ABSTRACT
CO2 and bicarbonate are required for carboxylation reactions, which are essential in most bacteria. To provide the cells with sufficient CO2, there exist two dissolved inorganic carbon supply (DICS) systems: the membrane potential-generating system (MpsAB) and the carbonic anhydrase (CA). Recently, it has been shown that MpsAB is a bicarbonate transporter that is present not only in photo- and autotrophic bacteria, but also in a diverse range of nonautotrophic microorganisms. Since the two systems rarely coexist in a species but are interchangeable, we investigated what advantages the one system might have over the other. Using the genus Staphylococcus as a model, we deleted the CA gene can in Staphylococcus carnosus and mpsABC genes in Staphylococcus aureus. Deletion of the respective gene in one or the other species led to growth inhibition that could only be reversed by CO2 supplementation. While the S. carnosus Δcan mutant could be fully complemented with mpsABC, the S. aureus ΔmpsABC mutant was only partially complemented by can, suggesting that MpsAB outperforms CA. Interestingly, we provide evidence that mucus biofilm formation such as that involving polysaccharide intercellular adhesin (PIA) impedes the diffusion of CO2 into cells, making MpsAB more advantageous in biofilm-producing strains or species. Coexpression of MpsAB and CA does not confer any growth benefits, even under stress conditions. In conclusion, the distribution of MpsAB or CA in bacteria does not appear to be random as expression of bicarbonate transporters provides an advantage where diffusion of CO2 is impeded.
IMPORTANCE CO2 and bicarbonate are required for carboxylation reactions in central metabolism and biosynthesis of small molecules in all bacteria. This is achieved by two different systems for dissolved inorganic carbon supply (DICS): these are the membrane potential-generating system (MpsAB) and the carbonic anhydrase (CA), but both rarely coexist in a given species. Here, we compared both systems and demonstrate that the distribution of MpsAB and/or CA within the phylum Firmicutes is apparently not random. The bicarbonate transporter MpsAB has an advantage in species where CO2 diffusion is hampered—for instance, in mucus- and biofilm-forming bacteria. However, coexpression of MpsAB and CA does not confer any growth benefits, even under stress conditions. Given the clinical relevance of Staphylococcus in the medical environment, such findings contribute to the understanding of bacterial metabolism and thus are crucial for exploration of potential targets for antimicrobials. The knowledge gained here as exemplified by staphylococcal species could be extended to other pathogenic bacteria.
KEYWORDS: Staphylococcus aureus, Staphylococcus carnosus, Firmicutes, MpsAB, carbonic anhydrase, biofilm
INTRODUCTION
CO2 and bicarbonate (HCO3−) are simple carbon molecules and yet are so important in the life of prokaryotes and eukaryotes. The classical CO2 fixation is carried out by many autotrophic organisms/organelles, such as chloroplasts and the cyanobacterial carboxysomes. They possess ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), which catalyzes the fixation of inorganic CO2 to ribulose-1,5-bisphosphate (RuBP) to form two molecules of 3-phosphoglycerate (1). Since CO2 is gaseous and thus can diffuse in and out of the cell, it must be trapped in the cell to be available in sufficient quantity for carboxylation reactions. Many autotrophic and also nonautotrophic microorganisms use carbonic anhydrase (CA) enzymes as a dissolved inorganic carbon supply (DICS) system (2, 3). CAs are ubiquitous enzymes that can be found in the mitochondria, cytoplasm, periplasm, membrane, or cell-wall-associated carboxysome and also chloroplast in plants (3, 4). Most of these enzymes have a Zn-binding domain that catalyzes the reversible interconversion of CO2 and bicarbonate (CO2 + H2O ↔ HCO3− + H). CA is important for inorganic carbon-fixing enzymes that utilize either CO2 or HCO3− by interconverting these species to ensure a sufficient concentration in the cytoplasm. To date, CAs from eight evolutionary distinct families have been reported (α, β, γ, δ, ζ, η, θ, and ι) (5–8). Many prokaryotes possess putative CA genes of two or more families, whereas some even possess multiple genes from the same family, suggesting that this enzyme plays an important role in prokaryotic physiology and fitness (3).
Apart from the CA-catalyzed cytoplasmic interconversion of CO2 to HCO3−, there is another mechanism, which is based on a membrane-localized HCO3− transporter. Such a transporter transports HCO3− from the external environment over the membrane into the cytoplasm, where the imported HCO3− is consumed by the carboxylation reactions. The continuous consumption of HCO3− in the cytoplasm could induce a suction power to keep the transporter running. In the exterior milieu, the transporter is continuously removing HCO3− from the CO2/HCO3− equilibrium, resulting in a permanent replenishment of HCO3−. Unlike CO2, HCO3− is charged, which prevents an immediate back-diffusion over the membrane. In this regard, most cellular systems depend on dedicated transporters to facilitate the movement of membrane-impermeant HCO3− across the cell membrane (9). However, such transporters have until recently been described only in autotrophic bacteria, such as in cyanobacteria, where the mechanisms for DIC transport are well characterized. DIC refers to the total amount of CO2, HCO3−, and CO32− in water. Given the absence of a classical bicarbonate uptake system, questions arise as to how other autotrophs and some nonautotrophic bacteria lacking these transporters and/or CAs manage to concentrate intracellular HCO3−.
A recent resurgence of interest in bicarbonate transporters appears to be driven largely by the discovery by the group of Kathleen M. Scott of a novel two-component transporter that facilitates dissolved inorganic carbon (DIC) uptake (10). They were the first to describe that these transporters possess DIC uptake activity in Hydrogenovibrio crunogenus, a chemolithoautotroph from gammaproteobacteria and confirmed to transport DIC (11). Subsequently, these homologs which were named DABs, were also reported to accumulate HCO3− in a sulfur-oxidizing gammaproteobacterial chemolithoautotroph, Halothiobacillus neapolitanus (12). Surprisingly, DAB homologs were already described earlier in the nonautotrophic bacterium Staphylococcus aureus, where they were referred to as MpsAB (membrane-potential generating system) because they contribute to the membrane potential generation (13). Further studies showed that MpsAB represents an HCO3−-concentrating system, possibly acting as a sodium bicarbonate cotransporter (14), the first example of such transporters in the phylum Firmicutes. Subsequently, we used an S. aureus HG001 mpsABC deletion mutant for most of the experiments, but follow-up studies showed that mpsC is not a functional part of the mpsAB operon. It was further found that all examined representatives of the species S. aureus possess only the MpsAB transporter, but no CA.
In our previous study, we reported that MpsAB and CA represent a CO2/bicarbonate-concentrating system, each of which can functionally replace the other (14). Against this background, we aimed to understand the link between both the systems and to investigate if one system is superior than the other by using staphylococcal strains. In S. aureus HG001, MpsAB is essential for growth under atmospheric air while no CA-encoding gene is present. On the other hand, a gene annotated as encoding a putative CA from Staphylococcus carnosus TM300 has yet to be investigated for its physiological function or enzymatic activity.
Here, we report that the distribution of MpsAB and/or CA within the phylum Firmicutes is apparently not random. The bicarbonate transporter MpsAB has an advantage in species where CO2 diffusion is hampered—for instance, in mucus and biofilm-forming bacteria.
RESULTS
The distribution of MpsAB and/or CA in selected Firmicutes species.
First, we wanted to get an overview of the distribution of the two completely different DICS systems, MpsAB and/or CA, within the phylum Firmicutes. A selection of families within this phylum carrying MpsAB, CA(s), or both based on the presence and/or absence of Pfam motif is shown in Table 1. Due to the huge numbers of strains available in the database, we limited our search to only the finished genomes in the IMG/M database (15), and only one representative strain per species is shown in Table S1 in the supplemental material. Almost all of the species representatives have at least one system—either MpsAB or CA (Table 1; Table S1). Nevertheless, quite a number of species, particularly in the Bacillaceae family, have both systems. A similar observation was seen only in S. sciuri (Staphylococcaceae) and Sulfobacillus acidophilus (Clostridiaceae). It is interesting to note that many species have two copies of CA genes, of either the prokaryotic (Pfam00484) or eukaryotic (Pfam00194) type of CA. We use the terms β-CA for Pfam00484 and α-CA for Pfam00194 in order to more adequately describe the evolutionary history of these enzymes. It seems that CO2-concentrating systems play an important role in bacteria, and the presence of two systems is probably advantageous in certain habitats.
TABLE 1.
The presence of MpsAB and/or CA in selected Firmicutes
| Family | Species | Presence of MpsAB or CA (PFam)a |
||
|---|---|---|---|---|
| MpsAB | β-CA (Pfam00484) | α-CA (Pfam00194) | ||
| Bacillaceae | Anoxybacillus sp. strain B2M1 | + | − | − |
| Bacillus anthracis Ames | + | + | − | |
| Bacillus anthracis BF1 | − | + | − | |
| Bacillus subtilis ATCC 13952 | − | ++ | − | |
| Bacillus subtilis spizizenii W23 | + | ++ | − | |
| Bacillus thuringiensis 97-27 | + | + | − | |
| Geobacillus kaustophilus HTA426 | + | − | − | |
| Lysinibacillus macroides DSM 54 | − | + | − | |
| Oceanobacillus iheyensis CHQ24 | − | + | − | |
| Listeriaceae | Listeria monocytogenes NCTC7973 | − | − | + |
| Staphylococcaceae | Macrococcus caseolyticus IMD0819 | − | + | − |
| Staphylococcus aureus aureus USA300_FPR3757 | + | − | − | |
| Staphylococcus carnosus TM300 | − | + | − | |
| Staphylococcus epidermidis RP62A | + | − | − | |
| Staphylococcus pseudintermedius ED99 | − | + | − | |
| Staphylococcus sciuri SNUSD-18 | (+) | + | − | |
| Enterococcaceae | Enterococcus faecalis KB1 | − | − | + |
| Enterococcus faecium ERS2704476 | − | − | + | |
| Lactobacillaceae | Lacticaseibacillus casei ATCC 334 | − | − | + |
| Lactiplantibacillus plantarum 16 | − | − | + | |
| Lactobacillus acidophilus FSI4 | − | − | − | |
| Lactobacillus delbrueckii JCM 17838 | − | − | + | |
| Streptococcaceae | Lactococcus lactis subsp. lactis 14B4 | − | − | − |
| Lactococcus piscium CMTALT02 | − | − | ++ | |
| Streptococcus pneumoniae NCTC 7466 | − | + | − | |
| Streptococcus pyogenes M28PF1 | − | + | − | |
| Streptococcus salivarius 57.I | − | + | + | |
| Streptococcus suis DN13 | − | + | − | |
| Streptococcus thermophilus LMG 18311 | − | + | + | |
| Clostridiaceae | Clostridium botulinum 111 | − | + | − |
| Clostridium perfringens CP15 | − | + | − | |
| Sulfobacillus acidophilus TPY | + | + | − | |
The presence of the proteins was inferred based on the following Pfam domain search from finished bacterial genomes in the Integrated Microbial Genomes & Microbiomes (IGM/G) database: MpsAB (Pfam00361 and Pfam10070, respectively), prokaryotic type-carbonic anhydrase (Pfam00484), and eukaryotic-type CA (Pfam00194). Other Pfam domains, such as Pfam08936 for carboxysome shell carbonic anhydrase (CsoSCA), Pfam18484 for cadmium CA repeat, and Pfam10563 for a putative CA-like domain, were also searched within the above Firmicutes, but these domains were not found. The terms β-CA for Pfam00484 and α-CA for Pfam00194 are used in the table in order to more adequately describe the evolutionary history of these enzymes. The symbols + and − indicate the presence or absence, respectively, of the protein domains. The symbol +/− indicates the presence or absence of the protein domains, with + indicates one and ++ indicate two protein domains. The symbol (+) indicates that in S. sciuri SNUSD-18, MpsA and MpsB appear to be truncated.
To gain an insight into their genome organization, we then looked at the comparative synteny maps of various Firmicutes species (see Fig. S1 in the supplemental material). Examples of staphylococcal strains harboring only MpsAB are S. aureus, S. epidermidis, and S. haemolyticus (14). In these genomes, a hypothetical protein, MpsC, is located immediately downstream of MpsAB (Fig. S1A). MpsC was initially thought to be part of the Mps operon, but further analysis revealed that it is not a functional part of it (14). Next, we investigated the CA localization in genomes containing both MpsAB and β-CA (Fig. S1B). In S. sciuri, Bacillus anthracis, and Bacillus subtilis, a putative CA is located immediately downstream of MpsAB homologs. The other CAs of S. sciuri and B. subtilis located elsewhere in the genome shared a much higher identity with the S. carnosus CA (Fig. 1B). In the two Bacillus strains, the second CA gene is located between luxS and cydA genes, whereas B. subtilis has a third CA gene located elsewhere. In the third part, genomes harboring only CAs can be further divided into those with β- or α-CAs (Fig. S1C). Among the β-CAs, the Staphylococcaceae family members share a similar location for CA. It is always located downstream of a gene (labeled as mp in Fig. S1C) that encodes a membrane protein of unknown function. No common synteny was observed in the other group of CAs, suggesting that CA localization in the genomes is conserved only in closely related species or genera.
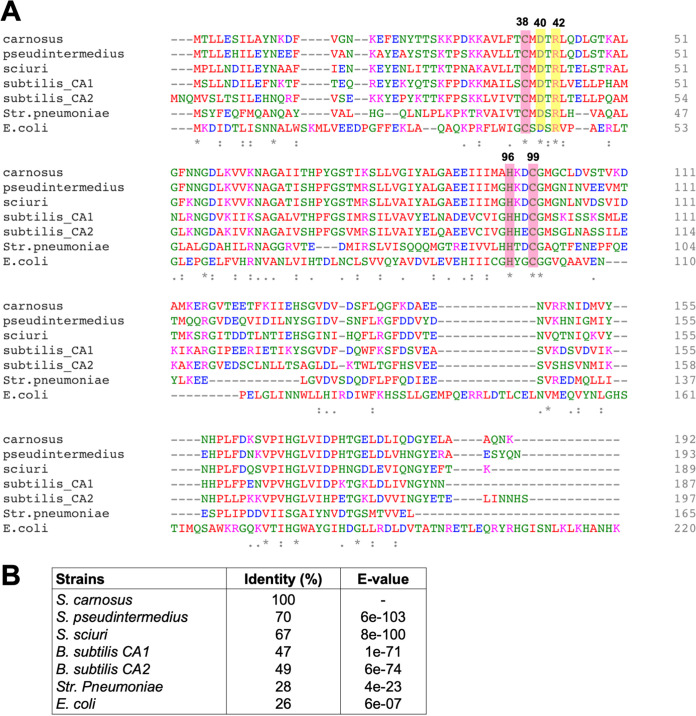
FIG 1.
Multiple protein sequence alignment for CA. (A) The alignment was carried out for the CAs from S. carnosus TM300 (carnosus), S. pseudintermedius ED99 (pseudintermedius), S. sciuri FDAARGOS_285 encoded by locus tag Ga0225916_842 with Pfam00484 (sciuri_CA), Bacillus subtilis subtilis 168 encoded by locus tag BSU30690 with Pfam00484 (subtilis_CA1), B. subtilis 168 encoded by locus tag BSU34670 with Pfam00484 (subtilis_CA2), Str. pneumoniae TIGR4 encoded by locus tag SP0024 (pneumoniae), and E. coli MG1655 encoded by locus tag b0126 (E. coli). All sequences were obtained from the IMG/M database. The deduced sequences were aligned using Clustal Omega (56). The numbers correspond to the S. carnosus sequence. Highlighted in red are residues 38 (cysteine), 96 (histidine) and 99 (cysteine), which are ligands to zinc. Residues 40 and 42 highlighted in yellow are an Asp/Arg dyad that is important for the proton transfer step of catalysis. (B) The percentages of identity of the CA protein from S. carnosus to those of the other sequences were compared using BLASTp (51).
The CA from S. carnosus and its functional complementation in E. coli Δcan.
As we identified the staphylococcal CA genes based on Pfam domain, we first need to establish whether the identified genes encode active CA enzymes. We used S. carnosus TM300 and S. pseudintermedius ED99 as our model strains to investigate CA in more detail. The annotation of the genome of S. carnosus TM300 (hereafter S. carnosus) and S. pseudintermedius ED99 (hereafter S. pseudintermedius) revealed that a single putative CA-encoding gene, SCA_1457 and SPSE_0869, respectively, is present in each (16, 17). To verify that no other potential CAs are present, the deduced can gene product of the respective staphylococcal strains were subjected to a BLAST search (BLASTp) against their own genome sequences. No significant homology to any other proteins was found, implying that only a single β-CA is present in each strain. The 192-amino-acid CA from S. carnosus shares 70% identity with the CA from S. pseudintermedius (193 amino acids) and has a comparable identity of 28% to Streptococcus pneumoniae TIGR4 and, to a lesser extent, E. coli MG1655 at 26% (Fig. 1B). For S. carnosus and S. pseudintermedius genomes, we did not find any other homology (BLASTp) with α-CAs from Enterococcus faecium, Helicobacter pylori, Neisseria gonorrhoeae, and Vibrio cholerae and human CA1 and CA2, as well as γ-CAs from Enterococcus faecium, Escherichia coli, Halobacterium salinarum, and Methanosarcina thermophila.
According to the NCBI Conserved Domain database, the S. carnosus CA belongs to the β-class and D clade (cd03379). Likewise, Streptococcus pneumoniae and E. coli are also members of the β-CAs (18, 19). Multiple-sequence alignment revealed that S. carnosus, along with the bacteria in Fig. 1A, shares a highly conserved motif. Such distinctive motif is consistent with those found in β-CAs, namely, the HXXC motif (where H is histidine, C is cysteine, and X is any residue), which binds to the active site metal ion, and the CXDXR motif (where C is cysteine, D is aspartic acid, R is arginine, and X is any residue), which completes the active site (20). A metal ion binding site that consists of one His and two Cys residues was found at residues 38, 96, and 99, a characteristic feature of all β-class CAs (21). This feature facilitates the ligation of the zinc active site, with sulfur atoms of the two Cys residues as well as a nitrogen atom from His. Additionally, two residues numbered 40 and 42 were detected, which corresponds to the Asp/Arg dyad. The pair is important to ensure that water is the fourth ligand to zinc and contributes to the proton transfer step of catalysis (22).
To screen for the functional activity of CA, we utilized E. coli EDCM636, which is a can deletion mutant (E. coli Δcan) and therefore cannot grow in atmospheric air (19) but only grows under high-CO2 (5%) conditions. This mutant was used as a recipient strain for complementation with staphylococcal can genes. The expression of the can gene from S. carnosus (can-Sc) or S. pseudintermedius (can-Sp) enabled it to grow under low-atmospheric-CO2 conditions (Table 2), indicating that the probable CA in both strains is functional. Although the E. coli CA shows a protein identity of only 26% to the staphylococcal CAs, full complementation was achieved. E. coli Δcan strains harboring the respective empty plasmids were used as a negative control.
TABLE 2.
Growth of the deletion mutant and its complemented strains
| Strains | Description | Growth ina: |
|
|---|---|---|---|
| Atmospheric air | 5% CO2 | ||
| E. coli | |||
| Δcan | CA deletion mutant | − | + |
| Δcan(pRB473can-Sc) | Mutant complemented with CA from S. carnosus | + | + |
| Δcan(pRB473) | Mutant complemented with empty plasmid (control) | − | + |
| Δcan(pRB473can-Sp) | Mutant complemented with CA from S. pseudintermedius | + | + |
| S. carnosus | |||
| Δcan | CA deletion mutant | − | + |
| Δcan(pRB473can-Sc) | Mutant complemented with its own CA | + | + |
| Δcan(pRB473) | Mutant complemented with empty plasmid (control) | − | + |
| S. pseudintermedius | |||
| Δcan | CA deletion mutant | − | + |
| Δcan(pCtufcan-Sp) | Mutant complemented with its own CA | + | + |
| Δcan(pCtuf) | Mutant complemented with empty plasmid (control) | − | + |
| Δcan(pRB473mpsABC) | Mutant complemented with mpsABC from S. aureus | + | + |
| S. aureus ΔmpsABC(pCtufcan-Sp) | mpsABC deletion mutant complemented with CA from S. pseudintermedius | + | + |
+ indicates growth on agar plates after overnight incubation under the respective conditions, while − indicates no growth.
The can gene from S. carnosus and S. pseudintermedius is required for growth under atmospheric CO2.
In order to determine the importance of the can gene for staphylococcal growth, we constructed can deletion mutants (Δcan) in S. carnosus and S. pseudintermedius. The deletion of can-Sc in S. carnosus and can-Sp in S. pseudintermedius led to a severe growth defect (Fig. 2A and Table 2), a phenotype similar to those of the E. coli Δcan strain and also the S. aureus ΔmpsABC mutant and which requires a high concentration of CO2 for growth. Complementation of these staphylococcal Δcan mutants reversed the phenotype, enabling their growth under atmospheric conditions without the need for high CO2, indicating that the can gene is the only gene required for growth under atmospheric air conditions (Table 2). As negative controls, both of the deletion mutants carrying empty plasmids did not grow under atmospheric air conditions, whereas all of the strains grew under 5% CO2.
FIG 2.
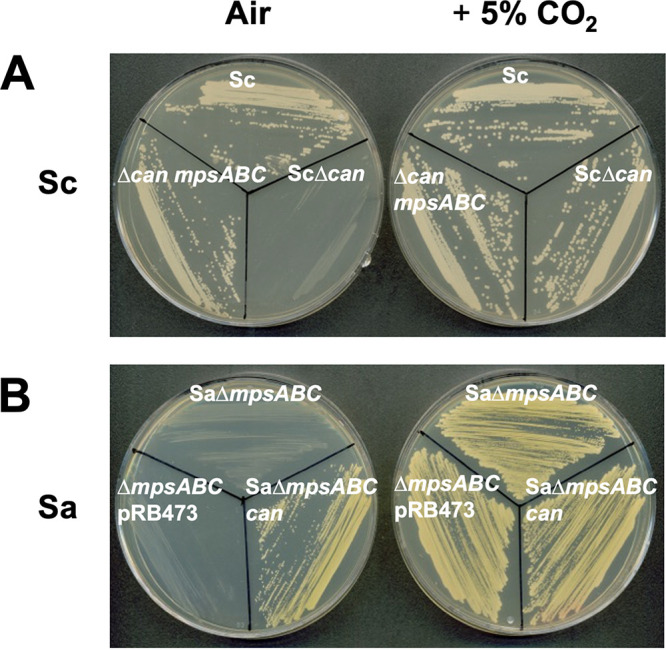
The S. carnosus CA deletion mutant cannot grow in atmospheric air and is interchangeable with mpsABC from S. aureus. (A) The S. carnosus CA deletion mutant cannot grow in atmospheric air (0.04% CO2) but can be restored at 5% CO2 and can also be complemented with mpsABC from S. aureus. Clockwise from top: S. carnosus TM300 wild type (Sc), S. carnosus TM300 CA deletion mutant (ScΔcan), and ScΔcan complemented with plasmid pRB473 carrying mpsABC-Sa (Δcan mpsABC). Likewise, CA from S. carnosus can also complement S. aureus ΔmpsABC. (B) Clockwise from top: S. aureus HG001 ΔmpsABC (SaΔmpsABC), S. aureus HG001 ΔmpsABC complemented with plasmid pRB473 carrying CA from S. carnosus (SaΔmpsABC can), and S. aureus HG001 ΔmpsABC carrying empty plasmid pRB473 as a control (ΔmpsABC pRB473). Plates were incubated in atmospheric air (left) and 5% CO2 (right).
S. carnosus and S. pseudintermedius Δcan mutants can be complemented by S. aureus mpsABC and vice versa.
Earlier we reported that MpsAB and CA represent a CO2/bicarbonate-concentrating system in which each can functionally replace the other (14). Here, we wanted to address this hypothesis with reciprocal complementation within the staphylococcal species. We transformed both the S. carnosus Δcan and S. pseudintermedius Δcan mutants with plasmid pRB473mpsABC, containing the S. aureus-specific mpsABC genes. Both the Δcan strains could be complemented by mpsABC and grew like its parental counterparts under atmospheric conditions (Table 2). Likewise, reciprocal complementation showed that the can gene also rescued the growth of S. aureus ΔmpsABC mutants under atmospheric air, indicating the interchangeable relationship between MpsAB and CA (Fig. 2B).
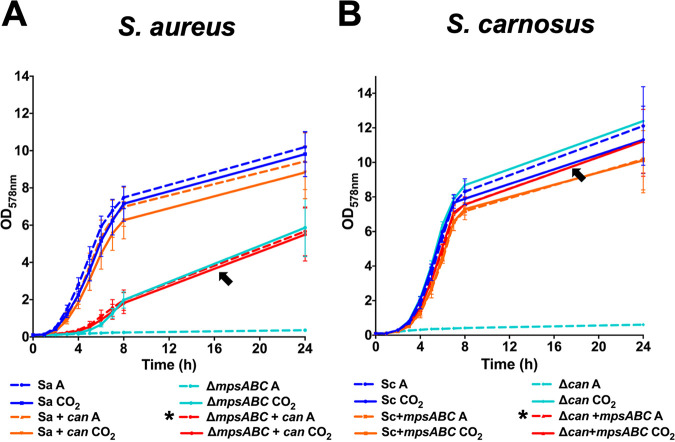
MpsAB outperforms CA with regard to CO2/bicarbonate concentration.
Given the interchangeable relationship of MpsAB and CA, we sought to examine if one system is superior to the other at concentrating CO2/bicarbonate. We coexpressed MpsAB in S. carnosus and CA in S. aureus so that the strains have an extra CO2/bicarbonate concentration system in addition to its own. The S. aureus ΔmpsABC and S. carnosus Δcan strains cannot grow at atmospheric CO2 levels, suggesting that mpsAB and can are not only essential in their respective species, but also represent the sole CO2/bicarbonate concentration system. When the can gene from S. carnosus was coexpressed in S. aureus(pRB473can-Sc), there was no evident increase in the growth compared to that of the wild type under atmospheric conditions (Fig. 3A). In contrast, the growth of the coexpressed strains seemed to decrease slightly compared to the wild type, and the decrease was more evident under CO2 conditions. It appears that too much CO2/bicarbonate attenuates the growth rather than enhances it. As no growth benefit was seen, we investigated the growth in the absence of MpsAB using the S. aureus ΔmpsABC(pRB473can-Sc) reciprocal complemented strain. The growth of this strain was similar under both atmospheric and CO2 conditions. The cloning of can-Sc could not fully complement the growth of the S. aureus ΔmpsABC strain, but only to the levels of the deletion mutant grown under 5% CO2 (shown by the arrow in Fig. 3A).
FIG 3.
Effect of coexpression of both MpsAB and CA on the growth of S. aureus and S. carnosus. The growth of S. aureus HG001 and S. carnosus TM300 strains under atmospheric air (A, dashed lines) and 5% CO2 (CO2, solid lines) conditions in 24 h. Coexpression of CA in S. aureus harboring MpsAB did not show any growth benefit, while coexpression of CA in S. aureus ΔmpsABC (marked with an asterisk in the figure key) could only partially complement the mutant to the same levels as in 5% CO2, as shown by the arrow in panel A. (A) The growth of the S. aureus wild type (Sa), S. aureus complemented with CA from S. carnosus (Sa+can), S. aureus mpsABC deletion mutant (ΔmpsABC), and S. aureus mpsABC deletion mutant complemented with CA from S. carnosus (ΔmpsABC+can) under both atmospheric and CO2 conditions. Likewise, coexpression of MpsAB in S. carnosus harboring CA did not show any growth benefit. However, the coexpression of MpsAB in S. carnosus Δcan (marked with an asterisk in the figure key) could fully complement the mutant to almost the wild-type levels and 5% CO2, as shown by the arrow in panel B. (B) The growth of S. carnosus wild type (Sc), S. carnosus complemented with mpsABC from S. aureus (Sc+mpsABC), and the S. carnosus CA deletion mutant (Δcan), the S. carnosus can deletion mutant complemented with mpsABC from S. aureus (Δcan+mpsABC) under both conditions. Each point in the graph is the mean ± standard deviation (SD) from three independent biological replicates.
Likewise, the same observation was seen for the S. carnosus wild type (Fig. 3B). However, complementation of the S. carnosus Δcan mutant with mpsABC or 5% CO2 fully restored the growth of the S. carnosus Δcan mutant to wild-type levels (shown by the arrow in Fig. 3B). This is in stark contrast to what we observed with the S. aureus ΔmpsABC mutant. Unlike the reciprocal complemented S. aureus strain, the addition of mpsABC could restore the growth of the S. carnosus Δcan mutant, suggesting that MpsAB outperforms CA with regard to CO2/bicarbonate concentration, as reflected by the higher growth under low-atmospheric-CO2 conditions. This observation led us to the question of why the growth of the S. aureus ΔmpsABC mutant can be fully restored neither by cloning of can nor by 5% CO2. Therefore, we hypothesized that in contrast to S. carnosus, the diffusion of CO2 in S. aureus is hindered.
Does biofilm hinder the diffusion of CO2 into the cell?
Compared to S. carnosus, S. aureus and S. epidermidis produce polysaccharide intercellular adhesin (PIA), which is composed of linear β-1,6-linked glucosaminylglycans (23–25). PIA is of slimy consistency, loosely surrounding the cells, and is the basis for tight biofilm formation (26). Therefore, PIA could have a negative effect on the diffusion of CO2 into the cell. Following on to this, we searched for the occurrence of MpsAB, CA, and the intercellular adhesion gene (ica)-encoded protein cluster, which mediates the formation of biofilm in selected staphylococcal species. Table 3 shows the presence of such genes/proteins based on Pfam domains of each ica gene. Most of the strains can be divided into four categories of having MpsAB and Ica (group i), CA only (group ii), MpsAB only (group iii), and CA and Ica (group iv). The first two categories, groups i and ii, support our hypothesis that on the one hand, MpsAB and Ica are correlated, and on the other hand, CA predominates in those species lacking ica genes. However, groups iii and iv are exceptions. To provide more evidence for the hypothesis, we examined S. aureus Δica mutants and the expression of icaADBC genes in S. carnosus.
TABLE 3.
Occurrence of MpsAB, CA, and intercellular adhesion gene (ica)-encoded protein cluster in selected staphylococcal species
| Protein(s) and species | Presence of MpsAB, CA, or Ica proteina |
||||
|---|---|---|---|---|---|
| MpsAB | CA | IcaA | IcaB | IcaC | |
| Group i: MpsAB and Ica | |||||
| Staphylococcus argenteus BN75 | + | − | + | + | + |
| Staphylococcus aureus subsp. aureus MSHR1132 | + | − | + | + | + |
| Staphylococcus aureus subsp. aureus USA300_FPR3757 | + | − | + | + | + |
| Staphylococcus capitis AYP1020 | + | − | + | + | + |
| Staphylococcus epidermidis RP62A | + | − | + | + | + |
| Staphylococcus lugdunensis C_33 | + | − | + | + | + |
| Staphylococcus simiae NCTC 13838 | + | − | + | + | + |
| Staphylococcus xylosus SMQ121 | + | − | + | + | + |
| Staphylococcus saprophyticus 883 | + | − | ++ | ++ | ++b |
| Staphylococcus cohnii SNUDS-2 | + | − | + | − | + |
| Staphylococcus sciuri SNUSD-18 | (+) | + | ++ | − | ++ |
| Group ii: CA only | |||||
| Staphylococcus agnetis 908 | − | + | − | − | − |
| Staphylococcus carnosus TM300 | − | + | − | − | − |
| Staphylococcus felis ATCC 49168 | − | + | − | − | − |
| Staphylococcus hyicus ATCC 11249 | − | + | − | − | − |
| Staphylococcus lutrae ATCC 700373 | − | + | − | − | − |
| Staphylococcus muscae NCTC 13833 | − | + | − | − | − |
| Staphylococcus schleiferi 1360-13 | − | + | − | − | − |
| Group iii: MpsAB only | |||||
| Staphylococcus equorum KS1039 | + | − | − | − | − |
| Staphylococcus haemolyticus JCSC1435 | + | − | − | − | − |
| Staphylococcus hominis subsp. hominis K1 | + | − | − | − | − |
| Staphylococcus nepalensis JS1 | + | − | − | − | − |
| Staphylococcus pasteuri SP1 | + | − | − | − | − |
| Staphylococcus succinus 14BME20 | + | − | − | − | − |
| Staphylococcus warneri SG1 | + | − | − | − | − |
| Group iv: CA and Ica | |||||
| Staphylococcus condimenti DSM 11674 | − | + | + | + | + |
| Staphylococcus pettenkoferi FDAARGOS_288 | − | + | +c | + | + |
| Staphylococcus piscifermentans NCTC 13836 | − | + | + | + | + |
| Staphylococcus pseudintermedius ED99 | − | + | + | + | + |
| Staphylococcus stepanovicii NCTC 13839 | − | + | + | + | + |
| Staphylococcus simulans FDAARGOS_124 | − | + | + | − | + |
The presence of the proteins was inferred based on the following Pfam domain searches from finished bacterial genomes in the Integrated Microbial Genomes & Microbiomes (IGM/G) database: MpsAB was from Pfam00361 and PFam10070, respectively, carbonic anhydrase (CA) was based on prokaryotic type-carbonic anhydrase (Pfam00484) and eukaryotic-type CA (Pfam00194), and the biofilm-associated proteins are based on the Ica-encoding genes icaA, which has the domain glycosyl transferase family 2 (Pfam00535), icaB, which contains the domain polysaccharide deacetylase (Pfam01522), and icaC, which has the domain acyltransferase family (Pfam01757). The symbols + and − indicate the presence or absence, respectively, of the protein domains. The symbol +/− indicates the presence or absence of the protein domains, with + indicates one and ++ indicate two protein domains. The symbol (+) indicates that in S. sciuri SNUSD-18, MpsA and MpsB appear to be truncated.
Two sets of operons of ica genes in different locations were detected in the genome of S. saprophyticus 883.
icaA was found to contain Pfam13641 which is annotated as glycosyltransferase-like family 2, but the protein has 76% similarity (BLASTp) to the IcaA from S. aureus.
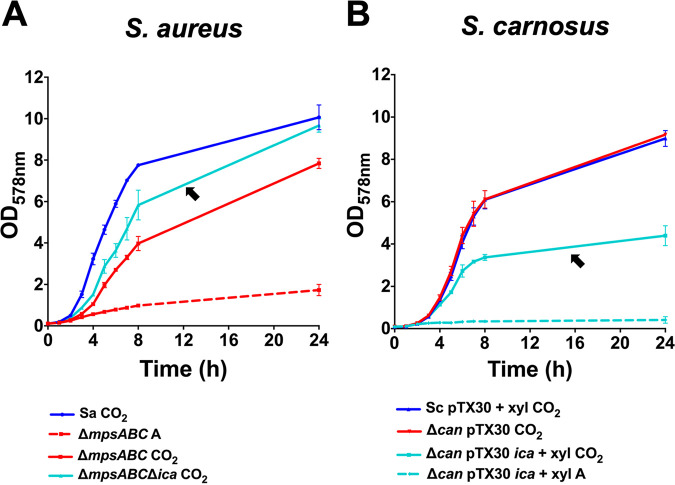
Deletion of the ica operon in the S. aureus ΔmpsABC Δica mutant increased growth in the presence of CO2.
In S. aureus, we deleted the ica operon, including its regulator in the background of the S. aureus ΔmpsABC mutant. Indeed, under CO2 conditions, the S. aureus ΔmpsABC Δica mutant grew to a higher optical density (OD) than the ΔmpsABC mutant, although not quite to the wild-type levels (indicated by the black arrow in Fig. 4A).
FIG 4.
The influence of biofilm mediated by polysaccharide intercellular adhesin (PIA) encoded by the ica operon in the growth of staphylococcal strains. (A) The growth of the S. aureus HG001 wild type (Sa), HG001 mpsABC deletion mutant (ΔmpsABC), and HG001 mpsABC and ica deletion mutant (ΔmpsABC ica) under atmospheric (A, dashed lines) and 5% CO2 (CO2, solid lines) conditions for 24 h. The arrow shows the ΔmpsABC Δica double mutant in which the biofilm-associated genes were deleted and which grew to a higher OD than the ΔmpsABC mutant alone in CO2. (B) The growth of the S. carnosus TM300 wild type (Sc) and TM300 carbonic anhydrase gene deletion mutant (Δcan) carrying empty plasmid pTX30 (as a control) under atmospheric (A, dashed lines) and 5% CO2 (CO2, solid lines) conditions for 24 h. The arrow shows the Δcan(pTX30) strain carrying the ica genes required for biofilm formation, which grew to a much lower OD than the Δcan strain alone in CO2 when induced by 0.7% xylose (xyl). All growth studies were performed using TSB. Each point in the graph is the mean ± SD from three independent biological replicates.
Expression of the ica operon in the S. carnosus Δcan mutant decreases growth in the presence of CO2.
To confirm the effect of PIA, we transformed plasmid pTX30icaADBC, which encodes PIA biosynthesis into the S. carnosus Δcan mutant. In this plasmid, the ica expression is xylose inducible. Under inducing conditions and in the presence of 5% CO2, the growth of the S. carnosus Δcan(pTX30icaADBC) strain was significantly reduced, constituting only half of the S. carnosus Δcan mutant grown with CO2 and the wild type (indicated by the black arrow in Fig. 4B).
As controls, the addition of xylose and the presence of the empty plasmid (pTX30) did not influence the growth of the S. carnosus Δcan mutant or wild type under both atmospheric and CO2 conditions (see Fig. S2A, B, D, and E in the supplemental material). In addition, induction of PIA expression in wild-type S. carnosus(pTX30icaADBC) did not affect growth (Fig. S2C). Taken together, these observations support our hypothesis that PIA formation hinders the diffusion of CO2 into the cells with pronounced reduction of growth in the S. carnosus Δcan mutant.
Coexpression of both MpsAB and CA does not confer any growth benefits under stress conditions.
Next, we proceeded to find out if having both the CO2 and bicarbonate concentration systems confers any advantage under different environmental stress. To confirm a potential growth benefit in all backgrounds, we used three strains of S. aureus and S. carnosus, respectively, which included the wild type, strains coexpressing both systems, and reciprocal complemented strains. Cells were exposed to different concentrations of NaCl ranging from 0% to 7.5% (see Fig. S3 in the supplemental material), to pHs from 5.5 to 8.5 (see Fig. S4 in the supplemental material), or to iron limitation by adding the iron chelator 2,2-dipyridyl (DIP) (see Fig. S5 in the supplemental material). We also tested the effect of temperature at 25, 37, and 42°C (see Fig. S6 in the supplemental material). However, we observed no growth benefit when both systems were present under any of the applied stress conditions. Collectively, these results indicate that the coexpression of MpsAB and CA did not confer any growth benefits under the stress conditions tested.
Phylogenetic analysis of CA.
The large number of strains listed in Table S1 necessitated further phylogenetic analysis to explore the relationship regarding the distribution of CA in Firmicutes. Since we confirmed in this study that the S. carnosus CA is functional, we used the protein sequence as a query sequence to search for homologs in NCBI database, and the resulting sequences were used to construct a phylogenetic tree. The CA homologs are widespread in the phylum Firmicutes, with a high degree of conservation among Staphylococcaceae (see Fig. S7 in the supplemental material). Among the main genera seen are Planomicrobium, Bacillus, Clostridium, Streptococccus, and Oceanobacillus, with Lactobacillus delbrueckii being the most distantly related to the CA protein of S. carnosus. Some Streptococcus species, such as Str. parauberis, Str. didelphis, and Str. suis, form a distinct clade from the rest of the Firmicutes. A closer look at the family Staphylococcaceae revealed that CA is further split into two subclades: the first consists of Staphylococcus and Macrococcus and the second of Jeotgalicoccus and Salinicoccus (Fig. S7B). With respect to the S. carnosus CA, the most closely related species is S. simulans, whereas the most distantly related species is Auricoccus indicus. Of note, a few of the Staphylococcus species, S. stepanovicii, S. lentus, and S. sciuri, split and form a separate clade from the others in the same genus.
All completely sequenced S. aureus genomes contain only the mpsAB operon but no can gene.
According to the IMG/G database, there are 209 finished genomes of S. aureus (accessed 20 October 2020). We checked all of these genomes for the presence of putative CA homologs to S. carnosus, but none was found in any of these genomes. On the basis of the Pfam motif of CA, no potential CA homologs was present. In addition, a search in all these genomes for any protein annotated as CA also yielded no result. Furthermore, we did a BLAST search of the S. carnosus β-CA against the NCBI database and found two protein sequences from an S. aureus strain with low similarity to S. carnosus CA. However, the sequences were derived from an unfinished genome (permanent draft) and may not be properly sequenced/annotated. In addition, we also did not find significant homology (BLASTp) with α-CAs from Enterocococus faecium, Helicobacter pylori, Neisseria gonorrhoeae, and Vibrio cholerae and human CA1 and CA2, as well as γ-CAs from Enterococcus faecium, Escherichia coli, Halobacterium salinarum, and Methanosarcina thermophila. Given the extensive search performed, it is extremely unlikely that CA is present in S. aureus.
DISCUSSION
In the present study, we have followed up on our previous work on the interchangeable roles of MpsAB and CA in CO2/bicarbonate-concentrating systems and described a previously unreported CA activity in Staphylococcus. Firmicutes harboring MpsAB or CA have adapted to diverse habitats from soil, animals, and plants to deep ocean (Table 1; Table S1). However, the ecological and metabolic diversity of these Firmicutes renders it difficult to group them based solely on the presence of MpsAB and CA. A first look at the distribution of MpsAB and CA within Firmicutes families shows a seemingly arbitrary distribution in the respective species: some have only MpsAB or CA, some have both, and others have two or more genes for β- or α-CA. At the moment, we have no clear explanation on the distribution of MpsAB and CA among the members of the Firmicutes.
Phylogenetic analyses of CA in Firmicutes, particularly in Staphylococcaceae, revealed that only related genera and species are clustered. However, no conclusion could be made about the evolution of CAs since we do not know the common ancestor of these phyla. On another note, there are only some bacteria in which the β-CA activity was thoroughly described to date (3) such as in E. coli (19), Streptococcus pneumoniae (18), or Clostridium autoethanogenum (27). In other classes, the α-CA includes studies in Helicobacter pylori (28), Neisseria gonorrhoeae (29), Mesorhizobium loti (30), and Rhodopseudomonas palustris (31), while γ-CA was well characterized in Methanosarcina thermophila (32). Different classes of CAs (α and β) have been reported in the same bacteria, such as Thiomicrospira crunogena (33). To the best of our knowledge, no credible study has investigated the activity and role of CA in staphylococci. For this reason, we selected S. carnosus and S. pseudintermedius for a more detailed study. S. carnosus is a nonpathogenic food-grade staphylococcus strain that does not possess ica genes and therefore does not produce biofilm, while S. pseudintermedius is a canine pathogen carrying the ica genes (Table 3). Both species possess only one CA gene (can), and when this gene was deleted, the mutants were not able to grow in ambient air. Such a phenotype was also seen in other CA mutant strains of bacteria like E. coli and Str. pneumoniae, further emphasizing the importance of CO2/bicarbonate concentration systems.
Given that some genes have no detectable fitness effects under normal conditions and only exert their effects under certain stress situations (34), we repeated the experiments under various environmental stress stimuli, but the coexpression of both systems did not confer any significant growth benefits under these conditions (Fig. S3 to S6). As with all growth studies shown here (Fig. 3; Fig. S3), we observed that the presence of both MpsAB and CA did not improve growth but instead slightly reduced it, particularly under CO2 conditions. In principle, it does not really make sense if bacteria have both an HCO3− transporter and a CA because the HCO3− transported into the cell will be converted by CA to CO2, which can escape the cells by diffusion. Thus, the benefit of a transporter is mitigated by the presence of a CA. This is exactly what we observed experimentally by coexpressing can in S. aureus(pRB473can-Sc), which caused decreased growth compared to the wild type. Indeed, most of the bacterial species have only one or the other system. Nevertheless, there are a few species which have both systems, like some endospore-forming bacilli and clostridia (Table 1). In such cases where microorganisms undergo morphological differentiation, it might be advantageous to have both systems, where both systems are active in different cell compartments.
An interesting observation in this study is that the growth of the S. aureus ΔmpsABC mutant could only be partially complemented by can or 5% CO2, while the growth of the S. carnosus Δcan mutant complemented with mpsABC or 5% CO2 could be almost fully restored (Fig. 3). One possible explanation for this finding could be that CO2 diffuses less easily into the cell in S. aureus than in S. carnosus. While bacteria with CA depend on the diffusion of CO2, MpsAB-harboring bacteria are independent of CO2 diffusion because they transport bicarbonate from outside into the cell. In this context, we hypothesized that in S. aureus, the diffusion of CO2 is impaired, and therefore the CO2 levels entering the cells are insufficient to fully complement the growth in the S. aureus ΔmpsABC mutant by either 5% CO2 or cloning of can. We assumed that the diffusion barriers are found in the cell envelope, and its major differences between S. aureus and S. carnosus may affect CO2 diffusion in S. aureus.
The S. aureus cell wall is much richer in cell-wall-anchored adhesin proteins such as protein A, fibronectin, and fibrinogen or collagen binding proteins (35), which are not present in S. carnosus (16, 36, 37). Another important difference between the two species is that S. aureus has mucus substances attached to its cell surface, which are not present in S. carnosus. One of the mucus substances is PIA (26), which is encoded by the ica genes (23–25). The abundance of adhesins and the mucilage could possibly impair the diffusion of CO2 in S. aureus. Moreover, most S. aureus strains produce type 5 and 8 capsular polysaccharides (38, 39), which might further contribute to the impairment.
The first two groups in Table 3 were indeed consistent with our hypothesis. For group i, it seems to be advantageous to have a bicarbonate transporter (MpsAB) instead of CA since the PIA produced by these species form the basis of biofilm. For group ii, CA appears to be fully adequate since there is no PIA to interfere with the CO2 diffusion. Only groups iii and iv do not seem to agree with our assumption. The lack of a strict correlation between the last two groups could be attributed to acquisition of mpsAB or ica by horizontal gene transfer. Furthermore, we have no indication if the ica genes are expressed at all in this group. Another possible explanation may be the interaction of the microbial community in the natural environment. Some bacteria that rely on high levels of CO2 but lack CA can still grow in commensal situations in this context (40).
Deletion of ica genes in the S. aureus ΔmpsABC mutant, although not fully, improved the growth in 5% CO2. Incomplete restoration could be due to the capsule polymers that are still present. Conversely, the cloning of ica genes into the S. carnosus Δcan mutant decreased its growth as this strain cannot be fully supplied with CO2 in the presence of PIA. We are aware that the ica expression could lead to cell aggregation, which could falsify the OD measurements. Therefore, we always vortexed the samples rigorously, and appropriate controls were made by using all the clones as shown in Fig. S2. Clearly, these are only indirect results. However, it should be noted that a direct measurement of CO2 diffusion in aqueous medium is almost impossible due to its high dissociation capability. Nevertheless, biophysical calculations and measurements suggest that gas diffusion is impaired in the biofilm mode of growth due to sorption to the biofilm matrix (41, 42).
Recently we showed that MpsAB homologs are widespread in the Firmicutes phylum (14). Among them are pathogens such as Bacillus anthracis, possessing capsule and S-layer proteins, Vibrio cholerae, harboring a capsular layer, or Legionella pneumophila, which has its S-layer proteins. All of these pathogens are distinguished by a surface mucilage that may retard CO2 diffusion and thus making a bicarbonate transporter more advantageous than CA. Other pathogens like Streptococcus pyogenes, Enterococcus faecalis, Mycobacterium tuberculosis, or E. coli have only CA homologs (14). Interestingly, the lipid bilayer of M. tuberculosis should also retard CO2 diffusion; however, this might be compensated for by possessing three CA homologs (14).
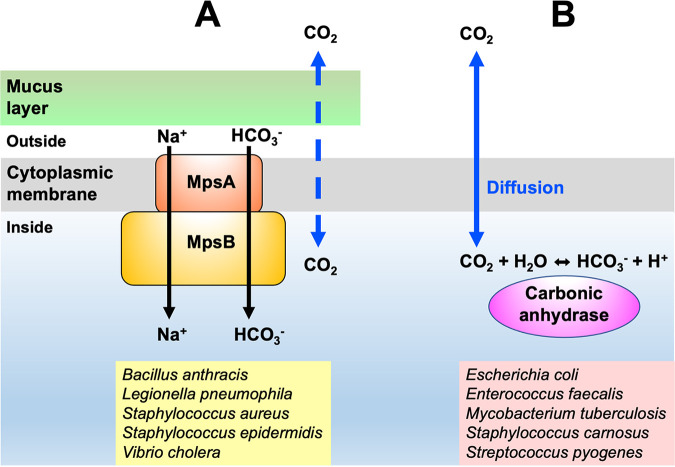
Finally, the aforementioned findings and that >200 fully sequenced S. aureus genomes harbor only MpsAB and no CA diminish the possibility that CA is present in S. aureus. These findings are in contradiction with other publications about the presence of CA in S. aureus, which must be reconsidered. In conclusion, we underlined the importance of CO2/bicarbonate-concentrating systems in Firmicutes that depend on either MpsAB or CA. Despite the apparent lack of growth benefits when both are coexpressed in the same strain, we found that MpsAB outperforms CA on the basis of growth restoration. Our findings suggest that in certain species, the CO2 diffusion is hindered by mucilage, slime, capsules, or other polymers. For these species, it is advantageous if they have an MpsAB-bicarbonate transporter. With such a transporter, the cells are not dependent on CO2 diffusion for their carboxylation reactions (Fig. 5). Against this background, it is thus conceivable that S. aureus and other bacterial species have a membrane-localized bicarbonate/CO2 transporter instead of a cytoplasmic CA.
FIG 5.
Model of the restricted CO2 diffusion in mucoid bacteria and the advantage of having a bicarbonate transporter. (A and B) Biofilm-producing bacteria such as S. aureus and S. epidermidis (A) utilize a membrane-localized transporter (MpsAB) to facilitate the movement of membrane-impermeant HCO3− across the cell membrane, as well as (B) non-biofilm-producing bacteria such as S. carnosus and S. felis (B), whose cytoplasmic carbonic anhydrase (CA) activity is dependent on good CO2 diffusion into the cell. The dashed blue arrow represents restricted CO2 diffusion, and the solid blue arrow indicates unrestricted CO2 diffusion. Additional examples of bacterial species in the MpsAB and CA group are listed at the bottom of the figure.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in this study are listed in Table S2 in the supplemental material. For cloning procedures, all E. coli and staphylococcal strains were cultivated in at 37°C with shaking at 150 rpm in basic medium (BM), unless otherwise specified. The BM consists of 1% soy peptone, 0.5% yeast extract, 0.5% NaCl, 0.1% glucose, and 0.1% K2HPO4 at pH 7.2. All cultures were grown in 10 ml medium using baffled 100-ml shake flasks, except for growth studies, in which cells were grown in 15 ml medium. When necessary, the culture medium was supplemented with the following antibiotics used at the indicated concentrations: 10 μg/ml chloramphenicol for staphylococcal strains and 100 μg/ml ampicillin and 30 μg/ml kanamycin for E. coli strains.
Construction of staphylococcal deletion mutants.
All oligonucleotides used in this study are listed in Table S3 in the supplemental material. The nucleotide and amino acid sequences were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG). The S. carnosus TM300 Δcan (KEGG accession no. SCA_1457) and S. pseudintermedius ED99 Δcan (KEGG accession no. SPSE_0869) deletion mutants were constructed as markerless deletions using allelic replacements as described by Bae and Schneewind (43). Briefly, approximately 1-kb fragments of upstream and downstream of the carbonic anhydrase-encoding gene (can) were amplified from the chromosomal DNA of S. carnosus TM300 and S. pseudintermedius ED99, respectively. The amplified fragments were assembled with linearized plasmid pBASE6 (EcoRV restriction site) (44) via Gibson assembly (45) using Hi-Fi DNA assembly master mix (New England Biolabs). The resulting plasmid was transformed into chemically competent E. coli DC10B (46). The respective clones harboring the right genes were then transformed into S. carnosus TM300 via electroporation and into S. pseudintermedius ED99 by protoplast transformation (47). Deletion of the can genes in both of the strains was confirmed by PCR and sequence analysis.
For the construction of S. aureus HG001 ΔmpsABC, the ica operon genes consisting of the transcriptional regulator, icaR, as well as icaA, icaD, icaB, and icaC (corresponding to KEGG accession no. SAOUHSC_03001, SAOUHSC_03002, SAOUHSC_03003, SAOUHSC_03004, and SAOUHSC_03005, respectively) were deleted. Approximately 1,500-kb fragments upstream and downstream of the Ica operon-encoding genes were amplified from the chromosomal DNA of S. aureus HG001, and the amplified fragments were assembled with linearized plasmid pBASE6 (SmaI restriction site) via Gibson assembly. The remaining steps were performed as described above.
Construction of complementation vectors.
The complementation for S. carnosus CA (can-Sc) was carried out using plasmid pRB473 (48). Along with its putative native promoter, the CA-encoding gene (can-Sc) was amplified from the chromosomal DNA of S. carnosus TM300 before being assembled into linearized pRB473 (SmaI restriction site) by Gibson assembly. The putative promoter regions were determined by DNA sequence analysis. As for the construction of complementation of S. pseudintermedius ED99 CA (can-Sp), the plasmid pCtufamp (49) was used. The CA-encoding gene (can) was amplified from the chromosomal DNA of S. pseudintermedius ED99 and then assembled into linearized pCtufamp (PacI and HindIII restriction sites) by Gibson assembly. The constructed plasmids were first introduced into E. coli DC10B and then into the S. carnosus TM300 Δcan and S. pseudintermedius ED99 Δcan strains accordingly. Both of the recombinant plasmids (pRB473can-Sc and pCtufcan-Sp) were also introduced into S. aureus RN4220 before being transformed into the S. aureus HG001 wild type and S. aureus ΔmpsABC mutant (14). Additionally, both of the recombinant plasmids were also transformed into EDCM636 (E. coli Δcan) which is an E. coli MG1655 derivative harboring a kanamycin resistance marker replacing a deletion of the carbonic anhydrase-encoding gene can (19). The strain was purchased from E. coli Genetic Stock Center, Yale University. All of the staphylococcal strains and the E. coli Δcan mutant were also transformed with the empty plasmids pRB473 and pCtufamp as controls.
For the complementation of S. carnosus TM300 Δcan with ica genes, plasmid pTX30 containing the entire ica operon (icaADBC) under the control of a xylose-inducible promoter (24) was used and transformed into S. carnosus TM300 Δcan. As negative controls, the empty plasmid pTX30 was transformed into S. carnosus TM300 and S. carnosus TM300 Δcan.
Reciprocal complementation.
To investigate if the MpsAB and CA could complement reciprocally, plasmid pRB473 carrying mpsABC from S. aureus HG001(pRB473mpsABC) from our previous study (14) was used for transformation into the S. carnosus TM300 Δcan and S. pseudintermedius ED99 Δcan, respectively, via protoplast transformation.
Growth and complementation studies.
Tryptic soy broth (TSB) (Sigma-Aldrich) and tryptic soy agar (TSA) were used for growth studies involving staphylococcal strains. For growth characterization on solid medium, all staphylococcal strains were cultured for 24 h under atmospheric conditions and continuous shaking at 37°C, except for the S. aureus ΔmpsABC complemented strain, which was precultured in 5% CO2 due to its slow growth. The 5% CO2 condition was achieved in a CO2 incubator (Heraeus Instruments). The cultures were subsequently adjusted to an OD578 of 5 and then streaked on agar plates and incubated overnight in atmospheric and 5% CO2 conditions. Images of the agar plates were taken using an image scanner (Epson). The complementation experiments of E. coli EDCM636 were also performed as described above, except that BM agar was used instead of TSA.
For growth characterization of staphylococcal strains in liquid medium, the strains were precultured in TSB as described above. The main cultures were inoculated to an OD578 of 0.1 and grown under atmospheric and 5% CO2 conditions under continuous shaking. Aliquots were taken for OD578 measurements at 0 h and every 1 h until 8 h and then at 24 h. Growth studies were performed in three independent biological replicates. For growth studies of S. carnosus involving plasmid pTX30, xylose at a final concentration of 0.7% was added to the TSB. For bacterial cultures with the presence of pTX30 ica, the flasks were vortexed vigorously before the OD measurements were taken to disrupt clumps due to possible biofilm formation.
Occurrence of CA and MpsAB homologs and Ica based on Pfam domains.
The occurrence of CA and MpsAB protein homologs was inferred from a Pfam domain (50) search from finished bacterial genomes found in the Integrated Microbial Genomes & Microbiomes (IGM/G) database (accessed 1 September 2020) (15). MpsA and MpsB belong to Pfam00361 and Pfam10070, respectively, while Pfam00484 and Pfam00194 are members of the prokaryotic and eukaryotic-type CAs, respectively. Other Pfam domains, such as Pfam08936 for carboxysome shell carbonic anhydrase (CsoSCA), Pfam18484 for cadmium CA repeat, and Pfam10563 for a putative CA-like domain, were also searched within the Firmicutes species, but yielded no results. Results are shown as the presence or absence of the respective Pfam domains and the frequency in a particular species if the domain is present.
For Ica-encoding genes, icaA belongs to Pfam00535, which has the domain glycosyl transferase family 2, while icaB and icaC are members of the domains Pfam01522 (polysaccharide deacetylase) and Pfam01757 (acyltransferase family), respectively. Results are presented as the presence and absence of the related Pfam domains and were divided into four categories.
Phylogenetic trees.
Homologs of the S. carnosus TM300 CA (NCBI Protein ID accession no. CAL28362) were identified from the NCBI database (51) using Protein BLAST (accessed 17 September 2020). Searches were conducted within the bacterial Firmicutes phylum and bacterial family Staphylococcaceae. Selected protein sequences of mostly one strain per species in the Firmicutes phylum and Staphylococcaceae family were aligned using ClustalW (52). The multiple sequence alignments comprising 103 taxa in Firmicutes and 50 taxa in Staphylococcaceae, respectively. Phylogenetic trees were constructed using the maximum likelihood method and JTT matrix-based model (53). Results were assessed using 500 bootstrap replicates conducted in MEGAX (54, 55). The trees with the highest log likelihood (−18,048.25 for Firmicutes and −6,526.83 for Staphylococcaceae) are shown. The percentage of trees in which the associated taxa clustered together is displayed next to the branches. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analyses involved 103 and 50 amino acid sequences, with a total of 208 and 194 positions in the final data set for Firmicutes and Staphylococcaceae, respectively.
Growth studies for stress tolerance.
To evaluate if MspAB or CA confers any benefits under environmental stress, we exposed the bacterial cells to different stresses using NaCl, pH, iron limitation, and temperature and measured the growth in terms of OD578 using a multiplate reader (Varioskan Lux; Thermo Scientific). For all stress conditions, six bacterial strains were used: the S. aureus HG001 wild type, S. aureus HG001(pRB473can-Sc), S. aureus HG001 ΔmpsABC complemented with pRB473can-Sc, S. carnosus TM300 wild type, S. carnosus TM300(pRB473mpsABC) and S. carnosus TM300 Δcan complemented with pRB473mpsABC. For salt stress tolerance studies, cells were precultured in LB without NaCl. Overnight cultures were washed once with LB (without NaCl) before being resuspended in the same medium. NaCl was added to LB with final concentrations of 2.5, 5, and 7.5% and with no NaCl (0%) as a negative control. Cells were added to the LB medium at a final OD of 0.01 in a total volume of 500 μl each in a 48-well microplate. The microplate was sealed with Parafilm to prevent evaporation and incubated at 37°C with shaking in a multiplate reader. OD readings were recorded every 30 min for 24 h. For pH stress, cells were precultured in LB medium at pH 7.2, and overnight cultures were washed once with the LB before being resuspended in the same medium. Washed cells were added to LB medium adjusted to pHs 5.5, 6.5, 7.5, and 8.5, and the plate was incubated as with the same protocols as NaCl studies. For iron limitation studies, cells were precultured in TSB, and overnight cultures were washed and resuspended in TSB. The iron chelator 2,2-dipyridyl (DIP) was dissolved in ethanol and added to TSB at final concentrations of 250 μM, 500 μM, 1 mM, and 2 mM. Cells at a final OD of 0.01 were added to the TSB, and growth studies were carried out as with the NaCl studies. All growth studies were performed in three independent biological replicates. For temperature stress, overnight cultures of the cells were adjusted to an OD of 0.5 and then streaked on LB agar plates prior to incubation at room temperature, 37°C, and 42°C for 24 h.
Data visualization.
Multiple-sequence alignment of the protein sequences in Fig. 2 were obtained from IMG/M database and aligned using Clustal Omega (56). The percentages of identity of the CA protein from S. carnosus to the other sequences were compared using BLASTp (51). The growth curves were visualized using GraphPad Prism 6.0 software.
Data availability statement.
The main data supporting the findings of this work are available within the article and its supplemental material files or from the corresponding author upon request.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the Deutsche Forschungsgemeinschaft the Germany’s Excellence Strategy—EXC 2124—390838134 “Controlling Microbes to Fight Infections” and the Ministry for Science, Research and the Arts of Baden-Wuerttemberg (MWK) “AntibioPPAP.” S.-H.F. received a Ph.D. fellowship from the German Academic Exchange Service (DAAD). L.H. was supported by the Chinese Scholarship Council. We acknowledge support by the Open Access Publishing Fund of the University of Tübingen.
F.G. and S.-H.F. conceived the idea and designed the study. S.-H.F. performed most of the experiments. M.M., L.H., and P.M.T. performed some of the cloning experiments. F.G. and S.-H.F. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Friedrich Götz, Email: friedrich.goetz@uni-tuebingen.de.
Cezar M. Khursigara, University of Guelph
REFERENCES
- 1.Tabita FR, Hanson TE, Satagopan S, Witte BH, Kreel NE. 2008. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos Trans R Soc B 363:2629–2640. doi: 10.1098/rstb.2008.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badger M. 2003. The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth Res 77:83–94. doi: 10.1023/A:1025821717773. [DOI] [PubMed] [Google Scholar]
- 3.Smith KS, Ferry JG. 2000. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 24:335–366. doi: 10.1111/j.1574-6976.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 4.Moroney JV, Bartlett SG, Samuelsson G. 2001. Carbonic anhydrases in plants and algae. Plant Cell Environment 24:141–153. doi: 10.1111/j.1365-3040.2001.00669.x. [DOI] [Google Scholar]
- 5.Del Prete S, Vullo D, Fisher GM, Andrews KT, Poulsen SA, Capasso C, Supuran CT. 2014. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—the eta-carbonic anhydrases. Bioorg Med Chem Lett 24:4389–4396. doi: 10.1016/j.bmcl.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Kikutani S, Nakajima K, Nagasato C, Tsuji Y, Miyatake A, Matsuda Y. 2016. Thylakoid luminal theta-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci U S A 113:9828–9833. doi: 10.1073/pnas.1603112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMario RJ, Machingura MC, Waldrop GL, Moroney JV. 2018. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci 268:11–17. doi: 10.1016/j.plantsci.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Jensen EL, Clement R, Kosta A, Maberly SC, Gontero B. 2019. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J 13:2094–2106. doi: 10.1038/s41396-019-0426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey JR. 2006. Why bicarbonate? Biochem Cell Biol 84:930–939. doi: 10.1139/o06-184. [DOI] [PubMed] [Google Scholar]
- 10.Mangiapia M, Usf M, Brown TW, Chaput D, Haller E, Harmer TL, Hashemy Z, Keeley R, Leonard J, Mancera P, Nicholson D, Stevens S, Wanjugi P, Zabinski T, Pan C, Scott KM. 2017. Proteomic and mutant analysis of the CO2 concentrating mechanism of hydrothermal vent chemolithoautotroph Thiomicrospira crunogena. J Bacteriol 199:e00871-16. doi: 10.1128/JB.00871-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott KM, Leonard JM, Boden R, Chaput D, Dennison C, Haller E, Harmer TL, Anderson A, Arnold T, Budenstein S, Brown R, Brand J, Byers J, Calarco J, Campbell T, Carter E, Chase M, Cole M, Dwyer D, Grasham J, Hanni C, Hazle A, Johnson C, Johnson R, Kirby B, Lewis K, Neumann B, Nguyen T, Nino Charari J, Morakinyo O, Olsson B, Roundtree S, Skjerve E, Ubaldini A, Whittaker R. 2019. Diversity in CO2-concentrating mechanisms among chemolithoautotrophs from the genera Hydrogenovibrio, Thiomicrorhabdus, and Thiomicrospira, ubiquitous in sulfidic habitats worldwide. Appl Environ Microbiol 85. doi: 10.1128/AEM.02096-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmarais JJ, Ai F, Blikstad C, Dugan EJ, Laughlin TG, Oltrogge LM, Chen AW, Wetmore K, Diamond S, Wang JY, Savage DF. 2018. Genome-wide screening reveals a novel class of carbonic anhydrase-like inorganic carbon pumps in chemoautotrophic bacteria. bioRxiv. https://www.biorxiv.org/content/10.1101/476713v2.
- 13.Mayer S, Steffen W, Steuber J, Götz F. 2015. The Staphylococcus aureus NuoL-like protein MpsA contributes to the generation of membrane potential. J Bacteriol 197:794–806. doi: 10.1128/JB.02127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan SH, Ebner P, Reichert S, Hertlein T, Zabel S, Lankapalli AK, Nieselt K, Ohlsen K, Gotz F. 2019. MpsAB is important for Staphylococcus aureus virulence and growth at atmospheric CO2 levels. Nat Commun 10:3627. doi: 10.1038/s41467-019-11547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen IA, Chu K, Palaniappan K, Pillay M, Ratner A, Huang J, Huntemann M, Varghese N, White JR, Seshadri R, Smirnova T, Kirton E, Jungbluth SP, Woyke T, Eloe-Fadrosh EA, Ivanova NN, Kyrpides NC. 2019. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res 47:D666–D677. doi: 10.1093/nar/gky901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenstein R, Nerz C, Biswas L, Resch A, Raddatz G, Schuster SC, Götz F. 2009. Genome analysis of the meat starter culture bacterium Staphylococcus carnosus TM300. Appl Environ Microbiol 75:811–822. doi: 10.1128/AEM.01982-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Zakour NL, Bannoehr J, van den Broek AH, Thoday KL, Fitzgerald JR. 2011. Complete genome sequence of the canine pathogen Staphylococcus pseudintermedius. J Bacteriol 193:2363–2364. doi: 10.1128/JB.00137-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burghout P, Cron LE, Gradstedt H, Quintero B, Simonetti E, Bijlsma JJ, Bootsma HJ, Hermans PW. 2010. Carbonic anhydrase is essential for Streptococcus pneumoniae growth in environmental ambient air. J Bacteriol 192:4054–4062. doi: 10.1128/JB.00151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlin C, Masters M, McAteer S, Coulson A. 2003. Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 185:6415–6424. doi: 10.1128/JB.185.21.6415-6424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowlett RS. 2010. Structure and catalytic mechanism of the beta-carbonic anhydrases. Biochim Biophys Acta 1804:362–373. doi: 10.1016/j.bbapap.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Rowlett RS, Chance MR, Wirt MD, Sidelinger DE, Royal JR, Woodroffe M, Wang YF, Saha RP, Lam MG. 1994. Kinetic and structural characterization of spinach carbonic anhydrase. Biochemistry 33:13967–13976. doi: 10.1021/bi00251a003. [DOI] [PubMed] [Google Scholar]
- 22.Kumar RS, Hendrick W, Correll JB, Patterson AD, Melville SB, Ferry JG. 2013. Biochemistry and physiology of the beta class carbonic anhydrase (Cpb) from Clostridium perfringens strain 13. J Bacteriol 195:2262–2269. doi: 10.1128/JB.02288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. doi: 10.1128/IAI.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerke C, Kraft A, Sussmuth R, Schweitzer O, Götz F. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem 273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 25.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 26.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pander B, Harris G, Scott DJ, Winzer K, Kopke M, Simpson SD, Minton NP, Henstra AM. 2019. The carbonic anhydrase of Clostridium autoethanogenum represents a new subclass of beta-carbonic anhydrases. Appl Microbiol Biotechnol 103:7275–7286. doi: 10.1007/s00253-019-10015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus EA, Moshfegh AP, Sachs G, Scott DR. 2005. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol 187:729–738. doi: 10.1128/JB.187.2.729-738.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elleby B, Chirica LC, Tu C, Zeppezauer M, Lindskog S. 2001. Characterization of carbonic anhydrase from Neisseria gonorrhoeae. Eur J Biochem 268:1613–1619. doi: 10.1046/j.1432-1327.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- 30.Kalloniati C, Tsikou D, Lampiri V, Fotelli MN, Rennenberg H, Chatzipavlidis I, Fasseas C, Katinakis P, Flemetakis E. 2009. Characterization of a Mesorhizobium loti alpha-type carbonic anhydrase and its role in symbiotic nitrogen fixation. J Bacteriol 191:2593–2600. doi: 10.1128/JB.01456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puskas LG, Inui M, Zahn K, Yukawa H. 2000. A periplasmic, alpha-type carbonic anhydrase from Rhodopseudomonas palustris is essential for bicarbonate uptake. Microbiology (Reading) 146:2957–2966. doi: 10.1099/00221287-146-11-2957. [DOI] [PubMed] [Google Scholar]
- 32.Alber BE, Ferry JG. 1994. A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc Natl Acad Sci U S A 91:6909–6913. doi: 10.1073/pnas.91.15.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobrinski KP, Boller AJ, Scott KM. 2010. Expression and function of four carbonic anhydrase homologs in the deep-sea chemolithoautotroph Thiomicrospira crunogena. Appl Environ Microbiol 76:3561–3567. doi: 10.1128/AEM.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa AM, Machado I, Pm O. 2011. Phenotypic switching: an opportunity to bacteria thrive, p 252–262. In Mendez-Vilas A (ed), Science against microbial pathogens: communicating current research and technological advances, vol 1. Formatexa, Badajoz, Spain. [Google Scholar]
- 35.Foster TJ. 2019. Surface proteins of Staphylococcus aureus. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenstein R, Götz F. 2013. What distinguishes highly pathogenic staphylococci from medium- and non-pathogenic? Curr Top Microbiol Immunol 358:33–89. doi: 10.1007/82_2012_286. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstein R, Götz F. 2010. Genomic differences between the food-grade Staphylococcus carnosus and pathogenic staphylococcal species. Int J Med Microbiol 300:104–108. doi: 10.1016/j.ijmm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Poutrel B, Sutra L. 1993. Type 5 and 8 capsular polysaccharides are expressed by Staphylococcus aureus isolates from rabbits, poultry, pigs, and horses. J Clin Microbiol 31:467–469. doi: 10.1128/jcm.31.2.467-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueda K, Tagami Y, Kamihara Y, Shiratori H, Takano H, Beppu T. 2008. Isolation of bacteria whose growth is dependent on high levels of CO2 and implications of their potential diversity. Appl Environ Microbiol 74:4535–4538. doi: 10.1128/AEM.00491-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart PS. 2003. Diffusion in biofilms. J Bacteriol 185:1485–1491. doi: 10.1128/JB.185.5.1485-1491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart PS. 1998. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng 59:261–272. doi:. [DOI] [PubMed] [Google Scholar]
- 43.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 46.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Götz F, Kreutz B, Schleifer KH. 1983. Protoplast transformation of Staphylococcus-carnosus by plasmid DNA. Mol Gen Genet 189:340–342. doi: 10.1007/BF00337828. [DOI] [Google Scholar]
- 48.Brückner R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187–192. doi: 10.1016/0378-1119(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 49.Ebner P, Prax M, Nega M, Koch I, Dube L, Yu W, Rinker J, Popella P, Flötenmeyer M, Götz F. 2015. Excretion of cytoplasmic proteins (ECP) in Staphylococcus aureus. Mol Microbiol 97:775–789. doi: 10.1111/mmi.13065. [DOI] [PubMed] [Google Scholar]
- 50.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD. 2019. The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O'Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, et al. 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stecher G, Tamura K, Kumar S. 2020. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol Biol Evol 37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM00305-21_Supp_1_seq7.pdf, PDF file, 1.9 MB (1.9MB, pdf) .
Data Availability Statement
The main data supporting the findings of this work are available within the article and its supplemental material files or from the corresponding author upon request.