Dear Editor,
Shmueli et al. analysed the immune response to two doses of the Pfizer (BNT162b2) vaccine in 129 patients treated for cancer [1]. They reported that 84% of these patients were seropositive after the second injection, but that the seropositivity rate after each dose was significantly lower than that in the 348 controls.
Similar results have been published for a larger prospective study comparing 232 patients and 261 public health workers in good health, which reported anti-SARS-CoV-2 antibody development after the first dose in 84% of the control group but only 29% of cancer patients [2]. However, after the second dose, 86% of the cancer patients were seropositive. Several studies have confirmed the insufficiency of a single injection in patients with cancer and a weaker, more heterogeneous and less durable immune response following the second injection, attributable to the immunosuppressive effects of cancer itself or of the treatments administered particularly for cytotoxic treatments [3,4].
The aim of the study by Shmueli et al. was to investigate the clinical characteristics potentially associated with seronegativity, including age, BMI, type of cancer, stage (localised, metastatic), comorbid conditions and type of treatment [1]. In our view, the degree of lymphopenia is an important factor missing from this list. In June 2021, before the proposal of a third dose of the vaccine for this high-risk population, the French Directorate General for Health recommended a third dose of the vaccine for patients with lymphopenia without specifying a threshold value.
In this context, we performed a prospective single-centre study at Foch Hospital in France, with the aim of evaluating antibody titers in patients with a solid tumour (other than lung cancers) who had received two doses of vaccine and were treated as outpatients and to assess the correlation between these titers and lymphopenia. The study was approved by the institutional review board (IRB) of the hospital (00012437). The blood sample for antibody and lymphocyte determinations was taken during the month of July, on the day of chemotherapy or immunotherapy treatment, from a cohort of 237 patients who had received two doses of the Pfizer-BioNTech vaccine (mostly between March and April 2021). The SARS-CoV-2 IgG II Quant assay was used for the detection of anti-spike antibodies.
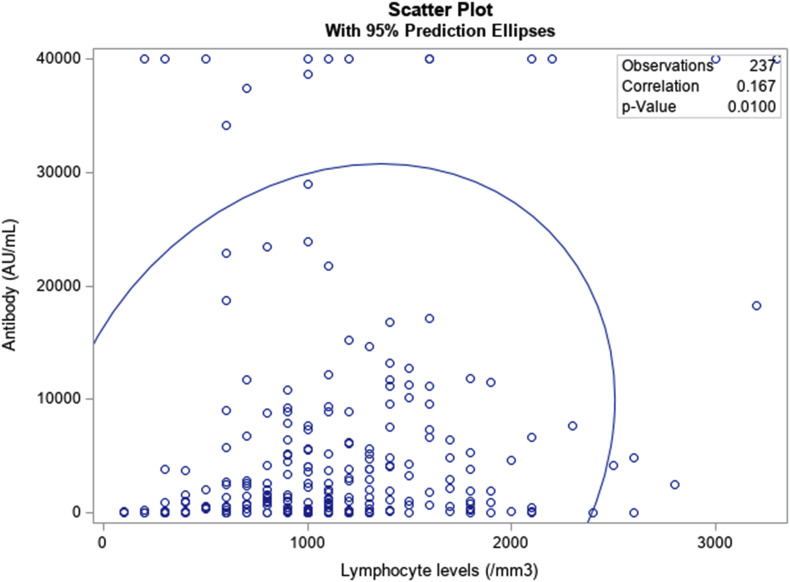
The median antibody level was 1596 AU/ml, and the median lymphocyte count was 1100/mm3. We found a significant correlation between antibody and lymphocyte levels (correlation test; p = 0.01) (Fig. 1 ). Lymphocyte counts appear to be a simple potential criterion that could be integrated into evaluations, alongside serological tests, to improve the selection of patients requiring an additional booster dose in the future.
Fig. 1.
Correlation between antibody and lymphocyte levels.
The type of treatment was not significantly correlated with antibody levels (p = 0.06) in our analysis but may nevertheless be a useful criterion to take into account.
The question of the acceptability to patients of a third injection remains in a situation in which the clinical standards have yet to be scientifically established. We are working on the development of appropriate information documents for the patients concerned to help limit the number of refusals [5].
Funding
N/A.
Conflict of interest statement
None declared.
References
- 1.Shmueli E.S., Itay A., Margalit O., Berger R., Halperin S., Jurkowicz M., et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy – a single centre prospective study. Eur J Cancer. 2021;157:124–131. doi: 10.1016/j.ejca.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goshen-Lago T., Waldhorn I., Holland R., Szwarcwort-Cohen M., Reiner-Benaim A., Shachor-Meyouhas Y., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoeklé H.-C., Sekkate S., Angellier E., Hervé C., Beuzeboc P. Refusal of anti-coronavirus disease 2019 vaccination in cancer patients: is there a difference between the sexes? Eur J Cancer. 2021;155:54–55. doi: 10.1016/j.ejca.2021.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]