Abstract
Objective:
Long-Segment Hirschsprung Disease (LSHD) differs clinically from short-segment disease. This review article critically appraises current literature on the definition, management, outcomes, and novel therapies for patients with LSHD.
Methods:
Four questions regarding the definition, management, and outcomes of patients with LSHD were generated. English-language articles published between 1990 and 2018 were compiled by searching PubMed, Scopus, Cochrane Central Register of Controlled Trials, Web of Science, and Google Scholar. A qualitative synthesis was performed.
Results:
66 manuscripts were included in this systematic review. Standardized nomenclature and preoperative evaluation for LSHD are recommended. Insufficient evidence exists to recommend a single method for the surgical repair of LSHD. Patients with LSHD may have increased long-term gastrointestinal symptoms, including Hirschsprung-associated enterocolitis (HAEC), but have a quality of life similar to matched controls. There are few surgical technical innovations focused on this disorder.
Conclusions:
A standardized definition of LSHD is recommended that emphasizes the precise anatomic location of aganglionosis. Prospective studies comparing operative options and long-term outcomes are needed. Translational approaches, such as stem cell therapy, may be promising in the future for the treatment of long-segment Hirschsprung disease.
Level of Evidence:
Level 3, 4
Keywords: Hirschsprung, aganglionosis, long-segment, surgery, outcome
INTRODUCTION
Hirschsprung disease (HD) is a congenital disorder of the enteric nervous system. The disordered caudal migration of neural crest cells results in a lack of intrinsic innervation (neuronal ganglion cells and enteric glial cells) in the affected intestine.[1] While the transition from aganglionic to ganglionated bowel occurs most commonly in the rectosigmoid colon, a significant proportion of patients will have “long-segment” Hirschsprung disease (LSHD). Patients with LSHD have aganglionosis proximal to the sigmoid colon, which may involve the more proximal colon, the entire colon, and even a significant portion of the small intestine. With a greater degree of bowel involvement, patients with LSHD present with distinct problems related to their diagnosis and require alternate surgical treatments and potentially more aggressive long-term management compared to those with short-segment disease.[2–5] While the goals of surgical intervention are similar for all types of HD, patients with LSHD require specific operative considerations and can present unique challenges postoperatively.
LSHD is variably defined and can be difficult to determine through preoperative evaluations. There is no consensus on the optimal surgical approach and variation exists in reported long-term outcomes. Currently there are potential novel therapies. The purpose of this systematic review from the APSA Outcomes and Evidence-Based Practice Committee is to refine the definition of LSHD and to summarize the available literature on surgical approaches, long-term outcomes, novel techniques, and future strategies for treatment of patients with LSHD.
METHODS
Research Questions
The membership of the APSA Outcomes and Evidence-Based Practice Committee developed four questions a priori for the systematic review through an iterative process:
What is the definition of Long-Segment Hirschsprung Disease and how is it best determined?
What is the preferred method for surgical repair of Long-Segment Hirschsprung disease?
What are the long-term outcomes for patients with Long-Segment Hirschsprung disease?
What novel techniques exist, and what future strategies are being developed for the treatment of Long-Segment Hirschsprung disease?
Search Strategy
Electronic searches were created and completed by a health sciences librarian experienced with systematic searches in the following databases: PubMed [NLM], Scopus [Elsevier], Cochrane Central Register of Controlled Trials [Wiley], Web of Science [Thomson Reuters], and Google Scholar. The librarian developed a search strategy in PubMed, and then translated that strategy for each database platform as appropriate. MeSH terms and keywords were used to search the concepts and related concepts of Hirschsprung disease and pediatrics. Results were restricted to January 1, 1990 to December 31, 2018, human-only studies, and English language. Appendix A contains the PubMed search strategy. All searches were updated by the librarian in April 2019.
Study Selection for Inclusion/Exclusion
Screening of studies for inclusion or exclusion followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology [FIGURE 1]. Screening of titles and abstracts was performed in quadruplicate (AG, AK, SS, YG) with conflicts resolved by re-review and group consensus. The full-text manuscript review was performed in a similar fashion and reasons for exclusion noted. Each phase of screening for inclusion/exclusion was performed using Rayyan.[6] [https://rayyan.qcri.org] Studies were assigned to the four research questions; some articles addressed more than one study question. Level of evidence was classified according to Oxford Centre for Evidence-Based Medicine (OCEBM) guidelines.[7]
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology.
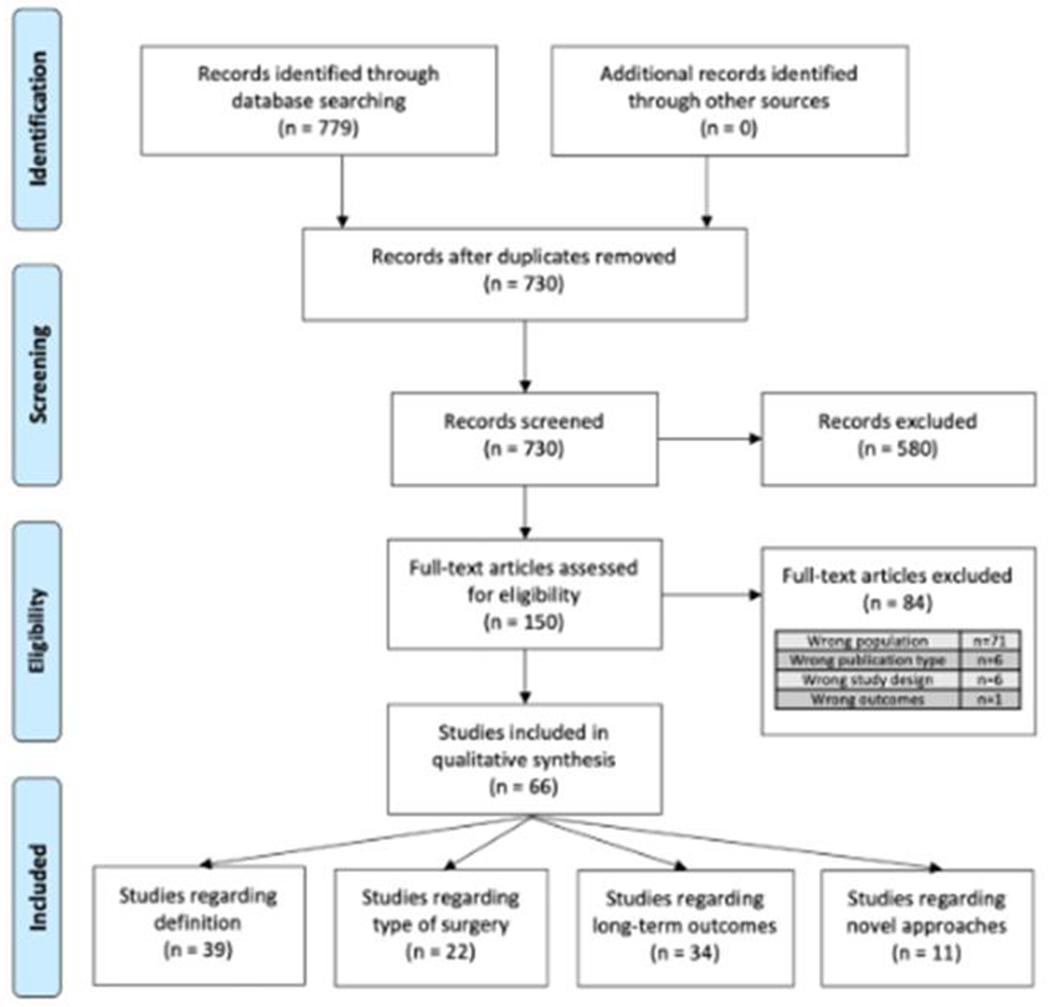
779 records were identified through database searches and 730 records remained after duplicates were removed. 150 full-text articles were assessed for inclusion and 66 articles were included in the qualitative synthesis. Some articles addressed more than one of the pre-identified questions
RESULTS
Search Strategy
The search strategy revealed 730 non-duplicated titles. Of these, 150 abstracts were deemed relevant or possibly relevant and the full text obtained for review. Eighty-four records were excluded after full-text review, most commonly due to focusing on the wrong patient population. Sixty-six records were included in the qualitative synthesis and assigned to one or more of the pre-defined questions [FIGURE 1].
Question 1. What is the definition of long-segment Hirschsprung disease and how is it best determined?
The definition of LSHD varies widely in the literature. Historically, patients with Hirschsprung disease have been categorized by multiple duplicative terms, such as “long-segment,” “standard,” “short-segment,” “total-colon,” “ultra-short,” and others. A standardized definition for the subsets of Hirschsprung disease has never been developed.
Thirty-three articles explicitly defined LSHD in their methodology [Figure 1]. The majority of these articles (22/33) defined LSHD as disease proximal to the rectosigmoid colon while a minority (3/33) defined LSHD as disease proximal to the splenic flexure. Additionally, 8/33 studies utilized the precise anatomic location of transition from ganglionated to aganglionic bowel (e.g. descending colon, splenic flexure, transverse colon, hepatic flexure, ascending colon, cecum, small intestine). Twenty-five studies described both LSHD and total colonic Hirschsprung disease (TCHD), of which four studies included TCHD in the definition of LSHD. Twenty-one studies defined TCHD by documenting intestinal aganglionosis including the entire colon, possibly extending a short distance into the small intestine. The majority of studies (16/21) defined small-intestine Hirschsprung disease (SIHD) as a lack of ganglion cells in the entire colon and portion of the small bowel. Some of these studies (11/21) provided more detailed information about the length of aganglionosis present in the small bowel (either measured length or region).
The studies defining LSHD also included definitions of short-segment Hirschsprung Disease (SSHD), variably calling it “rectosigmoid,” “rectum or rectosigmoid,” “short,” “classic,” “classical,” “standard,” or providing precise anatomic locations (e.g. rectum, mid-sigmoid, upper-sigmoid). A total of 22 studies provided detailed information on length of aganglionosis for all of their patients (n=2350). From this group of studies, the median reported type of aganglionosis was SSHD in 72% (range 64-83%), LSHD in 15% (range 5-24%), TCHD in 8% (range 1-20%), and small intestinal Hirschsprung disease (SIHD) in 0% (0-11%) [Figure 2B].
Figure 2. Anatomic definitions of Long-segment Hirschsprung disease.
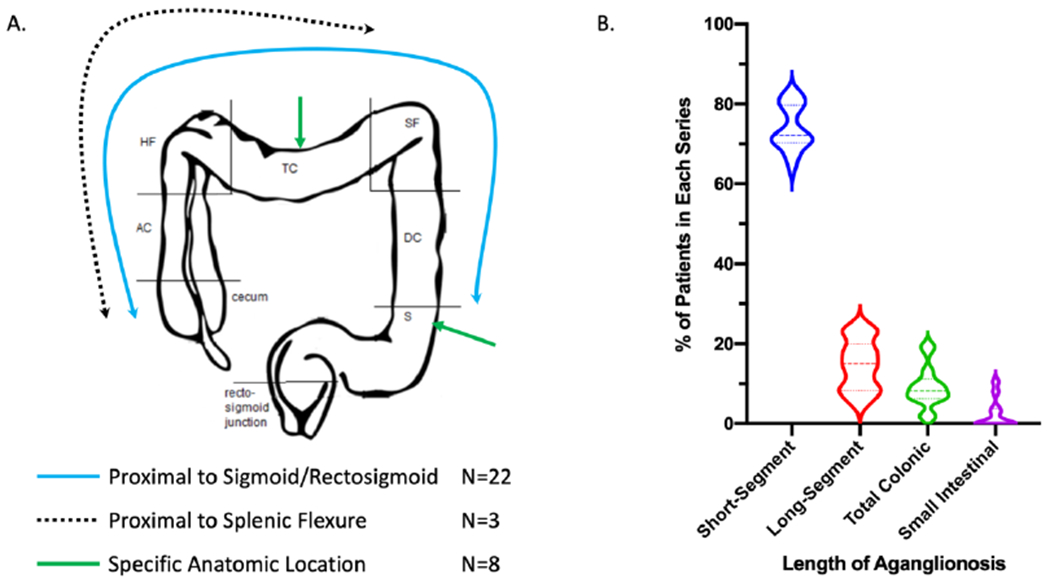
(A) Of 33 manuscripts, 3 defined LSHD as proximal to the splenic flexure (dotted line), 22 defined LSHD as proximal to the sigmoid/rectosigmoid (blue line) and 8 preferred to define the anatomic location of the most proximal level of aganglionosis (e.g. green arrows). (S = sigmoid, DC= descending colon, SF= splenic flexure, TC = transverse colon, HF= hepatic flexure, AC = ascending colon) (B) N=22 studies provided detailed information on length of aganglionosis for a total of 2450 patients. From this group of studies, the median length of aganglionosis was Short-Segment in 72% (range 64–83%), Long-Segment in 15% (range 5–24%), Total Colonic in 8% (range 1–20%), and Small Intestinal in 0% (0–11%). Data are shown as violin plots, with heavy dotted lines for the median, and light dotted lines for the 25% and 75% quartiles.
In order to understand the impact of the length of aganglionosis on surgical and functional outcomes, surgeons need to use standardized nomenclature. This will help with both communication amongst providers and with comparing outcomes when studies on patients with LSHD are performed. We therefore recommend noting in the operative note the specific anatomic location of aganglionosis AND the level of intestinal transection in the gastrointestinal tract (e.g. rectum, sigmoid, descending, transverse, ascending, cecum) to identify the specific extent of disease. If the patient is found to have small intestinal HD, the distance of aganglionosis from the ileocecal valve should be measured and recorded. If the patient is found to have total intestinal HD, the length of ganglionated bowel distal to the ligament of Treitz should be measured and recorded. The APSA Hirschsprung Disease Interest Group has recently made recommendations for synoptic reporting of surgery and pathology of Hirschsprung disease, and our recommendations are compatible with that reporting schema.[8]
Because of heterogeneity in the literature and a paucity of literature that separates TCHD, SIHD, and TIHD as distinct entities, for the remainder of the manuscript, “LSHD” will refer to any combination of these entities, unless otherwise specified. For future publications, we recommend separating categories of HD by the anatomic location of aganglionosis.
Based on the studies described above and the consensus opinion of the APSA OEBP Committee and Hirschsprung Disease Interest Group, we make the following recommendations for standard nomenclature [TABLE 1]:
Table 1.
Recommended Hirschsprung Disease Nomenclature.
| Nomenclature | Definition |
|---|---|
| Ultrashort-segment | No clear definition and usage of this term should be abandoned |
| Short-Segment HD | Aganglionosis up to the sigmoid colon-descending colon junction |
| Long-Segment HD | Aganglionosis from sigmoid colon-descending colon junction up to the cecum (ganglion cells present in colon) |
| Total Colonic HD | Aganglionosis of the entire colon and <5 cm of terminal ileum |
| Small Intestinal HD | Aganglionosis extending beyond the terminal ileum (>5 cm) |
| Total Intestinal HD | <20 cm of ganglionated intestine beyond the Ligament of Treitz. |
| Anatomic Location of extent of aganglionosis | rectum, sigmoid colon, descending colon, transverse colon, ascending colon, cecum, ileum, jejunum |
Ultrashort-segment: No clear definition and usage of this term should be abandoned.
Short-Segment HD (SSHD): Aganglionosis up to the sigmoid colon-descending colon junction
Long-Segment HD (LSHD): Aganglionosis proximal to the sigmoid colon-descending colon junction, but with ganglion cells present in some portion of the colon
Total Colonic HD (TCHD): Aganglionosis of the entire colon and <5 cm of the terminal ileum.
Small Intestinal HD (SIHD): Aganglionosis extending proximal to >5cm of the terminal ileum.
Total Intestinal HD (TIHD): Aganglionosis of nearly the entire intestine, with <20 cm of ganglionated small intestine beyond the Ligament of Treitz.
Level of evidence 4, Grade of Recommendation D
How is long-segment Hirschsprung disease best determined pre-operatively?
Four studies addressed how to identify LSHD pre-operatively. Two studies described features on contrast enema that are associated with LSHD.[9,10] These radiographic features include normal appearance, small/micro-colon, meconium plug, comma-shaped colon, and flattened hepatic/splenic flexures. In identifying LSHD, two additional studies evaluated the utility of the recto-sigmoid index (RSI), which is the widest diameter of the rectum, divided by the widest diameter of the sigmoid colon, when both are fully distended with contrast. The RSI is normally >1 and can be <1 in the setting of HD. These studies reported that the RSI was not suggestive of HD in any case of LSHD.[11,12]
A retrospective study of 75 patients evaluating the utility of the radiographic transition zone (RTZ) seen on contrast enema in predicting the presence of LSHD found that 89% (67/75) had a transition zone suggestive of Hirschsprung disease. Of the 60 patients whose contrast enema diagnosed a rectosigmoid transition zone, 90% of patients had short-segment disease and 8% actually had long-segment disease with a more proximal level of aganglionosis. In the remaining 7 patients whose contrast enema demonstrated a long-segment RTZ, no patients had short-segment disease, 5 patients (71%) had disease matching diagnosis, and 2 (29%) had TCHD. Finally, in the 8 patients with no RTZ, 6 (75%) had short-segment disease, 1 (12.5%) had long-segment disease, and 1 (12.5%) had TCHD. For patients with LSHD, the contrast enema had a RTZ that correctly predicted the level of aganglionosis in only 31% of patients.[11] One study additionally recommended consideration of delayed (24-48 hours) abdominal films to evaluate for retained colonic contrast, which may be suggestive of HD.[9] A normal recto-sigmoid index or radiographic transition zone can be misleading in the preoperative diagnosis of LSHD and TCHD as the studies may not correlate well with the length of aganglionosis. For this reason, one should not solely rely on preoperative imaging and that either trans-umbilical or laparoscopic biopsies be performed to confirm the intended level of pull-through before starting anorectal dissection.[11]
Recommendations and Observations:
While a contrast enema should be obtained preoperatively, a normal Rectum-Sigmoid Index (RSI) and absence of a radiographic transition zone (RTZ) does not exclude LSHD or TCHD.
The radiographic transition zone (RTZ) poorly correlates with length of aganglionosis in LSHD and TCHD.
Due to the inaccuracy of contrast enema in predicting the length of aganglionosis for LSHD and TCHD, leveling biopsies should first be obtained to confirm the level of aganglionosis prior to anorectal dissection.
Level of evidence 4, Grade of Recommendation D
Question 2. What is the preferred method for surgical repair of long-segment Hirschsprung disease?
Multiple operations have been described for the management of Hirschsprung disease. We identified 24 studies that included a description of Long-Segment or TCHD disease and detailed the operative technique, all of which were observational and retrospective. In the majority of studies, the patients had either previously undergone a leveling diversion procedure prior to definitive surgery, or it was not specified. The Swenson procedure involves complete resection of the aganglionic bowel and an end-to-end anastomosis of normal bowel to the remaining rectum. For the modern Soave procedure (AKA Soave-Boley), a sleeve of mucosa and submucosa of the distal rectum are removed leaving a cuff rectal muscle. The normal bowel is brought through the cuff of variable length aganglionic muscle and anastomosed to the remaining mucosa. In a standard Duhamel procedure, the normal bowel is brought down in a retro-rectal position, the aganglionic bowel other than the rectum is resected, and the normal proximal bowel and aganglionic rectum are brought together in a side-to-side manner. A Martin procedure is similar to an extended Duhamel procedure, where the aganglionic remaining colon extends to the entire left colon. With a Kimura procedure for longer segment disease, the aganglionic right colon is anastomosed side-to-side with the ileum in an attempt to improve water absorption. Other procedures include a total colectomy with ileoanal anastomosis with a pouch (IPAA), colectomy with straight ileoanal pullthrough (IAPT), permanent ileostomy/colostomy, and intestinal transplantation. The choice of operation may depend on anatomic issues as well as surgeon/institution experience/preference [TABLE 2].
Table 2.
Types of surgery for LSHD
| Technique | Papers (N=24)* |
Patients (N=449)** |
Comments |
|---|---|---|---|
| Duhamel | 11(45%) | 228 (50%) | • Papers do not differentiate LSHD from TCA • >40% incidence of incontinence (not unique to Duhamel) |
| Left colon patch (Martin) | 5 (20%) | 94 (21%) | • Technically difficult vs. Duhamel or Soave • Long-term dilation of colon pouch requiring revisional surgery |
| Soave/Swenson | 6 (25%) | 60 (13%) | • Outcomes similar to Duhamel • Stool frequency decreases after ileal adaption |
| Right colon patch (Kimura) | 4 (18%) | 19 (4%) | • Primarily used for Small Intestinal HD • Long-term dilation requiring revisional surgery |
| Ileoanal +/− Pouch | 4 (16%) | 40 (8 %) | • High failure rates/need for revision |
| Small bowel transplantation | 2 (8%) | 8 (1.8%) | • Rare due to advances in intestinal rehabilitation • Only appropriate for total intestinal HD |
denotes number of articles, some articles contain multiple types of surgical procedures
denotes number of total patients that had surgery for HD, and excludes if cases can’t be assigned to a specific operation, i.e., some studies grouped patients without identifying the numbers of cases of the specific operation.
The most commonly performed operation for LSHD or TCHD is the Duhamel procedure. The benefits of a Duhamel can be less liquid stool and decreased stool frequency, but must be balanced with the risk of later pouch dilation and stool stasis [TABLE 2]. In a retrospective study of 260 cases comparing Duhamel to Soave and Swenson procedures, the authors concluded that long term continence and function were better with Duhamel in all lengths of aganglionosis.[9] In a separate series of 15 patients with LSHD and 11 with TCHD who underwent a Duhamel, functional outcomes were similar to recto-sigmoid disease.[13] In a long-term follow up of 48 children with TCHD, where 38 underwent Duhamel, 13 Martin, and 3 Soave, the authors found the Martin modification has no advantage in continence and a significant proportion of these patients required multiple operations. They concluded that the Duhamel was the procedure of choice for TCHD due to the technical difficulty and higher complication rate of the Martin procedure.[14] In a series of 26 TCHD patients where 18 patients underwent Duhamel procedure and 2 underwent transplantation; no specific comparison could be made to another type of operation. The patient outcomes were dependent on the level of aganglionosis and 15/18 patients who underwent Duhamel in this series were able to be weaned off TPN.[15] In a series of 36 patients with TCHD treated via Duhamel with Kimura patch for intestinal aganglionosis, 18 required TPN post-operatively, 8 developed short bowel syndrome, 6 required home TPN, and there was almost universal perianal excoriation following pull-through procedures.[10] There have been multiple additional case series describing positive outcomes with Duhamel procedure for TCHD as the preferred surgical procedure for its relative ease in terms of surgical technique, including when performed laparoscopically. [16–22]
A Martin operation was the second most common operation identified among the papers selected for this review as a surgical treatment for LSHD or TCHD.[23] In a series of 5 children with TCHD; 3 underwent Duhamel with Martin modification, and 2 had a Soave procedure. Their report predominantly compared Soave to Duhamel for short segment disease, with their LSHD and TCHD numbers too small to determine a significant difference.[24] In a report of 9 children with LSHD where there were 3 Martin, 2 Swenson-Deloyers (complete right colon mobilization with preservation of the ileocolic artery and full-thickness coloanal anastomosis), 2 Swenson, and 2 ileostomy-alone, they found no technique to be satisfactory, with a preference towards the Martin procedure.[25] In a series of 48 TCHD patients, the average number of additional surgical procedures under anesthetic was 3.7 following a Soave operation (n=3), 1.4 after a Martin procedure (n=10), and 1.0 after standard Duhamel procedure (n=14).[26] The authors concluded that there was no advantage to Martin procedure over Duhamel procedure with a higher need for further surgery and similar outcomes.[26] In a series of 35 children with TCHD comparing 20 cases of Duhamel, 4 Martin-Duhamel, 5 Soave and 6 Kimura patch, the authors documented worse outcomes with more complications for the Martin procedure or Soave, thus favoring Duhamel as the procedure of choice.[10]
Remaining methods included Soave, Kimura patch, straight pull through (Swenson), and ileoanal pouch procedures, none of which had significant numbers or strong recommendations as the preferred procedure. All of these cohorts of patients had complications requiring multiple operations.[14,16,17,24,25,27–30] Nonetheless, some more recent manuscripts have described the use of a straight ileoanal pull through (IAPT) for the management of LSHD and TCHD. These patients, with either Soave or Swenson straight pull-throughs, were reported to have improving function with adaptation over time. A retrospective comparison of straight IAPT to the Martin procedure for patients with TCHD and found similar outcomes, with initial liquid stools and perineal excoriation, but the majority improved with eventual full continence.[31]
Traditionally, LSHD and TCHD have been treated in a staged fashion, with a leveling colostomy and later pull-through procedure with no consensus on the timing of definitive pull-through and timing of stoma closure. One study suggested delaying stoma closure until after children are older (2.5-3 years of age) and able to control their own urination and potentially stool, thus decreasing the risk of severe perianal dermatitis.[32] Several studies did report the safe utilization of a primary pull-through for both LSHD and TCHD without the use of a protective stoma.[33–36] One small study specifically described primary pull-through for both long segment HD and TCHD compared to staged procedures. In a comparison of 4 primary laparoscopic endorectal pull-throughs (PLEP) with 3 staged procedures, the staged procedure resulted in normal bowel function at a mean follow up of 13 years, with 1-3 bowel movements per day and normal continence. All 4 patients with PLEP had initial perianal excoriation and required local treatment and antidiarrheal medications. At a mean follow up of 3.5 years, all patients still required antidiarrheal medications with 3-6 bowel movements per day. [37] Another group discussed the use of the outpatient trans-anal decompression tube to decompress the normal bowel and allow for a delayed primary pull-through for LSHD at 17-137 days of life.[38]
Recommendations and Observations:
While the Duhamel procedure is the most commonly reported surgery for LSHD and TCHD, there is insufficient evidence to recommend a preferred or a superior method for the surgical repair of LSHD.
While a staged approach is the standard surgical method for LSHD and TCHD, a primary pull-through has been performed in few select patients.
Prospective studies are needed to compare operative options based on relevant long-term outcomes, including patient-reported outcome measures.
Level of evidence 3-4; Grade of Recommendation D
Question 3. What are the long-term outcomes for patients with long-segment Hirschsprung disease?
To answer this question, we identified studies that evaluated incontinence and soiling, constipation, Hirschsprung-associated enterocolitis (HAEC), and quality of life (QOL) in patients who had surgery for LSHD and TCHD. While many studies included outcomes of both LSHD and SSHD patients, the majority of studies reported on either only SSHD or a combination of SSHD and LSHD without separate subgroups analyses. We identified nine studies that reported specifically on outcomes for LSHD. As most manuscripts differentiated LSHD from TCHD, we have reported outcomes for the TCHD cohort separately. Ten studies reported on outcomes specifically for patients with TCHD [Table 3]. All of the studies were retrospective reviews or case series providing level 3 or level 4 data.
Table 3.
Summary of Outcomes for patients with Total Colonic Hirschsprung Disease (TCHD)
| Author | Year | N/total | Surgery type | Constipation | Normal | Soiling | Incont | BM/day | BM Meds | HAEC | Ileostomy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoehner | 1998 | 27/29 | 11 different variations on surgical correction of TCA | 30% | * | 19% | 23% with >5 | 30% | 42% | 4% | |
| Tsuji | 1999 | 27/48 | 94 % Duhamel +/− Martin 6% Soave |
* | * | 82, 57, 33% @ 5, 10, 15y |
4.6@ 5y 3.0 @15y |
* | 54% | 22% | |
| Dodero | 2001 | 24/24 | 100% Soave | * | * | 17% | 0% | 10–12 initially 3.6 @ 2y |
4% | 4% | 0% |
| Wildhaber | 2005 | 18/25 | 16% Primary ERPT 12% Martin/ Duhamel 52% Swenson |
* | 62% | 38% | 4 | 17% | 55% | 5% | |
| Escobar | 2005 | 29/36 | 21% Kimura 14% Martin 69% Duhamel 17% Soave |
* | 81% | 19% | 4–6 | * | 31% | 1% | |
| Choe | 2008 | 7/17 | 90% Martin 10% Soave |
0% | 16% | 28% | 56% | 5.5 @ 6mo 4.4 @ 12mo 3.5 @ 18mo |
0 | 54% | 0% |
| Menezes | 2008 | 42/58 | 62% Duhamel 29% Soave 14% Swenson |
* | 52% | 48% | 5.2 @ 5y 3.4 @ 15y |
NR | 55% | 7% | |
| Raboei | 2008 | 8/12 | 100% Duhamel | * | * | * | 1–4 | NR | 38% | 0% | |
| Stenström | 2017 | 25/27 | 24% Swenson 32% Soave 24% J pouch 12% Duhamel 8% Rehbein |
0% | 75% | 24% | 5 overall | NR | 45% | 20% | |
| Roorda | 2018 | 18/35 | 67% Duhamel 33% Rehbein |
76% | 0 | 53% | 23% | variable | 29% | 58% | 17% |
= not measured
N/total = number of TCHD/ total number of patients reported
Incont = incontinence
BM/day = bowel movements per day
BM Meds = medications used to modify bowel movements
HAEC = Hirschsprung-associated entercolitis
Ileostomy = permanent ileostomy created
Incontinence and Soiling
While definitions varied in the literature, fecal incontinence/accidents were most often defined as involuntary passage of stools, and fecal soiling was defined as stool staining on underwear.[39,40] Measurement tools of bowel function included the standardized Paediatric Defecation and Faecal Continence (P-DeFeC), the Baylor Continence Scale, the Cleveland Clinic Constipation scoring system, the Vancouver Dysfunctional Elimination Syndrome Survey, and other adapted questionnaires that were specific to bowel function.
Incontinence and Soiling in long-segment Hirschsprung disease
Four studies noted more soiling and incontinence in patients with LSHD compared to SSHD. One group noted increased stool frequency and worse stooling scores in patients with aganglionosis proximal to the rectosigmoid.[41] Another found that for patients undergoing a single-stage trans-anal endorectal pull-through, patients with LSHD had a higher rate of soiling compared to SSHD.[42] In a 2007 study, both LSHD and SSHD patients had approximately six bowel movements per week and patients with LSHD were more likely to have liquid or pasty, rather than formed, bowel movements. Soiling became less frequent with time and was more common in the younger compared to older patients. The LSHD patients experienced a higher rate of night-time soiling, at a rate of 50% versus 15% of patients with SSHD.[43] In a study of 115 HD patients over 4 years of age using 3 different measurements of anorectal function, the authors found that 34% of LSHD and less than 10% of SSHD patients had “less than optimal” anorectal function.[44] Conversely, five studies found no difference in soiling or incontinence scores between patients with LSHD and SSHD, with rates ranging from 19 to 82 percent of patients. [7], [40],[45] ,[46] When comparing LSHD disease to SSHD, one study found no difference with 26% incontinence for SSHD and 28% incontinence for LSHD. Another study did not differentiate outcomes specifically for LSHD vs. SSHD, but reported that the incidence of soiling and incontinence decreases over time. [25] The age at follow up for these patients was after the expected age of toilet training, with a median age at assessment ranging from 5-15 years.
Whether LSHD or SSHD, patients with Down’s syndrome or other neurological impairment were found to have a higher rate of incontinence than neurologically normal children.[47] A recent study found that syndrome-associated disease was the only risk factor for soiling and constipation in patients with HD.[48]
Incontinence and Soiling in Total Intestinal Hirschsprung disease
Bowel function results varied widely in TCHD patients. [TABLE 3] Bowel movements have reported as “normal” in 0-81% of patients implying the definitions of normal must be widely variant. Patients with TCHD had reported soiling and incontinence rates ranging from 19 to 82%.[10,49,50],[18,51],[39] Nocturnal soiling was particularly common.[31],[44] In a comparison of the long-term outcomes of 18 children with TCHD who were treated with Martin procedure to 15 that underwent a straight ileoanal pull through, the authors found that both groups had similar outcomes with satisfactory continence, but children who were treated with Martin had fewer overall bowel movements.[31] Another study showed a decrease in the rate of soiling and incontinence over five-year increments- decreasing from 82% at 5 years, 57% at 10 years, to 33% at 15 years of age. Similarly, the number of bowel movements was the highest in the first three months following definitive pull-through, but decreased as the patients got older, ranging from one to five per day.[14,17,50] Five studies reported 0-30% of patients with TCHD needing to continue regular medications to optimize bowel function.[27,39,49,50,52] Seven studies included patients with a permanent ileostomy, ranging from 0-22%.[10,14,17,39,49,51,52] A permanent ileostomy was used to treat severe perianal excoriation, high stool output with dehydration or electrolyte anomalies, and otherwise was chosen for some neurologically impaired children.
Recommendations and Observations:
Patients with LSHD and TCHD should be followed long-term as a significant percentage of patients continue to have difficulty with soiling and incontinence, even though both generally improve over time.
Prospective studies with precise definitions of incontinence and soiling are needed.
A permanent ileostomy can be used to treat LSHD and TCHD if soiling and/or incontinence is not manageable.
Level of evidence 3-4, grade of recommendation D
Constipation
Constipation in Long-Segment Hirschsprung disease
Constipation defined by Rome IV criteria includes straining, large and hard stools, incomplete evacuation, and less than two spontaneous bowel movements per week.[53] Constipation was poorly defined in the included studies and was associated with the need for medications, dietary modifications, and enema therapy. One group reported constipation in 5 out of 12 (42%) children with LSHD, albeit mild for most patients.[54] For LSHD, constipation seems to be similar to the general population when a strict definition is used to survey patients after surgery for Hirschsprung disease.[43]
Constipation in Total Colonic Hirschsprung disease
Of the studies reporting long-term outcomes for TCHD, four papers reported specifically on constipation. The incidence of constipation in this patient population is widely variable, without a precise definition of constipation. Two studies noted no TCHD patients with constipation. [50,51] [TABLE 3] One study reported 30% of 27 patients had constipation[49] while another found a 76% constipation rate in 18 patients with TCHD.[39]
Recommendations and Observations:
Patients with LSHD and TCHD should be followed long-term to evaluate their bowel habits.
A prospective analysis of constipation, with precise definitions, in patients with LSHD and TCHD is needed.
Level of evidence 4, Grade of Recommendation D
Hirschsprung Associated Enterocolitis (HAEC)
Hirschsprung associated enterocolitis (HAEC) is an inflammatory condition of the intestine that can occur both preoperatively and postoperatively in patients with Hirschsprung disease. Symptoms of HAEC can include fever, abdominal distension, diarrhea, bloody bowel movements, lethargy, and shock.[55–57] While many manuscripts reported rates of HAEC among their patients, the criteria for diagnosis of HAEC was not well-defined in most of the studies.
HAEC is common after surgical correction of both LSHD and TCHD, with rates ranging from 31-80%. In a study of 146 patients, an increased risk of recurrent HAEC was noted in patients with LSHD (80%) compared to SSHD (37%).[40] Another study found a small, but not significant, increase in the rate of HAEC in their review of 9 patients with LSHD and 44 SSHD, with an overall HAEC rate of 29%.[25] In contrast, in a 1999 study of 63 patients with HD, the level of aganglionosis did not significantly affect the HAEC rate.[58] In a multi-institutional case series of 245 SSHD and 35 LSHD, the level of aganglionosis did not alter the HAEC rate, with an overall rate of 37%.[59] For patients with total colonic aganglionosis (TCHD), reported rates of HAEC were reported as more common in patients with TCHD, generally ranging from 31-58%, although one study reported only 4%.[2,27] In a separate study of 25 patients with TCHD, the type of surgical pull-through performed did not significantly affect the rate of HAEC in patients.[52]
Recommendations and Observations:
The HAEC rate is high in patients with both LSHD and TCHD, careful follow-up and patient/family education about the signs and symptoms of HAEC is warranted.
Standardized definitions of HAEC, along with standardized treatment plans, should be developed.
Level of evidence 3-4, Grade of Recommendation D
Quality of Life (QOL)
When examining QOL for patients with Hirschsprung disease, a variety of measurement tools were utilized, including the previously-validated Pediatric Quality of Life Inventory (PedsQL), Fecal Incontinence and Constipation (FICQOL), Ditesheim and Templeton QOL Scoring System, the World Health Organization QOL (WHO QOL-100), Child Health Questionnaire, the Hirschsprung Disease/ Anorectal Malformation Quality of Life (HAQL), as well as other unvalidated quality of life surveys. Ten studies reported on quality of life after surgery for a cohort of HD patients that included LSHD and TCHD with 7 of the studies reporting specifically on quality of life in patients with LSHD and TCHD and 3 of the studies specifically for TCHD.
Quality of life in long-segment Hirschsprung disease
For quality of life, seven studies reported data specifically for LSHD. Using the Ditesheim and Templeton QOL scoring system, one group found that LSHD patients were more likely to have fair, rather than good QOL scores. The QOL scores were correlated with continence scores. Patients with LSHD were more likely to have increased school absences and have an integration aide.[60] In a contrasting study that used the FIC QOL, the length of aganglionosis did not affect the QOL. When compared to healthy controls, patients with either SSHD or LSDH have increased incontinence and decreased psychosocial scores, but equivalent overall QOL.[61] In a study using the GIQOL, no difference was found in the QOL for patients with rectosigmoid HD compared to those with LSHD. However, they did note that the longer the aganglionic segment, the higher the risk of the HD affecting the patient’s QOL in adulthood.[62] In a combined cohort with 282 SSHD, 29 LSHD, and 25 TCHD children and adults with HD, the HD patients had higher rates of incontinence when compared to normal controls. While the incontinence rate was not related to the level of aganglionosis in children, incontinence correlated with lower QOL scores for behavior, self-esteem, and general health when compared to normal controls. Although incontinent adults with HD also had lower scores in the domain of physical health, adults with HD had higher overall QOL scores when compared to normal controls.[63] Similarly, global QOL scores of 49 SSHD, 4 LSHD, and 2 TCHD patients aged 11-17 years were comparable to those of normal controls. However, worse functional scores did correlate with lower QOL scores.[64] In a study using a non-validated QOL survey on 209 SSHD, 31 LSHD, and 19 TCHD patients, the majority of patients had good general health. However, 24% of patients stated that problems with their bowel movements did affect their general well-being and social activities.[65] Finally, a study using an HD-specific patient survey found that LSHD was associated with a lower QOL than age-matched controls for fecal continence and psychosocial aspects.[66]
Quality of Life in total colonic Hirschsprung disease
For TCHD, three studies specifically addressed QOL. One found TCHD patients had an overall good quality of life, with nearly all living normal lives- with no or only limited dietary restrictions, good school integration, normal or near-normal leisure activities, and successful academic or personal lives.[31] In a study of 24 primary Soave pull-throughs for TCHD, parental descriptions for quality of life were 80% “greater than satisfactory”, 17.5% “satisfactory”, and 2.5% “fair”.[27] The third study found that patients with TCHD had significantly lower generic QOL scores for overall health perception, physical functioning, and bodily pain/discomfort, but had similar psychological and social scores when compared to normal controls. Disease-specific QOL was most influenced by diarrhea and other physical symptoms when compared to normal controls.[39]
Recommendations and Observations:
While patients with LSHD and TCHD have an overall good self-reported QOL, they should be followed long-term into adulthood as they have lower scores on domains of physical health, predominantly related to bowel function.
Patients with LSHD and TCHD need a transition of care plan into adulthood as their bowel function may continue to affect their QOL.
Level 3-4 evidence, Grade of Recommendation D.
Question 4. What novel techniques exist and what future strategies are being developed for the treatment of long-segment Hirschsprung disease?
Surgical techniques
Despite an extensive review of the literature, we were unable to identify true new innovations in the surgical management of LSHD and TCHD. There are no reported significant modifications of the procedures that have already been described - Duhamel, Martin, Swenson, and Soave. All procedures involve either complete removal of the aganglionic bowel or anastomosis of normal ganglionated bowel to a segment of retained aganglionic bowel.
Observation:
There are no new surgical techniques to recommend for the treatment of LSHD and TCHD.
Probiotics
Hirschsprung-associated enterocolitis (HAEC) is one of the more serious complications in patients with Hirschsprung disease. The etiology remains unknown, but theories include stasis due to partial obstruction, altered microbiome, and loss of normal intestinal mucosal defense.[67],[68] Probiotics, which are non-pathogenic microorganisms, have been hypothesized as one possible way to decrease the rate of HAEC. Previous studies have shown probiotics to be beneficial in the treatment of diarrhea, inflammatory bowel disease, and Helicobacter pylori infection.[69–71] In a prospective, double-blinded, placebo-controlled, randomized study, 62 patients received either probiotic therapy or placebo following a definitive pull-through procedure for Hirschsprung disease. Patients were followed at 1, 3, 6, and 12 months postoperatively and monitored for signs of Hirschsprung-associated enterocolitis (HAEC).[72] There was an overall rate of 28.3% HAEC. The reported rate was 43.8% in the 16 patients with “long segment” disease vs. 20.8% in the 48 patients with “standard” rectosigmoid disease, with a relative risk of 2.95. However, the administration of probiotics did not significantly change the rate of HAEC. In a 2015 prospective, multi-center, randomized, controlled trial of 60 children with Hirschsprung disease, children were given probiotics or a placebo for four weeks and then followed for 3 months. The children in the probiotic group had a lower rate of HAEC (10% vs. 33%, P<0.05)), less severe symptoms, lower pro-inflammatory markers such as TNFα, IFNα, and IL-6, but higher levels of the anti-inflammatory IL-10. In this study 35% of the patients had “long-segment” HD, but there was not a specific mention if the probiotics were effective in this group.[73]
Recommendations and Observations:
There is insufficient evidence to recommend the routine use of probiotics for the prevention of HAEC in patients with LSHD and TCHD.
Level of evidence 2, Grade of Recommendation C
Stem cell therapies for long-segment Hirschsprung disease
While our search strategy did not identify any human studies of stem cell-based therapy for LSHD, the corresponding authors felt it important to include mention of this promising approach. Cell-based therapy, in which enteric nervous system (ENS) progenitor cells are transplanted into the bowel, has emerged as a promising approach to ENS regeneration.[74] Recent studies have demonstrated both endogenous and pluripotent stem-cell-derived ENS progenitor transplantation into mice.[75],[76–81] Each of these approaches has advantages and disadvantages[74]; however, endogenous ENS progenitors have an established long-term safety profile following transplantation.[75,76] Additionally, multiple groups are investigating the use of scaffolds, which consist of naturally-derived decellularized matrices that preserve extracellular matrix composition (ECM) and native tissue architecture or bioengineered scaffolds composed of synthetic materials and/or natural ECM components, for intestinal replacement.[82] While studies are currently limited to pre-clinical models, these types of approaches may hold promise for techniques to establish innervation of the distal bowel in order to restore normal function, reduce patient morbidity and mortality, reduce indications for intestinal transplantation, and improve overall patient quality of life.
CONCLUSIONS
This review summarize a heterogenous literature about Long-segment Hirschsprung disease. The overall scientific quality of the literature was poor, with predominantly Level of Evidence of 3-4 and Grade of Recommendation D. This speaks to the need for prospective, multicenter studies to better understand this rare condition.
Uniform definitions and precise anatomic description of the location of aganglionosis should be utilized for the reporting and classification of HD. While the Duhamel procedure is the most commonly reported operation to treat LSHD, there is insufficient evidence to recommend a preferred or a superior method for the surgical repair of LSHD. Both LSHD and TCHD are uncommon conditions that would require a prospective multi-institutional study to be able to identify factors that affect outcomes. Patients with LSHD have a good overall QOL, but they may continue to have difficulty with soiling and incontinence throughout their lifetime. Continence generally improves with patient age, but patients should continue to be followed into adulthood due to these concerns. There have been few advances in surgical techniques for LSHD, but stem cell therapy appears to be a promising approach.
ACKNOWLEDGEMENTS
The authors would like to thank the members of the APSA Hirschsprung Disease Interest Group, in particular Allan Goldstein, MD, Jack Langer, MD and Marc Levitt, MD, for their input into the question regarding nomenclature of Long-Segment Hirschsprung Disease.
FUNDING:
This work was supported by grants from the National Institutes of Health, USA (DK098271, DK114543 and DK125047 to AG).
LIST OF ABBREVIATIONS:
- APSA
American Pediatric Surgical Association
- ENS
Enteric nervous system
- HAEC
Hirschsprung-associated enterocolitis
- HD
Hirschsprung Disease
- IAPT
Ileoanal pullthrough
- IPAA
Ileal pouch anal anastomosis
- LSHD
Long-segment Hirschsprung disease
- OCEBM
Oxford Centre for Evidence-Based Medicine
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QOL
Quality of Life
- RSI
Recto-sigmoid index
- RTZ
Radiographic transition zone
- SIHD
Small Intestinal Hirschsprung disease
- SSHD
Short-segment Hirschsprung disease
- TCHD
Total Colonic Hirschsprung disease
- TIHD
Total Intestinal Hirschsprung disease
Appendix A. PubMed final search strategy.
(((((“hirschsprung disease”[MeSH Terms] OR “Hirschsprung Disease”[All Fields] OR “Hirschsprung’s Disease”[All Fields] OR “Hirschsprungs Disease”[All Fields] OR “Congenital Megacolon”[All Fields] OR “Aganglionic Megacolon”[All Fields] OR “Rectosigmoid Colon Aganglionosis”[All Fields] OR “Rectosigmoid Aganglionosis”[All Fields] OR “Congenital Intestinal Aganglionosis”[All Fields] OR “Colonic Aganglionosis”[All Fields] OR “Total Colonic Aganglionosis”[All Fields])) AND (Adolescent[MeSH Terms] OR Adolescent[All Fields] OR Adolescents[All Fields] OR “Young Adult”[MeSH Terms] OR “Young adult”[All Fields] OR “Young Adults”[All Fields] OR “Child”[MeSH Terms] OR “Child”[All Fields] OR “Children”[All Fields] OR “Infant”[MeSH Terms] OR “Infant”[All Fields” OR “Infant, newborn”[MeSH Terms] OR “Infant, newborn”[All Fields] OR “Infants”[All Fields] OR “Teen”[All Fields] OR “Teens”[All Fields] OR “Teenager”[All Fields] OR “Teenagers”[All Fields] OR “Kid”[AllFields] OR “Kids”[All Fields] OR “Baby”[All Fields] OR “Babies”[All Fields] OR “Juvenile”[All Fields] OR “Juveniles”[All Fields] OR “Minor”[All Fields] OR “Minors”[MeSH Terms] OR “Minors”[All Fields] OR “Toddler”[All Fields] OR “Toddlers”[All Fields] OR “Newborn”[All Fields] OR “Newborns”[All Fields])))) NOT “Animals”[Mesh]) NOT ((“Animals”[Mesh] AND “Humans”[Mesh])) AND ((“1990/01/01”[PDAT] : “2018/05/07”[PDAT]) AND English[lang])
REFERENCES
- [1].Ambartsumyan L, Smith C, Kapur RP. Diagnosis of Hirschsprung Disease. Pediatr Dev Pathol 2020;23:8–22. 10.1177/1093526619892351. [DOI] [PubMed] [Google Scholar]
- [2].Moore SW. Total colonic aganglionosis in Hirschsprung disease. Semin Pediatr Surg 2012. 10.1053/j.sempedsurg.2012.07.004. [DOI] [PubMed] [Google Scholar]
- [3].Reding R, de Ville de Goyet J, Gosseye S, Clapuyt P, Sokal E, Buts JP, et al. Hirschsprung’s disease: a 20-year experience. J Pediatr Surg 1997;32:1221–5. [DOI] [PubMed] [Google Scholar]
- [4].Puri P, Gosemann JH. Variants of Hirschsprung disease. Semin Pediatr Surg 2012. 10.1053/j.sempedsurg.2012.07.005. [DOI] [PubMed] [Google Scholar]
- [5].Nakamura H, Henderson D, Puri P. A meta-analysis of clinical outcome of intestinal transplantation in patients with total intestinal aganglionosis. Pediatr Surg Int 2017;33:837–41. 10.1007/s00383-017-4107-2. [DOI] [PubMed] [Google Scholar]
- [6].Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].OCEBM Levels of Evidence Working Group, Durieux N, Pasleau F, Howick J. The Oxford 2011 Levels of Evidence. Group 2011;1:5653. [Google Scholar]
- [8].Veras L V, Arnold M, Avansino JR, Bove K, Cowles RA, Durham MM, et al. Guidelines for synoptic reporting of surgery and pathology in Hirschsprung disease. J Pediatr Surg 2019:1–7. 10.1016/j.jpedsurg.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rescorla FJ, Morrison AM, Engles D, West KW, Grosfeld JL. Hirschsprung’s Disease: Evaluation of Mortality and Long-term Function in 260 Cases. Arch Surg 1992. 10.1001/archsurg.1992.01420080068011. [DOI] [PubMed] [Google Scholar]
- [10].Escobar MA, Grosfeld JL, West KW, Scherer LR, Rouse TM, Engum SA, et al. Long-term outcomes in total colonic aganglionosis: A 32-year experience. J Pediatr Surg 2005. 10.1016/j.jpedsurg.2005.03.043. [DOI] [PubMed] [Google Scholar]
- [11].Proctor ML, Traubici J, Langer JC, Gibbs DL, Ein SH, Daneman A, et al. Correlation between radiographic transition zone and level of aganglionosis in Hirschsprung’s disease: Implications for surgical approach. J. Pediatr. Surg, 2003. 10.1016/jpsu.2003.50165. [DOI] [PubMed] [Google Scholar]
- [12].Falcone R a, Daugherty M, Schweer L, Patterson M, Brown RL, Garcia VF. Multidisciplinary pediatric trauma team training using high-fidelity trauma simulation. J Pediatr Surg 2008;43:1065–71. 10.1016/j.jpedsurg.2008.02.033. [DOI] [PubMed] [Google Scholar]
- [13].Baillie CT, Kenny SE, Rintala RJ, Booth JM, Lloyd DA. Long-term outcome and colonic motility after the Duhamel procedure for Hirschsprung’s disease. J. Pediatr. Surg, 1999. 10.1016/S0022-3468(99)90201-4. [DOI] [PubMed] [Google Scholar]
- [14].Tsuji H, Spitz L, Kiely EM, Drake DP, Pierro A. Management and long-term follow-up of infants with total colonic aganglionosis. J. Pediatr. Surg, 1999. 10.1016/S0022-3468(99)90248-8. [DOI] [PubMed] [Google Scholar]
- [15].Fouquet V, De Lagausie P, Faure C, Bloch J, Malbezin S, Ferkhadji L, et al. Do prognostic factors exist for total colonic aganglionosis with ileal involvement? J Pediatr Surg 2002. 10.1053/jpsu.2002.29430. [DOI] [PubMed] [Google Scholar]
- [16].Sauer CJE, Langer JC, Wales PW. The versatility of the umbilical incision in the management of Hirschsprung’s disease. J Pediatr Surg 2005. 10.1016/j.jpedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- [17].Menezes M, Prato AP, Jasonni V, Puri P. Long-term clinical outcome in patients with total colonic aganglionosis: a 31-year review. J Pediatr Surg 2008. 10.1016/j.jpedsurg.2008.01.072. [DOI] [PubMed] [Google Scholar]
- [18].Raboei EH. Long-term outcome of total colonic aganglionosis. Eur J Pediatr Surg 2008. 10.1055/s-2008-1038495. [DOI] [PubMed] [Google Scholar]
- [19].Urla C, Lieber J, Obermayr F, Busch A, Schweizer R, Warmann SW, et al. Surgical treatment of children with total colonic aganglionosis: Functional and metabolic long-term outcome. BMC Surg 2018. 10.1186/s12893-018-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Widyasari A, Pravitasari WA, Dwihantoro A, Gunadi. Functional outcomes in Hirschsprung disease patients after transabdominal Soave and Duhamel procedures. BMC Gastroenterol 2018. 10.1186/s12876-018-0783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shu CZ, Yu ZB, Wang W, Wei LW. Clinical outcome in children after transanal 1-stage endorectal pull-through operation for Hirschsprung disease. J Pediatr Surg 2005;40:1307–11. 10.1016/j.jpedsurg.2005.05.016. [DOI] [PubMed] [Google Scholar]
- [22].Pini Prato A, Arnoldi R, Sgrò A, Felici E, Racca F, Nozza P, et al. Hirschsprung disease and Down syndrome: From the reappraisal of risk factors to the impact of surgery. J Pediatr Surg 2019. 10.1016/j.jpedsurg.2019.01.053. [DOI] [PubMed] [Google Scholar]
- [23].Martin LW. Total colonic aganglionosis preservation and utilization of entire colon. J Pediatr Surg 1982. 10.1016/S0022-3468(82)80125-5. [DOI] [PubMed] [Google Scholar]
- [24].Fortuna RS, Weber TR, Tracy TF, Silen ML, Cradock T V. Critical analysis of the operative treatment of Hirschsprung’s disease. Arch. Surg, 1996. 10.1001/archsurg.1996.01430170066013. [DOI] [PubMed] [Google Scholar]
- [25].Reding R, De Ville De Goyet J, Gosseye S, Clapuyt P, Sokal E, Buts JP, et al. Hirschsprung’s disease: A 20-year experience. J Pediatr Surg 1997;32:1221–5. 10.1016/S0022-3468(97)90686-2. [DOI] [PubMed] [Google Scholar]
- [26].Tsuji H, Spitz L, Kiely EM, Drake DP, Pierro A. Management and long-term follow-up of infants with total colonic aganglionosis. J Pediatr Surg 1999;34:158–62. 10.1016/S0022-3468(99)90248-8. [DOI] [PubMed] [Google Scholar]
- [27].Dodero P, Magillo P, Scarsi PL. Total colectomy and straight ileo-anal Soave endorectal pull-through: Personal experience with 42 cases. Eur J Pediatr Surg 2001. 10.1055/s-2001-18549. [DOI] [PubMed] [Google Scholar]
- [28].Fonkalsrud EW, Thakur A, Beanes S. Ileoanal pouch procedures in children. J Pediatr Surg 2001. 10.1053/jpsu.2001.27961. [DOI] [PubMed] [Google Scholar]
- [29].Ademuyiwa AO, Bode CO, Lawal OA, Seyi-Olajide J. Swenson’s pull-through in older children and adults: Peculiar peri-operative challenges of surgery. Int J Surg 2011. 10.1016/j.ijsu.2011.08.006. [DOI] [PubMed] [Google Scholar]
- [30].Granström AL, Husberg B, Nordenskjöld A, Svensson PJ, Wester T. Laparoscopic-assisted pull-through for Hirschsprung’s disease, a prospective repeated evaluation of functional outcome. J. Pediatr. Surg, 2013. 10.1016/j.jpedsurg.2013.07.017. [DOI] [PubMed] [Google Scholar]
- [31].Barrena S, Andres AM, Burgos L, Luis AL, Hernandez F, Martinez L, et al. Long-term results of the treatment of total colonic aganglionosis with two different techniques. Eur J Pediatr Surg 2008;18:375–9. 10.1055/s-2008-1038895. [DOI] [PubMed] [Google Scholar]
- [32].Huang WK, Li XL, Zhang J, Zhang SC. Prevalence, Risk Factors, and Prognosis of Postoperative Complications after Surgery for Hirschsprung Disease. J Gastrointest Surg 2018. 10.1007/s11605-017-3596-6. [DOI] [PubMed] [Google Scholar]
- [33].Langer JC, Durrant AC, de la Torre L, Teitelbaum DH, Minkes RK, Caty MG, et al. One-Stage Transanal Soave Pullthrough for Hirschsprung Disease. Trans . Meet Am Surg Assoc 2003. 10.1097/01.sla.0000089854.00436.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yokota K, Uchida H, Tainaka T, Tanaka Y, Shirota C, Hinoki A, et al. Single-stage laparoscopic transanal pull-through modified Swenson procedure without leaving a muscular cuff for short- and long-type Hirschsprung disease: a comparative study. Pediatr Surg Int 2018;34:1105–10. 10.1007/s00383-018-4318-1. [DOI] [PubMed] [Google Scholar]
- [35].Georgeson KE, Cohen RD, Hebra A, Jona JZ, Powell DM, Rothenberg SS, et al. Primary laparoscopic-assisted endorectal colon pull-through for Hirschsprung’s disease: A new gold standard. Ann. Surg, vol. 229, 1999, p. 678–83. 10.1097/00000658-199905000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cobellis G, Noviello C, Cruccetti A, Romano M, Mastroianni L, Amici G, et al. Staged laparoscopic-assisted endorectal pull-through for long segment Hirschprung’s disease and total colonic aganglionosis. Minerva Pediatr 2011. [PubMed] [Google Scholar]
- [37].Cheung ST, Tam YH, Chong HM, Chan KW, Mou WC, Sihoe DYJ, et al. An 18-year experience in total colonic aganglionosis: from staged operations to primary laparoscopic endorectal pull-through. J Pediatr Surg 2009. 10.1016/j.jpedsurg.2009.07.057. [DOI] [PubMed] [Google Scholar]
- [38].Mochizuki K, Shinkai M, Kitagawa N, Take H, Usui H, Hosokawa T, et al. Continuous transanal decompression for infants with long- and total-type Hirschsprung’s diseases as a bridge to curative surgery: a single-center experience. Surg Case Reports 2017. 10.1186/s40792-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roorda D, Witvliet MJ, Wellens LM, Schulten D V., Sloots CEJ, de Blaauw I, et al. Long-term outcome and quality of life in patients with total colonic aganglionosis in the netherlands. Color Dis 2018;20:719–26. 10.1111/codi.14095. [DOI] [PubMed] [Google Scholar]
- [40].Neuvonen MI, Kyrklund K, Rintala RJ, Pakarinen MP. Bowel function and quality of life after transanal endorectal pull-through for hirschsprung disease. Ann Surg 2017;265:622–9. 10.1097/SLA.0000000000001695. [DOI] [PubMed] [Google Scholar]
- [41].Kim AC, Langer JC, Pastor AC, Zhang L, Sloots CEJ, Hamilton NA, et al. Endorectal pull-through for Hirschsprung’s disease-a multicenter, long-term comparison of results: transanal vs transabdominal approach. J Pediatr Surg 2010. 10.1016/j.jpedsurg.2010.02.087. [DOI] [PubMed] [Google Scholar]
- [42].Dahal GR, Wang JX, Guo LH. Long-term outcome of children after single-stage transanal endorectal pull-through for Hirschsprung’s disease. World J Pediatr 2011;7:65–9. 10.1007/s12519-011-0247-y. [DOI] [PubMed] [Google Scholar]
- [43].Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence after surgery for Hirschsprung’s disease. J Gastroenterol Hepatol 2007. 10.1111/j.1440-1746.2006.04750.x. [DOI] [PubMed] [Google Scholar]
- [44].Moore SW, Albertyn R, Cywes S. Clinical outcome and long-term quality of life after surgical correction of Hirschsprung’s disease. J Pediatr Surg 1996. 10.1016/S0022-3468(96)90164-5. [DOI] [PubMed] [Google Scholar]
- [45].Sood S, Lim R, Collins L, Trajanovska M, Hutson JM, Teague WJ, et al. The long-term quality of life outcomes in adolescents with Hirschsprung disease. J Pediatr Surg 2018;53:2430–4. 10.1016/j.jpedsurg.2018.08.036. [DOI] [PubMed] [Google Scholar]
- [46].Stensrud KJ, Emblem R, Bjørnland K. Functional outcome after operation for Hirschsprung disease-transanal vs transabdominal approach. J Pediatr Surg 2010;45:1640–4. 10.1016/j.jpedsurg.2010.02.065. [DOI] [PubMed] [Google Scholar]
- [47].Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence in patients with Hirschsprung’s disease and Down syndrome. J Gastroenterol Hepatol 2006. 10.1111/j.1440-1746.2005.03996.x. [DOI] [PubMed] [Google Scholar]
- [48].Bjørnland K, Pakarinen MP, Stenstrøm P, Stensrud KJ, Neuvonen M, Granström AL, et al. A Nordic multicenter survey of long-term bowel function after transanal endorectal pull-through in 200 patients with rectosigmoid Hirschsprung disease. J Pediatr Surg 2017;52. 10.1016/j.jpedsurg.2017.01.001. [DOI] [PubMed] [Google Scholar]
- [49].Hoehner JC, Ein SH, Shandling B, Kim PCW. Long-term morbidity in total colonic aganglionosis. J. Pediatr. Surg, 1998. 10.1016/S0022-3468(98)90515-2. [DOI] [PubMed] [Google Scholar]
- [50].Choe EK, Moon SB, Kim HY, Lee SC, Park KW, Jung SE. Outcomes of surgical management of total colonic aganglionosis. World J Surg 2008;32:62–8. 10.1007/s00268-007-9270-5. [DOI] [PubMed] [Google Scholar]
- [51].Stenström P, Brautigam M, Borg H, Graneli C, Lilja HE, Wester T. Patient-reported Swedish nationwide outcomes of children and adolescents with total colonic aganglionosis. J Pediatr Surg 2017;52:1302–7. 10.1016/j.jpedsurg.2016.11.033. [DOI] [PubMed] [Google Scholar]
- [52].Wildhaber BE, Teitelbaum DH, Coran AG. Total colonic Hirschsprung’s disease: A 28-year experience. J Pediatr Surg 2005. 10.1016/j.jpedsurg.2004.09.033. [DOI] [PubMed] [Google Scholar]
- [53].Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Van Tilburg M. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016;150:1456–1468.e2. 10.1053/j.gastro.2016.02.015. [DOI] [Google Scholar]
- [54].Teitelbaum DH, Drongowski RA, Chamberlain JN, Coran AG. Long-term stooling patterns in infants undergoing primary endorectal pull-through for Hirschsprung’s disease. J. Pediatr. Surg, 1997. 10.1016/S0022-3468(97)90397-3. [DOI] [PubMed] [Google Scholar]
- [55].Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC. Development of a standardized definition for Hirschsprung’s-associated enterocolitis: a Delphi analysis. J Pediatr Surg 2009. 10.1016/j.jpedsurg.2008.10.052. [DOI] [PubMed] [Google Scholar]
- [56].Frykman PK, Short SS. Hirschsprung-associated enterocolitis: Prevention and therapy. Semin Pediatr Surg 2012;21:328–35. 10.1053/j.sempedsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gosain A, Frykman PK, Cowles RA, Horton J, Levitt M, Rothstein DH, et al. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr Surg Int 2017. 10.1007/s00383-017-4065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Foster P, Cowan G, Wrenn EL. Twenty-five years’ experience with Hirschsprung’s disease. J Pediatr Surg 1990;25:531–4. 10.1016/0022-3468(90)90566-R. [DOI] [PubMed] [Google Scholar]
- [59].Kim AC, Langer JC, Pastor AC, Zhang L, Sloots CEJ, Hamilton N a., et al. Endorectal pull-through for Hirschsprung’s disease-a multicenter, long-term comparison of results: transanal vs transabdominal approach. J Pediatr Surg 2010;45:1213–20. 10.1016/j.jpedsurg.2010.02.087. [DOI] [PubMed] [Google Scholar]
- [60].Catto-Smith AG, Trajanovska M, Taylor RG. Long-term continence after surgery for Hirschsprung’s disease. J Gastroenterol Hepatol 2007. 10.1111/j.1440-1746.2006.04750.x. [DOI] [PubMed] [Google Scholar]
- [61].Collins L, Collis B, Trajanovska M, Khanal R, Hutson JM, Teague WJ, et al. Quality of life outcomes in children with Hirschsprung disease. J Pediatr Surg 2017;52. 10.1016/j.jpedsurg.2017.08.043. [DOI] [PubMed] [Google Scholar]
- [62].Gunnarsdóttir A, Sandblom G, Arnbjörnsson E, Larsson L-T. Quality of life in adults operated on for Hirschsprung disease in childhood. J Pediatr Gastroenterol Nutr 2010;51:160–6. 10.1097/MPG.0b013e3181cac1b6. [DOI] [PubMed] [Google Scholar]
- [63].Meinds RJ, van der Steeg AFW, Sloots CEJ, Witvliet MJ, de Blaauw I, van Gemert WG, et al. Long-term functional outcomes and quality of life in patients with Hirschsprung’s disease. Br J Surg 2019;106:499–507. 10.1002/bjs.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sood S, Lim R, Collins L, Trajanovska M, Hutson JM, Teague WJ, et al. The long-term quality of life outcomes in adolescents with Hirschsprung disease. J Pediatr Surg 2018. 10.1016/j.jpedsurg.2018.08.036. [DOI] [PubMed] [Google Scholar]
- [65].Menezes M, Corbally M, Puri P. Long-term results of bowel function after treatment for Hirschsprung’s disease: A 29-year review. Pediatr Surg Int 2006;22:987–90. 10.1007/s00383-006-1783-8. [DOI] [PubMed] [Google Scholar]
- [66].Tran VQ, Mahler T, Dassonville M, Truong DQ, Robert A, Goyens P, et al. Long-Term Outcomes and Quality of Life in Patients after Soave Pull-Through Operation for Hirschsprung’s Disease: An Observational Retrospective Study. Eur J Pediatr Surg 2018. 10.1055/s-0037-1604115. [DOI] [PubMed] [Google Scholar]
- [67].Bastard F, Podevin G. Hirschsprung disease, diagnosis and management. Colon and Rectum 2015. 10.1007/s11725-015-0593-3. [DOI] [Google Scholar]
- [68].Heuckeroth RO. Hirschsprung disease - Integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 2018. 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]
- [69].Abraham BP, Quigley EMM. Probiotics in Inflammatory Bowel Disease. Gastroenterol Clin North Am 2017. 10.1016/j.gtc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [70].Patel A, Shah N, Prajapati JB. Clinical application of probiotics in the treatment of Helicobacter pylori infection--a brief review. J Microbiol Immunol Infect 2014. 10.1016/j.jmii.2013.03.010. [DOI] [PubMed] [Google Scholar]
- [71].Szajewska H, Canani RB, Guarino A, Hojsak I, Indrio F, Kolacek S, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr 2016. 10.1097/MPG.0000000000001081. [DOI] [PubMed] [Google Scholar]
- [72].El-Sawaf M, Siddiqui S, Mahmoud M, Drongowski R, Teitelbaum DH. Probiotic prophylaxis after pullthrough for Hirschsprung disease to reduce incidence of enterocolitis: A prospective, randomized, double-blind, placebo-controlled, multicenter trial. J Pediatr Surg 2013. 10.1016/j.jpedsurg.2012.10.028. [DOI] [PubMed] [Google Scholar]
- [73].Wang X, Li Z, Xu Z, Wang Z, Feng J. Probiotics prevent Hirschsprung’s disease-associated enterocolitis: A prospective multicenter randomized controlled trial. Int J Colorectal Dis 2014;30:105–10. 10.1007/s00384-014-2054-0. [DOI] [PubMed] [Google Scholar]
- [74].Stamp LA. Cell therapy for GI motility disorders: Comparison of cell sources and proposed steps for treating hirschsprung disease. Am J Physiol - Gastrointest Liver Physiol 2017. 10.1152/ajpgi.00018.2017. [DOI] [PubMed] [Google Scholar]
- [75].Hotta R, Stamp LA, Foong JPP, McConnell SN, Bergner AJ, Anderson RB, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest 2013. 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cooper JE, McCann CJ, Natarajan D, Choudhury S, Boesmans W, Delalande JM, et al. In vivo transplantation of enteric neural crest cells into mouse gut; Engraftment, functional integration and long-term safety. PLoS One 2016. 10.1371/journal.pone.0147989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cheng LS, Hotta R, Graham HK, Belkind-Gerson J, Nagy N, Goldstein AM. Postnatal human enteric neuronal progenitors can migrate, differentiate, and proliferate in embryonic and postnatal aganglionic gut environments. Pediatr Res 2017. 10.1038/pr.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Stamp LA, Gwynne RM, Foong JPP, Lomax AE, Hao MM, Kaplan DI, et al. Optogenetic Demonstration of Functional Innervation of Mouse Colon by Neurons Derived From Transplanted Neural Cells. Gastroenterology 2017. 10.1053/j.gastro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- [79].Becker L, Peterson J, Kulkarni S, Pasricha PJ. Ex Vivo Neurogenesis within Enteric Ganglia Occurs in a PTEN Dependent Manner. PLoS One 2013. 10.1371/journal.pone.0059452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 2017. 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 2016. 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bitar KN, Zakhem E. Bioengineering the gut: Future prospects of regenerative medicine. Nat Rev Gastroenterol Hepatol 2016. 10.1038/nrgastro.2016.124. [DOI] [PubMed] [Google Scholar]


