Abstract
Objective
To evaluate the muscle strength and functional level of patients discharged from intensive care unit (ICU) in relation to the swimmer position as a nurse intervention during pronation.
Methods
Prospective study conducted in the hub COVID-19 center in Milan (Italy), between March and June 2020. All patients with COVID-19 discharged alive from ICU who received invasive mechanical ventilation were included. Forward continuation ratio model was fitted to explore the statistical association between muscle strength grades and body positioning during ICU stay.
Results
Over the 128 patients admitted to ICU, 87 patients were discharged alive from ICU, with available follow-up measures at hospital discharge. Thirty-four patients (39.1%) were treated with prone positioning as rescue therapy, for a total of 106 pronation cycles with a median duration of 72 (IQR 60–83) hours. Prone positioning did not influence the odds of showing particular level of muscle strength, in any of the evaluated districts, namely shoulder (OR 1.34, 95%CI:0.61–2.97), elbow (OR 1.10, 95%CI:0.45–2.68) and wrist (OR 0.97, 95%CI:0.58–1.63). Only in the shoulder district, age showed evidence of association with strength (OR 1.06, 95%CI:1.02–1.10), affecting people as they get older. No significant sequalae related to swimmer position were reported by physiotherapists or nurses.
Conclusion
Swimmer position adopted during prone ventilation is not associated with worse upper limb strength or poor mobility level in COVID-19 survivors after hospital discharge.
Keywords: COVID-19, Intensive Care Units, Nursing, Physiotherapy, Prone position
Implications for clinical practice.
-
•
Pronation with arms placed in swimmer position was not associated with shoulder muscle strength at ICU discharge.
-
•
Swimmer position performed by nurses is feasible in ICU.
-
•
Multi-professional ICU team was the key to support at best patients admitted to ICU during the first months of pandemic.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that has spread globally at the beginning of 2020 (Huang et al., 2020). SARS-CoV-2 infection affects the respiratory system causing an acute respiratory distress syndrome (ARDS) that can require extended periods of invasive mechanical ventilation (Richardson et al., 2020). In particular, many patients required also prone positioning (Langer et al., 2021) as a rescue therapy, which does not come without complications, like pressure ulcers (Binda et al., 2021a, Douglas et al., 2021, Ibarra et al., 2020, Lucchini et al., 2020, Shearer et al., 2021) and musculoskeletal injuries (Goettler et al., 2002, Malik et al., 2020).
The guidelines from United Kingdom recommend to use the alternate swimmer position for the upper limbs of patients sedated and mechanically ventilated requiring pronation (Bamford et al., 2019). Prolonged prone positioning in patients with ARDS, which is also recommended by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine (Alhazzani et al., 2020, Mitchell and Seckel, 2018), increases the risk of exposure to localized compression in specific part of the body and can be responsible for nerve damage and plexopathy (Brugliera et al., 2021, Simpson et al., 2020). The head rotation on the side of the abducted arm advised within the swimmer position is used by nurses to maintain neutral alignment of the cervical spine, becoming also a relief for the emerging roots from intervertebral foramen (Bogduk and Mercer, 2000, Mihara et al., 2018). However, current evidence shows that patients mechanically ventilated have high risk of developing also limbs muscles weakness (Medrinal et al., 2020), and the differentiation between the post critical care weakness and the prone positioning plexopathy can be difficult (Shepherd et al., 2017). Considering the severity of COVID-19, it is reasonable to expect that some patients with ARDS undergoing prone positioning may experience upper limbs weakness. So far, early physiotherapy mobilization has been recommended to prevent muscles weakness and limit further sequelae potentially due to prone positioning (Eggmann et al., 2021, Thomas et al., 2020), thus making physiotherapy an essential part of the complex management of patients in ICU.
The primary aim of this study was to evaluate the levels of muscle strength of patients discharged from ICU, and to report any adverse event related to the swimmer position adopted during the ICU stay. As a secondary aim, we explored how muscle strength and functional measures of independence changed over time, and if these were influenced by prone positioning.
Methods
Study design
This prospective single-centre study was conducted at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, an academic tertiary-level hospital in Milan (Italy), during the COVID-19 pandemic between March and June 2020. We included all adult patients with laboratory-confirmed SARS-CoV-2 infection admitted to ICU and discharged alive from ICU who received invasive mechanical ventilation. Considering the repeated-measure design, patients were excluded if died before the ICU discharge. The study was approved by the local ethics committee of our Institution (ethics approval number 667_2020).
Nursing and physiotherapy interventions
The prone positioning maneuver was used for all patients as a rescue measure in case of severe impairment of gas exchanges (PaO2/FiO2 ≤100), after having optimized the ventilatory strategy in supine position (Foti et al., 2020). Enteral nutrition was initiated early after ICU admission using a high protein liquid formula. Before the maneuver, the gastric content was suctioned to avoid inhalation and enteral nutrition was continued, monitoring episode of intolerance (Bruni et al., 2020).
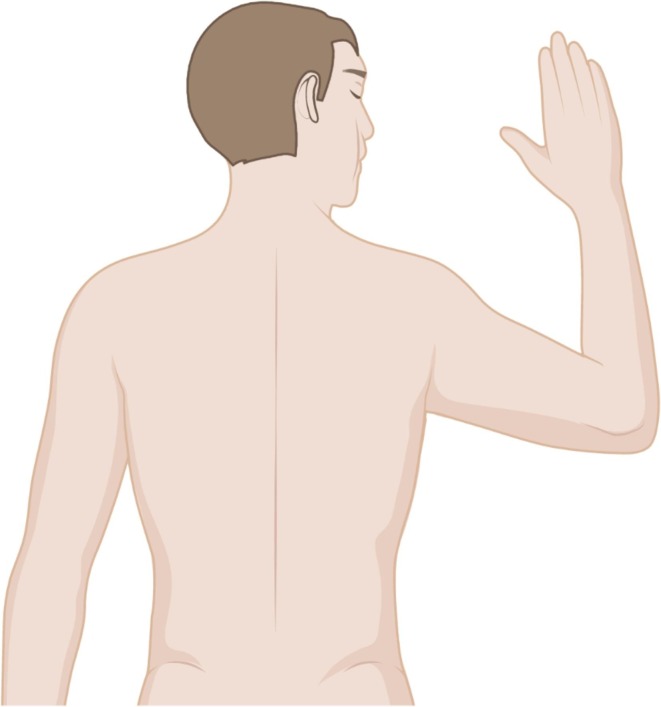
Four nurses and one experienced team leader were necessary to perform a safety maneuver following a strict protocol, as already reported by our group elsewhere (Binda et al., 2021b). Patients were rolled into prone position, and upper limbs were carefully placed in the swimmer position. This involves the rotation of the head on the side of the abducted arm and the other arm placed by the patient side. The shoulder should be abducted to 80° and the elbow and forearm should be slightly flexed and pronated so as to not create a stretching force along the median and ulnar nerves (Bamford et al., 2019, Nee et al., 2010) (Fig. 1 ). The upper arms and head rotation were scheduled every 2–4 hours, after fixing the endotracheal tube (Bamford et al., 2019). The use of thoracic pelvic supports and other special measures (foam head support or pillows) during prone positioning was avoided (Chiumello et al., 2006).
Fig. 1.
Prone patient in swimmer position. The position involves raising one arm on the same side to which the head is facing whilst placing the other arm by the patient side. The figure was created with permission of BioRender.com.
After withdrawal from neuromuscular blocking agents (NMBA), all patients were evaluated by physiotherapists. A progressive rehabilitation programme aimed to maintain or restore functional range of movement, strength, and functional capacity was implemented. Physiotherapists delivered passive and active mobilization, muscle strengthening exercise, balance training and progressive re-training of postural steps, with the overall goal to recover functional activities of daily living (ADL) through the entire length of hospitalization. Specifically, considering the swimmer position adopted when patients were prone, physiotherapists mostly focused on early recognition of nerve injuries and treatment of potential muscle weakness, as described in a recent study (Miller et al., 2021).
Data collection
Strength and functional measures were evaluated at ICU and hospital discharge. Physiotherapists used Manual Muscle Testing (MMT) to assess muscles strength, assigning a score depending on how much one patient was able to resist the pressure, measured on a five-point scale (Ciesla et al., 2011). Although relying on a subjective assessment, MMT returns reliable results (Bohannon, 2019). Muscles were tested during shoulder abduction, elbow flexion and wrist extension. These muscles were selected based on the standard approach for evaluating patients for ICU-acquired weakness used in prior publications (Ali et al., 2008, De Jonghe et al., 2002). Physiotherapists also recorded the Manchester Mobility Score (MMS), a seven-point scale used for assessing mobility levels in critical care setting (McWilliams et al., 2016). Before hospital discharge, ADL were assessed using the Barthel index, an ordinal scale that included ten variables describing mobility and independence (Mahoney and Barthel, 1965).
Baseline characteristics, including demographics, days of invasive mechanical ventilation, days of NMBA administration, tracheostomy insertion, use of prone positioning, ward- and ICU-length of stay were obtained from medical records. All study data were collected using the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico secure REDCap database (Research Electronic Data Capture, version 11.0.3) (Harris et al., 2009).
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical features. We presented patients’ characteristics stratified by the body positioning adopted during ICU stay as median (interquartile range, IQR) and count (percentage). Difference between continuous or categorical variables were tested with Wilcoxon rank sum test or Fisher test. Correlation between continuous measures was explored using Spearman correlation coefficient (ρ), and the Benjamini-Hochberg method was applied to correct for multiple testing. Considering that muscle strength was measured on an ordinal five-point scale, we estimated the conditional odds of showing a particular strength, given that an individual has reached that level of strength or above. Therefore, a forward continuation ratio (Agresti, 2012) model was fitted to explore the statistical association between muscle strength grades and body positioning during ICU stay, adjusted for the number of days under NMBA, length of hospital stay and Sequential Organ Failure Assessment (SOFA) score. Based on the lowest Akaike information criteria, age entered in the model as linear. Every model was fitted separately for each body district (i.e., shoulder, elbow, and wrist). We used the same approach to explore the association between selected variables and the scores of the MMS scale. Results from these models are reported as odds ratio (OR) and 95% confidence intervals (CI).
For all analyses, p-values were two-sided, and p < 0.05 was considered statistically significant. All of the current analyses were performed using R Core Team (R Core Team, 2019), version 3.6.2 with package GLMMadaptive added.
Results
Over the 128 patients admitted to our ICU in the considered period, 35 patients died before weaning and other 6 were treated only with noninvasive ventilation. This analysis focused on the 87 patients who were discharged alive from ICU, with available follow-up measures at hospital discharge.
Table 1 reports patients’ characteristics at ICU discharge.
Table 1.
General characteristics of COVID-19 ICU survivors.
| Demographic characteristics | Overall (N = 87) | Supine (N = 53) | Prone (N = 34) |
|---|---|---|---|
| Age (years) | 58.0 (50.0–64.0) | 58.0 (50.0–64.0) | 58.0 (50.0–65.0) |
| Sex (female) | 23 (26.4%) | 15 (28.3%) | 8 (23.5%) |
| Sex (male) | 64 (73.6%) | 38 (71.7%) | 26 (76.5%) |
| Ethnicity | |||
| Caucasian | 77 (88.5%) | 46 (86.8%) | 31 (91.2%) |
| Black | 4 (4.6%) | 3 (5.7%) | 1 (2.9%) |
| Hispanic | 3 (3.4%) | 3 (5.7%) | 0 (0%) |
| Arab | 2 (2.3%) | 1 (1.9%) | 1 (2.9%) |
| Asian | 1 (1.1%) | 0 (0%) | 1 (2.9%) |
| Clinical characteristics | |||
| Body Mass Index | |||
| Normal weight (≤24.9) | 24 (27.6%) | 16 (30.2%) | 8 (23.5%) |
| Overweight (25.0–29.9) | 40 (46.0%) | 24 (45.3%) | 16 (47.1%) |
| Obesity (≥30.0) | 23 (26.4%) | 13 (24.5%) | 10 (29.4%) |
| SOFA score at ICU admission | 5.0 (4.0–8.0) | 5.0 (3.0–7.0) | 6.5 (4.0–9.5) |
| NMBA therapy (days) | 11.0 (5.0–19.0) | 7.0 (2.0–12.0) | 18.0 (11.0–22.0) |
| Length of IMV (days) | 16.0 (10.0–28.0) | 12.0 (7.0–21.0) | 24.0 (14.0–48.0) |
| Length of ICU stay (days) | 17.0 (12.0–32.0) | 14.0 (9.0–32.0) | 27.0 (17.0–50.0) |
| Tracheostomy | 22 (25.3%) | 7 (13.2%) | 15 (44.1%) |
Data are presented as counts (%) or median (IQR).
Abbreviations: ICU, Intensive Care Unit; IMV, Invasive Mechanical Ventilation; NMBA, Neuro Muscular Blocking Agents; SOFA, Sequential Organ Failure Assessment Score.
Prone position was used in 39.1% (34/87) of patients as rescue therapy, for a total of 106 pronation cycles with a median duration of 72 (IQR 60–83) hours. In particular, 64.7% (22/34) of patients were positioned prone for longer than ≥ 16 hours. No significant adverse events related to swimmer position were reported by physiotherapists or nurses.
Table 2 reports the functional characteristics of patients when they were discharged from ICU. Neither strength nor MMS score showed evidence of statistical differences between groups. Using a clinical cut-off of MMS < 3, almost 85.1% of patients in both groups could not even be hoisted to chair.
Table 2.
Functional characteristics stratified by body position at ICU discharge.
| Physiotherapy metrics | Supine (N = 53) | Prone (N = 34) | P-value |
|---|---|---|---|
| Manual Muscle Testing | |||
| Shoulder | 0.819 | ||
| ≤2 | 18 (34.0%) | 13 (38.0%) | |
| ≥3 | 35 (66.0%) | 21 (62.0%) | |
| Elbow | 0.296 | ||
| ≤2 | 9 (17.0%) | 9 (26.5%) | |
| ≥3 | 44 (83.0%) | 25 (73.5%) | |
| Wrist | 0.101 | ||
| ≤2 | 4 (7.5%) | 7 (20.6%) | |
| ≥3 | 49 (92.5%) | 27 (79.4%) | |
| Manchester Mobility Score | 0.925 | ||
| 1 – In bed interventions | 22 (41.5%) | 14 (41.2%) | |
| 2 – Sit on edge of bed | 23 (43.4%) | 15 (44.1%) | |
| 3 – Hoisted to chair | 1 (1.9%) | 0 (0%) | |
| 4 – Standing practice | 0 (0%) | 0 (0%) | |
| 5 – Transfers with assistance | 3 (5.7%) | 4 (11.8%) | |
| 6 – Mobilizing with or without assistance | 3 (5.7%) | 1 (2.9%) | |
| 7 – Mobilizing > 30 m | 1 (1.9%) | 0 (0%) | |
Data are presented as counts (%).
On average, patients who were supine in ICU spent 12 days less in hospital compared to patients treated with prone position, respectively 38 (IQR 29–53) and 50.5 (IQR 36–81.75) days (P = 0.010). Barthel index was not statistically different between patients treated prone or supine (P = 0.746), when evaluated at hospital discharge. However, patients discharged to home (46/87) scored at Barthel index 10.0 points higher than those discharged to other hospital facilities (34/87), like rehabilitation units (P < 0.001). Regarding MMS, no evidence of difference was found between groups at hospital discharge (P = 0.073).
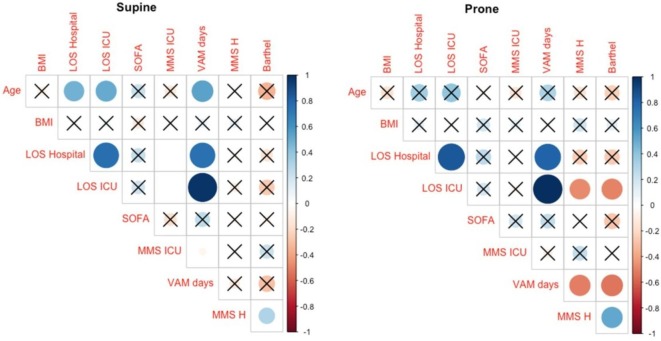
The analysis of ICU survivors revealed a statistically significant correlation between few variables between prone and supine groups (Fig. 2 ). Particularly, Barthel index and MMS administered at hospital discharge showed a moderate positive correlation (ρ = 0.62, P = 0.001) in the prone group; however, the strength of such relationship was weaker for patients who were supine (ρ = 0.37, P = 0.038). When patients were prone, correlation between age and length of hospital stay or days spent mechanically ventilated disappeared compared to patients in supine position. At hospital discharge, the same pattern was seen between the Barthel index and MMS score with the length of ICU stay or days of mechanical ventilation.
Fig. 2.
Correlation plot in patients treated with supine or prone position. Correlations not surviving correction for multiplicity are crossed. Direction and strength of correlation is displayed both by colors and circles areas.
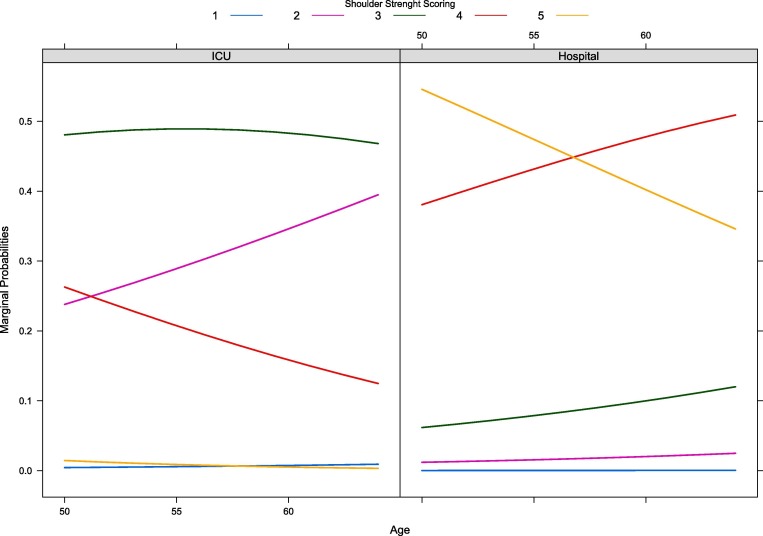
From the fitted models, prone positioning did not influence the odds of showing particular level of strength, in any of the evaluated districts, namely shoulder (OR 1.34, 95%CI:0.61–2.97, P = 0.468), elbow (OR 1.10, 95%CI:0.45–2.68, P = 0.839) and wrist (OR 0.97, 95%CI:0.58–1.63, P = 0.916). Only in the shoulder district, age showed evidence of association with the odds of having a particular strength (OR 1.06, 95%CI:1.02–1.10) rather than higher one. In other words, the older were the patients, the smaller the odds of showing higher levels of strength. Marginal probabilities of each strength level for the shoulder are reported in Fig. 3 . At ICU discharge, patients were more likely than patients at hospital discharge to stop out at a particular strength (shoulder and elbow: ORs 0.03, 95%CI:0.01–0.08; wrist OR 0.22, 95%CI: 0.14–0.35). This also applies to MMS (OR 0.01, 95%CI: 0.00–0.03). Particularly, the number of days under NMBA influenced the odds of showing a particular MMS level (OR 1.06 95%CI:1.00–1.12) rather than higher one. SOFA and ICU length of stay did not show evidence of association with muscle strength or MMS.
Fig. 3.
Forward continuation ratio model for shoulder strength. Lines depict marginal probabilities for each strength level. For example, when discharged from ICU, a patient aged 60 years shows greater probability of having strength equal to 3 rather than 4 or 5. Before leaving the hospital, the probability of one patient of the same age to show strength equal to 4 is higher than showing a normal muscle strength (i.e., 5).
Discussion
This study describes the clinical course of 87 critically ill patients who presented with ARDS due to COVID-19 and survived to ICU. The results showed that a large proportion of COVID-19 survivors had low MMS score at ICU discharge, with no substantial difference between prone and supine individuals, also in terms of shoulder muscle strength. Nearly 4 patients over 10 underwent prone positioning and prolonged period of mechanical ventilation with a high use of NMBA, however their functional outcomes were comparable between considered groups.
During COVID-19 pandemic, the extensive use of prone positioning and the heterogeneity of arms position could have had unfavourable consequences, like weakness and pain due to potential nerve damage (Miller et al., 2021). The swimmer position is traditionally adopted to reduce the difficulties in positioning patient’s head and endotracheal tube, to reduce the risk of developing facial pressure ulcers and to ensure a safe intravenous access line, as implemented also in our clinical practice. Alternate positioning of the arms is a nursing intervention aimed to prevent both pressure ulcers and nerve/muscle injuries. As suggested, arms should be cycled every 2–4 hours (Bamford et al., 2019), however these recommendations are not always feasible in patients who are mechanically ventilated in prone position, especially throughout the COVID-19 pandemic, considering also the undertrained staff not familiar with this procedure (Peko et al., 2020). Unfortunately, the asymmetrical arm position used in the maneuver may cause traction and compression of the plexus of the adducted arm: for this reason, upper limb nerve injuries related to brachial plexus neuropathy have been associated with the prone position (Kwee et al., 2015). Suspicion should be raised if a patient reports signs of neuropathic pain or loss of sensation in a peripheral nerve distribution or demonstrates focal muscle wasting or loss of power. Physiotherapists and ICU nurses are directly involved with patient positioning, and they can early recognize who deserves more care. Due to the severity of the COVID-19, which required deep sedation and NMBA administration, physiotherapy interventions started on average after 11 days of mechanical ventilation. The goal of physiotherapists intervention was to facilitate the weaning from mechanical ventilation and to shorten the ICU length of stay. Besides the recognized benefits of a reduced ICU stay (Hashem et al., 2016), early ICU discharge was also one of the solutions to manage the paucity of ICU beds during the first pandemic wave in Italy (Grasselli et al., 2020).
It is interesting to compare our findings with other clinical experiences available worldwide. For example, patients from our ICU were mechanically ventilated for a median of 16 days, and the 85.1% scored below 3 at MMS when discharged from ICU; by the contrary, a study from the United Kingdom showed that only 13.6% of patients had MMS < 3, and these were also ventilated for a longer time (McWilliams et al., 2021). Our clinical approach included patient extubation and a quick transfer to a high dependency unit right after stepping out from the most critical phase of the disease. Such approach was also feasible thanks to the cooperation between ICU nurses and physiotherapists performing intensive rehabilitation program. This could also explain the different length of stay in ICU: 17.0 (IQR 12.0–32.0) days compared to 20.0 (IQR 15.0–27.5) days for patients from United Kingdom (McWilliams et al., 2021).
With regards to MMS, we found that only age and the number of days under NMBA administration were statistically associated to MMS score, which increased after ICU discharge. Long period of NMBA administration is notably associated with a higher risk of ICU acquired weakness and post intensive care syndrome, which have meaningful impact on patient recovery (Needham and Brindley, 2012). Also, it is worth of noting that only in patients treated with prone positioning, days of mechanically ventilation were negatively correlated with MMS at hospital discharge, corroborating the impact of intensive care on patients’ prognosis.
Patients included in the present analysis presented with incomplete strength recovery at hospital discharge. As we showed, this aspect is influenced by age, and patients admitted to our ICU were also older compared to the study from United Kingdom (McWilliams et al., 2021). Whichever is the causative mechanism, lower muscle strength could also affect the overall hospital stay and the functional outcomes at hospital discharge, especially in patients with COVID-19 (Medrinal et al., 2021). The literature reports that physical complications after critical illness affected several ARDS survivors before pandemic. Many studies document that a large proportion of ARDS survivors had limb muscle weakness at hospital discharge, associated with impairment of walking ability, ADL and quality of life (Chiumello et al., 2016, DiSilvio et al., 2019, Herridge et al., 2016).
In some patients, COVID-19 causes symptoms over weeks or months after the infection has gone, now defined as post-COVID-19 syndrome (Mahase, 2020). Disease severity, length of mechanical ventilation and NMBA administration are known risk factors associated with development of this syndrome (Crook et al., 2021). For this reason, patients with severe COVID-19 surviving to ICU may require a throughout and multidisciplinary follow up (Sykes et al., 2021). As witnessed by a small group of patients discharged to rehabilitative facilities in our study, 39.1% (34/87) needed longer recovery.
Barthel index and the MMS turned to be useful tools to monitoring levels of functioning in COVID-19 survivors, considering the positive correlation found in the present study. This is supported also by another work that shows how MMS is positively correlated with the Barthel index (Mcwilliams et al., 2015). Only in the prone group, both MMS and Barthel index at discharge were negatively correlated with the number of days spent on mechanical ventilation. Future scenarios may consider MMS to early stratify patients to different rehabilitative needs.
Strengths and limitations
Our study is characterized by unique strengths. This is one of the few studies investigating the muscle strength of upper limbs following prone positioning in patients with COVID-19. Prone position-related upper limb muscle weakness has not been widely discussed in the literature, and it is therefore impossible to retrieve a real-world estimate of the prevalence of such sequelae. Moreover, despite we used a convenience sampling, a study of this size, with a desired significance of 0.05 and a desired power of 0.90, can reliably detect an effect size of about 0.7 (Cohen’s d) between proportions of patients with MMS < 3.
However, several limitations also merit acknowledgments. Due to this unprecedented health emergency and elevated critical care bed occupancy, we screened the muscle strength using MMT, although the gold standard to measure muscle strength is hand-held dynamometer. It is also worth to be mentioned that we did not record exposure to corticosteroids, which may have a role in muscle deterioration in older COVID-19 survivors (Sagarra-Romero and Viñas-Barros, 2020). Also, neurologists were not involved in the peripheral nervous assessment of the upper limbs. Another important aspect is that timing of upper limbs postural changes is missing. These were supposed to occur every 2–4 hours according to internal protocol but they were not recorded on a routine basis, making impossible to determine an association between muscle strengths and exposure.
Conclusion
Given the exceptional times during which nurses and physiotherapists have carried out their activities, both professionals delivered the best possible care, aiming to give patients the same chance to leave ICU at the top of their possibilities. Prone positioning was a challenge for the ICU staff, and the present findings show that swimmer position adopted during prone ventilation is not associated with worse upper limb strength or poor mobility level in COVID-19 survivors.
Funding Source
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical Approval
The study was approved by ethical committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico – Milan, Italy (approval 667_2020).
Authors’ contribution
FB, VR and AG designed the study, coordinate data collection, data curation and writing the original draft. SG performed formal analysis of data and reviewed the manuscript. FM and MS made the data collection. IA, EP, GG and DL gave expert content and reviewed the manuscript. GG and DL also contributed to study supervision. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agresti, A., 2012. Categorical Data Analysis, 3rd ed. New York.
- Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., Oczkowski S., Levy M.M., Derde L., Dzierba A., Du B., Aboodi M., Wunsch H., Cecconi M., Koh Y., Chertow D.S., Maitland K., Alshamsi F., Belley-Cote E., Greco M., Laundy M., Morgan J.S., Kesecioglu J., McGeer A., Mermel L., Mammen M.J., Alexander P.E., Arrington A., Centofanti J.E., Citerio G., Baw B., Memish Z.A., Hammond N., Hayden F.G., Evans L., Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N.A., O'Brien J.M., Hoffmann S.P., Phillips G., Garland A., Finley J.C.W., Almoosa K., Hejal R., Wolf K.M., Lemeshow S., Connors A.F., Marsh C.B. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am. J. Respir. Crit. Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- Bamford P., Denmade C., Newmarch C., Shirley P., Singer B., Webb S., Whitmore D. Guidance for: prone positioning in adult critical care. Intensive Care Soc. 2019:1–39. [Google Scholar]
- Binda F., Galazzi A., Marelli F., Gambazza S., Villa L., Vinci E., Adamini I., Laquintana D. Complications of prone positioning in patients with COVID-19: a cross-sectional study. Intensive Crit. Care Nurs. 2021;67:103088. doi: 10.1016/j.iccn.2021.103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda F., Marelli F., Galazzi A., Pascuzzo R., Adamini I., Laquintana D. Nursing management of prone positioning in patients with COVID-19. Crit. Care Nurse. 2021;41:27–35. doi: 10.4037/ccn2020222. [DOI] [PubMed] [Google Scholar]
- Bogduk N., Mercer S. Biomechanics of the cervical spine. I: Normal kinematics. Clin. Biomech. (Bristol, Avon) 2000;15(9):633–648. doi: 10.1016/S0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Bohannon, R.W., 2019. Considerations and practical options for measuring muscle strength: A narrative review. Biomed. Res. Int. https://doi.org/10.1155/2019/8194537. [DOI] [PMC free article] [PubMed]
- Brugliera L., Filippi M., Del Carro U., Butera C., Bianchi F., Castellazzi P., Cimino P., Capodaglio P., Monti G., Mortini P., Pradotto L.G., Priano L., Spina A., Iannaccone S. Nerve compression injuries after prolonged prone position ventilation in patients with SARS-CoV-2: A case series. Arch. Phys. Med. Rehabil. 2021;102:359–362. doi: 10.1016/j.apmr.2020.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A., Garofalo E., Grande L., Auletta G., Cubello D., Greco M., Lombardo N., Garieri P., Papaleo A., Doldo P., Spagnuolo R., Longhini F. Nursing issues in enteral nutrition during prone position in critically ill patients: A systematic review of the literature. Intensive Crit. Care Nurs. 2020;60:102899. doi: 10.1016/j.iccn.2020.102899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumello D., Coppola S., Froio S., Gotti M. What’s next after ARDS: long-term outcomes. Respir. Care. 2016;61:689–699. doi: 10.4187/respcare.04644. [DOI] [PubMed] [Google Scholar]
- Chiumello D., Cressoni M., Racagni M., Landi L., Li Bassi G., Polli F., Carlesso E., Gattinoni L. Effects of thoraco-pelvic supports during prone position in patients with acute lung injury/acute respiratory distress syndrome: a physiological study. Crit. Care. 2006;10:R87. doi: 10.1186/cc4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla N., Dinglas V., Fan E., Kho M., Kuramoto J., Needham D. Manual muscle testing: a method of measuring extremity muscle strength applied to critically ill patients. J. Vis. Exp. 2011 doi: 10.3791/2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook, H., Raza, S., Nowell, J., Young, M., Edison, P., 2021. Long covid—mechanisms, risk factors, and management. BMJ n1648. https://doi.org/10.1136/bmj.n1648. [DOI] [PubMed]
- De Jonghe, B., Sharshar, T., Lefaucheur, J.-P., Authier, F.-J., Durand-Zaleski, I., Boussarsar, M., Cerf, C., Renaud, E., Mesrati, F., Carlet, J., Raphaël, J.-C., Outin, H., Bastuji-Garin, S., Groupe de Réflexion et d’Etude des Neuromyopathies en Réanimation, 2002. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288, 2859–67. https://doi.org/10.1001/jama.288.22.2859. [DOI] [PubMed]
- DiSilvio B., Young M., Gordon A., Malik K., Singh A., Cheema T. Complications and outcomes of acute respiratory distress syndrome. Crit. Care Nurs. Q. 2019;42:349–361. doi: 10.1097/CNQ.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Douglas I.S., Rosenthal C.A., Swanson D.D., Hiller T., Oakes J., Bach J., Whelchel C., Pickering J., George T., Kearns M., Hanley M., Mould K., Roark S., Mansoori J., Mehta A., Schmidt E.P., Neumeier A. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure*. Crit. Care Med. 2021;49:490–502. doi: 10.1097/CCM.0000000000004818. [DOI] [PubMed] [Google Scholar]
- Eggmann S., Kindler A., Perren A., Ott N., Johannes F., Vollenweider R., Balma T., Bennett C., Silva I.N., Jakob S.M. Early physical therapist interventions for patients with COVID-19 in the acute care hospital: A case report series. Phys. Ther. 2021;101:1–9. doi: 10.1093/ptj/pzaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti G., Giannini A., Bottino N., Castelli G.P., Cecconi M., Grasselli G., Guatteri L., Latronico N., Langer T., Monti G., Muttini S., Pesenti A., Radrizzani D., Ranucci M., Russotto V., Fumagalli R. Management of critically ill patients with COVID-19: suggestions and instructions from the coordination of intensive care units of Lombardy. Minerva Anestesiol. 2020;86 doi: 10.23736/S0375-9393.20.14762-X. [DOI] [PubMed] [Google Scholar]
- Goettler C.E., Pryor J.P., Reilly P.M. Brachial plexopathy after prone positioning. Crit. Care. 2002;6:540–542. doi: 10.1186/cc1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem M.D., Parker A.M., Needham D.M. Early mobilization and rehabilitation of patients who are critically ill. Chest. 2016;150:722–731. doi: 10.1016/j.chest.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge M.S., Moss M., Hough C.L., Hopkins R.O., Rice T.W., Bienvenu O.J., Azoulay E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42:725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra G., Rivera A., Fernandez-Ibarburu B., Lorca-García C., Garcia-Ruano A. Prone position pressure sores in the COVID-19 pandemic: The Madrid experience. J. Plast. Reconstr. Aesth. Surg. 2020;74:2141–2148. doi: 10.1016/j.bjps.2020.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee, M.M., Ho, Y.H., Rozen, W.M., 2015. The prone position during surgery and its complications: A systematic review and evidence-based guidelines. Int. Surg. https://doi.org/10.9738/INTSURG-D-13-00256.1. [DOI] [PMC free article] [PubMed]
- Langer T., Brioni M., Guzzardella A., Carlesso E., Cabrini L., Castelli G., Dalla Corte F., De Robertis E., Favarato M., Forastieri A., Forlini C., Girardis M., Grieco D.L., Mirabella L., Noseda V., Previtali P., Protti A., Rona R., Tardini F., Tonetti T., Zannoni F., Antonelli M., Foti G., Ranieri M., Pesenti A., Fumagalli R., Grasselli G. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit. Care. 2021;25:128. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini A., Bambi S., Mattiussi E., Elli S., Villa L., Bondi H., Rona R., Fumagalli R., Foti G. Prone position in acute respiratory distress syndrome patients: A retrospective analysis of complications. Dimens. Crit. Care Nurs. 2020;39:39–46. doi: 10.1097/DCC.0000000000000393. [DOI] [PubMed] [Google Scholar]
- Mahase E. Covid-19: What do we know about “long covid”? BMJ. 2020;370 doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- Mahoney F.I., Barthel D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965;14:61–65. [PubMed] [Google Scholar]
- Malik G.R., Wolfe A.R., Soriano R., Rydberg L., Wolfe L.F., Deshmukh S., Ko J.H., Nussbaum R.P., Dreyer S.D., Jayabalan P., Walter J.M., Franz C.K. Injury-prone: peripheral nerve injuries associated with prone positioning for COVID-19-related acute respiratory distress syndrome. Br. J. Anaesth. 2020;125:e478–e480. doi: 10.1016/j.bja.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams, D., Atkins, G., Hodson, J., Boyers, M., Lea, T., C, S., 2016. Feasibility and reliability of the Manchester Mobility Score as a measure of physical function within the Intensive Care Unit. J. ACPRC 48, 26–33.
- Mcwilliams D., Atkins G., Hodson J., Boyers M., Lea T., Snelson C. Is the Manchester mobility score a valid and reliable measure of physical function within the intensive care unit. Intensive Care Med. Exp. 2015;3:A553. doi: 10.1186/2197-425X-3-S1-A553. [DOI] [Google Scholar]
- McWilliams D., Weblin J., Hodson J., Veenith T., Whitehouse T., Snelson C. Rehabilitation levels in patients with COVID-19 admitted to intensive care requiring invasive ventilation an observational study. Ann. Am. Thorac. Soc. 2021;18:122–129. doi: 10.1513/AnnalsATS.202005-560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrinal C., Combret Y., Hilfiker R., Prieur G., Aroichane N., Gravier F.E., Bonnevie T., Contal O., Lamia B., Ali N., Carrié C., Cottereau G., Demoule A., Dres M., Dubé B.P., Goligher E., Hermans G., Hussein A.M., Spadaro S., Tenza-Lozano E.M., Wieske L., Witteveen E. ICU outcomes can be predicted by noninvasive muscle evaluation: A meta-analysis. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.02482-2019. [DOI] [PubMed] [Google Scholar]
- Medrinal C., Prieur G., Bonnevie T., Gravier F.-E., Mayard D., Desmalles E., Smondack P., Lamia B., Combret Y., Fossat G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021;21:64. doi: 10.1186/s12871-021-01274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara A., Kanchiku T., Nishida N., Tagawa H., Ohgi J., Suzuki H., Imajo Y., Funaba M., Nakashima D., Chen X., Taguchi T. Biomechanical analysis of brachial plexus injury: Availability of three-dimensional finite element model of the brachial plexus. Exp. Ther. Med. 2018;15:1989–1993. doi: 10.3892/etm.2017.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., O’Sullivan J., Jeffrey J., Power D. Brachial plexus neuropathies during the COVID-19 pandemic: A retrospective case series of 15 patients in critical care. Phys. Ther. 2021;101 doi: 10.1093/ptj/pzaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.A., Seckel M.A. Acute respiratory distress syndrome and prone positioning. AACN Adv. Crit. Care. 2018;29:415–425. doi: 10.4037/aacnacc2018161. [DOI] [PubMed] [Google Scholar]
- Nee R.J., Yang C.-H., Liang C.C., Tseng G.F., Coppieters M.W. Impact of order of movement on nerve strain and longitudinal excursion: A biomechanical study with implications for neurodynamic test sequencing. Man. Ther. 2010;15:376–381. doi: 10.1016/j.math.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Needham C.J., Brindley P.G. The role of neuromuscular blocking drugs in early severe acute respiratory distress syndrome. Can. J. Anesth. 2012;59:105–108. doi: 10.1007/s12630-011-9615-2. [DOI] [PubMed] [Google Scholar]
- Peko L., Barakat‐Johnson M., Gefen A. Protecting prone positioned patients from facial pressure ulcers using prophylactic dressings: A timely biomechanical analysis in the context of the COVID-19 pandemic. Int. Wound J. 2020;17:1595–1606. doi: 10.1111/iwj.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing.
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagarra-Romero L., Viñas-Barros A. COVID-19: short and long-term effects of hospitalization on muscular weakness in the elderly. Int. J. Environ. Res. Public Health. 2020;17:8715. doi: 10.3390/ijerph17238715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer, S.C., Parsa, K.M., Newark, A., Peesay, T., Walsh, A.R., Fernandez, S., Gao, W.Z., Pierce, M.L., 2021. Facial pressure injuries from prone positioning in the COVID‐19 era. Laryngoscope lary.29374. https://doi.org/10.1002/lary.29374. [DOI] [PubMed]
- Shepherd S., Batra A., Lerner D.P. Review of critical illness myopathy and neuropathy. The Neurohospitalist. 2017;7:41–48. doi: 10.1177/1941874416663279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A.I., Vaghela K.R., Brown H., Adams K., Sinisi M., Fox M., Quick T. Reducing the risk and impact of brachial plexus injury sustained from prone positioning—A clinical commentary. J. Intensive Care Med. 2020;35:1576–1582. doi: 10.1177/0885066620954787. [DOI] [PubMed] [Google Scholar]
- Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Baldwin C., Bissett B., Boden I., Gosselink R., Granger C.L., Hodgson C., Jones A.Y.M., Kho M.E., Moses R., Ntoumenopoulos G., Parry S.M., Patman S., van der Lee L. Physiotherapy management for COVID-19 in the acute hospital setting: Recommendations to guide clinical practice. Pneumon. 2020;33:32–35. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]