Abstract
Background & Aim
Patients with inflammatory bowel diseases (IBD), specifically those treated with anti–tumor necrosis factor (TNF)α biologics, are at high risk for vaccine-preventable infections. Their ability to mount adequate vaccine responses is unclear. The aim of the study was to assess serologic responses to messenger RNA–Coronavirus Disease 2019 vaccine, and safety profile, in patients with IBD stratified according to therapy, compared with healthy controls (HCs).
Methods
Prospective, controlled, multicenter Israeli study. Subjects enrolled received 2 BNT162b2 (Pfizer/BioNTech) doses. Anti-spike antibody levels and functional activity, anti-TNFα levels and adverse events (AEs) were detected longitudinally.
Results
Overall, 258 subjects: 185 IBD (67 treated with anti-TNFα, 118 non–anti-TNFα), and 73 HCs. After the first vaccine dose, all HCs were seropositive, whereas ∼7% of patients with IBD, regardless of treatment, remained seronegative. After the second dose, all subjects were seropositive, however anti-spike levels were significantly lower in anti-TNFα treated compared with non–anti-TNFα treated patients, and HCs (both P < .001). Neutralizing and inhibitory functions were both lower in anti-TNFα treated compared with non–anti-TNFα treated patients, and HCs (P < .03; P < .0001, respectively). Anti-TNFα drug levels and vaccine responses did not affect anti-spike levels. Infection rate (∼2%) and AEs were comparable in all groups. IBD activity was unaffected by BNT162b2.
Conclusions
In this prospective study in patients with IBD stratified according to treatment, all patients mounted serologic response to 2 doses of BNT162b2; however, its magnitude was significantly lower in patients treated with anti-TNFα, regardless of administration timing and drug levels. Vaccine was safe. As vaccine serologic response longevity in this group may be limited, vaccine booster dose should be considered.
Keywords: COVID-19, Vaccine, mRNA-BNT162b2, Serologic Response
Abbreviations used in this paper: 5-ASA, 5-aminosalicylic acid; Ab, antibodies; ACE2, angiotensin converting enzyme2; ADA, adalimumab; AEs, adverse events; anti-TNFα, anti–tumor necrosis factor α; AU, activity units; CD, Crohn’s disease; CI, confidence intervals; COVID-19, Coronavirus Disease 2019; CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assay; GMC, geometric mean concentration; HC, healthy controls; IBD, inflammatory bowel diseases; IFX, infliximab; Ig, immunoglobulin; IQR, interquartile range; N, nucleocapsid; RBD, receptor binding domain; S, spike; SAE, severe adverse event; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UC, ulcerative colitis
Graphical abstract
Patients with inflammatory bowel diseases treated with anti–tumor necrosis factor-α had lower serologic response to Coronavirus Disease 2019 vaccine compared with those treated with other medications and healthy controls. Vaccine was safe.
What You Need to Know.
Background and Context
Patients with inflammatory diseases, specifically those treated with anti–tumor necrosis factor-α, may have attenuated vaccine immune responses. Data regarding response to Coronavirus Disease 2019 vaccines are scarce and conflicting.
New Findings
Seroconversion demonstrated after 2, but not 1, BNT162b2 doses. Significantly lower serologic responses in patients treated with anti–tumor necrosis factor-α, irrespective of drug levels and drug-vaccine intervals. Vaccine was safe.
Limitations
Evaluation of 1 vaccine type (BNT162b2). Short follow-up (∼4 weeks) after the second vaccine dose.
Impact
Two doses of BNT162b2 in patients with inflammatory bowel diseases are safe and effective; however, patients treated with anti–tumor necrosis factor-α biologics had lower serologic response, supporting specific vaccination schedules for this population. No requirement to coordinate timing of vaccination and drug administration.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) resulted in a worldwide pandemic.1 To face the immense morbidity and mortality burden, accelerated vaccine development programs and mass vaccination campaigns were conducted. Vaccine studies included healthy adults or those with stable chronic diseases.2 , 3 Patients with immune-mediated diseases such as inflammatory bowel diseases (IBD), both Crohn's disease (CD) and ulcerative colitis (UC), were specifically excluded from trials.2 , 3 These patients are often treated with immunomodulators and/or biologic therapy such as anti–tumor necrosis factor (TNF) α, potentially associated with an increased risk of infection.4, 5, 6 Although guidelines recommend vaccination per standard immunization schedules,4 , 7 , 8 patients’ ability to mount an adequate immune response to certain vaccines or infections is doubted.6 , 9, 10, 11, 12, 13, 14, 15, 16, 17 This was even less clear for the new messenger RNA (mRNA)-based vaccines against SARS-CoV-2. Concerns regarding adverse events (AEs), including IBD exacerbation, further underscored the need for vaccine responses assessment in these patients, which may also be relevant to patients with other immune-mediated inflammatory disorders using similar medications.
A massive vaccination campaign against COVID-19 started in Israel on December 19, 2020, with mRNA-based COVID-19 vaccine (BNT162b2; Pfizer/BioNTech, New York, NY), administered in 2 doses 3 weeks apart.18 We conducted a prospective multicenter Israeli study to assess serologic responses to BNT162b2 in patients with IBD stratified according to therapy, compared with healthy controls (HCs).
Methods
Study Design and Participants
A prospective, observational, multicenter study was conducted to assess serologic responses to the mRNA-based COVID-19 vaccine BNT162b2, their dynamics, predictors of response and safety, in patients with IBD compared with HCs. A call for patient referral was distributed to all Israeli gastroenterologists and patients with IBD on December 28, 2020. Patients aged ≥18 years were recruited. IBD diagnosis was defined by accepted criteria. The HC group included volunteers (health care professionals and their relatives) without known gastrointestinal diseases. Patients with past COVID-19 infection proved by SARS-CoV-2 polymerase chain reaction test and pregnant women were excluded. Patients with IBD were stratified at baseline into those treated with anti-TNFα, or those not treated with anti-TNFα but by other IBD treatments (ie, 5-aminosalicylic acid [5-ASA], immunomodulators, steroids, ustekinumab, and JAK inhibitors) who were included in a "non–anti-TNFα" group together with patients who were completely "untreated" (ie, they were not treated with anti-TNFα or any other agent). All participants received two 30-μg BNT162b2 vaccine doses intramuscularly, administered 21 to 28 days apart, as per the manufacturers’ recommendations. The study was approved by the local institutional review boards at the Rabin, Shaare Zedek, Emek, and Soroka Medical Centers (1072-20-RMC, 0557-20-SZMC, 0247-20-EMC, 0568-20-SOR, respectively). MOH number: 2020-12-30_009617. All participants signed an informed consent form before any study procedure.
Study Procedure
Eligible participants were evaluated at 4 time points: (1) visit 1, before the first vaccine dose; (2) visit 2, 14 to 21 days after the first and before the second vaccine dose; (3) phone call a week after the second vaccine dose to report adverse events (AEs); and (4) visit 3, 21 to 35 days after the second vaccine dose (see Figure 1 A). At enrollment, patients were assessed for baseline demographic and IBD characteristics. Specifically, medical treatment, duration, and dose were registered, including date of biologics injections/infusions as well as interval between biologics administration and vaccination. Each visit clinical evaluation was performed using IBD specific questionnaires: Harvey-Bradshaw Index,19 Simple Clinical Colitis Activity Index,20 and Pouch Disease Activity Index21 for CD, UC, and patients with an ileal pouch, respectively. Postvaccination AEs22 were evaluated by standard questionnaires, specifically referring to pain or swelling at injection site, fever, headache, shivering, nausea, dizziness, fatigue, muscle soreness, joint pain, allergic reaction, other AEs,2 , 22 and severe AEs (SAEs; anaphylactic reaction, hospitalization, death). Safety measures also included assessment of IBD clinical activity as well as inflammatory biomarkers.
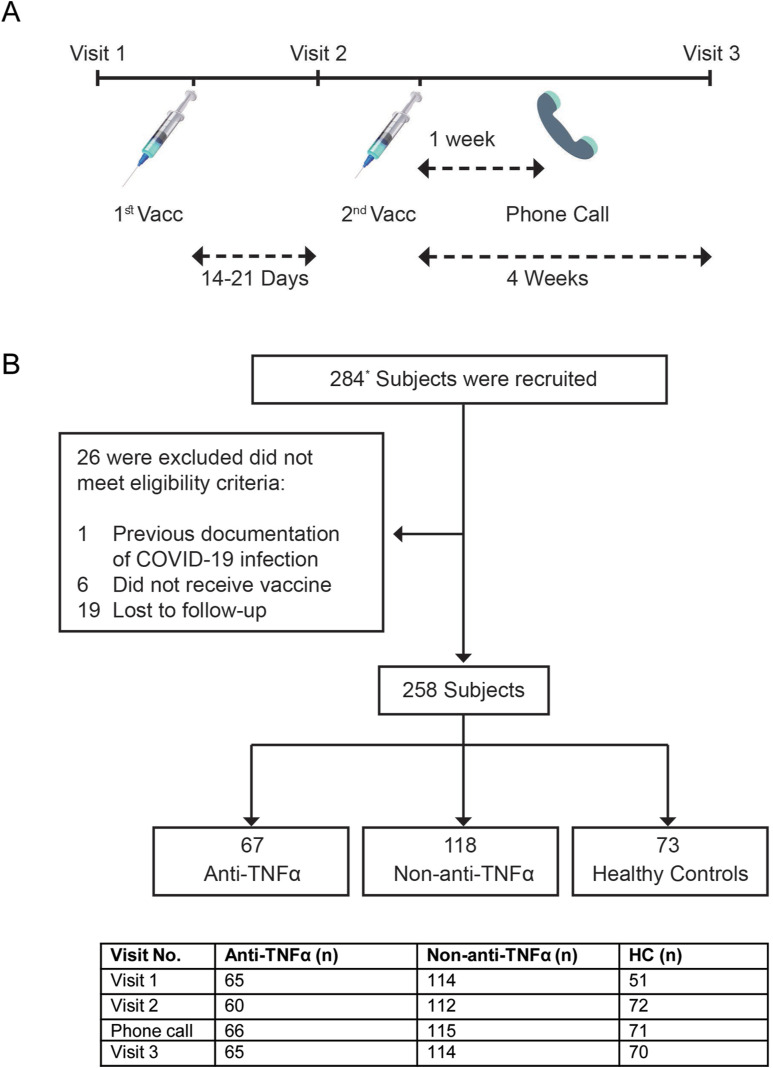
Figure 1.
(A) Study protocol. Patients were enrolled at visit 1, before the first vaccine dose. Visit 2 was 14 to 21 days after the first but before the second vaccine dose. A week after the second vaccine dose, a phone call was made to evaluate AEs, and a visit 3 was 4 weeks after the second vaccine dose. In each visit, laboratory tests were performed, and questionnaires regarding disease severity and AEs were filled. (B) Patient disposition. The diagram represents all enrolled participants who were recruited before vaccination. ∗28 subjects were recruited at the second visit (after first vaccine dose but before the second one), mainly for logistic reasons. Most of them (22) were HCs. Number of subjects at each visit is detailed in the table below the diagram. Vacc, vaccine dose.
Laboratory tests were performed at each visit, including complete blood count, C-reactive protein (CRP), COVID-19 serology, and functional neutralization and inhibition assays. Anti-TNFα drug levels and anti-TNFα antibodies were measured. Serum was separated from collected blood, aliquoted, and stored at −80°C until further analyses.
Outcomes
The primary endpoint was seropositivity rate and magnitude of the serologic response (levels of binding immunoglobulin [Ig]G antibodies to SARS-CoV-2 spike [S] antigen and neutralizing and inhibitory antibodies functionality) following BNT162b2 in patients with IBD with or without anti-TNFα treatment, or HCs, at visit 3. Secondary endpoints were serologic response dynamics induced after the first and second vaccine doses; and AEs, specifically local and systemic reactions and IBD exacerbation.
Laboratory Methods
SARS-CoV-2 IgG II quantitative testing was performed using the Abbott architect i2000sr platform in accordance with manufacturer’s instructions.23 Values ≥50 activity units (AU)/mL are considered positive.
SARS-CoV-2 nucleocapsid (N) IgG testing was performed semiquantitatively using enzyme-linked immunosorbent assay (ELISA) plates coated with N protein in accordance with manufacturer’s instructions (EUROIMMUN, Lubeck, Germany). Values ≥1.1 units are considered positive. Anti-N was assessed in all subjects at visit 3 (after second vaccine dose). For all those testing positive, existence of anti-N antibodies was assessed at visits 1 and 2.
Anti-TNFα drug and anti-drug antibody levels were assessed for adalimumab (ADA and ADA-antibodies [Abs]) and infliximab (IFX and IFX-Abs) using Lisa-Tracker ELISA in accordance with the manufacturer’s instructions (Theradiag, Beaubourg, France). Range for drug levels: 0.3 to 20 μg/mL. Range for Abs levels: 10 to 160 ng/mL and 10 to 200 ng/mL for ADA-Abs and IFX-Abs, respectively.
Receptor binding domain (RBD): angiotensin converting enzyme (ACE)2 inhibition ELISA24 was performed as described25 using RBD-serum mix incubated with ACE2-coated plates. Inhibition percentage was calculated for each well by the following formula:
Negative results, indicating no inhibition, were set as 0% inhibition.
Preparation of SARS-CoV-2 spike pseudoparticles and neutralization assay: detailed in the Supplementary Methods.
Statistical Analysis
Data were collected in a secured Web-based platform (REDCap) and analyzed using SPSS version 27 (IBM, Armonk, NY).
All tests were 2-tailed, and P < .05 was considered significant. Anti-S antibody concentrations are expressed as geometric mean concentrations (GMCs) with 95% confidence intervals (CIs). Other continuous data are reported as median and interquartile range (IQR) unless otherwise stated. Counts and percentages were employed for categorical variables. Univariate analyses, using independent samples t test, 1-way analysis of variance with Bonferroni multiple-comparison correction or Kruskal-Wallis nonparametric test of ln-transformed anti-S antibody concentration and Spearman’s rank correlation coefficients, were used to identify demographic, disease, vaccine, and treatment-related factors associated with anti-S levels. We used multivariate stepwise linear regression models to identify factors independently associated with ln anti-S levels. Standardized Beta coefficients were obtained from linear regression.
Sample Size Calculation
We estimated a sample size of 70 subjects needed in each of the 3 study groups to detect a GMC Ratio of 2.0 between study groups. The null hypothesis was that all vaccine groups would have the same GMC Ratio. This estimated sample size assumed a standard deviation of 0.5 in the log values of antibodies at 90% power and alpha 2.5%.
Results
Study Population
Subjects were recruited in IBD centers located in central (Rabin); Northern (Emek); Eastern and Jerusalem (Shaare Zedek); and Southern (Soroka) Israel, between December 29, 2020, and May 5, 2021. Participants' baseline characteristics are presented in Table 1 . Of 258 subjects, 185 had IBD (122 CD, 53 UC, 6 ileal pouch anal-anastomosis, 4 IBD-Unclassified [U]) and 73 were HCs. Average age (years) in the IBD (37.9 ± 14.3) and HC groups (36.6 ± 12.4) was comparable. There were 60.6% men in the IBD and 27.4% in the HC group. Patients with IBD were divided to 2 separate groups according their medical treatment: those who were treated with anti-TNFα (anti-TNFα group, 67 subjects) and those who had other medical treatment or no medical treatment at all (non–anti-TNFα group, 118 subjects). Most (56 of 67) patients treated with anti-TNFα had CD. Patient disposition is presented in Figure 1 B. In the anti-TNFα group, concomitant therapy included immunomodulators (8), 5-ASA (5), and steroids (1). Therapy in the non–anti-TNFα group included 5-ASA (37), non–anti-TNFα biologics (34, mostly vedolizumab), immunomodulators (8), steroids (7), and tofacitinib (3). This group also included 38 patients (26 CD, 8 UC, 4 ileal pouch anal-anastomosis) without medical treatment. Most subjects (230 of 258, 89%) were recruited within a median of 1 (interquartile range [IQR] 0–4) day before the first vaccine dose (visit 1), and 28 of 258 (10.8%) within 21 (IQR 20–21) days post first vaccine dose (visit 2) mainly due to logistic reasons (Figure 1 B). Median interval between first and second vaccine doses was 21 (IQR 20–24) days. Median interval between the second vaccine dose and visit 3 blood sampling was 30 (IQR 28–33) days. Baseline laboratory results, including blood counts and CRP were comparable among the 3 study groups (Supplementary Table 1).
Table 1.
Baseline Demographic Characteristics of Participants
| Characteristic | Anti-TNFα n = 67 | Non–anti-TNFα n = 118 | HC n = 73 | P value |
|---|---|---|---|---|
| Mean age, y (SD) | 37.8 (14.3) | 38.2 (14.3) | 36.6 (12.4) | .744 |
| Female, n (%) | 24 (35.8) | 49 (41.5) | 53 (72.6) | <.001 |
| Origin, n (%) | ||||
| Ashkenazi | 31 (46.3) | 49 (41.5) | 36 (49.3) | .558 |
| Non-Ashkenazi | 36 (53.7) | 69 (58.5) | 37 (50.7) | |
| Mean BMI, kg/m2 (SD) | 25 (4.0) | 24.4 (5.2) | 25.7 (6.4) | .354 |
| Smoking status, n (%) | ||||
| Present | 8 (11.9) | 15 (12.7) | 7 (9.5) | .299 |
| Past | 9 (13.4) | 7 (5.9) | 3 (4.1) | |
| No | 50 (74.6) | 89 (75.4) | 63 (86.3) | |
| Comorbidities,a n (%) | 8 (11.9) | 11 (9.3) | 5 (6.8) | |
| IBD phenotype, n (%) | ||||
| CD | 56 (83.6) | 66 (55.9) | — | <.001 |
| UC | 8 (11.9) | 45 (38.1) | — | <.001 |
| IPAA | 2 (3) | 4 (3.4) | — | |
| IBD-U | 1 (1.5) | 3 (2.5) | — | |
| Disease activity,b n (%) | ||||
| Remission | 46 (68.6) | 74 (62.7) | — | 1.000 |
| Active | 21 (31.4) | 44 (37.3) | — | |
| Current medication, n (%) | ||||
| IFX | 34 (50.7) | — | — | |
| ADA | 33 (49.3) | — | — | |
| Vedolizumab | — | 26 (22.03) | — | |
| Ustekinumab | — | 5 (4.23) | ||
| 5-ASA | 5 (7.4) | 37 (31.3) | — | |
| Steroids | 1 (1.5) | 7 (5.9) | — | |
| Immunomodulatorsc | 8 (11.9) | 8 (6.7) | — | |
| JAK inhibitor | — | 3 (2.5) | ||
| No medical treatment | — | 38 (32.2) | — |
BMI, body mass index; IBD-U, IBD-unclassified; IPAA, ileal pouch anal-anastomosis.
Comorbidities were present in 21 patients overall and included mainly asthma (6), diabetes (5), high blood pressure (5), and celiac disease (2). The rest were fatty liver disease, hypothyroidism, ankylosing spondylitis, and prostate cancer.
Disease activity was quantified clinically by validated questionnaires.
Including 6-mercatopurine, azathioprine, and methotrexate.
All Patients With IBD Achieve Seropositivity After the Second Vaccine Dose, However Those Treated With Anti-TNFα Have Significantly Lower Antibody Titers
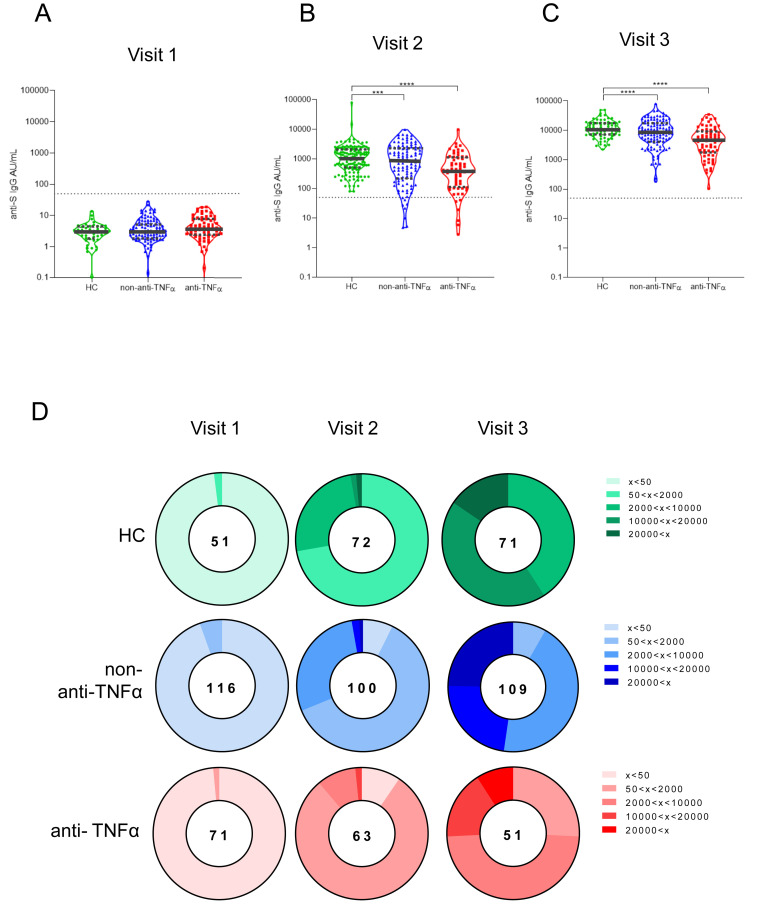
SARS-CoV-2 anti-S IgG antibodies were positive in all subjects after the second vaccine dose (visit 3). This suggests that neither IBD itself nor anti-TNFα treatment abolish the ability to mount serologic response to 2 BNT162b2 doses. However, anti-TNFα treatment was associated with significantly lower antibody levels. Specifically, prevaccination (visit 1), anti-S IgG GMCs were negligible in all subjects (Figure 2 A; Supplementary Table 2). Visit 2 GMCs (95% CI) were 2- to 3-fold lower in the anti-TNFα–treated compared with non–anti-TNFα–treated patients and HC groups: 340 (221–523), 710 (509–991), and 1039 (797–1355), P = .012 and P < .001, respectively (Figure 2 B). GMC increase after the second vaccine dose was robust and similar (around 10-fold) in all study groups maintaining the 2- to 3-fold differences between the groups. Visit 3 GMCs (95% CI) were 3787 (2732–5249), 8320 (6630–10441), and 10979 (9396–12829), P < .001 and P < .001, in the anti-TNFα–treated compared with the non–anti-TNFα–treated patients and HC groups, respectively (Figure 2 C).
Figure 2.
Patients with IBD treated with anti-TNFα have significantly reduced levels of anti-S antibodies. (A–C) Levels of anti-S antibodies in sera from HCs (shown in green), patients with IBD receiving non–anti-TNFα treatment (non–anti-TNFα, shown in blue), and patients with IBD receiving anti-TNFα treatment (anti-TNFα, shown in red). Antibodies were measured by the Abbott quantitative anti-S IgG kit. Visit 1 was before vaccination, visit 2 and visit 3, after first and second vaccine doses, respectively. Statistical analysis was carried out using independent-samples Kruskal-Wallis test. ∗∗∗P < .0005, ∗∗∗∗P < .0001. Black solid line denotes median, black dashed lines denote IQR 25–75. Dotted line represents the threshold for seroconversion (50 AU/mL). Specific GMCs and P values are in Supplementary Table 2. (D) Pie charts representing the fractions of patients at timepoint visits 1, 2, and 3, with anti-SARS-CoV-2 antibody levels as designated in the legend (A–C). Numbers in the middle of pies denote the total number of subjects tested in each group for every timepoint.
Importantly, although all HCs were seropositive at visit 2 (no subject <50 AU/mL), 14 patients with IBD were still seronegative, of whom 6 were treated and 8 untreated with anti-TNFα (Figure 2 D, Supplementary Table 3). We also assessed the GMC fold rise of anti-S antibodies in the individual sera of subjects in the 3 study groups from baseline (visit 1) to either visit 2 and 3, and between visit 2 to 3, demonstrating that similarly to anti-S levels, GMC fold rise is significantly lower in the anti-TNFα group.
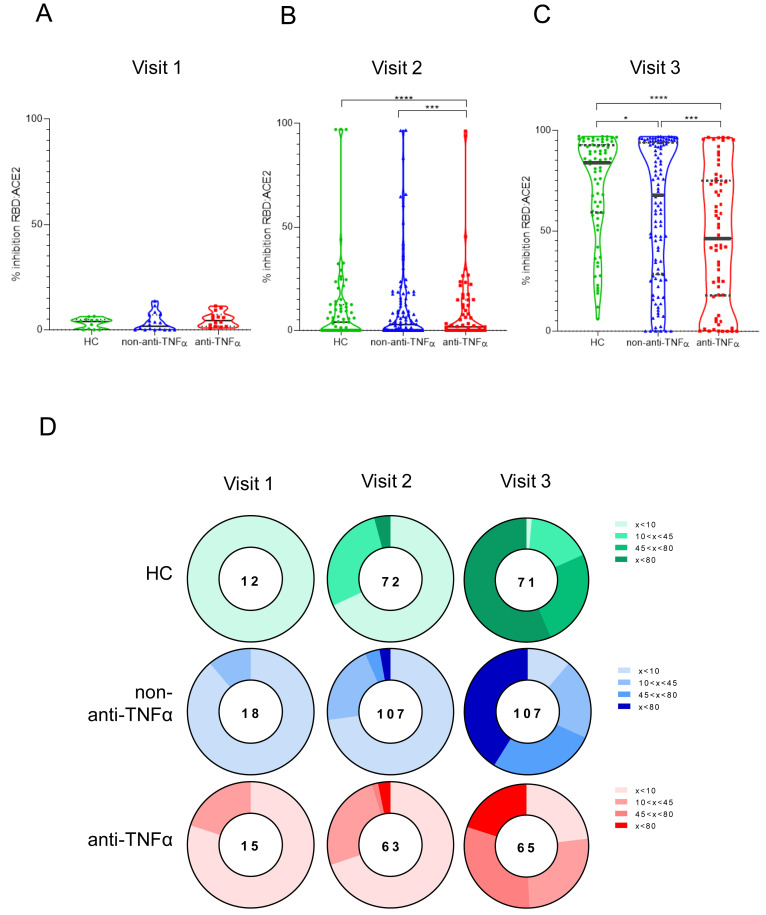
We next assessed neutralizing antibodies, considered critical for patient survival and virus control.26 Using competitive ELISA, we show that although at visit 1 inhibition activity was low and comparable between the groups (Figure 3 A–C, Supplementary Table 4), at visit 2 the anti-TNFα–treated group had significantly lower ability to inhibit RBD:ACE2 binding compared with HCs (P < .05). This was even more prominent at visit 3 (P < .001). Notably, significant differences in inhibition activity were apparent between patients with IBD, regardless of treatment regimen, and HCs (Figure 3 C). We observed a positive correlation between anti-S titers and inhibition activity in visit 2, which increased even further in visit 3, suggesting that after 2 vaccine doses, the proportion of anti-S IgG antibodies with inhibitory function increases (Supplementary Figure 1).
Figure 3.
Patients with IBD treated with anti-TNFα have significantly reduced levels of anti-SARS-CoV-2 inhibiting antibodies. (A–C) Ability of serum from HCs (shown in green), patients with IBD receiving non–anti-TNFα treatment (non–anti-TNFα, shown in blue), and patients with IBD receiving anti-TNFα treatment (anti-TNFα, shown in red) to inhibit SARS-CoV-2 RBD binding to ACE2 receptor. Values measured by ELISA are presented as % inhibition (y axis), following vaccination. Visit 1 was before vaccination, and visit 2 and visit 3 were after first and second vaccine doses, respectively. Zero inhibition was set as the value of RBD without added sera. Statistical analysis was carried out using independent-samples Kruskal-Wallis test. ∗∗∗P < .0005, ∗∗∗∗P < .0001. At least 3 repetitions for every sample. Black solid line denotes median, black dashed lines denote IQR 25–75. Median percentage of inhibitions are in Supplementary Table 4. (D) Pie charts representing the fractions of patients at timepoint visits 1, 2, and 3, who developed none (<20%), low (20%<x<50%), medium (50%<x<80%), and high (>80%) SARS-CoV-2 RBD:ACE2 inhibition, based on (A–C). The numbers in the middle of the pies denote the total number of subjects tested in each group for every timepoint. Correlation between anti-S and inhibition responses are in Supplementary Figure 1.
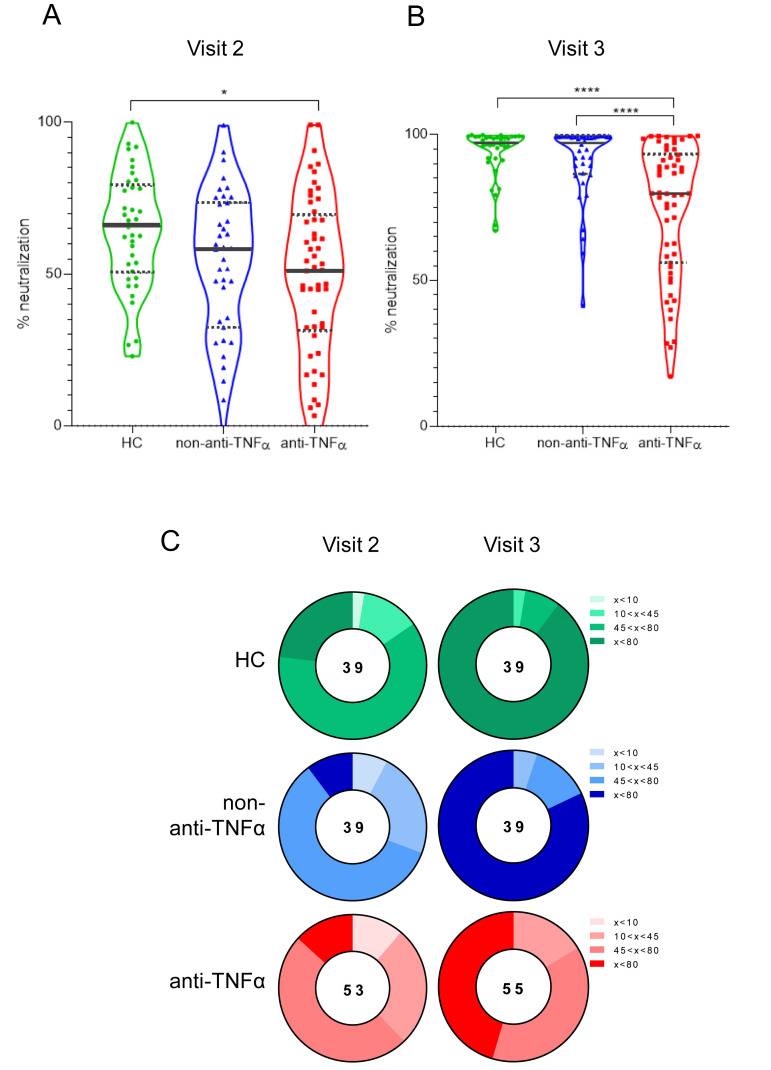
Finally, we assessed vaccine functional activity using SARS-CoV-2 spike pseudoparticle neutralization assays. Serum from patients in all groups did not neutralize infection in visit 1, and was used for normalizing neutralization at visit 2 and visit 3. At visit 2, HC serum had a 65% neutralization capability, contrasting with significantly reduced activity in the anti-TNFα group (51%, P < .05; Supplementary Table 5). Furthermore, at visit 3, serum from the HC and the non–anti-TNFα–treated groups had significantly higher neutralization activity compared with serum from patients in the anti-TNFα group (97%, 96%, and 79%, respectively, P < .0001; Figure 4 A and B). Neutralization activity highly correlated with both anti-S titers and inhibition measures (Supplementary Figures 2 and 3), suggesting that anti-S IgG assays may be indicative of the functional serological anti-SARS-CoV-2 antibody activity.
Figure 4.
Patients with IBD treated with anti-TNFα have significantly reduced levels of anti-SARS-CoV-2 neutralizing antibodies. (A, B) Sera, diluted to a final concentration of 1:200, were incubated with vesicular stomatitis virus-spike pseudo-particles (VSVΔGGFPSΔ19) for 1 hour at 37°C, before infecting ACE2 expressing human embryonic kidney 293 cells for 24 hours. The number of green fluorescent protein–positive cells was normalized and converted to a neutralization percentage in each sample, compared with the average of control samples. Visit 1 was before vaccination, visit 2 and visit 3 were after first and second vaccine doses, respectively. Statistical analysis was carried out using independent-samples Kruskal-Wallis test. ∗∗∗P < .0005, ∗∗∗∗P < .0001. Black solid line denotes median, black dashed lines denote IQR 25–75. (C) Pie charts representing the fractions of patients in timepoints visit 2 and visit 3, who developed none (<20%), low (20%<x<50%), medium (50%<x<80%), and high (>80%) SARS-CoV-2 RBD neutralizing antibodies, based on (A and B). Correlations between anti-S and neutralization, and between inhibition and neutralization, are in Supplementary Figures 2 and 3, respectively. HEK, human embryonic kidney; VSV; vesicular stomatitis virus.
Importantly, non–anti-TNFα IBD therapies did not significantly modify seroconversion or magnitude of response. Specifically, in patients treated with vedolizumab (n = 27, 51.8% UC) or 5-ASA (n = 35, 76.9% UC), vaccine responses were comparable to those of HCs (P = .288, P = .191, respectively). We further analyzed responses of specific subgroups within the non–anti-TNFα group according to treatment. Specifically, 5-ASA (35), vedolizumab (27), no medical treatment (38), and other IBD medications that were used by lower numbers of patients (steroids [7], immunomodulators [8], ustekinumab [5] and JAK inhibitors [3], see Table 1). Measuring serologic and functional responses of the 5-ASA, vedolizumab, no medical treatment, and "other" groups, comparable responses were found, further supporting the decision to include them in the same group (Supplementary Figure 4).
Anti-N, reflecting infection with COVID-19, was positive after the second vaccine dose in <2% of study participants and comparable between the groups. Specifically, anti-N Abs were detected after vaccination in 2 HCs, 2 non–anti-TNFα (1 treated with vedolizumab and 1 untreated), and 1 anti-TNFα (infliximab)–treated patients. Importantly, subjects were asymptomatic and were not aware of having been infected (Supplementary Table 6). In addition, there were 6 subjects who had a positive anti-N already at visit 1. Interestingly, baseline anti-S IgG levels were negative in 4 of them, and positive in 2 (Supplementary Table 6), potentially suggesting differences in the dynamics of serologic responses, although numbers are too small for further analysis. These subjects were not excluded from analysis, given the equal distribution between the groups and the comparability to uninfected patients’ anti-S titers.
Older Age is an Additional Predictor of Lower Vaccine Serologic Response
In univariate analysis (Supplementary Table 7), factors such as older age, male sex, and elevated white blood cell count (WBC) were also associated with a lower serologic response after the first vaccine dose (male sex and WBC values, P = .012 and P = .026, respectively) and after both vaccine doses (older age, P < .001).
In the multivariate linear regression model, only anti-TNFα treatment and older age maintained a significant distinct association with lower IgG anti-S response (P < .001, Table 2 ).
Table 2.
Factors Associated With Serologic Response (Multivariate Linear Regression)
|
Variable |
Visit 2 |
Visit 3 |
|||
|---|---|---|---|---|---|
| Ba (95% CI) | P value | Ba (95% CI) | P value | ||
| Treatment | Anti-TNFα | −0.25 (−1.4 to 0.4) | <.001 | −0.32 (−1.2 to 0.46) | <.001 |
| Non–anti-TNFα | −0.09 | .270 | −0.128 | .141 | |
| HC | Reference | Reference | |||
| Gender | Male | −0.09 | .198 | −0.039 | .602 |
| Female | Reference | Reference | |||
| Age (y) | −0.43 (−0.07 to −0.03) | <.001 | −0.27 (−0.03 to −0.01) | <.001 | |
| IBD current medication | ADA | 0.157 | .074 | 0.081 | .382 |
| IFX | −0.137 | .12 | −0.032 | .732 | |
| Other | 0.007 | .935 | −0.114 | .262 | |
| None | Reference | Reference | |||
| White blood cells (K/μL) | –0.038 | .591 | −0.104 | .159 | |
Standardized Beta coefficients were obtained from linear regression.
The inverse correlation between older age and lower IgG anti-S antibodies levels in the 3 study groups after the first and second vaccine doses is displayed in Supplementary Figure 5, and Supplementary Table 8 shows the consistently lower GMCs in subjects 40 years and older compared with younger ones in all study groups after the first and second vaccine doses.
Anti-TNFα Drug Levels Do Not Affect Serologic Responses
We next asked whether anti-TNFα drug levels mediated lower vaccine serologic responses in this group. Importantly, anti-TNFα drug level measurement was not assessed at trough (ie, immediately before anti-TNFα drug administration), but at the time of serologic assessment at each visit. No correlation between drug levels and serologic responses was observed, using Spearman’s correlation (Supplementary Table 9).
We further asked whether lower responses in patients treated with anti-TNFα were affected by the interval between anti-TNFα drug administration and vaccination. Importantly, no such correlation was observed either when anti-TNFα drugs were administered before the first or second vaccine doses. Specifically, in Supplementary Figure 6, serologic responses for 3 time intervals between drug and vaccine administration are presented, showing no difference in serologic responses in the different time intervals (using Independent-Samples Kruskal-Wallis Test). Finally, only 2 patients had anti-IFX and 2 anti-ADA drug antibodies. Those did not correlate with vaccine serologic responses (Supplementary Table 10).
Vaccine Is Safe in Patients With IBD and Is Not Associated With Increased IBD Activity
Immediate and short-term AEs were detected using phone call and accepted questionnaires, respectively. We further evaluated IBD exacerbation using clinical and laboratory variables. To this end, no SAEs were registered. The most common AEs were local pain (<70%) and headache (∼30%), with more AEs after the second compared with first vaccine dose (Supplementary Table 11). AEs were not in excess or more prominent in patients treated with anti-TNFα who had higher drug levels during vaccination (P = .722 and P = .909 after first and second vaccine dose, respectively, using Pearson’s correlation). Finally, baseline IBD activity was comparable in patients treated with anti-TNFα or not and remained comparable after the first and second vaccine doses (Supplementary Table 12, Supplementary Figure 7). Neither CRP levels nor WBC count were increased following vaccination in both groups.
Discussion
Patients with IBD treated with immunomodulators and/or anti-TNFα biologics are at an increased risk of vaccine-preventable diseases, and vaccination programs are recommended. Patients with chronic diseases were more prone to COVID-19 complications and death.27, 28, 29 Vaccination campaigns encouraged patients with IBD to vaccinate,30 , 31 despite their exclusion from phase-3 trials.2 , 3 Here, we aimed to prospectively evaluate serologic responses and safety of the BNT162b2 vaccine in patients with IBD.
Our results show that all subjects, regardless of medical treatment, seroconverted after the second vaccine dose, consistent with recent reports.32 , 33 However, patients treated with anti-TNFα had significantly lower serologic responses, represented by 2- to 3-fold decreased anti-S levels, compared with patients not treated with anti-TNFα and HCs. Furthermore, impaired immune function was demonstrated by significantly lower RBD:ACE2 inhibition and significantly lower capability to neutralize SARS-CoV-2 in a pseudoviral assay.According to recent population-based reports, patients with IBD treated with anti-TNFα had a lower serologic response to COVID-19 infection 34, 35, 36 and vaccination,35 , 37 , 38 in line with previous reports regarding other vaccines.15, 16, 17 , 39 , 40 Our study is the first to prospectively and comprehensively demonstrate the profound impairment in functional serologic responses in anti-TNFα–treated patients with IBD, which may be relevant for other immune-mediated diseases as well.25 Reassuringly, the rate of anti-N Abs was low and comparable in all groups, suggesting that protection was enough to prevent short-term infection. However, recent evidence of declining serologic responses post infection,35 , 41 as well as the data presented herein demonstrating significantly lower serologic responses, in patients with IBD treated with anti-TNFα, observed already 4 weeks after the second vaccine dose, raise the question of, although may not directly prove, lower durability of response in patients treated with anti-TNFα compared with non–anti-TNFα–treated patients or HCs. If indeed both longevity and neutralizing activity of anti–SARS-CoV-2 antibodies is reduced, the consequence may be reduction in infection protection, supporting earlier booster vaccination for this patient subpopulation, as already considered for severely immunocompromised patients such as those with certain cancers and chemotherapy.42 , 43
The prospective nature of our study enabled evaluation of serologic responses dynamics. Importantly, approximately 10% of patients treated with anti-TNFα were still seronegative after the first vaccine dose, and an additional 18% had a low level (50–150 AU/mL) of anti-S antibodies. This supports maintenance of thorough COVID-19 precautions for them and their household members until after the second vaccine dose. Notably, after the first vaccine dose, there were also 8 (7%) seronegative patients in the non–anti-TNFα–treated group, pointing to additional patient factors that may modify seronegativity.
In this regard, we found that age was an independent predictor of lower vaccine serologic responses, regardless of IBD treatment. Although our patients were mostly young (∼37 years), a continuous decline in serology with age was noticed. As older age is also a risk factor for severe COVID-19,44 , 45 these patients should be at highest priority for booster vaccine doses. A recent report from the US Veteran Affairs database demonstrating only 80.4% vaccine effectiveness in a patient population with a median age of 68 supports our finding.46
Our study, the first specifically designed to address vaccine timing relative to anti-TNFα drug administration did not demonstrate such correlation. Moreover, in 14 patients vaccinated during anti-TNFα induction, responses were comparable to those vaccinated during maintenance. Anti-TNFα drug levels during vaccination were unrelated to impaired responses. In a recent publication36 focusing on patients with IBD after SARS-CoV-2 infection, those having undetectable anti-TNFα drug levels were more likely to be seropositive. Although no data were provided regarding time interval between infection and anti-TNFα measurement, and the dichotomous correlations rather than quantitative assessment performed in our study, further data regarding anti-TNFα drug levels and serologic response to infection and vaccination are anticipated. Altogether, our findings lend evidence to the empiric recommendation to vaccinate patients with IBD regardless of anti-TNFα administration timing.30
Importantly, no SAEs were reported in the week following vaccination. Although our study included only 10 subjects younger than 21 years, it is reassuring that no cardiac AEs, specifically myocarditis,47 were detected. AEs were similar in all subjects: mainly local pain, and resolved within a few days. No IBD exacerbation was observed, regardless of disease activity during vaccination. This is specifically reassuring, as approximately a third of patients were not in remission. Because anti-TNFα is a common treatment for other immune-mediated inflammatory diseases, those results are highly relevant to multiple patient populations and diseases.
There are several strengths to our study. This is the first prospective multicenter study comprehensively investigating multiple aspects of BNT162b2 vaccine serologic responses in patients with IBD. Most subjects were recruited before the first vaccine dose, allowing longitudinal evaluation of the dynamics of serologic response development. While focusing on anti-TNFα therapy, all other IBD therapies, or no therapy were included; thus, enabling differentiation between disease and treatment effects. Finally, this is the first study addressing timing of vaccination and anti-TNFα drug administration, levels, or anti-drug antibodies, showing a lack of correlation. Another meaningful strength is assessment of vaccine safety including IBD activity, as previous reports in other immune-mediated diseases suggested disease exacerbation postvaccination.48, 49, 50, 51, 52, 53
Our study, including 67 patients treated with anti-TNFα, was powered to demonstrate significant differences, which indeed were apparent, between them and to patients without anti-TNFα treatment. As a non–anti-TNFα group was specifically designed to include multiple IBD therapies, we were able to address serologic response of these subgroups as well, demonstrating its comparability to HCs (Supplementary Figure 4), although the small numbers of patients treated with steroids and immunomodulators limit findings in these specific subgroups.33 , 34 , 38 , 54 Limitations include difference in gender ratio between IBD and HC groups at baseline, the relatively young age of participants (although this reflects typical IBD populations), and the use of only 1 vaccine type. Evaluation of vaccine efficacy is limited, as infection rate in Israel during the study period was low. Finally, observation was limited to 4 weeks after the second vaccine.
To conclude, our study provides prospective, controlled evidence for the efficacy and safety of the COVID-19 BNT162b2 vaccine in patients with IBD stratified according to therapy. We demonstrate the dynamics of development of functional serologic response and the factors causing impairment, specifically anti-TNFα therapy and older age. The lack of correlation with timing of anti-TNFα therapy or drug levels, enables important clinical guidance to patients and their caregivers.
As immune response longevity in this group may be limited, vaccine booster dose should be considered.
Long-term outcomes and the mechanism of decreased serologic responses should be evaluated.
Acknowledgments
We thank study participants for their effort and time. The Israeli IBD Society and Israeli GI Association are thanked for their support, and Dr Naim Abu-Freha for specific efforts to promote the study. The study was partially supported by a generous grant from The Leona M. and Harry B. Helmsley Charitable Trust. The Crohn's and Colitis Foundation of Israel, the European Federation of Crohn's and Colitis Associations, and the AMICI group are thanked for partially funding the study and supporting recruitment efforts. We thank Kawsar Kaboub and Hanan Abu Taha from the Mucosal Immunology Laboratory at the Felsenstein Medical Research Center for their scientific contribution. This study was performed in collaboration with the Israeli Ministry of Health. Natalia T. Freund acknowledges the kind support of L. Cohen and the Milner Foundation.
CRediT Authorship Contributions
Hadar Edelman-Klapper, MD, PhD (Data curation: Lead; Formal analysis: Supporting; Methodology: Supporting; Writing – original draft: Lead)
Eran Zittan, MD (Data curation: Lead)
Ariella Bar-Gil Shitrit, MD (Data curation: Lead)
Keren Masha Rabinowitz, PhD (Conceptualization: Supporting; Formal analysis: Lead; Methodology: Supporting; Writing – review & editing: Supporting)
Idan Goren, MD (Data curation: Supporting)
Irit Avni-Biron, MD (Data curation: Supporting)
Jacob E. Ollech, MD (Data curation: Supporting)
Lev Lichtenstein, MD (Data curation: Supporting)
Hagar Banai-Eran, MD (Data curation: Supporting)
Henit Yanai, MD (Data curation: Supporting)
Yifat Snir, MD (Data curation: Supporting)
Maor H. Pauker, PhD (Conceptualization: Supporting; Data curation: Supporting;
Methodology: Supporting; Project administration: Lead)
Adi Friedenberg, BPT (Conceptualization: Supporting; Data curation: Equal; Project administration: Equal)
Adva Levy-Barda, PhD (Data curation: Supporting)
Arie Segal, MD (Data curation: Supporting)
Yelena Broitman, MD (Data curation: Supporting)
Eran Maoz, MD (Data curation: Supporting)
Baruch Ovadia, MD (Data curation: Supporting)
Maya Aharoni Golan, MD (Data curation: Supporting)
Eyal Shachar, MD (Data curation: Supporting)
Shomron Ben-Horin, MD (Data curation: Supporting)
Tsachi-Tsadok Perets, PhD (Data curation: Supporting)
Haim Ben Zvi, PhD (Data curation: Supporting)
Rami Eliakim, MD (Data curation: Supporting)
Sophy Goren, BSc (Data curation: Equal; Formal analysis: Supporting)
Michal Navon, BMs (Data curation: Supporting; Formal analysis: Supporting)
Noy Krugliak, BSc (Data curation: Supporting; Formal analysis: Supporting)
Michal Werbner, PhD (Data curation: Supporting; Formal analysis: Supporting)
Joel Alter, PhD (Data curation: Supporting; Formal analysis: Supporting)
Moshe Dessau, PhD (Data curation: Supporting; Formal analysis: Supporting)
Meital Gal-Tanamy, PhD (Data curation: Supporting; Formal analysis: Equal; Funding acquisition: Supporting; Methodology: Equal; Writing – review & editing: Supporting)
Natalia T. Freund, PhD (Data curation: Supporting; Formal analysis: Equal; Funding acquisition: Supporting; Methodology: Equal; Writing – review & editing: Supporting)
Dani Cohen, PhD (Data curation: Supporting; Formal analysis: Lead; Writing – review & editing: Supporting)
Iris Dotan, MD (Conceptualization: Lead; Funding acquisition: Lead; Methodology: Lead; Supervision: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest Iris Dotan: Consultation/advisory board: Abbvie, Arena, Cambridge Healthcare, Celltrion, Celgene/BMS, Ferring, Janssen, MSD, Neopharm, Pfizer, Rafa laboratories, Roche/Genentech, and Takeda; and Speaking/teaching: Abbvie, Celltrion, Celgene/BMS, Ferring, Falk Pharma, Janssen, MSD, Neopharm, Pfizer, Rafa laboratories, Roche/Genentech, Sandoz, and Takeda. Ariella Bar-Gil Shitrit: Grant support from Takeda and Janssen, consultancy and lectures fees from Takeda, Janssen, Abbvie, Pfizer, Neopharm, and BMS. Eran Zittan has received research support and consulting fees from Janssen, Abbvie, Takeda, Neopharm, Celgene, and Pfizer. The remaining authors disclose no conflicts.
Funding The study was partially supported by a generous grant from The Leona M. and Harry B. Helmsley Charitable Trust # G-2101-04950 (Iris Dotan). The Crohn's and Colitis Foundation of Israel and the European Federation of Crohn's and Colitis Associations partially supported the study. Natalia T. Freund and Meital Gal-Tanamy are funded by Israel Science Foundation (ISF) Coronavirus grant #3711/20. Natalia T. Freund is funded by ISF grant #1422/18. Meital Gal-Tanamy is funded by ISF grant #2475/19. Moshe Dessau is funded by ISF grant #401/18.
Author names in bold designate shared co-first authorship.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.10.029.
Supplementary Material
References
- 1.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim Guidance. World Health Organization; 2020.
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahier J.F., Magro F., Abreu C., et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohn’s Colitis. 2014;8:443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka K., Kobayashi T., Ueno F., et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford A.C., Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–1276. doi: 10.1038/ajg.2013.138. [DOI] [PubMed] [Google Scholar]
- 7.Farraye F.A., Melmed G.Y., Lichtenstein G.R., et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 8.Rubin L.G., Levin M.J., Ljungman P., et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 9.Dotan I., Werner L., Vigodman S., et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. 2012;18:261–268. doi: 10.1002/ibd.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melmed G.Y., Agarwal N., Frenck R.W., et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. doi: 10.1038/ajg.2009.523. [DOI] [PubMed] [Google Scholar]
- 11.Melmed G.Y., Ippoliti A.F., Papadakis K.A., et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen H.J., Mortensen T., Holten-Andersen M., et al. Increased levels of specific leukocyte- and platelet-derived substances during normal anti-tetanus antibody synthesis in patients with inactive Crohn disease. Scand J Gastroenterol. 2001;36:265–269. doi: 10.1080/003655201750074537. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.K., Kim H.S., Ye B.D., et al. Patients with Crohn’s disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohn’s Colitis. 2014;8:384–391. doi: 10.1016/j.crohns.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Shu H.J., Lu H., et al. Long-term risk of infection in patients with Crohn’s disease on anti-TNF treatment: a prospective single-center cohort study in China. J Dig Dis. 2017;18:388–394. doi: 10.1111/1751-2980.12499. [DOI] [PubMed] [Google Scholar]
- 15.Fiorino G., Peyrin-Biroulet L., Naccarato P., et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. 2012;18:1042–1047. doi: 10.1002/ibd.21800. [DOI] [PubMed] [Google Scholar]
- 16.Shirai S., Hara M., Sakata Y., et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082–1091. doi: 10.1093/ibd/izx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launay O., Abitbol V., Krivine A., et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two-year prospective study. J Crohns Colitis. 2015;9:1096–1107. doi: 10.1093/ecco-jcc/jjv152. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous The COVID-19 vaccine operation launched today | Ministry of Health. https://www.gov.il/en/Departments/news/19122020-02 Available at:
- 19.Harvey R.F., Bradshaw J.M. A simple index of Crohn’s-disease activity. Lancet. 1980;315:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 20.Walmsley R.S., Ayres R.C.S., Pounder R.E., et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandborn W.J., Tremaine W.J., Batts K.P., et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. doi: 10.1016/s0025-6196(12)61634-6. [DOI] [PubMed] [Google Scholar]
- 22.Norquist J.M., Khawaja S.S., Kurian C., et al. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA Toxicity Grading Scale Guidance. Hum Vaccines Immunother. 2012;8:1208–1212. doi: 10.4161/hv.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anonymous. SARS-CoV-2 Immunoassay | Abbott Core Laboratory. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2 Available at:
- 24.Mor M., Werbner M., Alter J., et al. Multi-clonal SARS-CoV-2 neutralization by antibodies isolated from severe COVID-19 convalescent donors. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagin D., Freund T., Navon M., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dispinseri S., Secchi M., Pirillo M.F., et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun. 2021;12:1–12. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robilotti E.V., Babady N.E., Mead P.A., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Fang X., Cai Z., et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020 doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alyammahi S.K., Abdin S.M., Alhamad D.W., et al. The dynamic association between COVID-19 and chronic disorders: an updated insight into prevalence, mechanisms and therapeutic modalities. Infect Genet Evol. 2021;87 doi: 10.1016/j.meegid.2020.104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel C.A., Melmed G.Y., McGovern D.P.B., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander J.L., Moran G.W., Gaya D.R., et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6:218–224. doi: 10.1016/S2468-1253(21)00024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jena A., Mishra S., Deepak P., et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmune Rev. 2021 doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kappelman M.D., Weaver K.N., Boccieri M., et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology. 2021;161:1340–1343. doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy N.A., Goodhand J.R., Bewshea C., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 35.Dailey J., Kozhaya L., Dogan M., et al. Antibody responses to SARS-CoV-2 after infection or vaccination in children and young adults with inflammatory bowel disease. Inflamm Bowel Dis. 2021 doi: 10.1093/ibd/izab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chanchlani N., Lin S., Chee D., et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11422 biologic-treated patients. J Crohn’s Colitis. 2021 doi: 10.1093/ecco-jcc/jjab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong S.-Y., Dixon R., Pazos V.M., et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161:715. doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy N.A., Lin S., Goodhand J.R., et al. Inflammatory bowel disease Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70:1884–1893. doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 39.Debruyn J., Fonseca K., Ghosh S., et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2015;22:638–647. doi: 10.1097/MIB.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 40.Pratt P.K., David N., Weber H.C., et al. Antibody response to hepatitis B virus vaccine is impaired in patients with inflammatory bowel disease on infliximab therapy. Inflamm Bowel Dis. 2018;24:380–386. doi: 10.1093/ibd/izx001. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan G.G., Ma C., Charlton C., et al. Antibody response to SARS-CoV-2 among individuals with IBD diminishes over time: a serosurveillance cohort study. Gut. 2021 doi: 10.1136/gutjnl-2021-325238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia Medscape Continuing Medical Education online. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF Antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang W., Liang H., Ou L., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan N., Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a Veterans Affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161:827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall M., Ferguson I.D., Lewis P., et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 48.Waheed S., Bayas A., Hindi F., et al. Neurological complications of COVID-19: Guillain-Barre Syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13 doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akinosoglou K., Tzivaki I., Marangos M. Covid-19 vaccine and autoimmunity: awakening the sleeping dragon. Clin Immunol. 2021;226 doi: 10.1016/j.clim.2021.108721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bril F., Diffalha S. A.l., Dean M., et al. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:221–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee E.J., Cines D.B., Gernsheimer T., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruddy J.A., Connolly C.M., Boyarsky B.J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.