Abstract
Background
The use of nasopharyngeal (NP) swabs as a specimen collection method to diagnose SARS-CoV-2 infection is frequently perceived as uncomfortable by patients and requires trained personnel. In this study, detection rate of SARS-CoV-2 in mouthwash samples and buccal swabs were compared in both children and adults.
Material and methods
In patients admitted to hospital with confirmed COVID-19 within the previous 72 hours, NP and buccal swabs as well as mouthwash samples were collected. RT-qPCR was performed on all samples.
Results
In total, 170 samples were collected from 155 patients (137 adults and 18 children). Approximately 91.7% of the collected NP swabs were positive in RT-PCR compared to 63.1% of mouthwash samples and 42.4% of buccal swabs. Compared to NP swabs, the sensitivity of using mouthwash was 96.3% and 65.4% for buccal swabs in NP swab samples with a CT value <25. With increasing CT values, sensitivity decreased in both mouthwash and buccal swabs. The virus load was highest during the first week of infection, with a continuous decline observed in all three collection methods over time.
Discussion
Mouthwash presents an alternative collection method for detecting SARS-CoV-2 in the case of unfeasible NP swab sampling. Buccal swabs should not be used due to their low sensitivity.
Key Words: SARS-CoV-2, Mouth wash, Buccal swab, COVID-19
Introduction
Infection rates with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and subsequently morbidity and mortality remain high. Rapid identification and isolation of infected individuals are crucial for targeted infection control and mitigation measures. The current gold standard to diagnose early infection is the nucleic acid-based polymerase chain reaction (PCR) performed on naso-oropharyngeal swabs (NP). However, NP swab sampling is accompanied by several disadvantages. It is frequently perceived as uncomfortable by patients and is therefore not well tolerated particularly by children.1, 2, 3 Furthermore, trained healthcare personnel with protective equipment are required for sampling.4 , 5 If sampling is not performed adequately its sensitivity declines.6 In patients with complex anatomy or cases of improper sampling, adverse events, such as cerebrospinal fluid leakage, temporomandibular joint dislocation and swab brea1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 kage in the nasopharyngeal area, may occur.7 , 8 Furthermore, especially in children, patients with mental disability or preexisting naso- or pharyngeal disease, NP swabs might not be obtainable. Additionally, sneezing or coughing during NP swab sampling might generate aerosols which again could increase SARS-CoV-2 transmission.
Since viral replication of SARS-CoV-2 has been shown to be highest in the upper respiratory tract tissues,9 the collection and evaluation of other respiratory specimens for SARS-CoV-2 detection should be considered. In a recent study, a high viral load of SARS-CoV-2 in sputum and saliva was reported.10 However, critically ill adults or children are often unable to spontaneously produce sufficient amounts of saliva or sputum.10 Findings of previous studies concerned with detection of SARS-CoV-2 in saliva specimens were limited by small sample size and diverse methodology.11, 12, 13, 14 Whether PCR from saliva mouthwash is an accurate method to detect SARS-CoV-2 in symptomatic patients remains, therefore, yet to be determined.
This study aims to compare the sensitivity and specificity of saliva samples collected as mouthwash and buccal swabs (using a standardized collection method) with the gold standard, NP swabs, in adults and children. Mouthwash and buccal swabs facilitate producing and collecting saliva and avoid discomfort for the patient, with the potential for self-collection.
Methods
The study took place at two different hospitals in Vienna, Austria. Pediatric and adolescent patients were recruited at one site (Clinic Ottakring, Vienna), adult patients at another (Clinic Favoriten, Vienna). The study was approved by the local ethics committee of the Vienna Health Care Group (EK-20-190-0820).
Material collection and processing
Mouthwash
The Saliva Collection System from Greiner Bio-One15 was used to collect saliva samples. Collection occurred through thorough rinsing of the oral cavity for 2 minutes using the saliva extraction solution Balanced-Salt-Solution buffer. Subsequently, the solution was expelled into the small empty collection beaker of the Greiner Bio-One system.
Buccal and NP swabs
Buccal swab sampling was performed on both buccal sides. NP swab sampling was performed on both nostrils and oropharynx sampling using a single swab. FLOQSwab (Modell) swab preserved in universal transport medium (Copan UTM system, both, CA) was used.
Study participants
Adult patients admitted to the Department of Infectious Diseases and Tropical Medicine, Clinic Favoriten due to COVID-19 (confirmed by RT-qPCR within the previous 72 hours) were asked to participate in the study. After obtaining written consent, mouthwash samples were collected by the patient under supervision by a heathcare personel. NP and buccal swab sampling were performed by a trained health care staff member.
At the second site, the Department of Pediatrics and Adolescent Medicine, Clinic Ottakring, patients above the age of 7 years were asked to participate in the trial. Written informed consent was obtained by patients (age 14-18 years) or by patients and legal guardians (age 7-13 years). Due to small number of eligible pediatric inpatients with COVID-19, specimens were obtained either from pediatric in- and outpatient to facilitate recruitment. All inpatients were previously tested positive for SARS-CoV-2 by NP swab within 72 hours. Outpatients eligible for study entry either had to have contact with a known COVID-19 case and symptoms of any kind or signs and symptoms highly suspicious of SARS-CoV-2 infection (such as anosmia or atypical pneumonia) without known contact. Only outpatients who were tested positive for SARS-CoV-2 by in-house PCR at study entry, were included in data evaluation.
Processing
The NP and buccal swabs were stored in virus transport medium. All sample types were immediately sent to the laboratory, where RT-qPCR was performed within 24 hours after specimen collection. The reference method for comparing cycle threshold (CT values) comprised a diagnostic SARS-CoV-2 RT-qPCR test. RNA was extracted from 200 µl of NP or throat wash supernatants using the MagnaPure 24 platform with MagNA Pure 24 Total NA Isolation Kit (Roche Diagnostics GmbH, Austria), according to a standard protocol, and was eluted in 50 µl. Detection of SARS-CoV-2 RNA was performed using a commercial primer/probe mix (LightMix ModularDx Kit: SARS and Wuhan CoV E-gene, TIB Molbiol, Germany) and LC Multiplex RNA Virus Master (Roche Diagnostics GmbH, Austria) on a z480 real-time PCR instrument (Roche Diagnostics GmbH, Austria). Nuclease-free water and a synthetic RNA control provided with the primer/probe mix were included as respective no-template control and positive control. Ten µl of the probe and master mix were pooled, respectively, and RT-qPCR was performed according to the manufacturer's instructions.
Statistics
Continuous variables are presented as means with standard deviations, and categorical variables as counts and percentages. The McNemar test was used to calculate differences in dichotomous dependent samples and the Chi-Square test independent variables. For small sample numbers (n < 5), Fisher's exact test was used. The sensitivity and specificity were calculated using a 2 × 2 table. Pearson's correlation calculation was used to evaluate the correlation between cycle threshold (CT value) and time since symptom onset or outcome (death in hospital vs discharge). A P-value <.05 was considered statistically significant. IBM SPSS Statistics 26 was used for statistical analysis.
Results
Basic demographics and outcome
At total of 170 samples were collected in 155 patients (137 adult and 18 pediatric patients). One patient refused a NP swab, in two patients mouthwash samples were not collected, two pediatric patients only tolerated oropharyngeal swab. In general, the sample collection method mouthwash and buccal swab was tolerated well from all participants.
The basic demographics and outcome parameter are listed in Table 1 .
Table 1.
Basic demographics and outcome parameter of adult and minor participants
| Characteristic | All | Adult | Pediatric | P-value |
|---|---|---|---|---|
| Number of patients (%) | 155 | 137 (88.39) | 18 (11.61) | |
| Age (years ± SD) | 51.46 (±23.39) | 60.98 (±17.67) | 13.55 (±3.01) | <.0001 |
| Female sex (%) | 65 (41.94) | 53 (38.69) | 12 (66.67) | .024 |
| Time since symptom onset (days ± SD) | 7.80 (±4.60) | 8.18 (± 4.33) | 2.80 (± 1.72) | <.0001 |
| Length of hospital stay (days) (min-max) * | 9 (1-58) | 10 (2-58) | 5 (1-13) | .006 |
| Asymptomatic at sampling (%) | 10 (6.45) | 2 (1.46) | 8 (44.44) | <.0001 |
| Disease severity | ||||
| Mild (%) | 61 (39.35) | 43 (31.39) | 18 (100) | <.0001 |
| Pneumonia, no supplemental oxygen (%) | 34 (21.94) | 34 (24.82) | 0 (0%) | .0135 |
| Pneumonia, requiring supplemental oxygen (%) | 55 (35.48) | 55 (40.15) | 0 (0%) | .0003 |
| ARDS/ MOF (%) | 5 (3.23) | 5 (3.65) | 0 (0%) | 1.000 |
| Course & outcomes | ||||
| Discharge | 146 (94.19) | 128 (93.43) | 18 (100) | .5995 |
| ICU admission | 9 (5.81) | 9 (6.57) | .600 | |
| Death (%) | 9 (5.81) | 9 (6.57) | 0 (0%) | 1.000 |
| Samples | ||||
| Number (%) | 170 | 152 (89.41) | 18 (10.59) | |
| NP swab (CT ± SD) | 30.15 (±7.75) | 30.31 (±7.72) | 28.72 (±8.19) | .362 |
| Mouthwash (CT ± SD) | 35.44 (±6.21) | 35.40 (±6.36) | 35.81 (±4.76) | .931 |
| Buccal swab (CT ± SD) | 36.95 (±10.21) | 37.17 (±10.61) | 34.58 (±3.47) | .108 |
ARDS, acute respiratory distress syndrome; CT, cycle threshold; min, minimum; max, maximum; MOF, multi organ failure; NP, nasopharyngeal swab SD, standard deviation.
Three outpatients in the pediatric group without hospital admission.
Performance of mouthwash and buccal swab samples
Of the collected NP swabs, 91.7% (155 of 170) were positive in RT-qPCR compared to 63.1% (106 of 168) of mouthwash samples and 42.4% (72 of 170) of buccal swab samples. In three patients, RT-qPCR results of mouthwash samples were positive, while the NP swab was negative, with a mean CT value of 40.89. One patient showed a positive result in the buccal swab (CT value of 40.99) and was negative in the NP swab sample.
Compared to the gold standard, the NP swab, the overall sensitivity of mouthwash samples was 67.3% compared to 45.8% in buccal swabs. Among pediatric patients, 83.3% of NP swabs were positive, compared to 55.5% of mouthwash samples, and 33.3% of buccal swabs, respectively. CT values were lowest in NP swabs (mean 28.7, SD 8.2) compared to mouthwash samples (mean 35.1, SD 4.8), and buccal swabs (mean 34.6, SD3.5).
The overall sensitivity with different CT values is shown in Table 2 .
Table 2.
Sensitivity of qRT-PCR mouthwash and buccal swab compared to qRT-PCR of NP swab depending on the CT value
|
NP swab |
|||||
|---|---|---|---|---|---|
| Overall | CT <30 | CT <22 | CT 22-25 | CT 25-30 | |
| Mouthwash | 67.3 % (103 of 153) | 90.3 % (65 of 72) | 100.0 % (23 of 23) | 90.9 % (20 of 22) | 81.5 % (22 of 27) |
| Buccal swab | 45.8 % (71 of 155) | 76.7 % (56 of 73) | 82.6 % (19 of 23) | 77.3 % (17 of 22) | 71.4 % (20 of 28) |
CT, cycle threshold; min, minimum; max, maximum; NP, nasopharyngeal swab.
Mouthwash was shown to be significantly more sensitive compared to buccal swabs regarding the overall sensitivity (P < .001) and in NP swab samples with a CT value less than 30 (P = .021). As seen in Figure 1 , the mean CT value in NP positive swab samples was 30.16 (SD 7.05, range 16.34-40.99), in mouthwash 35.4 (SD 5.2, range 22.89-40.99), and in buccal swabs 37.05 (SD 5.2, range 22.89-40.99).
Fig 1.
Median with interquartile range of CT value in qRT-PCR of NP swab, buccal swab and mouthwash.
The difference of the mean CT value was demonstrated to be significant between NP swab and mouthwash samples (P < .001), NP and buccal swab samples (P < .001), and mouthwash and buccal swab samples (P < .001).
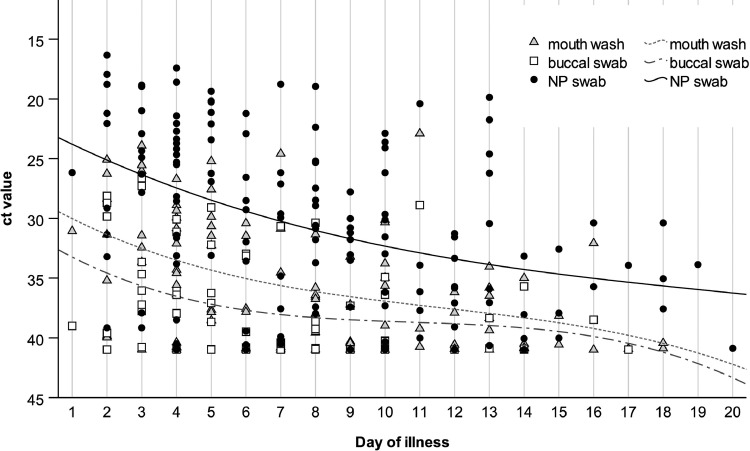
A significant correlation between time from symptom onset and CT value was calculated using NP swabs (r = 0.420, P < .001), mouthwash (r = 0.442, P < .001), and buccal swabs (r = 0.315, P = .009). As depicted in Figure 2 , there is a peak at the start of symptoms, followed by a continuous decline.
Fig 2.
Trend of CT value in the course of time since symptom onset.
Compared to NP swabs, the sensitivity of mouthwash and buccal swab samples is highest during the first week since symptom onset. Subsequently, a strong decline in the sensitivity is observed for buccal swabs. The difference is demonstrated to be significant on days 7-12 (P = .057) and day >13 (P = .039) since symptom onset. The difference at up to day six since symptom onset was not observed to be significant (P = .057); however, a tendency was found.
Outcome
The CT value in NP swab (r = -0.288, P = .004) and mouthwash samples (r = -0.249, P = .010) were demonstrated to correlate negatively with hospital mortality. There was no correlation found using buccal swabs (r = -0.128, P = .284).
Discussion
Our data demonstrate that sample collection via mouthwash is a potential method that can be used if NP swabbing is not possible. Although CT values were shown to be higher in mouthwash samples and the overall sensitivity was significantly lower than in NP swab samples, the sensitivity in patients with high virus load (CT value ≤25) is 95.5% compared to NP swabs. In three patients, mouthwash samples were demonstrated to have a higher sensitivity than NP swabs.
Regarding buccal swabs, our findings are consistent with other studies3 , 16 that demonstrate the detection of SARS-CoV-2 via RT-qPCR in buccal specimens and a high viral load during the first week of disease or diagnosis. Similar to our findings, the authors report significantly higher CT values in buccal swab and lower sensitivity compared to NP swab.
Other reports on the use of saliva for SARS-CoV-2 detection emerged in the last months.11 , 14 However, these were criticized for the small sample size and that, unlike sampling with nasopharyngeal swabs, collection and processing methods for saliva specimens differed broadly among studies.
Mouthwash, that we used in our study, using the Saliva Collection System from Greiner Bio-One and following the given construction, enables standardized collection.
Interestingly, not only does the viral load influences the sensitivity (the higher the CT value, the less sensitivity), but the time from symptom onset was also found to play a role. The highest sensitivity was observed during the first week, correlating with the highest detected viral load in mouthwash samples.
Up to now, especially data from pediatric patients on SARS-CoV-2-detection in saliva specimens are scarce.17 In recent trial, authors report a sensitivity of 87.7% when compared to NP swabs in children presenting for COVID-19 screening.18 Guzmán-Ortiz et al.19 report a sensitivity of 82.3% in saliva specimens compared to NP swabs from pediatric patients with COVID-19. Sensitivity was lower in this trial, which might be attributable to different methodology and a high-rate of asymptomatic patients in our pediatric population. In contrast to our study, in these studies saliva was collected by spitting in a collection tube with and without gargeling first.
Caulley et al. demonstrated that in paired NP and saliva samples taken from adults, in 20% of patients SARS-CoV-2-PCR was detected only in saliva specimens.20 Cases of children that test positive for SARS-CoV-2 by PCR only in saliva samples have been reported in other trials.18 , 19 Similarly, among our pediatric patient population, one patient only tested positive by mouthwash while remaining negative in NP and buccal swab. In our adult population three patients only tested positive by mouthwash. Testing saliva samples additionally to NP swabs in symptomatic patients could potentially increase diagnostic yield while minimizing discomfort. We postulate that collecting saliva samples via mouthwash might increase sensitivity compared to spitting saliva in a collection tube.
Mouthwash collection was well tolerated by all participants in the present study, even by young children. Collection could be self-performed, minimizing the transmission risk for healthcare personnel. In our protocol, patients only had to rinse the fluid in their mouths and not gargle the solution, therefore no aerosol droplets were produced. Furthermore, samples by mouthwash can be obtained at home, enabling broad application.
No adverse events (such as aspiration, nausea, or vomiting) or abortion of mouth rinsing was observed during the study period. This study demonstrates that using mouth washes to detect SARS-CoV-2 could be both save and feasible, even in patients with dyspnea or children and adolescents with limited compliance.
Limitations and strengths
Although several studies have evaluated saliva or sputum for SARS-CoV-2 detection, this is the first known trial utilizing mouthwash samples without gargling. The strength of the present study is the inclusion of both minors and adults, as well as symptomatic and asymptomatic individuals. However, the rate of asymptomatic patients was only 5.8%, therefore, data validity in this group is limited. Further studies are required to evaluate the sensitivity of mouthwash samples in individuals with asymptomatic or mild COVID-19. We included a high number of adults, but only 18 pediatric patients participated in the trial. Many children and/or legal guardians denied study participation due to fear of additional NP sampling. Another strength of the study is the standardized sample collection using a collection system.
Conclusion
Similar to previous studies, our data shows that detection of SARS-CoV-2 in saliva specimens is possible. However, due to the low sensitivity and higher CT values compared to the NP swabs, buccal swabbing does not represent a suitable screening modality for COVID-19.
Though, RT-qPCR of mouthwash samples appears to be a reliable, easy-to-use collection method for the detection of SARS-CoV-2. Despite a lower sensitivity compared to NP swabs overall, especially in the first week of symptom onset, in low resource settings (ie, few NP sampling equipment, shortage of trained personnel), and patients, where NP sampling is not feasible (ie, children, reduced collaboration, underlying medical conditions) detection of SARS-CoV-2 in mouthwash samples is a suitable alternative.
Footnotes
Funding: None
Conflicts of interest: None to report.
Availability of data and material (data transparency): All data is available.
Code availability (software application or custom code): Not applicable.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals: Not applicable.
Ethics approval: The study was approved by the local ethics committee of the Vienna Health Care Group (EK-20-190-0820).
Consent to participate: Written informed consent was obtained from adults. Written informed consent was obtained by patients (age 14 to 18 years) or by patients and legal guardians (age 7-13 years).
Consent for publication: All authors gave consent for publication.
References
- 1.Westra AE, van Gils EJ, Aarts F, et al. Perceived discomfort levels in healthy children participating in vaccine research. J Empir Res Hum Res Ethics. 2013;8:66–72. doi: 10.1525/jer.2013.8.3.66. [DOI] [PubMed] [Google Scholar]
- 2.Frazee BW, Rodriguez-Hoces de la Guardia A, Alter H, et al. Accuracy and discomfort of different types of intranasal specimen collection methods for molecular influenza testing in emergency department patients. Ann Emerg Med. 2018;71:509–17e1. doi: 10.1016/j.annemergmed.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Ku CW, Shivani D, Kwan JQT, et al. Validation of self-collected buccal swab and saliva as a diagnostic tool for COVID-19. Int J Infect Dis. 2021;104:255–261. doi: 10.1016/j.ijid.2020.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marty FM, Chen K, Verrill KA. How to obtain a nasopharyngeal swab specimen. N Engl J Med. 2020;382:e76. doi: 10.1056/NEJMvcm2010260. [DOI] [PubMed] [Google Scholar]
- 6.Higgins TS, Wu AW, Ting JY. SARS-CoV-2 nasopharyngeal swab testing-false-negative results from a pervasive anatomical misconception. JAMA Otolaryngol Head Neck Surg. 2020;146:993–994. doi: 10.1001/jamaoto.2020.2946. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan CB, Schwalje AT, Jensen M, et al. Cerebrospinal fluid leak after nasal swab testing for coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146:1179–1181. doi: 10.1001/jamaoto.2020.3579. [DOI] [PubMed] [Google Scholar]
- 8.Foh B, Borsche M, Balck A, et al. Complications of nasal and pharyngeal swabs: a relevant challenge of the COVID-19 pandemic? Eur Respir J. 2021;57 doi: 10.1183/13993003.04004-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 10.Khiabani K, Amirzade-Iranaq MH. Are saliva and deep throat sputum as reliable as common respiratory specimens for SARS-CoV-2 detection? A systematic review and meta-analysis. Am J Infect Control. 2021;49:1165–1176. doi: 10.1016/j.ajic.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abasiyanik MF, Flood B, Lin J, et al. Sensitive detection and quantification of SARS-CoV-2 in saliva. Sci Rep. 2021;11:12425. doi: 10.1038/s41598-021-91835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan D, Sze S, Rogers B, et al. Serial simultaneously self-swabbed samples from multiple sites show similarly decreasing SARS-CoV-2 loads in COVID-19 cases of differing clinical severity. J Infect. 2020;81:979–997. doi: 10.1016/j.jinf.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaur R, Verma DK, Mohindra R, et al. Buccal swabs as non-invasive specimens for detection of severe acute respiratory syndrome coronavirus-2. J Int Med Res. 2021;49 doi: 10.1177/03000605211016996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan SH, Allicock O, Armstrong-Hough M, Wyllie AL. Saliva as a gold-standard sample for SARS-CoV-2 detection. Lancet Respir Med. 2021;9:562–564. doi: 10.1016/S2213-2600(21)00178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speichelentnahmesystem [June 30th, 2021]. Available from: https://shop.gbo.com/en/row/products/preanalytics/saliva-collection-system/. Accessed January 6, 2022.
- 16.Kam KQ, Yung CF, Maiwald M, et al. Clinical utility of buccal swabs for severe acute respiratory syndrome coronavirus 2 detection in coronavirus disease 2019-infected children. J Pediatric Infect Dis Soc. 2020;9:370–372. doi: 10.1093/jpids/piaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford V, Curtis N. Saliva testing for SARS-CoV-2 in children. Clin Microbiol Infect. 2021;27:1199–1201. doi: 10.1016/j.cmi.2021.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27:1330–1335. doi: 10.1016/j.cmi.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzmán-Ortiz AL, Nevárez-Ramírez AJ, López-Martínez B, et al. Sensitivity of the molecular test in saliva for detection of COVID-19 in pediatric patients with concurrent conditions. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.642781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caulley L, Corsten M, Eapen L, et al. Salivary detection of COVID-19. Ann Intern Med. 2021;174:131–133. doi: 10.7326/M20-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]