Abstract
Introduction
The first wave of COVID-19 pandemic has disrupted almost all areas of the health care services to some extent throughout the world. Although the negative impact of COVID-19 on patients with autoimmune diseases has also been recognized, available data in this regard are limited. In the current study of the European Autoimmunity Standardisation Initiative (EASI) we aimed to provide reliable data on the extent of the impact of COVID-19 pandemic on test requests for different autoantibodies in European countries.
Methods
Data on test numbers and on the number of positive results were collected in 97 clinical laboratories from 15 European countries on a monthly basis for the year before (2019) and the year during (2020) the COVID-19 pandemic.
Results
A reduction in the number of autoantibody tests was observed in all European countries in the year 2020 compared to 2019. The reduction affected all autoantibody tests with an overall decrease of 13%, ranging from 1.4% (Switzerland) to 25.5% (Greece). In all countries, the decrease was most pronounced during the first wave of the pandemic (March–May 2020) with an overall decrease in those three months of 45.2%. The most affected autoantibodies were those commonly requested by general practitioners (anti-tTG IgA (−71%), RF IgM (−66%) and ACPA (−61%)). In the second wave of the pandemic (October–December 2020) the decrease was less pronounced (6.8%).
With respect to the rate of positive results, subtle differences were observed for distinct autoantibodies during the pandemic, but the total rate of positive results was similar in both years.
Conclusions
Our study demonstrated a strong decrease in autoantibody requests during the first wave of the COVID-19 pandemic in 15 European countries. The second wave was characterized by a less pronounced impact, with some participating countries hardly affected, while some other countries experienced a second decline. The decrease was clearly associated with the level of lock-down and with the required adjustments in the health care systems in different countries, supporting the importance of an effective strategy for the coordination of autoimmune testing in challenging situations as the COVID-19 pandemic.
Keywords: Autoimmune diseases, Autoantibody testing, COVID-19 pandemic
1. Introduction
In Europe, the first patient with SARS-CoV-2 (COVID-19) infection was diagnosed on January 24, 2020, in France. Besides other incidental cases in several countries, clusters of cases were reported in the Northern regions of Italy on February 22, 2020. From then on, the pandemic gradually spread over the European continent. Italy was the first country to implement strict regulations to prevent further spreading of the SARS-CoV-2 on March 8, soon followed in the weeks thereafter by most other countries. After a couple of months, the infection rate declined and, hence, containment measures were reduced. However, in the autumn of 2020 the number of infections gradually increased again and in combination with the spreading of more infectious SARS-CoV-2 variants, new containment measures and restrictions were installed (see timeline of the European Centre for Disease Prevention and Control (ECDC) on www.ecdc.europa.eu).
The first wave of COVID-19 had an enormous impact on the health care system of the affected countries. In many countries general practices were closed and due to the high demand on intensive care facilities and to prevent spreading towards patients with other diseases, the health service in hospitals was scaled down and specialist referrals delayed or postponed [[1], [2], [3], [4], [5], [6], [7], [8]]. As a consequence of scarce resources during the pandemic, diagnoses for non-COVID-19 conditions may have been missed or delayed. During the second wave of COVID-19 the health care system was better prepared to face the challenges and, therefore, the measures taken in general practices and hospitals were less drastic than during the first wave. In this respect, in most countries attention was at first focused on patients with malignancies [9]. The problem of the fewer cancer diagnoses was faced in April 2020 by the Netherlands Comprehensive Cancer Organization who highlighted how the management of low-risk malignancies (e.g., many skin cancers) might have slightly affected the quantity and quality of life, while the management of high risk malignancies (e.g., acute leukaemias) could not be postponed [10].
Although the problem was also recognized for patients with chronic conditions such as autoimmune diseases, data illustrating this problem are limited to the Rare Diseases (RDs) [11]. RDs networks (such as the European Reference Networks) tried to manage the negative impact on the vulnerable patients with RDs developing plans to ensure the appropriate care also in case of future health emergencies.
On one hand, there was a great scientific interest whether patients with autoimmune diseases were more at risk of having COVID-19 [[12], [13], [14]] and on the other hand, whether SARS-CoV-2 virus could trigger the onset of autoimmune diseases [[15], [16], [17], [18], [19], [20], [21], [22], [23]]. In particular, the observation that patients with COVID-19 have an increased risk for thrombotic arterial and venous occlusions has hinted at the possible induction of the antiphospholipid syndrome (APS). In line with this hypothesis, some studies revealed that about half of the hospitalized patients become at least transiently positive for antiphospholipid (aPL) antibodies [24], although this was not confirmed by other studies [reviewed in 22]. At first, Gatto et al. showed that even if thrombosis is a frequent manifestation of COVID-19 infection, aPL antibodies or lupus anticoagulant (LAC) were not associated with thrombosis [25]. This has been confirmed by Borghi et al. who displayed also how aPL antibodies in COVID-19 patients are mainly directed against β2glycoprotein I (β2GPI domain 1 and 4–5) with an epitope specificity different from antibodies in APS [26]. Nevertheless, the possible relation between COVID-19 and induction of autoimmune diseases, especially APS, may have increased the number of requests for detection of autoantibodies, like aPL antibodies.
In the current study of the European Autoimmunity Standardisation Initiative (EASI) we examined the effect of the COVID-19 pandemic on the requesting behavior for autoantibodies in 15 European countries. For this purpose clinical laboratories were addressed to provide the number of requests per month for 10 autoantibodies in the year before (2019) and the year during (2020) the COVID-19 pandemic. The selection of the type of autoantibodies was based on being among those more frequently requested by general practitioners (such as rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA) and anti-tissue transglutaminase (anti-tTG)), being relevant for diagnosis and follow-up of severe autoimmune diseases like systemic lupus erythematosus (SLE) and small vessel vasculitis, and being part of the APS-associated antibodies.
2. Methods
EASI conducted a pilot survey in October 2020 in three European laboratories (Hungary, Italy and the Netherlands) revealing a notable decrease in autoantibody testing in all three laboratories during the first wave of the COVID-19 pandemic. The results of this survey were presented at the EASI meeting held on-line on 27th November 2020, together with the proposal to extend this survey to other European laboratories. The study plan and the list of autoantibodies to be included in the extended survey were prepared in agreement with the representatives of the national EASI groups during the meeting. Autoantibodies for the survey were selected according the following criteria: their relevance in diagnosis and/or monitoring of severe autoimmune diseases like SLE and small vessel vasculitis (e.g. anti-double stranded-DNA - dsDNA; anti-neutrophil cytoplasmic antibodies - ANCA); as possible markers of COVID-19 associated autoimmune diseases (e.g. aPL antibodies, ANCA); tests widely requested by general practitioners in daily clinical practice (e.g. RF IgM, tTG IgA). Since the hypothesized association between the occurrence of aPL antibodies and the COVID-19 associated coagulopathy may have influenced the evolution of aPL antibody requests, the inclusion of aPL antibodies was also warranted. According to the above mentioned selection criteria, the list of the autoantibodies included in the study was: anti-dsDNA, anti-myeloperoxidase (MPO-ANCA) and anti-proteinase 3 (PR3-ANCA), ACPA, RF IgM, anti-cardiolipin IgG (aCL IgG), anti-cardiolipin IgM (aCL IgM), anti-β2GPI IgG, anti-β2GPI IgM, and anti-tTG IgA.
With respect to the study coordination, a contact person from each national EASI group (n = 20) was addressed to gather the data. In some countries, lacking an official national EASI team, activities were covered by other societies (e.g., FIRMA in Italy, SSAI in Switzerland, or GEAI in Spain).
Data on test numbers and on the number of positive results have been collected for the years 2019 and 2020 on a monthly basis for the above listed autoantibodies. In addition, information related to the test type and the cut-off level for the respective autoantibody could also be provided (Supplementary fig.1 ). A section with some additional questions regarding the lockdown time interval and the main causes of disruption with multiple choices was also included in the template. The inability to provide the number of positive results, although inclusion of such data was highly encouraged, was not considered an exclusion criterion.
The data collection template accompanied by a guide with instructions was distributed mainly to laboratories associated with an EASI member. The excel-files completed by the participating laboratories were collected by the representatives of the national EASI teams and sent to the study coordinators. Data were checked for consistency upon receiving, followed by a second survey with targeted questions in order to remove the possible biases caused by changes of organization (e.g., consolidation of laboratories) or by other interfering factors (e.g., change in the method or testing algorithm, participation in various research studies) during the study period.
Descriptive statistics was performed using the statistical function of excel 2010 (Microsoft).
Test numbers and the number of positive results were summarized for each country and for all participating countries as well, separately for individual autoantibodies and for all autoantibodies together. The impact on the test numbers was examined by comparing the number of requests per months of 2020 to the average number of monthly requests in 2019 and on a monthly basis between the two years in the whole cohort, but also for two separate three-month periods, corresponding to the first and the second pandemic waves (March–April-May and October–November-December). The rate of positive results was analyzed in the same approach.
3. Results
3.1. Data acquisition
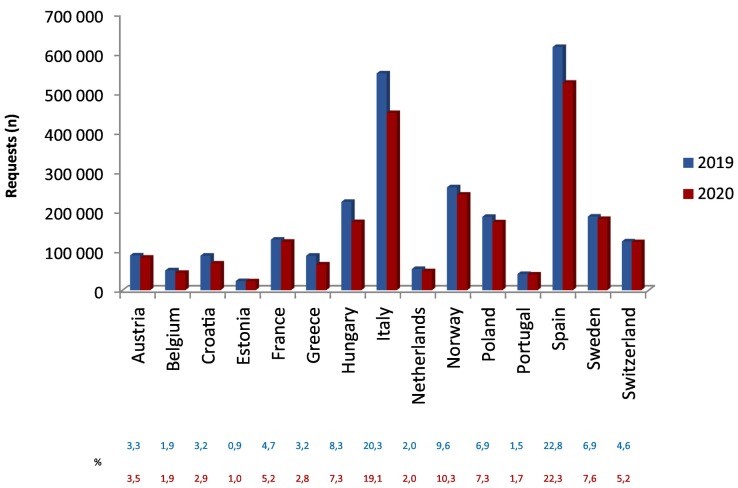
Out of the 20 countries addressed, 15 returned the data collection template, revealing an overall response rate of 75%. In total, 99 laboratories provided their data. Data verification followed by the short survey to eliminate biases revealed the effect of other factors than COVID-19 in three cases. For one participant, a significant increase in test requests was observed in 2020 caused by merging of autoimmune tests from several laboratories; therefore, the data for the two years couldn't be compared. For this reason, this laboratory was excluded from the study. One more participant was excluded due to incomplete data provision. Finally, for another laboratory, due to partial consolidation of autoimmune tests from other laboratories, only data for RF IgM and ACPA were applicable. Thus, data from 97 European laboratories were processed in total. The number of laboratories varied between countries, with a range of 1 (Portugal) to 23 (Italy). The list of participating countries with laboratories providing test numbers for each autoantibody are summarized in the S upplementary table 1. This resulted in an unequal distribution of reported numbers of requests over the participating countries with Italy and Spain representing about 40% of the total test requests (Fig. 1 ). Also the number of test requests for the distinct autoantibodies showed large variation ( S upplementary table 2 ). Tests for anti-tTG IgA (21%) and RF IgM (18%) were requested most frequently, while tests for IgG and IgM anti-β2GPI were ordered with the lowest frequency (~5%).
Fig. 1.
Reported numbers and relative distribution of requests over the participating countries for 2019 (blue column and upper row) and for 2020 (red column and bottom row).
3.2. Changes in total test request over time in participating countries
In all European countries a reduction in the number of tests for the 10 antibody tests considered was observed in the year 2020 compared to the previous year. The reduction affected all antibody tests with an overall decrease of 13%, ranging from 10 to 16%, except for anti-β2GPI IgG and IgM antibodies which recorded a smaller decrease (5–6%) (Table 1 ). The biggest drop was recorded in Greece (25.5%), followed by Hungary (23%), Croatia (22.5%) and Italy (18.3%). The smallest drop was recorded in Switzerland (1.4%), Estonia (2.5%), Sweden (3.4%), Portugal (3.7%) and France (4.4%). In all countries, however, the decline was most marked in the period March–May 2020 (first wave of the pandemic) with an overall decrease in those three months of 45.2% (Table 2A ). In Hungary, Italy, Croatia and Spain the overall decrease during the first wave even exceeded 50%, while in Sweden and Switzerland the decrease was less than 25%. Within the first wave, the nadir was observed in April 2020, reaching a mean value of −60.9% for the total number of requests. In the last quarter of the year (second wave of the pandemic) the overall decrease was only 6.8%. During the second wave Greece, Hungary and Croatia were most affected with a decrease in test requests of about 20–30%, while in Estonia (8%) and Portugal (10%) an overall increase was reported in October to December 2020 (Table 2B ).
Table 1.
Percent variation of test number between the year 2020 and the year 2019, per antibody and per country.
| Country (no. laboratories) | Austria (4) | Belgium (4) | Croatia (4) | Estonia (2) | France (6) | Greece (7) | Hungary (8) | Italy (23) | Netherlands (6) | Norway (5) | Poland (3) | Portugal (1) | Spain (12) | Sweden (4) | Switzerland (8) | Total (97) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| anti-dsDNA | 2.7 | −14.1 | −24.7 | 9.8 | −12.3 | −27.6 | −22.0 | −18.6 | −12.0 | −10.7 | −27.4 | −6.7 | −13.2 | −6.1 | −0.7 | −14.3 |
| MPO-ANCA | −4.7 | −18.9 | −32.9 | −2.7 | −3.1 | −23.5 | −20.8 | −17.3 | −13.3 | −7.4 | 36.8 | −9.9 | −12.7 | −4.1 | 0.0 | −11.8 |
| PR3-ANCA | −4.8 | −19.1 | −32.8 | −2.3 | −2.5 | −19.2 | −21.5 | −15.3 | −13.7 | −7.2 | −32.6 | −12.5 | −13.7 | −4.1 | −0.2 | −12.2 |
| ACPA | −6.8 | −16.4 | −21.6 | −3.2 | −5.4 | −27.6 | −24.3 | −16.3 | −6.3 | −6.6 | −12.6 | 2.9 | −17.6 | −3.5 | −5.9 | −12.8 |
| RF IgM | −9.0 | −16.1 | −20.8 | −6.0 | −4.3 | −28.9 | −25.0 | −21.1 | −8.2 | −5.6 | −18.4 | −7.2 | −17.3 | −0.1 | −7.2 | −14.8 |
| aCL IgG | −4.2 | −0.2 | −19.5 | 4.9 | 0.8 | −25.8 | −22.5 | −10.6 | −3.0 | −4.3 | −11.7 | 0.5 | −9.7 | 0.7 | 4.9 | −10.0 |
| aCL IgM | −15.1 | 9.1 | −16.9 | 5.4 | 4.0 | −25.8 | −22.5 | −11.1 | −1.9 | −5.8 | −14.2 | −1.0 | −7.3 | 0.6 | 2.0 | −10.2 |
| anti-β2GPI IgG | −8.9 | 37.6 | 0.5 | 8.7 | 1.9 | −21.6 | −24.3 | −10.0 | −0.1 | −3.6 | 4.4 | 6.8 | 3.0 | 3.5 | 6.7 | −5.1 |
| anti-β2GPI IgM | −12.6 | 31.5 | −4.2 | 5.1 | 6.3 | −21.6 | −24.3 | −10.5 | 15.3 | −5.6 | −6.4 | 5.1 | 2.4 | 4.4 | 6.8 | −6.2 |
| anti-tTG IgA | −14.7 | −17.6 | −27.3 | −7.8 | −17.7 | −30.5 | −22.7 | −26.2 | −16.6 | −8.6 | 29.2 | −5.1 | −20.0 | −5.5 | −9.8 | −16.1 |
| Total | −6.5 | −12.5 | −22.5 | −2.5 | −4.4 | −25.5 | −23.0 | −18.3 | −10.4 | −7.2 | −7.5 | −3.7 | −14.7 | −3.4 | −1.4 | −13.0 |
Abbreviations: ACPA, anti-citrullinated protein antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; β2GPI, β2glycoprotein I; aCL, anti-cardiolipin; dsDNA, double stranded-DNA; MPO, myeloperoxidase; PR3, proteinase 3; RF, rheumatoid factor; tTG, tissue transglutaminase.
Table 2A.
Percent variation in test numbers per antibody and per country in March–May 2020 (first pandemic wave).
| Country (no. laboratories) | Austria (4) | Belgium (4) | Croatia (4) | Estonia (2) | France (6) | Greece (7) | Hungary (8) | Italy (23) | Netherlands (6) | Norway (5) | Poland (3) | Portugal (1) | Spain (12) | Sweden (4) | Switzerland (8) | Total (97) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| anti-dsDNA | −22.4 | −40.3 | −58.8 | −27.9 | −46.6 | −51.7 | −56.3 | −58.3 | −38.6 | −26.4 | −56.6 | −46.0 | −45.3 | −24.3 | −21.3 | −44.8 |
| MPO-ANCA | −30.2 | −42.2 | −62.6 | −39.3 | −25.7 | −45.0 | −50.7 | −47.3 | −31.8 | −21.4 | −5.9 | −33.1 | −44.1 | −22.3 | −16.4 | −36.8 |
| PR3-ANCA | −30.4 | −43.2 | −62.4 | −38.4 | −26.6 | −42.3 | −52.2 | −43.9 | −36.1 | −21.2 | −50.8 | −39.7 | −44.9 | −22.1 | −15.9 | −36.8 |
| ACPA | −34.9 | −50.2 | −56.6 | −35.0 | −38.2 | −53.6 | −58.5 | −60.2 | −35.2 | −26.4 | −40.7 | −42.3 | −56.5 | −25.4 | −26.2 | −44.5 |
| RF IgM | −38.2 | −49.0 | −52.8 | −42.5 | −40.1 | −51.0 | −59.2 | −58.2 | −38.4 | −25.2 | −58.2 | −51.2 | −58.3 | −21.0 | −24.4 | −49.1 |
| aCL IgG | −19.4 | −35.9 | −49.5 | −11.4 | −30.8 | −50.0 | −58.3 | −45.0 | −39.8 | −18.7 | −47.4 | −41.4 | −43.9 | −16.3 | −10.1 | −41.5 |
| aCL IgM | −43.4 | −30.5 | −56.4 | −12.0 | −25.0 | −50.0 | −58.1 | −46.4 | −38.7 | −19.4 | −48.3 | −40.4 | −40.2 | −18.2 | −12.6 | −42.0 |
| anti-β2GPI IgG | −17.2 | 3.1 | −31.0 | −7.1 | −27.6 | −48.5 | −58.5 | −46.3 | −37.9 | −15.9 | −35.4 | −34.2 | −34.5 | −13.9 | −11.2 | −37.1 |
| anti-β2GPI IgM | −25.1 | 4.7 | −37.2 | −7.8 | −19.9 | −48.5 | −58.5 | −46.5 | −33.3 | −17.7 | −34.8 | −34.8 | −34.8 | −15.6 | −11.3 | −37.8 |
| anti-tTG IgA | −44.4 | −50.7 | −57.3 | −45.3 | −54.7 | −57.0 | −60.9 | −69.6 | −53.3 | −30.9 | −31.5 | −56.5 | −61.5 | −27.3 | −36.9 | −53.1 |
| Total | −33.3 | −44.2 | −55.3 | −35.8 | −36.4 | −49.7 | −57.4 | −56.7 | −40.5 | −25.7 | −42.8 | −46.7 | −52.8 | −23.7 | −19.7 | −45.2 |
(Abbreviations: see legend of Table 1).
Table 2B.
Percent variation in test numbers per antibody and per country in October–December 2020 (second pandemic wave).
| Country (no. laboratories) | Austria (4) | Belgium (4) | Croatia (4) | Estonia (2) | France (6) | Greece (7) | Hungary (8) | Italy (23) | Netherlands (6) | Norway (5) | Poland (3) | Portugal (1) | Spain (12) | Sweden (4) | Switzerland (8) | Total (97) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| anti-dsDNA | −6.9 | −8.9 | −12.2 | 21.4 | −9.9 | −29.8 | −26.2 | −11.3 | −9.1 | −8.8 | −36.3 | 1.8 | −3.7 | 2.1 | 0.8 | −9.9 |
| MPO-ANCA | −2.6 | −20.1 | −31.9 | 15.2 | −3.7 | −33.5 | −28.3 | −11.7 | −18.5 | −2.3 | 44.3 | 2.1 | −0.2 | 6.8 | 2.5 | −8.6 |
| PR3-ANCA | −2.6 | −19.8 | −31.9 | 14.5 | −1.5 | −25.5 | −28.5 | −9.4 | −16.9 | −2.1 | −36.6 | −3.3 | −3.0 | 6.2 | 0.3 | −9.3 |
| ACPA | 1.6 | −4.0 | −24.8 | 1.2 | 5.1 | −33.5 | −31.1 | −8.4 | −6.4 | 4.8 | −15.9 | 18.7 | −4.4 | 3.4 | −6.6 | −7.8 |
| RF IgM | −2.7 | −6.3 | −24.9 | 1.6 | 5.1 | −39.5 | −27.9 | −23.4 | −7.5 | 6.4 | −15.8 | 11.4 | −4.9 | 6.0 | −10.3 | −8.6 |
| aCL IgG | 5.9 | 17.3 | −19.0 | 12.6 | 0.7 | −27.9 | −24.4 | 2.9 | 8.5 | −3.7 | −16.9 | 11.8 | 3.9 | 6.4 | 5.3 | −3.2 |
| aCL IgM | −11.2 | 33.6 | −18.1 | 12.6 | 3.9 | −27.9 | −24.4 | 3.7 | 9.5 | −4.6 | −19.4 | 14.1 | 7.7 | 9.5 | 4.9 | −2.7 |
| anti-β2GPI IgG | −3.9 | 52.3 | −9.7 | 24.3 | 1.9 | −20.5 | −26.7 | −0.2 | 10.3 | −4.5 | 24.4 | 16.7 | 19.7 | 8.4 | 17.9 | 2.4 |
| anti-β2GPI IgM | −9.0 | 41.2 | −8.8 | 18.5 | 5.8 | −20.5 | −26.7 | −0.1 | 18.0 | −6.3 | −16.6 | 21.1 | 19.6 | 12.2 | 18.3 | 1.0 |
| anti-tTG IgA | −17.8 | −4.0 | −32.2 | 6.4 | −12.7 | −41.4 | −26.9 | −19.0 | −8.4 | 1.7 | 38.2 | 8.0 | −5.8 | 0.5 | −2.9 | −7.6 |
| Total | −5.6 | −2.8 | −22.6 | 8.3 | 0.04 | −30.3 | −27.2 | −11.2 | −7.3 | 1.0 | −8.3 | 9.6 | −1.4 | 3.6 | 1.2 | −6.8 |
(Abbreviations: see legend of Table 1).
3.3. Changes in test request for distinct autoantibodies over time in participating countries
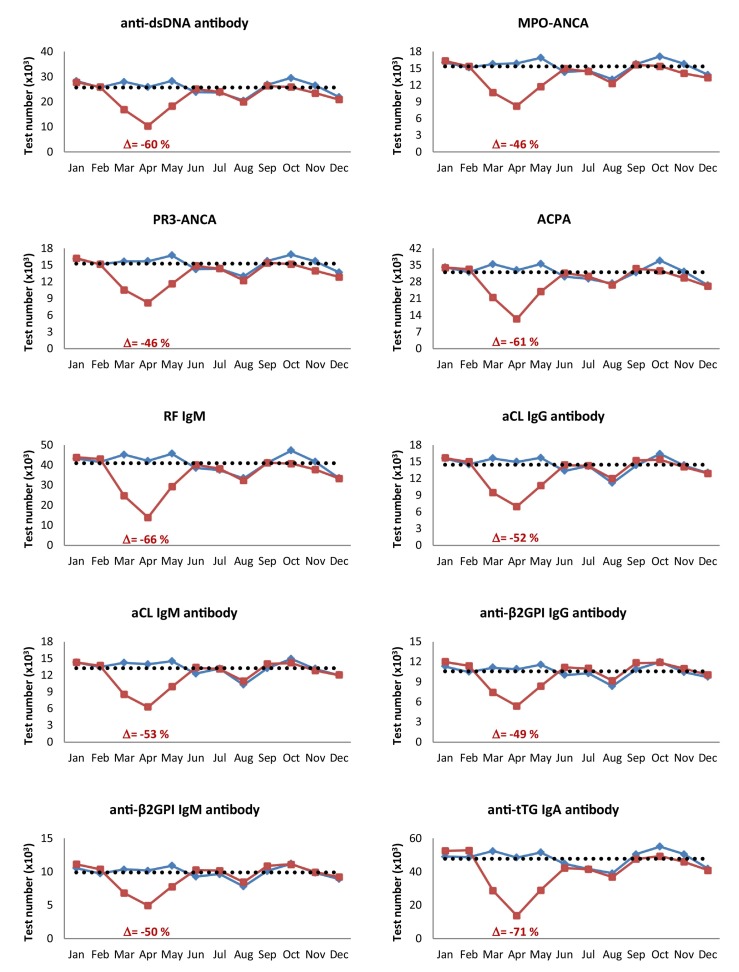
When taking all data from the participating countries together, the strong decrease in the first wave of the pandemic was evident for all autoantibodies included in this study (Table 2A). For all individual autoantibodies the nadir was in April 2020 and varied between 46 and 71% ( Fig. 2 ). The most affected antibodies were those commonly requested by general practitioners, i.e. anti-tTG IgA (−71%), RF IgM (−66%) and ACPA (−61%), but also requests for anti-dsDNA antibodies and ANCA were strongly reduced. In the second wave there was more diversity between countries and type of autoantibodies (Table 2B). Interestingly, in several countries the requests for aPL antibodies, in particular IgM and IgG anti-β2GPI antibodies, increased, while this was not or less the case for the other autoantibodies included in our study. This was most evident in Belgium, Estonia, Portugal, the Netherlands, Spain, and Switzerland.
Fig. 2.
Number of monthly requests for individual autoantibodies in 2019 (blue line) and 2020 (red line). Delta values represents the most pronounced decreases (%) compared to the average test numbers in 2019 (dashed line). (Abbreviations: see legend of Table 1).
3.4. Changes in the relative amount of positive results
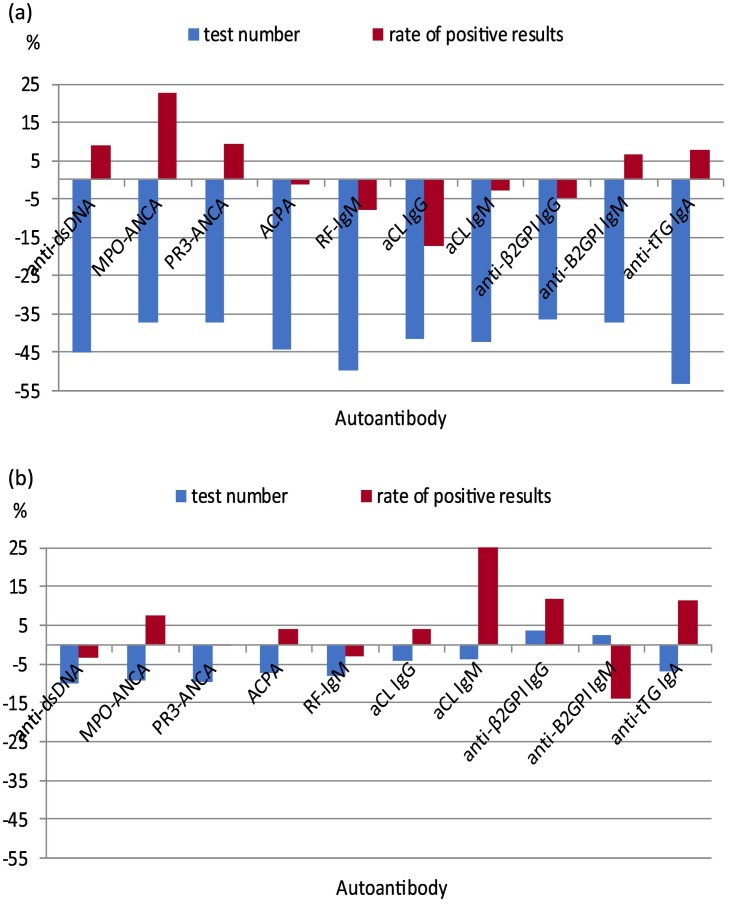
Since it can be reasoned that patients with the most severe clinical manifestation were still allowed access to the health care system, this could have resulted in a selection of patients with a higher pre-test probability and, hence, a relative increase in positive results for autoantibody tests. The vast majority of laboratories also collected data on positive results for the selected autoantibodies. When overall effects on the requesting numbers for those laboratories were compared to the whole set of participating laboratories, essentially the same patterns were observed for individual autoantibodies and individual countries (data not shown). From these data it was concluded that there was no substantial bias for the analyses of proportion of positive results. Though the total rate of positive results did not change between the two years, being 8.87% in 2019 and 8.91% in 2020, this finding differed according to the type of the antibody test. In the first wave a clear increase in the positive result rate was seen for MPO-ANCA, and to a lesser extend for anti-dsDNA, PR3-ANCA, anti-β2GPI IgM and anti-tTG IgA, while RF IgM and aCL IgG antibodies showed a more marked decrease (Fig. 3A). In the second wave a clear increase in the proportion of positive results was seen for aCL IgM antibodies, and to a lesser extend for MPO-ANCA, anti-β2GPI IgG and anti-tTG IgA. For anti-β2GPI IgM the proportion of positive results strongly decreased during the second wave (Fig. 3B). Typically, the effects on the positive rate of aPL antibodies were opposite in the first wave as compared to the second wave with anti-β2GPI IgM antibodies deviating from the other three autoantibodies in terms of the direction of the effect.
Fig. 3.
Percent variation in test number (blue columns) and in the rate of positive results (red columns) for each autoantibody during: A) the first wave of the pandemic (March–May 2020) and B) the second wave of the pandemic (October–December 2020) compared to the same period of 2019. (Abbreviations: see legend of Table 1).
3.5. Changes in the organization of the health care system
With respect to the main causes of healthcare disruption, 86 of the 97 participating laboratories reported one or multiple factors, the most frequent being the closure or limitation of outpatient disease specific consultations and the closure of outpatient health care services by government decree (84.9% and 52.3%, respectively). The clinical or laboratory staff redeployment due to COVID-19 and the inadequacy of clinical or laboratory staff were estimated as lower (39.5% and 23.3%), while inaccessible inpatient services and other causes were reported less frequently (9.3% and 8.1%, respectively).
4. Discussion
The current EASI-study investigated the effect of the COVID-19 pandemic on the number of requests for autoantibodies. Data were acquired from 97 laboratories in 15 European countries. Results revealed, in particular during the first wave of the pandemic, i.e., March – May 2020, a strong decline in the number of requests. All participating countries and included autoantibodies were affected, but clear differences between countries and autoantibodies were observed. The second wave of the pandemic, i.e., October – December 2020, only affected a restricted number of countries and also the effect on the number of requests was less as compared to the first wave. Overall, these data seem to be related to the degree the respective countries were affected by the pandemic and to the adaptations made in the health care system to contain the pandemic.
Autoantibodies play an important role both in the diagnostic work-up for autoimmune diseases as well as in the follow-up of patients with established autoimmune diseases [27]. Therefore, it is tempting to speculate that the strong decline in autoantibody testing has a severe impact on patients with clinical manifestations associated with autoimmune diseases. First, diagnoses will be delayed in patients suspected of autoimmune diseases. The strongest declines were observed for autoantibodies often requested by general practitioners (IgM RF, ACPA, and IgA tTG). General practitioners often request these tests to exclude a disease, eventually resulting in rather low rates of positive results [28,29]. Consequently, the effect on missed diagnoses may be limited. Furthermore, the associated autoimmune diseases, being rheumatoid arthritis (RA) and celiac disease, normally do not have an acute onset. The diagnostic delay of several months may have only limited effects on irreversible damage due to the ongoing disease. SLE, ANCA-associated vasculitis (AAV), and APS, on the other hand, may have an acute onset. In these diseases a delayed diagnosis may have severe, and even life threatening, consequences. Evidently, the number of requests for the autoantibodies associated with these diseases were also strongly affected in the participating laboratories. Although it was anticipated that the proportion of positive results would increase because the remaining autoantibody requests were expected to be for patients with more severe clinical manifestations and, hence, a higher pre-test probability, this effect was limited. Data on the number of delayed diagnoses for these diseases are currently lacking, but detection of anti-dsDNA antibodies and ANCA is also relevant for monitoring disease activity and predicting clinical relapses in the respective diseases. For prediction of clinical relapses in AAV, at least four ANCA measurements per year are considered optimal [30], but this number might have been reduced due to limited admission to the hospital. It is questionable if solid data on the effect of the COVID-19 pandemic on the clinical outcome of patients with autoimmune diseases will ever become available, because of multiple confounders. Due to immunosuppressive treatment, these patients may have an increased risk for becoming infected with SARS-CoV-2 and, depending on the type of treatment, may have an altered risk for admittance to an intensive care unit. For instance, treatment with anti-TNF biologicals in RA may reduce the risk on systemic inflammation, treatment with anti-coagulation in APS may reduce the risk on thrombotic complications, while treatment with cyclophosphamide or rituximab in AAV may prevent adequate clearance of the virus. Next, vaccination strategies in these patients may have resulted in adaptation of their therapy. In patients treated with rituximab, return of B-cells in the peripheral blood is considered important for adequate vaccination responses [[31], [32], [33]], but this contains the risk of increased disease activity, let aside the potential risk due to immune activation by the vaccine itself. Altogether, although concise clinical information in our study is lacking, our data strongly indicate that the COVID-19 pandemic not only affected patients with malignancies, but also patients with (suspected) autoimmune diseases.
As elaborated upon in the introduction, the observation of thrombotic events in patients infected with the SARS-CoV-2 virus has raised the possibility of a relation with the APS [reviewed in 22]. Although our data are not conclusive, it is striking that in several countries the number of requests for aPL antibodies increased in the second wave of the COVID-19 pandemic. This suggests that the awareness of a possible relation with APS has increased the number of requests for these autoantibodies. The increase in number of requests was associated with a higher proportion of aCL IgM antibodies, but a lower proportion of anti-β2GPI IgM antibodies. The clinical significance of these findings remains to be determined. Overall, IgM aPL antibodies are the least associated with APS and it has not been determined if positive results are persistent over time. Therefore, these data should not be over-interpreted.
Our study on the effect of the COVID-19 pandemic on autoantibody testing has several limitations. First, there might have been a bias in the selection of participating laboratories. On the one hand, there was an imbalance in the number of participating laboratories and number of requests per country. In particular, Italy and Spain represented about 40% of the total dataset. On the other hand, the laboratories selected for eventual participation were largely based on the national EASI-network (or equivalent thereof). Since EASI-members most often work in university laboratories specialized in autoimmune diagnostics, the results may not be representative for the whole country. Nevertheless, the data obtained seem to be rather consistent between laboratories of the same country and this also holds for the overall effects reported. Second, some of the autoantibodies investigated in our study are part of a testing algorithm. For instance, when there is clinical suspicion of SLE, testing for anti-dsDNA antibodies is advised if the HEp-2 indirect immunofluorescence (IIF) test reveals a nuclear homogeneous pattern [34]. Similarly, although the international consensus on ANCA testing in AAV has been revised in 2017 [35], laboratories may not have implemented yet this new consensus and perform MPO- and PR3-ANCA as second-level tests after a positive ANCA IIF. Therefore, the number of anti-dsDNA test requests reported may represent patients that are not suspected of having SLE (low pre-test probability), while the number of MPO- and PR3-ANCA requests may represent patients that already have been selected based on a positive ANCA IIF (high pre-test probability). Obviously, this also will influence the proportion of positive results. However, it is to be expected that these effects will be equally distributed over the years 2019 and 2020, since laboratories were questioned about possible changes in their testing algorithms during the study period. Third, in our current study we did not include data on the clinical background of the requesting physician, on the eventual diagnosis of the patient, or if the request was for diagnostic or follow-up purposes. Therefore, the interpretation and extrapolation of the data presented includes several assumptions. However, it is inevitable that general declines in autoantibody requests of ~60% at the nadir in April 2020 may have had a strong impact on the care for patients with clinical manifestations related to autoimmune diseases. Finally, only general information about the national lock-down measures and related adjustments in the health care system were asked for. The extent to which admission to general practitioners and hospitals was blocked, or re-allocation of clinicians specialized in autoimmune diseases to the care of patients with SARS-CoV-2 infections occurred, will have been different between countries. Actually, this could be an interesting study item because during the second wave several countries did not experience a decline in the number of test requests for autoantibodies, while some other countries again revealed a significant drop in test requests. Although countries may have experienced different numbers of infections, knowledge of the adjustments made in the organization of the health care system in the first countries could be of help for the latter countries to keep-up the care of patients with (suspected) autoimmune diseases during a pandemic.
In conclusion, our study demonstrates a strong decrease in autoantibody requests during the first wave of the COVID-19 pandemic in 15 European countries. This decrease was clearly associated with the level of lock-down in the different countries and with the required adjustments in the health care systems. During the second wave of the pandemic, the decrease in autoantibody requests was less pronounced, but some countries were hardly affected, while other countries experienced a second decline. If future studies will analyze the reason for this difference, lessons could be learned about optimal organization of the health care system upon challenge by a pandemic. Certainly, what we have already learned is that in critical situations such as a pandemic, we should develop and apply priority criteria, establishing which antibodies based on the underlying disease are of an urgent nature and should therefore be sought even in emergency situations, and those which have no immediate clinical relevance and whose search can be deferred.
Declaration of Competing Interest
The authors declare that they have nothing to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.autrev.2021.102985.
Appendix A. List of collaborators
Ulrike Demel, Clinical Department for Rheumatology and Immunology, University Hospital of Graz, Austria;
Andrea Griesmacher, Central Institute of Clinical and Chemical Laboratory Diagnostics, University Hospital of Innsbruck, Austria;
Jörg Hofmann, Institut für Labormedizin, Sozialmedizinisches Zentrum Ost, KAV Wien, Austria;
Carolien Bonroy, Department of Laboratory Medicine, Ghent University Hospital, Ghent, Belgium; Lieve Van Hoovels, Department of Laboratory Medicine, Onze-Lieve-Vrouw Hospital, Aalst, Belgium;
Marc Jacquemin, Centre for Molecular and Vascular Biology, KU Leuven, Leuven; Belgium;
Martine Vercammen, Department of Laboratory Medicine, AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium;
Lovorka Đerek, Clinical Department of Laboratory Diagnostics, University Hospital Dubrava, Zagreb, Croatia;
Andrea Tešija Kuna, Department of Clinical Chemistry, Sestre Milosrdnice University Hospital Center, Zagreb, Croatia;
Jasna Pavela, Institute of Clinical Laboratory Diagnostics, Osijek University Hospital, Osijek, Croatia;
Kaja Metsküla, United Laboratories, Tartu University Hospital, Tartu, Estonia;
Maarit Veski, Central Laboratory, East Tallinn Central Hospital, Tallinn, Estonia;
Marie-Alexandra Alanyakan, Department of Immunology, Centre Hospitalier Necker-Enfants malades, Paris, France;
Marie-Agnès Dragon-Durey, Laboratory of Immunology, Hôpital Européen Georges Pompidou, APHP, Université de Paris, Paris, France;
Chantal Dumestre-Perard, Laboratory of Immunology, Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France;
Pascale Ghillani-Dalbin, Department of Immunology, Pitie-Salpêtrière Hospital, Paris, France;
Laurence Guis-Cabanne, Laboratory of Immunology, Laboratoire Eurofins-Biomnis, Ivry Sur Seine, France;
Marie Senant, Laboratory of Immunology, plateau technique Cerballiance IDFS, Lisses, France;
Fylaktou Asimina, National Peripheral Histocompatibility Center, Immunology Department, Hippokration General Hospital Thessaloniki, Greece;
Dimitrios Bogdanos, Department of Rheumatology and Clinical Immunology, Faculty of medicine, School of Health Sciences, University of Thessaly, Larissa, Greece;
Kontou Elisavet, Immunology-Histocompatibility Department, “Evangelismos” General Hospital of Athens, Greece;
Gounari Evdoxia, Department of Immunology-Laboratory of Microbiology, AHEPA University Hospital, Thessaloniki, Greece;
Anastasia Giannakou, Immunology-Histocompatibility Department, Papageorgiou General Hospital, Thessaloniki, Greece;
Christiana Kaliouli-Antonopoulou, Immunology-Histocompatibility Department, “Agios Panteleimon” General Hospital of Nikaia, Greece;
Kalliroi Tsalimalma, Immunology and Histocompatibility Department, “LAIKO” General Hospital Athens, Greece;
Christina Tsigalou, Democritus University of Thrace, Alexandroupolis, Greece;
Gábor Nagy, Department of Laboratory Medicine, Faculty of Medicine, University of Debrecen, Debrecen;
Zsuzsanna Beleznay, Department of Laboratory Medicine, Semmelweis University, Budapest;
Tímea Berki, Department of Immunology and Biotechnology, Medical School, University of Pécs, Pécs;
Bertalan Fodor, Borsod-Abaúj-Zemplén County Hospital, Department of Laboratory Medicine, Miskolc;
Krisztina Jost, Institute of Laboratory Medicine, University of Szeged, Szeged;
Katalin Miklós, Central Laboratory, Medical Centre, Hungarian Defence Forces, Budapest;
Julianna Németh, Medicover Diagnostic Center, Budapest;
Antonio Antico, Lab Analisi ULSS 4, Santorso (VI), Italy;
Giuseppina Barberio, Medicina di Laboratorio, Azienda ULSS n 2 Marca Trevigiana, Treviso, Italy;
Chiara Bonaguri, Laboratorio Diagnostica Ematochimica, Azienda Ospedaliero-Universitaria, Parma, Italy;
Teresa Carbone, UOC Patologia Clinica, ASM, Matera, Italy;
Caterina Castiglione, Laboratorio Patologia Clinica P.O. Santo Spirito, Pescara, Italy;
Luigi Cinquanta, IRCCS S.D.N., Napoli, Italy;
Andrea Costantini, AOU Ospedali Riuniti, Ancona, Italy;
Gaia Deleonardi, Laboratorio Unico Metropolitano, Ospedale Maggiore, Bologna, Italy;
Nicoletta Gallo, Azienda Ospedaliera Università di Padova, Dipartimento Strutturale Medicina di Laboratorio, UOC Medicina di Laboratorio, Padova, Italy;
Emirena Garrafa, Dipartimento di Medicina Molecolare e Traslazionale, Università di Brescia and.
Dipartimento di Diagnostica di Laboratorio, ASST, Spedali Civili, Brescia, Italy;
Martina Fabris, Istituto di Patologia Clinica, Dipartimento di Medicina di Laboratorio, Azienda Sanitaria Universitaria Integrata, Udine, Italy;
Franco Franceschini, Dipartimento di Scienze Cliniche e Sperimentali, Università di Brescia and Reumatologia e Immunologia Clinica, ASST Spedali Civili, Brescia, Italy;
Mariangela Manfredi, Laboratorio Immunologia Allergologia, Dipartimento di Medicina di Laboratorio, Ospedale San Giovanni di Dio Azienda, Usl Toscana Centro, Florence, Italy;
Vito Pafundi, Laboratorio di Immunopatologia, Ospedale San Carlo, Potenza, Italy;
Eleonora Palella, U.O. Patologia Clinica, Ospedale S. Chiara,Azienda Provinciale per i Servizi Sanitari, Trento, Italy;
Boaz Palterer, Dipartimento di Medicina Sperimentale e Clinica, Università degli Studi di Firenze, Florence, Italy;
Giampaola Pesce, Laboratorio Diagnostico di Autoimmunologia, IRCCS Ospedale Policlinico San Martino, and Dipartimento di Medicina Interna e specialità mediche (DIMI), Università di Genova, Genova, Italy;
Stefan Platzgummer, Laboratorio Centrale, Ospedale Civile, Merano (BZ), Italy;
Brunetta Porcelli, Dipartimento Biotecnologie Mediche, Università degli Studi di Siena, and Laboratorio Patologia Clinica, Policlinico S. Maria alle Scotte, AOU Senese, Siena, Italy;
Giulia Previtali, ASST Papa Giovanni XXIII, Bergamo, Italy;
Valeria Riccieri, Dipartimento di Scienze Cliniche, Internistiche, Anestesiologiche e Cardiovascolari,
“Sapienza” Università di Roma, Azienda Ospedaliero Universitaria Policlinico Umberto I, Roma, Italy;
Maria-Cristina Sacchi, Laboratorio Analisi, Alessandria, Italy;
Maria-Teresa Trevisan, Laboratorio, Ospedale G. Fracastoro, Verona, Italy;
Marco Di Tola, Patologia Clinica, Ospedale San Giovanni Addolorata, Roma, Italy;
Danilo Villalta, Allergologia e Immunologia clinica, Presidio Ospedaliero S. Maria degli Angeli, Pordenone, Italy;
Henny G Otten, Central Diagnostic Laboratory (CDL) / Center of Translational Immunology (CTI), University Medical Center, Utrecht, The Netherlands;
Caroline Roozendaal, Laboratory Medical Immunology, Department of Laboratory medicine, University Medical Center, Groningen, The Netherlands;
Marco WJ Schreurs, Department of Immunology, Erasmus Medical Center, Rotterdam, The Netherlands;
Renate G van der Molen, Department Laboratory Medicine, Medical Immunology laboratory, Radboud University Medical Center, Nijmegen, The Netherlands;
Livia Bajelan, Autoantibodies and allergy, Section for medical immunology, Department for immunology and transfusion medicine, Oslo University Hospital, Oslo, Norway;
Morten Haugen, Division of Immunology and Transfusion Medicine, Department of Blood Center and Medical Biochemistry, Innlandet Hospital Trust, Norway;
Silje Helland Kaada, Department of Immunology and Transfusion Medicine, Haukeland University Hospital, Bergen, Norway;
Christine Torsvik Steinsvåg, Department of Clinical immunology and Transfusion Medicine, Sørlandet Hospital, Kristiansand, Norway;
Danuta Kozłowska, Diagnostyka Laboratoria Medyczne, Kraków, Poland;
Włodzimierz Pawłowski, Zakład Diagnostyki Laboratoryjnej i Mikrobiologicznej, Szpital Wojewódzki w Poznaniu, Poznan, Poland;
Agata Strukow, ALAB Laboratoria, Warsaw, Poland;
Concha González Rodríguez, Laboratorio de Autoinmunidad, Hospital Universitario Virgen Macarena de Sevilla, Spain;
Aurora Jurado Roger, Jefe de Sección de Inmunología, UGC Inmunología y Alergología, Hospital Universitario Reina Sofía de Córdoba, Spain;
Goitzane Marcaida, Servicio de Laboratorio, Hospital General de Valencia, Spain;
Laura Martínez Martínez, Servicio de Inmunología, Hospital de la Santa Creu i Sant Pau, Barcelona Spain;
Jesús Ontañon Rodriguez, Unidad de inmunología Servicio de AACC, Hospital General Universitario de Albacete, Spain;
Alvaro Prada Iñurrategui, Sección de Inmunología UGC Laboratorios Gipuzkoa, Hospital Universitario Donostia, Spain;
Carmen Rodriguez, Servicio de Inmunología, mHospital Universitario Puerta del Mar Cádiz, Spain;
Ricardo Rojo Amigo, Sección de Inmunología, Complejo Hospitalario Universitario A Coruña, Spain;
Garbiñe Roy Ariño, Servicio de Inmnología, Hospital Universitario Ramon y Cajal, Madrid, Spain;
Mª del Carmen Vegas Sánchez, Servicio de Inmunología, Fundación Jiménez Díaz, Madrid, Spain;
M. Luisa Vargas, Servicio de Inmunología y Genética Hospital Universitario Badajoz, Spain;
Catharina Eriksson, Department of Clinical Microbiology/clinical immunology, Umea University, Sweden;
Johan Rönnelid, Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden;
Rui Da Silva Rodrigues, Department of Clinical Immunology and Transfusion Medicine, Karolinska University Hospital, Stockholm, Sweden;
Lionel Arlettaz, Immunology and Allergology, Hospital of Valais, Sion, Switzerland;
Vincent Aubert, Division of Immunology, Lausanne University Hospital, Lausanne, Switzerland;
Luca Bernasconi, Institute of laboratory Medicine, Kantonsspital Aarau AG, Aarau, Switzerland;
Pascale Bruyère-Cerdan, Laboratory of Immunology and Allergy, Department of Diagnostics, Geneva University Hospital, Geneva, Switzerland;
Michael P. Horn, Department of Clinical Chemistry, University Hospital of Bern, Bern, Switzerland.
Franco Keller, Institute of Laboratory Medicine, Ente Ospedaliero Cantonale, Bellinzona, Switzerland;
Elsbeth Probst, Department of Immunology, University Hospital Zurich, Zurich, Switzerland;
Appendix B. Supplementary data
Supplementary material
References
- 1.Durant T.J.S., Peaper D.R., Ferguson D., Schulz W.L. Impact of COVID-19 pandemic on laboratory utilization. J Appl Lab Med. 2020;5:1194–1205. doi: 10.1093/jalm/jfaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y.N., Chen Y., Wang Y., Li F., Pender M., Wang N., et al. Reduction in healthcare services during the COVID-19 pandemic in China BMJ. Global Health. 2020;5 doi: 10.1136/bmjgh-2020-003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y., Ahn E., Lee S., Lim D.H., Kim A., Lee S.G., et al. Changing patterns of medical visits and factors associated with no-show in patients with rheumatoid arthritis during COVID-19 pandemic. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini M., Roda M., Lupardi E., Di Geronimo N., Giannaccare G., Schiavi C. The impact of COVID-19 pandemic on ophthalmological emergency department visits. Acta Ophthalmol. 2020;98:e1058–e1059. doi: 10.1111/aos.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joy M., McGagh D., Jones N., Liyanage H., Sherlock J., Parimalanathan V., et al. Reorganisation of primary care for older adults during COVID-19: a cross-sectional database study in the UK. Br J Gen Pract. 2020;70:e540–e547. doi: 10.3399/bjgp20X710933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendzerska T., Zhu D.T., Gershon A.S., Edwards J.D., Peixoto C., Robillard R., et al. The effects of the health system response to the COVID-19 pandemic on chronic disease management: a narrative review. Risk Manag Health Policy. 2021;14:575–584. doi: 10.2147/RMHP.S293471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moynihan R., Sanders S., Michaleff Z.A., Scott A.M., Clark J., To E.J., et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schäfer I., Hansen H., Menzel A., Eisele M., Tajdar D., Lühmann D., et al. The effect of COVID-19 pandemic and lockdown on consultation numbers, consultation reasons and performed services in primary care: results of a longitudinal observational study. BMC Fam Pract. 2021;22:125. doi: 10.1186/s12875-021-01471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earnshaw C.H., Hunter H.J.A., McMullen E., Griffiths C.E.M., Warren R.B. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792–794. doi: 10.1111/bjd.19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talarico R., Marinello D., Cannizzo S., Gaglioti A., Ticciati S., Carta C., et al. Shaping the future of rare diseases after a Global Health emergency: Organisational points to consider. Int J Environ Res Public Health. 2020;17:8694. doi: 10.3390/ijerph17228694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020;13 doi: 10.1136/annrheumdis-2020-218946. annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q., Liu J., Shao R., Han X., Su C., Lu W. Risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population: a systematic review and meta-analysis. Rheumatol Int. 2021;41:851–861. doi: 10.1007/s00296-021-04803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmi G., Bettiol A., Mattioli I., Silvestri E., Di Scala G., Urban M.L., et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19:102575. doi: 10.1016/j.autrev.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smatti M.K., Cyprian F.S., Nasrallah G.K., Al Thani A.A., Almishal R.O., Yassine H.M. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caso F., Costa L., Ruscitti P., Navarini L., Del Puente A., Giacomelli R., et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talotta R., Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621–3644. doi: 10.12998/wjcc.v8.i17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpert G., Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19:102695. doi: 10.1016/j.autrev.2020.102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Z.W., Wang X., Lin F., Dong K. The correlation between SARS-CoV-2 infection and rheumatic disease. Autoimmun Rev. 2020;19:102557. doi: 10.1016/j.autrev.2020.102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novelli L., Motta F., De Santis M., Ansari A.A., Gershwin M.E., Selmi C. The JANUS of chronic inflammatory and autoimmune diseases onset during COVID-19 - a systematic review of the literature. J Autoimmun. 2021;117:102592. doi: 10.1016/j.jaut.2020.102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatto M., Perricone C., Tonello M., Bistoni O., Cattelan A.M., Bursi R., et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol. 2020;38:754–759. [PubMed] [Google Scholar]
- 26.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol. 2020;11:584241. doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damoiseaux J., Andrade L.E., Fritzler M.J., Shoenfeld Y. Autoantibodies 2015: from diagnostic biomarkers toward prediction, prognosis and prevention. Autoimmun Rev. 2015;14:555–563. doi: 10.1016/j.autrev.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Avery T.Y., van de Cruys M., Austen J., Stals F., Damoiseaux J.G. Anti-nuclear antibodies in daily clinical practice: prevalence in primary, secondary, and tertiary care. J Immunol Res. 2014;2014:401739. doi: 10.1155/2014/401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damoiseaux M., van Doorn W., van Lochem E., Damoiseaux J. Testing for IgA anti-tissue transglutaminase in routine clinical practice: requesting behaviour in relation to prevalence of positive results. J Transl Autoimmun. 2020;3:100045. doi: 10.1016/j.jtauto.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemna M.J., Damoiseaux J., Austen J., Winkens B., Peters J., van Paassen P., et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. 2015;26:537–542. doi: 10.1681/ASN.2013111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bijlsma J.W. EULAR December 2020 view points on SARS-CoV-2 vaccination in patients with RMDs. Ann Rheum Dis. 2021;80:411–412. doi: 10.1136/annrheumdis-2020-219773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases – version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 34.Agmon-Levin N., Damoiseaux J., Kallenberg C., Sack U., Witte T., Herold M., et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 35.Bossuyt X., Cohen Tervaert J.W., Arimura Y., Blockmans D., Flores-Suárez L.F., Guillevin L., et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017;13:683–692. doi: 10.1038/nrrheum.2017.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material