Abstract
Traditional Chinese medicine (TCM) has been successfully applied worldwide in the treatment of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the pharmacological mechanisms underlying this success remain unclear. Hence, the aim of this review is to combine pharmacological assays based on the theory of TCM in order to elucidate the potential signaling pathways, targets, active compounds, and formulas of herbs that are involved in the TCM treatment of COVID-19, which exhibits combatting viral infections, immune regulation, and amelioration of lung injury and fibrosis. Extensive reports on target screening are elucidated using virtual prediction via docking analysis or network pharmacology based on existing data. The results of these reports indicate that an intricate regulatory mechanism is involved in the pathogenesis of COVID-19. Therefore, more pharmacological research on the natural herbs used in TCM should be conducted in order to determine the association between TCM and COVID-19 and account for the observed therapeutic effects of TCM against COVID-19.
Keywords: Traditional Chinese medicine, SARS-CoV-2, COVID-19, Antivirus, Anti-inflammation, Immune regulation
1. Introduction
As of 25 April 2021, more than 147 million people have been diagnosed with the viral disease [1], which has negatively impacted health, economic development, and social stability [2]. However, effective clinical drugs for coronavirus disease 2019 (COVID-19) are still lacking.
Traditional Chinese medicine (TCM) has shown success in treating viral infectious pneumonia. It has also exhibited therapeutic effects against infectious diseases, such as severe acute respiratory syndrome (SARS) and COVID-19. On 7 February 2020, the National Health Commission of the People’s Republic of China and the National Administration of Traditional Chinese Medicine recommended the Qingfei Paidu decoction (QFPD), the Huashi Baidu formula (HSBD), the Xuanfei Baidu decoction (XFBD), the Jinhua Qinggan granule, the Lianhua Qingwen capsule/granule (LHQW), and the Xuebijing injection (XBJ) for the treatment of COVID-19† , as these medications were found to have clinical efficacy and were used as the classical prescriptions for the treatment of flu-like symptoms, asthma, inflammation, tonsillitis, and sore throat [3], [4], [5], [6], [7], [8]. However, pharmacological evidence is needed to elucidate the anti-SARS coronavirus 2 (anti-SARS-CoV-2) effect of TCM. Here, we briefly discuss the potential therapeutic targets of COVID-19 in clinics, followed by the application of the theory of TCM in the treatment of COVID-19. This information provides the fundamental ideas behind the design and experimental assays that have been performed to elucidate the pharmacological mechanisms of the herbs used in the treatment of COVID-19. We screen existing studies and explain the mechanisms of TCM in combatting viral infections, immune modulation, anti-inflammation, and the suppression of lung injury and fibrosis, with the aim of providing a holistic understanding of TCM mechanisms against COVID-19.
2. The pathogenesis of COVID-19
2.1. Replication of and infection with SARS-CoV-2
SARS-CoV-2 is an enveloped single-stranded RNA virus that belongs to the coronavirus family [9]. It consists of four major structural proteins: the membrane protein (M protein), spike protein (S protein), envelope protein (E protein), and nucleocapsid protein (N protein) [10]. The M protein plays a pivotal role in determining the shape of the viral envelope. The S protein is a key mediator in the attachment of the virus to host cells, making it essential for viral entry into the host cell [11]. The E protein is an important M protein involved in the formation of the viral envelope and in viral budding and assembly [12]. The N protein binds to the viral RNA genome. Similar to the E protein, the N protein is also involved in the budding and assembly of the virus [11]. In addition to these structural proteins, the non-structural proteins RNA-dependent RNA polymerase (RdRP) and 3-chymotrypsin-like cysteine protease (3CLpro) play vital roles in the life cycle of SARS-CoV-2. RdRP is the core component of the replication-and-transcription complex of the virus [13], while 3CLpro is responsible for post-translational modifications of the viral polyproteins. Thus, RdRP and 3CLpro are both indispensable for viral replication and infection [14].
In the human body, angiotension-converting enzyme 2 (ACE2) is responsible for the regulation of blood pressure. ACE2 is widely expressed in the nasal mucosa, bronchus, lungs, heart, esophagus, kidneys, stomach, bladder, and ileum, all of these organs are vulnerable to SARS-CoV-2 [15]. In addition, ACE2 has been identified as the receptor of SARS-CoV-2 on the human cell membrane. ACE2 mediates the entry of SARS-CoV-2 into host cells by interacting with the S protein. Hence, ACE2 plays a key role in viral infection [16].
2.2. Pathological damage after SARS-CoV-2 invasion
SARS-CoV-2 is transmitted predominantly via respiratory droplets or direct contact. Primary viral replication is presumed to occur in the mucosal epithelium of the upper respiratory tract, while further multiplication occurs in the lower respiratory tract [17]. The initial clinical manifestations in adults infected with SARS-CoV-2 are fever, cough, shortness of breath, and fatigue. The pathophysiology of COVID-19 involves alveolar damage, infiltration with a high number of macrophages and lymphocytes manifesting as interstitial pneumonia, and pulmonary fibrosis, which emerge as the disease progresses [18]. In severe cases, patients exhibit extremely complicated symptoms including acute respiratory distress syndrome (ARDS), acute liver and heart injury, kidney failure, secondary infections, and septic shock, which imply multiple organ involvement [19].
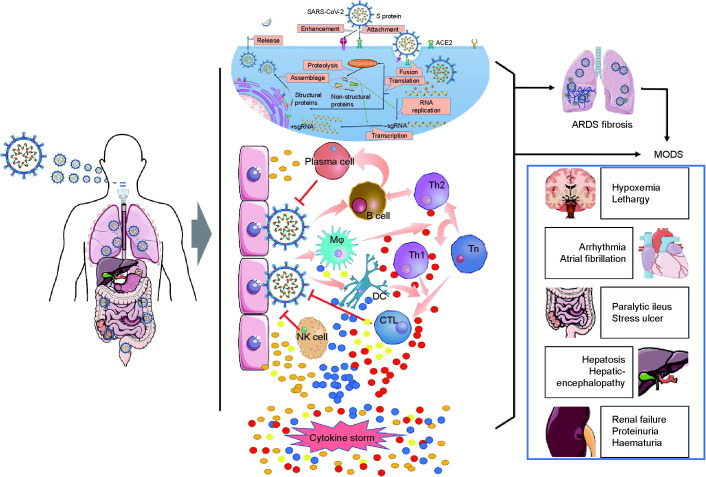
As a response to SARS-CoV-2 infection, macrophages and dendritic cells trigger an initial immune response that includes lymphocytosis and cytokine release. However, the inflammatory response results in the destruction of lymphocytes that attempt to kill SARS-CoV-2 [20], which then results in lymphopenia. In addition, cytokine production becomes rapidly dysregulated, culminating in a cytokine storm [21]. The damage caused by inflammation results in the disruption of the epithelial barrier in the lungs, which leads to further pulmonary inflammation. This damage can then spread to other organs, including the kidneys, heart, blood vessels, and brain [22] (Fig. 1 ).
Fig. 1.
Pathogenesis of COVID-19. After entering the human body, SARS-CoV-2 invades cells by binding with ACE2 and begins to replicate in the cytoplasm of the host cells. The virus is presented to B and T cells by macrophages and dendritic cells, while the host immune system is simultaneously activated. B and T cells are differentiated into effector immune cells, inducing the production of proinflammatory cytokines and chemokines. However, continuous activation of the immune system and accumulation of cytokines can lead to uncontrolled cytokine storm, causing the lungs and other organs to be seriously injured by SARS-CoV-2 infection. MODS: multiple organ dysfunction syndrome; TMPRSS2: transmembrane protease serine 2; Th: T helper cell; CTL: cytotoxic T lymphocyte; DC: dendritic cell; NK: natural killer; Tn: naive T cell; Mφ: macrophage; sgRNA: single guide RNA.
2.3. Pathobiology of SARS-CoV-2 in comparison with other viruses
As a member of the coronavirus family, SARS-CoV-2 shares approximately 80.0% and 50.0% genetic similarity with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), respectively [23]. SARS-CoV-2 also shares similar epidemiological and biological characteristics with the two aforementioned viruses. These coronaviruses cause severe respiratory tract infection with cytokine storm that can lead to viral pneumonia and ARDS [24]. However, there are differences among the three viruses, especially in their various affinities toward the host receptors and in their pathogeneses. The entry of a coronavirus into host cells involves the binding of its S proteins with the host receptors, followed by proteolytic cleavage of the S protein to expose the S2 fusion domain and subsequent membrane fusion [25], [26]. Dipeptidyl peptidase 4, the receptor of MERS-CoV, is a multifunctional cell-surface protein that is widely expressed not only in the kidneys, small intestine, liver, prostate epithelial cells, and activated white blood cells, but also in the epithelium of the upper respiratory tract in humans [27], [28]. ACE2, which acts as the receptor of SARS-CoV, is widely expressed in the alveolar epithelial cells, trachea, bronchi, bronchial serous glands, alveolar monocytes, and macrophages in the respiratory tract [29]. Similar to SARS-CoV, SARS-CoV-2 has ACE2 as its receptor, which facilitates the entry of the virus into the host cells. However, SARS-CoV-2 has a higher binding affinity with the ACE2 of host cells than SARS-CoV [30], which may explain the higher transmission rate of SARS-CoV-2 in comparison with SARS-CoV. Moreover, the monoclonal antibodies (mAbs) that are raised against the SARS-CoV receptor-binding domain (RBD) do not effectively bind with the S protein RBD of SARS-CoV-2 [30], which indicates that mAbs raised against SARS-CoV have a limited cross-reactivity with SARS-CoV-2.
There are differences in the tissue expression of the viral receptors and the activating proteases of SARS-CoV-2 and SARS-CoV, which may contribute to their unique pathophysiologies. Aside from respiratory symptoms, MERS-CoV-infected patients may present with muscle soreness and gastrointestinal manifestations. The main clinical complications include renal failure and severe respiratory distress syndrome with shock [31]. In terms of lung pathological changes, the pulmonary fibrosis and consolidation of SARS-CoV-2 are not as serious as those caused by SARS-CoV, but the exudative reaction of SARS-CoV-2 is much more apparent than that of SARS-CoV [32]. In particular, SARS-CoV-2 mainly damages the small airways and alveoli, which results in a large amount of sticky mucus and sputum plugs that block the airways, thereby resulting in secondary infections, ARDS, and respiratory failure [9], [33].
3. TCM for COVID-19 treatment
According to historical records, China has experienced more than 500 plagues in the past 3000 years [34]. Thus, the people of China have rich experience in the prevention and treatment of diseases that cause epidemics. Based on this experience, a TCM theory for treating plagues has been developed. Based on this theory, SARS-CoV-2 belongs to the category of “plague” [1]. Most COVID-19 patients present with low-grade fever, cough, fatigue, inappetence, and a thick and greasy tongue at the onset of the disease [19]. The characteristics of dampness pathogenicity affect the function of both the lungs and the gastrointestinal tract [35]. In addition, SARS-CoV-2 possesses the classic characteristic of producing dampness toxins that often cause prolonged disease duration and the functional impairment of multiple organs. Such toxins are refractory to treatment due to the viscous characteristic of the dampness, which can impede the Qi circulation. Moreover, as the disease progresses, the syndrome elements of SARS-CoV-2 transform into different subtypes, including dryness, heat, cold, or deficiency syndrome. According to the pathogenic environment and variations in the clinical manifestations of COVID-19 patients, COVID-19, as a typical “dampness-toxicity plague,” has the pathogenetic characteristics described as “dampness, heat, toxin, stasis, deficiency, and closure” [36]. Therefore, based on the theory of TCM, the treatment for COVID-19 patients should involve prescriptions of herbals that can relieve the dampness-toxicity pathogenesis. In general, such treatment strategies involve the resolution of diaphoresis to relieve heat during clinical observation, stimulation of ventilation, clearance of phlegm in the early stages of treatment, opening blocked airways in the later stages of treatment, and detoxification. These processes reverse the pathological effects of the virus in severe cases, replenish the Qi circulation, and facilitate the development of the Yin during convalescence. However, the mechanism underlying these processes remains unclear.
4. The scientific foundations of TCM as a treatment for COVID-19
As there are still no effective drugs for the treatment of COVID-19 as of the date of writing this review, the search for potential anti-coronavirus agents that can be applied to human cells is paramount [37]. Considering the pathophysiology of COVID-19, therapeutic strategies should include combatting viral infections, immune regulation, inhibition of inflammation, and prevention of lung fibrosis and injury. To investigate the multi-constituent, multi-target mechanism of TCM in the treatment of COVID-19, a network pharmacological approach has been widely applied to predict and explore the main ingredients and effective targets of various TCMs, as well as to analyze the correlation between TCM targets and COVID-19 [38], [39], [40], [41], [42], [43], [44]. Typically, the compounds of herbs have been comprehensively collected from databases (e.g., the Encyclopedia of Traditional Chinese Medicine (ETCM) and Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP)) based on their absorption, distribution, metabolism, and excretion (ADME) parameters. The compound targets of anti-COVID-19 TCM and disease targets were obtained from databases (e.g., GeneCards and Online Mendelian Inheritance in Man (OMIM) database) by adopting the keyword “new coronavirus.” Next, the genes of the compound targets from the anti-COVID-19 TCM were overlapped with those of the COVID-19 targets, and a target network model and major modules were established. The main biological targets and pathways regulated by TCM were then evaluated by Kyoto Encyclopedia of Genes and Genomes (KEGG), gene ontology (GO), and protein–protein interaction (PPI) analysis. In most reports, the findings showed a multi-herb, multi-constituent, multi-target pattern involving interference with viral infection, energy metabolism, immunity and inflammation, parasites, and bacterial infection. However, more experiments are needed to confirm the network pharmacology prediction. Here, the scientific foundations for the use of TCM in COVID-19 treatment are summarized according to the three main processes in COVID-19 treatment—namely, combatting viral infections, immune modulation and anti-inflammation, and the suppression of lung injury and fibrosis—by screening and identifying effective TCM formulas, herbs, and their active compounds.
4.1. Combatting viral infections
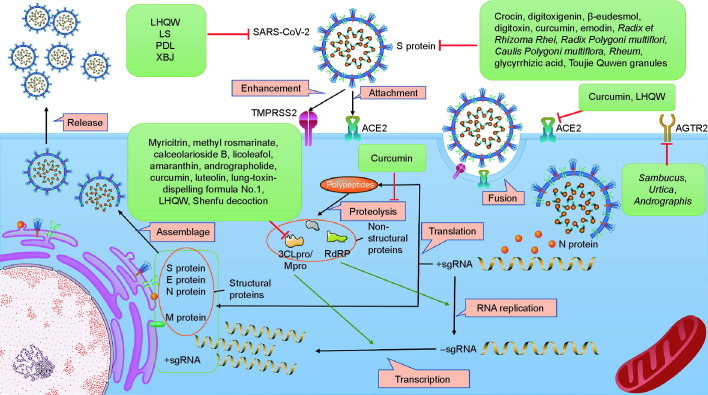
ACE2 interaction with the S protein of SARS-CoV-2, RdRP, and 3CLpro are the three main targets [45]. Blockage of the binding of cell-surface receptors with SARS-CoV-2 or inhibition of the enzyme activities of RdRP and 3CLpro are therefore potential mechanisms of TCM candidates (Fig. 2 ).
Fig. 2.
Antiviral action by TCM. TCM inhibits infection of SARS-CoV-2 through multiple mechanisms, including prevention of the activity of ACE2 and the S protein by directly binding with them, downregulating the expression of the receptors of the S protein (e.g., ACE2 and angiotensin II receptor type 2 (AGTR2)), and inhibiting viral replication by suppressing the expression of RdRP and 3CLpro. The representative TCMs related to each mechanisms are listed in the green boxes. Mpro: main protease.
4.1.1. Compounds
The results of molecular docking analysis demonstrated that several compounds with relatively low binding energies and inhibition constants can bind to the target receptors and can therefore have potential inhibitory effects on SARS-CoV-2 [2], [46]. Tahir ul Qamar et al. [14] analyzed the sequence of 3CLpro, constructed its three-dimensional (3D) homology model, and screened it against a medicinal plant library containing 32 297 potential antiviral phytochemicals and TCM compounds. The study revealed that the top nine hits, including 5,7,3′,4′-tetrahydroxy-2′-(3,3-dimethylallyl) isoflavone, myricitrin, methyl rosmarinate, 3,5,7,3′,4′,5′-hexahydroxy flavanone-3-O-β-D-glucopyranoside, (2S)-eriodictyol 7-O-(6″-O-galloyl)-β-D-glucopyranoside, calceolarioside B, myricetin 3-O-β-D-glucopyranoside, licoleafol, and amaranthin are potential lead molecules that could be developed into drugs to combat SARS-CoV-2 [14]. The compounds andrographolide, curcumin, and epigallocatechin-3-gallate were predicted to be potential inhibitors of levels of main protease (Mpro)/3CLpro of SARS-CoV-2. In silico studies should include molecular docking, target analysis, toxicity prediction, and prediction of TCM absorption, distribution, metabolism, and excretion [47], [48], [49] to further explore the potential effects of TCM against SARS-CoV-2. Luteolin, the main flavonoid in honeysuckle, was also predicted to bind with the main protease of SARS-CoV-2 and thereby prevent the entry of the virus into host cells [50]. Three natural-origin components—namely, crocin, digitoxigenin, and β-eudesmol—have been predicted to be inhibitors of S protein binding of SARS-CoV-2. This conclusion is based on an energy analysis from molecular docking experiments [51]. In addition, digitoxin is predicted to bind with the S protein of SARS-CoV-2 to inhibit interaction between the virus and human cells [52]. Flavaglines are widely used TCMs for the treatment of cough, diarrhea, fever, and inflammation. A recent study has reported that flavaglines can suppress SARS-CoV-2 infection by inhibiting the levels of the eukaryotic initiation factor 4A (eIF4A) and prohibitins-1/2 (PHB1/2), which are the key proteins used by SARS-CoV-2 to infect the human body [53].
Based on the premise that the responses against SARS-CoV-2 are similar to those of the other coronaviruses, several active compounds of TCM have exhibited antiviral activity, and may be therapeutic options for COVID-19 [20]. Curcumin is a well-known natural compound with antiviral effects. It blocks the entry of viruses to cells by targeting critical steps in the viral replication cycle [48], including alteration of the structures of surface proteins in viruses, competitive inhibition of viruses by binding with cell receptors, and inhibition of the expression of ACE2 receptor [54], [55]. Moreover, the S protein level of SARS-CoV-2 [55] and the plaque numbers of the coronavirus porcine epidemic diarrhea virus (PEDV) in infected cultured cells were quantified [54]. The results of these quantification assays showed that curcumin inhibited viral replication. In addition, the anti-coronaviral activity of saikosaponins (A, B2, C, and D) has been demonstrated. In particular, saikosaponins inhibited human coronavirus 229E infection at concentrations of 0.25–25 mmol·L–1, with saikosaponin B2 exhibiting the strongest activity after its addition at various times including at pre-infection (−4 to −1 h), co-infection (0 h), and post-infection (1–4 h) [56]. Furthermore, the results of the in silico analysis revealed that saikosaponin effectively bound with critical inflammation-related targets, including the interleukin-6 receptor (IL-6R), Janus kinase-3 (JAK3), and nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase 5 (NOX5) [57]. Furthermore, emodin, an anthraquinone compound derived from Rheum and Polygonum, significantly blocked S protein and ACE2 receptor interaction and reduced the pathogenicity of a S protein-pseudotyped retrovirus in Vero E6 cells in a dose-dependent manner [58].
4.1.2. Herbs
Many herbs have exhibited therapeutic potential against viral diseases. For example, peel extracts of Citrus sinensis significantly reduced the viral load in HeLa-CEACAM1a cells inoculated with mouse hepatitis virus (MHV)-A59 [59]. Moreover, Cimicifuga foetida L. (Sheng Ma), Melia azedarach L. (Ku Lian Pi), Coptis chinensis Franch. (Huang Lian), Phellodendron chinense Schneid. (Huang Bai), and Sophora flavescens Ait. (Ku Shen) decreased the replication and suppressed the expression of intracellular RNA and proteins of MHV, vesicular stomatitis virus (VSV), and PEDV at a half maximal effective concentration (EC50) of 2.0–27.5 μg·mL−1 [60]. Furthermore, Rheum officinale Baill. (Da Huang), Polygonum multiflorum Thunb. (He Shou Wu), and Caulis Polygoni multiflori (Shou Wu Teng) potentially inhibited the interaction between SARS-CoV-2 and ACE2 at a half maximal inhibitory concentration (IC50) of 1–10 μg·mL−1 [58]. Moreover, TCM herbs such as Sambucus williamsii Hance (Jie Gu Mu), Urtica fissa E. Pritz. (Xun Ma), and Andrographis paniculata (Burm. f.) Nees (Chuan Xin Lian) can potentially block the entry of the virus to human cells by downregulating the expression of angiotensin II receptor type 2 (AGTR2), which is a lung-specific protein with a high affinity for the S protein of SARS-CoV-2 [61]. The results of a network pharmacology analysis revealed that Ephedra sinica Stapf. (Ma Huang)–Prunus armeniaca L. (Ku Xing Ren), a common couplet medicine in anti-COVID-19 prescriptions, exhibited anti-COVID-19 activity [62]. In particular, β-sitosterol, estrone, and stigmasterol, which were isolated from ephedra and bitter almond, were found through a molecular docking analysis to have a high binding affinity to 3CLpro and ACE2 [62]. An ACE2/cell membrane chromatography analysis also revealed that the components of ephedra may bind to ACE2 [63]. Another classic couplet medicine, Ephedra sinica Stapf. (Ma Huang)–Glycyrrhiza uralensis Fisch. (Gan Cao), was also found to have a potential role in the treatment of COVID-19. The results of a network pharmacology analysis revealed that Gan Cao regulated cyclic adenosine monophosphate (cAMP) levels and the phosphatidylinositol-3-kinase–protein kinase B (PI3K–Akt) and JAK–signal transducer and activator of transcription (STAT) pathways and inhibited viral replication by targeting 3CLpro or the S protein [64].
4.1.3. Formula
Based on clinical results, TCM formulas have been applied to treat COVID-19, and their effects have been remarkable. Experimental studies have focused on the potential antiviral effects of classical formulas. For example, the HSBD has been recommended by the National Health Commission of the People’s Republic of China for the treatment of COVID-19 patients with mild and severe symptoms [1]. Cai et al. [65] identified 223 active ingredients in HSBD that potentially interact with 84 COVID-19-related target genes, such as ACE2, estrogen receptor 1, adrenergic receptor α1, and histone deacetylase 1. The results of GO and KEGG analyses revealed that these target genes were enriched in nuclear factor (NF)-κB, renin–angiotensin-related signaling, and the adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling pathways, which indicates that HSBD plays a beneficial role in the treatment of COVID-19 by regulating various pathways [65].
QFPD is another formula recommended by the National Health Commission of the People’s Republic of China for treating COVID-19. Zhou et al. [66] analyzed the major components of QFPD via ultrahigh‐performance liquid chromatography and mass spectrometry (UHPLC-LTQ-Orbitrap-MS), and characterized 87 compounds, including flavonoids, alkaloids, triterpenoid saponins, sesquiterpene, and phenolic acid. After the administration of QFPD to mice, a total of 12 compounds were detected in the plasma [67]. The results of in silico analysis showed that the mechanisms involved were regulation of oxidoreductase activity, lipid metabolism, lipid binding, small-molecule metabolism, and homeostasis [68]. Furthermore, the results of a molecular docking experiment showed that the compounds in QFPD exerted antiviral effects by directly binding with the key proteins of SARS-CoV-2 and host proteins [69]. Thus, QFPD may effectively inhibit infection with and replication of SARS-CoV-2. Moreover, a study revealed that early treatment with QFPD was associated with viral shedding, rapid recovery, and a reduced hospital stay [70]. Similarly, the lung-toxin-dispelling formula No. 1, also referred to as the respiratory detox shot, has been used in the prevention and treatment of COVID-19 [71]. Molecular docking data showed that 118 constituents from 1071 known chemical constituents in the formula exhibited a high binding affinity to 3CLpro in SARS-CoV-2. In addition, 22 more chemical constituents were further validated by performing an in vitro study [71].
Maxing Shigan decoction (MXSGD) is a classic prescription for lung diseases that is composed of Ephedra sinica Stapf. (Ma Huang), Prunus armeniaca L. (Ku Xing Ren), Gypsum fibrosum (Shi Gao), and Glycyrrhiza uralensis Fisch. (Gan Cao). A total of 97 components were identified from MXSGD via liquid chromatography–electrospray ionization–tandem mass spectrometry (LC–ESI–MS/MS) [72]. The results of the KEGG analysis revealed that the potential signaling pathways targeted in COVID-19 treatment with MXSGD were mainly enriched in the immune system and viral infection. An in vitro study also found that MXSGD effectively inhibited IL-6-stimulated JAK–STAT activation and inhibited lung epithelial damage by increasing B-cell lymphoma-2 (Bcl-2) expression in rat lung epithelial type II cells (RLE-6TN) [73].
Another TCM formula, LHQW, a commercial drug that is widely used to treat influenza, exhibits a broad-spectrum antiviral effect against influenza viruses [74]. The results of a network pharmacology analysis suggested that LHQW regulated ACE2 expression [75]. Furthermore, pharmacokinetic data showed that its active compounds, such as rhein, forsythoside A, forsythoside I, and neochlorogenic acid, suppressed the activity of ACE2 [76]. In addition, the antiviral activity of LHQW against the novel SARS-CoV-2 virus was observed in Vero E6 cells via cecal ligation and puncture (CLP) and a plaque reduction assay [77]. Similarly, the Liu Shen capsule (LS) significantly inhibited SARS-CoV-2 replication and thus reduced the number of viral particles in Vero E6 cells [78]. Moreover, the Shenfu decoction was used in the treatment of patients with severe COVID-19 [79]. Data from in silico analysis was used to screen 43 active components of the Shenfu decoction, which predicted that their antiviral effect involved the suppression of SARS-CoV-2 replication, mainly through the inhibition of levels of viral Mpro, RdRP, and the S protein [80]. In addition, Pudilan Xiaoyan oral liquid (PDL) was reported to clear the heat and detoxify, which explained its antiviral and antibacterial effects. Recently, it was found that PDL effectively suppressed viral replication in Vero E6 cells inoculated with SARS-CoV-2. In addition, PDL significantly reduced the number of viral RNA copies in the lungs of SARS-CoV-2-infected human angiotensin-converting enzyme 2 (hACE2) transgenic mice [81].
Another Chinese patent medicine, the XBJ, was approved for the treatment of sepsis, systemic inflammatory response syndrome, multiple organ failure, and severe and critical SARS-CoV-2 [77]. The results of a network pharmacology analysis demonstrated that 22 components from XBJ acted via 54 SARS-CoV-2 infection-related targets, including IL-6, tumor necrosis factor (TNF), mitogen-activated protein kinase 1 (MAPK1), glyceraldehyde-3-phosphate dehydrogenase, and tumor protein p53 which are mainly expressed in the heart, lungs, liver, intestine, trachea, pancreas, and kidneys. The multicellular biological processes of the critical targets included antioxidant and anti-inflammatory activities. Moreover, results of a molecular docking analysis revealed that the components of XBJ had a high affinity with the 3CLpro hydrolase of SARS-CoV-2 and the ACE2 receptors of the host cells. These results indicate that one of the mechanisms of XBJ in the treatment of pneumonia associated with SARS-CoV-2 infection includes the essential protein of the virus and its human ACE2 receptor, producing an antiviral effect [82].
4.2. Immune modulation and anti-inflammation
Viral pathogens cause malignant diseases in humans and animals, resulting in considerable mortality, morbidity, and economic losses worldwide [83], [84]. Although great progress has been made in the treatment of infectious viral diseases, there are still no specific drugs for several mutant viral strains [85]. Thus, new treatment strategies are urgently needed.
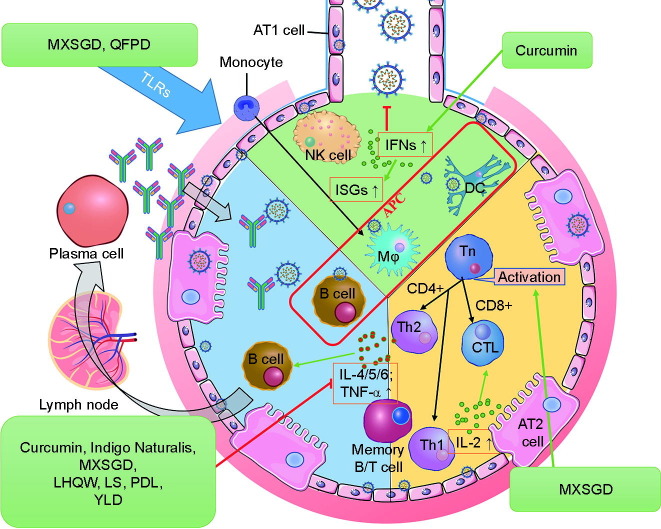
Strengthening the immune system helps to build resistance against viral infections and to control the response of the immune system after significant viral replication. Several agents enhance the immune system, including the natural herbs of TCM. In addition, the treatment principle of TCM focuses on the whole body of the patient rather than only on the eradication of the pathogen. Therefore, natural herbs and TCM are considered to be effective pretreatment therapies for the prevention of diseases and the alleviation of mild symptoms (Fig. 3 ).
Fig. 3.
TCM immune modulation and anti-inflammation. TCM treats SARS-CoV-2 infection by modulating the host immune system, which includes increasing the production of interferons (IFNs) at the onset of the disease, inhibiting Toll-like receptor (TLR)-mediated inflammatory response, suppressing the production of proinflammatory cytokines, and inducing the differentiation of T and B cells into effector immune cells. The representative TCMs acting on each pathway are listed in the green boxes. CD: cluster of differentiation; ISG: interferon-stimulated gene; AT: alveolar type; YLD: Yidu-toxicity blocking lung decoction.
One of the key processes that reduces the fatality of SARS-CoV-2 may be the activation of the innate immune responses that trigger interferon (IFN) production at the onset of the disease. This can be achieved through the administration of agents that can increase the synthesis of IFNs [86], [87]. There is growing evidence that curcumin can induce the production of IFNs in different viral diseases [88], [89], [90]. This finding was supported by a study that showed that cationic carbon dots of curcumin resulted in the suppression of coronavirus replication by stimulating the expression of interferon-stimulating genes and by decreasing IL-6 and IL-8 cytokine levels in Vero cells when the innate immunity of the host was triggered [66].
TCM formulas exhibit advantages in the modulation of the immune system. Several classical formulas aim to remove dampness, heat, toxins, stasis, deficiency, and closure, and are therefore strongly recommended for the treatment of COVID-19. MXSGD is recommended as a basic prescription and is already widely applied in the clinical treatment of COVID-19. Using network pharmacology results, a total of 97 active ingredients of MXSGD were screened, and 169 targets were predicted. The results revealed that the altered biological processes that occurred after taking MXSGD were closely related to the acute inflammatory response, chemokine production, vascular permeability, response to oxygen radicals, oxidative stress-induced apoptosis, T cell differentiation, immune globulin secretion, and extracellular matrix disassembly [91]. Experimental data confirmed that MXSGD exerted its anti-inflammatory effect via the suppression of levels of Toll-like receptors (TLRs) in macrophages and rats with pneumonia induced with liposaccharide (LPS) [79].
The TCM formula LHQW was reported to markedly reduce the production of proinflammatory cytokines such as TNF-α, IL-6, C–C motif ligand 2 (CCL-2)/monocyte chemotactic protein 1 (MCP-1), and chemokine C–X–C motif ligand 10 (CXCL-10)/interferon-inducible protein 10 (IP-10) [77]. The LS also exhibited an antiviral effect against SARS-CoV-2 in regulating the host immune response. The LS significantly inhibited the production of proinflammatory cytokines such as TNF-α, IL-6, IL-1β, IL-8, CCL-2/MCP-1, and CXCL-10/IP-10 via modulation of the NF-κB/MAPK signaling pathways [78]. Another TCM formula, the PDL, alleviated SARS-CoV-2-induced pneumonia in hACE2 mice. Mice treated with PDL exhibited reduced numbers of infiltrated inflammatory cells in lung tissues. Furthermore, the results of bioinformatics and network pharmacology analyses indicated that PDL reduced the expression levels of proinflammatory cytokines such as IL-10 and TNF-α [81]. Another TCM that is widely used to treat COVID-19 patients is QFPD. This formula was developed based on the classic formula of MSXGD. A target network analysis isolated the active compounds of QFPD and predicted their targets. According to the data, the TLR signaling pathway was found to be an important pathway regulated by QFPD. In addition, the XFBD is a formula that was developed based on the interpretation of COVID-19 in Chinese medicine theory. Clinical trials on XFBD showed that, when used in combination with conventional medicines, XFBD significantly suppressed the expression of inflammatory factors and provided symptomatic relief [4]. Another TCM prescription, the Yidu-toxicity blocking lung decoction (YLD), was created especially for the treatment of COVID-19. It was reported that YLD showed an anti-inflammatory effect in COVID-19 patients. Moreover, the expression levels of IL-6 and TNF-α were found to be significantly reduced in patients treated with YLD in combination with western medicine [92].
4.3. Suppression of lung injury and fibrosis
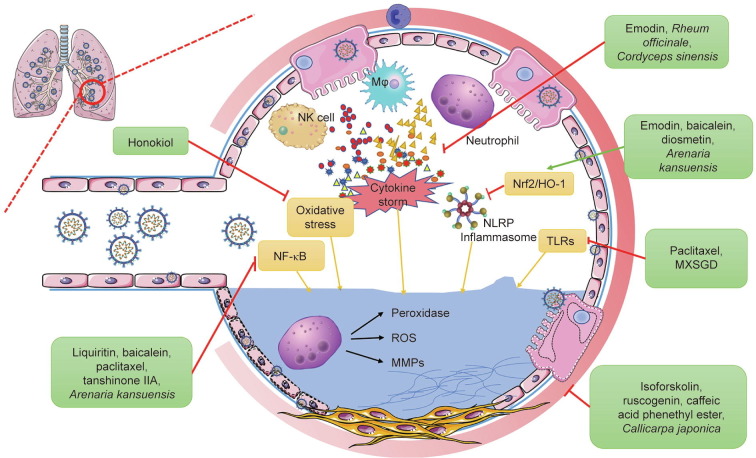
Acute lung injury (ALI) is one of the manifestations of COVID-19, and is described as increased capillary permeability and acute inflammatory disorder caused by endotoxemia [93]. One of the most serious forms of ALI is ARDS, which leads to high mortality due to over-activation of the immune system, which results in the production of a cascade of proinflammatory mediators [94], [95]. Although considerable progress has been made in understanding the pathogenesis of ARDS, little progress has been made in the development of specific therapies to combat injury and inflammation. Recently, several natural products were studied in experimental models and were shown to inhibit multiple inflammatory pathways associated with ALI and ARDS at a molecular level (Fig. 4 ).
Fig. 4.
Suppression of lung injury and fibrosis by TCM. TCM reduces lung injury and pulmonary fibrosis by inhibiting inflammation-related and oxidation-related pathways, such as by downregulating the expression of cytokines, inflammasomes, and reactive oxygen species (ROS); suppressing alveolar neutrophil infiltration; inhibiting NF-κB, nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1), and TLR-mediated pathways; and reducing oxidative stress. The representative TCMs acting through each pathway are listed in the green boxes. NLRP: nucleotide-binding domain leucine-rich repeat (NLR) and pyrin domain containing receptor; MMP: matrix metalloproteinase.
Honokiol, which is derived from Magnolia, alleviates sepsis-associated ALI via the inhibition of oxidative stress in mice [96]. Several natural compounds, including isoforskolin, ruscogenin, and caffeic acid phenethyl ester, exhibited inhibitory effects against lung injury, as demonstrated in mice and rats with ALI induced by LPS [97], [98], [99], [100], [101]. It was reported that a variety of TCM herbs and their active compounds contribute to the suppression of ALI. Arenaria kansuensis Maxim. (Xue Ling Zhi) was reported to play a protective role in pulmonary fibrosis through activation of the nuclear factor E2-related factor 2 (Nrf2) pathway and inhibition of the NF-κB/transforming growth factor (TGF)-β1/Sma- and Mad-related protein 2/3 (Smad2/3) axis [102]. Licorice, the root of Glycyrrhiza uralensis Fisch. (Gan Cao), has been widely used in China as an anti-inflammatory and antitussive herb. It was found that its main flavonoid, liquiritin, which was isolated from Glycyrrhiza uralensis Fisch. (Gan Cao), prevented lung damage and suppressed inflammation by inhibiting the NF-κB, transient receptor potential vanilloid 1 (TRPV1), and transient receptor potential cation (TRPC) channel subfamily A member 1 signaling pathways [103]. Rheum officinale Baill. (Da Huang) has been used for the treatment of pulmonary diseases in China for over a thousand years, as it can ameliorate inflammatory responses. Emodin [104], one of its major active components, improves pathological conditions and decreases the number of infiltrated inflammatory cells by regulating the mechanistic target of rapamycin kinase(mTOR)/hypoxia inducible factor 1 subunit α (HIF-1α)/vascular endothelial growth factor (VEGF) signaling pathways in LPS-induced ALI. Another report showed that emodin effectively protected rats against acute pancreatitis-associated lung injury by inhibiting pyrin domain-containing protein 3 (NLRP3) inflammasome activation through Nrf2/heme oxygenase-1 (HO-1) signaling [105]. Emodin also exerted protective effects against lung injury in septic rats via inactivation of the p38 MAPK pathway and reduction of oxidative stress and anti-inflammatory response during sepsis [106]. Baicalein, a phenolic flavonoid extracted mainly from the root of Scutellaria baicalensis Georgi (Huang Qin), demonstrated a protective effect against LPS-induced ALI in rats [107]. The underlying mechanism included inhibition of the NF-κB-mediated inflammatory response and upregulation of the Nrf2/HO-1 pathway. In addition, curcumin was found to regulate the IL-35 level by activating the differentiation of regulatory T (Treg) cells to control inflammation in CLP-induced ALI [108]. Diosmetin, an active component in Chinese herbs, had inhibitory effects on an LPS-induced ALI model via activation of Nrf2 and inhibition of NLRP3 inflammasome activation [109]. Paclitaxel, a classic anticancer drug isolated from the bark of the Taxus brevifolia tree, significantly alleviated ALI in CLP-induced septic mice by activating mucin 1 (MUC1) and suppressing the TLR-4/NF-κB pathway [110]. Moreover, β-sitosterol (24-ethyl-5-cholestene-3-ol), a common phytosterol in Chinese medical plants, ameliorated influenza A virus(IVA)-induced inflammatory response and ALI in mice by disrupting the cross-talk between retinoic acid-inducible gene-I (RIG-I) and IFN/STAT signaling [111]. Tanshinone IIA, one of the major active components in Salvia miltiorrhiza Bge. (Dan Shen), exhibited protective effects against LPS-induced lung injury in mice through the inhibition of NF-κB level [112]. Cordyceps sinensis extract (Dong Chong Xia Cao (DCXC)) reduced the degree of histopathological injury, wet/dry weight ratio, and myeloperoxidase (MPO) activity in experimental ALI mice [113]. The numbers of total cells, neutrophils, and macrophages in the bronchoalveolar lavage fluid (BALF) were also significantly inhibited by DCXC treatment. The levels of TNF-α, IL-1β, IL-6, and nitric oxide in BALF after LPS administration were significantly reduced by DCXC. In particular, DCXC exhibited anti-inflammatory and antioxidation effects on LPS-induced ALI by inhibiting NF-κB p65 phosphorylation and the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in the lungs.
Polysaccharides are a group of substances with active pharmacological effects that have been widely identified in Chinese medicine. Several studies have revealed that polysaccharides play important immune regulatory, antitumor, antiviral, and antipulmonary fibrosis roles [114]. Recently, Chen et al. [115] identified 16 kinds of polysaccharides extracted from herbal plants, algae, and fungi that possess significant antipulmonary fibrosis activities. The study reported that these polysaccharides improved pulmonary histopathological changes and collagen deposition in animal models induced by bleomycin (BLM). The study also summarized the antipulmonary fibrotic effect of these polysaccharides, which was mainly exerted through the TGF-β1/Smad2/3 and differentiation antagonizing non-protein coding RNA (DANCER)/AU-rich element binding factor1 (AUF-1)/forkhead box O3 (FOXO3) regulatory axes [115], suggesting polysaccharides as potential effective substances for the prevention and treatment of pulmonary fibrosis in COVID-19 patients.
5. Discussion
As a newly emerging and severe acute respiratory infectious disease, COVID-19 has caused great losses in global health, economy, and social stability. Thus far, few effective drugs have been used specifically for the treatment of COVID-19. Based on experience from treating SARS and MERS, several well-known antiviral agents have been considered as potential candidates for the treatment of patients infected with SARS-CoV-2. The first category of these antiviral medications function by inhibiting the level of RdRP of the virus. This mechanism is exhibited by remdesivir [116], ribavirin [117], and favipiravir [118]. All these drugs have shown potential therapeutic effects in treating COVID-19 patients [119]. Antiviral medications in another category function by inhibiting the levels of viral replication-related key proteases and by arresting replication of the virus [120]. This is the mechanism of lopinavir/ritonavir, which has been shown to improve the condition of COVID-19 patients. Thus, these medications have been recommended by the National Health Commission of the People’s Republic of China and the Infectious Diseases Society of America for the treatment of COVID-19 patients [1], [2]. Aside from these viral targets, the inflammatory cytokines produced by critically ill COVID-19 patients have also been suggested as a therapeutic target, as these cytokines can trigger a serious inflammatory response [25]. Based on this consideration, some mAbs (e.g., Tocilizumab, which suppresses IL-6-mediated inflammatory response by blocking its receptor [121]) are also used to treat COVID-19 patients. However, a particular medicine that can address all the symptoms of COVID-19 is still not available at the moment. Because TCM has shown excellent clinical effects in treating infectious diseases, an exploration of effective intervention strategies from TCM for the prevention and treatment of COVID-19 is warranted.
TCM has a history of more than 2000 years in the prevention and treatment of epidemics and plagues, with remarkable clinical therapeutic efficacy. In the last few decades, several new viruses have emerged, including SARS-CoV, MERS-CoV and, most recently, SARS-CoV-2. These viruses have caused epidemics and pandemics, and the pathogens associated with these diseases are all coronaviruses. TCM does not only focus on the eradication of the etiologic viral agent; it also considers the overall state and internal environment of the body by promoting vital Qi and by enhancing the body’s resistance to viruses. This treatment strategy is guided by the holistic concept and syndrome differentiation of TCM theory, which may be one of the reasons explaining the therapeutic efficacy of TCM against various viral diseases that have caused epidemics. Although SARS-CoV-2 is not exactly the same as SARS-CoV and MERS-CoV, these three coronaviruses have partially similar pathological and clinical manifestations. Therefore, in the process of clarifying the therapeutic strategies of TCM for SARS-CoV-2 treatment, the effects of TCM on other coronaviruses have provided important and meaningful references.
The strengths and weaknesses of Chinese medicines in the treatment of COVID-19 are an important and broad issue, which many TCM doctors and pharmacologists are continually attempting to figure out. At present, in order to fight against COVID-19 in an efficient way, various drug–drug or drug–herb combinations have been recommended for treating COVID-19 in clinical settings. The tireless efforts of the scientific community have led to an increased accumulation of knowledge and data about the disease and relevant TCM formulas. Clinical data show that the integrative combination of TCM and western medicine provides comprehensive therapy for COVID-19 [122]. In brief, TCM mainly focuses on the patients, with the aim of enhancing the natural activity of the human body in a dialectical way. In comparison, western medicine focuses on the symptoms of disease. The advantages of TCM lie in its individualized and comprehensive consideration of treatment. However, TCM prescriptions are generally complicated and contain several ingredients. The weakness of TCM may be the difficulty in target identification; furthermore, the mechanism involved may not be easily and completely elucidated, even though efforts to uncover the pharmacological mechanisms of TCM are ongoing. Increasing amounts of data have shown that the effectiveness of TCM in the treatment of COVID-19 is due to the multiple targets and signaling pathways mentioned above. Published results are providing a new research basis for a better understanding of the mechanisms of TCM treatment of COVID-19. Recently, the three Chinese patent medicines and three TCM prescriptions that have been recommended by National Administration of Traditional Chinese Medicine for the COVID-19 treatment have been systematically investigated to uncover their potential mechanisms using modern pharmacological technologies [69], [123], [124], [125]. The pharmacokinetics of TCM in COVID-19 treatment have also been extensively studied to illustrate the pharmacodynamic material basis [67], [126], [127]. However, some active components of TCM are difficult to detect in serum, which indicates that they may exert efficacy in other ways, such as via modulation of the gut microbiota and metabolomics. Given the complexity of the compositions of TCM prescriptions and the pathogenesis of COVID-19, the advantages of TCM may lie not only in its regulation of immunity, but also in its holistic regulation of metabolism and the intestinal environment [126]. Furthermore, establishing an in vivo model that combines TCM theory with the SARS-CoV-2 virus infection may help in improving the reliability of nonclinical models in better simulating clinical complexities. However, the number of existing P3-grade labs cannot meet the research requirements. Moreover, in order to align a model with the features of TCM, it is necessary to superimpose the infection onto an ideal syndrome model, which presents another challenge for TCM researchers.
In this review, we summarized the potential targets of the natural compounds, herbs, and formulas from TCM that address SARS-CoV-2 infection, based on reported network pharmacology and molecular docking results. The experimental antivirus effects are mainly characterized by the direct inhibition of virus replication. In regard to the immune system destruction, inflammatory cytokine storm, and lung damage caused by COVID-19, some classic TCM formulas and proprietary Chinese medicines may regulate the immune system, reduce inflammatory responses, and suppress lung fibrosis and injury (Table 1 [4], [47], [51], [54], [55], [56], [58], [60], [69], [71], [74], [76], [77], [78], [80], [81], [96], [98], [99], [100], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [124], [127], [128], [129], [130], [131], [132], [133], [134]).
Table 1.
Potential anti-SARS-CoV-2 effects of TCM.
| Categories of TCM effect | Natural compound/herbal medicine/formula | Targets/pathway | Cell/animal model | Function | References |
|---|---|---|---|---|---|
| Diaphoretic drug | Saikosaponins (Bupleurum chinense DC.) (Chai Hu) | — | MRC-5 cells | Inhibits absorption and penetration of viral HCoV-229E | [56] |
| MXSGD | TLR signaling pathway | LPS-induced pneumonia in rats | Suppresses LPS-induced inflammatory response by inhibiting TLR signaling in LPS-induced pneumonia in rats | [127] | |
| Shen Zhu San | — | — | Network pharmacology shows that Shen Zhu San has the ability to suppress cytokine storms, protect the pulmonary alveolar-capillary barrier, regulate the immune response, and mediate cell death and survival | [133] | |
| Toujie Quwen granules | — | — | Quercetin and isoquercitrin have high affinity with the SARS-CoV-2 S protein, while astragaloside IV and rutin have high affinity with ACE2 | [134] | |
| Heat-clearing drug | Andrographolide (Andrographis paniculata (Burm. f.) Nees) (Chuan Xin Lian) | Mpro | — | Inhibits the main protease of SARS-CoV-2 | [47] |
| Baicalein (Scutellaria baicalensis Georgi) (Huang Qin) | NF-κB and Nrf2/HO-1 | LPS-induced acute lung injury in rat | Suppresses LPS-induced acute lung injury | [107] | |
| Cimicifuga foetida L. (Sheng Ma), Melia azedarach L. (Ku Lian Pi), Coptis chinensis Franch. (Huang Lian), Phellodendron chinense Schneid. (Huang Bai), and Sophora flavescens Ait. (Ku Shen) | MHV proteins | Mouse cell line—delayed brain tumor cells | Decreases MHV, VSV, and PEDV production and intracellular viral RNA and protein expression | [60] | |
| Indigo Naturalis (Polygonum tinctorium Ait.) (Qing Dai) | — | Peritoneal macrophages, IAV-induced acute lung injury in BALB/c mice | Alleviates IAV-induced ALI in mice by its anti-influenza, anti-inflammatory (TNF-α, IL-6) and antioxidation (MPO, MDA) properties | [129] | |
| β-sitosterol (Taraxacum mongolicum Hand.-Mazz., Chrysanthemum morifolium Ramat., Forsythia suspensa (Thunb.) Vahl) (Pu Gong Ying, Ju Hua, Lian Qiao) | RIG-I and IFN/STAT | Acute lung injury in mice | Ameliorates IAV-induced proinflammatory response and acute lung injury in mice | [111] | |
| LHQW | — | Vero E6 cells | Inhibits replication of SARS-CoV-2 and decreases expression of proinflammatory cytokines in Vero E6 cells | [77] | |
| — | — | Four key components (quercetin, luteolin, wogonin, and kaempferol) showed a high binding affinity with 3CLpro of SARS-CoV-2 | [130] | ||
| LHQW | — | — | Rhein, forsythoside A, forsythoside I, neochlorogenic acid, and its isomers exhibited high inhibitory effect on ACE2 | [76] | |
| — | — | Six active compounds of LHQW can enter the active pocket of Akt1 exerting potential therapeutic effects in COVID-19 | [131] | ||
| NF-κB and Raf/MEK/ERK signaling | MDCK cells, BALB/c mice | Inhibits propagation of influenza viruses and decreases expression of proinflammatory cytokines by suppressing NF-κB activation | [74] | ||
| LS | — | Vero E6 cells | Inhibits SARS-CoV-2 replication in Vero E6 cells and reduces the number of virus particles | [78] | |
| NF-κB/MAPK | Huh-7 cells | Suppresses inflammatory response by inhibiting the production of proinflammatory cytokines (TNF-α, IL-6, IL-1β, IL-8, CCL-2/MCP-1, and CXCL-10/IP-10) | [78] | ||
| Lung-toxin dispelling formula No. 1 | — | — | One hundred and eighteen constituents of respiratory detox shot (RDS) showed a high binding affinity with 3CLpro of SARS-CoV-2 | [71] | |
| PDL | — | Vero E6 cells | Suppresses replication of SARS-CoV-2 in Vero E6 cells | [81] | |
| — | SARS-CoV-2 infected hACE2 transgenic mice | Reduces replication of SARS-CoV-2 in hACE2 transgenic mice; suppresses inflammatory response by inhibiting expression of IL-10 and TNF-α | [81] | ||
| QFPD | NF-κB/MAPK | — | Inhibits expression of proinflammation factors, such as TNF-α, IL-1β, IL-8 | [4] | |
| Production of cytokines and chemokines | RAW264.7 cells | Suppresses production of proinflammatory cytokines and chemokines, such as IL-6, CCL-2, and TNF-α, and induces the expression of IL-10 | [69] | ||
| Blood-activating and stasis-dissolving drug | Curcumin (Curcuma Longa L., Curcuma phaeocaulis Val.) (Jiang Huang, E Zhu) | SARS-CoV-2 protease, spike glycoprotein-RBD, and PD-ACE2 | — | Inhibits the expression of ACE2 and viral replication | [54], [55], [128] |
| β-actin | Vero cells | Suppresses viral replication and changes the structure of the surface protein in viruses, thereby inhibiting viral entry | [54] | ||
| Differentiation of Treg cells | CLP-induced acute lung injury in mouse | Controls inflammation in CLP-induced acute lung injury | [108] | ||
| Isoforskolin (Sparganium stoloniferum Buch.-Ham.) (San Leng) | — | LPS-induced acute lung injury in mice and rats | Suppresses LPS-induced acute lung injury and decreases the expression of TNF-α, IL-6, IL-8 and IL-1β | [99] | |
| Tanshinone IIA (Salvia miltiorrhiza Bge.) (Dan Shen) | MIF and NF-κB | LPS-induced lung injury in mice | Protects against LPS-induced lung injury in mice | [112] | |
| XBJ | — | — | The active ingredients of XBJ regulate different genes, act on different pathways, and synergistically produce anti-inflammatory and immune regulatory effects | [132] | |
| Crocin (Crocus sativus L.) (Zang Hong Hua), Digitoxigenin (Nerium oleander L.) (Jia Zhu Tao), and β-eudesmol (Laurus nobilis, Commiphora myrrha Engl.) (Yue Gui, Mo Yao) | — | — | Suppresses the replication of SARS-CoV-2 by inhibiting the main protease of SARS-CoV-2 | [51] | |
| Purging drug | Emodin (Rheum officinale Baill.) (Da Huang) | SARS-CoV-2 S protein | Vero E6 cells infected with protein-pseudotyped retrovirus | Blocks the S protein and ACE2 interaction and the infectivity of S-protein-pseudotyped retrovirus to Vero E6 cells in a dose-dependent manner | [58] |
| Emodin (Rheum officinale Baill., Polygonum cuspidatum Sieb. et Zucc.) (Da Huang, Hu Zhang) | mTOR/HIF-1α/VEGF | LPS-induced acute lung injury in rat | Improves the pathological conditions and decreases the number of infiltrated inflammatory cells in LPS-induced ALI | [104] | |
| Nrf2/HO-1 | Rat with acute pancreatitis | Protects rats against acute pancreatitis-associated lung injury by inhibiting NLRP3 inflammasome activation | [105] | ||
| p38 MAPK | Rat with sepsis | Protects lung injury in septic rats by suppressing inflammatory response | [106] | ||
| Tonify deficiency medicine | Polygonum multiflorum Thunb. (He Shou Wu) and Caulis Polygoni multiflora (Shou Wu Teng) | SARS-CoV-2 S protein | Vero E6 cells infected with protein-pseudotyped retrovirus | Blocks the S protein and ACE2 interaction and the infectivity of S-protein-pseudotyped retrovirus to Vero E6 cells in a dose-dependent manner | [58] |
| Ruscogenin (Ophiopogon japonicus (L.f) Ker-Gawl) (Mai Dong) | — | LPS-induced acute lung injury in rats | Suppresses LPS-induced acute lung injury | [100] | |
| Glycyrrhizic acid (Glycyrrhiza uralensis Fisch.) (Gan Cao) | — | HEK293 cells | Inhibits the SARS-CoV-2 RBD interaction with ACE2 | [124] | |
| Arenaria kansuensis Maxim. (Xue Ling Zhi) | Nrf2 pathway and NF-κB/TGF-β1/Smad2/3 pathway | Paraquat-induced pulmonary fibrosis animal model | Protects pulmonary fibrosis though activation of the Nrf2 pathway and inhibition of the NF-κB/TGF-β1/Smad2/3 pathway | [102] | |
| Liquiritin (Glycyrrhiza uralensis Fisch.) (Gan Cao) | NF-κB, TRPV1, and TRPA1 | LPS-induced acute lung injury in mice | Alleviates lung injury and suppresses inflammation | [103] | |
| Cordyceps sinensis extract (Cordyceps sinensis (Berk.) Sacc.) (DCXC) | NF-κB, COX-2, and iNOS | LPS-induced acute lung injury in mice | Alleviates LPS-induced ALI | [113] | |
| Shenfu decoction | — | — | The effective ingredients showed a high docking affinity with Mpro, RdRP, and S proteins | [80] | |
| Dampness-resolving drug | Honokiol (Magnolia officinalis Rehd. et Wils.) (Hou Po) | NF-κB | Sepsis in mice | Alleviates sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress in mice | [96] |
| Paclitaxel (Taxus cuspidata Sieb. et Zucc.) (Zi Shan) | MUC1 and TLR-4/NF-κB | CLP-induced sepsis in mice | Alleviates acute lung injury in CLP-induced septic mice | [110] | |
| Peels of Citrus sinensis (Citrus reticulata Blanco.) (Chen Pi) | TRPA1, TRPC4, TRPM6, TRPM7, TRPM8, and TRPV4 | HeLa-CEACAM1a cells | Decreases the virus load in HeLa-CEACAM1a cells inoculated with MHV-A59 | [58] | |
| Qi-regulating drug | Diosmetin (Citrus aurantium L.) (Zhi Shi) | Nrf2 | RAW264.7, A549 cells LPS-induced ALI in mice | Inhibits LPS-induced ALI via activation of Nrf2 and inhibition of NLRP3 inflammasome | [109] |
| Cold-dispelling drug | Caffeic acid phenethyl ester (Cinnamomum cassia Presl.) (Rou Gui) | TNF, iNOS, and NF-κB p65 | LPS-induced acute lung injury in mice | Suppresses LPS-induced acute lung injury | [98] |
MRC-5: human fetal lung fibroblasts; MDA: malondialdehyde; Akt1: V-akt murine thymoma viral oncogene homologue 1; Raf: serine/threonine kinase; MEK: mitogen-activated protein kinase kinase; ERK: extracellular signal-regulated kinase; MDCK: Madin–Darby canine kidney; MIF: macrophage migration inhibitory factor; TRPA: transient receptor potential ankyrin; TRPM: transient receptor potential melastatin; TNF: tumour necrosis factor.
However, there are some limitations in the research progress on TCM for COVID-19 treatment. First, most of the published data were from virtual screening or network pharmacological prediction. It is thus urgent and necessary to provide direct experimental evidence of the targets or mechanisms of TCM in treating COVID-19. Second, although some experimental evidence was found showing that TCM affects immune system modification and the anti-inflammatory response and inhibits lung injury, the experimental models in vivo and in vitro were insufficient based on SARS-CoV-2 infection. Although diseases may share similar pathological stages or processes with COVID-19, more experiments directly related to COVID-19 infection are strongly warranted.
Acknowledgments
Acknowledgments
This work was supported by the National Key Research and Development Project of China (2020YFA0708004 and 2020YFA0708000); the Tianjin Natural Science Fund for Distinguished Young Scholars (20JCJQJC00070); and the International Cooperation Study on the Mechanism of Xuanfei Baidu Decoction Against COVID-19 Pneumonia (2021YFE0200300).
Compliance with ethics guidelines
Lin Li, Yuzheng Wu, Jiabao Wang, Huimin Yan, Jia Lu, Yu Wang, Boli Zhang, Junhua Zhang, Jian Yang, Xiaoying Wang, Min Zhang, Yue Li, Lin Miao, and Han Zhang declare that they have no conflict of interest or financial conflicts to disclose.
Footnotes
References
- 1.Wei P.F. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Chin Med J. 2020;133(9):1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Edwards KM, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020;ciaa478. [DOI] [PMC free article] [PubMed]
- 3.Zhou L., Wang X.N., Liu X.K., Fei X., Liu L., Liu Z.L., et al. Case report of Xuanfei Baidu decoction for curing severe cases of COVID-2019. Tianjin J Tradit Chin Med. 2021;38(5):556–559. Chinese. [Google Scholar]
- 4.Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr Med Res. 2020;9(3):100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung E.H., Pan H.D., Huang Y.F., Fan X.X., Wang W.Y., He F., et al. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering. 2020;6(10):1099–1107. doi: 10.1016/j.eng.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi N., Guo L., Liu B., Bian Y., Chen R., Chen S., et al. Efficacy and safety of Chinese herbal medicine versus lopinavir–ritonavir in adult patients with coronavirus disease 2019: a non-randomized controlled trial. Phytomedicine. 2021;81:153367. doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Lu C., Li H., Qi W., Ruan L., Bian Y., et al. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan. China. J Ethnopharmacol. 2021;277:113888. doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical corona virus disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol Res. 2020;160:105073. doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42(1):3–11. [PubMed] [Google Scholar]
- 12.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020;10(4):313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Zhou W., Yang L.i., You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni Y., Alu A., Lei H., Wang Y., Wu M., Wei X. Immunological perspectives on the pathogenesis, diagnosis, prevention and treatment of COVID-19. Mol Biomed. 2021;2(1):1. doi: 10.1186/s43556-020-00015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174–184. [PubMed] [Google Scholar]
- 24.Mackman N., Antoniak S., Wolberg A.S., Kasthuri R., Key N.S. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40(9):2033–2044. doi: 10.1161/ATVBAHA.120.314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinke L.M., Spiegel M., Plegge T., Hartleib A., Nehlmeier I., Gierer S., et al. Different residues in the SARS-CoV spike protein determine cleavage and activation by the host cell protease TMPRSS2. PLoS ONE. 2017;12(6):e0179177. doi: 10.1371/journal.pone.0179177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Author correction: characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2021;12(1):2144. doi: 10.1038/s41467-021-22614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonacker E., Van Noorden C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82(2):53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 29.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohd H.A., Memish Z.A., Alfaraj S.H., McClish D., Altuwaijri T., Alanazi M.S., et al. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14(5):464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z., et al. Gross examination report of a COVID-19 death autopsy. J Forensic Med. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. Chinese. [DOI] [PubMed] [Google Scholar]
- 33.Tian S., Xiong Y., Liu H., Niu L.i., Guo J., Liao M., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong S. Changes of the temporal-spatial distribution of epidemic disasters in 770BC-AD1911 China. Acta Geogr Sin. 2003;58(6):870–878. [Google Scholar]
- 35.Chen S., Zhou Z. On the strategy and therapy of TCM diagnosis and treatment to COVID-19. Jiangsu J Tradit Chin Med. 2020;52(4):34–38. Chinese. [Google Scholar]
- 36.Zheng W., Zhang J., Yang F.W., Huang M., Miao Q., Qi W.S., et al. Treatment of coronavirus disease 2019 (COVID-19) from perspective of dampness-toxicity plagues. J Tradit Chin Med. 2020;61(22):1020–1028. [Google Scholar]
- 37.Rodríguez-Morales A.J., MacGregor K., Kanagarajah S., Patel D., Schlagenhauf P. Going global—travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H., Song H.X., Wang D.F., Ma X.R., Zou D.X., Miao J.X., et al. Potential mechanism of Xuanfei Baidu formula in treating new coronavirus pneumonia on network pharmacology and molecular docking. J Hainan Med Coll. 2020;26(18):1361–1372. [Google Scholar]
- 39.Wang Y., Li X., Zhang J.H., Xue R., Qian J.Y., Zhang X.H., et al. Mechanism of Xuanfei Baidu tang in treatment of COVID-19 based on network pharmacology. China J Clin Mater Med. 2020;45(10):2249–2256. doi: 10.19540/j.cnki.cjcmm.20200325.401. Chinese. [DOI] [PubMed] [Google Scholar]
- 40.Pan H.D., Yao X.J., Wang W.Y., Lau H.Y., Liu L. Network pharmacological approach for elucidating the mechanisms of traditional Chinese medicine in treating COVID-19 patients. Pharmacol Res. 2020;159:105043. doi: 10.1016/j.phrs.2020.105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y.W., Yan X.F., Ye T.J., Hu J., Wang X.L., Qiu F.J., et al. Analyzing the potential therapeutic mechanism of Huashi Baidu decoction on severe COVID-19 through integrating network pharmacological methods. J Tradit Complement Med. 2021;11(2):180–187. doi: 10.1016/j.jtcme.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao Q., Du J., Li X., Zeng J., Tan B., Xu J., et al. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev Ind Pharm. 2020;46(8):1345–1353. doi: 10.1080/03639045.2020.1788070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren Y., Yin Z.H., Dai J.X., Yang Z., Ye B.B., Ma Y.S., et al. Evidence-based complementary and alternative medicine exploring active components and mechanism of Jinhua Qinggan granules in treatment of COVID-19 based on virus–host interaction. Nat Prod Commun. 2020;15(9) 1934578X2094721. [Google Scholar]
- 44.Zhang Y., Yao Y., Yang Y., Wu H. Investigation of anti-SARS, MERS, and COVID-19 effect of Jinhua Qinggan granules based on a network pharmacology and molecular docking approach. Nat Prod Commun. 2021;16(5) 1934578X2110206. [Google Scholar]
- 45.Drexler J.F., Gloza-Rausch F., Glende J., Corman V.M., Muth D., Goettsche M., et al. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol. 2010;84(21):11336–11349. doi: 10.1128/JVI.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khaerunnisa S., Aminah N.S., Kristanti A.N., Kuswarini S., Wungu C.D.K., Soetjipto S., et al. Isolation and identification of a flavonoid compound and in vivo lipid-lowering properties of Imperata cylindrica. Biomed Rep. 2020;13(5):38. doi: 10.3892/br.2020.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J Biomol Struct Dyn. 2021;39(9):3092–3098. doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34(11):2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du A., Zheng R., Disoma C., Li S., Chen Z., Li S., et al. Epigallocatechin-3-gallate, an active ingredient of traditional Chinese medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int J Biol Macromol. 2021;176:1–12. doi: 10.1016/j.ijbiomac.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu R., Chen L., Lan R., Shen R., Li P. Computational screening of antagonists against the SARS-CoV-2 (COVID-19) coronavirus by molecular docking. Int J Antimicrob Agents. 2020;56(2):106012. doi: 10.1016/j.ijantimicag.2020.106012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aanouz I., Belhassan A., El-Khatabi K., Lakhlifi T., El-ldrissi M., Bouachrine M. Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: computational investigations. J Biomol Struct Dyn. 2021;39(8):2971–2979. doi: 10.1080/07391102.2020.1758790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei T.Z., Wang H., Wu X.Q., Lu Y., Guan S.H., Dong F.Q., et al. In silico screening of potential spike glycoprotein inhibitors of SARS-CoV-2 with drug repurposing strategy. Chin J Integr Med. 2020;26(9):663–669. doi: 10.1007/s11655-020-3427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nebigil C.G., Moog C., Vagner S., Benkirane-Jessel N., Smith D.R., Désaubry L. Flavaglines as natural products targeting eIF4A and prohibitins: from traditional Chinese medicine to antiviral activity against coronaviruses. Eur J Med Chem. 2020;203:112653. doi: 10.1016/j.ejmech.2020.112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ting D., Dong N., Fang L., Lu J., Bi J., Xiao S., et al. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl Nano Mater. 2018;1(10):5451–5459. doi: 10.1021/acsanm.0c00970. [DOI] [PubMed] [Google Scholar]
- 55.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 56.Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin Exp Pharmacol Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chikhale R., Sinha S.K., Wanjari M., Gurav N.S., Ayyanar M., Prasad S., et al. Computational assessment of saikosaponins as adjuvant treatment for COVID-19: molecular docking, dynamics, and network pharmacology analysis. Mol Divers. 2021;25(3):1889–1904. doi: 10.1007/s11030-021-10183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho T., Wu S., Chen J., Li C., Hsiang C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., et al. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep. 2014;41(3):1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H.Y., Shin H.S., Park H., Kim Y.C., Yun Y.G., Park S., et al. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, Cimicifuga rhizoma, Meliae cortex, Coptidis rhizoma, and Phellodendron cortex. J Clin Virol. 2008;41(2):122–128. doi: 10.1016/j.jcv.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui C., Huang C., Zhou W., Ji X., Zhang F., Wang L., et al. AGTR2, one possible novel key gene for the entry of SARS-CoV-2 into human cells. IEEE/ACM Trans Comput Biol Bioinform. 2021;18(4):1230–1233. doi: 10.1109/TCBB.2020.3009099. [DOI] [PubMed] [Google Scholar]
- 62.Gao K., Song Y.P., Song A. Exploring active ingredients and function mechanisms of Ephedra-bitter almond for prevention and treatment of corona virus disease 2019 (COVID-19) based on network pharmacology. BioData Min. 2020;13(1):19. doi: 10.1186/s13040-020-00229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lv Y., Wang S., Liang P., Wang Y., Zhang X., Jia Q., et al. Screening and evaluation of anti-SARS-CoV-2 components from Ephedra sinica by ACE2/CMC-HPLC-IT-TOF-MS approach. Anal Bioanal Chem. 2021;413(11):2995–3004. doi: 10.1007/s00216-021-03233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X., Qiu Q., Li M., Lin H., Cao S., Wang Q., et al. Chemical composition and pharmacological mechanism of Ephedra–Glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19) Aging. 2021;13(4):4811–4830. doi: 10.18632/aging.202622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai Y., Zeng M., Chen Y.Z. The pharmacological mechanism of Huashi Baidu formula for the treatment of COVID-19 by combined network pharmacology and molecular docking. Ann Palliat Med. 2021;10(4):3864–3895. doi: 10.21037/apm-20-1759. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y.Y., Gao W.Y., Gu X.R., Chen Z.Q., Zhao H.Y., Bian B.L., et al. Identification and attribution of chemical constituents of Qingfei Paidu decoction based on UHPLC-LTQ-Orbitrap-MS technology. China J Clin Mater Med. 2020;45(13):3035–3044. doi: 10.19540/j.cnki.cjcmm.20200423.202. Chinese. [DOI] [PubMed] [Google Scholar]
- 67.Liu W., Ge G.B., Wang Y.L., Huang K., Chen J.M., Wang C.H., et al. Chemical constituent and tissue distribution study of Qingfei Paidu decoction in mice using UHPLC-Q-Orbitrap HRMS. Chin Tradit Herbal Drugs. 2020;51(8):2035–2045. Chinese. [Google Scholar]
- 68.Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., et al. Protection against COVID-19 injury by Qingfei Paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed Pharmacother. 2020;129:110281. doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., et al. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. 2021;85:153315. doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., et al. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: a retrospective multicenter cohort study. Pharmacol Res. 2020;161:105290. doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z.J., Wu W.Y., Hou J.J., Zhang L.L., Li F.F., Gao L., et al. Active constituents and mechanisms of Respiratory Detox Shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: network-molecular docking-LC-MSE analysis. J Integr Med. 2020;18(3):229–241. doi: 10.1016/j.joim.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Q., Bai C., Yang R., Xing W., Pang X., Wu S., et al. Deciphering the pharmacological mechanisms of Ma Xing Shi Gan decoction against COVID-19 through integrating network pharmacology and experimental exploration. Front Pharmacol. 2020;11:581691. doi: 10.3389/fphar.2020.581691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Chu F., Li P., Johnson N., Li T., Wang Y., et al. Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J Ethnopharmacol. 2021;271:113854. doi: 10.1016/j.jep.2021.113854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., et al. The Chinese prescription Lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement Altern Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng S., Baak J.P., Li S., Xiao W., Ren H., Yang H., et al. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine. 2020;79:153336. doi: 10.1016/j.phymed.2020.153336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X., Wu Y., Chen C., Gu Y., Zhu C., Wang S., et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B. 2021;11(1):222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Q., Pan W., Li R., Liu B., Li C., Xie Y., et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol Res. 2020;158:104850. doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pang W.T., Jin X.Y., Pang B., Yang F.W., Wang H., Liu C.X., et al. Analysis on pattern of prescriptions and syndromes of traditional Chinese medicine for prevention and treatment of COVID-19. China J Clin Mater Med. 2020;45(6):1242–1247. doi: 10.19540/j.cnki.cjcmm.20200218.502. Chinese. [DOI] [PubMed] [Google Scholar]
- 80.Li X., Lin H., Wang Q., Cui L., Luo H., Luo L. Chemical composition and pharmacological mechanism of Shenfu decoction in the treatment of novel coronavirus pneumonia (COVID-19) Drug Dev Ind Pharm. 2020;46(12):1947–1959. doi: 10.1080/03639045.2020.1826510. [DOI] [PubMed] [Google Scholar]
- 81.Deng W., Xu Y., Kong Q., Xue J., Yu P., Liu J., et al. Therapeutic efficacy of Pudilan Xiaoyan oral liquid (PDL) for COVID-19 in vitro and in vivo. Signal Transduct Target Ther. 2020;5(1):66. doi: 10.1038/s41392-020-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y., Liu Z., Zhu X.Q., Wang B.L. To Investigation of the mechanism of Xuebijing injection in COVID-19 treatment based on network pharmacology and molecular docking. Chin J Comp Med. 2020;30(7):57–64. Chinese. [Google Scholar]
- 83.Thompson D.A., Cormier E.G., Dragic T. CCR5 and CXCR4 usage by non-clade B human immunodeficiency virus type 1 primary isolates. J Virol. 2002;76(6):3059–3064. doi: 10.1128/JVI.76.6.3059-3064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindahl J.F., Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol. 2015;5(1):30048. doi: 10.3402/iee.v5.30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115(7):1688–1698. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao J., Wohlford-Lenane C., Zhao J., Fleming E., Lane T.E., McCray P.B., Jr, et al. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J Virol. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumaki Y., Salazar A.M., Wandersee M.K., Barnard D.L. Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model. Antiviral Res. 2017;139:1–12. doi: 10.1016/j.antiviral.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jasso-Miranda C., Herrera-Camacho I., Flores-Mendoza L.K., Dominguez F., Vallejo-Ruiz V., Sanchez-Burgos G.G., et al. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect Drug Resist. 2019;12:1833–1852. doi: 10.2147/IDR.S210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mounce B.C., Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 90.Sordillo P.P., Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo. 2015;29(1):1–4. [PubMed] [Google Scholar]
- 91.Wang Y.X., Ma J.R., Wang S.Q., Zeng Y.Q., Zhou C.Y., Ru Y.H., et al. Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan decoction in treating COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(6):3360–3384. doi: 10.26355/eurrev_202003_20704. [DOI] [PubMed] [Google Scholar]
- 92.Zhao J., Yang X., Wang C., Song S., Cao K., Wei T., et al. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-CoV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-α. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kao S.J., Su C.F., Liu D.D., Chen H.I. Endotoxin-induced acute lung injury and organ dysfunction are attenuated by pentobarbital anaesthesia. Clin Exp Pharmacol Physiol. 2007;34(5–6):480–487. doi: 10.1111/j.1440-1681.2007.04598.x. [DOI] [PubMed] [Google Scholar]
- 94.Johnson E.R., Matthay M.A. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–252. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Standiford T.J., Ward P.A. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167(1):183–191. doi: 10.1016/j.trsl.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weng T.I., Wu H.Y., Kuo C.W., Liu S.H. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Med. 2011;37(3):533–541. doi: 10.1007/s00134-010-2104-1. [DOI] [PubMed] [Google Scholar]
- 97.Shin N.R., Shin I.S., Song H.H., Hong J.M., Kwon O.K., Jeon C.M., et al. Callicarpa japonica Thunb. reduces inflammatory responses: a mouse model of lipopolysaccharide-induced acute lung injury. Int Immunopharmacol. 2015;26(1):174–180. doi: 10.1016/j.intimp.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 98.Koksel O., Ozdulger A., Tamer L., Cinel L., Ercil M., Degirmenci U., et al. Effects of caffeic acid phenethyl ester on lipopolysaccharide-induced lung injury in rats. Pulm Pharmacol Ther. 2006;19(2):90–95. doi: 10.1016/j.pupt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Yang W., Qiang D., Zhang M., Ma L., Zhang Y., Qing C., et al. Isoforskolin pretreatment attenuates lipopolysaccharide-induced acute lung injury in animal models. Int Immunopharmacol. 2011;11(6):683–692. doi: 10.1016/j.intimp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Sun Q., Chen L., Gao M., Jiang W., Shao F., Li J., et al. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int Immunopharmacol. 2012;12(1):88–93. doi: 10.1016/j.intimp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 101.Patel V.J., Biswas Roy S., Mehta H.J., Joo M., Sadikot R.T. Alternative and natural therapies for acute lung injury and acute respiratory distress syndrome. BioMed Res Int. 2018;2018:1–9. doi: 10.1155/2018/2476824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cui Y., Xin H., Tao Y., Mei L., Wang Z. Arenaria kansuensis attenuates pulmonary fibrosis in mice via the activation of Nrf2 pathway and the inhibition of NF-κB/TGF-β1/Smad2/3 pathway. Phytother Res. 2021;35(2):974–986. doi: 10.1002/ptr.6857. [DOI] [PubMed] [Google Scholar]
- 103.Liu Z., Wang P., Lu S., Guo R., Gao W., Tong H., et al. Liquiritin, a novel inhibitor of TRPV1 and TRPA1, protects against LPS-induced acute lung injury. Cell Calcium. 2020;88:102198. doi: 10.1016/j.ceca.2020.102198. [DOI] [PubMed] [Google Scholar]
- 104.Li X., Shan C., Wu Z., Yu H., Yang A., Tan B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm Res. 2020;69(4):365–373. doi: 10.1007/s00011-020-01331-3. [DOI] [PubMed] [Google Scholar]
- 105.Gao Z., Sui J., Fan R., Qu W., Dong X., Sun D. Emodin protects against acute pancreatitis-associated lung injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1 signaling. Drug Des Devel Ther. 2020;14:1971–1982. doi: 10.2147/DDDT.S247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin J.T., Wan B., Liu D.D., Wan S.X., Fu H.Y., Wan Y., et al. Emodin alleviates lung injury in rats with sepsis. J Surg Res. 2016;202(2):308–314. doi: 10.1016/j.jss.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 107.Tsai C.L., Lin Y.C., Wang H.M., Chou T.C. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014;153(1):197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y.Q., Chai Y.S., Xie K., Yu F., Wang C.J., Lin S.H., et al. Curcumin promotes the expression of IL-35 by regulating regulatory T cell differentiation and restrains uncontrolled inflammation and lung injury in mice. Inflammation. 2020;43(5):1913–1924. doi: 10.1007/s10753-020-01265-2. [DOI] [PubMed] [Google Scholar]
- 109.Liu Q., Ci X., Wen Z., Peng L. Diosmetin alleviates lipopolysaccharide-induced acute lung injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Biomol Ther. 2018;26(2):157–166. doi: 10.4062/biomolther.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y.M., Ji R., Chen W.W., Huang S.W., Zheng Y.J., Yang Z.T., et al. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway. Drug Des Devel Ther. 2019;13:3391–3404. doi: 10.2147/DDDT.S222296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou B.X., Li J., Liang X.L., Pan X.P., Hao Y.B., Xie P.F., et al. β-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling. Acta Pharmacol Sin. 2020;41(9):1178–1196. doi: 10.1038/s41401-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y., Zhang B., Xu D.Q., Li W.P., Xu M., Li J.H., et al. Tanshinone IIA attenuates seawater aspiration-induced lung injury by inhibiting macrophage migration inhibitory factor. Biol Pharm Bull. 2011;34(7):1052–1057. doi: 10.1248/bpb.34.1052. [DOI] [PubMed] [Google Scholar]
- 113.Fu S, Lu W, Yu W, Hu J. Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice. Biosci Rep 2019;39(6):BSR20190789. [DOI] [PMC free article] [PubMed]
- 114.Chen X., Han W., Wang G., Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int J Biol Macromol. 2020;164:331–343. doi: 10.1016/j.ijbiomac.2020.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen R.R., Li Y.J., Chen J.J., Lu C.L. A review for natural polysaccharides with anti-pulmonary fibrosis properties, which may benefit to patients infected by 2019-nCoV. Carbohydr Polym. 2020;247:116740. doi: 10.1016/j.carbpol.2020.116740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. e00221-e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, et al. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically Ill patients with influenza virus infection. J Infect Dis 2020;221(10):1688–98. [DOI] [PubMed]
- 119.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin X., Pang B., Zhang J., Liu Q., Yang Z., Feng J., et al. Core outcome set for clinical trials on coronavirus disease 2019 (COS-COVID) Engineering. 2020;6(10):1147–1152. doi: 10.1016/j.eng.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang F., Huang J., Liu W., Wang C.R., Liu Y.F., Tu D.Z., et al. Inhibition of drug-metabolizing enzymes by Qingfei Paidu decoction: implication of herb-drug interactions in COVID-19 pharmacotherapy. Food Chem Toxicol. 2021;149:111998. doi: 10.1016/j.fct.2021.111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu S., Zhu Y., Xu J., Yao G., Zhang P., Wang M., et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine. 2021;85:153364. doi: 10.1016/j.phymed.2020.153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei W.L., Wu S.F., Li H.J., Li Z.W., Qu H., Yao C.L., et al. Chemical profiling of Huashi Baidu prescription, an effective anti-COVID-19 TCM formula, by UPLC-Q-TOF/MS. Chin J Nat Med. 2021;19(6):473–480. doi: 10.1016/S1875-5364(21)60046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu G.S., Zhong J., Zheng N.N., Wang C.R., Jin H.L., Ge G.B., et al. Investigation of modulating effect of Qingfei Paidu decoction on host metabolism and gut microbiome in rats. China J Clin Mater Med. 2020;45(15):3726–3739. doi: 10.19540/j.cnki.cjcmm.20200609.201. Chinese. [DOI] [PubMed] [Google Scholar]
- 127.Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., et al. Chemical composition and pharmacological mechanism of Qingfei Paidu decoction and Ma Xing Shi Gan decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res. 2020;157:104820. doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Utomo R, Ikawati M, Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprint 2020.
- 129.Tu P., Tian R., Lu Y., Zhang Y., Zhu H., Ling L., et al. Beneficial effect of Indigo Naturalis on acute lung injury induced by influenza A virus. Chin Med. 2020;15(1):128. doi: 10.1186/s13020-020-00415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X., Gao R., Zhou Z., Tang X., Lin J., Wang L., et al. A network pharmacology based approach for predicting active ingredients and potential mechanism of Lianhuaqingwen capsule in treating COVID-19. Int J Med Sci. 2021;18(8):1866–1876. doi: 10.7150/ijms.53685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia Q.D., Xun Y., Lu J.L., Lu Y.C., Yang Y.Y., Zhou P., et al. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Prolif. 2020;53(12):e12949. doi: 10.1111/cpr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng W.J., Yan Q., Ni Y.S., Zhan S.F., Yang L.L., Zhuang H.f., et al. Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology. BioData Min. 2020;13(1):17. doi: 10.1186/s13040-020-00227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y., Ru Y., Zhuo G., Sheng M., Wang S., Ma J., et al. Investigation of the potential mechanism governing the effect of the Shen Zhu San on COVID-19 by network pharmacology. Evid Based Complement Alternat Med. 2020;2020:1–23. doi: 10.1155/2020/8468303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ye M., Luo G., Ye D., She M., Sun N., Lu Y.J., et al. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen granules against coronavirus disease 2019 pneumonia. Phytomedicine. 2021;85:153401. doi: 10.1016/j.phymed.2020.153401. [DOI] [PMC free article] [PubMed] [Google Scholar]