ABSTRACT
Extended-spectrum-beta-lactamase (ESBL)-producing Enterobacterales continue to pose a major threat to human health worldwide. Given the limited therapeutic options available to treat infections caused by these pathogens, identifying additional effective antimicrobials or revisiting existing drugs is important. Ceftriaxone-resistant Escherichia coli and Klebsiella pneumoniae containing CTX-M-type ESBLs or AmpC, in addition to narrow-spectrum OXA and SHV enzymes, were selected from blood culture isolates obtained from the MERINO trial. Isolates had previously undergone whole-genome sequencing (WGS) to identify antimicrobial resistance genes. Cefotetan MICs were determined by broth microdilution (BMD) testing with a concentration range of 0.125 to 64 mg/liter; CLSI breakpoints were used for susceptibility interpretation. BMD was performed using an automated digital antibiotic dispensing platform (Tecan D300e). One hundred ten E. coli and 40 K. pneumoniae isolates were used. CTX-M-15 and CTX-M-27 were the most common beta-lactamases present; only 7 isolates had coexistent ampC genes. Overall, 98.7% of isolates were susceptible, with MIC50s and MIC90s of 0.25 mg/liter and 2 mg/liter (range, ≤0.125 to 64 mg/liter), respectively. MICs appeared higher among isolates with ampC genes present, with an MIC50 of 16 mg/liter, than among those containing CTX-M-15, which had an MIC50 of only 0.5 mg/liter. Isolates with an ampC gene exhibited an overall susceptibility of 85%. Presence of a narrow-spectrum OXA beta-lactamase did not appear to alter the cefotetan MIC distribution. Cefotetan demonstrated favorable in vitro efficacy against ESBL-producing E. coli and K. pneumoniae bloodstream isolates.
IMPORTANCE Carbapenem antibiotics remain the treatment of choice for severe infection due to ESBL- and AmpC-producing Enterobacterales. The use of carbapenems is a major driver of the emergence of carbapenem-resistant Gram-negative bacilli, which are often resistant to most available antimicrobials. Cefotetan is a cephamycin antibiotic developed in the 1980s that demonstrates enhanced resistance to beta-lactamases and has a broad spectrum of activity against Gram-negative bacteria. Cefotetan holds potential to be a carbapenem-sparing treatment option. Data on the in vitro activity of cefotetan against ESBL-producing Enterobacterales remain scarce. Our study assessed the in vitro activity of cefotetan against ceftriaxone-nonsusceptible blood culture isolates obtained from patients enrolled in the MERINO trial.
KEYWORDS: extended-spectrum beta-lactamase, ampC beta-lactamase, antimicrobial susceptibility testing, AmpC, cefotetan, Enterobacterales
INTRODUCTION
Among Enterobacterales, resistance to third-generation cephalosporins mediated by extended-spectrum beta-lactamase (ESBL) and AmpC beta-lactamase is a major contemporary threat to the health and well-being of individuals globally (1, 2). Approximately 200,000 infections and 9,000 deaths due to ESBL-producing Enterobacterales infection in U.S. hospitals occur annually (3). Treatment options for ESBL-producing Gram-negative pathogens are limited compared to those for non-ESBL producers. Indeed, coexisting non-beta-lactamase resistance genes are often identified in these isolates (e.g., gyrA and parC mutations mediating quinolone resistance in Escherichia coli ST131) (4). Carbapenems have been regarded as the treatment of choice for infection due to ESBL-producing Enterobacterales (5). The MERINO trial failed to demonstrate noninferiority, with respect to 30-day all-cause mortality, of piperacillin-tazobactam compared to meropenem for treatment of bloodstream infection due to ceftriaxone-resistant E. coli and Klebsiella pneumoniae (6). Rising use of carbapenems, paired with a rising incidence of carbapenem-resistant organisms globally, has prompted a search for suitable therapeutic alternatives to treat these infections (7).
Cefotetan is a cephamycin antibiotic developed in the 1980s (8). Its unique structure confers enhanced resistance to beta-lactamases and a broad spectrum of activity against Gram-negative bacteria. It is administered via the intravenous and intramuscular routes and has been approved for use in urinary tract, lower respiratory tract, skin and soft tissue, gynecologic, intra-abdominal, and bone and joint infections. Early in vitro studies indicated that cefotetan achieved an MIC90 of 4 mg/liter against enterobacteria (9). Moreover, a randomized clinical trial of cefotetan versus cefoxitin or moxalactam for treatment of intra-abdominal infection demonstrated superior infection clearance and bacteriologic response with cefotetan (10). Cephamycins, including cefotetan, are unable to be efficiently hydrolyzed by ESBLs and may prove to be a therapeutic alternative to carbapenems. Data on the in vitro activity of cefotetan against ESBL-producing Enterobacterales remain scarce (11). We aimed to assess the in vitro activity of cefotetan against ceftriaxone-nonsusceptible blood culture isolates obtained from patients enrolled in the MERINO trial (6).
RESULTS
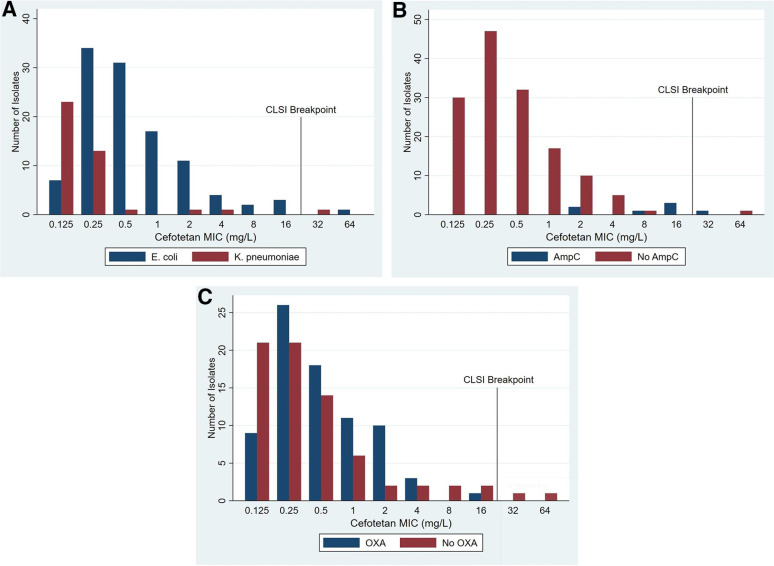
One hundred fifty isolates (110 E. coli and 40 K. pneumoniae) from the MERINO trial were collected, and their cefotetan MICs were determined by broth microdilution (BMD). Overall, 98.7% were susceptible according to the CLSI cefotetan susceptible breakpoint, with MIC50s and MIC90s of 0.25 mg/liter and 2 mg/liter (range, ≤0.125 to 64 mg/liter), respectively. Table 1 presents the cefotetan MIC distribution and percent susceptible according to species and beta-lactamase type. The MIC distributions of E. coli and K. pneumoniae isolates appeared similar, each registering one resistant isolate (64 mg/liter and 32 mg/liter, respectively). The resistant E. coli isolate had blaCTX-M-27 identified, and the intermediate K. pneumoniae isolate had blaSHV-106 and blaDHA-1 present. Overall, MICs appeared higher among isolates with ampC genes present, with an MIC50 of 16 mg/liter, than among those containing CTX-M-15, which had an MIC50 of only 0.5 mg/liter. Indeed, isolates with an ampC gene exhibited an overall susceptibility of 85%. Presence of an OXA beta-lactamase did not appear to alter the cefotetan MIC distribution (Fig. 1). The MICs for all the trays testing ATCC strains fell within acceptable ranges. Purity and colony count checks demonstrated pure growth and colony counts ranging from 1 to 9 colonies.
TABLE 1.
Cefotetan MIC frequency distribution against ESBL-producing E. coli and K. pneumoniae isolates according to species and beta-lactamase type
| Organism | No. of isolates with MIC (mg/liter) |
% susceptible (CLSI breakpoint) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | Total | ||
| All | 30 | 47 | 33 | 16 | 12 | 5 | 3 | 4 | 1 | 1 | 150 | 98.7 |
| E. coli | 7 | 34 | 32 | 16 | 11 | 4 | 3 | 4 | 1 | 110 | 99.1 | |
| K. pneumoniae | 23 | 13 | 1 | 1 | 1 | 1 | 40 | 97.5 | ||||
| ESBL only (n = 67) | ||||||||||||
| CTX-M-3 | 4 | 4 | 100 | |||||||||
| CTX-M-14 | 3 | 2 | 5 | 100 | ||||||||
| CTX-M-15 | 14 | 5 | 4 | 3 | 1 | 2 | 29 | 100 | ||||
| CTX-M-24 | 1 | 1 | 100 | |||||||||
| CTX-M-27 | 3 | 7 | 7 | 2 | 1 | 20 | 95 | |||||
| CTX-M-55 | 1 | 2 | 2 | 1 | 6 | 100 | ||||||
| CTX-M-134 | 1 | 1 | 100 | |||||||||
| CTX-M-174 | 1 | 1 | 100 | |||||||||
| ESBL + OXA (n = 74) | ||||||||||||
| CTX-M-15 + OXA-1 | 8 | 24 | 17 | 10 | 9 | 3 | 71 | 100 | ||||
| SHV-12 + OXA-9 | 1 | 1 | 100 | |||||||||
| CTX-M-15 + OXA-10 | 1 | 1 | 2 | 100 | ||||||||
| ESBL + ampC (n = 7) | ||||||||||||
| CTX-M-15 + CMY-2 | 1 | 1 | 100 | |||||||||
| CTX-M-55 + CMY-2 | 1 | 1 | 2 | 100 | ||||||||
| CTX-M-15 + CMY-138 | 1 | 1 | 2 | 100 | ||||||||
| CTX-M-14 + DHA-1 | 1 | 1 | 100 | |||||||||
| SHV-106 + DHA-1 | 1 | 1 | 0 | |||||||||
| ESBL + ampC + OXA (n = 2) | ||||||||||||
| CTX-M-15 + CMY-2 + OXA-1 | 1 | 1 | 100 | |||||||||
| CTX-M-15 + CMY-138 + OXA-1 | 1 | 1 | 100 | |||||||||
FIG 1.
Cefotetan MICs determined by broth microdilution (BMD) of 150 ESBL-producing E. coli and K. pneumoniae isolates by (A) species, (B) AmpC beta-lactamase, and (C) narrow-spectrum OXA beta-lactamase.
DISCUSSION
We demonstrated that almost all ESBL-producing E. coli and K. pneumoniae isolates from our study were susceptible to cefotetan in vitro. Unsurprisingly, ampC-carrying isolates showed higher MICs overall; in vitro resistance to cefoxitin is used as a phenotypic marker to infer the presence of ampC, and there exists a structural similarity between cefotetan and cefoxitin. Among AmpC producers, cefoxitin MICs are generally higher than those of cefotetan (12). Isolates harboring the DHA-1 enzyme appeared to have higher cefotetan MICs than those harboring CMY enzymes, although isolate numbers were small. It is unclear whether there is a biological or clinically significant difference in relation to cephalosporinase activity between the two enzyme types.
Isolates producing common CTX-M and narrow-spectrum OXA-type beta-lactamases appeared highly susceptible to cefotetan. Although not a new antimicrobial class, cephamycins have demonstrated promising in vitro potency and clinical efficacy against invasive isolates that are resistant to third-generation cephalosporins (13–15). Previous concerns have been put forward over the use of cephamycins for infections with ESBL-producing organisms and development of outer membrane protein (OMP) mutations and/or plasmid-encoding AmpC enzymes during exposure (11). The true significance of this finding from case reports remains uncertain. Cefotetan may be a suitable carbapenem-sparing treatment option for multidrug-resistant Enterobacterales, especially those not harboring an ampC enzyme. This agent could also be formulated with an inhibitor to mitigate the effect of ampC. Cefotetan achieves high plasma levels after intravenous and intramuscular injection and is typically administered twice daily as a 30-min infusion. It achieved a mean plasma concentration of 158 mg/liter at 30 min after a 1-g dose given intravenously to healthy adults. Cefotetan has shown very little in vitro activity against Pseudomonas and Acinetobacter species (MIC90s, >32 and >32 to 256 mg/liter, respectively) and wide variation in susceptibility against Enterobacter and Serratia species (MIC90s, 2 to 256 mg/liter and 0.5 to 64 mg/liter, respectively) (8). The lack of activity seen against non-lactose-fermenting Gram-negative organisms may explain why it has not been widely adopted for treatment of urinary tract infection. In the era of emerging multidrug-resistant bacteria, use of pathogen-directed therapies (as opposed to a “cure-all” approach with a single agent) based on species or resistance type may be a useful strategy.
There are a few limitations to this study. Selection of bacterial isolates was restricted to include a subset of nonrandomly selected representative isolates obtained from the MERINO trial. These isolates may not be truly representative of all the resistance mechanisms seen in third-generation-cephalosporin-resistant E. coli and K. pneumoniae globally. Antimicrobial susceptibility testing was performed using an automated digital antibiotic dispensing platform (Tecan D300e; Tecan Trading AG, Switzerland). In precision studies assessing the performance of this platform in Enterobacteriaceae, essential and categorial agreement levels were 96.8% and 98.3%, respectively (16). This finding supports the accuracy of this approach for use in BMD testing. The clinical efficacy of cefotetan for infection due to ESBL producers remains uncertain but warrants further study.
Conclusion.
Cefotetan demonstrated favorable in vitro efficacy against ESBL-producing E. coli and K. pneumoniae bloodstream isolates with MIC50s and MIC90s of 0.25 mg/liter and 2 mg/liter (range, ≤0.125 to 64 mg/liter), respectively. Higher MICs were seen in isolates coharboring an ampC beta-lactamase. Cefotetan may have a place for therapeutic use as a carbapenem-sparing therapy for infection due to these organisms.
MATERIALS AND METHODS
Bacterial isolates.
The MERINO trial recruited patients with bloodstream infections due to third-generation-cephalosporin-nonsusceptible E. coli and K. pneumoniae in nine countries from February 2014 to July 2017 (6). All blood culture isolates from enrolled patients were stored and had previously undergone whole-genome sequencing (WGS) to detect antimicrobial resistance genes. A subset of isolates that had at least one ESBL gene identified were chosen to be included in this study. Ultimately, isolates containing different combinations of CTX-M ESBLs, narrow-spectrum OXA and SHV enzymes, and AmpC beta-lactamases were used. Each isolate was subjected to broth microdilution (BMD) testing for cefotetan MIC determination.
Antibiotic preparation.
Cefotetan powder (Glentham Life Sciences, GA5476) was dissolved in DMSO (Thermo Fisher, D/4121/PB08) at a concentration of 10,000 mg/liter. This stock solution was loaded directly to the Tecan D300e (Tecan Trading AG, Switzerland) T8 print cartridge.
Broth microdilution tray preparation.
The concentration range (0.125 to 64 mg/liter) were chosen to include both recommended reference strains, CLSI breakpoints (susceptible, ≤16 mg/liter; intermediate, 32 mg/liter; resistant, ≥64 mg/liter) (see Table 2A in reference 17), and attainable therapeutic concentration (128 mg/liter). The tray layout was designed in Tecan D300e Control software. Prepared antibiotic was dispensed into labeled 96-well trays (Thermo Scientific, 262162) which were inoculated within 20 min of printing.
Quality control.
Two ATCC strains were used to check the performance of each batch of trays: Escherichia coli ATCC 25922 (target MIC, 0.125 mg/liter) and Staphylococcus aureus ATCC 29213 (target MIC, 8 mg/liter) (see Table 5A-1 in reference 17). A separate tray was prepared to check E. coli ATCC 25922 at lower concentrations, ranging from 0.004 to 2 mg/liter.
Isolate preparation.
Test and reference isolates were stored in brain heart infusion (BHI) broth (BD, Bacto 237500) containing 30% glycerol (Chem-Supply, GA010) at −80°C. A scraping from the frozen vials was streaked onto 5% Columbia horse blood agar (HBA) (Edwards, MM1085) and incubated at 37°C in ambient atmosphere for 18 to 24 h. A single colony of each was subcultured to fresh HBA and incubated under the same conditions. Two or three colonies of each isolate were collected using a sterile rayon swab and resuspended in sterile normal saline (0.9% NaCl; Chem-Supply, US008779). Turbidity was adjusted to a 0.5 McFarland standard as read using DensiCHEK Plus (bioMérieux, France). Five microliters of inoculated saline was added to 1 ml of cation-adjusted Mueller-Hinton broth (CAMHB) (BD, BBL 211322) and vortexed, to achieve an approximate concentration of 5 × 105 CFU/ml. Fifty microliters of inoculated broth was dispensed into each into each well of a single row on the BMD tray using an electronic repeat-dispense pipette. Purity and colony count checks were performed by collecting a 1-μl loop of broth from the positive-control well for each isolate and streaking onto half of an HBA plate. A second 1-μl sample from the same well was diluted in 100 μl of sterile saline, and 1 μl was streaked on the other half of the plate. Plates showing pure growth on the undiluted streak and 1 to 10 colonies on the diluted streak passed purity and colony count checks.
ACKNOWLEDGMENTS
Sandoz Australia (Novartis) provided funding for this project.
D.L.P. has received funding from AstraZeneca, Pfizer, Shionogi, Leo Pharmaceuticals, Bayer, GlaxoSmithKline (GSK), Cubist, Entasis, Sumitomo, QPex, Venatorx, bioMérieux and Accelerate; reports board membership from Entasis, Qpex, Merck, Shionogi, Achaogen, AstraZeneca, Leo Pharmaceuticals, Bayer, GSK, Cubist, Venatorx, and Accelerate, reports grants/grants pending from Shionogi and Merck, and has received payment for lectures, including service on speaker’s bureaus from Pfizer and Merck, outside the submitted work. P.N.A.H. has received research grants from MSD, Sandoz, and Shionogi, as well as speaker’s fees from Pfizer, and has served on an advisory board for Sandoz outside the submitted work.
We declare no conflicts of interest.
Contributor Information
Adam G. Stewart, Email: adam.stewart@uq.edu.au.
S. Wesley Long, Houston Methodist Hospital.
REFERENCES
- 1.Bush K. 2018. Past and present perspectives on beta-lactamases. Antimicrob Agents Chemother 62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belley A, Morrissey I, Hawser S, Kothari N, Knechtle P. 2021. Third-generation cephalosporin resistance in clinical isolates of Enterobacterales collected between 2016–2018 from USA and Europe: genotypic analysis of beta-lactamases and comparative in vitro activity of cefepime/enmetazobactam. J Glob Antimicrob Resist 25:93–101. doi: 10.1016/j.jgar.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States: 2019. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 4.Matsumura Y, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S, Kyoto-Shiga Clinical Microbiology Study Group. 2013. Association of fluoroquinolone resistance, virulence genes, and IncF plasmids with extended-spectrum-beta-lactamase-producing Escherichia coli sequence type 131 (ST131) and ST405 clonal groups. Antimicrob Agents Chemother 57:4736–4742. doi: 10.1128/AAC.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 6.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, Merino Trial Investigators, the Australasian Society for Infectious Disease Clinical Research Network. 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elshamy AA, Aboshanab KM. 2020. A review on bacterial resistance to carbapenems: epidemiology, detection and treatment options. Future Sci OA 6:FSO438. doi: 10.2144/fsoa-2019-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward A, Richards DM. 1985. Cefotetan. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 30:382–426. doi: 10.2165/00003495-198530050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Phillips I, King A, Shannon K, Warren C. 1983. Cefotetan: in-vitro antibacterial activity and susceptibility to beta-lactamases. J Antimicrob Chemother 11(Suppl):1–9. doi: 10.1093/jac/11.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SE, Boswick JA, Jr, Duma RJ, Echols RM, Jemsek JG, Lerner R, Lewis RT, Najem AZ, Press RA, Rittenbury MS. 1988. Cephalosporin therapy in intraabdominal infections. A multicenter randomized, comparative study of cefotetan, moxalactam, and cefoxitin. Am J Surg 155:61–66. doi: 10.1016/S0002-9610(88)80215-0. [DOI] [PubMed] [Google Scholar]
- 11.Tamma PD, Rodriguez-Bano J. 2017. The use of noncarbapenem beta-lactams for the treatment of extended-spectrum beta-lactamase infections. Clin Infect Dis 64:972–980. doi: 10.1093/cid/cix034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippon A, Arlet G, Jacoby GA. 2002. Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother 46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guet-Revillet H, Emirian A, Groh M, Nebbad-Lechani B, Weiss E, Join-Lambert O, Bille E, Jullien V, Zahar JR. 2014. Pharmacological study of cefoxitin as an alternative antibiotic therapy to carbapenems in treatment of urinary tract infections due to extended-spectrum-beta-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 58:4899–4901. doi: 10.1128/AAC.02509-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y, Yamamoto M, Nagao M, Komori T, Fujita N, Hayashi A, Shimizu T, Watanabe H, Doi S, Tanaka M, Takakura S, Ichiyama S. 2015. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-beta-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother 59:5107–5113. doi: 10.1128/AAC.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S. 2016. In vitro activities and detection performances of cefmetazole and flomoxef for extended-spectrum beta-lactamase and plasmid-mediated AmpC beta-lactamase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 84:322–327. doi: 10.1016/j.diagmicrobio.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Smith KP, Kirby JE. 2016. Verification of an automated, digital dispensing platform for at-will broth microdilution-based antimicrobial susceptibility testing. J Clin Microbiol 54:2288–2293. doi: 10.1128/JCM.00932-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2021. M100: performance standards for antimicrobial susceptibility testing, 31st ed. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]