figure 1 shows the changes in radiotherapy dose per fraction for patients who did and did not have their treatment changed. A higher proportion of the change to treatment group were treated with 3–5.9 Gy/fraction across all stages compared with the no change group (27.2% versus 5.1%). No patient in the change to treatment group received a radiotherapy schedule with <2 Gy/fraction.
Fig 1.
Bubble plot of radiotherapy dose per fraction by stage for patients who had standard of care treatment and those who had their treatment changed.
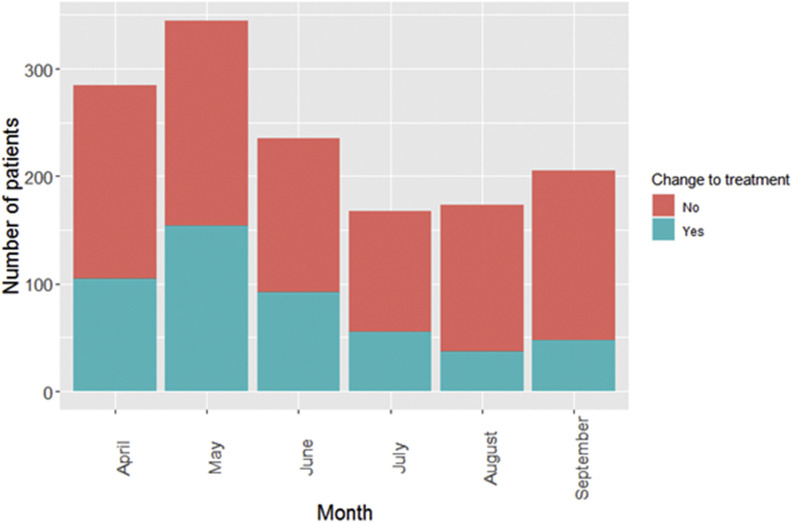
Figure 2 shows how the changes to treatment varied between April and October 2020. In April 2020, 105 of 180 (37%) patients had their treatment changed; in May 2020, 154 of 345 (45%) had their treatment changed. The total number of patients treated and the proportion of patients who had a change to their treatment decreased from June to August 2020.
Fig 2.
Monthly number of patients referred for radical radiotherapy for lung cancer and the number who had a change to their treatment from April to September 2020.
Lymphocyte Count at End of Radiotherapy
Lymphocyte count in the final week of radiotherapy was available for 90 patients with stage I–II lung cancer (10%) and 210 patients with stage III lung cancer (32.6%). Sixty patients for whom counts were available had sequential chemoradiotherapy and 114 had concurrent chemoradiotherapy. The median lymphocyte count in the last week of radiotherapy for patients who had any chemotherapy was 0.6 × 109/l (0.2–2.4 × 109/l) and in patients who did not have chemotherapy the median lymphocyte count was 0.7 × 109/l (0.2–2.3 × 109/l). Fourteen patients out of 33 who had a diagnosis of COVID-19 had a lymphocyte count from the last week of radiotherapy available for analysis and nine of these patients had a lymphocyte count ≤0.5 × 109/l.
Seventy-eight patients in COVID-RT Lung received G-CSF during treatment. Sixty-one patients who received G-CSF underwent concurrent chemoradiotherapy; 43 for NSCLC and 18 for SCLC. Three patients who received G-CSF had a diagnosis of COVID-19 during their radiotherapy.
COVID-19 Diagnosis and Treatment Delays
Thirty-three (2.1%) patients had a diagnosis of COVID-19, 26 of whom had RT-PCR swab confirmation. Twelve patients were diagnosed with COVID-19 prior to starting treatment for lung cancer, six were diagnosed during radiotherapy and 15 were diagnosed after the end of radiotherapy. Of the 21 patients who had COVID-19 during or after radiotherapy, seven patients had stage I–II lung cancer (0.8% of all early stage patients) and 14 had stage III disease (2.2% of all stage III patients). Six patients died with COVID-19 at a median of 175 days (21–279 days) after the start of radiotherapy.
The median duration of treatment interruption for patients with confirmed or suspected COVID-19 was 4 days (1–16 days). In total, 83 patients (5.3%) had an interruption to radiotherapy for any reason, 18 of whom had their treatment stopped early. No patient with a diagnosis of COVID-19 had their treatment stopped early. The most common reasons for treatment interruptions, apart from COVID-19, were other infections (10 patients) or treatment toxicity (10 patients). One patient (who had RT-PCR-confirmed COVID-19) had a radiotherapy delay compensation with the addition of two extra fractions at the end of radiotherapy, making a total dose of 60.5 Gy in 22 fractions.
Discussion
This first analysis of the UK COVID-RT Lung cohort study has shown that the COVID-19 pandemic led to a subsequent change to treatment in a third of lung cancer patients referred for radical radiotherapy between April and October 2020. In addition, the diagnostic pathway was altered in 12% of patients of the same cohort.
The most common change to treatment was the use of a different radiotherapy dose/fractionation from the centre's usual standard of care in 17.5% of patients, resulting in a higher proportion of patients with lung cancer treated with hypofractionated radiotherapy. These changes are in line with a national UK guidance document of hypofractionated radiotherapy [6]. Greater use of hypofractionation in all cancers during the COVID-19 pandemic was reported in a population-based study analysing data from the UK National Radiotherapy Dataset [8]. The national dataset showed an increase in moderately hypofractionated radiotherapy for patients with lung cancer (2.5–4.9 Gy/fraction) but little change in ultra-hypofractionated radiotherapy (≥5 Gy per fraction). In COVID-RT Lung, there was an increase in the use of 3–5.9 Gy/fraction regimens in patients who had their treatment changed for all stages of lung cancer. This change reflects the increased use of 15-fraction schedules during the pandemic. The evidence for the use of 60 Gy in 15 fractions comes from a phase II trial in patients with T1-3 N0 M0 NSCLC, which reported an overall survival of 68.7% at 2 years with a low rate of grade 3+ toxicity [9].
Radiotherapy was suggested as an alternative to surgery at the start of the COVID-19 pandemic if there was pressure on operating and anaesthetic resources [8,10]. We found that radiotherapy was used as an alternative treatment to surgery in 9.5% of stage I–II and 2.8% of stage III operable patients between April and October 2020. The number of patients who had changes to treatment changed over time with, the largest number of treatment changes in April and May 2020. Our results show a fall in the number of referrals for radical radiotherapy in June, July and August 2020 which could be explained by the fall in suspected lung cancer referrals [11].
Multivariable analysis of baseline factors found that male gender, age ≥70 years and stage III lung cancer were associated with patients having a change to their treatment from their centre's standard of care. Spencer et al. [8] also found that there was more of a decrease in the number of radiotherapy treatment courses for all cancer patients in patients aged ≥70 compared with patients <70 years. Older age and male gender have consistently been associated with a higher morbidity and mortality with COVID-19 [3], leading to adjustment in treatments to mitigate the risk.
When interpreting the results of COVID-RT Lung it should be noted that standard of care treatment for stage I–III lung cancer varies considerably between UK centres. For this reason, the central question of the analysis was whether the patient's treatment had been changed from their centre's standard of care.
Results from the National Lung Cancer Audit 2016 reported that 65% of patients who received radical radiotherapy for stage III lung cancer had sequential or concurrent chemotherapy [12]. Only 55% of patients with stage III disease in COVID-RT Lung had chemotherapy in addition to radical radiotherapy and 10.7% had their chemotherapy omitted because of the pandemic. The lower rates of chemotherapy in our study may be due to the perceived risk of COVID-19 in patients who are immunosuppressed [13]. The combination of lower rates of chemotherapy and more hypofractionated radiotherapy resulted in patients with stage III disease having more changes to treatment than those with stage I disease. Furthermore, we found that patients with PS 2–3 were less likely to have their treatment changed. Patients with PS 2–3 often receive curative-intent radiotherapy alone rather than surgery or chemoradiotherapy [12,14], leaving less scope for their treatment to be changed as a result of the pandemic. No specific comorbidity was associated with a change in treatment, although patients with chronic kidney disease were more likely to have a change to their diagnostic investigations.
COVID-RT Lung demonstrates geographical variation in treatment changes, with the North of England having the greatest proportion of patients with changes to their treatment. These regions had some of the highest rates of COVD-19 infection in the UK [15]. Pre-pandemic regional treatment variations may also explain why some areas of the country recorded a lower proportion of patients with a change to treatment.
The incidence of COVID-19 infections in the COVID-RT Lung cohort was low (2.1% of patients in total and only 1.4% were infected during or after radiotherapy). Variations in COVID-19 testing policies between centres and over time, especially the slow roll out of COVID-19 testing nationally during the initial months of the pandemic, will influence the reported incidence rate in this study. Nevertheless, the low COVID-19 rate is reassuring given the high rates of lymphopenia reported during the last week of radiotherapy in a subgroup of patients. The low rates of COVID-19 infection and death may reflect the UK Government's shielding advice [4] for patients having thoracic radiotherapy. In addition the use of hypofractionated radiotherapy, as per UK and international guidance [6,16], may have reduced the patients' exposure to COVID-19.
Only six patients in our study died with COVID-19, in contrast to the high rate of death from COVID-19 reported in the TERAVOLT [17] registry. TERAVOLT was a small cohort of 200 patients, most with stage IV disease, and included a skewed population of symptomatic patients on active treatment who presented to oncological services. The UK Coronavirus Cancer Monitoring project is a larger UK-based registry of patients with COVID-19 and cancer, which did not find an increased case-fatality rate due to COVID-19 in patients with lung cancer [18]. There were also concerns in April 2020 that patients having radiotherapy for lung cancer would have treatment delays as a result of COVID-19 and therefore the Royal College of Radiologists produced guidance on compensating for treatment gaps [19]. We found that 5.3% of patients in the COVID-RT Lung cohort had a treatment gap, but this was more often due to treatment toxicity or an infection other than COVID-19. The median treatment gap for patients with suspected COVID-19 during treatment was 4 days (range 1–16 days), which implies that most patients continued their treatment during the self-isolation period, in order to maximise the potential for cure [20]. Nevertheless, it is surprising that only one patient in this cohort had treatment compensation for a gap in radiotherapy.
Our analysis has limitations as it only includes data from 30 UK radiotherapy centres across the whole of the UK and participating centres had not completed data collection on all treated patients at the time of this initial analysis. Consequently, the denominator of patients with lung cancer receiving radiotherapy during this period of the pandemic is not known. The analysis of lymphopenia following radiotherapy is limited by the small proportion of patients in COVID-RT Lung for whom lymphocyte count was available. COVID-RT Lung demonstrates the same pattern of radiotherapy hypofractionation as reported in national datasets during the pandemic [8], which indicates that it is probably representative of changes across the country. Our study provides more granular detail on the changes to diagnostic pathways, radiotherapy and systemic therapy in patients with lung cancer and specifically asks if the patient had a change to treatment compared with the local standard of care rather than inferring this from changes in national datasets over time.
We have shown that the risk of developing COVID-19 in lung cancer patients receiving radical radiotherapy was low during the first wave of the pandemic, showing that the measures put in place by radiotherapy departments to protect patients [16] were adequate. We have described the characteristics of patients who had changes to their centre's standard of care management and the regional differences in the management of patients with lung cancer. An important next step is to report the outcomes of patients treated during the pandemic in order to assess the effect of radiotherapy and chemotherapy adaptations on survival and toxicity. Outcome data are being collected as data matures. Given the current concerns regarding the cancer backlog and National Health Service pressures as a consequence of the pandemic, our study will provide valuable information to the oncology community to help guide optimal treatment for lung cancer patients going forward.
Conflicts of interest
C. Peedell reports a relationship with Elekta that includes: speaking and lecture fees; a relationship with AstraZeneca Pharmaceuticals LP that includes: speaking and lecture fees; a relationship with Boston Scientific Corp that includes: consulting or advisory and speaking and lecture fees. K. Banfill reports a relationship with AstraZeneca that includes: speaking and lecture fees. Kamalram Thippu Jayaprakash reports a relationship with AstraZeneca that includes: travel reimbursement. K.Thippu Jayaprakash acknowledges the following funding support outside the submitted work: research grant from the UK National Institute of Health Research and educational grants from Bayer UK, Janssen Oncology, Pfizer, Roche, and Takeda.Elizabeth Toy is a member of Lung Cancer Expert Reference Group and Clinical Lead GIRFT Lung Cancer workstream.Crispin Hiley acknowledges the following funding support outside the submitted work: research grant and speaking fees from AstraZeneca. Crispin Hiley reports a relationship with Roche that includes: speaking and lecture fees.
Acknowledgements
This work was supported by Cancer Research UK RadNet Manchester (grant number C1994/A28701) and NIHR Manchester Biomedical Research Centre (grant number BRC-1215-20007). The authors would like to acknowledge the following people for their assistance with this project: Jacqui Parker, Lee Whiteside, Lucy Davies, Josephine Sanders, Louise McHugh, Philip Teles Amaro, Amy Irwin, Yash Choudhary, Victoria Harrop, Rebekah Shingler, Emma Wingate, Liliam Ross, Lynn Bell, Jasima Latif, Chloe Wilkinson, Stephen Harrow, Adam Peters, Paula Robson, Keith Harland, Asia Sarwar, Jolyne O'Hare, Jonathan McAleese, Ruth Eakin, Linda Young, Nicola Hill, Charis Thompson, C.L. Lee, Hannah Bainbridge, Mike Bayne, Eleanor Weir, Sam Guglani, Hannah Lord, Dila Mokhtar, Lynne White, Sarah Treece, Jennifer Poole.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2021.10.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abravan A., Faivre-Finn C., Kennedy J., McWilliam A., van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol. 2020;15:1624–1635. doi: 10.1016/j.jtho.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nhs Digital Clinical inclusion criteria. 2020. https://digital.nhs.uk/coronavirus/shielded-patient-list/methodology/rule-logic Available at:
- 5.Cancer Research UK. Lung cancer treatment. Available at: statisticshttps://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/diagnosis-and-treatment#heading-Two. Accessed 20 April 2021.
- 6.Faivre-Finn C., Fenwick J.D., Franks K.N., Harrow S., Hatton M.Q.F., Hiley C., et al. Reduced fractionation in lung cancer patients treated with curative-intent radiotherapy during the COVID-19 pandemic. Clin Oncol. 2020;32:481–489. doi: 10.1016/j.clon.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer K., Jones C.M., Girdler R., Roe C., Sharpe M., Lawton S., et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22:309–320. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung P., Faria S., Ahmed S., Chabot P., Greenland J., Kurien E., et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst. 2014;106:dju164. doi: 10.1093/jnci/dju164. [DOI] [PubMed] [Google Scholar]
- 10.Antonoff M., Backhus L., Boffa D.J., Broderick S.R., Brown L.M., Carrott P., et al. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. Ann Thorac Surg. 2020;110:692–696. doi: 10.1016/j.athoracsur.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Kingdom Lung Cancer Coalition A review of the impact of COVID-19 on the lung cancer pathway and opportunities for innovation emerging from the health system response to the pandemic. 2021. https://www.uklcc.org.uk/wp-content/uploads/2020/10/UKLCC-COVID-19-Matters-Report-Oct-2020.pdf Available at:
- 12.Adizie J.B., Khakwani A., Beckett P., Navani N., West D., Woolhouse I., et al. Stage III non-small cell lung cancer management in England. Clin Oncol. 2019;31:688–696. doi: 10.1016/j.clon.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Derosa L., Melenotte C., Griscelli F., Gachot B., Marabelle A., Kroemer G., et al. The immuno-oncological challenge of COVID-19. Nat Cancer. 2020;1:946–964. doi: 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- 14.Phillips I., Sandhu S., Lüchtenborg M., Harden S. Stereotactic ablative body radiotherapy versus radical radiotherapy: comparing real-world outcomes in stage I lung cancer. Clin Oncol. 2019;31:681–687. doi: 10.1016/j.clon.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 15.UK Government . UK Government; 2021. Cases | coronavirus in the UK.https://coronavirus.data.gov.uk/details/cases Available at: [Google Scholar]
- 16.Guckenberger M., Belka C., Bezjak A., Bradley J., Daly M.E., DeRuysscher D., et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Radiother Oncol. 2020;146:223–229. doi: 10.1016/j.radonc.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiara Garassino M., Whisenant J.G., Huang L.-C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study 2020. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee L.Y.W., Cazier J.-B., Starkey T., Briggs S.E.W., Arnold R., Bisht V., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick J.D., Faivre-Finn C., Franks K.N., Hatton M.Q.F. Managing treatment gaps in radiotherapy of lung cancer during the COVID-19 pandemic. 2020. https://www.rcr.ac.uk/sites/default/files/managing-treatment-gaps-radiotherapy-lung-cancer-covid19.pdf Available at:
- 20.Cox J.D., Pajak T.F., Asbell S., Russell A.H., Pederson J., Emami B., et al. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1993;27:493–498. doi: 10.1016/0360-3016(93)90371-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.