Abstract
Difficulty in controlling SARS-CoV-2 transmission made the ability to inactivate viruses in aerosols and fomites to be an important and attractive risk reduction measure. Evidence that light frequencies have the ability to inhibit microorganisms has already been reported by many studies which, however, focused on ultraviolet (UV) wavelengths, which are known to induce potential injury in humans. In the present study, the effect on suspensions of SARS-CoV-2 of a Light Emitting Diode (LED) device capable of radiating frequencies in the non-hazardous visible light spectrum (VIS) was investigated. In order to evaluate the efficiency of viral inactivation, plaque assay and western blot of viral proteins were performed. The observed results showed a significant reduction in infectious particles that had been exposed to the LED irradiation of visible light. Furthermore, the analysis of the intracellular expression of viral proteins confirmed the inactivating effect of this irradiation technology. This in vitro study revealed for the first time the inactivation of SARS-CoV-2 through LED irradiation with multiple wavelengths of the visible spectrum. However additional and more in-depth studies can aim to demonstrate the data obtained during these experiments in different matrices, in mutable environmental conditions and on other respiratory viruses such as the influenza virus. The type of LED technology can decisively contribute on reducing virus transmission through the continuous sanitation of common environments without risks for humans and animals.
Keywords: COVID-19, LED irradiation, Inactivation, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) is a serious respiratory disease caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), a novel betacoronavirus similar to SARS-CoV (severe acute respiratory syndrome) and MERS-CoV (Middle East respiratory syndrome), the etiological agents of SARS and MERS, respectively. The SARS-CoV-2 genome is a single-stranded, positive-sense RNA, of size ∼ 29.9 kB, encoding 16 non-structural proteins (NSPs) and four structural proteins, comprising envelope (E), membrane (M), nucleocapsid (N), and spike glycoprotein (S) [1]. The ongoing COVID-19 pandemic represents the leading global health emergency, resulting in over 200 million cases and 4.887.600 deaths worldwide, as of 18 October 2021 [2].
Although inhalation of aerosolized droplets from infectious people is the main way of virus transmission, the persistence of infectious virus particles on surfaces for hours to days, depending on the type of material and environmental conditions [3], suggests that a relevant role in transmission of SARS-CoV-2 is played by fomites and contaminated surfaces [4,5]. In this context, the search for new methods aimed to inactivate virus in the environment, could represent a good weapon to counteract its diffusion.
The environmental survival of the virus depends on various factors including temperature, humidity, sunlight, and the ability of the virus to adhere to different types of matrices; and, therefore, it should be further investigated [6]. The development - and use - of systems for efficient environmental decontamination, with consequent reduction of the infectious viral load, can play a crucial role in the prevention of contact infections, which are considered an important route of transmission. The photodynamic inactivation of viruses has been demonstrated to be a useful sanitation method, in particular for surface disinfection [6]. In fact, different studies have reported the inactivation of microorganisms through light frequencies focusing mainly on ultraviolet wavelengths (UV), in particular in the ranges 200–280 nm (UVC), 280–315 nm (UVB) and 315–380 nm (UVA) [7]. Thus, the efficacy of ultraviolet germicidal irradiation (UVGI) based primarily on UVC at 253.7 nm has been demonstrated for the inactivation of a wide range of airborne pathogens [8,9]. Regarding SARS-CoV-2, previous in vitro studies, performed in different experimental conditions, demonstrated that irradiation with ultraviolet light-emitting of 254 nm (UVC) rapidly inactivates high-titers of virus. Heilingloh et al., demonstrated that virus, (titer of 5 × 106 TCID50/ml), exposed to a UVC dose of 1048 mJ/cm2 was reduced by 50% after 1.4 min exposition and a complete inactivation after 9 min [10]. Criscuolo et al. reported that the exposition of SARS-CoV-2 (titer of 1.5 × 106 TCID50/ml) to UVC doses of 4.4 mJ/cm2 efficiently inactivated virus (99.9% reduction) after 15 min [11,12]. Furthermore, it has been reported that a light emitting diode instrument producing deep ultraviolet light (DUV-LED), with wavelength of approximately 250–300 nm, effectively inactivates the influenza A virus [13]. Just as it was recently shown that representative of UVB levels of natural sunlight, accelerate SARS-CoV-2 inactivation on surfaces [14]. However, UV light spectrum represents a potentially harmful form of radiation to humans, particularly the eyes (photokeratoconjunctivitis) and skin (photodermatitis) and often requires the use of protective equipment for personal safety [9,15].
Photodynamic inactivation of microorganisms using non-hazardous light energy emitted in the visible spectrum region (VIS) has recently been validated as an emerging tool to inhibit the spread of multi-drug resistant bacterial species [16]. In fact, evidence has been reported of the microbicidal effect of white light on bacteria (H.pylori, P.mirabilis, P.aeruginosa), when irradiated both directly with a density of 180 J/cm2 and in association with photosensitizing compounds (methylene blu) [17]. And the use of specific frequency peaks in the blue-violet band (400–420 nm) without the addition of photosensitizing factors also highlighted the susceptibility of a wide range of bacterial species to lower values (36 J/cm2) [18]. Emerging evidence demonstrated the effectiveness of the blue light in the inactivation of different viruses among which flu virus, coronaviruses HCoV-229E and HCoV-OC43 and several others [19]. Therefore, these results implicate the possibility to mitigate the environmental spread of SARS-CoV-2 not only with social distancing and use of germicidal UV but also with a novel light-based technology for the continuous environmental sanitization without risks for human health. Thus, in the present study the effect of a device based on a given combination of visible light frequencies was evaluated on the infectivity of SARS-CoV-2 in an experimental in vitro model.
2. Materials and methods
2.1. Cells
Vero E6 cells were cultured in minimum essential medium (MEM) (Life Technologies, Carlsbad, CA) complemented with 10% v/v heat-inactivated fetal bovine serum (FBS) (Sigma Aldrich, Milan, Italy), 2 mM Glutamine (Life Technologies), and 1% v/v antibiotic solution (Life Technologies), at 37 °C and under 5% CO2.
2.2. Virus
SARS-CoV-2 (hCoV-19/Italy/CDG1/2020/EPI_ISL_412,973), isolated from a nasopharyngeal swab by Department of Infectious Diseases, National Institute of Health Rome, Italy, was propagated in Vero cells cultured in MEM containing 2% FBS. Past 72 h from the infection, supernatants containing the released viral particles were collected and centrifuged at 600 g for 5 min. The viral titer was determined by plaque assay. Virus stocks were kept at −80 °C until use. Likewise, a SARS-CoV-2 isolate (hCoV-19/Sweden/RV-FOI-9/2020, accession ID: EPI_ISL_548,962) from a nasopharyngeal swab isolated by Department of Clinical Microbiology, University hospital of Umeå, Sweden, handled in the same way, was kept at −80 °C until use. A third SARS-CoV-2 isolate (hCoV-19/Germany/BY-ChVir-929/2020/EPI_ISL_406,862) derived from a sputum sample of the German COVID-19 index patient was generated at the Bundeswehr Institute of Microbiology, Munich, and viral stocks were produced accordingly.
2.3. Exposure system
The experiments were performed using a LED strip supplied by Nextsense Srl and powered with Biovitae® technology (Fig. 1 A). The LED device under investigation tested uses a special combination of frequencies covering the visible spectrum, with energy humps at 400–420 nm, 430–460 nm, 500–780 nm, and a main peak at 413 nm ± 5 nm Fig. 1B). In order to prevent any sample heating during exposure, the lamp is set up with a heat sink for thermal management. The experiments were performed in a multicentric format involving the Bundeswehr Institute of Microbiology, Munich, Germany; FOI CBRN Defense and Security, Sweden and Scientific Department, Army Medical Center, Rome, Italy. All experiments were conducted in BSL-3 laboratories.
Fig. 1.
A. The strip used for the experiments, consisting of 13 Biovitae® LEDs emitting wavelengths in the 400–420 nm range and delivering three different peaks within, and 37 conventional Osram Oslon (R) Square GW CSSRM2.EM-MFN2-XXX5-1 white light LEDs. The LEDs are powered at a constant current of 500 mA, guaranteed by a TCI MP 80/500 SLIM power supply. B. Relative radiometric spectral distribution of the Biovitae® strip.
To evaluate the antiviral efficacy of irradiation by visible light, serial dilutions of SARS-Co-2 were spotted in 96-wells plate and irradiated with 4.67 mW/cm2 at a working distance of 25 cm over times (n = 2 each for 15, 30, 45 and 60 min). The temperature and relative humidity inside the chamber were maintained within a narrow range for testing, specifically 20 ± 4 °C and 19 ± 5%, respectively. After irradiation treatment, virus was inoculated (in duplicate and triplicate) in confluent monolayers of Vero cells seeded in a 12-well plate and incubated for 1 h. Then, cells were overlaid with MEM containing 1.5% Tragacanth and 2% FBS (final concentration). At FOI CBRN Defense and Security, the infections were performed using 6-well plates and an overlay of 1% CMC in DMEM supplemented with 2% FBS. Cells were incubated for 72-96 h in a CO2 incubator. Untreated SARS-CoV-2 was used as a positive control. To calculate plaque forming units (PFU), cells were washed with physiological solution, followed by staining with crystal violet solution. The rate of inhibition of LED irradiations was calculated as (NLED /N0 × 100) where NLED is the PFU count of the LED-irradiated sample, while N0 is the PFU count of the unirradiated sample.
2.4. Protein extraction and western blot analysis
Vero E6 infected with SARS-CoV-2 stock irradiated or not with the LED-device, were lysed in RIPA buffer [20 mM Tris–HCl pH 8, 150 mM NaCl, 1% Triton X-100, 0.5% sodium dodecyl sulfate (SDS) and 1% sodium deoxycholate] complemented with phenylmethylsulfonyl fluoride, protease inhibitor mixture, and phosphatase inhibitor (Sigma- Aldrich, Milan, Italy). Subsequently, cell lysates were centrifuged (13,000 rpm, 30 min, 4 °C) and the supernatants were diluted in SDS sample buffer containing DL-Dithiothreitol (DTT 0.1 M). The total extract was subjected to SDS-PAGE followed by western blotting. Non-fat dry milk (10%) in Tris-buffered saline containing 0.01% Tween-100, was used to block the membranes for 1 h at room temperature (RT). Primary antibodies, utilized at final concentration of 1 μg ml−1, comprised mouse monoclonal anti-spike antibody (S) (Gene Tex cat No. GTX632604), rabbit polyclonal anti-nucleocapsid (N) (Rockland code: 200-401-A50) and mouse monoclonal anti-actin (Sigma Aldrich, Milan, Italy). Bound antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, PA, USA) followed by Clarity Western ECL substrate (Bio-Rad, Hercules, CA, USA) [20].
3. Results
3.1. Plaque assay
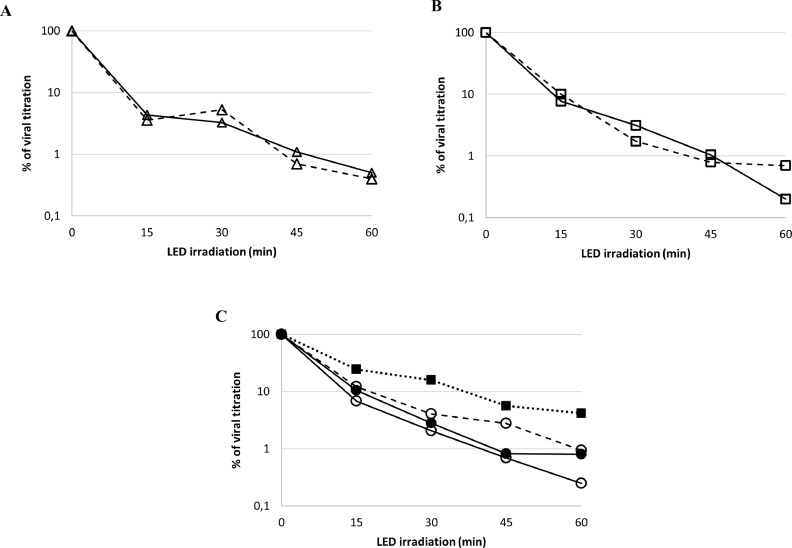
The plaque assay (Fig. 2 ) demonstrated that LED irradiation treatment with given wavelengths of the visible light spectrum (LED light) was able to significantly reduce the amount of infectious particle in standardized virus suspensions. Different concentrations of SARS-CoV-2 were irradiated at a distance of 25 cm over the course of 60 min with different subsampling time points (Fig. 3 ). Graphs A and B show the activity of LED light on viral concentrations of 8 × 101 PFU/ml and 8 × 102 PFU/ml, respectively. In both cases, it was observed that viral inactivation reaches more than 2-log reduction (99%) after 60 min of exposure. Graph C shows that even at higher concentration (7 × 103 PFU/ml and 3 × 104 PFU/ml) the LED light is still able to inactivate more than 99% of the viral particles. A viral inactivation of approximately 96% was also achieved using a dose of 1.7 × 105 PFU/ml.
Fig. 2.
Inhibition of SARS-CoV-2 (hCoV-19/Italy/CDG1/2020/EPI_ISL_412,973) by LED-irradiation with given wavelengths of the visible light spectrum (LED light). a–c I, Positive control. Plaque formation in Vero E6 cells. (a) 105 PFU/ml; (b) 104 PFU/ml; (c) 103 PFU/ml. d-f I+LED, Virus irradiated with LED light for 60 min and inoculated in Vero E6 cells. (d) 2 × 102 PFU/ml; (e) 10 PFU/ml; (f) 0 PFU/ml. As shown in d, the inhibition rate of the viral titer was 99.8%.
Fig. 3.
Viral inhibition of SARS-CoV-2 after 15, 30, 45 and 60 min of LED light exposition at 25 cm distance. Panel A: viral concentration 8 × 101 PFU/ml (∆); Panel B: viral concentration 8 × 102 PFU/ml (□); Panel C: viral concentrations 7 × 103 PFU/ml (○); 3 × 104 PFU/ml (●); 1,7 × 105 PFU/ml (■). Experiments were conducted by three different laboratories indicated as follows: Scientific Department, Italy (continuous line); FOI CBRN Defense and Security, Sweden (dashed line); Bundeswehr Institute of Microbiology, Germany (dotted line). Data points represent the average of duplicate samples from at least two single experiments.
3.2. Western blotting
The inactivating properties of LED light was also confirmed by analysis of the expression of the viral proteins. Vero E6 cells were infected with SARS-CoV-2 (hCoV-19/Italy/CDG1/2020/EPI_ISL_412,973), controls (6 × 103 PFU) that had been both exposed and unexposed to the LED light and harvested at 8 h and 24 h post infection (p.i.). The western blot assay, shown in Fig. 4 , revealed a time-dependent expression of nucleocapsid protein (N), which was first detected 8 h p.i., and the spike glycoprotein (S), which was detected for the first time only later (24 h p.i.). Notably, protein expression was not detectable in the same p.i. time points of the virus-infected cells exposed to LED light, thus confirming the inactivation of SARS-CoV-2 induced by the LED irradiation.
Fig. 4.
Western blot analysis of SARS-CoV-2 proteins (spike, S and nucleocapsid, N) in Vero E6 cells infected with viral stock exposed (I+LED) or not (I) to LED light. The expression of both proteins was analyzed at 8 h or 24 h p.i. Actin was used as loading control. Blot is representative of three experiments performed. CTR, Uninfected cells; I, positive control; I+LED, virus-irradiated infected cells.
4. Discussion
SARS-CoV-2 disease represents a global threat for both public health and economic outlook worldwide. Inhalation of aerosolized droplets from infectious people is considered to be the main way of virus transmission, but despite social distancing measures, virus circulation is still sustained. Thus, indirect infection through contact with contaminated surfaces seems to contribute to the route of transmission. Recent studies performed under controlled experimental conditions, have shown the stability of SARS-CoV-2 for days on high-touch surfaces [21], with short persistence on surfaces with low porosity as compared to other highly porous surfaces [22]. In this context, the search for new methods aimed to inactivate virus in the environment, could represent a good weapon to counteract SARS-Cov-2 diffusion.
The viral inactivation by UV irradiation based on lamps emitting UV radiation C (UVC) around 254 nm is one of the most adopted methods [23], although is known to cause harmful effects on humans with short- and long-term effects [9,15] and therefore it should be not used in inhabited environments.
A well-known method for the inactivation of pathogenic bacteria is based on a photodynamic approach involving the use of light energy emitted in the visible spectrum region.
Therefore, in the present study we investigated the inactivation of SARS-CoV-2 using given wavelengths of the visible spectrum emitted by a LED-device, in order to test its ability to reduce the transmission of infections through aerosols and contact.
Interestingly, our preliminary in vitro test results, demonstrated for the first time a significant decrease in the viability and infectivity of SARS-CoV-2 after exposure to a LED device emitting visible light in a given range of wavelengths. In particular, the plaque assay showed a marked reduction in the number of PFU in the cells infected with irradiated SARS-CoV-2 compared to what observed in those cells infected with non-irradiated virus. In fact, approximately 96% inhibition was observed at the highest viral titer tested 60 min after exposure. Furthermore, viral protein expression analysis showed greater inhibition of S and N proteins in the virus exposed to LED light compared to the unexposed virus. These results suggest that viral particles subjected to LED light treatment enter cells less efficiently.
Several studies described that LED devices emitting in the 400–420 nm, 430–460 nm or 500–780 nm ranges of the visible spectrum can inactivate a wide range of bacterial species (including both Gram-positive and Gram-negative bacteria) [18,24,25] through an oxygen-dependent process resulting from the photo-stimulation of endogenous porphyrin molecules: the reactive species (RS) produced by the process, interact with cellular components causing damage to the nucleic acids and the plasma membrane. However, to date the virucidal potential of visible light-based technologies is still a poorly investigated topic.
SARS-CoV-2 is an enveloped virus in which S protein, both responsible for the binding to cellular receptor and the main target for antigenic determinant, is inserted. Reactive oxygen species (ROS) can damage lipid structures or proteins of the viral envelope [26], but the virucidal effect obtained in our study through irradiation with visible spectrum wavelengths has to be elucidated to clarify the underlying virucidal mechanism(s) of visible light. It could be hypothesized that reactive species generated by LED irradiation may alter the viral membrane or S protein stability, thus affecting the virus ability to entry host cells. However, it is also known that cell culture medium itself can influence ROS levels: the common medium component riboflavin could lead/enhance photoinactivation through generation of ROS as its absorbance spectrum also includes peaks in the LED white light spectrum [27,28]. Therefore, for evaluating the effects of the culture medium on both light absorbance and SARS-CoV-2 infectivity, and exclude a possible role of riboflavin, an experiment was performed by exposing to LED light the virus diluted in PBS 1x. The results showed the same extent of inactivation (data not shown).
However, further studies are needed to clarify the virucidal underlying mechanism of visible light.
The use of lamps emitting in the UVC spectrum provides an efficient treatment of air, liquids and surfaces as demonstrated by studies showing complete viral inactivation in a time ranging from few seconds to 15 min depending on experimental conditions. But the limit of UVC disinfection systems is represented by its use only in unoccupied spaces. Therefore, it is noteworthy that these white light emitting devices can be used for continuous decontamination and viral reduction in virtually all human environments without the dangerous side effects and protective measures associated with the use of UV light frequencies [29]. Further additional and more in-depth studies can aim to demonstrate the data obtained during these experiments in different matrices, in mutable environmental conditions, and on other respiratory viruses such as the influenza virus.
This type of LED technology could decisively contribute to reducing virus transmission through the continuous sanitation of common environments without risks for humans and animals; and confirming the antiviral effects of such LED technology in common indoor environments will be an essential step to predict its potential impact on virus transmission via fomites.
Funding
This research received no external funding
CRediT authorship contribution statement
Riccardo De Santis: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Vincenzo Luca: Investigation, Writing – original draft, Writing – review & editing. Jonas Näslund: Investigation, Writing – review & editing. Rosina K. Ehmann: Investigation, Writing – review & editing. Marta De Angelis: Investigation, Writing – review & editing. Eva Lundmark: Investigation, Writing – review & editing. Lucia Nencioni: Writing – review & editing. Giovanni Faggioni: Conceptualization, Investigation, Writing – review & editing. Silvia Fillo: Writing – review & editing. Donatella Amatore: Investigation, Writing – review & editing. Elisa Regalbuto: Writing – review & editing. Filippo Molinari: Writing – review & editing. Giancarlo Petralito: Writing – review & editing. Roman Wölfel: Writing – review & editing. Paola Stefanelli: Writing – review & editing. Gianni Rezza: Writing – review & editing. Anna Teresa Palamara: Conceptualization, Writing – review & editing. Markus Antwerpen: Writing – review & editing. Mats Forsman: Writing – review & editing. Florigio Lista: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thanks Prof. Enrico Garaci for the supervision and useful suggestions.
References
- 1.Naqvi A.A.T., Fatima K., Mohammad T., Fatim U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/.
- 3.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R.B., Dong Y.Z., Chi X.Y., Zhang M.Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blatchley E.R., III; Peel M.M. In: Disinfection, Sterilization and Preservation. 4th ed. Block S.S., editor. Lea and Febiger; Philadelphia, PA: 1991. Disinfection by ultraviolet irradiation. 823-851. [Google Scholar]
- 7.Heßling M., Hönes K., Vatter P., Lingenfelder C. Ultraviolet irradiation doses for coronavirus inactivation - review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control. 2020;15(Doc08) doi: 10.3205/dgkh000343. May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nardell E.A., Bucher S.J., Brickner P.W., Wang C., Vincent R.L., Becan-McBride K., James M.A., Michael M., Wright J.D. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the tuberculosis ultraviolet shelter study. Public Health Rep. 2008;123:52–60. doi: 10.1177/003335490812300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadi J., Dunowska M., Wu S., Brightwell G. Control measures for SARS-CoV-2: a review on light-based inactivation of single-stranded RNA viruses. Pathogens. 2020;9(9):737. doi: 10.3390/pathogens9090737. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilingloh C.S., Aufderhorst U.W., Schipper L., Dittmer U., Witzke O., Yang D., Zheng X., Sutter K., Trilling M., Alt M., Steinmann E., Krawczyk A. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020;48(10):1273–1275. doi: 10.1016/j.ajic.2020.07.031. Octdoi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criscuolo E., Diotti R.A., Ferrarese R., Alippi C., Viscardi G., Signorelli C., Mancini N., Clementi M., Clementi N. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021;10(1):206–210. doi: 10.1080/22221751.2021.1872354. Decdoi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson E.I., Prince T., Anderson E.R., Casas-Sanchez A., Smith S.L., Cansado-Utrilla C., Solomon T., Griffiths M.J., Acosta-Serrano Á., Turtle L., Hughes G.L. Methods of inactivation of SARS-CoV-2 for downstream biological assays. J. Infect. Dis. 2020;222(9):1462–1467. doi: 10.1093/infdis/jiaa507. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishisaka-Nonaka R., Mawatari K., Yamamoto T., Kojima M., Shimohata T., Uebanso T.;., Nakahashi M., Emoto T., Akutagawa M., Kinouchi Y., Wada T., Okamoto M., Ito H., Yoshida K.I., Daidoji T., Nakaya T., Takahashi A. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. J. Photochem. Photobiol. B. 2018;189:193–200. doi: 10.1016/j.jphotobiol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K., Yeager J., Altamura L.A., Biryukov J., Yolitz J., Schuit M., Wahl V., Hevey M., Dabisch P. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaffina S., Camisa V., Lembo M., Vinci M.R., Tucci M.G., Borra M., Napolitano A., Cannatà V. Accidental exposure to UV radiation produced by germicidal lamp: case report and risk assessment. Photochem. Photobiol. 2012;88(4):1001–1004. doi: 10.1111/j.1751-1097.2012.01151.x. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 16.St Denis T.G., Dai T., Izikson L., Astrakas C., Anderson R.R., Hamblin M.R., Tegos G.P. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2:509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S.S., Lee H.K., Chae H.S. In vitro photodynamic antimicrobial activity of methylene blue and endoscopic white light against helicobacter pylori 26695. J. Photochem. Photobiol. B. 2010;101:206–209. doi: 10.1016/j.jphotobiol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Maclean M., MacGregor S.J., Anderson J.G., Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405 nm light-emitting diode array. Appl. Environ. Microbiol. 2009;75:1932–1937. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enwemeka C.S., Bumah V.V., Mokili J.L. Pulsed blue light inactivates two strains of human coronavirus. J. Photochem. Photobiol. B. 2021;222 doi: 10.1016/j.jphotobiol.2021.112282. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amatore D., Sgarbanti R., Aquilano K., Baldelli S., Limongi D., Civitelli L., Nencioni L., Garaci E., Ciriolo M.R., Palamara A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell. Microbiol. 2015;17:131–145. doi: 10.1111/cmi.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitagawa H., Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T., Ohge H. Effectiveness of 222 nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control. 2021;49(3):299–301. doi: 10.1016/j.ajic.2020.08.022. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13707. Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravanat J.L., Douki T., Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B. 2001;(1–3):88–102. doi: 10.1016/s1011-1344(01)00206-8. Oct; 63. [DOI] [PubMed] [Google Scholar]
- 24.Maclean M., MacGregor S.J., Anderson J.G., Woolsey G.A. The role of oxygen in the visible-light inactivation of staphylococcus aureus. J. Photochem. Photobiol. B. 2008 doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Maclean M., macgregor S.J., Anderson J.G., Woolsey G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of staphylococcus aureus. FEMS Microbiol. Lett. 2008;285:227–232. doi: 10.1111/j.1574-6968.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 26.Müller-Breitkreutz K., Mohr H., Briviba K., Sies H. Inactivation of viruses by chemically and photochemically generated singlet molecular oxygen. J. Photochem. Photobiol. B Biol. 1995;30:63–70. doi: 10.1016/1011-1344(95)07150-z. [DOI] [PubMed] [Google Scholar]
- 27.Larrea L., Calabuig M., Roldán V., Rivera J., Tsai H.M., Vicente V., Roig R. The influence of riboflavin photochemistry on plasma coagulation factors. Transfus. Apher. Sci. 2009;41(3):199–204. doi: 10.1016/j.transci.2009.09.006. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grzelak A., Rychlik B., Bartosz G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic. Biol. Med. 2001;30(12):1418–1425. doi: 10.1016/s0891-5849(01)00545-7. [DOI] [PubMed] [Google Scholar]
- 29.Thurman C.E., Muthuswamy A., Klinger M.M., Roble G.S. Safety evaluation of a 405 nm LED device for direct antimicrobial treatment of the murine brain. Comp. Med. 2019;69(4):283–290. doi: 10.30802/AALAS-CM-18-000126. Epub 2019 Aug 6. PMID: 31387666; PMCID: PMC6733165. [DOI] [PMC free article] [PubMed] [Google Scholar]