ABSTRACT
Dysfunctional accessory gene regulator (agr) is associated with unfavorable outcomes in invasive methicillin-resistant Staphylococcus aureus infections. However, it is unknown whether this association persists in methicillin-susceptible Staphylococcus aureus bacteremia (MSSA-B). This study evaluated the association between agr dysfunction and mortality in patients with MSSA-B. This retrospective cohort study included MSSA-B patients (≥15 years) enrolled from June 2014 to June 2019 and retrospectively collected their demographic and clinical information. Stored causative strains were measured for agr functionality by δ-hemolysin production assays. Among 244 MSSA-B patients, 91 (37.3%) and 153 (62.7%) had dysfunctional and functional agr MSSA-B, respectively. Ninety-day mortality occurred in 18.7% and 17.6% dysfunctional and functional groups, respectively (P = 0.97). Kaplan-Meier analysis showed that mortality due to dysfunctional agr MSSA-B was not significantly higher (P = 0.82). Age, sites, the severity of infection, and comorbidity adjusted hazard ratio (aHR) of the dysfunctional group for 90-day mortality was 1.303 (95% confidence interval [CI], 0.698 to 2.436, P = 0.41). Mortality due to MSSA-B with sequential organ failure assessment (SOFA) scores of 2 to 5 was significantly higher in the dysfunctional group (P = 0.03), and the dysfunctional agr aHR for 90-day mortality was 3.260 (95% CI, 1.050 to 10.118, P = 0.04). The agr dysfunction of causative organisms can have a significant effect on the outcomes of MSSA-B in patients with moderate severity (SOFA scores 2 to 5).
IMPORTANCE Few studies have examined the association between methicillin-susceptible Staphylococcus aureus (MSSA) infection and accessory gene regulator (agr) functionality. We evaluated the association between agr dysfunction and mortality in patients with MSSA bacteremia. Dysfunctional agr is associated with lower survival in MSSA bacteremia patients with moderately severe sequential organ failure assessment (SOFA) scores of 2 to 5. We found that the agr functionality of causative organisms may have an effect on patients’ outcomes in MSSA like in methicillin-resistant S. aureus.
KEYWORDS: MSSA, Staphylococcus aureus, agr, bacteremia, quorum sensing
INTRODUCTION
Staphylococcus aureus is a common and important cause of hospital-acquired and community-acquired bloodstream infection with high morbidity and mortality, despite prompt control of the source and appropriate antimicrobial therapy (1–3). S. aureus produces potential virulence factors, such as toxins, degradative enzymes, antimicrobial resistance genes, and immune evasion mechanisms, which contribute to pathogenicity and the ability to colonize the host (3, 4). Quorum sensing, which is a population density-dependent and environment-dependent gene regulation, is an important mechanism for the control of these virulence factors (5). The accessory gene regulator (agr) locus of S. aureus is responsible for quorum sensing and coordinates the expression of various virulence factors (6). The agr quorum-sensing system leads to the spread of infection by dispersion of the biofilm and increased production of exoproteins and murein hydrolases at high cell density (7, 8). Loss of agr activity has been associated with enhanced biofilm formation and decreased autolysis rates, which can contribute to the persistence of infection and poor outcomes (4).
Many clinical studies have demonstrated that a considerable proportion of invasive methicillin-resistant S. aureus (MRSA) infection is caused by the strain with dysfunctional agr (9–11).
Dysfunctional agr in invasive MRSA infection was associated with overall unfavorable outcomes, such as persistent bacteremia and high in-hospital mortality rates (10).
Dysfunctional agr is not uncommon even in invasive methicillin-susceptible S. aureus (MSSA) infections. However, few studies have examined the association between the outcomes of invasive MSSA infection and agr functionality, and the results of such studies are inconclusive (10).
The objective of this study was to evaluate the association between agr dysfunction and mortality in patients with MSSA bacteremia (MSSA-B), adjusting for important confounding factors, such as comorbid conditions and severity of the illness.
RESULTS
A total of 244 patients with MSSA-B were enrolled during the study period. The overall median age of the enrolled patients was 66.5 (interquartile range [IQR], 56 to 74) years, and 68.8% of these patients were males. Among the MSSA-B patients, 63.9% had more than one comorbidity, and the median Charlson comorbidity weighted index (CCWI) score was 4 (IQR, 2 to 6). The prevalence of community-onset bacteremia was 60.7%, and the lung (17.6%) was the most common site of infection, followed by the skin and soft tissues (16.8%). The median sequential organ failure assessment (SOFA) score of the patients was 3 (IQR, 1 to 5), and 73.4% of the patients had a SOFA score of ≥2.
Among the 244 MSSA-B patients, 91 (37.3%) were infected by MSSA with dysfunctional agr (the dysfunctional agr group) and 153 (62.7%) were infected by MSSA with functional agr (the functional agr group; Table 1). There were no significant differences in demographics and clinical manifestations between the dysfunctional and functional agr groups, although the dysfunctional agr group tended to have an initial severity lower than that of the functional agr group (SOFA score 0 to 1 [33.0% versus 22.9%], 2 to 5 [47.3% versus 50.3%], ≥6 [19.8% versus 26.8%], P = 0.18). More than 98% of patients in both groups received appropriate empirical antibiotics, and there was no significant difference in the rate of use of definitive antibiotics between the groups (Table 1). There was no significant difference in the 7-day, 30-day, and 90-day mortality rates between the dysfunctional and functional agr groups (Table 1).
TABLE 1.
Comparison of demographic, clinical, and microbiological characteristics according to the functionality of agr locus of MSSA bacteremiaa
| Characteristic | Value for group |
P value | |
|---|---|---|---|
| Functional agr (n = 153) | Dysfunctional agr (n = 91) | ||
| Median age, yrs (IQR) | 65 (56–74) | 67.0 (56–74.5) | 0.447 |
| Older age (≥65 yrs) | 78 (51.0%) | 52 (57.1%) | 0.424 |
| Male | 106 (69.3%) | 62 (68.1%) | 0.964 |
| Community-onset | 92 (60.1%) | 56 (61.5%) | 0.934 |
| Comorbidity | |||
| Cardiovascular disease | 16 (10.5%) | 8 (8.8%) | 0.841 |
| Central nervous disease | 23 (15.0%) | 11 (12.1%) | 0.652 |
| Chronic lung disease | 2 (1.3%) | 1 (1.1%) | >0.999 |
| Chronic liver disease | 12 (7.8%) | 5 (5.5%) | 0.662 |
| Chronic kidney disease | 18 (11.8%) | 10 (11.0%) | >0.999 |
| Connective tissue disease | 3 (2.0%) | 2 (2.2%) | >0.999 |
| Solid organ malignancy | 22 (14.4%) | 14 (15.4%) | 0.978 |
| Hematologic malignancy | 17 (11.1%) | 5 (5.5%) | 0.211 |
| Diabetes mellitus | 42 (27.5%) | 25 (27.5%) | >0.999 |
| HIV infection | 1 (0.7%) | 0 (0.0%) | >0.999 |
| Median CCWI, score (IQR) | 4 (2–6) | 3 (2–5.5) | 0.883 |
| High CCWI (CCWI ≥ 3) | 102 (66.7%) | 61 (67.0%) | >0.999 |
| Site of infection | |||
| Unknown | 34 (22.2%) | 16 (17.6%) | 0.481 |
| Central line infections | 22 (14.4%) | 16 (17.6%) | 0.628 |
| Infective endocarditis | 5 (3.3%) | 4 (4.4%) | 0.920 |
| Osteomyelitis | 24 (15.7%) | 17 (18.7%) | 0.669 |
| Septic arthritis | 5 (3.3%) | 6 (6.6%) | 0.373 |
| Skin and soft tissue | 30 (19.6%) | 11 (12.1%) | 0.180 |
| Lung | 28 (18.3%) | 15 (16.5%) | 0.852 |
| Combined deep-seated abscesses | 26 (17.0%) | 21 (23.1%) | 0.319 |
| agr genotype | 0.001 | ||
| I | 106 (69.3%) | 54 (59.3%) | |
| II | 15 (9.8%) | 5 (5.5%) | |
| III | 19 (12.4%) | 29 (31.9%) | |
| IV | 13 (8.5%) | 3 (3.3%) | |
| blaZ genotype | 0.009 | ||
| A | 16 (10.5%) | 17 (18.7%) | |
| B | 40 (26.1%) | 34 (37.4%) | |
| C | 60 (39.2%) | 18 (19.8%) | |
| D | 4 (2.6%) | 5 (5.5%) | |
| None | 33 (21.6%) | 17 (18.7%) | |
| Cefazolin inoculum effect | 13 (8.5%) | 12 (13.2%) | 0.342 |
| Vancomycin MIC | 0.321 | ||
| <0.5 mg/liter | 76 (49.7%) | 42 (46.2%) | |
| 1 mg/liter | 74 (48.4%) | 49 (53.8%) | |
| 2 mg/liter | 3 (2.0%) | 0 (0.0%) | |
| SOFA score | 0.178 | ||
| 0–1 | 35 (22.9%) | 30 (33.0%) | |
| 2–5 | 77 (50.3%) | 43 (47.3%) | |
| ≥6 | 41 (26.8%) | 18 (19.8%) | |
| Appropriate empirical antibiotics | 150 (98.0%) | 91 (100%) | 0.457 |
| Definitive antibiotics | 0.925 | ||
| Nafcillin | 54 (35.3%) | 31 (34.1%) | |
| Cefazolin | 39 (25.5%) | 22 (24.2%) | |
| Others | 60 (39.2%) | 38 (41.8%) | |
| Outcomes | |||
| Median duration of bacteremia, day (IQR) | 1 (1–4) | 1 (1–3) | 0.267 |
| Persistent bacteremia | 22 (14.4%) | 9 (9.9%) | 0.413 |
| 7-D death | 10 (6.5%) | 8 (8.8%) | 0.690 |
| 30-D death | 22 (14.4%) | 14 (15.4%) | 0.978 |
| 90-D death | 27 (17.6%) | 17 (18.7%) | 0.975 |
agr, accessory gene regulator; MSSA, methicillin-susceptible Staphylococcus aureus; IQR, interquartile range; HIV, human immunodeficiency virus; CCWI, Charlson comorbidity weighted index; SOFA, sequential organ failure assessment; D, day.
The 90-day mortality rate of MSSA-B was 18% (44 of 244 patients). In the bivariate analysis, a high CCWI (CCWI ≥ 3), a high SOFA score, sites of infection of endocarditis or pneumonia, and use of drugs other than nafcillin or cefazolin as definitive antibiotics were more likely to be associated with 90-day mortality, whereas community-onset bacteremia was less likely to be associated with the 90-day mortality (Table 2). In the logistic regression analysis, a high CCWI, a high SOFA score, and infective endocarditis were independently associated with mortality, whereas community-onset infection was negatively associated with mortality (Table 3).
TABLE 2.
Bivariate analysis of risk factors for 90-day mortality among patients with MSSA bacteremiaa
| Characteristic | Value for group |
P value | |
|---|---|---|---|
| Survived (n = 200) | 90-day mortality (n = 44) | ||
| Median age, yrs (IQR) | 66 (54.5–74) | 68 (56.5–75.5) | 0.23 |
| Older age (≥65 yrs) | 105 (52.5%) | 25 (56.8%) | 0.72 |
| Male | 135 (67.5%) | 33 (75.0%) | 0.43 |
| Community onset | 129 (64.5%) | 19 (43.2%) | 0.01 |
| Comorbidity | |||
| Cardiovascular disease | 19 (9.5%) | 5 (11.4%) | 0.92 |
| Central nervous disease | 28 (14.0%) | 6 (13.6%) | >0.999 |
| Chronic lung disease | 3 (1.5%) | 0 (0.0%) | 0.95 |
| Chronic liver disease | 11 (5.5%) | 6 (13.6%) | 0.11 |
| Chronic kidney disease | 23 (11.5%) | 5 (11.4%) | >0.999 |
| Connective tissue disease | 3 (1.5%) | 2 (4.5%) | 0.48 |
| Solid organ malignancy | 26 (13.0%) | 10 (22.7%) | 0.16 |
| Hematologic malignancy | 16 (8.0%) | 6 (13.6%) | 0.37 |
| Diabetes mellitus | 56 (28.0%) | 11 (25.0%) | 0.83 |
| HIV infection | 1 (0.5%) | 0 (0.0%) | >0.999 |
| Median CCWI, score (IQR) | 3 (2–5) | 4 (3–7) | 0.003 |
| High CCWI (CCWI ≥ 3) | 124 (62.0%) | 39 (88.6%) | 0.001 |
| Site of infection | |||
| Unknown | 37 (18.5%) | 13 (29.5%) | 0.15 |
| Central line infections | 33 (16.5%) | 5 (11.4%) | 0.53 |
| Infective endocarditis | 5 (2.5%) | 4 (9.1%) | 0.10 |
| Osteomyelitis | 37 (18.5%) | 4 (9.1%) | 0.20 |
| Septic arthritis | 9 (4.5%) | 2 (4.5%) | >0.999 |
| Skin and soft tissue infection | 38 (19.0%) | 3 (6.8%) | 0.08 |
| Pneumonia | 29 (14.5%) | 14 (31.8%) | 0.01 |
| Combined deep-seated abscesses | 40 (20.0%) | 7 (15.9%) | 0.68 |
| agr genotype | 0.43 | ||
| I | 129 (64.5%) | 31 (70.5%) | |
| II | 15 (7.5%) | 5 (11.4%) | |
| III | 43 (21.5%) | 5 (11.4%) | |
| IV | 13 (6.5%) | 3 (6.8%) | |
| Dysfunctional agr locus | 74 (37.0%) | 17 (38.6%) | 0.97 |
| blaZ genotype | 0.664 | ||
| A | 29 (14.5%) | 4 (9.1%) | |
| B | 57 (28.5%) | 17 (38.6%) | |
| C | 65 (32.5%) | 13 (29.5%) | |
| D | 8 (4.0%) | 1 (2.3%) | |
| None | 41 (20.5%) | 9 (20.5%) | |
| Cefazolin inoculum effect | 22 (11.0%) | 3 (6.8%) | 0.58 |
| Vancomycin MIC | 0.626 | ||
| <0.5 mg/liter | 98 (49.0%) | 20 (45.5%) | |
| 1 mg/liter | 99 (49.5%) | 24 (54.5%) | |
| 2 mg/liter | 3 (1.5%) | 0 (0.0%) | |
| SOFA score | <0.001 | ||
| 0–1 | 64 (32.0%) | 1 (2.3%) | |
| 2–5 | 105 (52.5%) | 15 (34.1%) | |
| ≥6 | 31 (15.5%) | 28 (63.6%) | |
| Appropriate empirical antibiotics | 197 (98.5%) | 44 (100.0%) | 0.95 |
| Definitive antibiotics | 0.04 | ||
| Nafcillin | 73 (36.5%) | 12 (27.3%) | |
| Cefazolin | 54 (27.0%) | 7 (15.9%) | |
| Others | 73 (36.5%) | 25 (56.8%) | |
MSSA, methicillin-susceptible Staphylococcus aureus; IQR, interquartile range; HIV, human immunodeficiency virus; CCWI, Charlson comorbidity weighted index; agr, accessory gene regulator; SOFA, sequential organ failure assessment.
TABLE 3.
Multivariate logistic regression analysis of risk factors for 90-day mortality among patients with MSSA bacteremiaa
| Characteristic | aOR (95% CI) | P value |
|---|---|---|
| Dysfunctional agr | 1.558 (0.669–3.653) | 0.30 |
| Cefazolin inoculum effect | 0.802 (0.141–3.402) | 0.78 |
| Community-onset bacteremia | 0.366 (0.147–0.870) | 0.03 |
| High CCWI (CCWI ≥ 3) | 6.260 (1.807–25.156) | 0.006 |
| SOFA score | ||
| 0–1 | 1 | |
| 2–5 | 8.0055 (1.453–150.494) | 0.05 |
| ≥6 | 52.276 (8.943–1,013.780) | <0.001 |
| Infective endocarditis | 8.131 (1.260–56.753) | 0.03 |
| Definitive antibiotics | ||
| Nafcillin | 1 | |
| Cefazolin | 0.929 (0.242–3.395) | 0.91 |
| Others | 1.443 (0.462–4.780) | 0.44 |
MSSA, methicillin-susceptible Staphylococcus aureus; aOR, adjusted odds ratio; CI, confidence interval; agr, accessory gene regulator; CCWI, Charlson comorbidity weighted index; SOFA, sequential organ failure assessment.
Ninety-day mortality rate was 1.5% (1 of 65 patients), 12.5% (15 of 120 patients), and 47.5% (28 of 59 patients) at SOFA scores of 0 to 1, 2 to 5, and ≥6, respectively. There was a tendency for MSSA-B in the dysfunctional agr group to have a mortality rate higher than that in the functional agr group at SOFA scores of 2 to 5, although there was no statistically significant difference between the two groups in the severity subgroup analysis (Table 4).
TABLE 4.
Mortality due to MSSA bacteremia stratified by the initial severity of infections between functional and dysfunctional agr groupsa
| Mortality rate for group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOFA 0–1 |
SOFA 2–5 |
SOFA ≥6 |
|||||||
| Time of mortality (day) | Fx agr (n = 35) | DysFx agr (n = 30) | P value | Fx agr (n = 77) | DysFx agr (n = 43) | P value | Fx agr (n = 41) | DysFx agr (n = 18) | P value |
| 7 | 0 (0%) | 0 (0%) | 0 (0%) | 4 (9.3%) | 0.03 | 10 (24.4%) | 4 (22.2%) | >0.999 | |
| 30 | 0 (0%) | 0 (0%) | 5 (6.5%) | 7 (16.3%) | 0.16 | 17 (41.5%) | 7 (38.9%) | >0.999 | |
| 90 | 1 (2.9%) | 0 (0%) | >0.999 | 6 (7.8%) | 9 (20.9%) | 0.07 | 20 (48.8%) | 8 (44.4%) | 0.98 |
MSSA, methicillin-susceptible Staphylococcus aureus; SOFA, sequential organ failure assessment; Fx agr, functional accessory gene regulator; DysFx agr, dysfunctional accessory gene regulator; D, day.
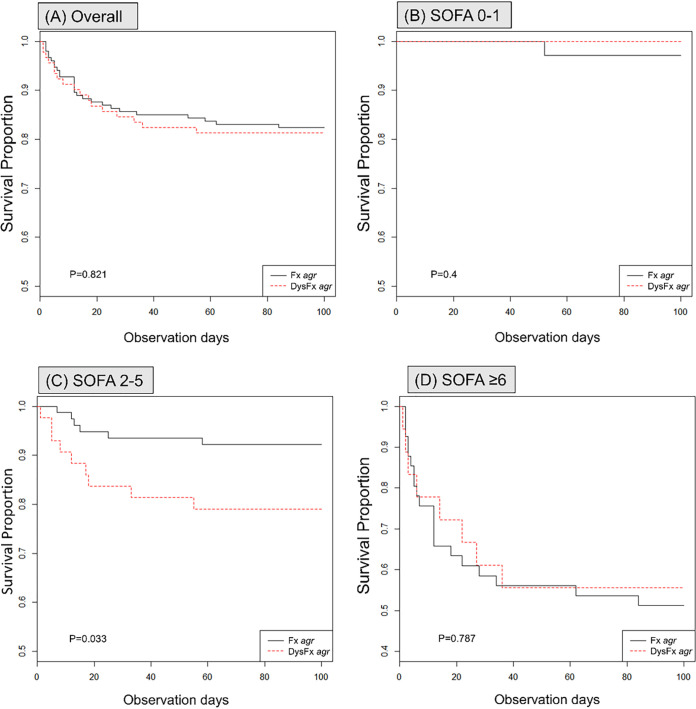
Kaplan-Meier analysis showed that the mortality of MSSA-B in the dysfunctional agr group was not significantly higher than that in the functional group (Fig. 1A; P = 0.82). In subgroup survival analysis with a SOFA score of 2 to 5, the mortality of MSSA-B was significantly higher in the dysfunctional agr group (Fig. 1C; P = 0.03) than in the functional agr group, while the mortality of MSSA-B was not significantly different between the two groups at SOFA scores of 0 to 1 (Fig. 1B; P = 0.4) and ≥6 (Fig. 1D; P = 0.79).
FIG 1.
Kaplan-Meier survival curve for 90-day mortality of MSSA-B with dysfunctional agr group or functional agr group according to the SOFA score overall (A), SOFA scores of 0 to 1 (B), SOFA score of 2 to 5 (C), SOFA scores of ≥6 (D).
In the Cox proportional hazards regression analysis of the entire population (n = 244) with age and sites and severity of infection, comorbidity adjusted hazard ratio (aHR) of dysfunctional agr MSSA-B for 90-day mortality was 1.303 (95% confidence interval [CI], 0.698 to 2.436, P = 0.406). The Cox proportional hazards regression analysis for the subgroup of patients with MSSA-B at SOFA scores of 2 to 5 revealed that dysfunctional agr (aHR, 3.26; 95% CI, 1.05 to 10.12) and a high CCWI (aHR, 9.50; 95% CI, 1.01 to 89.84) were independently associated with mortality.
DISCUSSION
S. aureus bacteremia (SAB) is a common bacterial infection but one of the most serious, with an associated mortality of about 20 to 30% despite advancing antibiotic treatments (3, 10, 12, 13). Risk factors affecting the high mortality include age, comorbid illness, the presence of metastatic foci, methicillin resistance, the severity of sepsis, and delayed appropriate antimicrobial therapy (3, 14, 15). S. aureus produces various potential virulence factors, which may cause poor clinical outcomes in SAB (3). Among these virulence factors, dysfunction of the agr locus has been studied frequently as a predictive factor of SAB, especially in MRSA.
In a recent meta-analysis, dysfunctional agr in invasive S. aureus infection was a significant risk factor for poor clinical outcomes, with the effect on the outcomes being more pronounced in invasive MRSA infection with dysfunctional agr than in that with functional agr (10). Contrastingly, invasive MSSA infection with dysfunctional agr was not associated with unfavorable outcomes and showed 30-day mortality lower than that with functional agr (10). However, the implications of agr dysfunction on the outcomes of invasive MSSA infection may not have been identified because of the insufficient number of the study subjects, the heterogeneity of the severity of infection, or the varied endpoints in previous studies (10). In this study, we evaluated the association between the outcomes of MSSA-B and agr functionality using stratified analysis to reduce the implications of the heterogeneity and severity of the infection. The findings of the present study provide useful information.
First, our study demonstrated that dysfunctional agr was associated with survival lower in patients with MSSA-B with a SOFA score of 2 to 5 than that in patients with other SOFA scores. As is generally known, the severity of disease at initial presentation is one of the strongest prognostic factors of SAB (3, 14, 16, 17). In accordance with previous studies, the 90-day mortality increased rapidly with increasing SOFA scores (1.5%, 12.5%, and 47.5% at SOFA scores 0 to 1, 2 to 5, and ≥6, respectively). We hypothesized that it would be difficult to assess the impact of other factors because of the impact of disease severity, which is the strongest risk factor. Therefore, we performed a stratified analysis using the SOFA score, which is useful for measuring disease severity and predicting the clinical outcomes (18). In the overall analysis of our data, there was no statistically significant difference in mortality between dysfunctional and functional agr groups. This finding is consistent with previous studies showing that MSSA-B with dysfunctional agr was not associated with higher mortality compared to that of MSSA-B with functional agr (10, 16, 19, 20). However, in patients with moderate illness at SOFA scores of 2 to 5, dysfunctional agr was an independent risk factor for mortality with MSSA-B. In contrast, patients with severe illness at a SOFA score of ≥6 had a high mortality rate, while patients with mild illness at SOFA scores of 0 to 1 showed a very low mortality rate regardless of the agr functionality. These findings suggest that the loss of agr function may enhance the adaptability of the causative organism for long-term survival in the host, contributing to chronic infection with increased long-term mortality, even in MSSA.
Second, our study demonstrated that approximately one-third of the patients with MSSA-B were infected with isolates with agr dysfunction. A considerable proportion of MSSA isolates possessed a dysfunctional agr, although this proportion is within the range reported by previous studies, from 8% to 48% of clinical MSSA isolates (16, 19–24). Previous MRSA studies have demonstrated that dysfunctional agr is associated with health care settings and multidrug resistance (11, 25–27). However, the strain included in our study was MSSA, and in most patients, the strain was neither multidrug-resistant nor hospital-acquired. Butterfield et al. (9) reported that prior beta-lactam and fluoroquinolone exposure are predictive factors of agr dysfunction. Therefore, the relatively high dysfunctional agr rate of MSSA in our study might be due to the relatively high consumption of antibiotics in Korea (28, 29).
Third, our study revealed that comorbidities, the severity of the infectious disease, and sites of infection were important factors affecting SAB outcomes. The risk factors associated with the 90-day mortality with MSSA-B included a high CCWI, a high SOFA score, and the occurrence of infective endocarditis, while the community-onset infection was negatively associated with mortality, which is consistent with previous studies (3, 14, 15, 30).
This study has several limitations. First, it was a single-center, retrospective study with a small sample size, and we cannot rule out the presence of unmeasured confounding factors. Second, data analyses were restricted to those with computerized medical records; some variables that may be associated with mortality were not available because of the lack of data. Third, we performed delta hemolysin assay using the isolates stored/frozen at −80°C. Freezing and long-term storage of the isolate might have affected the agr function of the isolates, although freezing is a very common method of preservation and storage of microorganisms (31).
In summary, agr dysfunctional MSSA-B was not uncommon. MSSA-B patients with moderate severity and SOFA scores between 2 to 5 and dysfunctional agr were associated with lower survival. The agr dysfunction in causative organisms may have a significant effect on the outcomes of patients with MSSA-B and MRSA-B. Dysfunction of agr might be useful as a biomarker for the outcomes of patients with MSSA bacteremia. Further studies are warranted to intensify our findings.
MATERIALS AND METHODS
Study design and population.
A retrospective cohort study was conducted to evaluate the comparative outcomes of MSSA-B with dysfunctional versus functional agr groups at a 1,300-bedded tertiary care hospital in Korea from June 2014 to June 2019. Patients (≥15 years of age) with MSSA-B were enrolled; patients with polymicrobial infection or suspected culture contamination were excluded. Only the first bloodstream culture isolate from each patient was included in the study. We assigned the patients with MSSA-B to dysfunctional or functional agr groups according to the agr functionality of causative MSSA isolates.
The study protocol was approved by the institutional review board (IRB) of Pusan National University Hospital (IRB no. H-2010-002-095), and the requirement of informed consent was waived.
Data collection and definitions.
We collected demographic data, clinical information, and antimicrobial susceptibility data of the participants retrospectively by reviewing the medical records. Infection was classified as community-onset if an S. aureus-positive specimen was obtained within 72 h of hospitalization (32). Infections were classified based on the site of infection as central line-associated bloodstream infection, infective endocarditis, pneumonia, osteomyelitis, septic arthritis, skin and soft tissue infections, deep-seated abscess, and unknown primary focus (33). The severity of the comorbidity was measured using the CCWI (34), while the severity of the bacteremia was measured using the sequential organ failure assessment (SOFA) score (18). Antimicrobial therapy was considered appropriate if the isolate was susceptible to the antibiotic chosen in vitro. Empirical and definitive antibiotics were defined as antibiotics administered within the first 48 h and those used after a report of antibiotic susceptibility of the isolates, respectively (35). Persistent bacteremia was defined as the first negative blood culture occurring >7 days after the first positive blood culture with appropriate antibiotic therapy (36).
Microbiological analyses.
Microbiological analyses were conducted using the isolates that were collected from patients and stored at −80°C. The functionality of the agr locus was measured through δ-hemolysin expression assays using the S. aureus strain RN4220 as an indicator, and the absence of or barely detectable synergistic hemolysis was defined as agr dysfunction (4). To determine the agr group genotype, agr group-specific multiplex PCR was performed using primers that have been described previously (37). The cefazolin inoculum effect was measured by comparing the MICs of the high inoculum (∼5 × 107 CFU/ml) with those of the standard inoculum (∼5 × 105 CFU/ml), as described previously (38, 39). Cefazolin inoculum effect was defined as an increase in MICs to ≥16 μg/ml at high inoculum from a susceptible range of MIC at standard inoculum (33).
Main outcome.
The main outcome of our study was the 90-day mortality rate. We also compared the rates of persistent bacteremia, 7-day mortality, and 30-day mortality. To analyze the difference in the effect of dysfunctional agr on the outcome of MSSA-B according to the severity of the initial infectious disease, we performed a stratified analysis according to the SOFA score (0 to 1, 2 to 5, ≥6).
Statistical analysis.
R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, and noncategorical variables were tested using the Mann-Whitney U test or Kruskal-Wallis test. Logistic regression analysis was used to determine the risk factors for 90-day mortality. Variables with a P value of <0.2 in bivariate analysis were included in a multivariate logistic regression model, and agr functionality and cefazolin inoculum effect were forced separately into the model to estimate their clinical implications. The Kaplan-Meier method was used to determine differences in survival between the dysfunctional and functional agr groups according to the initial SOFA score. Cox proportional hazards regression analysis was used to examine the association between agr functionality and mortality, where patients were censored if death occurred within 90 days after the first positive blood culture result was collected. The initial SOFA score (0 to 1, 2 to 5, ≥6) was stratified by agr functionality (functional and dysfunctional) during subgroup analysis. CCWI and definitive antibiotics were incorporated into the models as predictors of mortality. aHRs and 95% CIs were calculated using multivariate modeling. All tests of significance were 2-tailed, and the results were considered statistically significant at P < 0.05.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (Grant number NRF-2018R1A1A1A05079369). All data generated or analyzed during this study are included in this published article.
We declare no conflicts of interest.
Contributor Information
Shinwon Lee, Email: ebenezere.lee@gmail.com.
Daria Van Tyne, University of Pittsburgh School of Medicine.
REFERENCES
- 1.Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhoj P. 2007. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect 13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG, Hellmich M, Hopkins S, Kern WV, Llewelyn MJ, Rieg S, Rodriguez-Baño J, Scarborough M, Seifert H, Soriano A, Tilley R, Tőrők ME, Weiß V, Wilson APR, Thwaites GE, ISAC, INSTINCT, SABG, UKCIRG, and Colleagues. 2014. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68:242–251. doi: 10.1016/j.jinf.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. 2012. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelnour A, Arvidson S, Bremell T, Ryden C, Tarkowski A. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun 61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le KY, Otto M. 2015. Quorum-sensing regulation in staphylococci-an overview. Front Microbiol 6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN. 2011. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother 55:1082–1087. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimoto DF, Bayles KW. 1998. Opposing roles of the Staphylococcus aureus virulence regulators, agr and sar, in triton x-100- and penicillin-induced autolysis. J Bacteriol 180:3724–3726. doi: 10.1128/JB.180.14.3724-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Muir TW. 2016. Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem Biol 23:214–224. doi: 10.1016/j.chembiol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterfield JM, Tsuji BT, Brown J, Ashley ED, Hardy D, Brown K, Forrest A, Lodise TP. 2011. Predictors of agr dysfunction in methicillin-resistant Staphylococcus aureus (MRSA) isolates among patients with MRSA bloodstream infections. Antimicrob Agents Chemother 55:5433–5437. doi: 10.1128/AAC.00407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SO, Lee S, Lee JE, Song KH, Kang CK, Wi YM, San-Juan R, Lopez-Cortes LE, Lacoma A, Prat C, Jang HC, Kim ES, Kim HB, Lee SH. 2020. Dysfunctional accessory gene regulator (agr) as a prognostic factor in invasive Staphylococcus aureus infection: a systematic review and meta-analysis. Sci Rep 10:20697. doi: 10.1038/s41598-020-77729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shopsin B, Drlica-Wagner A, Mathema B, Adhikari RP, Kreiswirth BN, Novick RP. 2008. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis 198:1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- 12.Chong YP, Park SJ, Kim HS, Kim ES, Kim MN, Park KH, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2013. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine (Baltimore) 92:98–108. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskesen AN, Belle MA, Blomfeldt A. 2018. Predictors of one-year all-cause mortality and infection-related mortality in patients with Staphylococcus aureus bacteraemia. Infect Dis (Lond) 50:743–748. doi: 10.1080/23744235.2018.1470666. [DOI] [PubMed] [Google Scholar]
- 14.Sara EC, George S, Eli NP, Mitchell JS, Adolf WK, Yehuda C. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 15.Malani PN, Rana MM, Banerjee M, Bradley SF. 2008. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc 56:1485–1489. doi: 10.1111/j.1532-5415.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 16.Wi YM, Park YK, Moon C, Ryu SY, Lee H, Ki HK, Cheong HS, Son JS, Lee JS, Kwon KT, Kim JM, Ha YE, Kang CI, Ko KS, Chung DR, Peck KR, Song JH. 2015. The cefazolin inoculum effect in methicillin-susceptible Staphylococcus aureus blood isolates: their association with dysfunctional accessory gene regulator (agr). Diagn Microbiol Infect Dis 83:286–291. doi: 10.1016/j.diagmicrobio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Bassetti M, Peghin M, Trecarichi EM, Carnelutti A, Righi E, Del Giacomo P, Ansaldi F, Trucchi C, Alicino C, Cauda R, Sartor A, Spanu T, Scarparo C, Tumbarello M. 2017. Characteristics of Staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS One 12:e0170236. doi: 10.1371/journal.pone.0170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan SB, Austin ED, Stump S, Mathema B, Whittier S, Lowy FD, Uhlemann AC. 2017. Reduced vancomycin susceptibility of methicillin-susceptible staphylococcus aureus has no significant impact on mortality but results in an increase in complicated infection. Antimicrob Agents Chemother 61:e00316-17. doi: 10.1128/AAC.00316-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Cortes LE, Velasco C, Retamar P, del Toro MD, Galvez-Acebal J, de Cueto M, Garcia-Luque I, Caballero FJ, Pascual A, Rodriguez-Bano J. 2015. Is reduced vancomycin susceptibility a factor associated with poor prognosis in MSSA bacteraemia? J Antimicrob Chemother 70:2652–2660. doi: 10.1093/jac/dkv133. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Hidalgo N, Ribera A, Larrosa MN, Viedma E, Origuen J, de Alarcon A, Farinas MC, Saez C, Pena C, Munez E, Garcia Lopez MV, Gavalda J, Perez-Montarelo D, Chaves F, Almirante B. 2018. Impact of Staphylococcus aureus phenotype and genotype on the clinical characteristics and outcome of infective endocarditis. A multicentre, longitudinal, prospective, observational study. Clin Microbiol Infect 24:985–991. doi: 10.1016/j.cmi.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Gomes-Fernandes M, Laabei M, Pagan N, Hidalgo J, Molinos S, Villar Hernandez R, Dominguez-Villanueva D, Jenkins ATA, Lacoma A, Prat C. 2017. Accessory gene regulator (Agr) functionality in Staphylococcus aureus derived from lower respiratory tract infections. PLoS One 12:e0175552. doi: 10.1371/journal.pone.0175552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.San-Juan R, Perez-Montarelo D, Viedma E, Lalueza A, Fortun J, Loza E, Pujol M, Ardanuy C, Morales I, de Cueto M, Resino-Foz E, Morales-Cartagena MA, Fernandez-Ruiz M, Rico A, Romero MP, Fernandez de Mera M, Lopez-Medrano F, Orellana MA, Aguado JM, Chaves F. 2017. Pathogen-related factors affecting outcome of catheter-related bacteremia due to methicillin-susceptible Staphylococcus aureus in a Spanish multicenter study. Eur J Clin Microbiol Infect Dis 36:1757–1765. doi: 10.1007/s10096-017-2989-5. [DOI] [PubMed] [Google Scholar]
- 24.Sharma-Kuinkel BK, Ahn SH, Rude TH, Zhang Y, Tong SY, Ruffin F, Genter FC, Braughton KR, Deleo FR, Barriere SL, Fowler VG, Jr.. 2012. Presence of genes encoding panton-valentine leukocidin is not the primary determinant of outcome in patients with hospital-acquired pneumonia due to Staphylococcus aureus. J Clin Microbiol 50:848–856. doi: 10.1128/JCM.06219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong YP, Kim ES, Park SJ, Park KH, Kim T, Kim MN, Kim SH, Lee SO, Choi SH, Woo JH, Jeong JY, Kim YS. 2013. Accessory gene regulator (agr) dysfunction in Staphylococcus aureus bloodstream isolates from South Korean patients. Antimicrob Agents Chemother 57:1509–1512. doi: 10.1128/AAC.01260-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Oh IH, Lee HJ, Ihm CG, Son JS, Lee MS, Kim MN. 2013. Impact of reduced vancomycin MIC on clinical outcomes of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:5536–5542. doi: 10.1128/AAC.01137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoulas G, Moise PA, Rybak MJ. 2009. Accessory gene regulator dysfunction: an advantage for Staphylococcus aureus in health-care settings? J Infect Dis 199:1558–1559. doi: 10.1086/598607. [DOI] [PubMed] [Google Scholar]
- 28.Kim B, Myung R, Lee MJ, Kim J, Pai H. 2019. Trend of antibiotics usage for acute pyelonephritis in Korea based on national health insurance data 2010–2014. BMC Infect Dis 19:554. doi: 10.1186/s12879-019-4191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YA, Park YS, Youk T, Lee H, Lee K. 2018. Trends in South Korean antimicrobial use and association with changes in Escherichia coli resistance rates: 12-year ecological study using a nationwide surveillance and antimicrobial prescription database. PLoS One 13:e0209580. doi: 10.1371/journal.pone.0209580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitby M, McLaws ML, Berry G. 2001. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust 175:264–267. doi: 10.5694/j.1326-5377.2001.tb143562.x. [DOI] [PubMed] [Google Scholar]
- 31.van Griethuysen A, van Loo I, van Belkum A, Vandenbroucke-Grauls C, Wannet W, van Keulen P, Kluytmans J. 2005. Loss of the mecA gene during storage of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol 43:1361–1365. doi: 10.1128/JCM.43.3.1361-1365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim ES, Kim HB, Kim G, Kim KH, Park KH, Lee S, Choi YH, Yi J, Kim CJ, Song KH, Choe PG, Kim NJ, Lee YS, Oh MD, Korea INfectious Diseases (KIND) study group. 2014. Clinical and epidemiological factors associated with methicillin resistance in community-onset invasive Staphylococcus aureus infections: prospective multicenter cross-sectional study in Korea. PLoS One 9:e114127. doi: 10.1371/journal.pone.0114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song KH, Jung SI, Lee S, Park S, Kiem SM, Lee SH, Kwak YG, Kim YK, Jang HC, Kim YS, Kim HI, Kim CJ, Park KH, Kim NJ, Oh MD, Kim HB, Korea INfectious Diseases (KIND) study group. 2017. Characteristics of cefazolin inoculum effect-positive methicillin-susceptible staphylococcus aureus infection in a multicentre bacteraemia cohort. Eur J Clin Microbiol Infect Dis 36:285–294. doi: 10.1007/s10096-016-2799-1. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Kang CK, Cho JE, Choi YJ, Jung Y, Kim NH, Kim CJ, Kim TS, Song KH, Choe PG, Park WB, Bang JH, Kim ES, Park KU, Park SW, Kim NJ, Oh MD, Kim HB. 2015. agr dysfunction affects staphylococcal cassette chromosome mec type-dependent clinical outcomes in methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 59:3125–3132. doi: 10.1128/AAC.04962-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. 2007. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 167:1861–1867. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- 37.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. 2003. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol 69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Song KH, Jung SI, Park WB, Lee SH, Kim YS, Kwak YG, Kim YK, Kiem SM, Kim HI, Kim ES, Park KH, Kim NJ, Jang HC, Kim HB, Korea INfectious Diseases (KIND) study group. 2018. Comparative outcomes of cefazolin versus nafcillin for methicillin-susceptible Staphylococcus aureus bacteraemia: a prospective multicentre cohort study in Korea. Clin Microbiol Infect 24:152–158. doi: 10.1016/j.cmi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG, Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 53:3437–3441. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]