Abstract
Background
Coronavirus disease 2019 (COVID-19) has affected >210 million people worldwide. An optimal therapeutic approach for COVID-19 remains uncertain, to date. Since the history of cancer was linked to higher mortality rates due to COVID-19, the establishment of a safe and effective vaccine coverage is crucial in these patients. However, patients with cancer (PsC) were mostly excluded from vaccine candidates' clinical trials. This systematic review aims to investigate the current available evidence about the immunogenicity of COVID-19 vaccines in PsC.
Patients and methods
All prospective studies that evaluated the safety and efficacy of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were included, with immunogenicity after the first and the second dose as the primary endpoint, when available.
Results
Vaccination against COVID-19 for PsC seems overall safe and immunogenic after well-conducted vaccination schedules. Yet the seroconversion rate remains lower, lagged or both compared to the general population. Patients with hematologic malignancies, especially those receiving B-cell-depleting agents in the past 12 months, are the most at risk of poor seroconversion.
Conclusion
A tailored approach to vaccination may be proposed to PsC, especially on the basis of the type of malignancy and of the specific oncologic treatments received.
Key words: COVID19, Sars-CoV-2, vaccine, immunogenicity, cancer, seroconversion
Introduction
Since the first reports of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, coronavirus disease 2019 (COVID-19) has affected >210 million people worldwide.1 Besides oxygen therapy and positive pressure ventilation, glucocorticoids, especially dexamethasone, showed a mortality benefit in patients requiring respiratory support.2 Despite different therapeutic approaches being investigated, an optimal treatment of COVID-19 remains uncertain (Figure 1 ).3, 4, 5
Figure 1.
Investigated strategies against SARS-CoV-2.
Main mechanisms of viral entry into host cells are depicted, alongside anti-SARS-CoV-2 passive and active immune strategies. Strategy 1 and 2: soluble RBD mimetics or anti-ACE2 scFvs may hide ACE2 receptors from Spike proteins, preventing viral entry. RBD targeting may be achieved via either monoclonal Ab (i.e. casirivimab-imdevimab) or vaccine-induced Ab. In addition, vaccination also promotes the emergence of cellular anti-SARS-CoV2 adaptive immune responses, leading to killing of viral-infected cells.
Created with biorender.com.
Abs, antibodies; ACE2, Angiotensin-converting enzyme 2; mRNA, messenger ribonucleic acid; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; scFv, single-chain variable fragment; S protein, Spike protein.
Nevertheless, global efforts have established an effective vaccine strategy and, because history of cancer is linked to higher mortality rates due to COVID-19, an effective vaccine coverage is crucial in this population.6, 7, 8, 9, 10, 11, 12, 13 However, clinical trials investigating COVID-19 vaccine candidates mostly excluded patients with cancer (PsC). So, international COVID-19 vaccination guidelines for this population were initially based on expert opinions, on studies designed to test other vaccines and on initial real-world data reports.14 , 15
This systematic review aims to investigate the current available evidence about immunogenicity of COVID-19 vaccines currently administered to PsC.
Methods
A systematic review of the literature was carried out on 16 August 2021. The relevant studies were searched through Medline (via PubMed) and Embase, with no language or time restriction. The databases were searched (CC) using the mapped terms [‘cancer’ OR ‘tumor’ OR ‘malignancy’] AND ‘vaccine’ AND [‘COVID’ or ‘SARS-CoV-2’] and the exploded MeSH terms ‘COVID-19 Vaccines’. Two reviewers double-screened independently titles and abstracts (CC, GA). A third author functioned as a tiebreaker, in case of disagreements (GC). The reference lists of the most relevant papers were selected for snowballing (CC, GA). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology was applied, to depict the flow of studies through each phase (ph) of the review process.
We assessed the quality of included studies using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (NIH). Results were rated as FAIR, when a total of 5-10 points were assigned to the study and as GOOD if a total of ≥11 points were assigned on the basis of 14 quality assessment queries. A comprehensive summary and specifics of the quality assessment are provided in Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.10.014.
Data extraction was carried out by one reviewer (CC) and independently checked by two other authors (GA, GC). We included all prospective studies, evaluating as primary endpoint the immunogenicity of vaccines against SARS-CoV-2 in PsC. Findings which did not fulfil the aforementioned criteria were excluded. Safety was investigated as the secondary endpoint, as the incidence of adverse events (AEs), if reported in the clinical study.
We retrieved supplementary information about study design, population size and cancer types (if available). When a single study had resulted in multiple publications, we prioritized the most updated report, unless the reported endpoint was not relevant.
Substantial heterogeneity of study designs and outcome measures did not allow to perform a meta-analysis; therefore, a narrative synthesis was conducted without performing additional statistical or sensitivity analyses by a specific software or without additional feasibility assessment.
Results
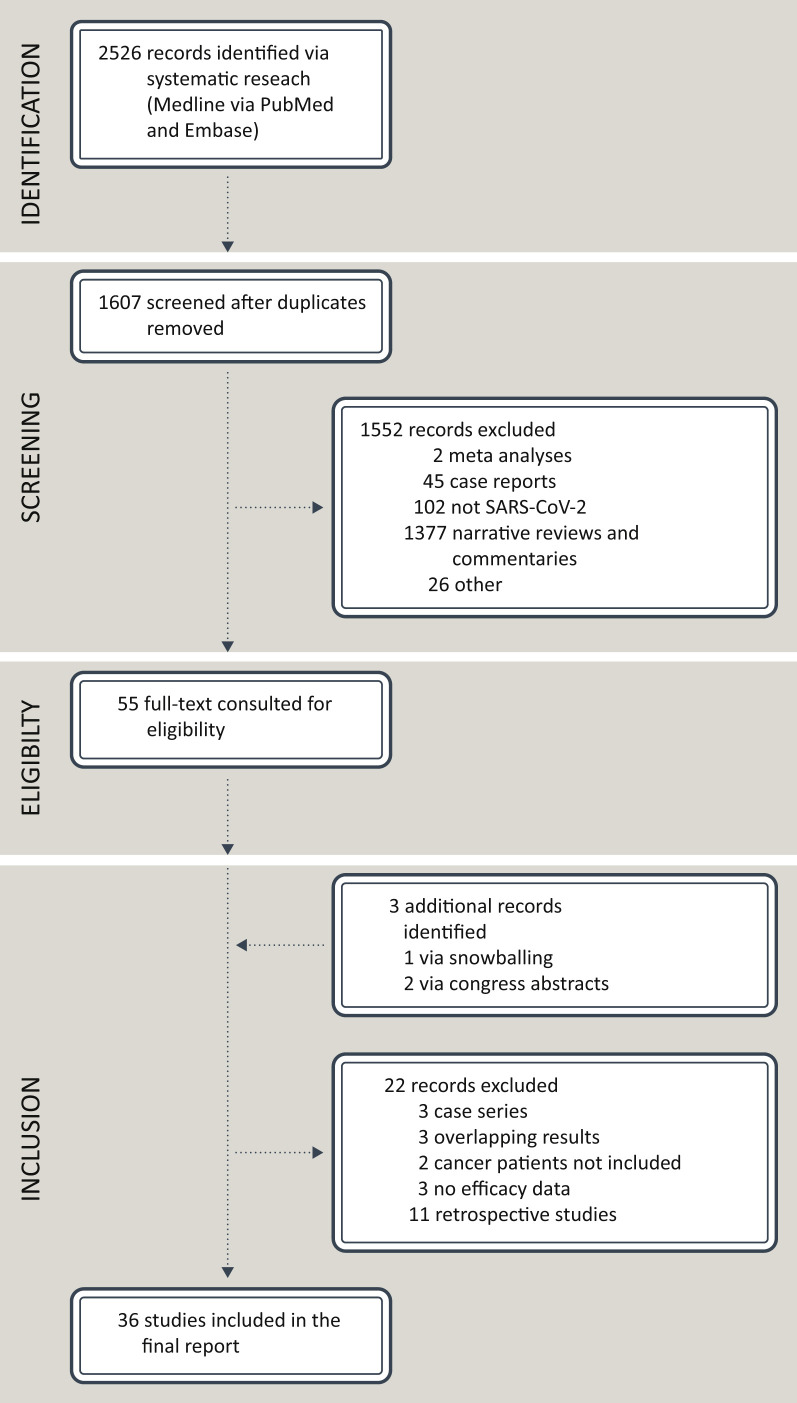
The systematic research of the literature returned 2526 records. After checking for duplicates, a total of 1607 records were obtained. After critical appraisal, a final amount of 36 studies met the aforementioned criteria, as depicted in Figure 2 . The investigations had been carried out in the World Health Organization (WHO) Region of the Americas (n = 7) and in the WHO European Region (i.e. United Kingdom, n = 8; Germany, n = 1; Denmark, n = 1; Italy, n = 3; France, n = 3; Greece, n = 3; Switzerland, n = 1; Israel, n = 6; Turkey, n = 1; Lithuania, n = 1; Netherlands, n = 1). The median and the mean number of PsC included in each study were 114.5 (minimum: 16; maximum: 1503) and 257.2, respectively, overall accounting for 9260 patients. As for vaccine platforms, Pfizer-BioNTech (BNT) and Moderna (MDN) vaccines were administered in 33 (91.6%) and in 13 (36.1%) clinical studies, respectively. Viral vector-based vaccines include Oxford/AstraZeneca (OxA) and Janssen vaccines. The former was administered in nine clinical studies (25%), whereas the latter was evaluated only in one study. The study conducted in Turkey administered CoronaVac, an inactivated COVID-19 vaccine.16
Figure 2.
PRISMA flow diagram of the study.
pts, patients; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Across all the studies, immunogenicity of COVID-19 vaccines was defined as the proportion of PsC who seroconverted to Spike proteins. Median anti-Spike antibody (Ab) titers, detection of neutralizing antibodies (NAbs) and cellular immune responses were investigated as secondary or exploratory endpoints.
Eight studies reported data only after the first dose; 20 studies reported data only after the second dose; 9 studies reported data after both the first and the second dose. The seroconversion rate ranged widely after the first dose, i.e. from 11% to 87.5%, overall; from 11% to 87.5% for hematologic patients; from 25% to 67% for patients with solid tumors. However, seroconversion data were collected at non-uniform time points after the first dose, across all the studies (median: 3 weeks; minimum: 1 week; maximum: 5 weeks).
As for the second dose, the seroconversion rate ranged widely as well, from 7.3% to 100%. Specifically, it ranged from 7.3% to 88.8%, for hematologic patients and from 47.5% to 100% for patients with solid tumors. Similarly, seroconversion data were collected at non-uniform time points after the second dose, across all the studies (median: 3 weeks; minimum: 1 week; maximum: 16 weeks).
When safety measures were described, the incidence of AEs was the most commonly reported outcome, even though 22 studies (61.1%) did not extensively report safety information. Conversely, two studies (5.56%) reported the incidence of AEs only after the first dose; five studies (13.9%) reported AEs only after the second dose. Finally, in seven studies (20.6%) AEs were reported after both the first and the second dose. Overall, COVID-19 vaccines were found to be safe and well tolerated, with no vaccine-related deaths. Any-grade AEs ranged between 9.7% and 87% after the first dose and between 23% and 85% after the second dose. The most commonly reported any-grade AEs were local pain (range: 7.4%-69%, I dose; range: 32.3%-67.2%, II dose) and fatigue (range: 4.2%-47.6%, I dose; range: 3%-23.4, II dose).
Patients with solid tumors were included in 15 studies (41.7%), whereas hematologic malignancies were represented in 28 studies (77.8%). Twenty-one studies (58.3%) were exclusively focused on hematologic patients. Six studies specifically focused on PsC on active treatments, namely cytotoxic agents, B-cell-depleting agents, Janus kinase inhibitors (JAKi) and immune checkpoint inhibitors (ICIs).17, 18, 19, 20, 21, 22 A comprehensive summary of the studies included is provided in Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014.
Cancer type
Available evidence suggests that vaccines, besides being generally safe and well tolerated, may have a compromised activity, especially in the case of hematologic malignancies (Figure 3 ).23 In this regard, a prospective observational study included 151 PsC (95 with a solid tumor and 56 with a hematologic malignancy), and 54 healthy controls (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014).24 In an interim analysis, the proportion of patients with positive anti-Spike IgG titers at ∼21 days following the first dose was 94% for the healthy controls, compared with 38% of those with solid tumors and 18% of those with hematologic malignancies.24 Considering patients with available blood samples 2 weeks after the second dose, 95% of the patients with solid tumors and 60% of those with hematologic malignancies showed seropositivity, in comparison with 100% of healthy controls. Another study evaluated 200 patients, of which 134 harbored solid tumors and the remaining 66 had a hematologic diagnosis. Vaccination was carried out with MDN (62/200), BNT (115/200) and Janssen (20/200). Although the overall seroconversion rate reached 94%, hematologic malignancies revealed a significantly lower rate (85%), particularly among those receiving B-cell-depleting therapies and following hematopoietic cell transplantation (HCT) (73%).21 For example, a detailed study highlighted that anti-CD20 Abs, Bruton tyrosine kinase inhibitors (BTKi), JAKi and B-cell lymphoma 2 (bcl-2) inhibitors seemed to electively impact on the Ab response to vaccination.23 Importantly, when vaccination was administered 12 months after the last treatment, serological responses improved.23 Consistently, initial findings from the CAPTURE study, a prospective longitudinal cohort study of SARS-CoV-2 infection and COVID-19 vaccine-induced immunity, were recently presented.25 Seroconversion rates for anti-Spike (S1) Abs following two doses were 85% and 54% for patients with solid tumors and hematologic malignancies, respectively. This study specifically focused on neutralizing antibodies (NAbs), describing lower detection rates and NAb titers in patients with hematologic malignancies in comparison with patients harboring solid tumors.25 Notably, after natural infection, neutralizing antibodies remained stable, unlike anti-Spike (S1) Abs that waned over time.25 Similar Ab production in PsC was shown in other studies (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014).19 , 23 , 26 , 27
Figure 3.
Estimated spectrum of COVID-19 vaccine efficacy for patients with cancer, according to cancer types and therapies.
Specific patient populations, especially those with hematologic malignancies receiving B-cell targeted agents, stem cell transplantation or CAR-T-cell treatment, may not mount a protective response. Further research is warranted to clarify the vaccine-induced immune response in a number of cancer types and regimens, particularly in those receiving targeted therapy and investigational drugs. # B-cell targeted agents include anti-CD20 agents (e.g. rituximab), anti-CD38 therapy, BCMA targeted agents, and Bruton tyrosine kinase inhibitors.
Created with biorender.com.
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CT, chemotherapy; ET, endocrine therapy; ICI, immune checkpoint inhibitors; JAKi, janus kinase inhibitor; MDS, myelodysplastic syndromes; MM, multiple myeloma; mo, months; MPN, myeloproliferative neoplasms; pts, patients; tx, therapies.
Multiple myeloma
Immunogenicity of COVID-19 vaccines has been investigated also in patients with specific hematologic conditions, such as multiple myeloma (MM). Among 103 patients (96 with active MM) who received mRNA-based vaccines, only 45% of active MM patients developed an adequate immune response, while 22% had a partial response, when stratified according to Ab titer.28 Conversely, smoldering MM patients (n = 7) responded better.28 Lower anti-Spike Ab levels were associated with older age, impaired renal function, low lymphocyte counts, reduced uninvolved immunoglobulin levels, > second line of treatment and absence of complete remission.28 The increased risk of poor seroconversion has been highlighted also in another study, though only focusing on the first vaccine dose.29 Other studies focusing on plasma cell disorders reported similar results.30 , 31
Consistently, two retrospective analyses investigated seroconversion in response to COVID-19 vaccines in a series of 320 and 23 fully immunized MM patients, respectively.32 , 33 In one study, individuals were assessed for serologic response at least 10 days after receiving the second dose of an mRNA-based vaccine. Although 84% of patients mounted a measurable Ab response, the serologic titer varied by three orders of magnitude (range: 5-7882 AU/ml, median: 149 AU/ml).32 Similarly, in the second study, the seroconversion rate reached 74% with a median anti-Spike titer or 4.9 UI/ml (range: 0-1028).33
Myelodysplastic and myeloproliferative neoplasms
Myeloproliferative neoplasms are associated with a pro-inflammatory state and dysregulation of pivotal natural killer cell (NK), regulatory T-cell (Tregs) and effector T-cell function.20 Two prospective studies evaluated the immune response after the first dose of COVID-19 vaccine in patients with MPNs. In one study, only BNT was administered.20 In the second study, both mRNA-based and viral vector-based vaccines were administered.34 After the first dose, patients with a diagnosis of myelofibrosis (MF) (n = 9) had significantly higher post-vaccine anti-Spike IgG half maximal effective concentration (EC50) as well as neutralizing Ab inhibitory dose (ID)50 titers, compared to patients with other MPN subtypes.20 Seroconversion measured >14 days after a single dose was only 58%, that is significantly lower than the one observed in health care professionals (HCPs) of similar age (97%). The median anti-Spike Ab titer was also significantly lower in MPN/myelodysplastic syndrome (MDS) patients (i.e. 630 versus 75 AU/ml, P < 0.0001).34 When focusing on disease subgroups, the seroconversion rate was highest in patients with chronic myeloid leukemia (CML, 75%), with no difference according to which vaccine was administered.34 Another study evaluated seroconversion rates at 5 weeks after the first BNT dose, thus also including patients receiving the second dose.35 Seroprotection rate at a cut-off of 15 AU/ml was 100% in controls compared to 88% in MPN patients (P = 0.038).35
Lymphoma
Patients diagnosed with lymphoma are at particular high risk of severe COVID-19.15 Recently, one prospective observational study evaluated the humoral immune response to BNT in a cohort of 148 patients harboring B-cell non-Hodgkin lymphoma (B-NHL). Of those, 47% displayed an aggressive disease, whereas 53% had an indolent malignancy.36 Of note, 37% of patients were receiving active treatment. Ab titer was measured 2-3 weeks after the second vaccine dose. Seroconversion was achieved in 49% of B-NHL patients versus a 98.5% rate achieved in healthy controls (P < 0.001).36
In the interim analysis of the PROSECO study, participants received either OxA or BNT, with two doses given 10-12 weeks apart. A total of 129 patients were enrolled. Of those, 12 patients (9%) had Hodgkin lymphoma (HL), 34 (26%) had aggressive B-NHL, 79 (61%) had indolent B-NHL and 4 (3%) had peripheral NK/T-cell lymphoma.37 Notably, 52 (44%) of 119 participants with lymphoma were on active treatment.37 Twenty-two (72%) of 31 participants after one dose of vaccine and 20/33 (61%) participants after two doses did not produce detectable anti-Spike IgG Abs. Among the lymphoma patients who were not on active treatment, 6/6 (100%) patients with HL and 13/16 (81%) with aggressive B-NHL developed an immune response comparable to that of healthy individuals.37 Thirty-two (89%) of 36 participants with an indolent B-NHL who were not on active treatment showed detectable Abs after two vaccine doses. However, their Ab titer was reduced in comparison with the levels observed in participants with HL and aggressive B-NHL that were either treatment-naïve or with completion of treatment >3 years before vaccination.
Chronic lymphocytic leukemia
Compared with other hematologic malignancies, the Ab response appears particularly impaired in chronic lymphocytic leukemia (CLL) patients. A prospective study that compared serologic response with BNT between matched cohorts of 52 patients and 52 healthy subjects showed that CLL patients had a lower serologic response rate (52% versus 100%) than healthy controls (P < 0.001).38 When focusing on the entire cohort of 167 CLL patients, the Ab response rate was only 39.5%, with younger age, lack of active treatment and early disease stage associated with better seroconversion rates. Other studies focusing on CLL reported similar results, suggesting that humoral response may be particularly affected by disease activity itself.33 , 39 , 40
Solid tumors
A prospective study investigated the serologic status of BNT in a cohort of patients with solid tumors on active treatment (n = 232), compared with age-matched HCPs (n = 261). In the patient group, 86/232 individuals were tested after the first vaccination dose and 218/232 were tested after the second dose. After the first dose, 25/86 (29%) patients were seropositive compared with 220/261 (84%) healthy controls (P < 0.001). After the second dose, the seropositive rate reached 86% (187/218) among the PsC.26 At the latest time point (4 weeks after the second dose), 14% of PsC were seronegative. Specifically, patients with breast cancer (BC) accounted for 29% of the seronegative group and 74% of these individuals were treated with diverse regimens of chemotherapy (CT).26 However, although specific CT agents may not be directly linked to impaired immunogenicity, the lymphosuppressive potential of some CT regimens may limit seroconversion.26
Another study including 95 patients with solid cancer and 66 healthy controls reported a seroconversion rate of 87% and 100% in patients and controls, respectively, after a median of 123 days from the second vaccination. However, a significantly lower median titer level in PsC was found, in comparison with the control group (417 AU/ml versus 1220 AU/ml, P < 0.001).41 In an exploratory multivariate analysis, the co-administration of CT plus immunotherapy (IO) or of IO plus a biological agent resulted in the only variable associated with lower IgG titers. A recent pooled analysis including 223 PsC with solid tumors highlighted a higher seroconversion rate (94%), with significantly lower anti-Spike Abs, compared to healthy controls, irrespective of the assay used.19 , 27 , 42
Type of treatment
A major unanswered question for PsC is whether vaccine immunogenicity is impacted by the concomitant use of specific drugs. Initial findings from the VOICE trial, focusing on solid tumors, have been recently presented.43 Among patients receiving IO, CT and CT-IO, anti-Spike (S1) IgG seroconversion rates were 99.3%, 97.4% and 100%, respectively. As the authors established a cut-off of 300 BAU/ml for adequate Ab response, seroconversion rates after two vaccine doses dropped to 93.1%, 83.8% and 88.8%, for patients receiving IO, CT and CT-IO, respectively.43 Thus, a significant minority of patients does not develop an adequate Ab response (6.9%, IO; 16.2%, CT; 11.2%, CT-IO). Few other studies focused on individual agents to fully elucidate any potential interaction with the ability to mount a protective immune response. Emerging data are clarifying that, in general, the protective role of two vaccine doses for PsC on certain active treatments may be suboptimal (Figure 3).16 , 19 , 34 , 37 , 44 , 45
Endocrine treatment
In a recent study, post-vaccination seroconversion rates in patients receiving endocrine treatment (ET) (n = 47) resulted high in comparison with other active treatments, reaching a 100% seropositivity rate (P = 0.04).21 Therefore, no major preventive measures or time windows should be implemented in current vaccination campaigns.
Cytotoxic chemotherapy
Increasingly consistent data suggest that among patients receiving systemic CT for solid tumors, there is a high proportion of weakly responsive and unresponsive patients after a single vaccine dose.19 In this regard, immune responses to BNT were evaluated in 52 solid tumor patients on active cytotoxic CT and compared to a control group of 50 healthy individuals.46 Neutralizing Abs were detected in 67% and in 80% of PsC after the first and the second dose, respectively.46 Similar trends were observed as for Abs against the receptor-binding domain (RBD) and the S2 regions of the Spike protein, although they were found to be reduced in comparison to healthy controls.46 Other studies showed similar results, consistent with a link between the lymphosuppressive potential of some CT regimens and a delay or reduction in an effective seroconversion.24 , 26 , 41 , 42 , 47
Targeted therapies
Evidence addressing the seroconversion rates in PsC on specific targeted agents is lacking.19 , 48 Compounds causing lymphopenia or specific B-cell-depleting agents are emerging as majorly responsible for an impaired protection from vaccines.23 However, the long-term immunologic effects of B-cell depletion and the characteristics of B-cell reconstitution, especially in lymphoid malignancies, are not well defined, despite the widespread usage of B-cell-directed therapies.45
B-cell-depleting therapies
Treatment with B-cell-directed agents—e.g. rituximab and obinutuzumab (anti-CD20), ibrutinib (BTKi)—may negatively impact the production of Abs in response to COVID-19 vaccines, especially in lymphoid malignancies, due to B-cell depletion and/or disruption of the B-cell receptor signaling pathway.45 In addition, the recovery of the memory B-cell pool has been shown to be delayed in lymphomas, remaining below normal controls at 1 year post rituximab.45 , 49
In a cohort of 149 B-NHL patients, 37% were actively treated with a rituximab/obinutuzumab (R/Obi)-based regimen for either induction or maintenance, whereas 44% had last been treated with R/Obi 6 months before COVID-19 vaccination. Seroconversion was achieved in 25/28 (89%) treatment-naïve patients, in 4/55 (7.3%) R/Obi patients and in 43/65 (66.7%) patients receiving the last dose of the B-cell-depleting regimen 6 months before vaccination. Multivariate analysis revealed that a longer-time window since the last R/Obi exposure and an absolute lymphocyte count ≥ 0.9 × 103/ml predicted seroconversion.36
An optimal window for vaccination in this patient population should be considered. In fact, in another study focused on lymphomas, seroconversion rates differed between patients who received the last infusion of an anti-B-cell agent within 9 months before the COVID-19 vaccine (6/52, 11%) compared to those who received the last infusion of a B-cell-depleting agent > 9 months before the vaccination (22/25, 88%).45 Consistently, in a cohort of CLL patients, none of the 22 individuals who received therapeutic anti-CD20 Abs in the 12 months before COVID-19 vaccination developed neutralizing Abs after two mRNA-based vaccinations, in comparison with 25/55 (46%) patients exposed to anti-CD20 therapy ≥12 months before vaccination.38 Similar results came from other recent reports, with a significant 14.2-fold increased risk of non-response to COVID-19 vaccination.23 , 50 , 51
Finally, serologic responses are also impaired in certain populations with MM receiving therapies against CD38 (e.g. daratumumab, isatuximab) and B-cell maturation antigen (anti-BCMA agents, e.g. belantamab mafodotin and idecabtagene vicleucel).29 , 30 , 32 , 35
JAKi
JAKi, such as ruxolitinib (RUXO), are currently approved for the treatment of MF and hydroxyurea-resistant/refractory polycythemia vera.17 RUXO is thought to have profound effects on different cell compartments of the immune system, including T cells, NK cells and dendritic cells. By inhibiting JAK-signal transducer and activator of transcription (STAT) signaling, a potential role in reducing inflammatory cytokine production is considered.17 Such features could explain the increased rate of infection in MPN patients receiving RUXO.17
A prospective study recently assessed the serologic response after the first COVID-19 mRNA-based vaccine injection in 30 consecutive MPN patients receiving RUXO at a median dose of 20 mg daily. The Ab response after a first vaccination dose was significantly lower in RUXO-treated patients compared to healthy controls and to patients not receiving RUXO. All 14 healthy controls were vaccine-responders (100%), whereas only 33.3% of RUXO-treated patients seroconverted to Spike protein (P < 0.001). As for patients not receiving RUXO, the seroconversion rate was 91.6%.17 Further studies, hopefully with larger sample sizes, are needed to address whether such unresponsive status persist after the second vaccine dose, as suggested by the 42% seropositivity rate described in MPN patients using JAKi, after completing the full mRNA-based vaccination schedule.52
Cyclin-dependent kinases 4/6 inhibitors
The involvement of the cyclin-dependent kinase (CDK)4/6 pathway in immune activation is well known.53 In a cohort of 23 BC patients receiving CDK4/6 inhibitors, neutralizing Ab titers in response to the first dose of vaccine were similar to healthy controls.54 However, anti-Spike Ab titers were low in another study assessing the response after full vaccination, even if the subset of patients was too small (n = 5) to draw solid conclusions.21
Tyrosine kinase inhibitors
To date, there is no evidence that any of the TKIs currently approved in clinical practice can interfere with effective immune responses against SARS-CoV-2.21 In spite of a small sample size, a recent report on CML patients receiving imatinib showed conserved seroconversion rates (5/6, 83%).34 Among patients who received other TKIs, namely nilotinib, bosutinib and dasatinib, the seroconversion rate was 66.7% (4/6).34
Immunotherapy
Chimeric antigen receptor T-cell therapy
Among patients with aggressive B-NHL receiving chimeric antigen receptor (CAR) T-cell therapy, 100% (3/3) had no detectable Abs after the first vaccination dose. Only one of these patients developed Abs after the second dose, even if the other two had yet to be tested at the time of the study report. These results were observed although these patients had completed CAR-T-cell treatment 11-23 months before vaccination.37
ICIs
An unresolved question for PsC is whether vaccine safety and/or immunogenicity are impacted by ICIs, which stimulate immune system function.22 , 55 As for safety, in a study enrolling 134 PsC receiving ICIs, either as monotherapy or in combination with CT, the AEs of COVID-19 vaccination seemed comparable with those of healthy controls. Of note, only the incidence of muscle pain was higher.56 However, there was no immune-related myositis, and COVID-19 vaccination did not appear to exacerbate or cause new immune-related AEs.56
Concerning immunogenicity, a recent clinical report documented that 15/59 (25%) versus 186/283 (65.7%) PsC developed neutralizing Ab titers after the first dose (P < 0.001).18 Conversely, in a second report, seroconversion reached 97%.21 Accordingly, the VOICE trial described seroconversion rates of 99.3% and 100% among patients receiving IO and CT-IO, respectively.43 However, a significant minority of patients did not develop an adequate Ab response (6.9%, IO; 16.2%, CT; 11.2%, CT-IO, with an established cut-off of 300 BAU/ml).43 As an exploratory finding, the CAPTURE trial highlighted a negative impact of ICIs on cellular immune responses, for which further research is warranted.25
Discussion
In the general population, the adaptive immune response to SARS-CoV-2 comprises B cells that produce different classes of Abs in order to neutralize the virus, as well as T cells that support Ab production while also directly killing virus-infected cells.15 Although memory B and T cells have been described both in individuals with a natural infection and in vaccinated populations, their specific roles in achieving protective immunity have to be defined yet.48 , 57 , 58 However, T cells are thought to play an important role in reducing COVID-19 severity.59, 60, 61 Several observations suggest that early SARS-CoV-2 T-cell responses are associated with milder COVID-19.59 , 60 In this regard, data from ph III clinical trials investigating COVID-19 vaccines suggest that protection may require low levels of neutralizing Abs and might involve other immune effector mechanisms, including non-neutralizing Abs, T cells and innate immunity.57 Circulating Ab titers did not result to be predictive of T-cell memory.58 , 61 , 62 Additionally, although real-world data indicate that vaccine protection against SARS-CoV-2 infection wanes over time, protection against hospitalization and severe disease appears to be preserved.63, 64, 65
For PsC, dissecting the complexity of a protective immune response against SARS-CoV-2 is challenging, considering both the biological differences among cancer types as well as the different treatments received.66 Moreover, since PsC were largely excluded from ph III clinical trials testing vaccine candidates, evidence about protective immune responses came from highly heterogeneous single-center observational studies (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014).14
Although emerging evidence suggests that simple serological tests for SARS-CoV-2 Abs may not reflect the complexity and durability of protective immunity against COVID-19, the primary endpoint of all the studies included was seroconversion to the anti-Spike protein.57 , 62 , 67
When cellular immunity was investigated, it was considered as an exploratory endpoint.25 , 43 Overall, T-cell immune responses seem generally maintained, although reduced, especially in patients with hematologic malignancies, in comparison to patients with solid tumors and to healthy individuals, both after COVID-19 infection and after a complete vaccination, with predominance of CD4+ responses over CD8+.21 , 25 , 47 , 51 , 58 Systemic therapies did not have a major effect on cellular responses, except for higher suppression rates of CD4+ activity among patients treated with ICIs.25 In the VOICE clinical trial, almost half of the vaccine non-responders and suboptimal responders to humoral immunity developed a Spike-specific T-cell response.43 Interestingly, in the preliminary report of the SOAP-2 vaccine study, the T-cell response appeared to be greater than the B-cell immune response after the first dose, but still lower compared to the control group (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014)24 , 48 Currently, data regarding the clinical efficacy of SARS-CoV-2 vaccines, defined as the incidence of symptomatic or severe COVID-19 in PsC, after full completion of the vaccination schedule, are still scarce.25 A longer follow-up is needed in order to address this crucial question.
With all the described limitations regarding the current understanding of immune responses to COVID-19 vaccines, the decreased seroconversion rates in PsC, especially in those on active treatment with B-cell-depleting agents, could not be neglected.33 , 48 , 68 Moreover, even if T-cell immunity could be thought to compensate for impaired humoral immunity, impaired T-cell responses in the event of COVID-19 have been observed in some patients under cancer treatment.25 , 43 , 67 , 69 For these reasons, different strategies to enhance vaccine-induced immunity have been proposed, such as heterologous prime-boost vaccination, a double-dose strategy and a third dose.48 , 70 In the first case, the non-inferiority design of the trial as well as the lack of PsC under active treatment included did not allow to draw conclusions about the feasibility of this approach.70 The double-dose strategy is based on literature data and current vaccination practices, particularly in immunocompromised patients vaccinated against hepatitis B and influenza viruses, although prospective randomized trials are needed.48 , 71, 72, 73, 74, 75, 76, 77 Finally, the role of a third dose is being investigated, especially in immunocompromised patients and the elderly population.78
To date, the largest study investigating the clinical efficacy of a booster shot of vaccine was conducted in Israel and included people >60 years of age.79 The rates of confirmed COVID-19 and severe illness were substantially lower among those who received the booster at least 5 months after the last dose.79 A number of other reports confirmed a benefit on seroconversion for a third mRNA-based vaccine dose, although longer follow-up and evaluation of cellular immune responses are needed to better characterize the impact of additional vaccine doses on the clinical outcomes in patients with an impaired immune system (e.g. OCTAVE DUO trial).51 , 68 , 76 , 78 , 80, 81, 82, 83, 84 Furthermore, in the CAPTURE clinical trial, previous SARS-CoV-2 infection boosted vaccine-induced responses, lending further support for a third dose in vulnerable populations.25
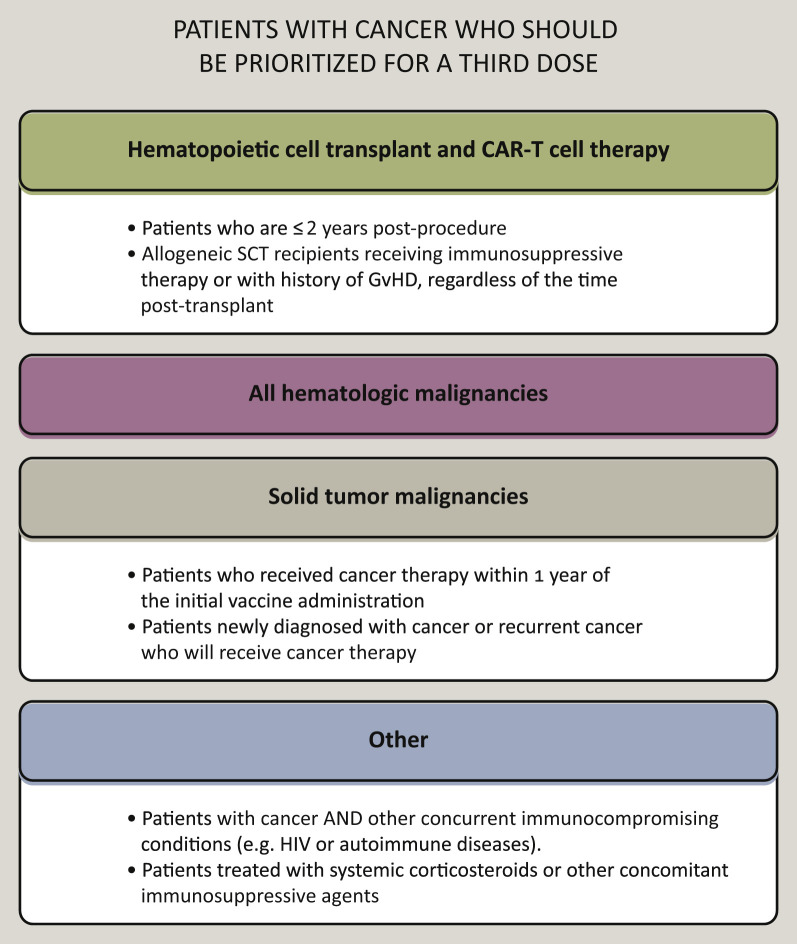
In line with these trends, many countries and institutions have already recommended that severely immunocompromised patients (e.g. transplant recipients, patients with hematologic malignancies or those receiving immunosuppressive agents) receive a third dose of COVID-19 vaccine (Figure 4 ).48 , 85 Moreover, on 12 August 2021, the Food and Drug Administration authorized an additional mRNA-based vaccine dose for certain immunocompromised individuals.86 On the other hand, the WHO notes that the benefit of a widespread use of booster vaccinations on morbidity and mortality from COVID-19 remains uncertain, also considering the alarming shortage of vaccine supplies in lower-income countries.87
Figure 4.
Consensus recommendations for patients with cancer who should be prioritized for a third dose.
Within each box, the subgroups which should receive a third dose with priority, as they may represent the most immunocompromised individuals per each macro category (bold font), are reported. The CDC recommends the additional dose of an mRNA COVID-19 vaccine be administered at least 4 weeks after a second dose of the Pfizer-BioNTech or Moderna vaccine. For people who received the Pfizer-BioNTech or Moderna COVID-19 vaccine series, a third dose of the same mRNA vaccine should be used if possible. If the same mRNA vaccine is not available for the third dose administration or is unknown, either mRNA COVID-19 vaccine may be used. The use of antibody titers to determine whether patients should receive the third dose is not recommended (outside of a research study).
Source: National Comprehensive Cancer Network (NCCN) COVID-19 Vaccination Advisory Committee. Version 4.0 08/30/2021. Created with biorender.com.
CDC, Centers for Disease Control and Prevention; GvHD, graft-versus-host disease; HIV, Human Immunodeficiency Virus; pts, patients; SCT, stem cell transplant.
Conclusion
Vaccination against COVID-19 for PsC seems overall safe and effective after well-conducted vaccination schedules.48 Yet seroconversion rates remain lower, lagged or both across some subgroups (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.10.014).19 , 26 , 27 , 48 Although complete absence of detectable Abs after vaccination likely equates to a lack of protection, no solid data are available to establish a correlation between the protective role of vaccination and the anti-Spike Ab titer.88 , 89
Therefore, provided that PsC are comprehensively counseled about the available information on vaccine effectiveness, a tailored approach may be proposed, considering the type of malignancy and of the specific oncologic treatment received (Figure 4).85 In general, for patients with solid tumors in remission, with MPN/MDS without treatment, receiving ET, ICIs or non-lymphosuppressive cytotoxic agents, no particular restrictions or time windows are advised.20 , 88 Patients with lymphoid malignancies that are candidates to B-cell-depleting agents and those with solid tumors that are candidates to lymphosuppressive cytotoxic agents should be vaccinated before starting the planned regimen, if feasible. If not, vaccine doses should be planned in a time window that considers the nadir of the expected CT-induced cytopenia. Patients with hematologic malignancies already under treatment with B-cell-depleting agents, CD19-directed CAR-T-cell therapies and HCT recipients showed very low seroconversion rates from vaccination, prompting concern that they are likely to have poor protection against COVID-19. A third vaccine dose may be proposed to such immunocompromised categories, possibly considering a time window consistent with the emerging evidence of an acceptable serological response between 9 and 12 months, after completing treatment (Figure 4).20 , 37 , 45 , 85 A similar approach may be warranted for individuals with active CLL and older patients with MM, although a case-by-case management is recommended.28 , 29 , 40 Findings from prospective clinical trials with a longer follow-up will further elucidate how to tailor vaccination in special populations.90, 91, 92, 93
A major obstacle to achieve herd immunity is vaccine hesitancy.94 In the specific population of PsC, vaccine acceptance is generally higher than in other patient populations.48 , 95 , 96 In an Italian report, of 914 patients eligible to the survey, only 102 refused vaccination (11.2%). The most frequently reported reasons to refuse the vaccine were concerns about vaccine-related AEs (48.1%). The identification of the reasons associated with vaccine hesitancy and refusal should be exploited to personalize educational approaches.97, 98, 99, 100
The reviewed data altogether suggest that PsC, especially those at the highest risk of poor seroconversion, should maintain strict preventive behaviors (e.g. FFP-2 masks), for at least 6-8 weeks after the first vaccine dose, and to not postpone the second dose, if possible.48 , 92 , 93 In addition, households and other close contacts of immunocompromised patients should be vaccinated.48 , 85
Acknowledgments
Funding
None declared.
Disclosure
FS reports consulting fees from AMGEN, Roche, Chugai, Mylan, Mundi Pharma, Leo Pharma, Pierre Fabre Oncology, Helsinn, MSD, Pfizer and BMS, all outside the submitted work. JPS has received personal fees as a speaker or consultant from Pfizer, AstraZeneca, Gilead, Leo Pharma, Daiichi, Mylan, BMS, MSD, Pierre Fabre, GSK (Abbvie), Roche, Novartis and Lilly. As part of the Drug Development Department (DITEP), J-M.M reports: principal/sub-Investigator of Clinical Trials for Abbvie, Adaptimmune, Adlai Nortye USA Inc., Aduro Biotech, Agios Pharmaceuticals, Amgen, Argen-X Bvba, Astex Pharmaceuticals, AstraZeneca Ab, Aveo, Basilea Pharmaceutica International Ltd., Bayer Healthcare Ag, Bbb Technologies Bv, Beigene, BicycleTx Ltd, Blueprint Medicines, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Ca, Celgene Corporation, Chugai Pharmaceutical Co, Clovis Oncology, Cullinan-Apollo, Curevac, Daiichi Sankyo, Debiopharm, Eisai, Eisai Limited, Eli Lilly, Exelixis, Faron Pharmaceuticals Ltd., Forma Tharapeutics, Gamamabs, Genentech, Glaxosmithkline, H3 Biomedicine, Hoffmann La Roche Ag, Imcheck Therapeutics, Innate Pharma, Institut De Recherche Pierre Fabre, Iris Servier, Iteos Belgium SA, Janssen Cilag, Janssen Research Foundation, Kura Oncology, Kyowa Kirin Pharm. Dev, Lilly France, Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Ricerche, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Molecular Partners Ag, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoethix, Oncopeptides, Orion Pharma, Ose Pharma, Pfizer, Pharma Mar, Pierre Fabre, Medicament, Roche, Sanofi Aventis, Seattle Genetics, Sotio A.S, Syros Pharmaceuticals, Taiho Pharma, Tesaro, Turning Point Therapeutics and Xencor; research grants from AstraZeneca, BMS, Boehringer Ingelheim, GSK, INCA, Janssen Cilag, Merck, Novartis, Pfizer, Roche and Sanofi; and non-financial support (drug supplied) from AstraZeneca, Bayer, BMS, Boringher Ingelheim, GSK, Medimmune, Merck, NH TherAGuiX, Pfizer and Roche. J.-M.M. also reports other support from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly Oncology, b. Hoffmann–La Roche Ltd, Merck, MSD, Pfizer and Regeneron, outside this work. FA reports grants from Novartis, grants from Pfizer, grants from Roche, grants from Daiichi, grants from Eli Lilly and grants from AstraZeneca, all outside the submitted work. GC served as consultant or advisor for Roche, Lilly and Bristol-Myers Squibb, served on the speaker's bureau for Roche, Pfizer and Lilly, received travel funding from Pfizer and Roche, and received honoraria from Roche, Pfizer, Lilly, Novartis, AstraZeneca and SEAGEN, all outside the submitted work. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.WHO Coronavirus disease (COVID-19) pandemic. Numbers at a glance. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at.
- 2.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. J Am Med Assoc. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marconi V.C., Ramanan A.V., de Bono S., et al. Efficacy and safety of baricitinib in patients with COVID-19 infection: Results from the randomised, double-blind, placebo-controlled, parallel-group COV-BARRIER phase 3 trial. MedRxiv. 2021 doi: 10.1016/S2213-2600(21)00331-3. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan H., Peto R., Henao-Restrepo A.M., et al. Repurposed antiviral drugs for covid-19 – interim WHO solidarity trial results. N Engl J Med. 2020;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkrief A., Desilets A., Papneja N., et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: a multicentre observational cohort study. Eur J Cancer. 2020;139:181–187. doi: 10.1016/j.ejca.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: a French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Joode K., Dumoulin D.W., Tol J., et al. Dutch Oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini K.S., Tagliamento M., Lambertini M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yekedüz E., Utkan G., Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92–104. doi: 10.1016/j.ejca.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti C., Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol. 2021;32(4):569–571. doi: 10.1016/j.annonc.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti C., Crimini E., Tarantino P., et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karacin C., Eren T., Zeynelgil E., et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021;17(33):4447–4456. doi: 10.2217/fon-2021-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmelli P., Mazzoni A., Maggi L., et al. Impaired response to first SARS-CoV-2 dose vaccination in myeloproliferative neoplasm patients receiving ruxolitinib. Am J Hematol. 2021;96:E408–E410. doi: 10.1002/ajh.26305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos E., Zagouri F., Liontos M., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021;14(1):86. doi: 10.1186/s13045-021-01099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington P., de Lavallade H., Doores K.J., et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;194:999–1006. doi: 10.1038/s41375-021-01300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatim N., Boussier J., Tetu P., et al. Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection. Sci Adv. 2021;7(34):eabg4081. doi: 10.1126/sciadv.abg4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maneikis K., Šablauskas K., Ringelevičiūtė U., et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd S., Fendler A., Au L., et al. 1557O - Adaptive immunity to SARS-CoV-2 infection and vaccination in cancer patients: the CAPTURE study. Ann Oncol. 2021;32:S1129–S1163. [Google Scholar]
- 26.Goshen-Lago T., Waldhorn I., Holland R., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palich R., Veyri M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32(8):1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stampfer S.D., Goldwater M.S., Jew S., et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021 doi: 10.1038/s41375-021-01354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpos E., Trougakos I.P., Gavriatopoulou M., et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674–3676. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghandili S., Schönlein M., Lütgehetmann M., et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers (Basel) 2021;13(15):3800. doi: 10.3390/cancers13153800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramasamy K., Sadler R., Jeans S., et al. COVID symptoms, testing, shielding impact on patient-reported outcomes and early vaccine responses in individuals with multiple myeloma. Br J Haematol. 2021 doi: 10.1111/bjh.17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Oekelen O., Gleason C.R., Agte S., et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Re D., Barrière J., Chamorey E., et al. Low rate of seroconversion after mRNA anti-SARS-CoV-2 vaccination in patients with hematological malignancies. Leuk Lymphoma. 2021:1–3. doi: 10.1080/10428194.2021.1957877. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury O., Bruguier H., Mallett G., et al. Impaired antibody response to COVID-19 vaccination in patients with chronic myeloid neoplasms. Br J Haematol. 2021;194(6):1010–1015. doi: 10.1111/bjh.17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimpinelli F., Marchesi F., Piaggio G., et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry C., Luttwak E., Balaban R., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim S.H., Campbell N., Johnson M., et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542–e544. doi: 10.1016/S2352-3026(21)00199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini O., Rokach L., Itchaki G., et al. Safety and efficacy of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2021 doi: 10.3324/haematol.2021.279196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parry H., McIlroy G., Bruton R., et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. doi: 10.1038/s41408-021-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eliakim-Raz N., Massarweh A., Stemmer A., Stemmer S.M. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palich R., Veyri M., Vozy A., et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated with chemotherapy for solid cancers. Ann Oncol. 2021;32(10):1294–1295. doi: 10.1016/j.annonc.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Veldt A.A.M., Oosting S.F., Dingemans A.C., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27(4):568–569. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 44.Heudel P., Favier B., Assaad S., Zrounba P., Blay J.Y. Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients. Ann Oncol. 2021;32(11):1443–1444. doi: 10.1016/j.annonc.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghione P., Gu J.J., Attwood K., et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell directed therapies. Blood. 2021;138(9):811–814. doi: 10.1182/blood.2021012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shroff R.T., Chalasani P., Wei R., et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. MedRxiv. 2021 Preprint. [Google Scholar]
- 47.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrière J., Re D., Peyrade F., Carles M. Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. Eur J Cancer. 2021;154:66–72. doi: 10.1016/j.ejca.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anolik J.H., Friedberg J.W., Zheng B., et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122(2):139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Benda M., Mutschlechner B., Ulmer H., et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol. 2021 doi: 10.1111/bjh.17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Re D., Seitz-Polski B., Carles M., et al. Brief communication: humoral and cellular responses after a third dose of BNT162b2 vaccine in patients with lymphoid malignancies. Research Square – Nature Portfolio. 2021 doi: 10.21203/rs.3.rs-727941/v1. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herzog Tzarfati K., Gutwein O., Apel A., et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laphanuwat P., Jirawatnotai S. Immunomodulatory roles of cell cycle regulators. Front Cell Dev Biol. 2019;7:23. doi: 10.3389/fcell.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zagouri F., Terpos E., Fiste O., et al. SARS-CoV-2 neutralizing antibodies after first vaccination dose in breast cancer patients receiving CDK4/6 inhibitors. Breast. 2021;60:58–61. doi: 10.1016/j.breast.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desage A.L., Bouleftour W., Rivoirard R., et al. Vaccination and immune checkpoint inhibitors: does vaccination increase the risk of immune-related adverse events? A systematic review of literature. Am J Clin Oncol. 2021;44(3):109–113. doi: 10.1097/COC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 56.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mairhofer M., Kausche L., Kaltenbrunner S., et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171–1172. doi: 10.1016/j.ccell.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan A.T., Linster M., Tan C.W., et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. doi: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 accessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27(7):1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 63.Nanduri S., Pilishvili T., Derado G., et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034e3.htm Available at. [DOI] [PMC free article] [PubMed]
- 64.Rosenberg E.S., Holtgrave D.R., Dorabawila V., et al. The Centers for Disease Control and Prevention (CDC): Morbidity and Mortality Weekly Report (MMWR) - New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034e1.htm Available at. [DOI] [PMC free article] [PubMed]
- 65.Tenforde M.W., Self W.H., Naioti E.A., et al. Centers for Disease Control and Prevention (CDC): Morbidity and Mortality Weekly Report (MMWR) - Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults 2021. https://stacks.cdc.gov/view/cdc/108947 Available at. [DOI] [PMC free article] [PubMed]
- 66.Garassino M.C., Giesen N., Grivas P., et al. ESMO Statements for Vaccination against COVID-19 in patients with cancer. https://bit.ly/3swNx9G 2020 Available at.
- 67.Bilich T., Roerden M., Maringer Y., et al. Preexisting and post-COVID-19 immune responses to SARS-CoV-2 in patients with cancer. Cancer Discov. 2021;11(8):1982–1995. doi: 10.1158/2159-8290.CD-21-0191. [DOI] [PubMed] [Google Scholar]
- 68.Gounant V., Ferré V.M., Soussi G., et al. Efficacy of SARS-CoV-2 vaccine in thoracic cancer patients: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. MedRxiv. 2021 doi: 10.1016/j.jtho.2021.10.015. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mansi L., Spehner L., Daguindau E., et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer. 2021;150:1–9. doi: 10.1016/j.ejca.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potsch D.V., Camacho L.A., Tuboi S., et al. Vaccination against hepatitis B with 4-double doses increases response rates and antibodies titers in HIV-infected adults. Vaccine. 2012;30(41):5973–5977. doi: 10.1016/j.vaccine.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 72.Branagan A.R., Duffy E., Gan G., et al. Tandem high-dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias. Blood Adv. 2021;5(5):1535–1539. doi: 10.1182/bloodadvances.2020003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sodhi J.S., Raja W., Zargar S.A., et al. The efficacy of accelerated, multiple, double-dose hepatitis B vaccine against hepatitis B virus infection in cancer patients receiving chemotherapy. Indian J Gastroenterol. 2015;34(5):372–379. doi: 10.1007/s12664-015-0595-y. [DOI] [PubMed] [Google Scholar]
- 74.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 75.Walsh E.E., Frenck R.W., Falsey A.R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.GOV.UK: OCTAVE DUO – New study to test third COVID-19 vaccine for people with weakened immune systems [press release, Available at https://bit.ly/3zTQpQm], 2021. Accessed September 2, 2021.
- 77.Kearns P., Siebert S., Willicombe M., et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE Trial. Lancet. 2021 doi: 10.2139/ssrn.3910058. Preprints. [DOI] [Google Scholar]
- 78.Karaba A.H., Zhu X., Liang T., et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. MedRxiv. 2021 doi: 10.1111/ajt.16933. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longlune N., Nogier M.B., Miedougé M., et al. High immunogenicity of a messenger RNA based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704–1709. doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Werbel W.A., Boyarsky B.J., Ou M.T., et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benotmane I., Gautier G., Perrin P., et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. J Am Med A. 2021;326(11):1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.NCCN – Recommendations of the National Comprehensive Cancer Network COVID-19 Vaccination Advisory Committee∗ Version 4.0 08/30/2021 30 August 2021. https://bit.ly/3ihKQoX Available at.
- 86.FDA Coronavirus (COVID-19) Update: The Food and Drug Administration Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals 2021. https://bit.ly/3gy5YX8 Available at.
- 87.World Health Organization (WHO) – Interim statement on COVID-19 vaccine booster doses 2021. https://bit.ly/3DzTpUX Available at.
- 88.Griffiths E.A., Segal B.H. Immune responses to COVID-19 vaccines in patients with cancer: promising results and a note of caution. Cancer Cell. 2021;39(8):1045–1047. doi: 10.1016/j.ccell.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 90.Loubet P., Wittkop L., Tartour E., et al. A French cohort for assessing COVID-19 vaccine responses in specific populations. Nat Med. 2021;27(8):1319–1321. doi: 10.1038/s41591-021-01435-1. [DOI] [PubMed] [Google Scholar]
- 91.Desai A., Gainor J.F., Hegde A., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18(5):313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Noia V., Pimpinelli F., Renna D., et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-21-2439. [DOI] [PubMed] [Google Scholar]
- 93.Mair M., Berger J., Berghoff A., et al. Humoral immune response in hematooncological patients and health care workers who received SARS-CoV-2 vaccinations. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lawrence G.L., Hull B.P., MacIntyre C.R., McIntyre P.B. Reasons for incomplete immunisation among Australian children. A national survey of parents. Aust Fam Physician. 2004;33(7):568–571. [PubMed] [Google Scholar]
- 95.Chun J.Y., Kim S.I., Park E.Y., et al. Cancer patients' willingness to take COVID-19 vaccination: a nationwide multicenter survey in Korea. Cancers (Basel) 2021;13(15):3883. doi: 10.3390/cancers13153883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barrière J., Gal J., Hoch B., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32(5):673–674. doi: 10.1016/j.annonc.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Opel D.J., Henrikson N., Lepere K., et al. Previsit screening for parental vaccine hesitancy: a cluster randomized trial. Pediatrics. 2019;144(5):e20190802. doi: 10.1542/peds.2019-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curigliano G., Eggermont A.M.M. Adherence to COVID-19 vaccines in cancer patients: promote it and make it happen! Eur J Cancer. 2021;153:257–259. doi: 10.1016/j.ejca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Noia V., Renna D., Barberi V., et al. The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: early data from a single-institute survey. Eur J Cancer. 2021;153:260–264. doi: 10.1016/j.ejca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spano J.P., Barre-Sinoussi F., Kieny M.P., Marcelin A.G., Blay J.Y. COVID-19 vaccination for cancer patients: medical and ethical need. Bull Cancer. 2021;108(3):225–227. doi: 10.1016/j.bulcan.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.