Abstract
Interleukin-1 (IL-1) plays an essential role in the immune pro-inflammatory process, which is regarded as one of many factors in the development of type 2 diabetes mellitus (T2DM). Several case-control studies have illustrated the association of the IL-1B (-511) (rs16944, Chr 2:112,837,290, C/T Intragenic, Transition Substitution) and IL-1RN (VNTR) (gene for IL-1 receptor antagonist, IL-1RA, 86 bp tandem repeats in intron 2) polymorphisms with T2DM risk. However, the results were inconsistent and inconclusive. We performed a meta-analysis (registry number: CRD42021268494) to assess the association of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk. Random-effects models were applied to calculate the pooled ORs (odds ratios) and 95% CIs (confidence intervals) to test the strength of the association in the overall group and subgroups stratified by ethnicity, respectively. Between-study heterogeneity and publication bias were evaluated by the Q-test, I2 statistic, Harbord test, and Peters test accordingly. Sensitivity analyses were also performed. A total of 12 publications evaluating the association of IL-1B (-511) and IL-1RN (VNTR) polymorphisms with the risk of T2DM development were included. The meta-analysis showed that IL-1RN (VNTR) was related to the increasing development of T2DM risk in the recessive model (OR = 1.62, 95% CI [1.09–2.42], Phet = 0.377, Pz = 0.018) and in the homozygous model (OR = 2.02, 95% CI [1.07–3.83], Phet = 0.085, Pz = 0.031), and the IL-1RN 2* allele was found a significant association with evaluated T2DM risk in all ethnicities (OR = 2.08, 95% CI [1.43–3.02], Phet < 0.001, Pz < 0.001) and in EA (OR = 2.01, 95% CI [1.53–2.66], Phet = 0.541, Pz < 0.001). Moreover, stratification by ethnicity revealed that IL-1B (-511) was associated with a decreased risk of T2DM in the dominant model (OR=0.76, 95% CI [0.59–0.97], Phet = 0.218, Pz = 0.027) and codominant model (OR = 0.73, 95% CI [0.54–0.99], Phet = 0.141, Pz = 0.040) in the East Asian (EA) subgroup. Our results suggest that the IL-1RN 2* allele and 2*2* homozygous polymorphism are strongly associated with increasing T2DM risk and that the IL-1B (-511) T allele polymorphism is associated with decreasing T2DM risk in the EA subgroup.
Keywords: IL-1B (-511), IL-1RN (VNTR), Polymorphism, Type 2 diabetes mellitus, Meta-analysis
Introduction
The latest data from the 9th edition of the IDF Diabetes Atlas show that approximately 463 million adults (20–79 years) have diabetes, and by 2045, the number will rise to 700 million. Type 2 diabetes mellitus (T2DM) is the most prevalent type of diabetes (>90% of diabetes). The proportion of T2DM patients is increasing in most countries, especially in low- and middle-income countries such as China and India (https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html). Growing evidence suggests that the risk of T2DM is associated with various factors, such as genetics, ethnicity, environment, and lifestyle. In particular, genetic susceptibility seems to play an essential role in the pathogenesis of T2DM (Hivert, Vassy & Meigs, 2014).
T2DM occurs when β-cell function progressively deteriorates and fails to compensate for insulin resistance, partly due to the demise of pancreatic β-cells through apoptosis (Donath & Halban, 2004). The chronic and low-grade inflammation in metabolic organs including the liver, brain, pancreas and adipose tissue is known as metaflammation (metabolism-induced inflammation), which can be widely observed in T2DM (Hotamisligil, 2006; Hotamisligil, 2017). Increasing evidence has shown that metaflammation plays a vital role in the development of T2DM and its cardiovascular complications (Hotamisligil, 2017) and pro-inflammatory cytokines, especially interleukin-1β (IL-1β), play a key role in this process (Dinarello, Donath & Mandrup-Poulsen, 2010; Fève & Bastard, 2009; Sathyapalan & Atkin, 2011). Moreover, the increased level of IL-1β in human pancreatic cells due to the elevated glucose concentrations and decreased level of IL-1 receptor antagonist (IL-1Ra) in islets of T2DM patients result in impaired insulin secretion, decreased cell proliferation, and apoptosis of β-cells (Böni-Schnetzler & Donath, 2013). A previous observational study demonstrated that a combined elevation of IL-1β and IL-6 was associated with a roughly threefold increased risk of T2DM, and IL-1β might induce insulin resistance via activating the IκB kinaseβ (Spranger et al., 2003). Furthermore, CANTOS and TRACK trials have evaluated the role of IL-1 inhibition in the treatment of several inflammatory disorders, such as coronary artery disease, T2DM and rheumatoid arthritis (Qamar & Rader, 2012; Ruscitti et al., 2019). According to the result from CANTOS trials, canakinumab (a fully humanized monoclonal antibody) could selectively block IL-1β, and its efficacy in suppressing both levels of inflammatory markers and clinical symptoms in patients with autoinflammatory disease such as T2DM and impaired glucose tolerance has been observed in this trial (Aday & Ridker, 2018; Rissanen et al., 2012). Therefore, the genetic polymorphisms that regulate the expression levels of IL-1β and IL-1Ra might have an essential impact on the interindividual differences in T2DM.
Consisting of three linked genes and mapping to chromosome 2q13-24, IL-1 cytokine genes encode IL-1α, IL-1β and IL-1Ra. Both IL-1α and IL-1β are pro-inflammatory cytokines, while IL-1Ra can inhibit inflammation by competing for receptor binding (Dinarello, 2000). In the promoter region, IL-1B (-511) (rs16944, Chr 2:112,837,290, C/T Intragenic, Transition Substitution) has a bi-allelic polymorphism at position -511, representing the C/T transition (Kristiansen et al., 2000). IL-1RN (VNTR) (gene for IL-1 receptor antagonist, IL-1RA, 86 bp tandem repeats in intron 2) has five different alleles: 1* (four repeats), 2* (two repeats), 3* (five repeats), 4* (three repeats) and 5* (six repeats) (Tarlow et al., 1993). IL-1RN 1* (four repeats) and IL-1RN 2* (two repeats) are the most common, whereas the others occur much less frequently (<5%) (Santtila, Savinainen & Hurme, 1998). Many genetic association studies have been performed to estimate the relationship of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk (Achyut et al., 2007; Muktabhant et al., 2013). Several results suggest that there is no relationship between them (Borilova Linhartova et al., 2019; Muktabhant et al., 2013), whereas Achyut et al., (2007) showed that both the IL-1B (-511) and IL-1RN (VNTR) polymorphisms were associated with susceptibility to T2DM as well as complications, and he indicated that T2 (IL-1β −511T/IL-1RN*2) haplotype was associated with a roughly twofold increased risk of T2DM. Considering the small sample sizes (n < 600) and varying population characteristics (ethnic differences) in different studies, the results are conflicting. To illustrate the potential association of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk, we conducted a meta-analysis including 12 reported publications.
Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines (Moher et al., 2009). A protocol was registered before commencing this review on PROSPERO (CRD42021268494).
The search strategy
The online databases we searched included PubMed, Web of Science, CNKI (China National Knowledge Infrastructure) and Wanfang. We identified relevant articles reporting the association of IL-1RN (VNTR) and IL-1B (-511) polymorphisms with the risk of T2DM in the medical literature through the end of September 12, 2020. This study used a combination of the following search terms: “Type 2 Diabetes Mellitus” or “T2DM” or “interleukin-1” or “IL-1” or “polymorphism” or “genetics” or “association”. In addition, we also conducted a hand search to identify relevant data in references from retrieved articles. Juan Jiao and Zhaoping Wang performed the Search Strategy. In case of disagreement, it will be settled by a third assessor’s evaluation and discussed until a consistent result was reached.
The inclusion and exclusion criteria
All studies included in this analysis met the following criteria: (1) the study revealed the relationship of IL-1B (-511) and IL-1RN (VNTR) with T2DM risk; (2) the design was a “case-control study”; (3) the research subjects were humans with T2DM; (4) the publication language was English or Chinese; and (5) the study provided sufficient data to estimate ORs (odds ratios) and 95% CIs (confidence intervals). The major exclusion criteria were as follows: (1) duplicate data; (2) randomly chosen controls; (3) non-research articles; and (4) insufficient reporting of data.
Data extraction
All data were systematically reviewed and extracted by two investigators (ZPW and DNG) according to a standardized form, and then all of the following information was collected in an electronic database: the first author’s name, year of publication, country of origin, ethnicity, total number of cases/controls, genotyping method, diagnostic criteria of T2DM, genotypic frequencies and language of the report. Disagreement was settled by a third assessor’s evaluation and discussed until a consistent conclusion was agreed.
Assessment of quality score
We assessed the quality score of identified publications based on the Newcastle Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). And the NOS involved “selection”, “comparability”, and “estimation of outcomes or exposures”. The scores ranged from 0 to 9, and the score of “high quality study” was ≥ 6 (detailed scores see Table S1) (Lo, Mertz & Loeb, 2014). And the STREGA (Strengthening the REporting of Genetic Association) system was also performed to assessed the methodological quality of all included studies, which includes 22 items with scoring from 0 to 22 (detailed scores see Table S2) (Little et al., 2009). And the score of high quality was 18–22; the score of moderate-high quality was 13–17; the score of low quality was 0–12 (Duan et al., 2018).
Data analysis
We conducted this analysis and entered data with Stata software, version 15.0 (Stata Corp., College Station, TX, USA). We used original genotypic distribution data without any adjustment to measure the strength of the association between IL-1B (-511)/IL-1RN and T2DM risk by ORs (odds ratios) with 95% CIs (confidence intervals) under dominant, recessive, additive (overdominant+codominant) and homozygous models. The distributions of genotype frequencies of controls were all consistent with Hardy-Weinberg Equilibrium (HWE) (P > 0.05, Table 1). Between-study heterogeneity among the studies was tested with Cochran’s Q and the I2 test statistics. I2 was calculated based on the formula I2 = 100% ×(Q−df)/Q. Heterogeneity was regarded as significant when Phet < 0.1 or I2 > 50%. For those with significant heterogeneity, we performed stratified analyses to examine the statistical significance of the difference in ORs according to ethnicity (East Asian, South Asian, North African and Caucasian). We used the random-effects model (DerSimonian and Laird method) which was combined by applying inverse variance-weighted meta-analysis to calculate the pooled OR and 95% CI, with Pz < 0.05 considered statistically significant (DerSimonian & Laird, 1986). When the heterogeneity was significant, sensitivity analyses were conducted to evaluate the influence of each single study by omitting one study at a time and checking the pool effect size for the remainder of the studies. Finally, the Harbord test and Peters test were used when fewer than ten articles were included, as they are more sensitive for assessing publication bias, with P < 0.1 assuming that the bias was statistically significant.
Table 1. Characteristics of Identified Studies on IL-1B (-511)/IL-1RN(VNTR) polymorphism and the risks of T2DM.
| Author | Year | Country/Ethnicity | Diagnostic criteria | Genotyping method | Number | Language | IL-1 gene | QA | HWE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||
| Zhang Jian | 2004 | China/EA | NA | PCR-RFLP | 106 | 198 | Chinese | IL-1RN | 7 | 1.00 |
| Zhang Ping-an | 2004 | China/EA | WHO, 1999 | PCR-RFLP | 106 | 247 | Chinese | IL-1RN | 8 | 0.70 |
| Zhou Jian-zhong | 2010 | China/EA | WHO, 1999 | PCR-RFLP | 72 | 97 | Chinese | IL-1RN | 7 | 0.23 |
| Petra Borilova Linhartova | 2019 | Czech Republic/Caucasian | NA | PCR-RFLP | 380 | 212 | English | IL-1RN | 8 | 1.00 |
| Alexandra I. F. Blakemore | 1995 | North of England/Caucasian | WHO, NA | PCR | 117 | 248 | English | IL-1RN | 7 | 0.61 |
| B.R Achyut | 2006 | North Indian/SA | WHO, 1999 | PCR-RFLP | 200 | 223 | English | IL-1RNIL-1B (-511) | 8 | 0.100.16 |
| Liu Chang | 2014 | China/EA | WHO, 1999 | PCR-RFLP | 583 | 366 | Chinese | IL-1B (-511) | 7 | 1.00 |
| Cao Yong | 2013 | China/EA | WHO, 1999 | PCR-RFLP | 268 | 263 | Chinese | IL-1B (-511) | 8 | 0.06 |
| Lin Neng-bo | 2016 | China/EA | WHO, 1999 | PCR-RFLP | 286 | 327 | Chinese | IL-1B (-511) | 8 | 0.08 |
| Natalie E. Doody | 2017 | North Indian/SA | WHO, 1999 | PCR-RFLP | 202 | 203 | English | IL-1B (-511) | 8 | 0.87 |
| Benja Muktabhant | 2013 | Thailand/SEA | NA | PCR-RFLP | 90 | 30 | English | IL-1B (-511) | 7 | 0.16 |
| Safaa I. Tayel | 2018 | Egypt/NA1 | ADA, NA | TaqMan | 50 | 30 | English | IL-1B (-511) | 8 | 1.00 |
Notes.
- T2DM
- Type 2 diabetes mellitus
- EA
- East Asian
- SA
- South Asian
- SEA
- South East Asian
- NA
- Not Available (the diagnosis of T2DM was originally based on the presence of clinical symptoms and biochemical)
- NA1
- North African
- ADA
- American Diabetes Association (fasting blood glucose ≥126 mg/dL or 2 h blood glucose after overload with 75 g of glucose ≥ 200 mg/dL in oral glucose tolerance test (OGTT) or glycated hemoglobin (HbA1c) ≥6.5 in patients with classic symptoms of hyperglycemia)
- WHO
- World Health Organization (fasting glucose levels [7.0 mmol/L or 126 mg/dL] after a minimum 12-h fast or 2-h post glucose level (oral glucose tolerance test or 2-h OGTT) [11.1 mmol/L or 200 mg/dL] on more than one occasion)
- QA
- Quality Assessment
- HWE
- Hardy-Weinberg Equilibrium
Results
Included studies
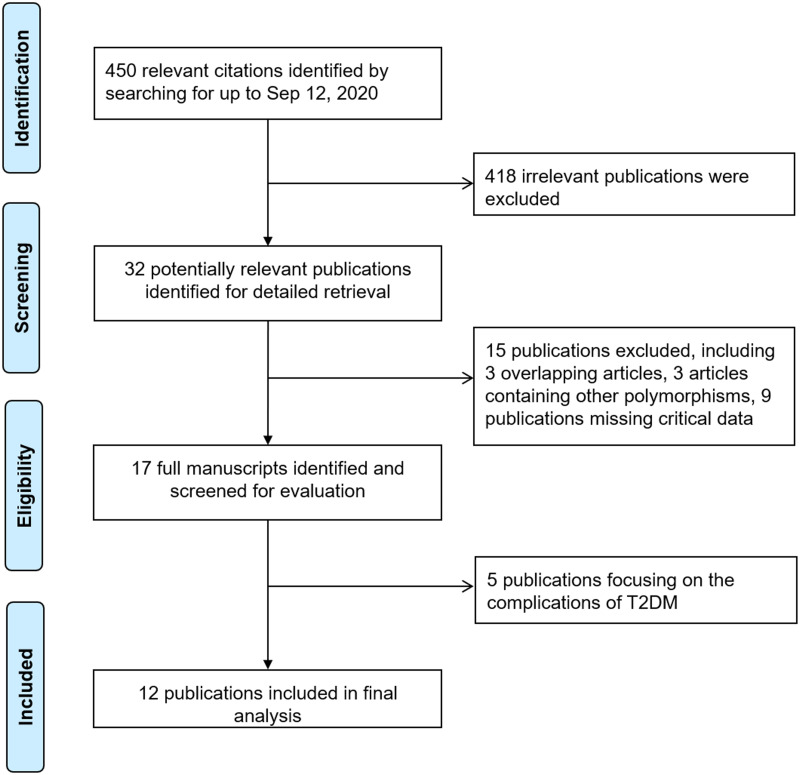
Figure 1 shows the detailed process of selecting and assessing eligible studies. We identified 450 publications via the initial keyword search. After screening, 12 publications met our inclusion criteria (Achyut et al., 2007; Blakemore et al., 1996; Borilova Linhartova et al., 2019; Cao et al., 2013; Doody et al., 2017; Lin et al., 2016; Liu et al., 2014; Muktabhant et al., 2013; Tayel et al., 2018; Zhang, Xiao & Li, 2004a; Zhang et al., 2004b; Zhou et al., 2010), and 438 studies were excluded for containing duplicate data, missing critical data, and being review papers or studies that were not related to T2DM. Table 1 illustrates the characteristics of all included publications in this meta-analysis. In terms of polymorphisms and diseases, six identified case-control studies, comprising 981 cases and 1,225 controls, regarded IL-1RN (VNTR) to evaluate its association with T2DM risk (Achyut et al., 2007; Blakemore et al., 1996; Borilova Linhartova et al., 2019; Zhang, Xiao & Li, 2004a; Zhang et al., 2004b; Zhou et al., 2010), and seven identified case-control studies, including 1,679 cases and 1,442 controls, tested the association between IL-IB (-511) and T2DM risk (Achyut et al., 2007; Cao et al., 2013; Doody et al., 2017; Lin et al., 2016; Liu et al., 2014; Muktabhant et al., 2013; Tayel et al., 2018). Stratified by ethnicity, six publications were performed in East Asian (EA) populations (Cao et al., 2013; Lin et al., 2016; Liu et al., 2014; Zhang, Xiao & Li, 2004a; Zhang et al., 2004b; Zhou et al., 2010), two studies were undertaken in South Asian (SA) populations (Achyut et al., 2007; Doody et al., 2017), two studies were conducted in Caucasian populations (Blakemore et al., 1996; Borilova Linhartova et al., 2019), and the remaining studies were completed in Southeast Asian (SA) (Muktabhant et al., 2013) and North African (NA1) populations (Tayel et al., 2018).
Figure 1. Flowchart of study identification process.
Individual polymorphism meta-analysis
The genotypic distributions of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms are shown in Table 2. Table 3 shows the meta-analysis results for these two polymorphisms.
Table 2. The IL-1 polymorphism distribution in cases and controls.
Table 2 shows the genotypic distributions of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms.
| Gene | Case | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-1B (-511) | C | T | CC | TC | TT | C | T | CC | TC | TT |
| Cao Yong | 302 | 270 | 78 | 129 | 61 | 332 | 322 | 52 | 147 | 64 |
| Liu Chang | 634 | 532 | 171 | 292 | 120 | 385 | 347 | 101 | 183 | 82 |
| B.R Achyut | 108 | 292 | 9 | 90 | 101 | 175 | 271 | 29 | 117 | 77 |
| Lin Neng-bo | 302 | 270 | 85 | 132 | 69 | 332 | 322 | 76 | 180 | 71 |
| Safaa I. Tayel | 55 | 45 | 16 | 23 | 11 | 21 | 39 | 4 | 13 | 13 |
| Natalie E. Doody | 186 | 218 | 40 | 106 | 56 | 133 | 273 | 21 | 91 | 91 |
| Benja Muktabhant | 90 | 90 | 23 | 44 | 23 | 33 | 27 | 11 | 11 | 8 |
| IL-1RN | 1* | 2* | 1*/1* | 1*/2* | 2*/2* | 1* | 2* | 1*/1* | 1*/2* | 2*/2* |
| B.R Achyut | 227 | 234 | 60 | 107 | 20 | 340 | 92 | 138 | 64 | 14 |
| Zhang Jian | 192 | 34 | 88 | 16 | 2 | 360 | 36 | 163 | 34 | 1 |
| Zhang Ping-an | 192 | 34 | 88 | 16 | 2 | 450 | 44 | 204 | 42 | 1 |
| Zhou Jian-zhong | 107 | 71 | 36 | 35 | 1 | 153 | 41 | 58 | 37 | 2 |
| Alexandra I. F. Blakemore | 154 | 106 | 55 | 44 | 18 | 372 | 124 | 141 | 90 | 17 |
| Petra Borilova Linhartova | 569 | 289 | 220 | 129 | 31 | 303 | 121 | 108 | 87 | 17 |
Table 3. Meta-analysis of the relationship of the IL-1 polymorphisms with the risks of T2DM.
| Gene | No. studies | Allele comparison | Genetic model comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P het | P z | OR (95% CI) | P het | P z | ||||
| IL-1B (-511) | |||||||||
| Total | 7 | 0.89 (0.68–1.17) | <0.001 | 0.406 | Dominant | 0.84 (0.57–1.25) | 0.001 | 0.395 | |
| Recessive | 0.89 (0.61–1.31) | <0.001 | 0.561 | ||||||
| Overdominant | 0.92 (0.74–1.14) | 0.065 | 0.422 | ||||||
| Codominant | 0.85 (0.6–1.21) | 0.011 | 0.375 | ||||||
| Codominant# | 0.96 (0.69–1.34) | 0.003 | 0.803 | ||||||
| Homozygote | 0.82 (0.48–1.39) | <0.001 | 0.451 | ||||||
| EA | 3 | 0.89 (0.79–1.01) | 0.606 | 0.072 | Dominant | 0.76 (0.59–0.97) | 0.218 | 0.027 | |
| Recessive | 0.97 (0.79–1.20) | 0.589 | 0.789 | ||||||
| Overdominant | 0.82 (0.64–1.04) | 0.162 | 0.094 | ||||||
| Codominant | 0.73 (0.54–0.99) | 0.141 | 0.040 | ||||||
| Codominant# | 1.07 (0.86–1.34) | 0.385 | 0.531 | ||||||
| Homozygote | 0.80 (0.62–1.03) | 0.571 | 0.081 | ||||||
| SA | 2 | 1.00 (0.33–2.98) | <0.001 | 0.997 | Dominant | 1.20 (0.18–7.84) | <0.001 | 0.853 | |
| SEA | 1 | 1.22 (0.68–2.20) | – | 0.503 | Dominant | 1.69 (0.70–4.07) | – | 0.245 | |
| NA | 1 | 0.44 (0.23–0.85) | – | 0.015 | Dominant | 0.33 (0.10–1.10) | – | 0.070 | |
| IL-1RN | |||||||||
| Total | 6 | 2.08 (1.43–3.02) | <0.001 | <0.001 | Dominant | 1.35 (0.79–2.31) | <0.001 | 0.275 | |
| Recessive | 1.62 (1.09–2.42) | 0.377 | 0.018 | ||||||
| Overdominant | 1.20 (0.72–1.99) | <0.001 | 0.485 | ||||||
| Codominant | 1.28 (0.73–2.24) | <0.001 | 0.398 | ||||||
| Codominant# | 1.38 (0.89–2.13) | 0.351 | 0.155 | ||||||
| Homozygote | 2.02 (1.07–3.83) | 0.085 | 0.031 | ||||||
| EA | 3 | 2.02 (1.53–2.66) | 0.541 | <0.001 | Dominant | 1.11 (0.78–1.59) | 0.525 | 0.558 | |
| Recessive | 2.30 (0.57–9.25) | 0.470 | 0.243 | ||||||
| Overdominant | 1.05 (0.72–1.54) | 0.337 | 0.790 | ||||||
| Codominant | 1.06 (0.74–1.53) | 0.371 | 0.749 | ||||||
| Codominant# | 2.25 (0.53–9.55) | 0.356 | 0.270 | ||||||
| Homozygote | 2.42 (0.60–9.78) | 0.554 | 0.216 | ||||||
| SA | 1 | 3.81 (2.84–5.11) | – | <0.001 | Dominant | 3.75 (2.48–5.67) | – | <0.001 | |
| Caucasian | 2 | 1.60 (0.998–2.58) | 0.02 | 0.051 | Dominant | 1.04 (0.54–2.02) | 0.017 | 0.901 | |
Notes.
- CI
- confidence interval
- IL-1
- interleukin-1
- OR
- odds ratio
- Phet
- P-value for heterogeneity
- Pz
- P-value for overall effect
- SA
- South Asian
- SEA
- South East Asian
- EA
- East Asian
- NA
- North Africa
For IL1B (-511) polymorphism: dominant (TT + TC vs CC), recessive (TT vs TC + CC), overdominant (CT vs CC+TT), codominant (CT vs CC), codominant # (CT vs TT) and homozygote (TT vs CC).
For IL1RN (VNTR) polymorphism: dominant (2*2* + 2*1* vs 1*1*), recessive (2*2* vs 2*1* + 1*1*), overdominant (2*1* vs 1*1*+2*2*), codominant (2*1* vs 1*1*), codominant # (2*1*vs 2*2*) and homozygote (2*2* vs 1*1*).
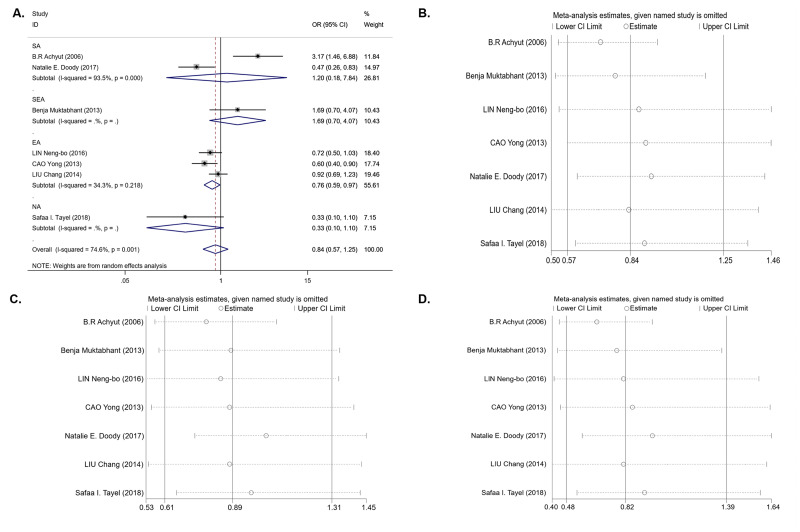
For the IL-1B (-511) polymorphism, seven studies with 1,679 cases and 1,442 controls were included in the meta-analysis. The results of the pooled analysis suggest that the IL-1B (-511) polymorphism is not significantly associated with T2DM risk in all study subjects under the dominant model (OR = 0.84, 95% CI [0.57–1.25], Phet = 0.001, Pz = 0.395) (Table 3 and Fig. 2A), recessive model (OR = 0.89, 95% CI [0.61–1.31], Phet < 0.001, Pz = 0.561) (Table 3), and homozygous model (OR = 0.82, 95% CI [0.48–1.39], Phet < 0.001, Pz = 0.451) (Table 3). In the stratification analyses by ethnicity, we found that there was a significant association between the IL-1B (-511) polymorphism and decreasing T2DM risk in the EA population under the dominant model (OR = 0.76, 95% CI [0.59–0.97], Phet = 0.218, Pz = 0.027) (Table 3 and Fig. 2A) and codominant model (OR = 0.73, 95% CI [0.54–0.99], Phet = 0.141, Pz = 0.040) (Table 3), but no association was observed under the recessive model (OR = 0.97, 95% CI [0.79–1.20], Phet = 0.589, Pz = 0.789) or homozygous model (OR = 0.80, 95% CI [0.62–1.03], Phet = 0.571, Pz = 0.081). In other ethnic groups, no association between the IL-1B (-511) polymorphism and T2DM risk was found. Between-study heterogeneity was found in the dominant, recessive and homozygous models (Phet = 0.001, Phet< 0.001, Phet< 0.001) (Table 3).
Figure 2. An overall forest plot of IL-1B (-511) associated with T2DM risk in the dominant model (TT + TC vs CC) and sensitivity analyses.
(A) Forest plot of IL-1B (-511) associated with T2DM in the dominant model; (SA: South Asian, SEA: South East Asian, EA: East Asian, NA: North Africa). (B) Sensitivity analysis for TT + TC vs CC associated with T2DM in the overall meta-analysis. (C) Sensitivity analysis for TT vs TC + CC associated with T2DM in the overall meta-analysis. (D) Sensitivity analysis for TT vs CC associated with T2DM in the overall meta-analysis.
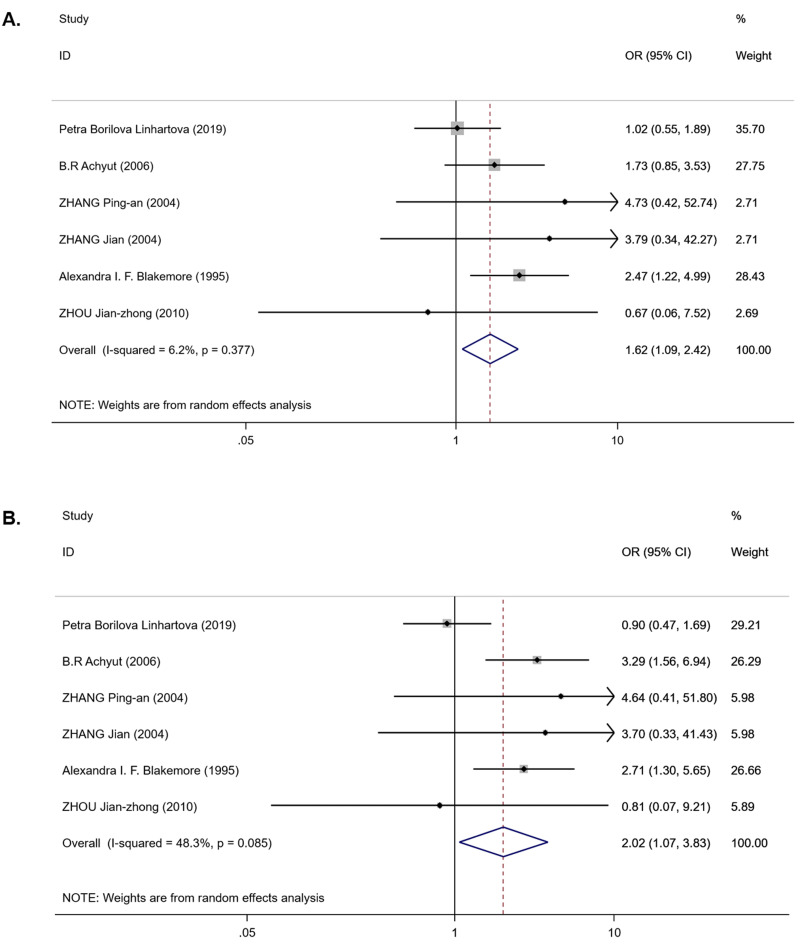
For the IL-1RN (VNTR) polymorphism, six publications were involved in the meta-analysis (980 cases and 1,225 controls). Overall, a significantly increased T2DM risk was found to be associated with 2* allele in all ethnicities (OR = 2.08, 95% CI [1.43–3.02], Phet < 0.001, Pz < 0.001) and in EA (OR = 2.01, 95% CI [1.53–2.66], Phet = 0.541, Pz < 0.001) (Table 3). Pooling data also revealed that this polymorphism is strongly associated with an increasing T2DM risk in the recessive model (OR = 1.62, 95% CI [1.09–2.42], Phet = 0.377, Pz = 0.018) (Table 3 and Fig. 3A) and homozygous model (OR = 2.02, 95% CI [1.07–3.83], Phet = 0.085, Pz = 0.031) (Table 3 and Fig. 3B), but there was no association in the dominant model (OR = 1.35, 95% CI [0.79–2.31], Phet < 0.001, Pz = 0.275) (Table 3). Between-study heterogeneity was found in the dominant model (Phet < 0.001) (Table 3).
Figure 3. Forest plot of meta-analysis on IL-1RN (VNTR) and the risk of T2DM in the recessive model ( 2*2* vs 2*1* + 1*1*) and homozygous model (2*2* vs 1*1*).
(A) Forest plot of IL-1RN (VNTR) associated with T2DM in the recessive model. (B) Forest plot of IL-1RN (VNTR) associated with T2DM in the homozygous model.
Sensitivity and publication bias analysis
We next performed sensitivity analysis on the association between IL-1β (-511) and T2DM. The results showed that Achyut’s study had an influence on the pooled OR in the IL-1β (-511)-dominant model (OR = 0.71, 95% CI [0.53–0.96]) and homozygous model (OR = 0.65, 95% CI [0.44–0.97]) (Figs. 2B, 2D) but did not affect the pooled OR in the recessive model (Fig. 2C). Achyut’s study was performed in a SA population; thus, there was no influence on the pooled OR in the EA population. Due to the lack of heterogeneity in IL-1RN recessive and homozygous models, sensitivity analysis was not performed.
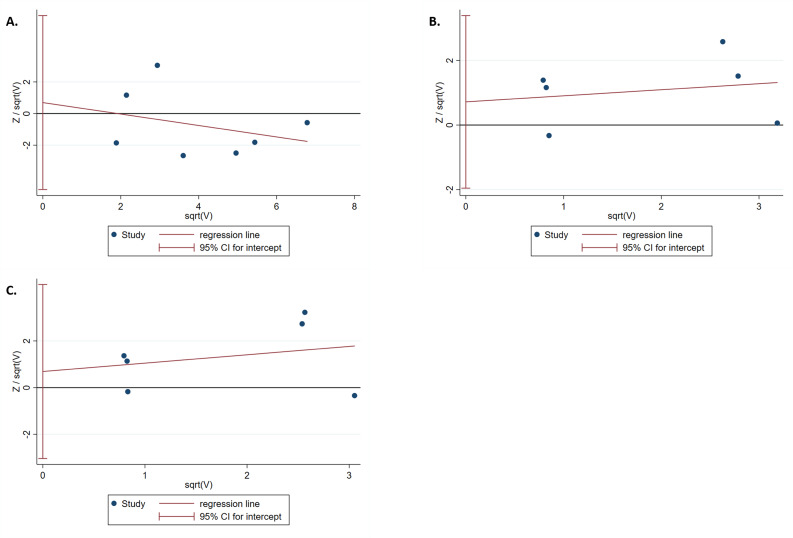
The results of the Harbord test and Peters test showed no publication bias for IL-1B (-511) in the dominant model (Phar = 0.759 and Ppet = 0.881, respectively) (Fig. 4A). Moreover, no evidence of publication bias was found for IL-1RN (VNTR) in the recessive (Phar = 0.498 and Ppet = 0.495, respectively) or homozygous models (Phar = 0.634 and Ppet = 0.481, respectively) (Figs. 4B, 4C).
Figure 4. Harbord test for publication bias testing.
(A) Publication bias of IL-1B (-511) associated with T2DM in the dominant model (TT + TC vs CC); (B) Publication bias of IL-1RN (VNTR) associated with T2DM in the recessive model (2*2* vs 2*1* + 1*1*); (C) Publication bias of IL-1RN (VNTR) associated with T2DM in the homozygous model(2*2* vs 1*1*).
Discussion
The IL-1B (-511) and IL-1RN (VNTR) polymorphisms have been reported to be related to the pathogenesis of T2DM (Ehses et al., 2009; Masters et al., 2010), and many case-control studies have illustrated the association of IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk. However, each individual study may not have been powerful, causing their results to be controversial. Given the above, we conducted the present meta-analysis to derive a more precise evaluation of the association of IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk. We found that (i) the T allele of IL-1B (-511) was related with a decreased T2DM risk in the EA subgroup; (ii) significantly elevated risk of developing T2DM was observed to be associated with the 2* allele in all ethnicities and EA subgroup, and its carriers of the IL-1RN (VNTR) polymorphism had an increased risk of developing T2DM among all ethnicities; (iii) compared to the 1*/1* homozygotes, the 2*/2* homozygotes of the IL-1RN (VNTR) polymorphism had an increased risk of T2DM development; and (iv) no significant association was observed between the IL-1RN (VNTR) polymorphism and T2DM risk in the EA subgroup. The results of sensitivity analysis showed that Achyut’s study affected the pooled ORs in the IL-1β (-511) dominant and homozygous models. However, Achyut’s study did not influence the pooled OR in the EA subgroup after stratification by ethnicity. Therefore, the result of the association between IL-1B (-511) and the decreased risk of T2DM in the dominant model in the EA subgroup was convincing.
First, IL-1β was considered a mediator of fever, and rapidly, it was found that IL-1β induced innate immunity to defend against pathogens. However, chronic overexpression of IL-1β has been related to multiple immune diseases, including T2DM (Mandrup-Poulsen, 1996). Eventually, it was suggested that IL-1β can induce the inflammatory microenvironment of islets, leading to impaired insulin secretion, decreased cell proliferation and apoptosis of β-cells, ultimately contributing to the development of T2DM (Rhodes, 2005). Donath, M Y et al. extensively described the development of islet inflammation in GK rats, suggesting that the expression of pro-inflammatory cytokines IL-1β and others (IL-6, TNFα) was increased in islets, while elevated expression levels of many chemokines (CXCL1/KC, MCP-1, MIP-1α) and infiltration of immune cells in islets were observed (Ehses et al., 2009). In all cases, macrophage infiltration is increased in the islet inflammatory process (Ehses et al., 2007). Moreover, two independent studies have now indicated that the numbers of islet-related CD68+ cells are increased in T2DM patients (Ehses et al., 2007; Richardson et al., 2009). In addition, data obtained from laser-captured β-cells from T2DM patients have shown that evaluated expression levels of IL-1β and chemokines possibly led to this immune cell infiltration (Böni-Schnetzler et al., 2008). Since it can regulate various inflammatory processes, any change in the level of IL-1β in blood or tissue possibly affects these processes. Larsen et al. suggested that blocking IL-1β is a potential therapy in the treatment of T2DM (Larsen et al., 2007). The IL-1B (-511) C allele is associated with higher expression of IL-1β and with severe inflammation in the liver, while the (-511) T allele is associated with lower levels of IL-1β (Hirankarn et al., 2006; Vishnoi et al., 2008). In this meta-analysis, we found that the IL-1B (-511) T allele had a decreased T2DM risk in the EA subgroup, suggesting an influence of the IL-1B (-511) polymorphism system in this ethnic group.
The IL-1RN 2* allele (two repeats) is associated with increased IL-1Ra levels (Danis et al., 1995), which can compete with an inhibitor of IL-1β by binding to the IL-1 receptor. The 2* allele significantly increases the IL-1β secretion in vitro and balances the expression of IL-1β and IL-1Ra (Santtila, Savinainen & Hurme, 1998), and their ratio (IL-1Ra/IL-1β) determines the severity of inflammation. It has been shown that the IL-1RN 2* allele is associated with a low ratio (IL-1Ra/IL-1β), thereby inducing a longer and more severe pro-inflammation (Witkin, Gerber & Ledger, 2002). The frequency of the 2* allele is increased in inflammatory conditions or autoimmune diseases (Tountas et al., 1999; Van der Paardt et al., 2002). Consistently, this meta-analysis suggested that the IL-1RN 2* allele and 2*2* homozygotes increased T2DM risk, which indicated that the IL-1RN 2* allele and 2*2* homozygote polymorphism play critical roles in the development of T2DM.
Although we have devoted considerable efforts and resources to testing the potential association of the IL-1B (-511) and IL-1RN (VNTR) polymorphisms with T2DM risk, this analysis still has some limitations. First, significant heterogeneity was found in pooled analyses between the IL-1B (-511) polymorphism and T2DM risk. Due to the limited number of included publications, we conducted further ethnic stratification analysis and meta-regression to identify the exact sources of between-study heterogeneity. The meta-regression results showed that ethnicity was not the source of heterogeneity (both values of tau2 were not much different (0.34 vs 0.40)). Some possible relevant factors (genotyping method, sex) may lead to heterogeneity. However, subgroup analysis can significantly reduce heterogeneity. Second, the literature articles were primarily published in English or Chinese; thus, some eligible publications may be missing, causing some bias. Third, the publications included have some confounders, such as age, sex, diet, and exercise, whereas we used only the raw data to conduct pooled analysis; therefore, we were unable to control these possible confounders or test the potential gene-environment interactions. Fourth, the number of included publications was limited; thus, additional studies with large sample sizes and a wider variety of ethnicities are needed in the future to evaluate the association.
In conclusion, our meta-analysis first indicated that the IL-1B (-511) T allele polymorphism is associated with decreased T2DM risk in the EA population and that the IL-1RN 2* allele and 2*2 * homozygote polymorphism are strongly associated with increased T2DM risk. Further well-designed studies including different ethnicities with large sample sizes are needed to verify this conclusion.
Supplemental Information
Abbreviations
- T2DM
type 2 diabetes mellitus
- IL-1
Interleukin-1
- IL-1Ra
IL-1 receptor antagonist
- IL-1β
interleukin-1β
- HWE
Hardy–Weinberg Equilibrium
- OR
odds ratios
- CIs
confidence intervals
- EA
East Asian
Funding Statement
This work was supported by the National Natural Science Foundation of China (81870552, 81400790, 81600622, 81872096, 81571385, 91849118, 91849132, and 81600622), the National Key R&D Program of China (2018YFC2000400), Beijing Hospital Doctoral Scientific Research Foundation (BJ-2018-024), Beijing Hospital Nova Project (BJ-2018-139), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018RC330003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Juan Jiao and Zhaoping Wang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yanfei Guo, Xiuqing Huang, Liang Sun, Xiaoquan Zhu and Qi Zhou analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Jie Liu and Ze Yang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xiaolin Ni and Danni Gao performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Huiping Yuan conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
References
- Achyut et al. (2007).Achyut BR, Srivastava A, Bhattacharya S, Mittal B. Genetic association of interleukin-1beta (-511C/T) and interleukin-1 receptor antagonist (86 bp repeat) polymorphisms with Type 2 diabetes mellitus in North Indians. Clinica Chimica Acta. 2007;377:163–169. doi: 10.1016/j.cca.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Aday & Ridker (2018).Aday AW, Ridker PM. Antiinflammatory therapy in clinical care: the CANTOS trial and beyond. Frontiers in Cardiovascular Medicine. 2018;5:62. doi: 10.3389/fcvm.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore et al. (1996).Blakemore AI, Cox A, Gonzalez AM, Maskil JK, Hughes ME, Wilson RM, Ward JD, Duff GW. Interleukin-1 receptor antagonist allele (IL1RN*2) associated with nephropathy in diabetes mellitus. Human Genetics. 1996;97:369–374. doi: 10.1007/bf02185776. [DOI] [PubMed] [Google Scholar]
- Borilova Linhartova et al. (2019).Borilova Linhartova P, Poskerova H, Tomandlova M, Bartova J, Kankova K, Fassmann A, Izakovicova Holla L. Interleukin-1 gene variability and plasma levels in Czech patients with chronic periodontitis and diabetes mellitus. International Journal of Dentistry. 2019;2019:6802349. doi: 10.1155/2019/6802349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler & Donath (2013).Böni-Schnetzler M, Donath MY. How biologics targeting the IL-1 system are being considered for the treatment of type 2 diabetes. British Journal of Clinical Pharmacology. 2013;76:263–268. doi: 10.1111/j.1365-2125.2012.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler et al. (2008).Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. Journal of Clinical Endocrinology and Metabolism. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2013).Cao Y, Wan Q, Tang XP, Song XQ, Chen Z, Chen F. The relationship between interleukin -1β- 511 (C/T) gene polymorphism and type 2 diabetes mellitus. Immunological Journal. 2013;29:503–506. [Google Scholar]
- Danis et al. (1995).Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clinical and Experimental Immunology. 1995;99:303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian & Laird (1986).DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dinarello (2000).Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. New England Journal of Medicine. 2000;343:732–734. doi: 10.1056/nejm200009073431011. [DOI] [PubMed] [Google Scholar]
- Dinarello, Donath & Mandrup-Poulsen (2010).Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1 beta in type 2 diabetes. Current Opinion in Endocrinology Diabetes and Obesity. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- Donath & Halban (2004).Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- Doody et al. (2017).Doody NE, Dowejko MM, Akam EC, Cox NJ, Bhatti JS, Singh P, Mastana SS. The Role of TLR4, TNF-α and IL-1β in Type 2 Diabetes Mellitus development within a North Indian population. Annals of Human Genetics. 2017;81:141–146. doi: 10.1111/ahg.12197. [DOI] [PubMed] [Google Scholar]
- Duan et al. (2018).Duan L, Xue Z, Ji H, Zhang D, Wang Y. Effects of CYP2R1 gene variants on vitamin D levels and status: a systematic review and meta-analysis. Gene. 2018;678:361–369. doi: 10.1016/j.gene.2018.08.056. [DOI] [PubMed] [Google Scholar]
- Ehses et al. (2009).Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses et al. (2007).Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Fève & Bastard (2009).Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nature Reviews Endocrinology. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- Hirankarn et al. (2006).Hirankarn N, Kimkong I, Kummee P, Tangkijvanich P, Poovorawan Y. Interleukin-1 beta gene polymorphism associated with hepatocellular carcinoma in hepatitis B virus infection. World Journal of Gastroenterology. 2006;12:776–779. doi: 10.3748/wjg.v12.i5.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hivert, Vassy & Meigs (2014).Hivert MF, Vassy JL, Meigs JB. Susceptibility to type 2 diabetes mellitus–from genes to prevention. Nature Reviews Endocrinology. 2014;10:198–205. doi: 10.1038/nrendo.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil (2006).Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil (2017).Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- Kristiansen et al. (2000).Kristiansen OP, Pociot F, Johannesen J, Bergholdt R, Dinarello CA, Nerup J, Mandrup-Poulsen T. Linkage disequilibrium testing of four interleukin-1 gene-cluster polymorphisms in Danish multiplex families with insulin-dependent diabetes mellitus. Cytokine. 2000;12:171–175. doi: 10.1006/cyto.1999.0546. [DOI] [PubMed] [Google Scholar]
- Larsen et al. (2007).Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New England Journal of Medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2016).Lin NB, Tang XP, Cao Y, Wan Q. Association between interleukin-1β-511 (C/T) gene polymorphism and early diabetic kidney disease. Chinese Journal of Diabetes. 2016:407–411. doi: 10.3969/j.issn.1006-6187.2016.05.005. [DOI] [Google Scholar]
- Little et al. (2009).Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, Scheet P, Gwinn M, Williamson RE, Zou GY, Hutchings K, Johnson CY, Tait V, Wiens M, Golding J, Van Duijn C, McLaughlin J, Paterson A, Wells G, Fortier I, Freedman M, Zecevic M, King R, Infante-Rivard C, Stewart A, Birkett N. Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE statement. PLOS Medicine. 2009;6:e22. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu C, Wang YX, Wu ZX, Zhao Y, Chi XM. Study on the correlation between IL-1β Rs16944 gene polymorphism and Type 2 Diabetes Mellitus in Han People of Liaoning Province. Journal of Clinical Research. 2014;3:426–428. doi: 10.3969/j.issn.1671-7171.2014.03.004. [DOI] [Google Scholar]
- Lo, Mertz & Loeb (2014).Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Medical Research Methodology. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen (1996).Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–1029. doi: 10.1007/bf00400649. [DOI] [PubMed] [Google Scholar]
- Masters et al. (2010).Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KHG, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LAJ. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1 beta in type 2 diabetes. Nature Immunology. 2010;11:897–U1501. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher et al. (2009).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muktabhant et al. (2013).Muktabhant B, Duangjinda M, Sanchaisuriya P, Kananit S, Schelp FP. Association of the polymorphism of interleukin-1B (-511C/T) with type 2 diabetes mellitus patients with and without family history of a first degree relative with diabetes mellitus. International Journal of Diabetes in Developing Countries. 2013;33:236–240. doi: 10.1007/s13410-013-0155-x. [DOI] [Google Scholar]
- Qamar & Rader (2012).Qamar A, Rader DJ. Effect of interleukin 1β inhibition in cardiovascular disease. Current Opinion in Lipidology. 2012;23:548–553. doi: 10.1097/MOL.0b013e328359b0a6. [DOI] [PubMed] [Google Scholar]
- Rhodes (2005).Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Richardson et al. (2009).Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- Rissanen et al. (2012).Rissanen A, Howard CP, Botha J, Thuren T. Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2012;14:1088–1096. doi: 10.1111/j.1463-1326.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- Ruscitti et al. (2019).Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, Cantatore FP, Carlino G, D’Abrosca V, Frassi M, Frediani B, Iacono D, Liakouli V, Maggio R, Mulè R, Pantano I, Prevete I, Sinigaglia L, Valenti M, Viapiana O, Cipriani P, Giacomelli R. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLOS Medicine. 2019;16:e1002901. doi: 10.1371/journal.pmed.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santtila, Savinainen & Hurme (1998).Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scandinavian Journal of Immunology. 1998;47:195–198. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Sathyapalan & Atkin (2011).Sathyapalan T, Atkin SL. Is there a role for immune and anti-inflammatory therapy in type 2 diabetes? Minerva Endocrinologica. 2011;36:147–156. [PubMed] [Google Scholar]
- Spranger et al. (2003).Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Tarlow et al. (1993).Tarlow JK, Blakemore AI, Lennard A, Solari R, Hughes HN, Steinkasserer A, Duff GW. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Human Genetics. 1993;91:403–404. doi: 10.1007/bf00217368. [DOI] [PubMed] [Google Scholar]
- Tayel et al. (2018).Tayel SI, Fouda EAM, Elshayeb EI, Eldakamawy ARA, El-Kousy SM. Biochemical and molecular study on interleukin-1β gene expression and relation of single nucleotide polymorphism in promoter region with Type 2 diabetes mellitus. Journal of Cellular Biochemistry. 2018;119:5343–5349. doi: 10.1002/jcb.26667. [DOI] [PubMed] [Google Scholar]
- Tountas et al. (1999).Tountas NA, Casini-Raggi V, Yang H, Di Giovine FS, Vecchi M, Kam L, Melani L, Pizarro TT, Rotter JI, Cominelli F. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999;117:806–813. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- Van der Paardt et al. (2002).Van der Paardt M, Crusius JB, García-González MA, Baudoin P, Kostense PJ, Alizadeh BZ, Dijkmans BA, Peña AS, van der Horst-Bruinsma IE. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms in ankylosing spondylitis. Rheumatology. 2002;41:1419–1423. doi: 10.1093/rheumatology/41.12.1419. [DOI] [PubMed] [Google Scholar]
- Vishnoi et al. (2008).Vishnoi M, Pandey SN, Choudhuri G, Mittal B. IL-1 gene polymorphisms and genetic susceptibility of gallbladder cancer in a north Indian population. Cancer Genetics and Cytogenetics. 2008;186:63–68. doi: 10.1016/j.cancergencyto.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Witkin, Gerber & Ledger (2002).Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clinical Infectious Diseases. 2002;34:204–209. doi: 10.1086/338261. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2004b).Zhang PA, Li Y, Xu P, Wen ZY. Study on the correlation of Interleukin-1 Receptor antagonist genotype with NIDDM. Journal of Clinical Research. 2004b:870–873. [Google Scholar]
- Zhang, Xiao & Li (2004a).Zhang J, Xiao Q, Li WN. Study of Interleukin-1 Receptor antagonist gene polymorphism in type 2 Diabetes mellitus. Journal of Laboratory Medicine. 2004a:15–17. [Google Scholar]
- Zhou et al. (2010).Zhou JZ, Zhou Y, Zhong WB, Li HW. Study on correlation between interleukin-1 receptor antagonist gene polymorphism and type 2 diabetes. Clinical Journal of Medical Officers. 2010;38:235–237. doi: 10.3969/j.issn.1671-3826.2010.02.028. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability: