Abstract
Background and aims
Vitamin C has been used as an anti-oxidant in various diseases including viral illnesses like coronavirus disease (COVID-19).
Methods
Meta-analysis of randomized controlled trials (RCT) investigating the role of vitamin C supplementation in COVID-19 was carried out.
Results
Total 6 RCTs including n = 572 patients were included. Vitamin C treatment didn't reduce mortality (RR 0.73, 95% CI 0.42 to 1.27; I2 = 0%; P = 0.27), ICU length of stay [SMD 0.29, 95% CI -0.05 to 0.63; I2 = 0%; P = 0.09), hospital length of stay (SMD -0.23, 95% CI -1.04 to 0.58; I2 = 92%; P = 0.57) and need for invasive mechanical ventilation (Risk Ratio 0.93, 95% CI 0.61 to 1.44; I2 = 0%; P = 0.76). Further sub-group analysis based on severity of illness (severe vs. non-severe), route of administration (IV vs. oral) and dose (high vs. low) failed to show any observable benefits.
Conclusion
No significant benefit noted with vitamin C administration in COVID-19. Well-designed RCTs with standardized control group needed on this aspect.
Keywords: Vitamin C, Ascorbic acid, l-ascorbate, Coronavirus, COVID 19, COVID penumonia, SARS-CoV-2
1. Introduction
The coronavirus (COVID-19) pandemic has wreaked havoc across the globe causing 226,844,344 cases till 14th September 2021 and nearly 4.7 crore deaths [1]. This virus leads to increase in pro-inflammatory cytokines and acute serum biomarkers (CRP, ferritin, D-dimer etc) [2]. The initial viral cytopathic effects, followed later on by ‘cytokine storm’ may bring about major health related adverse effects like acute respiratory distress syndrome (ARDS) [3,4]. Different anti-inflammatory interventions e.g. steroids, vitamins, micronutrients and immunomodulating agents have been tried till date. Vitamin C, also known as ascorbic acid, is well known for its anti-inflammatory and free radical scavenging properties [5,6]. It may also augment vasopressor and cortisol synthesis, influence leucocyte functioning via neutrophil extracellular traps (NET), thereby strengthening the armamentarium against various pathogens including viruses [[6], [7], [8]]. Previously vitamin C has been shown to reduce mortality, vasopressor usage and organ failure in sepsis patients [9,10], however, larger randomized trials failed to show any major health benefits [11,12]. Considerable controversy still exists regarding vitamin C supplementation among various systematic review and meta-analyses, owing to the diversified methodology of included studies. Though anecdotal reports suggested beneficial role of vitamin C in COVID [13], subsequent larger trials reported variable outcomes [[14], [15], [16], [17], [18], [19]]. Therefore, we endeavoured to evaluate the clinical efficacy of vitamin C administration in COVID infected patients.
2. Aims and objectives
The aim of this study was to find out the impact of Vitamin-C administration on major clinical outcomes (mortality, ICU admission, hospital stay, mechanical ventilation) in patients diagnosed with COVID-19.
3. Methodology
Preferred reporting items for systematic review and meta-analysis (PRISMA-2020) statement for conducting meta-analysis was followed [20]. This trial was prospectively registered in Prospero (CRD42021278213) [21].
3.1. Search strategy
A comprehensive literature search was carried out using the pre-defined search strategy ("ascorbic acid" OR "vitamin C" OR "Sodium Ascorbate" OR "L-ascorbic") AND ("coronavirus" OR "COVID 19" OR "COVID-19" OR "Corona" OR "COVID" OR "SARS-CoV2") published on PubMed, Embase, Scopus, Clinical Trial registry bodies [e.g. Clinical Trial Registry of India (CTRI)] and Google Scholar since inception till 18th September 2021 along with manual search to retrieve other articles. All the citing references were further checked and the first/corresponding author of registered trials were approached via emails. Those who replied with full published data were included. PRISMA 2021 statement was used for conducting meta-analysis.
3.2. Inclusion criteria
We wanted to evaluate the true role of vitamin C across all categories of adult COVID patients (irrespective of disease severity), hence, included RCTs which included vitamin C in the intervention arm. The control arm comprised of either standard treatment (without vitamin C) and/or placebo. We included articles which reported any of the following outcomes either as primary or secondary outcomes-mortality, duration of hospital/ICU stay, incidence of mechanical ventilation. Data for mortality were taken until the longest follow up period mentioned in individual studies.
3.3. Exclusion criteria
Articles were excluded if they had one of the following-
-
•
Included pediatric (<18 years) patients.
-
•
Articles published in language other than English.
-
•
Study protocols/trials without results.
-
•
Observational/retrospective studies.
-
•
Pre-print data (not peer reviewed).
-
•
Articles that didn't mention any of the above clinical outcomes.
3.4. Study selection
The articles were screened for title and abstract initially using the pre-defined search strategy by DR and AR. Duplicates or any other irrelevant articles not meeting the inclusion criteria were removed, followed by full text screening for the final inclusion in the present review. Any disagreement regarding the article was sought by SM.
3.5. Data extraction
Relevant data including study design, setting, duration, disease severity, mode of administration, dose of the intervention, duration of the intervention, ICU length of stay, duration of hospital stay, mortality and IMV incidence were extracted by DR and AR for each article included in the review. To resolve the conflict, SM was approached to reach a consensus. For converting data described in median (range) or median (Interquartile range) to mean (SD), appropriate validated methods by Luo and Shi et al. [median(IQR)] and Hozo et al. [mean(SD)] were used [[22], [23], [24]]. Data were tabulated using excel (2019) spreadsheet.
3.6. Quality assessment
Methodological quality was as per Cochrane Systematic Review Guidelines and GRADE-PRO approach was used for rating the quality of a body of evidence. The individual studies were evaluated separately by AR and DR and in case of dilemma regarding quality assessment, issues were resolved after discussion with SM.
3.7. Statistical analysis
Effect measure for variables described in rates/proportion (mortality, incidence of mechanical ventilation) was done by Risk Ratio (RR) and for continuous variables Standard Mean Difference (SMD) was used. I2 was used to test the heterogeneity among the included studies. P-value less than 0.05 was considered to be statistically significant. Methodological quality was assessed using Review Manager software, version 5.4.
4. Results
4.1. Study characteristics
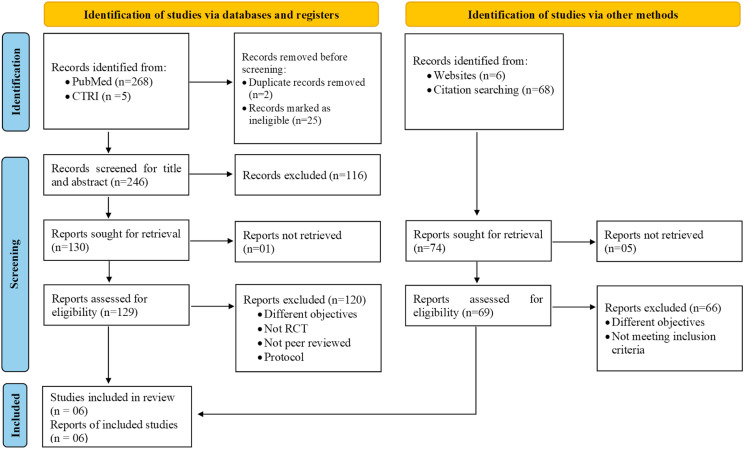
The initial search identified a total of 273 articles via database searching and 74 via manual searching. Authors of 7 trials were approached out of whom one replied with full published data [17]. After screening the title and abstract of all the retrieved articles followed by full text assessment, a total of 6 articles were included in the final review (Fig. 1 ; Table 1, Table 2, Table 3 ).
Fig. 1.
PRISMA 2020 flow diagram.
Table 1.
Study characteristics of all included studies (N = 6).
| S. No. | Author and Year | Study design | Study Setting | Duration | Disease severity | Sample Size (I/C) | Mean Age | Gender | Mode of administration | Dose of Vitamin-C | Intervention duration | Total cumulative dose of Vitamin-C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhang J et al., 2021 [14] | Multicenter, randomized trial | ICUs of 3 hospitals in Hubei, China Taihe Hospital | 14th February 14, 2020 to 29th March 2020 | Severe | 56 (27/29) | 66.7 ± 12.7 | Males: 66.1% | Central vein catheterization controlled by a pump (IV) | 24g/day | 7 days | 168g |
| 2 | Kumari P et al., 2020 [15] | Prospective, open-label RCT | A tertiary care hospital in Karachi, Pakistan | March to July 2020 | Severe | 150 (75/75) | I = 52 + 11; C = 53 + 12 | Males = 56.9%; Females = 43.1% | Intravenous | 50 mg/kg/day | Same as length of stay | 24g (∼) |
| 3 | Siahkali S et al., 2021 [19] | Open-label, non-blinded, randomized controlled trial | Ziaeian Hospital, Tehran, Iran | April and May 2020 | Severe | 60 (30/30) | C = 57.53 + 18.27; I = 61 + 15.90 | M = 50%; F = 50% | Intravenous | 6g/day | 5 days | 30g |
| 4 | Hakamifard A et al., 2021 [18] | Randomized controlled clinical trial | Amin hospital of Isfahan, Iran, affiliated to Isfahan University of Medical Sciences |
March to April 2020 | Non-severe | 72 (38/34) | I = 35.68; C = 37.41 | M = 63.9%; F = 36.1% | Oral | 1g/day | ||
| 5 | Darban M et al., 2021 [17] | Pilot single-center randomized, controlled, open-label, parallel-group trial | Kowsar Hospital, Semnan, Iran | Severe | 20 (10/10) | 59 ± 19 | M = 65%; F = 35% | Intravenous | 8g/day | 10 days | ||
| 6 | Thomas S et al., 2021 [16] | Multicenter, single health system factorial randomized open-label trial | Outpatient care in sites in Ohio and Florida | April 27, 2020, to October 14, 2020 | Non-severe | 214 (ASC vs. ST 48/50; ASC + Zn vs. Zn 58/58) | I = 47.15 + 14.65 (45.6 + 15 and 48.7 + 14.3); C = 42 + 14.6 | I = 64/106 female and 42/106 males; C = 31/50 female and 19/50 males | Oral | 8000 mg of ascorbic acid (to be divided over 2–3 times per day with meals) | 10 days |
Abbreviation: ASC = Ascorbic acid; ST=Standard.
Table 2.
PICO of all included studies (N = 6).
| S. No. | Author and Year | Population/Patient (P) | Intervention (I) | Comparator (C) | Outcome (O) | Standard Treatment |
|---|---|---|---|---|---|---|
| 1 | Zhang J et al., 2021 [14] | Patients with COVID pneumonia, having/at risk of multiple organs injury, P/F ratio <300 mmHg and admitted in the ICU, adults (age ≥18 and < 80 years) | Within 48 h after admission to the ICU high-dose intravenous vitamin C (24g/day): 12 g of vitamin C (diluted in 50 ml) BD for 7 days at a rate of 12 ml/h + standard therapy | Placebo: Bacteriostatic water for injection (same volume)+ Standard therapy | Primary: Invasive mechanical ventilation-free days in 28 days (IMVFD28). Secondary: (i)28-day mortality; (ii) organ failure [SOFA score]; (iii) inflammatory markers (IL-6, TLC, absolute neutrophil & lymphocyte counts, procalcitonin and CRP | Oseltamivir and azithromycin; LMWH; Piperacillin/tazobactan; hydrocortisone (1 mg/kg/day); Lung protective ventilation if IMV needed. |
| 2 | Kumari P et al., 2020 [15] | SARS-CoV-2 patients | 50 mg/kg/day of IV Vitamin C + standard therapy | Standard therapy | Treatment duration, hospital stay, need for invasive ventilation, mortality | Antipyretics, dexamethasone, and prophylactic antibiotics |
| 3 | Siahkali S et al., 2021 [19] | >18 year patients with confirmed (RTPCR based) or suspected COVID-19 [based on clinico-radiological pattern e.g. fever, dyspnea, dry cough and/or CT finding suggestive of COVID] and SpO2<93% at admission or >48h from the first COVID-19 treatment. | Vitamin C (1.5 g every 6 h, total 6 g daily)+standard therapy | Standard therapy alone | Primary: reduction in mortality, duration of hospital stay, and need for ICU admission. Secondary: Improvements in vitals (e.g. SpO2), clinical parameters | Oral lopinavir/ritonavir (400/100 mg) BD and single dose of oral hydroxychloroquine (400 mg) on the first day of hospitalization. |
| 4 | Hakamifard A et al., 2021 [18] | Adult patients with COVID based on lab (RT-PCR) test and/or CT scan. | Oral vitamin C 1 g daily and oral vitamin E (400 IU daily + standard treatment regimen. | Hydroxychloroquine 400 mg on the first day followed by 200 mg every 12 h. | Primary: Clinical response of at end of treatment in three ways: cure (complete elimination of clinical symptoms), improvement (elimination of some primary clinical symptoms), and failure (continued or exacerbated primary symptoms). Secondary: Duration of hospitalization, mortality, and change of lab variables. | Hydroxychloroquine or standard regimen as per national policy |
| 5 | Darban M et al., 2021 [17] | Adults with severe COVID-19 | IV vitamin C (2 g, every 6 hourly), oral melatonin (6 mg, 6 hourly), and oral zinc sulphate (50 mg, 6 hourly) for 10 days + standard therapy | Standard therapy alone | Changes in P/F ratio and inflammatory markers (LDH, ESR, CRP, ferritin) | Azithromycin (250 mg daily); lopinavir/ritonavir (100 mg/25 mg daily); glucocorticoids; Oxygen therapy. |
| 6 | Thomas S et al., 2021 [16] | Adult patients COVID-19; multiple treatment factorial trial | Three intervention groups: Group 1-zinc gluconate (50 mg), group 2-vitamin C (8 gm), Group 3: both agents along with standard care | Group 4: Standard care alone | Primary: Days needed to attain 50% reduction in symptoms based on questionnaire (Severity of each following symptoms: I)fever; II) cough; III) dyspnea; IV) fatigue rated on a 4-point scale). Secondary: Days needed to reach a total symptom severity score of 0, cumulative severity score at day 5, hospitalizations, mortality, adjunctive therapies, and adverse effects of the study supplements. | - |
Table 3.
List of all trials authors of whom were contacted and their reply.
| Trial/author(s) | Reply |
|---|---|
| IRCT20151228025732N52, Clinical trial registry of Iran | Received and study included∗ |
| IRCT20200516047468N1; Clinical trial registry of Iran | Study not published yet |
| ChiCTR2000032400; Clinical trial registry of China | No reply |
| NCT04363216; Clinical trials.gov.in | No reply |
| IRCT20200411047025N1; Clinical trial registry of Iran | No reply |
| IRCT20200324046850N5; Clinical trial registry of Iran | No reply |
| ChiCTR2000029768; Clinical trial registry of China | No reply |
4.2. Description of the included studies
All included studies in the present review had randomization involving 572 subjects, ranging from 20 to 214 subjects [[14], [15], [16], [17], [18], [19]]. Among the six included studies, three were from Iran [[17], [18], [19]], one from China [14], one from Pakistan(15) and one from United states [16]. Four studies were conducted on patients with disease severity as severe [14,15,17,19] and two as non-severe [16,18]. Route of administration was intravenous in fours studies [14,15,17,19] and oral in two studies [16,18]. Doses of vitamin C ranged from 50 mg/kg/day [15] to 24g/day [14]. In one study [16] patients were randomized in a 1:1:1:1 allocation ratio (zinc gluconate: ascorbic acid: both: standard). In this review, these 4 groups were sub divided into two groups for the purpose of analysis i.e., group 1: standard care vs ascorbic only and; group 2: zinc and ascorbic vs zinc.
4.3. Methodological quality of study
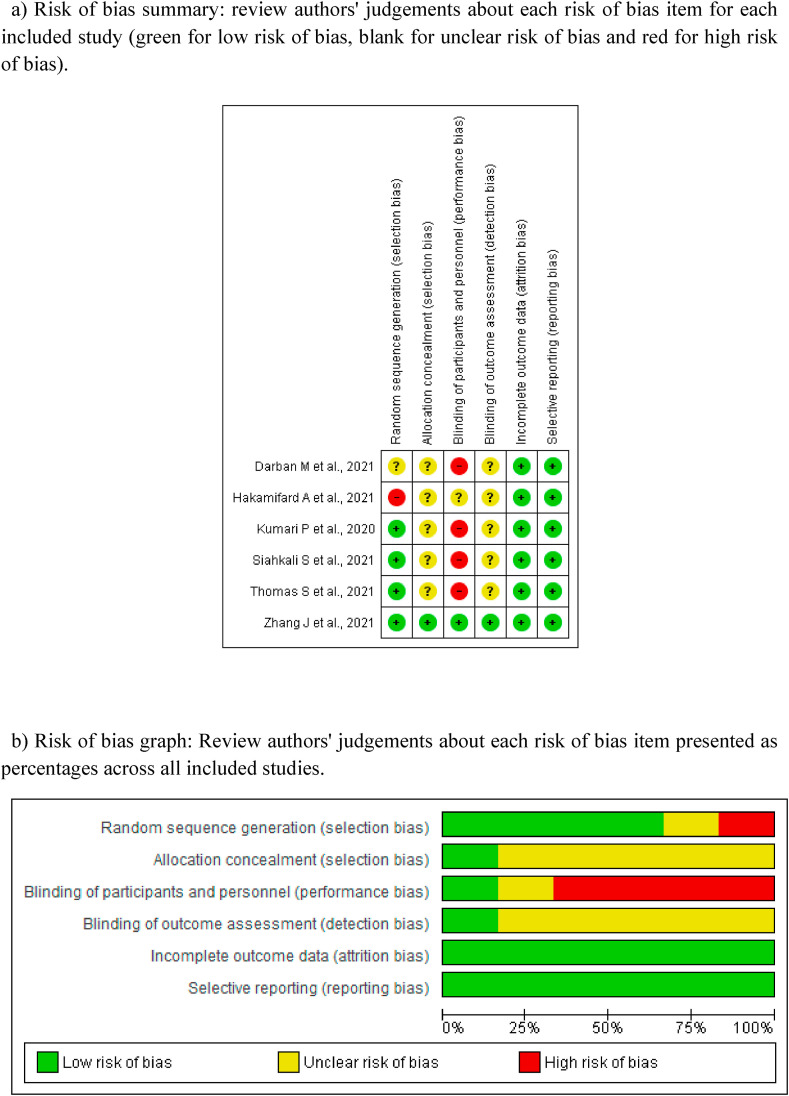
4.3.1) Risk of bias graph, review authors judgements about each risk of bias item are presented as percentages and risk of bias summary based on Cochrane Systematic Review Guidelines for each included study (green for low risk of bias, yellow for unclear risk of bias and red for high risk of bias) for randomised control trials is presented in Fig. 3a-3b.
Fig. 3.
[3a-3b]: Quality Assessment
3a) Risk of bias summary: review authors' judgements about each risk of bias item for each included study (green for low risk of bias, blank for unclear risk of bias and red for high risk of bias).
3b) Risk of bias graph: Review authors' judgements about each risk of bias item presented as percentages across all included studies.
Random sequence generation: Among all, Hakamifard A et al., 2021 [18] was rated as high risk due to absence of true randomization, Darban M et al., 2021 [17] as unclear risk of bias because the method of randomization was not stated and rest four [[14], [15], [16],19] at low risk of bias as Zhang J et al., 2021(14) used independent random numeric table generated by Microsoft Excel 2019 by the primary investigator alone and randomizer software was used by Kumari P et al., 2020 [15], whereas, Siahkali S et al., 2021(19) used block randomization and REDCap randomization software was used by Thomas S et al., 2021(16).
Allocation concealment: One study Zhang J et al., 2021 [14] was rated at low risk of bias, because the allocation was concealed adequately, the generated random list was stored by the principal investigator who was not involved in the treatment of patients and was hidden to the other investigators. Rest five were judged at unclear risk of bias [[15], [16], [17], [18], [19]] because either the method of allocation concealment was not mentioned [[16], [17], [18]] or it was unclear that who stored the generated random list [15,19].
Blinding of participants and personnel: Zhang J et al., 2021(14) was rated at low risk of bias because both the participants and trial personnel were blinded, the subjects were enrolled in the corresponding group according to the chronological order of ICU recruitment; also the grouping and intervention were unknown to the participants and investigator. Hakamifard A et al., 2021(18) did not mention about the blinding of participants and personnel, hence, was judged at unclear risk of bias. Rest four [[15], [16], [17],19] were open label trials, hence, were judged at high risk of bias.
Blinding of outcome assessment (Detection bias):: Zhang J et al., 2021 [14] was judged at low risk of bias as the grouping and intervention were unknown to the participants and investigators who were responsible for data collection and statistical analysis. Remaining five studies [[15], [16], [17], [18], [19]] were judged as unclear risk for bias as the blinding of outcome assessment were not mentioned.
Incomplete outcome data (Attrition bias): All included six studies [[14], [15], [16], [17], [18], [19]] reported data for all outcomes in results hence were judged at low risk of bias.
Selective reporting (Reporting bias): All included six studies [[14], [15], [16], [17], [18], [19]] were judged at low risk of bias for selective reporting.
4.3.2GRADE pro: The overall rating for the quality of evidence for the role of vitamin C supplementation in patients with COVID-19 is shown in the GRADE summary of finding Table 4 . GRADE summary reported the certainty of evidence as very low for the outcome duration of hospital stay and low for mortality, ICU admission and moderate for NIV incidence which means that any estimate of effect is very uncertain and we have little confidence in the effect.
Table 4.
The overall rating for the quality of evidence profile for COVID-19 related health outcomes based on the grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group methodology.
| Certainty assessment |
№ of patients |
Effect |
Certainty |
Importance |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Control | Relative (95% CI) | Absolute (95% CI) | ||||
| Mortality | ||||||||||||||
| 5 | randomised trials | serious a | not serious | serious b | not serious | none | 19/276 (6.9%) | 25/276 (9.1%) | RR 0.73 (0.42–1.27) | 24 fewer per 1,000 (from 53 fewer to 24 more) | ⨁⨁◯◯ LOW |
CRITICAL | ||
| ICU length of stay | ||||||||||||||
| 3 | randomised trials | not serious | not serious | very serious b | not serious | none | 67 | 69 | – | SMD 0.29 higher (0.05 lower to 0.63 higher) | ⨁⨁◯◯ LOW |
CRITICAL | ||
| Duration of hospital stay | ||||||||||||||
| 4 | randomised trials | serious a | very serious c | serious b | very serious d | none | 170 | 168 | – | SMD 0.23 lower (1.04 lower to 0.58 higher) | ⨁◯◯◯ VERY LOW |
CRITICAL | ||
| Invasive mechanical ventilation incidence | ||||||||||||||
| 3 | randomised trials | not serious | not serious | serious b | not serious | none | 28/132 (21.2%) | 31/134 (23.1%) | RR 0.93 (0.61–1.44) | 16 fewer per 1,000 (from 90 fewer to 102 more) | ⨁⨁⨁◯ MODERATE |
CRITICAL | ||
CI: Confidence interval; RR: Risk ratio; SMD: Standard mean difference.
Explanations.
True randomization not done (N = 1).
There were differences in the follow up time points to measure the outcomes along with vitamin C dose, route and duration.
I2 = 92%.
Confidence intervals are not narrow enough for us to be confident enough regarding the true effect of intervention.
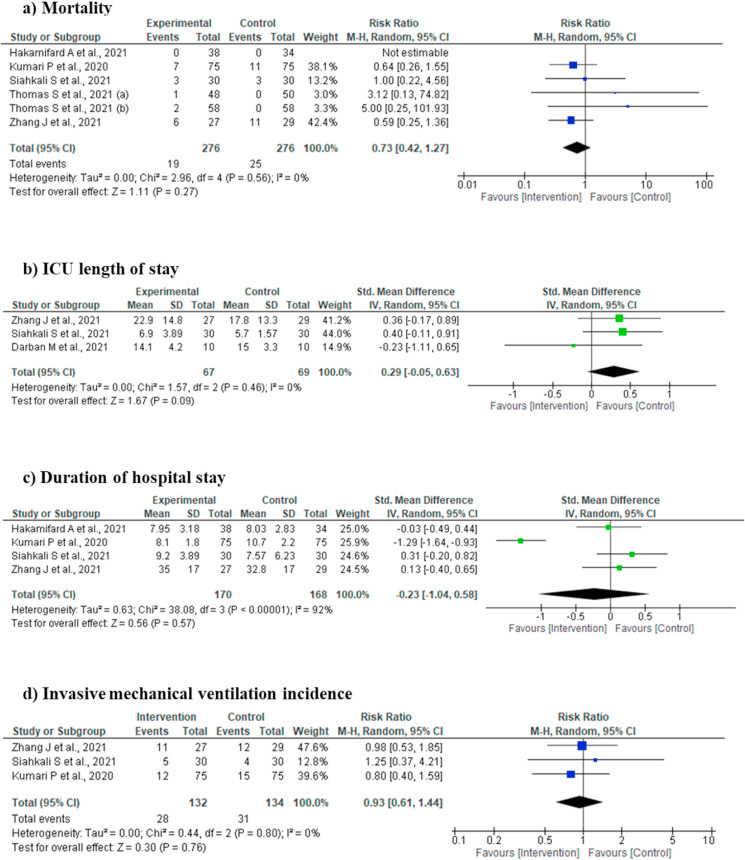
4.4. Efficacy outcomes (Fig. 2a-d)
Fig. 2.
[2a-2d]: Forest plot for various outcomes.
4.4.1. Mortality
Mortality was reported in five studies [[14], [15], [16],18,19], involving 552 subjects (276 intervention and 276 controls/placebo). In the pooled analysis, no statistically significant difference was observed as compared to controls/placebo (Risk Ratio 0.73, 95% CI 0.42 to 1.27; I2 = 0%; P = 0.27) (Fig. 2a).
4.4.2. ICU length of stay
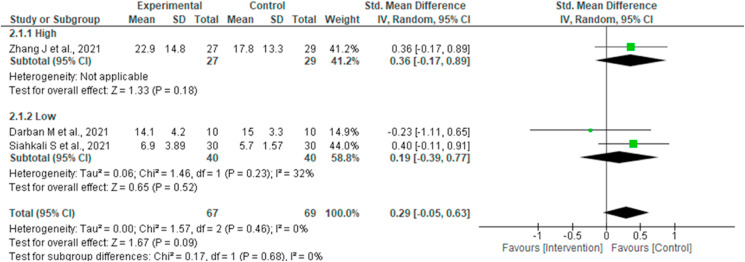
Three studies reported outcome ICU length of stay [14,17,19] involving 136 subjects (67 intervention and 69 controls/placebo). Pooled analysis did not show any statistically significant difference of the intervention for this outcome (SMD 0.29, 95% CI -0.05 to 0.63; I2 = 0%; P = 0.09) (Fig. 2b).
4.4.3. Duration of hospital stay
Four studies reported duration of hospital stay [14,15,18,19]. No statistically significant difference was observed in the pooled analysis (SMD -0.23, 95% CI -1.04 to 0.58; I2 = 92%; P = 0.57) (Fig. 2c).
4.4.4. Invasive mechanical ventilation (IMV) incidence
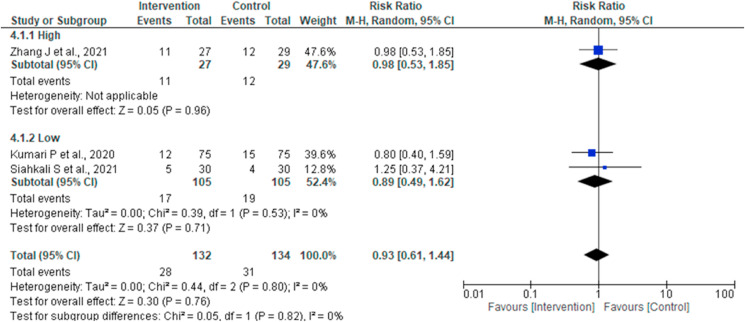
Three studies reported IMV incidence [14,15,19]. No statistically significant difference of the intervention for this outcome (Risk Ratio 0.93, 95% CI 0.61 to 1.44; I2 = 0%; P = 0.76) was noted in the pooled analysis (Fig. 2d).
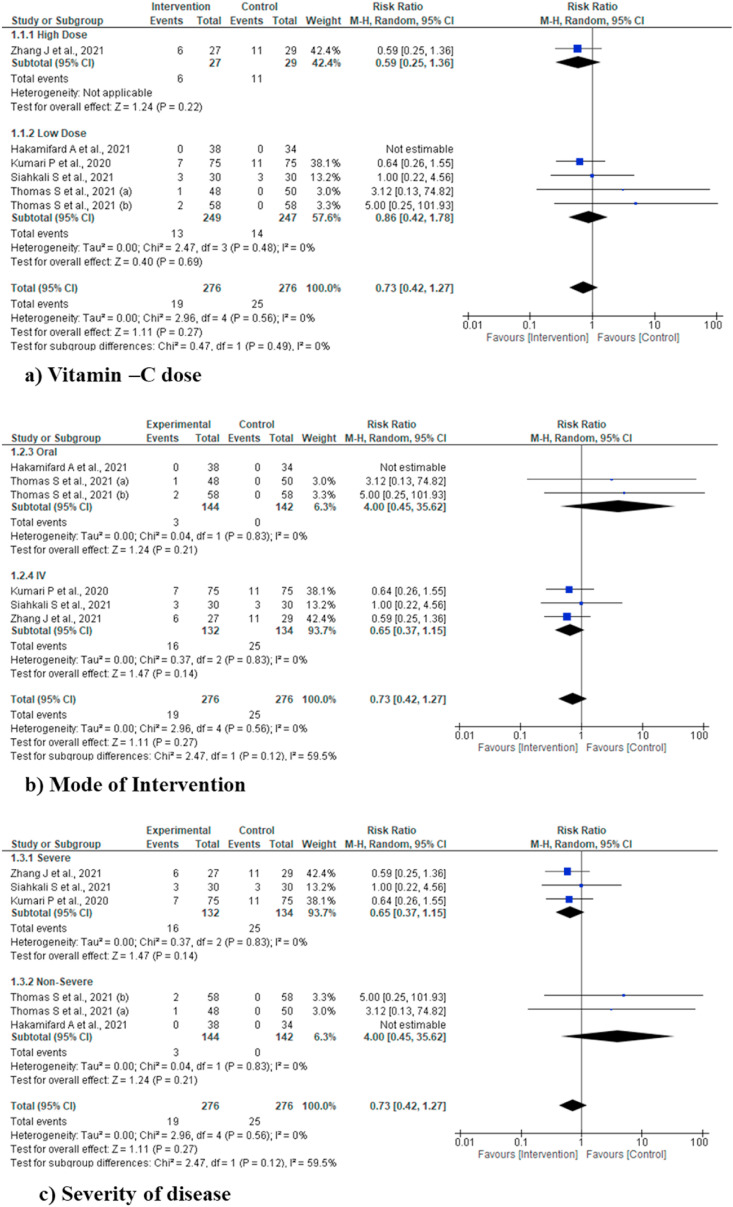
4.4.5. Sub-group analysis
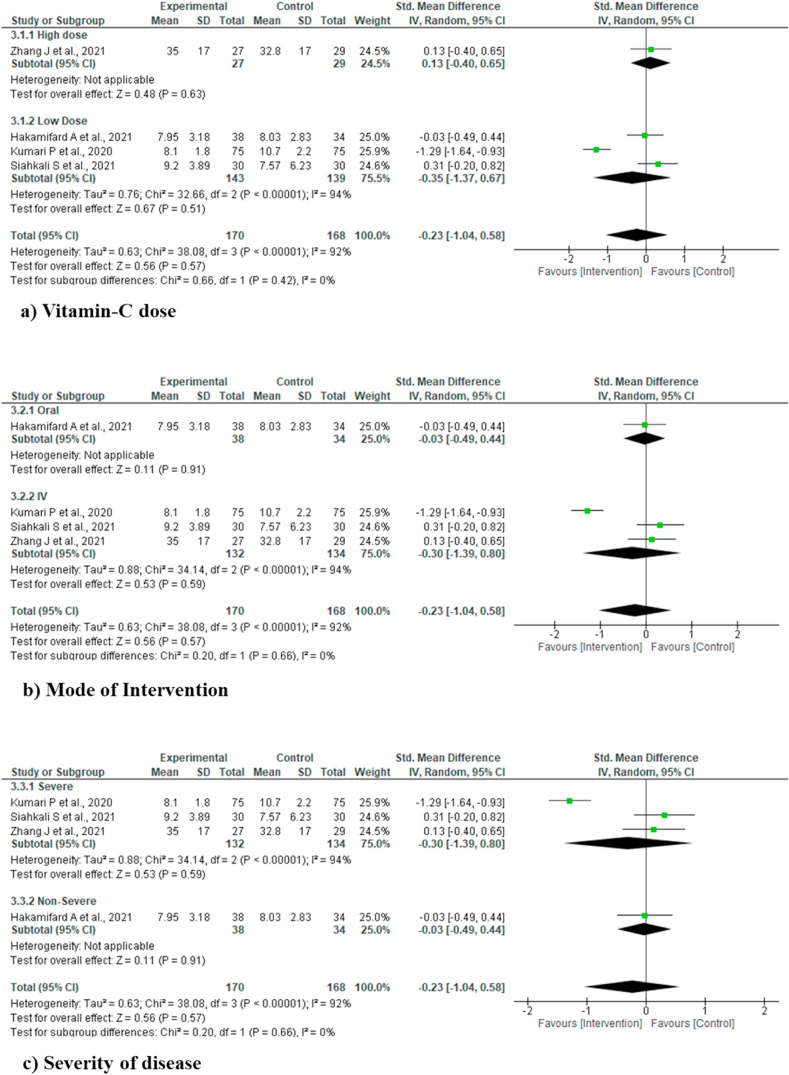
Sub-group analysis was performed for various outcomes including mortality, ICU length of stay, duration of hospital stay and IMV incidence for vitamin C dose (High and low dose), mode of intervention (oral and IV) and severity of disease (severe and non-severe), but did not find any statistically significant difference for any of the outcome and/or sub-groups. Due to clear cut lack of definition of high dose of vitamin C, we used the cut off value of >10 gm/day as high dose, based on two previous studies [10,25] (Fig. 4, Fig. 5, Fig. 6, Fig. 7 ).
Fig. 4.
Sub-group analysis for various outcomes (mortality).
Fig. 5.
Sub-group analysis for various outcomes (ICU length of stay).
Fig. 6.
Sub-group analysis for various outcomes (incidence of IMV).
Fig. 7.
Sub-group analysis for various outcomes (duration of hospital stay).
5. Discussion
The present meta-analysis showed that administration of vitamin C did not have any effect on major health outcomes in COVID infected patients, in comparison to either placebo/standard therapy. Sub-group analysis also revealed that irrespective of its dosage, route of administration and disease severity, it didn't have discernible benefit in such patients.
The SARS-CoV-2 may cause a pro-inflammatory state evidenced by raised serum levels of Interleukin-1,6 (IL-1,6) and tumor necrosis factor (TNF) etc., which may in turn lead to ‘cytokine storm’ and ARDS [2,26,27]. Various vitamins, anti-oxidants and immunomodulators have been investigated to curtail the disease progression. Vitamin C has been known to possess free radial oxygen and nitrogen scavenging properties along with anti-inflammatory effects [5]. Ascorbate augments synthesis of catecholamines [cofactor of enzyme Dopa beta-hydroxylase, which convert Dopa(Dihydroxy phenyl alanine) to Dopamine] and vasopressin [cofactor of peptidylglycine alpha-amidating mono-oxygenase(PAM)] [8]. In experimental animal models, it reduced organ dysfunction secondary to abdominal sepsis and gram negative bacteraemia [28,29], highlighting its role in stabilising leucocyte & NK cell function [7]. Meta-analysis of 9 trials which evaluated the effect of vitamin C in acute respiratory infections (ARI) showed that it reduced overall duration of symptoms and major complaints like chest pain and chills [30]. Various other case reports of high dose vitamin C halting disease progression in viral illnesses, highlighted its anti-viral properties [31]. In a retrospective propensity matched before after study carried out on (n = 94) patients with severe septic shock with elevated serum procalcitonin (>2 μg/ml) levels, combination therapy of 6 gm intravenous vitamin C (A), 200 mg hydrocortisone (H) and 400 mg thiamine (T) per day (HAT therapy) for 4 days reduced mortality by almost 5 times (8.5% with HAT therapy vs. 40.4% controls), as well as reduced vasopressor support, incidence of acute kidney injury (AKI) and serum procalcitonin levels [9]. In another phase I trial carried out on 24 patients, high dose (200 mg/kg/day) of IV vitamin C therapy showed promising results by reducing organ dysfunction (SOFA score) as compared to low dose(50 mg/kg/day) [10]. This study also highlighted possible role of vitamin C in attenuating sepsis induced endotheliopathy, evidenced by reduced thrombomodulin levels [10]. Another trial demonstrated reduction of dose and duration of vasopressor with 100 mg/kg/day of vitamin C therapy [32]. In burn patients (n = 31) with >30% body surface involvement, vitamin C therapy reduced volume of resuscitation fluid, wound edema and respiratory dysfunction [33].
Despite its promising role in preventing organ failure in sepsis, large trials failed to demonstrate improvement in major health related outcomes. In VITAMINS trial involving 211 septic shock patients, HAT therapy didn't reduce mortality or vasopressor free days as compared to hydrocortisone alone [11]. In a follow up phase II CITRIS-ALI trial on n = 167 patients, vitamin C didn't reduce organ failure score and inflammatory markers, despite reduction in 28 day mortality [12]. In a meta-analysis of heterogenous group of studies including those carried out in ICUs (n = 16) and cardiac surgery patients (n = 28), vitamin C reduced ICU & hospital stay along with incidence of post-operative atrial fibrillation, without having any effect on mortality [34]. Although findings of our meta-analysis matches with that of Putzu et al. [33], we included only RCTs in COVID infected patients. In another meta-analysis that included majorly cardiac surgery patients (13 out of 18 included studies), vitamin C reduced length of hospital stay and mechanical ventilation without any mortality benefit [35]. In a meta-analysis evaluating the effect of IV vitamin C that included 12 RCT and quasi RCTs, no mortality benefit was noted at doses >10 gm/day and <3 gm/day, though, reduction of vasopressor support and mechanical ventilation was seen. Interestingly, they showed a medium dose (3–10 gm/day) had mortality benefit, although no biologically plausible explanation was provided [30]. In comparison to our study, their population were functionally heterogenous including patients receiving vitamin C for prevention of contrast induced nephropathy, which could have contributed to this finding. A meta-analysis evaluating isolated effect of vitamin C on clinical outcomes showed reduced vasopressor dose and mechanical ventilation with similar mortality [36].
Findings of our study could be attributable to following reasons. Firstly, lack of universally accepted optimal dose and route of administration. Considering recommended dietary allowance of vitamin C to be 75–110 mg/day and a serum value of 50–70 μmol/L to be normal (<23 μmol/L deficient, <11 μmol/L very low) [37,38], as much as 40% ICU population may be vitamin C deficient, which in turn, is linked to excessive mortality [8,39]. Oral absorption of ascorbate becomes erratic due to saturation of transporter proteins in critical illness, so much so, that around 3 gm of intravenous replenishment is needed for attaining normal serum values [40,41]. Secondly, how ‘high’ should be considered high is not known. In this review, various dosages ranging from 1 to 24 gm/day have been used. For our convenience we assumed >10 gm/day as ‘high’ as in Fowler's study, 200 mg/kg/day (around 12 gm/day). Wang et al. also used the similar operational definition [25] for ‘high’ dose. Thirdly, as previously seen with meta-analysis of other vitamins/antioxidants in COVID, a theoretical benefit may not always extrapolate in to clinical benefit, as deficiency in particular vitamin may merely mark a disease process rather than its outcome [42]. Lastly, prospective administration after diagnosis of COVID, may not be as useful as physiologically replenished state prior to contracting the disease, echoing the notion ‘prevention is better than cure’.
6. Conclusion
Vitamin C therapy didn't reduce major health related outcomes in COVID patients. In sub-group analysis based on drug dose (high vs. low), route (IV vs. oral) and severity of illness (severe vs. non-severe) no significant benefit were observed. Hence, larger prospective randomized trials are needed in order to evaluate the effect of isolated vitamin C administration, separately for both vitamin C replete and deplete individuals. Treatment in the control group should be guided by prevailing standard of care for COVID infected patients.
7. Limitation
There are various limitations of this study. Firstly, heterogeneity of included population, drug dosages and route, which makes results inconclusive. However, sub-group analysis also revealed similar findings. Secondly combination therapy with other agents like vitamin E and melatonin etc. confounds our study finding, like it did in Marik's study [9]. Thirdly, the standard therapy in the control group were dissimilar, owing to their different period of completion. Steroids and anticoagulation have been considered as standard therapy for hospitalised patients requiring oxygen [43,44]. However, included studies conducted during the initial period of COVID, administered steroids to minority (8–30%) of patients requiring oxygen [14,15]. Fourth, due to lack of data, serious adverse events with high dose vitamin C could not be reported. Lastly, due to less number of studies (n < 10) meta regression could not be performed.
Funding
Not applicable.
Ethical approval
Not applicable.
Declaration of competing interest
None.
References
- 1.https://covid19.who.int/.
- 2.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020 Jun 1;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., et al. 2020. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19.https://www.researchsquare.com/article/rs-19346/v1 [Internet]. In Review. Mar [cited 2020 Jul 28]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr A.C. In: Oxidative stress and disease. Vissers M., Chen Q., editors. Boca Raton Taylor and Francis Group; 2019. Vitamin C in pneumonia and sepsis. In press. [Google Scholar]
- 6.Mousavi S., Bereswill S., Heimesaat M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019 Aug 16;9(3):73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng J., Pourmand A., Mazer-Amirshahi M. Vitamin C: the next step in sepsis management? J Crit Care. 2018 Feb;43:230–234. doi: 10.1016/j.jcrc.2017.09.031. Epub 2017 Sep 18. PMID: 28934705. [DOI] [PubMed] [Google Scholar]
- 8.Carr A.C., Shaw G.M., Fowler A.A., Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015 Nov 27;19:418. doi: 10.1186/s13054-015-1131-2. PMID: 26612352; PMCID: PMC4661979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. 2017. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study; p. 151. Chest [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 10.Fowler A.A., 3rd, Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., Gupta S. Medical Respiratory Intensive Care Unit Nursing, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014 Jan 31;12:32. doi: 10.1186/1479-5876-12-32. PMID: 24484547; PMCID: PMC3937164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii T., Luethi N., Young P.J., Frei D.R., Eastwood G.M., French C.J., Deane A.M., Shehabi Y., Hajjar L.A., Oliveira G., Udy A.A., Orford N., Edney S.J., Hunt A.L., Judd H.L., Bitker L., Cioccari L., Naorungroj T., Yanase F., Bates S., McGain F., Hudson E.P., Al-Bassam W., Dwivedi D.B., Peppin C., McCracken P., Orosz J., Bailey M., Bellomo R. VITAMINS trial investigators. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020 Feb 4;323(5):423–431. doi: 10.1001/jama.2019.22176. PMID: 31950979; PMCID: PMC7029761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler A.A., 3rd, Truwit J.D., Hite R.D., Morris P.E., DeWilde C., Priday A., et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019 Oct 1;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J., et al. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020 Dec;18(12):1259–1261. doi: 10.1080/14787210.2020.1794819. 2020/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021 Dec;11(1):5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, et al. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 12(11):e11779. [DOI] [PMC free article] [PubMed]
- 16.Suna K., Melahat U.Ş., Murat Y., Figen Ö.E., Ö Ayperi. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med Clin. 2021 May 11;S0025–7753(21) doi: 10.1016/j.medcli.2021.04.010. 00252-00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darban M., Malek F., Memarian M., Gohari A., Kiani A., Emadi A., et al. Efficacy of high dose vitamin C, melatonin and zinc in Iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial. J Cell Mol Anesth. 2021;6(2):164–167. [Google Scholar]
- 18.Hakamifard A., Soltani R., Maghsoudi A., Rismanbaf A., Aalinezhad M., Tarrahi J., et al. vol. 7. 2021. p. 7. (The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial). 2. [Google Scholar]
- 19.JamaliMoghadamSiahkali S., Zarezade B., Koolaji S., SeyedAlinaghi S., Zendehdel A., Tabarestani M., et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021 Dec;26(1):20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul 21;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.crd.york.ac.uk/prospero/.
- 22.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018 Jun;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 23.Shi J., Luo D., Weng H., Zeng X., Lin L., Chu H., et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020 Jul 25:1429. doi: 10.1002/jrsm.1429. jrsm. [DOI] [PubMed] [Google Scholar]
- 24.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005 Dec;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Lin H., Lin B.W., Lin J.D. Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care. 2019;9(1):58. doi: 10.1186/s13613-019-0532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silberstein M. Correlation between premorbid IL-6 levels and COVID-19 mortality: potential role for Vitamin D. Int Immunopharm. 2020 Nov 1;88:106995. doi: 10.1016/j.intimp.2020.106995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 Feb 20;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher B.J., Kraskauskas D., Martin E.J., Farkas D., Wegelin J.A., Brophy D., et al. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 29.Lankadeva Y.R., Peiris R.M., Okazaki N., Birchall I.E., Trask-Marino A., Dornom A., et al. Reversal of the pathophysiological responses to gram-negative sepsis by megadose vitamin C. Crit Care Med. 2021;49(2) doi: 10.1097/CCM.0000000000004770. https://journals.lww.com/ccmjournal/Fulltext/2021/02000/Reversal_of_the_Pathophysiological_Responses_to.37.aspx [Internet] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran L., Zhao W., Wang J., Wang H., Zhao Y., Tseng Y., et al. In: Pan H.-F., editor. BioMed Res Int; 2018. Extra dose of vitamin C based on a daily supplementation shortens the common cold: a meta-analysis of 9 randomized controlled trials; p. 1837634. 2018 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Mikirova N., Hunninghake R. Effect of high dose vitamin C on Epstein-Barr viral infection. Med Sci Monit. 2014 May 3;20:725–732. doi: 10.12659/MSM.890423. PMID: 24793092; PMCID: PMC4015650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabet M.H., Mohammadi M., Ramezani M., Khalili H. Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract. 2016 Apr-Jun;5(2):94–100. doi: 10.4103/2279-042X.179569. PMID: 27162802; PMCID: PMC4843590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka H., Matsuda T., Miyagantani Y., Yukioka T., Matsuda H., Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000 Mar 1;135(3):326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 34.Putzu A., Daems A.M., Lopez-Delgado J.C., Giordano V.F., Landoni G. The effect of vitamin C on clinical outcome in critically ill patients: a systematic review with meta-analysis of randomized controlled trials. Crit Care Med. 2019:47. doi: 10.1097/CCM.0000000000003700. [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 35.Hemilä Harri, Chalker Elizabeth. 2019. Vitamin C can shorten the length of stay in the ICU: a meta-analysis; p. 11. Nutrients [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M., Jativa D.F. vol. 6. SAGE Open Med; 2018 Oct 19. (Vitamin C supplementation in the critically ill: a systematic review and meta-analysis). 2050312118807615–2050312118807615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monsen E.R. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000 Jun 1;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 38.Lykkesfeldt J., Poulsen H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010 May;103(9):1251–1259. doi: 10.1017/S0007114509993229. Epub 2009 Dec 15. PMID: 20003627. [DOI] [PubMed] [Google Scholar]
- 39.Chiscano-Camón L., Ruiz-Rodriguez J.C., Ruiz-Sanmartin A., Roca O., Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020 Aug 26;24(1):522. doi: 10.1186/s13054-020-03249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. 2020 Jun 18. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med [Internet] Available from. [DOI] [PubMed] [Google Scholar]
- 41.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004 Apr 6;140(7):533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. PMID: 15068981. [DOI] [PubMed] [Google Scholar]
- 42.Rawat D., Roy A., Maitra S., Shankar V., Khanna P., Baidya D.K. Vitamin D supplementation and COVID-19 treatment: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2021 Jun 28:102189. doi: 10.1016/j.dsx.2021.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dexamethasone in hospitalized patients with covid-19 — preliminary report. N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. [Internet] [cited 2020 Sep 11]; Available from. [DOI] [Google Scholar]
- 44.https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/.