Abstract
Objectives
To investigate humoral responses and safety of mRNA SARS-CoV-2 vaccines in systemic autoimmune and autoinflammatory rheumatic disease (SAARD) patients subjected or not to treatment modifications during vaccination.
Methods
A nationwide, multicenter study, including 605 SAARD patients and 116 controls, prospectively evaluated serum anti-SARS-CoV-2 S1-protein IgG antibody titers, side-effects, and disease activity, one month after complete vaccination, in terms of distinct treatment modification strategies (none, partial and extended modifications). Independent risk factors associated with hampered humoral responses were identified by data-driven multivariable logistic regression analysis.
Results
Patients with extended treatment modifications responded to vaccines similarly to controls as well as SAARD patients without immunosuppressive therapy (97.56% vs 100%, p = 0.2468 and 97.56% vs 97.46%, p > 0.9999, respectively). In contrast, patients with partial or without therapeutic modifications responded in 87.50% and 84.50%, respectively. Furthermore, SAARD patients with extended treatment modifications developed higher anti-SARS-CoV-2 antibody levels compared to those without or with partial modifications (median:7.90 vs 7.06 vs 7.1, p = 0.0003 and p = 0.0195, respectively). Mycophenolate mofetil (MMF), rituximab (RTX) and methotrexate (MTX) negatively affected anti-SARS-CoV-2 humoral responses. In 10.5% of vaccinated patients, mild clinical deterioration was noted; however, no differences in the incidence of deterioration were observed among the distinct treatment modification SAARD subgroups. Side-effects were generally comparable between SAARD patients and controls.
Conclusions
In SAARD patients, mRNA SARS-CoV-2 vaccines are effective and safe, both in terms of side-effects and disease flares. Treatment with MMF, RTX and/or MTX compromises anti-SARS-CoV-2 antibody responses, which are restored upon extended treatment modifications without affecting disease activity.
Keywords: mRNA SARS-COV-2 vaccine, Systemic autoimmune rheumatic disease, Immunosuppressive treatment, Treatment modification, Anti-SARS-CoV-2 antibody response
Abbreviations: SAARD, systemic autoimmune and autoinflammatory rheumatic diseases; JAKi, JAK inhibitors; GC, glucocorticoids; MTX, methotrexate; RTX, rituximab; MMF, mycophenolate mofetil; GDPR, General Data Protection Regulation; EULAR, European Alliance of Associations for Rheumatology; ACR, American College of Rheumatology; OD, optical density; FCBF, fast correlation based feature; LR, logistic regression; TNFi, tumor necrosis factor inhibitors
1. Introduction
Phase II/III clinical trials evaluating the efficacy and safety of the mRNA SARS-CoV-2 vaccines either excluded or included a relatively small number of patients with systemic autoimmune and autoinflammatory rheumatic diseases (SAARD) [1,2]. Early in the vaccination era, both the European Alliance of Associations for Rheumatology (EULAR) and the American College of Rheumatology (ACR) recommended that SAARD patients should preferably get vaccinated when the disease is in remission or in low activity and, ideally, before the initiation of immunosuppressive therapy [3,4], despite that COVID-19-related morbidity and mortality did not seem to be significantly increased in SAARD patients [[5], [6], [7]]. They only recommended that SARS-CoV-2 vaccination should be performed either before the initiation of B cell depletion therapy or after its affect was eliminated [3,4]. In contrast, Moutsopoulos H. M [8]. and others [9] were more skeptical of the effect of immunosuppressive therapy on SARS-CoV-2 vaccine-induced immunogenicity, based on the precautions taken with the pneumococcal and influenza immunizations [[10], [11], [12]]. Concisely, he suggested that anti-metabolites, calcineurin and JAK inhibitors (JAKi), as well as cytokine inhibitors should be held for 7–10 days before and after each vaccine dose. As regards rituximab, it was suggested that vaccination should be performed either 6 months after the last or 1 month before the next drug administration [8].
Controversial results were published regarding humoral responses to SARS-CoV-2 in vaccinated patients with immune mediated inflammatory diseases. Indeed, glucocorticoids (GC), methotrexate (MTX), rituximab (RTX) and mycophenolate mofetil (MMF) have been found to reduce the immunogenicity of BNT162b2 mRNA vaccine to SARS-CoV-2 [[13], [14], [15], [16], [17], [18], [19], [20], [21]].
The absence of robust data on vaccination against SARS-CoV-2, prevents optimal management of SAARD patients and prompted this prospective, nationwide, multicenter study, which aims to assess the immunogenicity and safety of the mRNA SARS-CoV-2 vaccines, especially in the context of distinct strategies for treatment modification. In this article, the results on humoral responses and safety information among SAARD patients and immunocompetent participants one month after the 2nd dose of mRNA SARS- CoV-2 vaccines are presented.
2. Material & methods
2.1. Study design
The present study aims to evaluate the immunogenicity and safety of mRNA SARS-CoV-2 vaccines in Greek patients with SAARD. It was initiated in January 2021 at 10 tertiary medical centers across the country. Blood samples were drawn at baseline (within 2 weeks before vaccination) and 4 weeks after full vaccination. All patients were clinically evaluated using a specific questionnaire and physical examination. Parameters filled in the questionnaire and disease activity indices used by the contributing physicians are reported in Supplementary Materials and Methods in detail. Data on diagnosis and treatment modalities were also collected from patients’ medical records. Initiation of disease was based on clinical symptoms necessitating treatment. Thus, disease duration equals to treatment duration in this cohort of SAARD patients. All patients provided written informed consent prior to participation in the study. The study complied with the principles of the Declaration of Helsinki and the GDPR of the European Union and was approved initially by the Ethics Committee of School of Medicine, National and Kapodistrian University of Athens, Greece (leading partner; protocol no: 456) and subsequently by the ethics committees of the participating centers. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. One month after complete vaccination, all participants would be available for evaluation and, from the vaccine-safety standpoint, the study cohort would be homogenous enabling more accurate results. By including data in different timepoints, depending on vaccination dates, any associations would be difficult to interpret.
2.2. Study population
Consecutive SAARD patients followed up in the outpatient clinics of the participating medical centers were recruited based on the following inclusion criteria: (A) individuals >16 years-old, (B) vaccination with either the Pfizer-BioNTech BNT162b2 or the Moderna mRNA-1273 vaccine and (C) established diagnosis with SAARD as defined by international disease classification criteria [[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Exclusion criteria for all participants included: (A) history of SARS-CoV-2 infection, (B) acute illness resembling COVID-19 before vaccination and (C) pregnancy. Friend and/or family members of the patients and healthcare personnel of the participating medical centers were recruited to serve as immunocompetent controls with no history of SAARD or immunomodulatory/immunosuppressive therapy (n = 116) (Table 1 ).
Table 1.
Baseline characteristics and anti-SARS-CoV-2 antibody responses in SAARD patients and controls.
| FEATURES | SAARD Patients N = 605 (%) | Controls N = 116 (%) |
P-value |
|---|---|---|---|
| Demographics | |||
| Female | 432 (71.40) | 69 (59.48) | 0.0106 |
| Age, median (min-max), years | 58 (16–91) (600*) | 72 (24–90) | <0.0001 |
| Comorbidities | |||
| Diabetes mellitus | 58/598* (9.69) | 25 (21.55) | 0.0004 |
| Cardiovascular Disease | 96/598* (16.05) | 51 (43.96) | <0.0001 |
| Type of vaccine | |||
| Pfizer BioNTech BNT162b2 | 572 (94.54) | 87 (75) | <0.0001 |
| Moderna mRNA-1273 SARS-CoV-2 | 33 (5.45) | 29 (25) | <0.0001 |
| Other vaccines | |||
| Influenza | 528/591* (89.34) | 69 (59.48) | <0.0001 |
| Pneumococcus | 494/591* (83.58) | 45 (38.79) | <0.0001 |
| Treatment modifications | 222 (36.69) | N/A | N/A |
| Extended modifications | 118 (19,50) | N/A | N/A |
| Partial modifications | 104 (17,19) | N/A | N/A |
| Immune response to SARS-CoV-2 vaccines | |||
| Positive | 535 (88.42) | 116 (100) | <0.001 |
Abbreviations: *: Available data; N/A: Not applicable; Treatment modifications: as described in Materials and Methods.
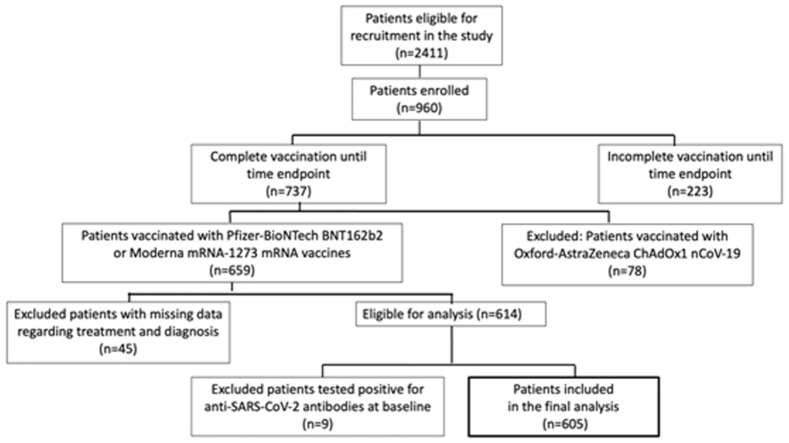
From February 1st, 2021, until June 30th, 2021, 2411 SAARD patients were eligible for recruitment in the study of whom 960 patients were finally enrolled. Among them, 737 had completed vaccination up to June 30th, 2021. According to the national SARS-CoV-2 vaccination campaign in Greece, SAARD patients had been prioritized for vaccination before the non-prioritized general population of similar age and sex, and due to availability of SARS-CoV-2 vaccines at that time in Greece, the majority of individuals were vaccinated with the mRNA vaccines. In the beginning of the prioritized vaccination campaign, Pfizer-BioNTech BNT162b2 was the only available vaccine in Greece and Moderna mRNA-1273 was introduced later, in lower quantities and fewer vaccination centers. Finally, vaccination appointments allocated to SAARD patients was based on citizens’ postal codes and therefore could be considered as random. Most of our patients (n = 659) were vaccinated with either Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 vaccines. Forty-five patients were excluded because of missing data on SAARD diagnosis and/or therapy and nine due to seropositivity for anti-SARS-CoV-2 antibodies prior to vaccination. Thus, 605 patients vaccinated with mRNA SARS-CoV-2 vaccines remained for final analysis. Analyzing each vaccine group separately, would not provide sufficient data for the mRNA-1273 vaccine, given the small sample size. On the other hand, based on their common technology, it was speculated that the possible effects of SAARD-related features on the immunogenicity would be the same (Fig. 1 ). The diagnoses of patients and the treatment regimens are shown in Supplementary Tables 1 and 2
Fig. 1.
SAARD population and study workflow.
2.3. Strategies for treatment modifications
Three distinct treatment modification strategies were followed according to physicians' judgment of the participating centers: (a) No treatment modifications according to ACR Guidance-Version 1 for COVID- 19 vaccination in patients with Rheumatic and Musculoskeletal Diseases [4], (b) Partial treatment modifications according to ACR Guidance-Version 2: Briefly, MTX, MMF, JAKi, abatacept and RTX treatment were withheld or postponed, accordingly [31] and (c) Extended treatment modifications as proposed by Moutsopoulos H.M [8]. In the beginning of the study, since no published guidance was available, patients did not modify their treatment. However, during the conduction of the study, since different treatment modification strategies were proposed in the literature and preliminary results from vaccine-induced immunogenicity studies were published, patients were, thereon, allocated either into the partial or the extended modification subgroup, according to physicians’ best judgment.
2.4. Measurement of anti-SARS-CoV-2 antibodies
Based on a previous report [32], an FDA-approved and independently validated commercial ELISA method (Euroimmun, Lübeck, Germany) was selected for the detection of anti-SARS-CoV-2 S1-protein IgG antibodies, according to the manufacturer's instructions [33]. An optical density (OD) index, defined as the value of the sample measured at 450 nm divided by the OD value of the provided calibrator, was used for the identification of the seropositive samples (OD index greater than 1.1). This OD index was considered as antibody titer throughout the manuscript, as previously [32]. An ELISA method detecting IgG anti-S1 antibodies was chosen over trimeric tests based on previous experience [34] and due to reports that the trimeric tests cross-react with antibodies against other CoV strains [35].
2.5. Statistical analysis
Statistical analysis for categorical data was performed using the chi-square test with Yates' correction or Fischer's exact test when cell counts were <5. For continuous variables, normality was tested with the Shapiro-Wilk test and Mann-Whitney U test (Wilcoxon rank-sum test) or t-test were applied appropriately. Comparison among several groups was based on Kruskal-Wallis with post hoc analysis, after testing the normality of the variables.
A data-driven analysis was performed, based on the combination of the Fast Correlation Based Feature (FCBF) selection method with the Logistic Regression (LR) algorithm and was applied on the unified dataset of all patients to identify risk factors for non-responder SAARD patients, minimizing potential selection bias, as previously described [36] (presented in detail Supplementary Materials and Methods). The implementation of the FCBF-based multivariable LR approach and the statistical analysis was performed using Python-v3.7, and GraphPad-v9. Unlike the classical statistical analysis which hampers the identification of hidden patterns within the variables in the data based on a target outcome, data-driven analysis is suitable for this classification task, since it involves automated methods for the extraction of hidden patterns within the variables in the data.
3. Results
3.1. Demographics, vaccine types and disease characteristics of SAARD patients and controls
Female predominance was recorded in both groups, with the SAARD group including significantly more females than the control group (71.40% and 59.48%, p = 0.0106, respectively). The median (minimum-maximum) age was 58 (16–91) years for the SAARD group and 72 (24–90) years for the control group, the latter being significantly older (p < 0.0001). Prevalence of diabetes mellitus and cardiovascular disease in the SAARD and the control group were 9.69% vs 21.55%, (p = 0.0004) and 16.05% vs 43.96% (p < 0.0001), respectively. The vast majority of SAARD patients and controls had been vaccinated with the Pfizer-BioNTech-BNT162b2 vaccine (n = 572, 94.54% and n = 87, 75%, respectively), while the remaining received the Moderna mRNA-1273 vaccine (n = 33, 5.45% and n = 29, 25%, respectively), both differences being statistically significant (p < 0.00001) (Table 1).
3.2. Anti-SARS-CoV-2 antibody responses to mRNA vaccines in SAARD patients and controls
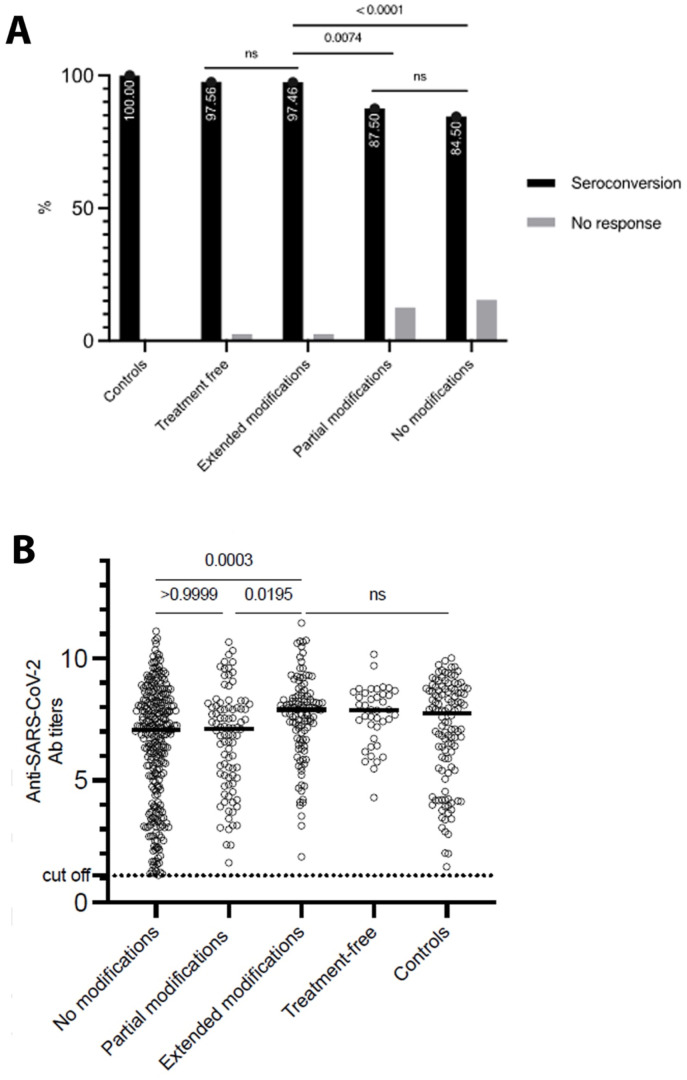
Forty-one (6.77%) of SAARD patients were not on immunosuppressive therapy during the vaccination period, 118 (19.50%) underwent extended treatment modifications and 104 (17.19%) partial modifications, while 342 (56.52%) did not modify their immunomodulatory therapy. The response rates among the above-mentioned subgroups were 97.56%, 97.46%, 87.50% and 84.50%, respectively, with the difference among them, being statistically significant (p < 0.001). Post-hoc analysis revealed that patients following extended treatment modifications responded to mRNA vaccines more frequently than patients who underwent partial or no modifications (97.46 vs 87.50%, p = 0.0074, OR = 5.476, 95%-CI:1.509–18.38 and 97.46 vs 84.50%, p < 0.0001, OR = 7.030, 95%-CI:2.278–21.86, respectively), but similar to the “treatment-free” SAARD subgroup (p > 0.9999) (Fig. 2 a). Among responders, anti-SARS-CoV-2 antibody titers were significantly higher in patients who underwent extended modifications compared to those who underwent partial modifications (median: 7.9 vs 7.1, p = 0.0195) or no modifications (median: 7.9 vs 7.06, p = 0.0003) and similar with treatment-free patients (median: 7.9 vs 7.88, p > 0.9999) (Fig. 2b).
Fig. 2.
Anti-SARS-CoV-2 antibody responses in SAARD patients with different treatment modification strategies and controls. (A) Comparison of seroconversion rates among the different treatment modification subgroups. Patients off treatment and patients with extended treatment modifications presented significantly higher response rates compared to patients with partial treatment modifications or patients without treatment modifications. (B) Comparison of anti-SARS-CoV-2 antibody titers among responders to vaccination. Antibody titers were significantly higher in patients with extended treatment modifications than those without treatment modifications or partial modifications (the black horizontal lines represent the medians). No modifications: No treatment modification during vaccination period; Partial modifications: Treatment modifications during vaccination period based on ref. [31]; Extended modifications: Treatment modifications during vaccination period based on ref. [8].
Overall, 535 out of the total of 605 SAARD patients (88.42%) and 116 (100%) of controls responded to mRNA vaccines - a difference that reached statistical significance (p < 0.001, OR:30.68 95%-CI:1.886–498.8).
3.3. Characteristics of responder and non-responder SAARD patients
Seventy out of 605 SAARD patients were non-responders. They were significantly older (median: 63.5 vs 57 years, p = 0.045), had more frequently comorbidities (45.71 vs 24.62%, p = 0.0003, OR = 2.578, 95%-CI:1.524–4.279) and suffered more often from systemic vasculitis [microscopic polyangiitis (n = 2), granulomatosis with polyangiitis (n = 5), polyarteritis nodosa (n = 2), giant cell arteritis (n = 2), cryoglobulinemic vasculitis (n = 1) and unclassified vasculitis (n = 5)] compared to responders (24.28 vs 9.34%, p = 0.0004, OR = 3.111, 95%-CI:1.685–5.678), while underlying SAARD duration did not differ between the two groups [median (range) duration in years:10 (0.2–47) vs 12 (1–40), p = 0.4254, respectively]. Non-responders more often belonged to the “no modification” treatment subgroup compared to responders (75.71 vs 54.01%, p = 0.0009, OR = 2.654, 95%-CI:1.497–4.831) and were treated more often with GC (57.14 vs 37.75%, p = 0.0028, OR = 2.198, 95%-CI:1.345–3.623), MMF (47.14 vs 8.03%, p < 0.0001, OR = 10.20, 95%-CI:5.699–17.54), RTX (30 vs 4.11%, p < 0.0001, OR = 9.994, 95%-CI:5.214–18.99) or CYC (4.28 vs 0.37%, p = 0.0125, OR = 11.93, 95%-CI:2.382–67.66). As far as the medication dose, SAARD patients receiving low dose of prednisone (<10 mg/day) or equivalent and MMF (<2000 mg/day) did not differ from patients receiving higher doses in terms of seroconversion (p = 0.4765 and p = 0.4993, respectively). More specifically, the median (range) dose (mg/day) of prednisone or equivalent was 5 (1.25–40) in responders and 5 (1.25–30) in non-responders; the difference being non-significant (p = 0.3590). Non-responders compared to responders received more frequently RTX treatment within 6 months before vaccination or 1 month after completion of vaccination (72.22 vs 27.77%, p = 0.0069, OR = 8.320, 95%-CI: 2.002–30.33).
On the other hand, responders underwent more frequently extended treatment modifications than non-responders (21.49 vs 4.28%, p = 0.0001, OR = 6.115, 95%-CI:2.033–18.91). The majority of SAARD patients treated with TNF inhibitors (TNFi; n = 130, 96.29%) seroconverted and only 5 SAARD patients (3.70%) did not (p = 0.002, OR = 4.173, 95%-CI:1.732–9.809). A more detailed comparison between responder and non-responder SAARD patients is presented in Table 2 , while detailed description of treatment modalities is shown in Supplementary Table 3.
Table 2.
Comparison of major baseline, disease and treatment characteristics between responders and non-responders SAARD patients.
| FEATURES | RESPONDERS N = 535 (%) |
NON-RESPONDERS N = 70 (%) | P-value |
|---|---|---|---|
| Demographics | |||
| Female gender | 384 (71.77) | 48 (68.57) | 0.6764 |
| Age median, min-max, years | 57, 16–91 (530*) | 63.5, 24-86 | 0.045 |
| Presence of any comorbidity | 130/528* (24.62) | 32 (45.71) | 0.0003 |
| Treatment status | |||
| No treatment | 40 (7.47) | 1 (1.42) | 0.0727 |
| Extended treatment modifications | 115 (21.49) | 3 (4.28) | 0.0001 |
| Partial treatment modifications | 91 (17.00) | 13 (18.57) | 0.8750 |
| No treatment modifications | 289 (54.01) | 53 (75.71) | 0.0009 |
| Diagnoses | |||
| Systemic vasculitis | 50 (9.34) | 17 (24.28) | 0.0004 |
| Rheumatoid arthritis | 152 (28.41) | 16 (22.85) | 0.4044 |
| Systemic lupus erythematosus | 101 (18.87) | 17 (24.28) | 0.3611 |
| Sjögren's syndrome | 51 (9.53) | 7 (10) | 0.9275 |
| Systemic sclerosis | 11 (2.05) | 3 (4.28) | 0.2135 |
| Idiopathic inflammatory myositis | 23 (4.29) | 5 (7.14) | 0.4457 |
| Seronegative arthritis | 121 (22.62) | 5 (7.14) | 0.0045 |
| Familial Mediterranean fever | 4 (0.74) | 0 (0) | 0.3799 |
| IgG4-related disease | 1 (0.18) | 1 (1.42) | 1.0000 |
| Antiphospholipid syndrome | 14 (2.61) | 2 (2.85) | 0.2182 |
| Polymyalgia rheumatica | 18 (3.36) | 0 (0) | 0.7067 |
| Behcet's disease | 4 (0.74) | 0 (0) | 0.2496 |
| Still's disease | 7 (1.30) | 0 (0) | 1.0000 |
| Mixed connective tissue diseases | 3 (0.56) | 1 (1.42) | 1.0000 |
| Disease Duration, median, min-max, years | 10, 0.2–47 (512*) | 12, 1–40 (65*) | 0.4254 |
| Treatment regimens | |||
| Glucocorticoids | 202 (37.75) | 40 (57.14) | 0.0028 |
| Mycophenolate mofetil | 43 (8.03) | 33 (47.14) | <0.0001 |
| Azathioprine | 36 (6.72) | 3 (4.28) | 0.6062 |
| Methotrexate | 169 (31.58) | 14 (20) | 0.0648 |
| Leflunomide | 31 (5.79) | 3 (4.28) | 0.7860 |
| Hydroxychloroquine | 92 (17.19) | 8 (11.42) | 0.2934 |
| TNF inhibitors | 130 (24.29) | 5 (7.14) | 0.0020 |
| Ustekinumab | 6 (1.12) | 0 (0) | 1,0000 |
| IL-1 inhibitors | 18 (3.36) | 0 (0) | 0.6147 |
| IL-6 inhibitors | 42 (7.85) | 2 (2.85) | 0.2158 |
| IL-17 inhibitors | 17 (3.17) | 1 (1.42) | 0.7094 |
| Rituximab | 22 (4.11) | 21 (30) | <0.0001 |
| JAK inhibitors | 4 (0.74) | 1 (1.42) | 0.4604 |
| Apremilast | 4 (0.74) | 0 (0) | 1.0000 |
| Abatacept | 4 (0.74) | 1 (1.42) | 0.4604 |
| Belimumab | 7 (1.30) | 3 (4.28) | 0.0981 |
| Colchicine | 5 (0.93) | 1 (1.42) | 0.5234 |
| Cyclosporine | 26 (4.85) | 3 (4,28) | 1.0000 |
| Cyclophosphamide | 2 (0.37) | 3 (4.28) | 0.0125 |
| Intravenous immunoglobulin G | 2 (0.37) | 1 (1.42) | 0.3089 |
| Disease activity | |||
| Remission | 404/530* (76.22) | 56 (80) | 0.5815 |
| Low | 93/530* (17.54) | 10 (14.28) | 0.6090 |
| Moderate | 29/530* (5.47) | 3 (4.28) | 1.0000 |
| High | 4/530* (0.75) | 1 (1.42) | 0.4634 |
Abbreviations: *: Available data; IgG4: Immunoglobulin G number 4; IL: Interleukin; JAK: Janus kinase.
3.4. Effect of different treatments on vaccine immunogenicity
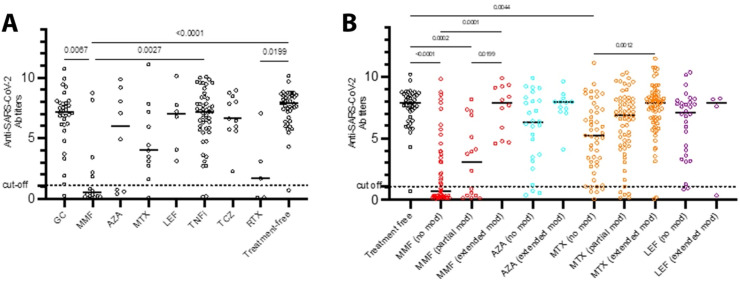
To evaluate the effect of each therapeutic regimen on the immunogenicity of mRNA SARS-CoV-2 vaccines, we analyzed patients receiving a single immunomodulatory agent. MMF and RTX monotherapies strongly inhibited antibody responses to mRNA vaccines. MTX was also found to negatively affect the response to mRNA vaccines but did not reach statistical significance, probably due to the small number of patients on MTX monotherapy (Fig. 3 a). On the contrary, GC, TNFi and tocilizumab did not affect anti-SARS-CoV-2 responses.
Fig. 3.
Anti-SARS-CoV-2 antibody responses among SAARD patients treated with different regimens. (A) Anti-SARS-CoV-2 antibody titers in SAARD patients receiving treatment with a single agent. Mycophenolate mofetil and rituximab were apparently the agents hampering responses to mRNA SARS-CoV-2 vaccines. Patients receiving methotrexate also showed lower anti-SARS-CoV-2 antibody titers (without reaching statistical significance) compared to other therapeutic regimens. (B) Anti-SARS-CoV-2 antibody titers in SAARD patients receiving different treatment regimens (as monotherapy and/or combinational therapy) with or without treatment modifications. SAARD patients who did not modify MMF or MTX-based treatment schedules developed significantly lower antibody titers compared to treatment free patients. SAARD patients who extensively modified MMF or MTX-based treatments developed comparable antibody titers with treatment-free patients and significantly higher than those who did not withhold the same agents (the black horizontal lines represent the medians). Abbreviations: GC:glucocorticoids;MMF:mycophenolate mofetil;AZA:azathioprine;MTX:methotrexate;LEF:leflunomide;TNFi:tumor necrosis factor inhibitors;TCZ:tocilizumabRTX:rituximab.
Subsequently, the effect of treatment regimens received as monotherapy or combinational therapy on antibody responses was analyzed among the treatment modification subgroups. SAARD patients who extensively withheld MTX or MMF-based therapies had comparable anti-SARS-CoV-2 antibody titers with treatment-free patients and higher than patients with no modifications (Fig. 3b).
3.5. Multivariable data driven logistic regression analysis for independent risk factors associated with poor anti-SARS-CoV-2 antibody responses
The working dataset included 605 SAARD patients and 43 features (variables) which are described in Table 3 . Forty-two missing values regarding age, SAARD duration, drug dose or SAARD activity representing 0.013% of total data were filled-in accordingly after imputation. The results of the FCBF-based multivariable logistic regression analysis, using non responder status as an outcome are presented in Table 3. The FCBF algorithm identified six potentially independent risk factors associated with poor immune responses: three (MMF, RXT, presence of comorbidities) displayed positive, while the rest (polymyalgia rheumatica, interleukin-1 inhibitors and ustekinumab) negative associations. Logistic regression analysis disclosed only MMF and RTX as independent negative modifiers of antibody response to SARS-Cov2. The performance of the data driven approach was favorable, yielding an average accuracy 0.80, sensitivity 0.80, specificity 0.90 and AUC 0.83, across the 100 random executions of the random down-sampling with replacement procedure (Supplementary Fig. 1). To study the effect of treatment dose on vaccine immunogenicity, another FCBF-multivariable LR model was conducted, with the various medication doses included as continuous variables. The dataset included only the 342 SAARD patients with no treatment modifications and 39 features described in Table 3; MMF and RTX were identified as independent risk factors for poor response following SARS-CoV-2 vaccination (data not shown).
Table 3.
FCBF-based multivariable logistic regression analysis for risk factors associated with poor immune responses to SARS-CoV-2 vaccination.
| Prominent featurea | Regression coefficient | Odds ratio | p-value | CI low | CI upper |
|---|---|---|---|---|---|
| Mycophenolate Mofetil** | 2.185 | 9.212, | <0.0001 | 3.46 | 24.759 |
| Rituximab** | 1.875 | 6.803 | 0.005 | 1.934 | 24.302 |
| Comorbidities | 0.664 | 2.0 | 0.139 | 0.942 | 4.254 |
| Polymyalgia Rheumatica | −0.466 | 0.644 | 0.765 | 0.094 | 26.61 |
| IL-1 inhibitors | −0.181 | 0.848 | 0.927 | 0.429 | 35.841 |
| Ustekinumab | −0.181 | 0.848 | 0.927 | 0.438 | 34.363 |
**<0.05 (95% confidence interval): final independent risk factors associated with non-responders.
***Logistic regression equation ( denotes the outcome).
Abbreviations: IL-: Interleukin.
The strongest potentially independent variables identified by the FCBF algorithm to construct the logistic regression model, after analyzing initially the following features included in the dataset: age, gender, duration of disease, disease activity before vaccination, presence of any comorbidity, no treatment, extended treatment modifications, partial treatment modifications, no treatment modifications, glucocorticoids, mycophenolate mofetil, azathioprine, methotrexate, leflunomide, hydroxychloroquine, TNF inhibitors, ustekinumab, IL-1 inhibitors, IL-6 inhibitors, IL-17 inhibitors, rituximab, JAK inhibitors, apremilast, abatacept, belimumab, colchicine, cyclosporine, cyclophosphamide, IVIG, systemic vasculitis, rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, systemic sclerosis, idiopathic inflammatory myositis, seronegative arthritis, familial mediterranean fever, IgG4-related diseases, antiphospholipid syndrome, polymyalgia rheumatica, Bechet's disease, Still's disease and mixed connective tissue diseases.
3.6. Safety of the mRNA SARS-CoV-2 vaccines among SAARD patients
Disease activity status as provided by the caring physician and disease course as self-reported by the patients before and after the vaccination is provided in Supplementary Figure 2. Considering disease activity, 64 (10.57%) SAARD patients experienced clinical deterioration (increase ≥1 in the physicians' scale defined in Supplementary Materials and Methods), while 96 patients (15%) reported that their disease course worsened after the SARS-CoV-2 vaccination (increase ≥1 in the patient's scale defined in Supplementary Materials and Methods). 64 patients showed clinical deterioration, of whom 59 (92.18%) were on immunosuppressive therapy and 5 (7.81%) were treatment-free. Among the 59 patients on immunosuppressive therapy, 26 (44.06%) modified and 33 (55.93%) did not modify their treatment. Among 64 patients with clinical deterioration, 54 (84.37%) responded to vaccination, while 10 (15.62%) patients did not respond. Similar were the findings from the 96 patients who self-reported that their SAARD course worsened after vaccination. 89 (92.70%) of them were on immunosuppressive therapy while 7 (7.29%) patients were treatment-free. Out of the 89 patients on immunosuppressive therapy, 48 (53.93%) modified their treatment during vaccination. Among 96 patients who felt a worsening of their disease, 83 (86.45%) responded to vaccination, while 13 (13.54%) patients did not respond. The median (range) SAARD duration of the 64 patients showing clinical deterioration was 11 (0.3–38) years, while the median (range) SAARD duration of the 96 patients experiencing a worsening of their disease was 12 (0.3–37) years; the difference being non-significant (p = 0.7684). Deterioration rates were similar among patients with distinct treatment modification approaches (12.5%, 9.7%, 12.6% and 10.9% in treatment-free, no, partial and extended modification groups, respectively, p = 0.8320).
The prevalence of mild adverse events was generally comparable in SAARD patients and controls. In detail, local pain at the injection site was observed in 63 (54.31%) of 116 controls vs 263 (43.83%) of 600 SAARD patients (p = 0.048), low or high grade fever in 27 (23.27%) and 99 (16.5%) (p = 0.1049), generalized pain in 13 (11.20%) and 62 (10.33%) (p = 0.9079), muscle weakness in 10 (8.62%) and 55 (9.16%) (p = 0.9913), fatigue in 19 (16.37%) and 132 (22.00%) (p = 0.2171), and headache in 14 (12.06%) and 88 (14.66%) (p = 0.5567), respectively. Fever was more common in SAARD patients with extended modifications (p = 0.0009), while injection site pain was more prevalent among patients not undergoing modifications (p = 0.0001). Rare side effects included hypertensive crisis (n = 2), lymphadenopathy (n = 6), generalized rash (n = 7), allergic reaction (n = 2) and herpes zoster (n = 1).
4. Discussion
To the best of our knowledge, this is the first study exploring the effect of different treatment modification strategies among SAARD patients in antibody responses induced by mRNA SARS-CoV-2 vaccines, including those recommended by ACR as well as an extended withhold of therapeutic regiments [4,8,31]. Our results substantiate and reinforce previous findings indicating that MMF, MTX and RTX reduce both the number of responders and the magnitude of antibody responses [[13], [14], [15],17,37,38]. In addition, it was shown that extended treatment modifications regarding MMF, MTX and RTX improve the immunogenicity of mRNA SARS-CoV-2 vaccines in SAARD patients, without significantly effecting the disease activity status.
Overall, SAARD patients developed anti-SARS-CoV-2 antibodies in 88% compared to 100% of non-SAARD controls, a finding described by other investigators [15,21]. This can be attributed to the large percentage of SAARD patients who did not undergo any treatment modification at the time of vaccination. Furthermore, it connotes that our control population despite the older age and comorbidities had an excellent response to mRNA vaccines. Treatment with MMF and RTX had a deleterious effect on vaccine-induced antibody responses and were identified as the only independent risk factors associated with poor humoral responses in the data driven multivariable analysis. Moreover, SAARD patients on MTX-based therapies demonstrated diminished antibody responses, compared to treatment-free patients, an effect minimized by the extended treatment modification. On the other hand, TNFi did not affect antibody responses, as previously reported [17,39]. Interestingly, poor humoral responses were not associated either with disease activity or the type of disease.
The novel finding of this study is that extensive treatment modifications [8] seem to fully restore immune responses to mRNA SARS-CoV-2 vaccines in SAARD patients without triggering significant disease exacerbation. Indeed, SAARD patients subjected to extended treatment modification developed significantly higher titers of anti-SARS-CoV-2 antibodies than those undertaking ACR recommendations or continuing treatment without changes [4,31]. Furthermore, these titers were similar to treatment-free SAARD patients and controls. Importantly, the prominent response to vaccination after extended treatment modification was not accompanied by significant disease flares. Theoretically, disease activity might be influenced by a) treatment modifications, even for a short period of time and b) the immunization itself by triggering both innate and adaptive immunity and subsequently activating autoreactive clones may induce autoinflammatory phenomena leading to disease flares [37]. Herein, a slight clinical deterioration of the underlying SAARD was observed in one out of ten patients. These phenomena were usually mild, not requiring hospitalization or radical therapeutic adjustments. Given the fact that at least some of these patients were expected to flare spontaneously during the study period, disease exacerbations after vaccination occur at low rates and are easily manageable. Finally, in consistency with previous studies [14,16,40,41], there were no major side effects following immunization and were generally comparable between patients and controls.
Our study had limitations. First, the non-SAARD controls were not age-matched; in fact, their age was older than the SAARD patients, accumulating enriched comorbidities and therefore favoring worse immune responses than an ideal age-matched control group. However, we considered this group as a sample of the general population with the same genetic background and even though they were expected to show defective responses to vaccination due to their demographic characteristics, all of them successfully seroconverted. Second, the prevalence of some disease groups and treatment modalities -especially anti-cytokine therapies-were limited among our SAARD group, attenuating the capacity to evaluate their effect on vaccine immunogenicity. Third, data regarding disease deterioration rates within a 6-month period before vaccination were missing because records only disease flares necessitating radical treatment changes were recorded in patients’ and as such a direct comparison between exacerbation rates before and after vaccination was not feasible. Fourth, the cellular component of immunity as an important element of immunogenicity, has not been evaluated in our patient group [42,43].
In summary, the present study supports that mRNA SARS-CoV-2 vaccines are immunogenic and safe in the majority of SAARD patients. Treatment with MTX, MMF and RTX were associated with hampered anti-SARS-CoV-2 antibody responses. However, extended treatment modifications significantly improved vaccine-induced immunogenicity compared to partial modifications. Adverse events following vaccination were comparable to controls and disease exacerbations presented in a few patients were rare and mild, providing evidence of overall good tolerance of the mRNA vaccines in SAARD patients. Concerns about waning vaccine-induced immunity are emerging [44] and the long-term sustainability of humoral responses and SAARD course are intended to be explored in a subsequent study.
Funding
This work was supported by the Institute for Autoimmune Systemic and Neurological Diseases, Athens, Greece.
Contributors
AGT, PGV, FNS and HMM designed and supervised the study, recruited and follow-up patients and wrote the paper. ADB, AVG, KB, IIC, LGC, ODA, AIV, MM, IES, PVV, CK, SK, SG, GK, CIS, AIG, SNL and CP recruited and followed-up the patients, collected the samples and clinical data. ADB and KB performed the experiments. ADB, AVG, KB, VP and DIF analyzed the data and performed statistical analyses. ADB, AVG and KB wrote the paper. AGT, PGV, HMM, AVG, ADB and KB had full access to the study's data. All authors have read and approved the submitted version of the manuscript.
Data availability statement
All data relevant to the study are included in the article and/or uploaded as supplementary information.
Author statement
Athanasios G. Tzioufas: Conceptualization, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding acquisition. Athanasios-Dimitrios Bakasis: Methodology, Formal analysis, Investigation, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization. Andreas V. Goules: Resources, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization. Kleopatra Bitzogli: Methodology, Formal analysis, Investigation, Resources, Writing - Original Draft, Visualization. Ilir I. Cinoku: Resources. Loukas G. Chatzis: Resources. Ourania D. Argyropoulou: Resources. Aliki I. Venetsanopoulou: Resources. Maria Mavrommati: Resources. Ioanna E. Stergiou: Resources. Vasilis Pezoulas: Formal analysis, Writing - Original Draft. Paraskevi V. Voulgari: Resources. Chaido Katsimpari: Resources. Spyridon Katechis: Resources. Souzana Gazi: Resources. Gkikas Katsifis: Resources. Charalampos I. Sfontouris: Resources. Athanasios I. Georgountzos: Resources. Stamatis-Nick Liossis: Resources. Charalampos Papagoras: Resources, Writing - Review & Editing. Dimitrios I. Fotiadis, Formal analysis. Fotini N. Skopouli: Conceptualization, Resources, Writing - Review & Editing, Visualization, Supervision. Panayiotis G. Vlachoyiannopoulos: Conceptualization, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding acquisition. Haralampos M. Moutsopoulos: Conceptualization, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Funding acquisition.
Declaration of competing interest
AGT has received research grants from Novartis, Pfizer, UCB, AbbVie and GSK pharmaceutical companies, through the National and Kapodistrian University of Athens, not relevant to the submitted work. The rest of the authors have no competing interests to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2021.102743.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijlsma J.W. EULAR December 2020 View points on SARS-CoV-2 vaccination in patients with RMDs. Ann. Rheum. Dis. 2021;80:411–412. doi: 10.1136/annrheumdis-2020-219773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheum. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serling-Boyd N., D'Silva K.M., Hsu T.Y., Wallwork R., Fu X., Gravallese E.M., et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann. Rheum. Dis. 2021;80:660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.F. R. S. S. S. C. I. consortium, contributors Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann. Rheum. Dis. 2020;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakasis A.D., Mavragani C.P., Boki K.A., Tzioufas A.G., Vlachoyiannopoulos P.G., Stergiou I.E., et al. COVID-19 infection among autoimmune rheumatic disease patients: data from an observational study and literature review. J. Autoimmun. 2021;123:102687. doi: 10.1016/j.jaut.2021.102687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moutsopoulos H.M. A recommended paradigm for vaccination of rheumatic disease patients with the SARS-CoV-2 vaccine. J. Autoimmun. 2021;121:102649. doi: 10.1016/j.jaut.2021.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.K., Lee E.B., Shin K., Sung Y.K., Kim T.H., Kwon S.R., et al. COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean college of rheumatology. J. Kor. Med. Sci. 2021;36:e95. doi: 10.3346/jkms.2021.36.e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg R.A., Jawad A.F., Boyer J., Maurer K., McDonald K., Prak E.T., et al. Rituximab-treated patients have a poor response to influenza vaccination. J. Clin. Immunol. 2013;33:388–396. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua C., Barnetche T., Combe B., Morel J. Effect of methotrexate, anti-tumor necrosis factor alpha, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 12.Rondaan C., Furer V., Heijstek M.W., Agmon-Levin N., Bijl M., Breedveld F.C., et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haberman R.H., Herati R., Simon D., Samanovic M., Blank R.B., Tuen M., et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun-Moscovici Y., Kaplan M., Braun M., Markovits D., Giryes S., Toledano K., et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021;80:1317–1321. doi: 10.1136/annrheumdis-2021-220503. [DOI] [PubMed] [Google Scholar]
- 15.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 16.Geisen U.M., Berner D.K., Tran F., Sumbul M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruddy J.A., Connolly C.M., Boyarsky B.J., Werbel W.A., Christopher-Stine L., Garonzik-Wang J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiera R., Jinich S., Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 19.Veenstra J., Wang J., McKinnon-Maksimowicz K., Liu T., Zuniga B., Hamzavi I., et al. Correspondence on 'Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021;80:e160. doi: 10.1136/annrheumdis-2021-220736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boekel L., Steenhuis M., Hooijberg F., Besten Y.R., van Kempen Z.L.E., Kummer L.Y., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in The Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021 Aug 6 doi: 10.1016/S2665-9913(21)00222-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., Demissie E.G., et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2 : a prospective cohort study. Ann. Intern. Med. 2021 Aug 31 doi: 10.7326/M21-1757. M21-1757. Online ahead of print In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 23.Petri M., Orbai A.M., Alarcon G.S., Gordon C., Merrill J.T., Fortin P.R., et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 25.Jennette J.C., Falk R.J., Andrassy K., Bacon P.A., Churg J., Gross W.L., et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 26.Taylor W., Gladman D., Helliwell P., Marchesoni A., Mease P., Mielants H., et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 27.Rudwaleit M., van der Heijde D., Landewe R., Listing J., Akkoc N., Brandt J., et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg I.E., Tjarnlund A., Bottai M., Werth V.P., Pilkington C., Visser M., et al. 2017 European League against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017;76:1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheum. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 31.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 2. Arthritis Rheum. 2021;73:e30–e45. doi: 10.1002/art.41877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon D., Tascilar K., Fagni F., Kronke G., Kleyer A., Meder C., et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlachoyiannopoulos P., Alexopoulos H., Apostolidi I., Bitzogli K., Barba C., Athanasopoulou E., et al. Anti-SARS-CoV-2 antibody detection in healthcare workers of two tertiary hospitals in Athens, Greece. Clin. Immunol. 2020;221:108619. doi: 10.1016/j.clim.2020.108619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y., Lian C., Chen Y., Wei D., Zhang X., Ling Y., et al. Sensitivity and specificity of SARS-CoV-2 S1 subunit in COVID-19 serology assays. Cell Discov. 2020;6:75. doi: 10.1038/s41421-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatzis L., Pezoulas V.C., Ferro F., Gandolfo S., Donati V., Binutti M., et al. Sjogren's syndrome: the clinical spectrum of male patients. J. Clin. Med. 2020;9 doi: 10.3390/jcm9082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman M.A., Curtis J.R., Winthrop K.L. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1255–1265. doi: 10.1136/annrheumdis-2021-221244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winthrop K.L., Whitley R.J., Aletaha D. SARS-CoV-2 and the rheumatology patient: the last 12 months and a boost in the future. Ann. Rheum. Dis. 2021;80:1249–1251. doi: 10.1136/annrheumdis-2021-221251. [DOI] [PubMed] [Google Scholar]
- 39.Mahil S.K., Bechman K., Raharja A., Domingo-Vila C., Baudry D., Brown M.A., et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connolly C.M., Ruddy J.A., Boyarsky B.J., Avery R.K., Werbel W.A., Segev D.L., et al. Safety of the firstdose ofmRNA SARS-CoV-2vaccines inpatients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80(10) doi: 10.1136/annrheumdis-2021-220231. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esquivel-Valerio J.A., Skinner-Taylor C.M., Moreno-Arquieta I.A., Cardenas-de la Garza J.A., Garcia-Arellano G., Gonzalez-Garcia P.L., et al. Adverse events of six COVID-19 vaccines in patients with autoimmune rheumatic diseases: a cross-sectional study. Rheumatol. Int. 2021:1–4. doi: 10.1007/s00296-021-05017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prendecki M., Clarke C., Edwards H., McIntyre S., Mortimer P., Gleeson S., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 44.Suresh L. Update on COVID-19 vaccines and autoimmune-mediated rheumatic diseases. Reumatologia. 2021;59:203–205. doi: 10.5114/reum.2021.108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article and/or uploaded as supplementary information.