Abstract
Background.
Frailty is a state of increased vulnerability to various health stressors but little information is summarized about frailty in patients with specific chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and asthma.
Objective.
We aimed to describe the burden of frailty on patients with chronic respiratory disorders and to discuss the need for multidisciplinary care services.
Methods.
PubMed and Cochrane Central databases were systematically reviewed for studies reporting outcomes associated with frailty in COPD, IPF, and asthma. Electronic databases were searched for relevant articles published in English from 2010 up to July 2020. Appraisal was carried out based on the Hierarchy of Evidence Rating System and the GRADE guidelines.
Results.
A total of 31 articles met all inclusion criteria with 24 of them at level IV, 1 at level V, and 6 at level VI. Frailty is likely to negatively affect quality of life and to increase the risk of mortality, especially in elderly with COPD, IPF and asthma. Each disease has a particular effect on the balance between health status, respiratory impairment and frailty. A greater understanding of frailty phenotype across different ages, as well as in a range of long-term conditions, is of great necessity in both clinical and research settings. Limited conformity was observed between different methodologies and nature of chronic diseases studied, leading to a further difficulty to extract homogeneous information.
Conclusion.
Literature shows that frailty is prevalent in COPD, IPF, and asthma, after adjusting for shared risk factors. Our findings suggest that frailty should be approached as an entity per se’, in order to assess real mortality risk, alongside respiratory disease severity and the presence of comorbidities. Health care professionals need knowledge, skills and multidisciplinary collaboration to buffer the impact of frailty on everyday practice.
Keywords: respiratory diseases, frailty, health care, quality of life
Introduction
Frailty is a syndrome which leads to decreased strength, endurance, and physiological function resulting to increased vulnerability, dependence and/or risk of dying (1). Frailty is an evolving concept in clinical practice, as frail patients are predisposed to falls, hospitalization, and institutionalization (2). Additionally, the presence of frailty has been shown to substantially increase dying risk (2-4). The prevalence of frailty is extremely high in some population groups and further increases with age (5-8). Aging (patients aged ≥65 years) and chronic disease can promote deterioration and increase the risk for, and severity of, frailty (6,9).
Chronic obstructive pulmonary disease (COPD) is a disease that shares risk factors with frailty; namely aging, smoking, and dysregulated inflammation and endocrine function (10). Along with asthma, COPD is one of the two more common chronic respiratory diseases (11). In 2017, 572 million persons were affected by COPD worldwide (11). COPD is the most frequently studied chronic respiratory disease with regard to frailty (12). Marengoni et al. (13) showed that COPD increases the risk of frailty twofold (13).
Frailty is also common in elderly patients with idiopathic pulmonary fibrosis (IPF) (14-16). IPF is a progressive, chronic respiratory disease, which mainly affects older adults in whom it early leads to activity-limiting dyspnea and debilitating fatigue (16).
The goal of our study was to synthesize available research evidence about the burden of frailty in patients with COPD, IPF, and asthma, which related with quality of life, respiratory dysfunction, mortality and age association. Based on our goal, we hope that revisiting this field, we can better understand the impact of frailty on patients with chronic respiratory morbidity and the need for an interdisciplinary care approach.
Methods
Search strategy
We conducted a systematic review based on recommendations by the Centre For Reviews And Dissemination (17), following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). We searched manually the PubMed electronic databases using combinations of the following medical subject heading (MeSH) terms and key words: (“idiopathic pulmonary fibrosis”[MeSH Terms] OR (“idiopathic”[All Fields] AND “pulmonary”[All Fields] AND “fibrosis”[All Fields]) OR “idiopathic pulmonary fibrosis”[All Fields]) AND (“frailty”[MeSH Terms] OR “frailty”[All Fields]); (“pulmonary disease, chronic obstructive”[MeSH Terms] OR (“pulmonary”[All Fields] AND “disease”[All Fields] AND “chronic”[All Fields] AND “obstructive”[All Fields]) OR “chronic obstructive pulmonary disease”[All Fields] OR “copd”[All Fields]) AND (“frailty”[MeSH Terms] OR “frailty”[All Fields]); (“asthma”[MeSH Terms] OR “asthma”[All Fields]) AND (“frailty”[MeSH Terms] OR “frailty”[All Fields]). We also applied additional filters, including peer reviewed articles in English, published between 2010 up to July 2020. To avoid any publication bias, we did not limit the type of publication (e.g. original research, review, or commentary). The same (medical subject) headings were applied to the Cochrane database. Studies that examined frailty in patients with COPD, IPF, and asthma were included. Articles without available full text online, studies reporting data with unclear associations, overlapping population groups or published in languages other than English were excluded.
Additionally, title/abstract and full-text screening was carried out by one author (AK) to determine inclusion based on the above criteria. A second author (EKS) cross-checked the list of the articles included and their content. Whenever there was a disagreement or uncertainty between the two authors, another author (KA) was consulted. When necessary, discussion among the three authors took place to reach a final decision. Last, the reference lists of all eligible studies were manually examined for any additional studies meeting inclusion criteria. The above strategy is depicted in the PRISMA(18) flow diagram Figure 1.
Figure 1.
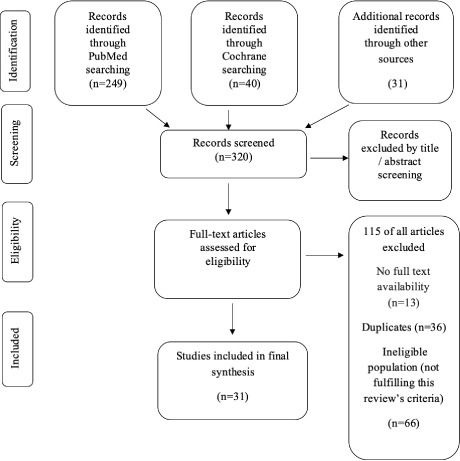
PRISMA flow diagram for database search and study selection process. (Based on Moher et al. (18))
Quality appraisal
All selected articles were appraised for type of study and level of evidence according to the adapted Hierarchy of Evidence Rating System, as it is configured from Oregon Health and Science University (19). Hierarchy of Evidence Rating System is assigned to studies based on the methodological quality of their design, validity, and applicability (19,20). As in a previous study (21), this appraisal step allows for interpretation of the usefulness and transferability of the review findings to practice and policy. In addition, we used the GRADE guidelines according to Balshemet al. (22) to rate the quality of the body of evidence. Table 1 presents the significance of the four GRADE levels of evidence: “high”, “moderate”, “low”, or “very low”.
Table 1.
Significance of the four quality levels*.
| Quality level | Definition |
| High | There is confidence that the true effect lies close to that of the estimate of the effect. |
| Moderate | There is moderate confidence in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | There is limited confidence in the effect estimate: The true effect may be substantially different from the estimate of the effect. |
| Very low | There is very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. |
| *Based on the GRADE guidelines:3(Balshem et al,2011). | |
Results
Thirty one were included in the final synthesis. The majority (n=13) were cohort studies; 10 prospective (3,6,7,15,24,26,28,38,40,44) and 3 retrospective (27,36,41), with evidence appraised at level IV. Eleven studies were appraised as level IV/cross-sectional study (8,10,29,30,31,32,34,35,37,41,42), one study was appraised as level V/ systematic review of descriptive statistics (13), one as level VI/ Quantitative descriptive study (33) and five studies as level VI/ Qualitative study (14,23,25,39,43). Study characteristics are provided in Table 2, subtopics, number of included studies, author(s), level of evidence, quality appraisal, methods and main findings.
Frailty and COPD
Twenty studies reported information on frailty in patients with COPD. In two of them cigarette smoking was identified as a major prevalence contributor between frailty and COPD (23,24). Furthermore, there was a study which reported a higher prevalence of frailty in COPD in males than in females, mostly due to higher smoking exposure and greater disease burden in males compared to females with COPD (23). In eight studies it was highlighted that frailty in COPD may particularly occur in elderly patients (6-8,13,25,26-28), and is strongly associated with dyspnea (30). Additionally, in two studies it was reported that frail patients with COPD have poorer quality of life than patients with COPD who are not frail (29,30). Frailty in COPD can also be mitigated through pulmonary rehabilitation (6-8,25,27), while patients with frailty and COPD showed lower levels of physical activity (31,32), and increased symptoms of anxiety and depression (6). In patients with COPD, frailty rose sharply after hospitalization, with a corresponding impact of exacerbations, in terms of patients’ physical status and disability (33). Patients with both COPD and frailty syndrome were at increased risk of death (34). It was also reported that frailty and COPD are expected to worsen patients’ quality of life (35,36). Finally, according to Park et al.(8) and Dias et al. (37), it was suggested that in patients with frailty and COPD there is a direct association between dyspnea and frailty (8,37).
Frailty and IPF
Five studies reported information on frailty in patients with IPF. Frailty was common in older patients with IPF (14, 15), strongly associated with dyspnea (38) and linked to reduced pectoralis muscle mass (15). As a consequence, this combination has considerable impact on patients’ quality of life (40) and survival (14,40). As IPF drug treatments could be more effective with an adequate clinical comorbidity management, more attention should be devoted to imbalanced nutrition, as well as to (early) occurrence of low muscle strength (dynapenia) and low physical performance (39).
Frailty and asthma
Six studies reported information on frailty in patients with asthma. Landré et al. (41) demonstrated that the patients with asthma had increased risk of frailty (41). Additionally, in four studies, asthma was related to frailty in community-dwelling older adults (aged >65 years) (3,42-44). Furthermore, a recent study showed that when asthma combined with the exposure to the toxic effects of smoke could increase the burden of respiratory dysfunction and make older individuals more vulnerable to developing frailty (44). Finally, one study highlighted that the prevalence of frailty and asthma was increased among cancer survivors and also this combination adds limitations to their usual activities (45).
Discussion
This systematic review identified 31 articles, mostly prospective cohort studies, related to the prevalence, severity, and impact of frailty in patients with chronic lung disease. Our review revealed some key findings. Firstly frailty can have a negative impact on pragmatic and perceived functionality and has also an effect to increase death risk, especially in older patients with COPD, IPF and asthma. Additionally, our review summarises that frailty, in these patients, with respiratory dysfunction, expressed as dyspnea worsening.
Frailty is significantly higher among older adults. Fried et al. (2) identified frailty in 3.9% of older adults aged 65 to 74 years, 11.6% among those aged 75 to 84 years, and 25% in individuals aged 85 years or older (2). Collard et al.(46) also detected a high frailty occurrence among older adults aged 80 to 84 years (15.7%) and among those older than 84 years of age (26.1%) (46). As the world’s population ages, frailty is likely to increase in prevalence. Thus, unfavourable health determinants as reduced physical capacity, increased risk of falls with their causal care episodes, impaired cognitive function, and poor nutrition (28) will rise in conjunction with frailty (15,34,47).
Frailty appears as a ‘satellite’ expression of an aging mechanism, but it is also associated with multiple chronic diseases and independently increases risk of disability, hospitalization, and mortality (26,32). According to recent evidence, frailty will affect more than half patients with interstitial lung diseases (ILD) and is strongly associated with respiratory impairment, accelerated biological aging, and the presence of comorbidities (48). Farooqi et al. (49) emphasize in their study that physical frailty is prevalent in patients with ILD and is independently associated with an increased risk of death (49). Assessment of physical frailty provides additional prognostic value to tested risk estimation tools, such as the ILD-GAP score, and may be seen as a modifiable target for future intervention (49).
Frailty is associated with quality of life (40) and survival in older patients (>65 years) with IPF (40,50) and not surprisingly, its prevalence is greater comapared to same age persons in general population groups (14,15,51). Additionally, the combination of comorbidities and complications, such as IPF with frailty and osteoporosis (14,52), can have a profound impact on patients’ quality of life (14). As expected, the incurable and progressive nature of IPF - as well as the burdensome symptoms of breathlessness, cough and fatigue - are additional factors that affect their lives (53). Supportive interventions could thus contribute to helping patients maintain their quality of life despite the increasing physical constraints (53).
The increased prevalence of frailty in patients with asthma is supported by little evidence. However, asthma may also interplay with other chronic diseases associated with frailty (53), especially in geriatric populations (8,54). Older patients diagnosed with asthma present unique characteristics, including severe symptoms, uncontrolled response to standard therapy, and higher mortality (54), which are different from those of younger asthma patients. As Kang et al. (55) proposed, major changes take place in the respiratory tract as a part of the ageing process (55). These changes include immunosenescence, which results in gradual and various alterations in the immune system brought on by advancing age (55). This is expressed through imbalance in lymphocyte subsets, with decreased production of new T cells, apoptosis and mitochondrial disregulation, and impaired function of immune regulatory cells (56). These age-related changes increase susceptibility to infections, and lead to a status of subclinical, sustained inflammation (56). Other alterations include age-associated changes in lung physiology (57), such as decreased strength of respiratory muscles (54), decreased lung recoil (8) and increased chest wall stiffness (58). As a result, elderly patients breathe at higher lung volumes than younger patients, which poses an additional load on their respiratory muscles (59,60). The abovementioned changes can affect the pathogenesis and the development of asthma in older people (55). Moreover, asthma is a comorbidity that can concur with COPD, affecting elderly patients as well (61,62). Some of the common comorbid disorders that complicate asthma and aging are osteoporosis (61) and frailty (41).
The results of our study are consistent with previous suggestions of an association between frailty, respiratory impairment, and health status. Results from recent studies demonstrated that frail patients with COPD had a poorer quality of life, suggesting that their health condition considerably impairs their everyday life (29-33,35). Lahousse et al. (27) suggested that frail elderly patients with COPD have an almost threefold increased risk of mortality (27). According to the literature, COPD is closely correlated to frailty, having shared risk factors such as aging and exposure to smoke, as well as common mechanisms of respiratory and endocrine dysfunction (24,25,29,44). Additionally Chen et al. in their results, highlight that frailty, which is common among patients with COPD with dyspnea, it does not only affect the maintenance of health-related quality of life but also increases the frequency of medical service utilization (35). A possible reason might be that aging-related changes affected pharmacokinetics (35), such as reduced drug clearance and increased drug accumulation (63) and disease-related distress increased the chances of taking multiple medications among COPD patients (64). Another effect of frailty and COPD in patients’ health, is that they are both associated with common systemic comorbidities, including osteoporosis (65-69). The high prevalence of osteoporosis in COPD patients is due to risk factors, such as older age, inactivity, smoking, systemic inflammation, vitamin D deficiency and use of oral or inhaled corticosteroids (65-67). Osteoporosis may cause fragility fractures such as rib cage and vertebral compression fractures, which further decrease mobility, reduce pulmonary function and thus increase morbidity and mortality in both women and men COPD patients (65-67). The parameter of coexisting frailty further increases the vulnerability of COPD patients for osteoporosis, as it acts as a predictor of decrease in bone mineral density and osteoporotic fractures (68,69). Early detection of osteoporosis in frail COPD patients is, therefore, important and can be based on routine screening for osteoporosis using dual photon absorptiometry measurements in hip and spine and risk assessment of fractures using tools such as the Fracture Risk Assessment Tool (FRAX), which takes into account bone mineral density, history of fragility fractures, and population-specific clinical features (67,69). These imaging and risk assessment tools will enable general physicians and pneumonologists to diagnose frail COPD patients with comorbid osteoporosis at an early stage and allow early prevention and treatment strategies to develop (67,69).
Moreover, patients suffering from chronic respiratory diseases, are immediately listed to be at risk for severe forms of COVID-19 (70,71). COVID-19 is responsible for various respiratory manifestations, from cough with dyspnea to acute respiratory distress syndrome (ARDS) in cases with most severe suffering (72). Implications for patients’ health may longly persist after infectius period. Wong et al. suggested the assessment need for quality of life, frailty, dyspnoea, mood and sleep in patients after hospitalisation for COVID-19 (73). Nalbandian et al. (74) describe a post-acute outpatient service established in Italy and report symptom persistence in 9 out of 10 recovered patients, following acute COVID-19 hospitalization, averagely two months after first symptom onset (74). Fatigue, dyspnea, joint pain and chest pain remain common symptoms, within almost 6 out of 10 patients, continuing to experience three or more symptoms, as another study suggests (75). In a Chinese study, most patients report at least one symptom; fatigue/muscular weakness was the most commonly described, followed by sleep difficulties and anxiety/depression (76). Addiotnally, COVID-19 infected, old and frail persons tend to experience substantially more severe symptoms and lethality (77,78). Effects of accelerated aging and the development of age-related disorders have been plausibly described as post-COVID-19 consequences (78). Moreover, post-infectious interstitial lung alterations may mimic tissue inflammation or fibrosing, ILD-like, patterns, deserving attention in pathophysiology terms and by treating them as a new source for building knowledge.
Returning to the general concept, at an interventional level, as summarized by Torres-Sanchez et al. (79), some systematic reviews (80,81) have provided evidence that exercise interventions have a benefit for frail older adults. There is evidence (79-81), that exercise improves cardiorespiratory function, muscle function, balance, performance of activities of daily living, and functional ability in frail older adults. Additionally, exercise interventions should be offered to older people during social isolation to reduce the risk of frailty, sarcopenia, cognitive and emotional impairment; and tele-rehabilitation may represent an option for people at home (82,83). Patients with frailty have to adapt to a number of limitations and participate in the treatment process. Upon frailty diagnosis and loss of health, patients have to go through multidimensional and continuous changes introduced in the scope of physical, mental, and social functioning (28). Decision making process should be, in parallel, focused on mental health and emotional status, since a psycho-eco-social background may play an important role in disease progression for several chronically built disorders (84,85). From this perspective, it is optimistic that consensus documents endorse frailty as an ‘entity’ to be integrated in the management of cardiovascular morbidity, opening new pathways within theragnostic choices and interdisciplinary care (86).
Limitations
The results of this review should be interpreted in the context of some limitations. In the general population, frailty has been associated with multimorbidity and the fact that fewer studies have reported frailty in patients with COPD, IPF and asthma, may imply an overlapping causality. However, our search strategy and data extraction were conducted using specific methodological tools, and we attempted a balanced approach based on our findings, to highpoint the need for well-designed proposals aimed to discriminate to what extend frailty is the cause, result or concurrence in the context of specific morbidity. Narrow conformity was also noticed between different methodologies and nature of chronic conditions, being parameters that deserve attention when collection and analysis of information might lead to strict conclusions. For this reason, we have tabulated some relevant descriptive information in order to offer more clarity.
Conclusion
This study shows that the link between frailty and chronic respiratory diseases (COPD, IPF and asthma) leads to a decline of functional ‘readiness’ and capacity in elderly. A greater understanding of the implications of ‘frail phenotype’ across different ages, as well as in a range of long-term conditions, is of great necessity. The early detection of frailty represents a needed strategy in the management of chronic respiratory diseases. A well designed plan of frailty-based tailored management for the older population groups, involving primary care and secondary respiratory care services, may help to prevent an overwhelming tertiary care demand. However, there is a need for a more thorough evaluation of frailty in order to identify this syndrome and intervene much earlier. Clinicians and policy makers are in need of an evidence base regarding the effective interventions so as to reduce frailty or buffer its effects. Future research should explore the consequences of frailty across a wider age range and in patients with multimorbidity by using specific research questions and advanced methodology. This will provide more evidence promoting interventions, which should be targeted at modifying or reversing the frailty installation as process. Interventions should be tailored to patients’ clinical contexts, as no single intervention is likely to be applicable to all those meeting the criteria for frailty. By intervening earlier, not only individuals, but also health-care systems, have more potential to gain benefits. All health professionals involved need evidence and mindful interventions to buffer the impact of frailty.
Authors’ contributions:
EKS conceived the study idea, invited team members and offered a guidance to search topic. AK performed the literature search and composed results section by appraising findings for the studies included. AK and EKS cross-checked the emerged literature information. KA critically reviewed literature information and offered clinical input when this was necessary. AK, EKS and KA contributed to the first draft preparation. ED and CJR offered intellectual input and contributed in the draft writing and revision based on the field of their expertise. All authors read and approved final manuscript
Acknowledgements:
We would like to thank Professor Jeffrey J. Swigris for his intellectual input and advice during writing the first draft.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Morley JE, Vellas B, Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty Consensus: A Call to Action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):323–332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 5.Limpawattana P, Putraveephong P, Inthasuwan P, Boonsawat W, Theerakulpisut D, Chindaprasirt J. Frailty syndrome in ambulatory patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1193–1198. doi: 10.2147/COPD.S134233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal N, Raj R, Islam EA, Nugent K. The frequency of frailty in ambulatory patients with chronic lung diseases. J Prim Care Community Health. 2016;7(1):10–15. doi: 10.1177/2150131915603202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006) Heart Lung. 2013;42(3):163–170. doi: 10.1016/j.hrtlng.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2011;27(1):39–52. doi: 10.1016/j.cger.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. doi: 10.1016/S0140-6736(18)32279-7. 2019 Jun 22;393(10190):e44]. Lancet. 2018; 392 (10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousquet J, Farrell J, Crooks G, Hellings P, Bel EH, Bewick M, et al. Scaling up strategies of the chronic respiratory disease programme of the European innovation partnership on active and healthy ageing (action plan B3: area 5) Clin Transl Allergy. 2016;6:29. doi: 10.1186/s13601-016-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty. Chest. 2018;154:21–40. doi: 10.1016/j.chest.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Meyer KC, Danoff SK, Lancaster LH, Nathan SD. Management of Idiopathic Pulmonary Fibrosis in the Elderly Patient: Addressing Key Questions. Chest. 2015;148:242–252. doi: 10.1378/chest.14-2475. [DOI] [PubMed] [Google Scholar]
- 15.Sheth JS, Xia M, Murray S, Martinez CH, Meldrum CA, Belloli EA, et al. Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis. Respir Med. 2019;148:6–12. doi: 10.1016/j.rmed.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centre for Reviews and Dissemination. York: University of York: Systematic reviews: CRD’s guidance for undertaking reviews in health care. 2009. [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oregon Health and Science University Evidence-Based Practice Course for Interprofessional Clinical Teams. [Internet]. Oregon Health and Science University library;[cited 2021 18 February]. Available from: https://libguides.ohsu.edu/c.php?g=693307&p=4912291 [Google Scholar]
- 20.Melnyk BM, Fineout-Overholt E. Evidence-based practice in nursing and healthcare: A guide to best practice. Philadelphia: Lippincott, Williams & Wilkins; 2005. pp. 6–10. [Google Scholar]
- 21.Kamekis A, Rachiotis G, Markaki A, Samara V, Symvoulakis EK. Employment and suicidal rates during economic recession: A country-targeted integrative review [published online ahead of print, 2020 Nov 2] Int J Soc Psychiatry. 2020:20764020969740. doi: 10.1177/0020764020969740. [DOI] [PubMed] [Google Scholar]
- 22.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Aryal S, Diaz-Guzman E, Mannino DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:1145–1154. doi: 10.2147/COPD.S54476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.G. Kojima G, Iliffe S, Jivraj S, Liljas A, Walters K. Does current smoking predict future frailty? The English 474 longitudinal study of ageing. Age Ageing. 2018;47:126–131. doi: 10.1093/ageing/afx136. [DOI] [PubMed] [Google Scholar]
- 25.Mirza S, Benzo R. Chronic Obstructive Pulmonary Disease Phenotypes: Implications for Care. Mayo Clin Proc. 2017;92(7):1104–1112. doi: 10.1016/j.mayocp.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziewierz A, Tokarek T, Kleczynski P, Sorysz D, Bagienski M, Rzeszutko L, et al. Impact of chronic obstructive pulmonary disease and frailty on long-term outcomes and quality of life after transcatheter aortic valve implantation. Aging Clin Exp Res. 2018;30(9):1033–1040. doi: 10.1007/s40520-017-0864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 28.Uchmanowicz I, Jankowska-Polanska B, Chabowski M, Uchmanowicz B. Fal AM. The influence of frailty syndrome on acceptance of illness in elderly patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2401–2407. doi: 10.2147/COPD.S112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ierodiakonou D, Kampouraki M, Poulonirakis I, Papadokostakis P, Lintovoi E, Karanassos D, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med. 2019;19:1–9. doi: 10.1186/s12890-019-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai K, Tanaka A, Homma T, Kaneko K, Uno T, Sato H, et al. Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2019;19:896–901. doi: 10.1111/ggi.13740. [DOI] [PubMed] [Google Scholar]
- 31.Valenza MC, Torres-Sanchez I, Cabrera-Martos I, Rodriguez-Torres J, Gonzalez-Jimenez E, Munoz-Casaubon T. Physical activity as a predictor of absence of frailty in subjects with stable COPD and COPD exacerbation. Respir Care. 2016;61(2):212–219. doi: 10.4187/respcare.04118. [DOI] [PubMed] [Google Scholar]
- 32.Kusunose M, Oga T, Nakamura S, Hasegawa Y, Nishimura K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir Res. 2017;4(1):e000196. doi: 10.1136/bmjresp-2017-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annegarn J, Meijer K, Passos VL, Stute K, Wiechert J, Savelberg HH, et al. Problematic activities of daily life are weakly associated with clinical characteristics in COPD. J Am Med Dir Assoc. 2012;13:284–290. doi: 10.1016/j.jamda.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Galizia G, Cacciatore F, Testa G, Della-Morte D, Mazzella F, Langellotto A, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res. 2011;23:118–125. doi: 10.1007/BF03351076. [DOI] [PubMed] [Google Scholar]
- 35.Chen PJ, Yang KY, Perng WC, Lin KC, Wang KY. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2463–2469. doi: 10.2147/COPD.S172694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty and Clinical Outcomes in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2019;16(2):217–224. doi: 10.1513/AnnalsATS.201803-175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias LS, Ferreira ACG, da Silva Junior JLR, Conte MB, Rabahi MF. Prevalence of Frailty and Evaluation of Associated Variables Among COPD Patients. Int J Chron Obstruct Pulmon Dis. 2020;15:1349–1356. doi: 10.2147/COPD.S250299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne KM, Kwan JM, Guler S, Winstone TA, Le A, Khalil N, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology. 2017;22:728–734. doi: 10.1111/resp.12944. [DOI] [PubMed] [Google Scholar]
- 39.Faverio P, Bocchino M, Caminati A, Fumagalli A, Gasbarra M, Iovino P, et al. Nutrition in Patients with Idiopathic Pulmonary Fibrosis: Critical Issues Analysis and Future Research Directions. Nutrients. 2020;12(4):1131. doi: 10.3390/nu12041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guler SA, Kwan JM, Leung JM, Khalil N, Wilcox PG, Ryerson CJ. Functional aging in fibrotic interstitial lung disease: the impact of frailty on adverse health outcomes. Eur Respir J. 2019;53(3) doi: 10.1183/13993003.00647-2019. [DOI] [PubMed] [Google Scholar]
- 41.Landré B, Nadif R, Goldberg M, Gourmelen J, Zins M, Ankri J, et al. Asthma is associated with frailty among community-dwelling adults: the GAZEL cohort. BMJ Open Respir Res. 2020;7(1):e000526. doi: 10.1136/bmjresp-2019-000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Jung HW, Won CW. What are the illnesses associated with frailty in community-dwelling older adults: the Korean Frailty and Aging Cohort Study. Korean J Intern Med. doi: 10.3904/kjim.2019.097. 2020:10.3904/kjim.2019.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8(9):1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevisan C, Rizzuto D, Maggi S, Sergi G, Welmer AK, Vetrano DL. Cross-Sectional and Longitudinal Associations between Peak Expiratory Flow and Frailty in Older Adults. J Clin Med. 2019;8(11):1901. doi: 10.3390/jcm8111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smitherman AB, Anderson C, Lund JL, Bensen JT, Rosenstein DL, Nichols HB. Frailty and Comorbidities Among Survivors of Adolescent and Young Adult Cancer: A Cross-Sectional Examination of a Hospital-Based Survivorship Cohort. J Adolesc Young Adult Oncol. 2018;7(3):374–383. doi: 10.1089/jayao.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collard RM, Boter H, Schoevers RA. Oude Voshaar RC. Prevalence of frailty in communitydwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 47.Brighton LJ, Evans CJ, Man WDC, Maddocks M. Improving Exercise-Based Interventions for People Living with Both COPD and Frailty: A Realist Review. Int J Chron Obstruct Pulmon Dis. 2020;15:841–855. doi: 10.2147/COPD.S238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guler SA, Ryerson CJ. Frailty in patients with interstitial lung disease. Curr Opin Pulm Med. 2020;26(5):449–456. doi: 10.1097/MCP.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 49.Farooqi M, O’Hoski S, Goodwin S, Makhdami N, Aziz A, Cox G, et al. Prevalence and prognostic impact of physical frailty in interstitial lung disease: A prospective cohort study. Respirology. 2021;26(7):683–689. doi: 10.1111/resp.14066. [DOI] [PubMed] [Google Scholar]
- 50.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, et al. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS One. 2016;11(3):e0151425. doi: 10.1371/journal.pone.0151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montgomery E, Macdonald PS, Newton PJ, Chang S, Jha SR, Hannu MK, et al. Frailty as a Predictor of Mortality in Patients With Interstitial Lung Disease Referred for Lung Transplantation. Transplantation. 2020;104(4):864–872. doi: 10.1097/TP.0000000000002901. [DOI] [PubMed] [Google Scholar]
- 52.King C, Nathan SD. Identification and treatment of comorbidities in idiopathic pulmonary fibrosis and other fi brotic lung diseases. Curr Opin Pulm Med. 2013;19(5):466–473. doi: 10.1097/MCP.0b013e328363f460. [DOI] [PubMed] [Google Scholar]
- 53.Antoniou K, Kamekis A, Symvoulakis EK, Kokosi M, Swigris JJ. Burden of idiopathic pulmonary fibrosis on patients’ emotional well being and quality of life: a literature review. Curr Opin Pulm Med. doi: 10.1097/MCP.0000000000000703. 2020:10.1097/MCP.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 54.Wuthrich B, Schmid-Grendelmeier P, Schindler C, Imboden M, Bircher A, Zemp E, et al. Prevalence of atopy and respiratory allergic diseases in the elderly SAPALDIA population. Int Arch Allergy Immunol. 2013;162:143–148. doi: 10.1159/000351416. [DOI] [PubMed] [Google Scholar]
- 55.Kang JY, Kim IK, Hur J, Kim SC, Lee SY, Kwon SS, et al. Expression of Muscarinic Receptors and the Effect of Tiotropium Bromide in Aged Mouse Model of Chronic Asthma. Tuberc Respir Dis (Seoul) 2019;82(1):71–80. doi: 10.4046/trd.2018.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis. 2017;8:162–175. doi: 10.14336/AD.2016.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardona V, Guilarte M, Luengo O, Labrador-Horrillo M, Sala-Cunill A, Garriga T. Allergic diseases in the elderly. Clin Transl Allergy. 2011;1:11. doi: 10.1186/2045-7022-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vignola AM, Scichilone N, Bousquet J, Bonsignore G, Bellia V. Aging and asthma: pathophysiological mechanisms. Allergy. 2003;58:165–175. doi: 10.1034/j.1398-9995.2003.02163.x. [DOI] [PubMed] [Google Scholar]
- 59.Tzortzaki EG, Proklou A, Siafakas NM. Asthma in the elderly: can we distinguish it from COPD? J Allergy. 2011:843543. doi: 10.1155/2011/843543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, et al. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132:1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 61.PG Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376:803–813. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 62.Bellia V, Scichilone N, Battaglia S. Asthma in the elderly. Eur Respir Mon. 2009;43:56–76. [Google Scholar]
- 63.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franssen FM, Spruit MA, Wouters EF. Determinants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:493. doi: 10.2147/COPD.S24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016;11:637–648. doi: 10.2147/COPD.S79638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okazaki R, Watanabe R, Inoue D. Osteoporosis Associated with Chronic Obstructive Pulmonary Disease. J Bone Metab. 2016;23(3):111–120. doi: 10.11005/jbm.2016.23.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139(3):648–657. doi: 10.1378/chest.10-1427. [DOI] [PubMed] [Google Scholar]
- 68.Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46. doi: 10.1186/s12891-017-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sternberg SA, Levin R, Dkaidek S, Edelman S, Resnick T, Menczel J. Frailty and osteoporosis in older women--a prospective study. Osteoporos Int. 2014;25(2):763–768. doi: 10.1007/s00198-013-2471-x. [DOI] [PubMed] [Google Scholar]
- 70.Beltramo G, Cottenet J, Mariet AS, Marjolaine G, Lionel P, Pascale TB, et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur Respir J. 2021 doi: 10.1183/13993003.04474-2020. in press (https://doi.org/10.1183/13993003.04474-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahrenfeldt LJ, Nielsen CR, Möller S, Christensen K, Lindahl-Jacobsen R. Burden and prevalence of risk factors for severe COVID-19 disease in the ageing European population - A SHARE-based analysis. Res Sq. 2020 doi: 10.1007/s10389-021-01537-7. doi:10.21203/rs.3.rs-73657/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020;56(5):2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carfi A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.She Q, Chen B, Liu W, Li M, Zhao W, Wu J. Frailty Pathogenesis, Assessment, and Management in Older Adults With COVID-19. Front Med (Lausanne) 2021;8:694367. doi: 10.3389/fmed.2021.694367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhavoronkov A. Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections. Aging (Albany NY) 2020;12(8):6492–6510. doi: 10.18632/aging.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torres-Sánchez I, Valenza MC, Cabrera-Martos I, López-Torres I, Benítez-Feliponi Á, Conde-Valero A. Effects of an Exercise Intervention in Frail Older Patients with Chronic Obstructive Pulmonary Disease Hospitalized due to an Exacerbation: A Randomized Controlled Trial. COPD. 2017;14(1):37–42. doi: 10.1080/15412555.2016.1209476. [DOI] [PubMed] [Google Scholar]
- 80.Weening-Dijksterhuis E, De Greef MH, Scherder EJ, Slaets JP, van der Schans CP. Frail institutionalized older persons: A comprehensive review on physical exercise, physical fitness, activities of daily living, and quality-of-life. Am J Phys Med Rehabil. 2011;90(2):156–168. doi: 10.1097/PHM.0b013e3181f703ef. [DOI] [PubMed] [Google Scholar]
- 81.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a metaanalysis. Arch Phys Med Rehabil. 2012;93(2):237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 82.Maltese G, Corsonello A, Di Rosa M, Soraci L, Vitale C, Corica F, et al. Frailty and COVID-19: A Systematic Scoping Review. J Clin Med. 2020;9(7):2106. doi: 10.3390/jcm9072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ceravolo MG, De Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid “living” review on rehabilitation needs due to covid-19: Update to march 31st 2020. Eur J Phys Rehabil Med. 2020;56(3):347–353. doi: 10.23736/S1973-9087.20.06329-7. [DOI] [PubMed] [Google Scholar]
- 84.Anyfantakis D, Symvoulakis E K, Linardakis M, Shea S, Panagiotakos D, Lionis C. Effect of religiosity/spirituality and sense of coherence on depression within a rural population in Greece: the Spili III project. BMC psychiatry. 2015;15:173. doi: 10.1186/s12888-015-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lionis C, Petelos E, Papadakis S, Tsiligianni I, Anastasaki M, Angelaki A, et al. Towards evidence-informed integration of public health and primary health care: Experiences from Crete. Public Health Panorama. 2018;4:491–735. [Google Scholar]
- 86.Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, et al. Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS) [published online ahead of print, 2021 Jul 16] Eur J Prev Cardiol. 2021:zwaa167. doi: 10.1093/eurjpc/zwaa167. [DOI] [PubMed] [Google Scholar]


