ABSTRACT
The bacterial cell envelope is not only a protective structure that surrounds the cytoplasm but also the place where a myriad of biological processes take place. This multilayered complex is particularly important for electroactive bacteria such as Shewanella oneidensis, as it generally hosts branched electron transport chains and numerous reductases for extracellular respiration. However, little is known about how the integrity of the cell envelope is established and maintained in these bacteria. By tracing the synthetic lethal effect of Arc two-component system and σE in S. oneidensis, in this study, we identified the lipopolysaccharide transport (Lpt) system as the determining factor. Both Arc and σE, by regulating transcription of lptFG and lptD, respectively, are required for the Lpt system to function properly. The ArcA loss results in an LptFG shortage that triggers activation of σE and leads to LptD overproduction. LptFG and LptD at abnormal levels cause a defect in the lipopolysaccharide (LPS) transport, leading to cell death unless σE-dependent envelope stress response is in place. Overall, our report reveals for the first time that Arc works together with σE to maintain the integrity of the S. oneidensis cell envelope by participating in the regulation of the LPS transport system.
IMPORTANCE Arc is a well-characterized global regulatory system that modulates cellular respiration by responding to changes in the redox status in bacterial cells. In addition to regulating expression of respiratory enzymes, Shewanella oneidensis Arc also plays a critical role in cell envelope integrity. The absence of Arc and master envelope stress response (ESR) regulator σE causes a synthetic lethal phenotype. Our research shows that the Arc loss downregulates lptFG expression, leading to cell envelope defects that require σE-mediated ESR for viability. The complex mechanisms revealed here underscore the importance of the interplay between global regulators in bacterial adaption to their natural inhabits.
KEYWORDS: lipopolysaccharide transport system, Arc regulatory system, σE, cell envelope, envelope stress response, regulation
INTRODUCTION
Bacteria live in unpredictable and harsh environments. As the outmost shell of the bacterial cell, the cell envelope constitutes the first line of defense against external assaults (1). The envelope of Gram-negative bacteria is composed of three principal layers: the outer membrane (OM), the peptidoglycan layer, and the cytoplasmic or inner membrane (IM) (2). The OM, an asymmetric phospholipid bilayer whose outside leaflet is composed of lipopolysaccharide (LPS) molecules, functions as a robust and outmost permeability barrier (1). Given its essentiality for cell viability, the biogenesis of the OM is a precisely regulated process, and perturbations and environmental threats affecting the integrity of their envelope are closely monitored (3). Under stressful conditions, envelope stress response (ESR) is activated to prevent unmanageable damages (2). Alternative sigma factor σE (RpoE), which is inactive under normal conditions because it is sequestered to the IM by its anti-sigma factor RseA, plays a primary role in mediating envelope biogenesis and ESR (4, 5). The σE signaling detects periplasmic stresses, including misfolded OM proteins and off-pathway lipopolysaccharides (LPS) input, and initiates a proteolytic cascade that results in the sequential degradation of RseA, amounting to the release of σE from the IM (6, 7). Freed cytoplasmic σE transcribes its regulon involved in synthesis, assembly, and homeostasis of OM proteins and LPS (3). In addition to σE, multiple phosphorelay systems have also been discovered to mediate ESR, including two-component systems (TCSs) CpxAR and BaeRS and complex signaling transduction system Rcs (3, 8).
Shewanella, a group of the gammaproteobacteria that inhabit the oxic-anoxic transition zones of water columns and aquatic sediments, are renowned for their respiratory versality (9, 10). These bacteria are able to utilize a variety of compounds as terminal electron acceptors (EAs), including oxygen, fumarate, trimethylamine N-oxide (TMAO), dimethyl sulfoxide (DMSO), nitrate, nitrite, sulfite/thiosulfate, and metals such as iron and manganese (11, 12). Reduction of all EAs is carried out outside the cytoplasm, dependent on various reduction systems, including terminal reductases and the electron transfer chains that supply electrons (13). Given that most, if not all, of the components of the reduction systems are located in the cell envelope, establishing and maintaining the integrity of the envelope is thus vital for these biological processes. Conceivably, the σE signaling pathway dictates maintaining the cell envelope integrity and mediating ESR in Shewanella oneidensis (14). While roles of CpxAR, BaeRS, and Rcs in mediating envelope integrity and ESR of S. oneidensis remain unexplored, the anoxic redox control (Arc, ArcBA) system is unambiguously involved.
Arc, best studied in Escherichia coli, is a conventional two-component system (TCS) mediating the metabolic shift from anaerobic to aerobic conditions (15, 16). The DNA-binding response regulator ArcA regulates the expression of genes in response to oxygen availability sensed by the sensor kinase ArcB in an indirect manner, mainly by repressing genes involved in aerobic respiration (17). The S. oneidensis Arc system is atypical, as there exists a connector (HptA), a protein similar to the histidine phosphotransfer domain (HPt) of ArcB in E. coli that establishes the regulatory link between the otherwise independent sensor kinase (ArcS) and ArcA (18–20). Moreover, overlaps in the ArcA regulons of these two species are surprisingly rare, implying that biological processes in which the S. oneidensis Arc system are involved differ from those established in other bacteria drastically (21–24).
In S. oneidensis, Arc was initially identified as a regulator involved in anaerobic respiration because it is required for transcription of the operon for the DMSO reductase and therefore is essential to DMSO respiration (18). Later on, the regulatory role of ArcA was extended to growth supported by aerobic respiration, although the underpinning mechanisms remain incompletely understood (13, 21) (Fig. 1A). Surprisingly, both transcriptomics and proteomics studies have revealed that the genes encoding a large number of membrane proteins, such as FadL (short-chain fatty acid transporter), PspA (phage shock protein), SO_2427 (TonB-dependent receptor), and CsgE (curli assembly/transport protein), to name a few, are among the most differentially expressed upon ArcA loss (21, 25). The involvement of ArcA in maintaining the cell envelope integrity is ultimately confirmed by the finding that its absence induces increased susceptibility to sodium dodecyl sulfate (SDS) (13, 21, 26) (Fig. 1B). More importantly, with respect to the cell envelope integrity and ESR, ArcA interplays with σE because σE and ArcA constitute a synthetic lethal pair in S. oneidensis (14). The goal of this study was to probe mechanisms underpinning synthetic lethality of σE and ArcA and to establish the link between these two transcriptional regulators.
FIG 1.
Cell envelope defects likely underpin the synthetic lethal phenotype resulting from the loss of ArcA and σE in S. oneidensis. (A) Growth of the wild type (WT) and mutants in liquid LB was measured by recording values of OD600. Both mutants were verified previously and again by genetic complementation as shown in Fig. S1. (B) Effects of arcA or rpoE deletion on growth and SDS susceptibility by spotting assays performed on LB agar plates. Cultures of each strain prepared to contain approximately 109 CFU/ml were regarded as the undiluted cultures (dilution factor, 0), which were subjected to 10-fold serial dilution. Five microliters of each dilution was dropped on indicated agar plates and photographed 30 h later. (C) Effects of arcA and rpoE double deletion on growth. Expression of both genes was driven by IPTG-inducible promoter Ptac, with IPTG at indicated concentrations. (D) Growth of ΔrpoEΔarcA/parcA strain in liquid LB with IPTG at indicated concentrations. The ΔrpoEΔarcA/parcA strain was grown overnight in LB liquid containing 0.2 mM IPTG, and the cells were collected by centrifugation, washed twice with fresh IPTG-free LB liquid, and transferred to LB liquid containing IPTG at indicated concentrations to an OD600 of 0.01. (E) Growth of ΔrpoEΔarcA/prpoE strain in liquid LB with IPTG at indicated concentrations. (F) Cell morphology of relevant strains in LB with IPTG at indicated concentrations by phase-contrast microscope. Cells of the exponential phase (∼0.4 of OD600) were fixed on a slice of LB agar. Scale bars, 2 μm. In all panels, experiments were performed at least three times, with either representative data or the means of replicate values ± standard deviations being presented.
RESULTS
Cell envelope defects likely underpin the synthetic lethal phenotype resulting from the loss of ArcA and σE.
To unravel the mechanisms underlying the synthetic lethality of the arcA and rpoE double deletion mutant (ΔrpoEΔarcA), we first comprehensively assessed physiological impacts of ArcA and σE produced at various levels on this strain. On LB agar plates without IPTG (isopropyl-β-D-thiogalactopyranoside), ΔrpoEΔarcA expressing a copy of arcA (ΔrpoEΔarcA/parcA) under the control of IPTG-inducible promoter Ptac, which is slightly leaky (Ptac, ∼30 Miller units) (27, 28), displayed severely impaired growth, whereas ΔrpoEΔarcA/prpoE was normal (Fig. 1C). This is expected because the activity of Ptac in the absence of the inducer is similar to that of the rpoE promoter (Ptac, ∼30 Miller units; PrpoE, ∼20 Miller units), whereas the arcA promoter is nearly 10 times stronger in the wild-type (WT) cells growing normally (14, 29). In LB, when IPTG was supplemented no more than 0.01 mM, the optical density (OD, 600 nm) of ΔrpoEΔarcA/parcA (the inocula were prepared from the culture grown in LB containing 0.2 mM IPTG by centrifugation, washing, and suspension with fresh medium) increased normally for about 4 h and was followed by a significant reduction (Fig. 1D). This pattern coincides with that observed from the S. oneidensis culture grown in the presence of β-lactam antibiotics, suggesting that a large portion of cells lyse during the OD reduction period (30) (see Fig. S2 in the supplemental material). Expression of arcA with 0.02 mM IPTG and above suppressed the lysis, and growth was fully restored with 0.2 mM IPTG compared to that of the ΔrpoE strain (Fig. 1C and D). In the case of σE, the best growth was observed from rpoE expression without IPTG (Fig. 1C and E). σE production induced by as low as 0.02 and 0.05 mM IPTG significantly impaired growth and nearly completely inhibited growth, respectively. Despite this, OD reduction was not observed in the presence of IPTG at all concentrations tested, implying that the overexpression of σE does not lead to cell lysis (Fig. 1E).
We then visualized the ΔarcAΔrpoE strains expressing arcA and rpoE at various levels using a phase-contrast microscope. The cell morphology of ΔrpoEΔarcA/parcA grown without IPTG was clearly altered, with most cells showing a variety of membrane defects, including distorted shape, formation of blebs, and division/separation failure (Fig. 1F). When arcA and rpoE were expressed at proper levels (ΔrpoEΔarcA/parcA, 0.2 mM IPTG; ΔrpoEΔarcA/prpoE, no IPTG), ΔrpoEΔarcA cells maintained a normal rod-shaped morphology. These observations suggest that the synthetic lethality resulting from the ArcA and σE double loss is due to cell envelope damages.
Altered expression of lptFG suppresses synthetic lethality of the arcA and rpoE mutant.
To identify suppressor genes of the synthetic lethal phenotype of ΔrpoEΔarcA, we performed transposon mutagenesis on ΔrpoEΔarcA/parcA with mariner-based transposon vector pFAC (31). ΔrpoEΔarcA/parcA was used as a parental strain because it has markedly different phenotypes with or without IPTG, allowing us to identify additional mutations that switch the phenotypes in the absence of IPTG. pFAC was chosen since it can be not only used for construction of transposon insertion libraries but also applicable for cryptic operon screening because of an embedded promoter in the transposable sequence (31, 32).
A library of ∼15,000 random mutants were screened for colonies formed on IPTG-free Km+Gm+ plates that were substantially larger than the average, and more than a hundred were obtained. However, a large majority of these isolates were unstable, losing viability during verification with spotting assays. In the end, only 3 isolates were found to grow well consistently in the absence of IPTG. All of them, which were indistinguishable from one another with respect to growth and SDS sensitivity, had transposon insertions that mapped to the region between pepA and lptF genes (250 bp upstream of lptFG gene), and to simplify description, we named these suppressor strains Tn-FG (Fig. 2A). The lptF gene is presumably cotranscribed with lptG, both of which encode components of the LPS transport system located on the IM of Gram-negative bacteria (Fig. 2B). LptFG, essential in E. coli, are responsible for the transport of LPS from the IM periplasmic side to LptC in the periplasm (33). Physiological characterization demonstrated that Tn-FG grew indistinguishably from ΔarcA but slower than ΔrpoE when grown in IPTG-free media (Fig. 2C), indicating that the suppression is on the synthetic lethal phenotype only without correcting the growth defect. This observation is understandable because the defects in growth and cell envelope resulting from the ArcA loss are independent of each other (14). Although Tn-FG was viable with normal cell morphology (see Fig. S4 in the supplemental material), it still carried a cell envelope defect (Fig. 2D), suggesting that the suppression in Tn-FG could not fully correct the envelope defect resulting from the ArcA and σE double loss.
FIG 2.
Altered expression of lptFG suppresses synthetic lethality of the arcA and rpoE mutant. (A) Screening for suppressor mutants from ΔrpoEΔarcA/parcA in the absence of IPTG was performed by transposon mutagenesis. The transposable element contains a promoter, aiming at expressing genes after the insertion in addition to the gene interruption. Genomic context of the lptABCDEFG operons in S. oneidensis and insertion sites in Tn-FG suppressor. By genetic mapping, 3 random mutants having insertions in the region upstream of the lptFG operon were obtained, which was drawn in scale. Arrows represent the approximate locations of transposon insertions. (B) The working model for LPS transport system based on the E. coli paradigm. (C) The growth of Tn-FG suppressor in IPTG-free LB, which was indistinguishable from ΔarcA. (D) Growth and SDS susceptibility of Tn-FG suppressor and ΔrpoEΔarcA/plptFG revealed by spotting assays on LB agar plates without or with SDS. In panels C and D, experiments were performed at least three times, with either representative data or the means of replicate values ± standard deviations being presented.
To validate the suppressing role of LptFG on the synthetic lethal phenotype resulting from the arcA and rpoE double deletion, we tried to construct ΔrpoEΔarcA while expressing lptFG from a plasmid and succeeded. The resulting strain, ΔrpoEΔarcA/plptFG, was hardly able to grow in IPTG-free media, similar to what was observed for the ΔrpoEΔarcA/parcA strain (Fig. 2D). When lptFG expression was increased with IPTG induction, ΔrpoEΔarcA/plptFG behaved similarly to Tn-FG, with respect to growth and morphology (Fig. 2D; Fig. S3). Notably, the ΔrpoEΔarcA/plptFG strain showed increased resistance to SDS, which is likely a result of LptFG overproduction (Fig. 2D). These data indicate that LptFG is crucially involved in the synthetic lethality of the arcA and rpoE mutation.
LptFG homeostasis is critical to the integrity of the cell envelope.
To explore the effects of LptFG on envelope integrity of the WT, ΔrpoE, and ΔarcA strains of S. oneidensis, we attempted to delete lptFG from them. However, attempts were not successful. Instead, the operon could be removed from strains expressing a copy of the lptFG operon under the control of Ptac. With respect to growth on plates and morphology, the ΔlptFG/plptFG strain appeared very similar to ΔrpoEΔarcA/parcA (Fig. 3A and B). In the absence of IPTG, cells of ΔlptFG/plptFG exhibited severely compromised viability and growth, apparently resulting from substantially impaired cell envelope. When produced sufficiently and even excessively (0.05 mM IPTG and above) (Fig. S4), all of the observed defects were fully corrected (Fig. 3A and B), indicating that the underproduced LptFG (from the leaky promoter, Ptac, ∼30 Miller units) underlies the phenotypes as well as implying that the overabundance of LptFG alone has no negative effects on the LPS transport in S. oneidensis. When IPTG was not added, ΔarcAΔlptFG/plptFG carried defects in viability and growth not significantly different from those of ΔlptFG/plptFG, but ΔrpoEΔlptFG/plptFG nearly lost viability completely (Fig. 3A). Consistently, ΔlptFG/plptFG and ΔarcAΔlptFG/plptFG cells had a similar morphology, suggesting that underproduced LptFG may act as a critical factor for the phenotypes of both strains. Furthermore, we found that the cell envelope of ΔrpoEΔlptFG/plptFG cells was impaired more severely, evidenced by more blebs and longer cell chains, than that observed in ΔrpoEΔarcA/parcA (Fig. 1F; Fig. 3B). These data manifest that σE is more important than ArcA in protecting cells from cell envelope damages introduced by LptFG in insufficient quantity.
FIG 3.
LptFG homeostasis is critical to the integrity of the cell envelope. (A) Growth and viability of relevant strains on LB agar plates without or with SDS. (B) Cell morphology of strains expressing lptFG at various levels. Scale bars, 2 μm. In both panels, experiments were performed at least three times, with either representative data or the means of replicate values ± standard deviations being presented.
Although the rpoE mutant grows indistinguishably from WT under normal conditions, it is more sensitive to SDS (Fig. 1B). To assess whether LptFG homeostasis is critical to the integrity of the cell envelope without σE, we examined the susceptibility of ΔrpoEΔlptFG/plptFG to SDS. As shown in Fig. 3A, when LptFG was produced minimally (without IPTG), cells died out on plates with 0.05% SDS. However, in the presence of 0.02 mM IPTG, ΔrpoEΔlptFG/plptFG already exhibited growth and viability improved compared to those of ΔrpoE. LptFG produced with IPTG at 0.1 mM and above completely corrected the cell envelope defect resulting from the σE loss (Fig. 1B; Fig. 3A). We also observed that increased LptFG production had no significant effect on SDS susceptibility of the ΔarcA strain (Fig. 1B; Fig. 3A). These data indicate that LptFG, crucial to the integrity of the cell envelope in general, is deeply involved in the envelope defect of the rpoE mutant in S. oneidensis.
The shortage of LptFG activates σE stress response.
Given that the increased LptFG abundance suppresses the synthetic lethal phenotype resulting from the ArcA and σE loss, we tested whether the depletion of ArcA and σE influences expression of lptFG with an integrative lacZ reporter system (34). In line with the prediction by two distinct sources (21, 24) that the lptFG operon belongs to the S. oneidensis ArcA regulons, the results of the promoter assay revealed that the activity of the lptFG promoter (PlptFG) was significantly lower in ΔarcA than in WT or ΔrpoE (Fig. 4A), suggesting that ArcA but not σE is required for maintaining normal expression of the lptFG operon. We also found that the lptFG expression in ΔrpoEΔarcA/parcA grown without IPTG was similar to that in ΔarcA, a result supporting that the loss of arcA but not rpoE significantly affects lptFG expression (Fig. 4A).
FIG 4.
The shortage of LptFG activates σE stress response. (A) lptFG promoter (PlptFG) activity assay. The activity in cells of the exponential phase (∼0.4 of OD600), assayed by integrative lacZ reporters, was presented as Miller Units for β-galactosidase activities. In strains expressing relevant genes, expression was controlled with IPTG at indicated concentrations. (B) Western blotting of active σE. Cytoplasmic fractions were prepared from S. oneidensis cells in the exponential growth phase, separated on 15% SDS-PAGE, and electrophoretically transferred to polyvinylidene difluoride (PVDF). σE was probed with polyclonal antibodies and detected by chemiluminescence. (C) degQ promoter (PdegQ) activity assay as carried out in panel A. In panels A and C, asterisks indicate statistically significant difference compared to the wild-type values (ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
We then examined whether the LptFG loss affects expression of rpoE given the central role of σE in maintaining the integrity of the cell envelope. Consistent with the previous findings (14), the rpoE promoter activities in ΔarcA, ΔrpoE, and ΔrpoEΔarcA/parcA were very low and rather stable (see Fig. S5 in the supplemental material). Importantly, the loss of LptFG, either alone or with ArcA and σE together, did not significantly influence the activity of the rpoE promoter (Fig. S5). Subsequently, the abundance of active σE (not membrane associated) was assessed with Western blotting because σE is activated by accumulation of off-pathway LPS (7). The results showed that the amount of σE increased in ΔlptFG/plptFG cells grown without IPTG relative to that in cells grown with IPTG at 0.1 mM or above (Fig. 4B). Importantly, the quantity of active σE was further elevated in ΔarcAΔlptFG/plptFG cells grown in IPTG-free media. Moreover, we validated the changes in the quantity of the active σE by using a lacZ reporter driven by the degQ promoter, which is a verified member of the σE regulon (14). As expected, the degQ promoter exhibited σE-dependent activity and the ArcA loss displayed an upregulating effect (Fig. 4C). When LptFG was present at minimal levels, the highest activity was observed, and the activity decreased with the LptFG amounts inversely (Fig. 4C). These data, collectively, suggest that LptFG at reduced levels triggers the σE stress response, which lessens the envelope defects resulting from the LptFG shortage. In line with this, the absence of σE abolishes this protective mechanism, leading to further aggravated cell envelope damage as seen in ΔrpoEΔlptFG/plptFG grown without IPTG.
LptD in excess impairs the cell envelope.
The Lpt system is composed of 7 components, including cytoplasmic LptB, LptFG, and LptC in the IM and periplasmic LptA and LptDE in the OM (35–37) (Fig. 2B). To explore whether Lpt components other than LptFG may interplay with ArcA and σE in S. oneidensis, we tested effects of their absence and overexpression on growth and viability. In strains expressing one of the lpt clusters on plasmids, each of them was successfully in-frame deleted, resulting in ΔlptCAB/plptCAB, ΔlptD/plptD, and ΔlptE/plptE. Characterization of these strains revealed that ΔlptCAB/plptCAB behaved similarly to ΔlptFG/plptFG in terms of growth and viability without and with IPTG (Fig. 5A). However, ΔlptD/plptD was normal in the absence of IPTG but showed severe defects in growth and viability with 0.5 mM IPTG, whereas the absence and overproduction of LptE did not exhibit significant influence (Fig. 5A). While results obtained from these strains grown on the plates containing SDS were similar in general, the sensitivity to LptD homeostasis became more evident, noticeable even with IPTG at 0.02 mM (Fig. 5A).
FIG 5.
LptD in excess impairs the cell envelope in S. oneidensis. (A) Effects of Lpt components in shortage and overexpression on growth, viability, and SDS susceptibility. (B) Growth of strains expressing lptD with IPTG at indicated concentrations in LB. (C) Cell morphology of indicated strains expressing lptD with IPTG at indicated concentrations. Scale bars, 2 μm. In all panels, experiments were performed at least three times, with either representative data or the means of replicate values ± standard deviations being presented.
The growth of ΔlptD/plptD in liquid media was then assayed. Clearly, when LptD was produced with IPTG at 0.1 mM and above, defects became significant (Fig. 5B). More importantly, LptD in excess by 0.5 mM IPTG induction caused growth curves resembling that of ΔarcAΔrpoE/parcA. These defects were likely a result of the substantially impaired cell envelope with excessive LptD (0.5 mM IPTG), evidenced by distorted shape, formation of blebs, dissolving cell membranes, and division failure, coinciding with the morphological changes observed from ΔarcAΔrpoE/parcA (Fig. 5C). Altogether, the data suggest that LptD is associated with the phenotypes resulting from the loss of the two regulators.
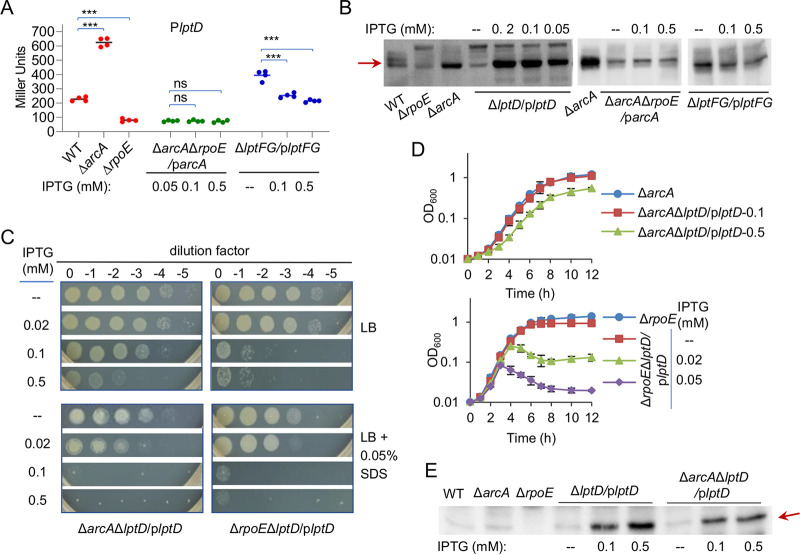
Enhanced lptD expression partially explains the cell envelope defects in ΔarcA and activates σE stress response.
According to the regulon prediction, the lptD operon, but not other lpt operons, is likely controlled by σE directly (14). Given that LptD homeostasis is critical to the cell envelope, we therefore hypothesized that regulation of lptD expression by σE, perhaps ArcA too, may be accountable for the synthetic lethal phenotype. To test this, we assayed activity of the lptD promoter (PlptD) in WT, ΔarcA, and ΔrpoE strains. As shown in Fig. 6A, the σE loss compromised PlptD activity significantly, approximately 30% relative to that of the WT, supporting that the lptD operon is a member of the σE regulon. In contrast, we observed substantially increased activity of PlptD in ΔarcA, up to 3-fold. In the absence of σE, the inducing effect of ArcA at any level under test vanished (ΔarcAΔrpoE/parcA) (Fig. 6A), supporting that the expression of lptD depends on σE. The LptD levels in the relevant strains were then assayed directly with Western blotting. As shown in Fig. 6B, LptD was present in significantly increased and decreased amounts in ΔarcA and ΔrpoE, respectively. In the absence of σE, the effects of ArcA on LptD levels became neglectable. Complementation of arcA and rpoE mutations validated that all these observations were due to the missing genes (see Fig. S6 in the supplemental material).
FIG 6.
Enhanced lptD expression partially explains the cell envelope defects in ΔarcA and activates σE stress response. (A) Effects of ArcA, σE, and LptFG on lptD expression by lacZ reporter assays. (B) Effects of ArcA, σE, and LptFG on LptD levels by Western blotting. (C) Effects of LptD at various levels on growth, viability, and SDS susceptibility revealed by spotting assays. (D) Effects of LptD at various levels on growth in LB liquid. (E) Activation of σE by excessive LptD revealed by Western blotting of cytoplasmic σE. In all panels, experiments were performed at least three times, with either representative data or the means of replicate values ± standard deviations being presented.
We then tested whether LptD in increased abundance might play a key role in the cell envelope defects of the arcA mutant. To this end, we generated ΔarcAΔlptD/plptD and monitored its growth and viability with IPTG at various concentrations. As shown in Fig. 6C, when LptD was overproduced with IPTG at 0.1 mM or above, ΔarcAΔlptD/plptD displayed reduced growth and viability, similar to those of ΔlptD/plptD (Fig. 5A). When grown with 0.1 mM IPTG in the presence of 0.05% SDS, the difference between ΔarcAΔlptD/plptD and ΔlptD/plptD became evident: ΔarcAΔlptD/plptD lost viability completely, whereas the growth and viability of ΔlptD/plptD were affected only modestly (Fig. 5A; Fig. 6C). The effects gained support from the results of growth in liquid media (Fig. 6D) and morphological visualization (Fig. 5C). These data, all together, suggest that the detrimental impact of excessive LptD on the cell envelope is likely, at least in part, accountable for the cell envelope defect of the arcA mutant.
The expression assay presented above revealed that LptD is produced at a very low level in the absence of σE, contrasting that in the absence of ArcA. To test how LptD at various levels affects the rpoE mutant, ΔrpoEΔlptD/plptD was generated and characterized. Different from the ΔarcAΔlptD/plptD strain, ΔrpoEΔlptD/plptD hardly survived in the presence of 0.1 mM IPTG, regardless of SDS (Fig. 6C). Consistently, in the liquid broth containing IPTG at 0.1 mM and above, ΔrpoEΔlptD/plptD showed a short period of growth and then lysed nearly completely (Fig. 6D). Thus, it is clear that σE is required for alleviating the detrimental effect of excessive LptD. Notably, the growth curve of ΔrpoEΔarcA/parcA without IPTG resembled that of ΔrpoEΔlptD/plptD with 0.05 mM IPTG, further supporting that the increased LptD levels are a critical factor causing the cell envelope defect of the arcA mutant.
Given that LptFG at insufficient levels triggers σE activation, we hypothesized that this is likely to be the case for LptD when it is overproduced. To test this, the levels of σE in ΔlptD/plptD and ΔarcAΔlptD/plptD strains were assessed. The results showed that σE was more abundant in ΔlptD/plptD and ΔarcAΔlptD/plptD strains with IPTG at 0.1 mM and above than in WT (Fig. 6E). However, when LptD was minimally produced, it did not introduce a significant difference in the amount of activated σE, a result consistent with the finding that LptD activates the σE stress response only when it is in overabundance. These observations were confirmed with the PdegQ-lacZ reporter (Fig. 4C). We then moved further to test whether activation of σE by underproduced LptFG and excessive LptD is intertwined. As shown in Fig. 4A, the expression of lptFG was not affected significantly when LptD was produced at various levels. In contrast, the minimal production of LptFG resulted in up to 2-fold induced production of LptD, and this effect vanished when it was produced normally or at increased levels (Fig. 6A and B). These data suggest an explanation for the effect of the LptFG shortage on the cell envelope, that is, the underproduced LptFG leads to overproduced LptD, which in turn triggers σE activation.
DISCUSSION
In many proteobacteria, the Arc system is a major transcriptional regulator for respiration in response to environmental cues that alter the redox status of the quinol pool, whereas σE mediates the cell envelope biogenesis and ESR. Seemingly, these two regulatory systems affect distinct physiological processes, and reports about the interplay between them are rare. However, in S. oneidensis, Arc and σE are functionally intertwined, amounting to the synthetic lethal phenotype in their simultaneous absence (14). In this study, we have unraveled the mechanisms underlying the phenotype, generating three contributions to the current understanding of the Arc and σE biology. First, we identified the Lpt system as the dictating factor responsible for the synthetic lethality. Second, we demonstrated that LptFG at underproduced levels per se, and its direct consequences, including the increased quantity of activated σE and subsequent LptD overproduction, largely underlies the cell envelope defect of the arcA mutant. Third, both the shortage of LptFG and overabundant LptD elicit ESR, during which σE is required to activate the damage-controlling system for viability.
By using a random mutagenesis to screen for suppressors, we related LptFG to the ArcA and σE synthetic lethality. In Gram-negative bacteria, Lpt is composed of seven essential lipopolysaccharide transport proteins (LptABCDEFG) that transport LPS from the IM to the cell surface (37) (Fig. 2B). LptB2FG form an atypical ABC transporter at the IM, in stable association with LptC, that extracts LPS from the periplasmic leaflet of the IM (38–41). Once extracted, the amphipathic LPS is transported across the aqueous periplasm through the periplasmic bridge formed by LptC, LptA, and LptD (42, 43). Our data indicate that the Lpt-mediated LPS transport pathway of S. oneidensis is highly conserved and each of the components appears to function the same as its counterpart in E. coli. S. oneidensis cells depleted of LptFG are defective in the transport of LPS to the cell surface, presumably leading to disordered OM structure and OM permeability defects the same as those in E. coli (33). In line with functional association, S. oneidensis LptCAB appears to affect LPS transport in a way similar to that of LptFG because reduced LptCAB creates a defect in LPS transport in the same manner as LptFG in E. coli (43). The complex of LptD and LptE at the OM functions in the final stages of assembling LPS into the outer leaflet of the OM. Moreover, S. oneidensis cells with the minimal expression of lptD, ΔlptD/plptD without IPTG, do not carry an OM defect, and this scenario can be readily explained by the finding that LptD is characterized as a low-abundance protein in E. coli (44, 45) (Fig. 5A). However, when produced excessively, LptD causes severe OM defects, consistent with the previous findings that the alteration of LptD structure or expression amount could affect not only the transport efficiency of LPS but also the OM permeability and membrane structures (46–49). In contrast, the physiological influence of excessive LptE is negligible because its activity is dependent on LptD (35, 50).
Given that it is increased expression rather than interruption of lptFG that recovers viability of the rpoE arcA mutant, it is clear that LptFG is underproduced in the absence of both ArcA and σE. Further investigations reveal that ArcA is responsible for the expression difference of LptFG because in both ΔarcA and ΔrpoEΔarcA, but not ΔrpoE, expression of lptFG is significantly lower than that in WT (Fig. 4A). This is not surprising, as the lptFG operon is a member of the S. oneidensis arcA regulon predicted by a combination of transcriptomic, in vitro DNA-protein interaction and bioinformatics analyses (21, 24, 29).
Unlike the lptFG operon, none of the remaining lpt operons is under the direct control of ArcA. Despite this, we found that the lptD gene is highly induced in the absence of ArcA and this induction depends on σE (Fig. 6A and B). This observation coincides with the notion that there is a compensatory mechanism in the Lpt system: if one component is disrupted, leading to impaired function of LPS translocation, other components undergo mutation and/or the altered activity to control/overcome the damage (51–53). For example, increased expression of the membrane-associated ABC protein LptB stabilizes C-terminally truncated LptC mutant proteins, thereby allowing the formation of a sufficient number of stable IM complexes to support growth (52). Certain mutations in the LptF periplasmic domain can compensate for defects in LPS transport conferred by the lack of LptC (51). Moreover, alterations in the LptFG coupling helices with the defective LPS transport can be rescued by changing a residue in LptB that is adjacent to functionally important residues in the groove region (53). Our data, however, suggest a twisted form of “compensation,” that is, when produced insufficiently, LptFG induces LptD production (Fig. 6B), which in turn, unfortunately, causes more profound damages on the cell envelope.
In Gram-negative bacteria, the activation of the σE stress response system is initiated by the presence of misfolded proteins (especially OM porins) in the periplasm, as well as off-pathway intermediates in LPS transport and assembly (7). The data presented here illustrate a close connection between the σE stress response and the Lpt system defect in S. oneidensis. Both reduced LptFG and excessive LptD result in OM defects that activate the σE stress response. Interestingly, among all lpt operons, lptA, lptB, and lptD belong to the σE regulon studied to date (54–56). This apparently holds true for S. oneidensis, as the lptD gene is predicted to be controlled by a σE-dependent promoter (14), and upon σE depletion the expression of lptD is no longer responsive to cell envelope stress imposed by SDS. Despite this, it should be noted that the minimal expression of lptD is allowed in the absence of σE because cells are viable without σE but not without LptD.
Based on the data herein, we propose that the cell envelope defect of the arcA mutant is due largely to the combined effects of both underproduced LptFG and overproduced LptD. In the presence of σE, ESR functions and the damages resulting from the ArcA loss can be controlled to some extent, allowing viability albeit being sensitive to SDS. In the arcA rpoE double mutant cells, although LptD could not be overproduced, LptFG at reduced levels leads to OM defects, which amount to killing because of the lack of the protection of functioning ESR. Thus, given the hypersusceptibility of S. oneidensis cells to LptD overdose, it is more likely that LptD in excess plays a larger role in the cell envelope defect of the arcA mutant whereas LptFG in an insufficient amount is responsible for the synthetic lethality of arcA and rpoE.
The involvement of ArcA in the regulation of LPS synthesis and modification has been reported in other bacteria. Transcription of genes encoding WzzSE and WzzfepE in Salmonella enterica serovar Enteritidis (S. Enteritidis), which control the long O antigen and the very long O antigen, respectively, is mediated by ArcA in response to oxygen availability (57). Additionally, ArcA of S. Enteritidis also modulates lpxO expression, resulting in changes in lipid A hydroxylation (58). In the plant pathogen Dickeya dadantii, ArcA activates transcription of dltB and phoS, whose products are implicated in modification of LPSs (59). In S. oneidensis, although the shortage of LptFG and excessive LptD are largely responsible for the cell envelope defect of the arcA mutant, many other membrane proteins may have a role, too. Omics analyses have revealed that membrane-bound proteins make up a large portion of the most downregulated proteins in the arcA mutant (21, 25). Eight and 23 out of the top 10 and 30 most downregulated are proteins outside the cytoplasm (see Table S1 in the supplemental material). Conceivably, these proteins (many are β-barrel outer membrane proteins) per se and their biogenesis may greatly affect the cell envelope integrity in S. oneidensis. Thus, it seems that the Arc system of S. oneidensis has shifted to modulate the envelope integrity rather than metabolism, as few metabolic genes of the E. coli Arc regulon are found to be controlled by Arc in S. oneidensis (21). More importantly, although the Arc systems of S. oneidensis and E. coli can respond to changes in the redox status to regulate the activity of ArcA, the former may perceive other signals (19, 60). This is inferred from the finding that the redox-sensing PAS domain within ArcS (CaChe-PAS-PAS-HisKA; E. coli ArcB, PAS-HisKA) is functionally dispensable (60). We envision that ArcS uses the Cache domain located in the periplasm to sense extracellular cues and that other domains may be involved. We are working to determine the precise nature of the inducing signal linked to the role of ArcA in modulation of the envelope integrity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Information for primers used for generating PCR products is available upon request. Chemicals were obtained from Sigma-Aldrich Co. unless otherwise noted. E. coli and S. oneidensis strains under aerobic conditions were grown in lysogeny broth (LB; Difco, Detroit, MI) medium at 37 and 30°C for genetic manipulation. When needed, the growth medium was supplemented with chemicals at the following concentrations: 2,6-diaminopimelic acid (DAP), 0.3 mM, ampicillin sodium, 50 μg/ml, kanamycin sulfate, 50 μg/ml, and gentamicin sulfate, 15 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Host for cloning | Lab stock |
| WM3064 | ΔdapA, donor strain for conjugation | W. Metcalf, UIUC |
| S. oneidensis strains | ||
| MR-1 | Wild type | Lab stock |
| HG1342 | ΔrpoE derived from MR-1 | 14 |
| HG3988 | ΔarcA derived from MR-1 | 21 |
| HG1342-3988c1 | ΔrpoEΔarcA/prpoE derived from MR-1 | 14 |
| HG1343-3988c2 | ΔrpoEΔarcA/parcA derived from MR-1 | This study |
| HG1342-3988c3 | ΔrpoEΔarcA/plptFG derived from MR-1 | This study |
| Tn-FG | Suppressor derived from ΔrpoEΔarcA/parcA | This study |
| HG1173c | ΔlptE/plptE derived from MR-1 | This study |
| HG1369-70c | ΔlptFG/plptFG derived from MR-1 | This study |
| HG3636c | ΔlptD/plptD derived from MR-1 | This study |
| HG3858-60c | ΔlptCAB/plptCAB derived from MR-1 | This study |
| HG1342-1369-70c | ΔrpoEΔlptFG/plptFG derived from MR-1 | This study |
| HG3988-1369-70c | ΔarcAΔlptFG/plptFG derived from MR-1 | This study |
| HG1342-3636c | ΔrpoEΔlptD/plptD derived from MR-1 | This study |
| HG3988-3636c | ΔarcAΔlptD/plptD derived from MR-1 | This study |
| Plasmid | ||
| pHGM01 | att-based suicide vector, Apr, Gmr, Cmr | 61 |
| pHGEI01 | Kmr, integrative lacZ reporter vector | 34 |
| pHGEN-Ptac | Kmr, IPTG-inducible expression vector | 28 |
| pBBR-Cre | Spr, helper plasmid for antibiotic cassette removal | 32 |
| pFAC | Gmr, vector containing transposable sequence | 31 |
| pHGEI01-PrpoE | PrpoE-lacZ fusion within pHGEI01 | 14 |
| pHGEI01-PlptFG | PlptFG-lacZ fusion within pHGEI01 | This study |
| pHGEI01-PlptD | PlptD-lacZ fusion within pHGEI01 | This study |
| pHGEI01-PdegQ | PdegQ-lacZ fusion within pHGEI01 | This study |
| pHGEN-Ptac-rpoE | Ptac-rpoE within pHGEN-Ptac | 14 |
| pHGEN-Ptac-lptFG | Ptac-lptFG within pHGEN-Ptac | This study |
| pHGEN-Ptac-lptD | Ptac-lptD within pHGEN-Ptac | This study |
| pHGEN-Ptac-lptE | Ptac-lptE within pHGEN-Ptac | This study |
| pHGEN-Ptac-lptCAB | Ptac-lptCAB within pHGEN-Ptac | This study |
Mutant construction and complementation.
In-frame deletion strains for S. oneidensis were constructed using the att-based fusion PCR method as described previously (61). In brief, two fragments flanking the gene of interest were amplified independently and then joined together by a second round of PCR. The resulting fusion fragment was introduced into suicide plasmid pHGM01 by site-specific recombination using the BP Clonase (Invitrogen), and the resulting mutagenesis vectors were maintained in E. coli DAP-auxotroph WM3064. The vectors were then transferred from E. coli into the relevant S. oneidensis strain by conjugation. Integration of the mutagenesis construct into the chromosome was selected by gentamicin resistance and confirmed by PCR. For most of mutants constructed in this study, plasmid pHGEN-Ptac expressing a copy of the target gene under the control of isopropyl β-d-1-thiogalactoside (IPTG)-inducible promoter Ptac was introduced to verified transconjugants before resolution (28). Such transconjugants were grown in LB with IPTG at proper concentrations in the absence of NaCl and plated on LB supplemented with 10% sucrose for resolution. Gentamycin-sensitive and sucrose-resistant colonies were screened by PCR for deletion of the target gene. Mutants were verified by sequencing the mutated regions.
For genetic complementation of the mutants and inducible gene expression, genes of interest generated by PCR were cloned into pHGEN-Ptac (28). After verification by sequencing, the resultant vectors in E. coli WM3064 were transferred into the relevant strains via conjugation.
Spotting assays.
Spotting assays were employed to evaluate viability, growth inhibition, and SDS susceptibility of relevant S. oneidensis strains on LB plates. Cells of the exponential phase (∼0.4 of OD600, the same throughout the study unless otherwise noted) were collected by centrifugation and adjusted to 108 CFU/ml, which was set as the undiluted culture (dilution factor 0). Ten-fold serial dilutions were prepared with fresh medium. Five microliters of each dilution was dropped onto LB plates without or with SDS. The plates were incubated for 30 h before being read. All experiments were conducted at least three times.
Transposon mutagenesis and screening.
A random mutation library for the ΔrpoEΔarcA/parcA strain was constructed with pFAC, which is a transposon vector with a promoter embedded in the transposable region (31, 32). Synthetic lethality of the arcA and rpoE suppressor strains were selected from a library of ∼15,000 random mutants for colonies formed on IPTG-free plates containing kanamycin and gentamicin that were substantially larger than the average. Spotting assays were then performed to verify these transposon mutants. Those that consistently grew well in the absence of IPTG were subjected to the mapping of the transposon insertion sites by using the arbitrary PCR (62).
Analysis of gene expression.
Activity of target promoters was assessed using a single-copy integrative lacZ reporter system as described previously (63). Briefly, fragments containing the sequence (∼300 bp) upstream of the target operon were amplified, cloned into the reporter vector pHGEI01, and verified by sequencing. The resultant vector in E. coli WM3064 was then transferred by conjugation into relevant S. oneidensis strains, in which it integrated into the chromosome, and the antibiotic marker was removed subsequently (32). Cells of the exponential phase under test conditions were harvested by centrifugation, washed with phosphate-buffered saline (PBS, pH 7.0), and lysed with the lysis buffer (0.25 M Tris/HCl [pH 7.5], 0.5% Triton X-100). We collected the resulting soluble protein after centrifugation and used it for enzyme assay by adding the aliquot of the o-nitrophenyl-β-d-galactopyranoside (ONPG) (4 mg/ml). β-Galactosidase activity was determined by monitoring color development at 420 nm using a Synergy 2 Pro200 multi-detection microplate reader (Tecan), and results were presented as Miller units.
Microscopic analysis.
Motic BA410E phase-contrast microscope was used to visualize the morphological changes of S. oneidensis cells. Cells of the exponential phase were fixed on a slice of LB agar and visualized in a time course manner. Micrographs were captured with a Moticam ProS5 Lite camera and Motic images plus 3.0 software.
Western blotting.
Rabbit polyclonal antibodies against S. oneidensis LptD, which were prepared using a synthesized fragment (amino acids [aa] 40 to 198) as the antigen in accordance with standard protocols provided by the manufacturer (GenScript, Nanjing, China), and antibodies against σE prepared previously (14) were used for immunoblotting analysis. Sample preparations, including cell cultivation and subcellular fractionation, were carried out as described before (14, 63). Throughout this study, the total protein concentration of the cell lysates was determined by the bicinchoninic acid assay (Pierce Chemical). The resulting samples for defection of LptD and σE were subjected to electrophoresis on 6% and 15% SDS polyacrylamide gels (PAGE), respectively. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes for 1 h at 60 V using a Criterion blotter (Bio-Rad). The blotting membrane was probed with specific antibodies, followed by a 1:10,000 dilution of goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate. The alkaline phosphatase was detected using a chemiluminescence Western blotting kit (Roche Diagnostics) in accordance with the manufacturer’s instructions. Images were visualized with Clinx Imaging System (Clinx, Shanghai, China).
Other analyses.
Experimental values were subjected to statistical analyses and presented as means ± standard error of the mean (SEM). Student’s t test was performed for pairwise comparisons of groups.
ACKNOWLEDGEMENTS
This research was supported by National Natural Science Foundation of China (31930003, 41976087) and by Ten Thousand Talent Program of China.
Footnotes
Supplemental material is available online only.
Contributor Information
Haichun Gao, Email: haichung@zju.edu.cn.
Jeffrey A. Gralnick, University of Minnesota
REFERENCES
- 1.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowicz M, Silhavy TJ. 2017. Envelope stress responses: an interconnected safety net. Trends Biochem Sci 42:232–242. doi: 10.1016/j.tibs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Las Peñas A, Connolly L, Gross CA. 1997. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol Microbiol 24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AM, Silhavy TJ. 2019. Envelope stress responses: balancing damage repair and toxicity. Nat Rev Microbiol 17:417–428. doi: 10.1038/s41579-019-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barchinger SE, Ades SE. 2013. Regulated proteolysis: control of the Escherichia coli σ(E)-dependent cell envelope stress response. Subcell Biochem 66:129–160. doi: 10.1007/978-94-007-5940-4_6. [DOI] [PubMed] [Google Scholar]
- 7.Lima S, Guo MS, Chaba R, Gross CA, Sauer RT. 2013. Dual molecular signals mediate the bacterial response to outer-membrane stress. Science 340:837–841. doi: 10.1126/science.1235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majdalani N, Gottesman S. 2005. The RCS phosphorelay: a complex signal transduction system. Annu Rev Microbiol 59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 9.Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JL, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 10.Myers CR, Nealson KH. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 11.Hau HH, Gralnick JA. 2007. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 12.Kostka JE, Nealson KH. 1995. Dissolution and reduction of magnetite by bacteria. Environ Sci Technol 29:2535–2540. doi: 10.1021/es00010a012. [DOI] [PubMed] [Google Scholar]
- 13.Liang H, Mao Y, Sun Y, Gao H. 2019. Transcriptional regulator ArcA mediates expression of oligopeptide transport systems both directly and indirectly in Shewanella oneidensis. Sci Rep 9:13839. doi: 10.1038/s41598-019-50201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang H, Zhang Y, Wang S, Gao H. 2021. Mutual interplay between ArcA and σ(E) orchestrates envelope stress response in Shewanella oneidensis. Environ Microbiol 23:652–668. doi: 10.1111/1462-2920.15060. [DOI] [PubMed] [Google Scholar]
- 15.Park DM, Akhtar MS, Ansari AZ, Landick R, Kiley PJ. 2013. The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genet 9:e1003839. doi: 10.1371/journal.pgen.1003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iuchi S, Lin EC. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA 85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234. doi: 10.1016/s0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 18.Gralnick JA, Brown CT, Newman DK. 2005. Anaerobic regulation by an atypical Arc system in Shewanella oneidensis. Mol Microbiol 56:1347–1357. doi: 10.1111/j.1365-2958.2005.04628.x. [DOI] [PubMed] [Google Scholar]
- 19.Lassak J, Henche A-L, Binnenkade L, Thormann KM. 2010. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl Environ Microbiol 76:3263–3274. doi: 10.1128/AEM.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shroff NP, Charania MA, Saffarini DA. 2010. ArcB1, a homolog of Escherichia coli ArcB, regulates dimethyl sulfoxide reduction in Shewanella oneidensis MR-1. J Bacteriol 192:3227–3230. doi: 10.1128/JB.01695-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao H, Wang X, Yang Z, Palzkill T, Zhou J. 2008. Probing regulon of ArcA in Shewanella oneidensis MR-1 by integrated genomic analyses. BMC Genomics 9:42. doi: 10.1186/1471-2164-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, De Wulf P. 2004. Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J Biol Chem 279:12588–12597. doi: 10.1074/jbc.M313454200. [DOI] [PubMed] [Google Scholar]
- 23.Salmon KA, Hung S-p, Steffen NR, Krupp R, Baldi P, Hatfield GW, Gunsalus RP. 2005. Global gene expression profiling in Escherichia coli K12: effect of oxygen availability and ArcA. J Biol Chem 280:15084–15096. doi: 10.1074/jbc.M414030200. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Gao H, Shen Y, Weinstock GM, Zhou J, Palzkill T. 2008. A high-throughput percentage-of-binding strategy to measure binding energies in DNA-protein interactions: application to genome-scale site discovery. Nucleic Acids Res 36:4863–4871. doi: 10.1093/nar/gkn477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J, Wei B, Lipton MS, Gao H. 2012. Impact of ArcA loss in Shewanella oneidensis revealed by comparative proteomics under aerobic and anaerobic conditions. Proteomics 12:1957–1969. doi: 10.1002/pmic.201100651. [DOI] [PubMed] [Google Scholar]
- 26.Wan F, Mao Y, Dong Y, Ju L, Wu G, Gao H. 2015. Impaired cell envelope resulting from arcA mutation largely accounts for enhanced sensitivity to hydrogen peroxide in Shewanella oneidensis. Sci Rep 5:10228. doi: 10.1038/srep10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Q, Dong Y, Chen H, Gao H. 2013. Mislocalization of Rieske protein PetA predominantly accounts for the aerobic growth defect of tat mutants in Shewanella oneidensis. PLoS One 8:e62064. doi: 10.1371/journal.pone.0062064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng Q, Liang H, Gao H. 2018. Roles of multiple KASIII homologues of Shewanella oneidensis in initiation of fatty acid synthesis and in cerulenin resistance. Biochim Biophys Acta Mol Cell Biol Lipids 1863:1153–1163. doi: 10.1016/j.bbalip.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Gao H, Wang X, Yang ZK, Chen J, Liang Y, Chen H, Palzkill T, Zhou J. 2010. Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS One 5:e15295. doi: 10.1371/journal.pone.0015295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin J, Gao H. 2011. Stress responses of shewanella. Int J Microbiol 2011:863623. doi: 10.1155/2011/863623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong SM, Mekalanos JJ. 2000. Genetic footprinting with mariner-based transposition in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 97:10191–10196. doi: 10.1073/pnas.97.18.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu H, Chen H, Wang J, Zhou G, Zhang H, Zhang L, Gao H. 2013. Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis. Environ Microbiol 15:2198–2212. doi: 10.1111/1462-2920.12091. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz N, Gronenberg LS, Kahne D, Silhavy TJ. 2008. Identification of two inner-membrane proteins required for the transport of lipopolysaccharide to the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 105:5537–5542. doi: 10.1073/pnas.0801196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu H, Jin M, Ju L, Mao Y, Gao H. 2014. Evidence for function overlapping of CymA and the cytochrome bc1 complex in the Shewanella oneidensis nitrate and nitrite respiration. Environ Microbiol 16:3181–3195. doi: 10.1111/1462-2920.12457. [DOI] [PubMed] [Google Scholar]
- 35.Hicks G, Jia Z. 2018. Structural basis for the lipopolysaccharide export activity of the bacterial lipopolysaccharide transport system. Int J Mol Sci 19:2680. doi: 10.3390/ijms19092680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz N, Kahne D, Silhavy TJ. 2009. Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat Rev Microbiol 7:677–683. doi: 10.1038/nrmicro2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperandeo P, Martorana AM, Polissi A. 2017. The lipopolysaccharide transport (Lpt) machinery: a nonconventional transporter for lipopolysaccharide assembly at the outer membrane of Gram-negative bacteria. J Biol Chem 292:17981–17990. doi: 10.1074/jbc.R117.802512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong H, Tang X, Zhang Z, Dong C. 2017. Structural insight into lipopolysaccharide transport from the Gram-negative bacterial inner membrane to the outer membrane. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1461–1467. doi: 10.1016/j.bbalip.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Orlando BJ, Liao M. 2019. Structural basis of lipopolysaccharide extraction by the LptB(2)FGC complex. Nature 567:486–490. doi: 10.1038/s41586-019-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens TW, Taylor RJ, Pahil KS, Bertani BR, Ruiz N, Kruse AC, Kahne D. 2019. Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 567:550–553. doi: 10.1038/s41586-019-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villa R, Martorana AM, Okuda S, Gourlay LJ, Nardini M, Sperandeo P, Dehò G, Bolognesi M, Kahne D, Polissi A. 2013. The Escherichia coli Lpt transenvelope protein complex for lipopolysaccharide export is assembled via conserved structurally homologous domains. J Bacteriol 195:1100–1108. doi: 10.1128/JB.02057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuda S, Freinkman E, Kahne D. 2012. Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli. Science 338:1214–1217. doi: 10.1126/science.1228984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran AX, Trent MS, Whitfield C. 2008. The LptA protein of Escherichia coli is a periplasmic lipid A-binding protein involved in the lipopolysaccharide export pathway. J Biol Chem 283:20342–20349. doi: 10.1074/jbc.M802503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botos I, Majdalani N, Mayclin SJ, McCarthy JG, Lundquist K, Wojtowicz D, Barnard TJ, Gumbart JC, Buchanan SK. 2016. Structural and functional characterization of the LPS transporter LptDE from Gram-negative pathogens. Structure 24:965–976. doi: 10.1016/j.str.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malojčić G, Andres D, Grabowicz M, George AH, Ruiz N, Silhavy TJ, Kahne D. 2014. LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc Natl Acad Sci USA 111:9467–9472. doi: 10.1073/pnas.1402746111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braun M, Silhavy TJ. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol 45:1289–1302. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaito C, Yoshikai H, Wakamatsu A, Miyashita A, Matsumoto Y, Fujiyuki T, Kato M, Ogura Y, Hayashi T, Isogai T, Sekimizu K. 2020. Non-pathogenic Escherichia coli acquires virulence by mutating a growth-essential LPS transporter. PLoS Pathog 16:e1008469. doi: 10.1371/journal.ppat.1008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperandeo P, Lau FK, Carpentieri A, De Castro C, Molinaro A, Deho G, Silhavy TJ, Polissi A. 2008. Functional analysis of the protein machinery required for transport of lipopolysaccharide to the outer membrane of Escherichia coli. J Bacteriol 190:4460–4469. doi: 10.1128/JB.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu T, McCandlish AC, Gronenberg LS, Chng SS, Silhavy TJ, Kahne D. 2006. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 103:11754–11759. doi: 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chimalakonda G, Ruiz N, Chng S-S, Garner RA, Kahne D, Silhavy TJ. 2011. Lipoprotein LptE is required for the assembly of LptD by the beta-barrel assembly machine in the outer membrane of Escherichia coli. Proc Natl Acad Sci USA 108:2492–2497. doi: 10.1073/pnas.1019089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedet M, Falchi FA, Puccio S, Di Benedetto C, Peano C, Polissi A, Dehò G. 2016. The lack of the essential LptC protein in the trans-envelope lipopolysaccharide transport machine is circumvented by suppressor mutations in LptF, an inner membrane component of the Escherichia coli transporter. PLoS One 11:e0161354. doi: 10.1371/journal.pone.0161354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martorana AM, Benedet M, Maccagni EA, Sperandeo P, Villa R, Dehò G, Polissi A. 2016. Functional interaction between the cytoplasmic ABC protein LptB and the inner membrane LptC protein, components of the lipopolysaccharide transport machinery in Escherichia coli. J Bacteriol 198:2192–2203. doi: 10.1128/JB.00329-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson BW, Owens TW, Orabella MJ, Davis RM, May JM, Trauger SA, Kahne D, Ruiz N. 2016. Identification of residues in the lipopolysaccharide ABC transporter that coordinate ATPase activity with extractor function. mBio 7:e01729-16. doi: 10.1128/mBio.01729-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dartigalongue C, Missiakas D, Raina S. 2001. Characterization of the Escherichia coli σE regulon. J Biol Chem 276:20866–20875. doi: 10.1074/jbc.M100464200. [DOI] [PubMed] [Google Scholar]
- 55.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol 4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sperandeo P, Cescutti R, Villa R, Di Benedetto C, Candia D, Deho G, Polissi A. 2007. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J Bacteriol 189:244–253. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva-Valenzuela CA, Velásquez F, Peñailillo J, Garcias-Papayani H, Fernández P, Tobar P, Contreras I, Santiviago CA, Álvarez SA. 2016. O-antigen chain-length distribution in Salmonella enterica serovar Enteritidis is regulated by oxygen availability. Biochem Biophys Res Commun 477:563–567. doi: 10.1016/j.bbrc.2016.06.074. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez PA, Velasquez F, Garcias-Papayani H, Amaya FA, Ortega J, Gomez S, Santiviago CA, Alvarez SA. 2018. Fnr and ArcA regulate lipid A hydroxylation in Salmonella Enteritidis by controlling lpxO expression in response to oxygen availability. Front Microbiol 9:1220. doi: 10.3389/fmicb.2018.01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pandin C, Caroff M, Condemine G. 2016. Antimicrobial peptide resistance genes in the plant pathogen Dickeya dadantii. Appl Environ Microbiol 82:6423–6430. doi: 10.1128/AEM.01757-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lassak J, Bubendorfer S, Thormann KM. 2013. Domain analysis of ArcS, the hybrid sensor kinase of the Shewanella oneidensis MR-1 Arc two-component system, reveals functional differentiation of its two receiver domains. J Bacteriol 195:482–492. doi: 10.1128/JB.01715-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin M, Jiang Y, Sun L, Yin J, Fu H, Wu G, Gao H. 2013. Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS One 8:e75610. doi: 10.1371/journal.pone.0075610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das S, Noe JC, Paik S, Kitten T. 2005. An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. J Microbiol Methods 63:89–94. doi: 10.1016/j.mimet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 63.Dong Y, Wang J, Fu H, Zhou G, Shi M, Gao H. 2012. A Crp-dependent two-component system regulates nitrate and nitrite respiration in Shewanella oneidensis. PLoS One 7:e51643. doi: 10.1371/journal.pone.0051643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM00690-21_Supp_1_seq1.pdf, PDF file, 0.3 MB (296.6KB, pdf)