Abstract
Attention is a central neural process enabling selective and efficient processing of visual information. Individuals can attend to specific visual information either overtly, by making an eye movement to an object of interest, or covertly, without moving their eyes. We review behavioral, neuropsychological, neurophysiological and computational evidence of presaccadic attentional modulations, occurring while preparing saccadic eye movements, and highlight their differences from those of covert spatial endogenous (voluntary) and exogenous (involuntary) attention. We discuss recent studies and experimental procedures on how these different types of attention impact visual performance and alter appearance; differentially modulate featural representation of basic visual dimensions–orientation and spatial frequency; engage different neural computations; and recruit partially distinct neural substrates. We conclude that presaccadic attention and covert attention are dissociable.
Keywords: exogenous attention, endogenous attention, eye movements, featural representation, orientation, spatial frequency, contrast
Presaccadic and covert attention are dissociable
Much of human visual experience results from moving our gaze to actively explore the visual world and gather information. By scanning the scene with saccadic eye movements [see Glossary] we bring relevant objects into our fovea, where visual information is processed with high precision. The link between eye movements and visual perception is so tight that perception is already facilitated before our gaze has reached a location of interest: right before an eye movement, while we prepare to saccade, presaccadic attention is deployed to the future gaze location, improving performance and altering stimulus appearance at that location.
Attention can also be deployed covertly toward specific locations, without moving the eyes. There are two types of covert attention. Exogenous attention is automatic, stimulus-driven (bottom-up), and transiently deployed within ~100 ms. By contrast, endogenous attention is voluntary, goal-driven (top-down), and deployed in a slower but sustained manner, typically reaching its full effect by ~300 ms [1,2]. Each type of covert attention improves performance and alters stimulus appearance at the attended location.
At a broader scale, the behavioral consequences and the neural correlates of presaccadic and covert attention appear to be similar. This resemblance led to the notion that covert attention may be a cognitive process subsidiary to oculomotor programming. Over a decade ago, some studies questioned such a proposal. Given advances in experimental design and characterizations of the attentional effects on performance and visual representation, recent studies have revealed novel dissociations between presaccadic and covert attention.
We bring together behavioral, neuropsychological, neurophysiological and computational evidence distinguishing the effects of presaccadic attention1 on visual performance and on featural representation from those of covert endogenous and exogenous attention.
Effects of presaccadic attention on visual performance
Linking saccadic eye movements and visual attention
Over the past three decades, different groups found that perceptual judgments right before eye movement onset are more accurate for stimuli presented at the saccade target (the upcoming eye fixation) than elsewhere [5–7]. Interest in the perceptual consequences of presaccadic attention has continuously increased and numerous studies have explored its spatial and temporal properties using variations of a typical dual-task protocol (Table 1).
Table 1.
Studies and experimental procedures investigating the spatial and temporal coupling between saccadic eye movements and visual attention.
| Task/protocol | Standard | Temporal focus | Spatial focus |
|---|---|---|---|
| Discrimination task | Kowler et al., 1995 [5] Hoffman & Subramaniam, 1995 [6] Deubel & Schneider, 1996 [7] Montagnini & Castet, 2007 [21] Zhao et al., 2012 [22] ★ Khan et al., 2015 [23] ★Li, Pan & Carrasco, 2019 [47] Kreyenmeier, Deubel & Hanning, 2020 [24] ▲ Parker, Heathcote & Finkenbeiner, 2020 [48] ★Li, Pan & Carrasco, 2021 [49] |
Castet et al., 2006 [12] Deubel, 2008 [13] Filali-Sacouk et al., 2010 [14] ★Rolfs & Carrasco, 2012 [15] White, Rolfs & Carrasco, 2013 [17] Harrison, Marringley & Remington, 2013 [16] ★Li, Barbot & Carrasco, 2016 [18] Jonikaitis, Klapetek & Deubel, 2017 [19] Hanning, Deubel & Szinte, 2019 [8] |
▲Ditterich, Eggert & Straube, 2000 [38] Doré-Mazars & Collins, 2005 [39] Ghahghaei & Verghese, 2017 [9] Puntiroli, Kerzel & Born, 2018 [10] Szinte, Puntiroli & Deubel, 2019 [11] |
| Eye-abduction protocol | Smith et al., 2010 [28] Smith, Schenk & Rorden, 2012 [29] ▲ Hanning & Deubel, 2020 [35] |
▲ Hanning, Szinte & Deubel, 2019 [34] | |
| Global-saccade task | ▲ Van der Stigchel & de Vries 2015 [41] | ▲ Wollenberg, Hanning & Deubel, 2020 [43] | ▲ Wollenberg, Deubel & Szinte, 2018 [42] |
| Anti-saccade task |
▲ Klapetek, Jonikaitis & Deubel, 2016 [44] Mikula et al., 2018 [45] |
Dissociations between attentional and oculomotor orienting
Dissociation between overt and covert attention
Spatial and temporal coupling of oculomotor planning and attentional orienting
By testing performance at different locations relative to the saccade target and/or at different times relative to eye movement onset, behavioral studies—with varying spatial and temporal resolution—have provided consistent evidence of a tight spatial and temporal link between oculomotor programming and attentional orienting (Table 1). Spatially, perceptual benefits are typically bound to the saccade target and do not spread to neighboring stimuli [5,7,8], with the specific distribution of presaccadic attention being shaped by the spatial configuration of the scene [9–11]. Temporally, presaccadic attention builds up during saccade preparation, gradually reaching its maximum within ~75 ms before saccade onset, which is within ~200 ms after the cue indicating the saccade goal [8,12–19]. Whereas the close linkage between oculomotor and attentional orienting is undisputed, the nature of this coupling, and whether it is mandatory, has been long debated (for a recent review see [20]).
Is the coupling mandatory?
Early presaccadic attention studies claimed that eye movements and attention are mandatorily coupled, i.e. shortly before saccade onset one cannot attend elsewhere than to the saccade target [5–7], a notion supported by the observation of worsened visual performance at non-saccade target locations. Whereas to some degree, visual performance can be maintained at locations other than the saccade target during the early stages of movement planning, right before saccade onset performance at non-target locations necessarily decreases [13,21]. In line with this presaccadic cost at non-saccade targets [22,23], studies have demonstrated that attention is deployed to the saccade target even when detrimental for the task [7,24], suggesting a strong functional coupling between eye movements and attention.
Associations
The premotor theory of attention [25] even equated oculomotor and attentional orienting. Based on the observation of relatively prolonged reaction times for detecting targets appearing in the other visual hemifield than a spatial pre-cue, the authors postulated that a saccadic motor plan is required for the (re)orienting of visual spatial attention (because reorienting the eyes across the midline also would take longer as it requires activating different sets of muscles). That is, covert shifts of attention are achieved by programming an eye movement, but not executing it. Accordingly, spatial attention and oculomotor processes are considered to rely on the same neural substrates; any shift of spatial attention—overt or covert—would be elicited by preceding motor activity in the oculomotor system.
Additional studies supporting a dependence of visual attention on the oculomotor system rely on restricted ability to execute eye movements, either due to a pathology of the eye movement system (e.g. [26,27]) or experimentally induced by a maximally rotated eye position that prevents healthy participants from moving their eyes to certain peripheral locations (Fig 1B) [28,29]. According to both lines of research, although endogenous visual spatial attention can be properly deployed toward locations outside the eyes’ reach, exogenous attention cannot [30].
Figure 1. Prominent experimental procedures used to investigate the effects of presaccadic attention on visual performance.
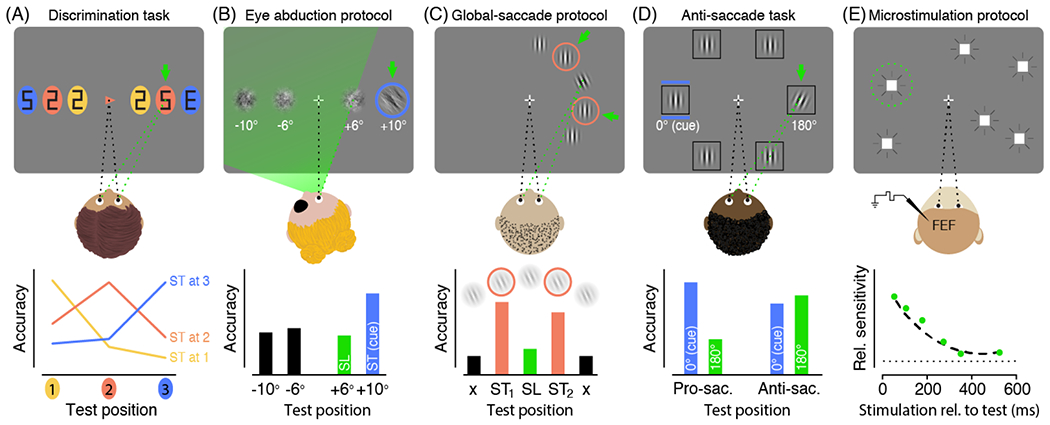
Top row: Depiction of the respective task (saccade targets indicated by green arrows, saccade endpoints indicated by dashed green lines). Bottom row: exemplary results. ST: Saccade target; SL: Saccade landing position; (A) Discrimination task. Participants prepare a saccade to an instructed saccade target (here the central arrow cue indicates the orange item on the right). A test stimulus (“E”) is presented briefly before eye movement onset along with several distractors (“2”s and “5”s), at a location coinciding with the saccade target or not. Participants are asked to discriminate the test stimulus (“E” versus “mirror-E”). While the eyes are still fixating the center, discrimination accuracy is selectively enhanced at the respective saccade target–indicating a presaccadic shift of attention. Adapted from [7]. (B) Eye abduction protocol. Participants view the display with one eye patched and their head rotated so that a portion of the display is still visible but outside the range reachable by the eyes (indicated by the green area). Saccades aimed at a cued target outside this range will fall short. Presaccadic discrimination accuracy (assessed with an oriented 1/f noise patch) is nonetheless highest at the unreachable saccade target (and not enhanced at the actual saccade landing)–demonstrating that attention is not limited to the eye movement range. Adapted from [34]. (C) Global-saccade protocol. Participants are instructed to saccade to one of two nearby items at free choice (either of the orange frames), in which case the eyes will frequently land in between the two salient targets. Presaccadic discrimination accuracy (assessed with an oriented Gabor patch) is not enhanced at the endpoint of such global-saccades, but instead equally enhanced at the two targets–showing that presaccadic attention is not necessarily coupled to the saccade endpoint. Adapted from [42]. (D) Anti-saccade task. Participants are instructed to saccade to the location in the opposite direction of a peripheral cue. Presaccadic discrimination accuracy (assessed with a tilted Gabor patch) before anti-saccades is equally enhanced at the cued location and the anti-saccade goal indicating attentional selection at both locations. Adapted from [44]. (E) Microstimulation protocol. Monkeys have to detect a luminance change of a peripheral target. Subthreshold frontal eye field stimulation increases visual sensitivity at the movement field (to which saccades would be evoked by stimulation currents above threshold; marked by the green circle), suggesting that saccade related mechanisms provide a source of spatial attention. Adapted from [46].
However, the vast majority of studies claiming evidence in favor of the premotor theory (e.g., [27,29]) (as well as some studies against it; e.g., [31–33]), have inferred attentional allocation from manual reaction times, following the rationale that stimuli are processed faster when presented within rather than outside the focus of attention. Yet, reaction times reflect the combined effect of detection-, decision-, criterion- and response-dependent processes, which can only be differentiated with special methods (e.g., speed-accuracy trade-off) and models (e.g., drift-diffusion model) not used in these studies. Studies measuring discrimination performance can isolate these components and better assess attention effects, as they can rule out speed-accuracy tradeoffs and/or rely on Signal Detection Theory, which indexes sensitivity and criterion separately [1,2].
Dissociations
Discrimination protocols have revealed that exogenous attention can be deployed at locations the eyes cannot reach. A salient visual cue equally attracts attention regardless of whether it was presented in-or outside the eyes’ reach, causing perceptual benefits at its location [34] and perceptual costs elsewhere [35]. These studies, as well as reports that pathological oculomotor restrictions (e.g., Moebius Syndrome) are not necessarily associated with corresponding attentional deficits [36], have revealed that covert attention can shift over the entire visual field range, independent of limitations imposed by the eye movement system [37].
This decoupling of attentional and oculomotor orienting is in line with studies evaluating presaccadic attention as a function of the saccade endpoint. Even when saccades undershoot their target, the attentional benefit occurs at the intended saccade target rather than the actual landing position [7,34]. Accordingly, when the amplitude of the executed saccade is modified via saccadic adaptation, the metrics of the presaccadic shift of attention remain unaffected [38]; however, when the saccade target is constant throughout the experiment, the shift of attention can be linked to the adapted saccade endpoint [39,40]. Moreover, visual attention is not coupled to the endpoint of global-saccades, which unintentionally land between two nearby potential saccade targets, but instead is allocated equally to both targets [41–43] (Fig 1C). Thus, whereas attentional orienting has often been considered dependent on oculomotor programming [25–29], converging behavioral evidence shows that presaccadic attention is tied to the intended motor goal rather than the executed motor program [7,34,38,41–43], providing evidence for a dissociation of attentional and oculomotor orienting.
Additionally, antisaccade tasks provide a unique opportunity to isolate and decouple the perceptual effects elicited by the visual stimulus and the final oculomotor command (Fig 1D). Visual spatial attention is simultaneously allocated to the cued location and the anti-saccade goal ([44,for similar results but a different interpretation see 45]), suggesting attentional selection at both locations. Were attention functionally equivalent to saccade preparation [25], it should have been primarily allocated to the anti-saccade goal. Following the same logic, deploying covert exogenous [33] or covert endogenous [31,32] attention does not speed up saccade initiation to the attended target, which would be expected if attention and oculomotor orienting were equivalent. In sum, these results show that covert attentional orienting is not the consequence of motor planning.
Modulations of visual representations by presaccadic attention and covert attention
The vast majority of presaccadic attention research has focused on its effects on performance (e.g., accuracy in discrimination tasks). Here we discuss recent advances that go beyond mere performance measures and reveal how presaccadic attention modulates featural representations; i.e., the processing of visual features, including orientation, spatial frequency and contrast. This assessment enables a differentiation of the cognitive, neural and computational processes underlying presaccadic and covert attention.
To characterize visual representation at the perceptual level, psychophysical procedures—e.g., adaptation, visual masking and reverse correlation—measure participants’ perceptual tuning functions. An adapter or a noise mask indexes how well human participants can detect or discriminate visual targets as a function of the distance between the target and the adaptor (or the mask) on a specific feature dimension (e.g., orientation, spatial frequency). The resulting perceptual tuning functions provide a gateway to compare perceptual effects resulting from different types of attention.
Orientation
Two studies employing psychophysical reverse correlation have investigated how presaccadic attention affects the perceptual orientation tuning function. In the first study [18], participants detected a visual target stimulus (a vertically oriented grating) presented at the saccade target. The target stimulus was embedded in random noise with rich orientation content, allowing for an estimation of the perceptual orientation tuning function. Compared to a neutral condition, without saccadic eye movements, the orientation tuning function at the saccade target exhibited both gain enhancement (increased sensitivity) and tuning width narrowing (higher selectivity) (Fig 2A; Table 2). These modulations emerged ~100 ms prior to saccade onset and reached maximum just before the eyes moved. Moreover, merely deploying covert attention within the same temporal interval without preparing a saccade did not alter performance. A follow-up study utilized dynamical noise and reported similar effects by presaccadic attention at the saccade target and lower gain and a wider tuning function (less selectivity) at a location opposite to the saccade direction just before saccade onset [50].
Figure 2. The effects of presaccadic and covert attention on featural representations.
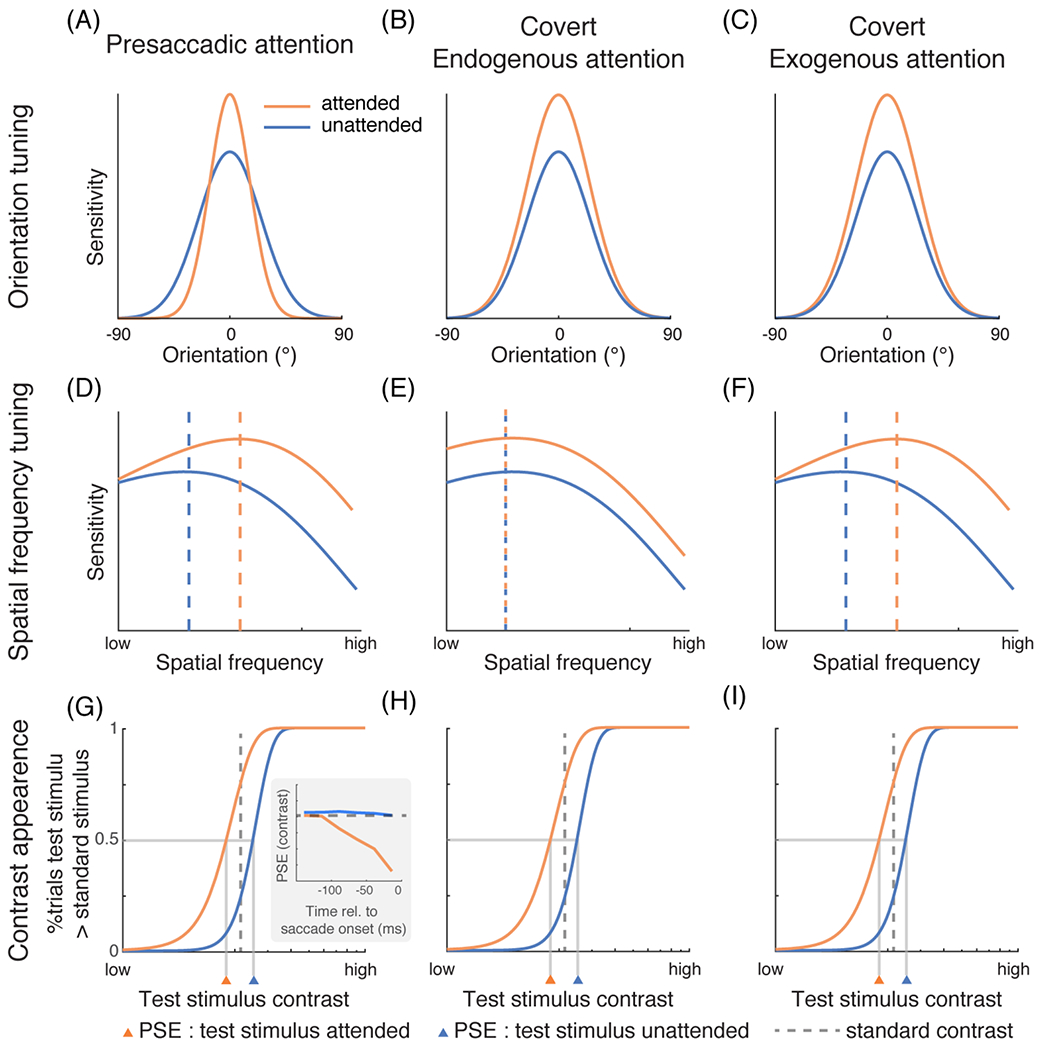
(A) In psychophysical experiments, participants were required to detect a grating at a fixed orientation (represented by 0° in the figure), and a reverse correlation technique was used to characterize the orientation tuning employed by the visual system. Presaccadic attention increases the gain and reduces the width of orientation tuning [18,50]. Adapted from [18]. (B-C) Covert endogenous and exogenous attention only increase the gain [51–55]. Adapted from [55]. (D-F) Presaccadic attention [18,47] and covert exogenous attention preferentially increase the sensitivity of high-SF information by shifting the SF tuning curve rightward [55,58,59,64]. whereas covert endogenous attention enhances a broad range of SFs uniformly [55,62–64]. The dashed vertical lines indicate the peak of the tuning functions. See details of the experimental protocols in BOX 1. (D) adapted from [18]. (E) and (F) adapted from [55]. (G-I) Subjective contrast appearance of visual stimuli can be estimated by measuring the point of subjective equality (PSE) in tasks requiring participants to compare the contrast of two stimuli. In the experiments, the contrast of a test stimulus typically varies across trials while the contrast of a standard stimulus is fixed. Here, the percentage of trials in which participants judge the test stimulus to have a higher contrast than the standard (y-axis) is plotted against the contrast of the test stimulus (x-axis). The orange curves represent the condition in which the test stimulus is cued (attended). Both presaccadic attention [15] as well as endogenous [74] and exogenous [66–71] covert attention enhance perceived contrast. The enhancement by presaccadic attention exhibits a gradual trend right before saccade onset (inset in G, adapted from [15]). (H) adapted from [65]. (I) adapted from [66].
Table 2.
Studies and experimental procedures investigating the modulation of visual representations by different types of attention in humans
| Feature | Method/Task | Presaccadic attentior | Covert endogenous attention | Covert exogenous attention |
|---|---|---|---|---|
|
| ||||
| Orientation gain | reverse correlation | ✓Li, Barbot & Carrasco, 2016 [18] | ✓Paltoglou & Neri, 2012 [52] | ✓Fernández, Li & Carrasc 2019 [54] |
| ✓Ohl, Kuper & Rolfs, 2017 [50] | ✓Wyart, Nobre & Summerfield, 2012 [53] | ✓Fernández, Okun & Carrasco, 2021 [55] | ||
| masking | ✓Baldassi & Bergheese, 2005 [51] | ✓Fernández, Okun & Carrasco, 2021 [55] | ||
|
| ||||
| Orientation tuning width | reverse correlation | ✓Li, Barbot & Carrasco, 2016 [18] | ✗Paltoglou & Neri, 2012 [52] | ✗Fernández, Li & Carrasc 2019 [54] |
| ✓Ohl, Kuper & Rolfs, 2017 [50] | ✗Wyart, Nobre & Summerfield, 2012 [53] | ✗Fernández, Okun & Carrasco, 2021 [55] | ||
| masking | ✗Baldassi & Verghese, 2005 [51] | ✗Fernández, Okun & Carrasco, 2021 [55] | ||
|
| ||||
| Enhancement of high spatial frequency | texture segmentation | ✗Jigo & Carrasco, 2018 [63] | ✓Yeshurun & Carrasco, 1998 [58] | |
| ✓Yeshurun & Carrasco, 2000 [75] | ||||
| ✓Talgar & Carrasco, 2002 [61] | ||||
| ✓Yeshurun & Carrasco, 2008 [60] | ||||
| texture segmentation + adaptation | ✗Barbot & Carrasco, 2018 [62] | ✓Carrasco, Loula & Ho, 2006 [59] | ✓Jigo & Carrasco, 2018 [63] | |
| reverse correlation | ✓Li, Barbot & Carrasco, 2016 [18] | ✗Fernández, Okun & Carrasco, 2021 [55] | ✓Fernández, Okun & Carrasco, 2021 [55] | |
| masking | ✓Li, Pan & Carrasco, 2019 [47] | ✗ Talgar, Pelli & Carrasco 2000 [103] | ||
| orientation discrimination | ✓Kroell & Rolfs, 2021 [57] | ✗Jigo & Carrasco, 2020 [64] | ✓Jigo & Carrasco, 2020 [64] | |
|
| ||||
| Contrast appearance | comparative judgement (PSE) + orientation discrimination | ✓Rolfs & Carrasco, 2012 [15] | ✓Liu, Abrams & Carrasco 2009 [74] | ✓Carrasco, Ling & Read, 2004 [66] |
| ✓Ling & Carrasco, 2007 [67] | ||||
| ✓Carrasco, Fuller & Ling, 2008 [68] | ||||
| ✓Störmer, McDonald & Hillyard, 2009 [69] | ||||
| ✓Anton-Erxleben, Abrams Carrasco, 2010 [70] | ||||
| ✓Cutrone, Heeger & Carrasco, 2014 [71] | ||||
| ✓Barbot & Carrasco, 2018 [72] | ||||
| ✓Zhou, Buetti, Lu & Cai, 2018 [73] | ||||
|
| ||||
| Contrast response function | orientation discrimination | + Li, Pan & Carrasco, 2021 [49] | * Ling & Carrasco, 2006 [81] | + Ling & Carrasco, 2006 [81] |
| * Pestilli, Ling & Carrascc 2009 [83] | + Pestilli, Viera & Carrasco, 2007 [82] | |||
| * or + : size dependent Herrmann et al., 2010 [84] | + Pestilli, Ling & Carrasco, 2009 [83] | |||
| ◆ Li, Pan & Carrasco, 2021 [49] | * or + : size dependent Herrmann et al., 2010 [84] | |||
| + Fernández & Carrasco, 2020 [104] | ||||
| ◆Li, Pan & Carrasco, 2021 [49] | ||||
| contrast discrimination | + Morrone, Denti & Spinelli, 2002 [78] | |||
| + Morrone, Denti & Spinelli, 2004 [79] | ||||
| ◆ Huang & Dobkins, 2005 [80] | ||||
the feature modulation indicated in the first column is observed
the feature modulation indicated in the first column is not observed
Contrast gain
Response gain
Mixture of both contrast gain and response gain
Unlike presaccadic attention, both covert endogenous and exogenous spatial attention increase the gain of perceptual orientation (or motion) tuning functions without affecting its tuning width [51–55] (Fig 2B and 2C; Table 2). Only when participants are instructed to attend to a particular orientation, covert feature-based attention reduces the width of orientation tuning [51,52].
Spatial frequency
Spatial frequency (SF) refers to the spatial scale (i.e., areas of relative light and dark in the visual scene) of visual inputs. The visual system’s SF selectivity varies across the visual field; sensitivity to higher SFs is higher at the fovea and decreases with eccentricity [1,2,56]. A study using reverse correlation revealed that presaccadic attention selectively increases sensitivity to higher SF information [18] (Fig 2D; Table 2). In this study, participants were required to detect a visual target stimulus (a vertically oriented grating embedded in random noise) presented at the saccade target. Compared to a neutral condition without saccadic eye movements, presaccadic attention increased the gain for higher SFs while leaving lower SFs unchanged, resulting in a rightward shift of SF tuning function. Merely deploying covert attention within the same temporal interval without preparing a saccade did not alter performance. A follow-up study has confirmed the shift of SF tuning towards higher SF with presaccadic attention [57]. Moreover, this modulation is compulsory [47] and cannot be explained by a shift of the tuning function toward the SF of the target [18,47]: Using a visual masking procedure, it has been shown that the rightward shift of SF tuning by presaccadic attention could move the SF tuning function away from (higher than) the target leading to an impairment on the task at hand (discriminating the orientation of the target) ([47], see details in BOX 1).
BOX 1.
Texture segmentation refers to the process by which the visual system isolates a region from its background based on its local structure. In the example depicted in Figure I, participants were required to detect the presence of the target patch whose line elements differ in orientation from the otherwise uniform background. Performance exhibits an inverted U-shape as a function of target eccentricity: higher performance for the target at midperiphery than at central and peripheral locations. This central performance drop (CPD) indicates that the visual system’s resolution, or sensitivity to high spatial frequency (SF) information, is too high for target detection. Accordingly, peak performance shifts to more peripheral or central locations when the texture scale increases or decreases respectively [58]. Selective adaptation to high-SF filters reduces participants’ sensitivity to high-SF, and correspondingly the CPD [59,62].
Figure I.
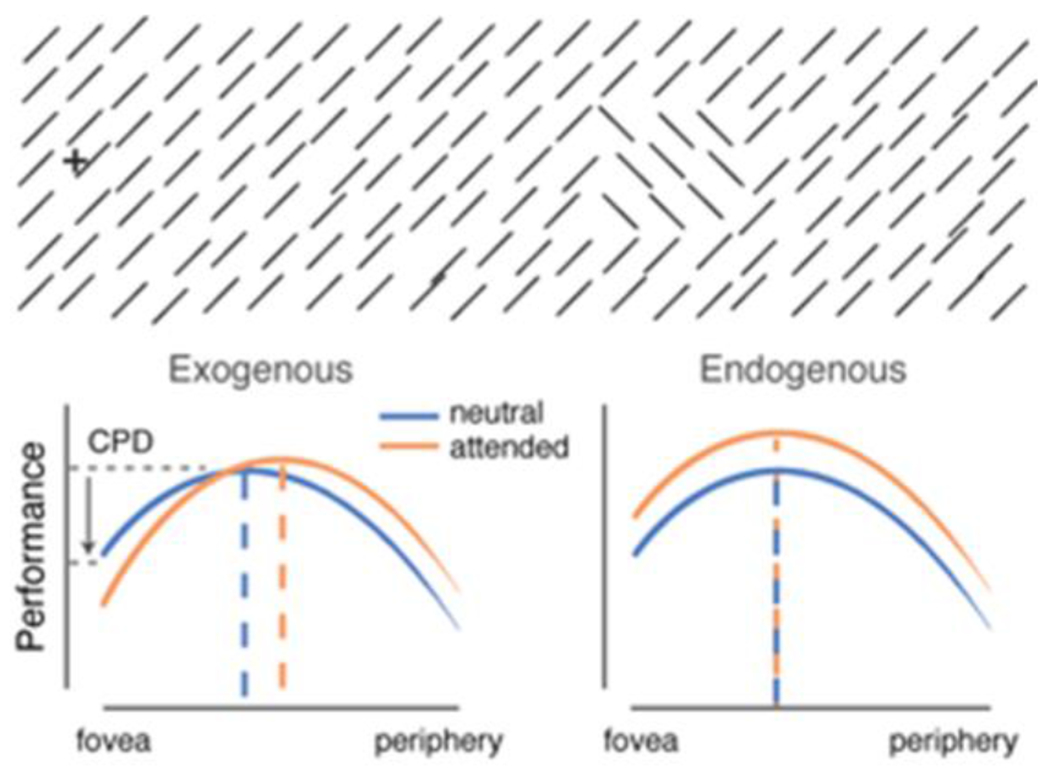
Texture segmentation tasks. Top: Example stimuli. Bottom: Exogenous attention impairs or improves performance as a function of stimulus eccentricity; endogenous attention improves performance throughout eccentricity. Adapted from [56].
Compared to a neutral baseline condition, exogenous attention impairs performance when the target patch is near the fovea, where visual resolution is already too high for the task but improves performance when the target patch is at periphery [58,59,61,63,75]. These results reveal that exogenous attention increases visual resolution, or the sensitivity to high SF, in a compulsory manner, even when detrimental for the task at hand. In the same task, endogenous attention adjusts resolution and improves performance regardless of target eccentricity [62,63]. Critically, selective adaptation to high-SF eliminates the central attentional impairment observed with exogenous attention [59] as well as the central improvement observed with endogenous attention [62].
Visual masking was utilized to test the effect of presaccadic attention on the processing of SF [47] (Figure II). A target at a fixed SF was superimposed with noise (mask) of different (lower, same or higher) SFs. In the neutral baseline condition, participants’ discrimination of the target orientation was lowest when the target was paired with the noise containing the same SF. Presaccadic attention enhanced the target’s discriminability when the noise had a lower or the same SF as the target but impaired its discriminability when the SF of the noise was higher than that of the target, presumably because it increased the suppressive effect elicited by the high-SF noise. Merely deploying covert endogenous attention within the same temporal interval did not alter performance. These results indicate that presaccadic attention enhances high SF information, even when detrimental for task performance.
Figure II.
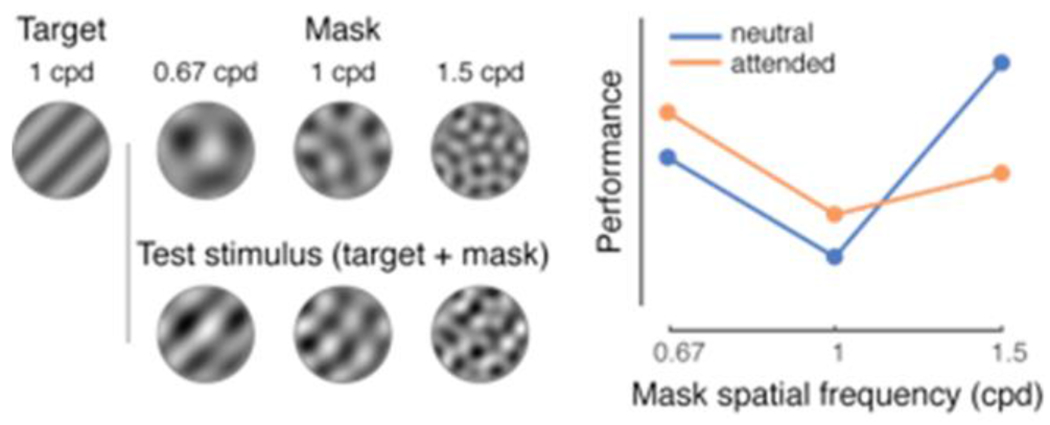
Masking experiments. Left: Example stimuli used in [47]. Right: Presaccadic attention impairs performance when the SF of the mask is higher that the SF of the target. Adapted from [47]
Interestingly, the compulsory increment of high-SF sensitivity by presaccadic attention is reminiscent of the modulations by covert exogenous attention. In texture segmentation tasks (Box 1), exogenous attention increases spatial resolution, mediated by high SF, even when it impairs performance [56,58–61]. In contrast, covert endogenous attention always improves performance in the same tasks, without preferentially enhancing high-SF information [62,63] (Fig 2E and 2F; Table 2).
Converging evidence indicates that covert exogenous attention and endogenous attention modulate SF processing differently: In orientation discrimination tasks, regardless of stimulus eccentricity, exogenous attention preferentially enhances SFs higher than the peak frequency in the baseline condition whereas endogenous attention enhances a broad range of lower and higher SFs [64]. Likewise, reverse correlation revealed that exogenous attention shifts peak sensitivity to higher SFs by enhancing the gain of SFs higher than the target’s SF, whereas endogenous attention enhances the gain of SFs below and above the target’s SF [55]. These changes in sensory tuning could underlie the differential performance effects of endogenous and exogenous attention in contrast sensitivity and texture segmentation tasks.
Contrast appearance
Covert attention changes the appearance of visual stimuli—i.e., how they appear subjectively to the participants—in a number of static (e.g., contrast, SF, size) and dynamic (e.g., flicker, motion coherence) visual dimensions [65]. Does presaccadic attention similarly affect appearance?
Appearance of visual stimuli can be assessed by a comparative procedure in which experimenters measure the point of subjective equality (PSE) of a standard stimulus. Both exogenous [66–73] and endogenous [74] covert spatial attention increase perceived contrast of the stimulus at the attended location, as indicated by a shift of the PSE to a lower contrast (Fig 2H and 2I; Table 2). A counterpart of this effect has been reported for presaccadic attention (Table 2). As time approaches saccade onset, perceived contrast and orientation discrimination increase concurrently and progressively (Fig 2G). A control experiment with a cue-to-stimulus interval matched to the presaccadic experiment demonstrated that endogenous attention cannot modulate contrast appearance in such a rapid fashion [15]. Thus, even though the perceptual modulations on contrast appearance are similar for presaccadic attention and covert endogenous attention, their temporal dynamics differ. These results link the dynamics of saccade preparation, visual performance and subjective experience and show that upcoming eye movements alter visual processing by increasing signal strength.
Contrast response functions and computational models
Computational models of attention aim to characterize attentional modulations on the input-output functions of visual neurons (e.g., contrast response functions: neural responses as a function of stimulus contrast). The neural activity as a function of stimulus contrast is either measured directly or is simulated by computational models and fit to behavioral performance. For covert attention, mainly two types of attentional modulation have been reported in both neurophysiological [76,77] and behavioral studies [78–84]: a contrast gain change, in which the response function shifts horizontally, as if attention scales the input stimulus contrast (e.g., Fig 3A), and a response gain change, in which the asymptotic response moves vertically, as if attention multiplicatively scales the neurons’ output response (Fig 3B). Additionally, human fMRI studies have reported an additive shift of response functions [85,86], or a combination of additive shift and contrast gain change by attention [87].
Figure 3. Attentional modulations on contrast response functions.
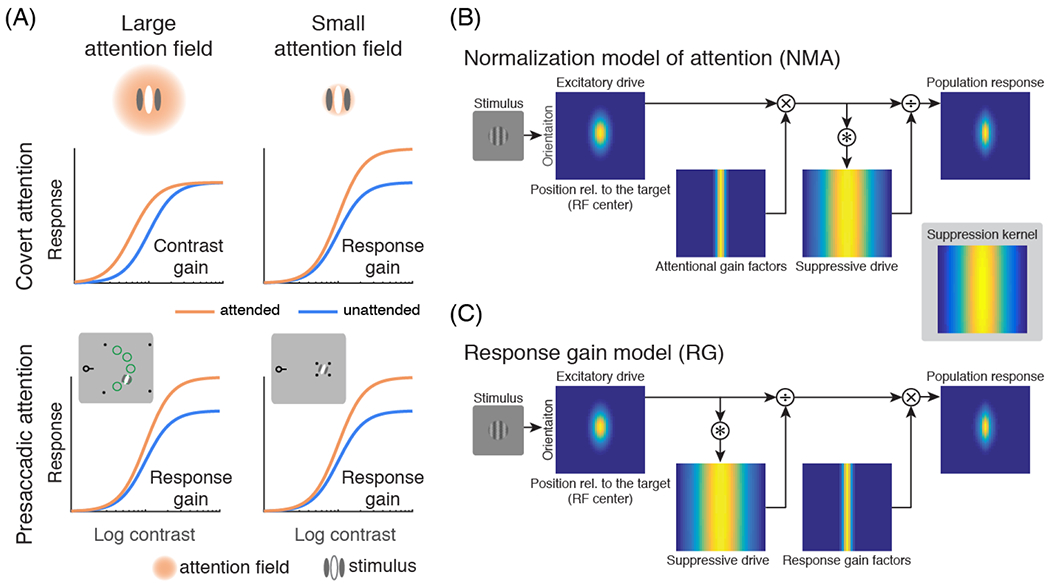
(A) Illustrations of how attentional field size (relative to the stimulus size) affects the form of attentional modulations. The left column represents the condition in which the size of the attentional field is large whereas the right column represents the condition in which the size of the attentional field is small. Covert attention (endogenous and exogenous; top row) generates contrast gain when the size of the attentional field is large and response gain when the size of the attentional field is small [77,84]. In contrast, presaccadic attention (bottom row) generates response gain changes regardless of the attentional field size [49]. The insets in the bottom row illustrate how the size of attention field was manipulated by the location uncertainty of the target in a study of presaccadic attention [49]. Only the fixation point with the pre-cue and the right half of the screen are shown. The left half of the screen contains similar stimuli (an aperture outlined by four black dots and a test stimulus). In the experiments, participants made a saccadic eye movement toward the center of the cued aperture (outlined by the four black dots). In the large attention field size condition, the aperture was large and the test stimulus (a grating) was presented at one of five possible locations (indicated by green circles for illustration purpose only) with equal probability. In the small attention field size condition, the test stimulus was presented at a fixed location [49,77,84] (attentional field size was also manipulated by location uncertainty in covert attention experiments [77,84]). (B) The Reynolds-Heeger normalization model of attention (NMA) explains these size-dependent gain changes. Blue-yellow 2-dimensional images represent a population of visual neurons selective for different orientations and receptive field centers. The response of visual neurons is computed by normalization: dividing neurons’ excitatory drive by their suppressive drive. The excitatory drive is determined by the preferred orientation and position of each neuron. The suppressive drive is computed by convolving the excitatory drive with the suppression kernel. The suppression kernel is uniform in the orientation domain. For simulating surround suppression of visual neurons, the suppression kernel is a Gaussian with a wide width in its spatial domain. In NMA, attention is modeled as attentional gain factors that multiplicatively modulate the excitatory drive before computing the suppressive drive and normalization. (C) The modulation of presaccadic attention is better explained by response gain factors that scale the neurons’ responses after normalization. In both (B) and (C), it is assumed that attention is deployed to the center of the target stimulus (the vertical grating). (A-C) adapted from [49].
Various computational studies have modeled attentional modulations on neural responses [88–91]. However, computational models of visual attention have either referred to covert attention or presaccadic attention exclusively, or have referred to spatial covert attention as a unitary construct. Here, we focus on a class of models that rely on normalization [92] to describe the computations underlying visual attention [76,77,93–95] as these normalization models of attention (NMA) have been widely applied to explain both behavioral [84,95,96] and neurophysiological [97–101] data in recent years. In normalization, the response of a visual neuron is modeled by the excitatory drive divided by the suppressive drive (normalization pool), computed as the excitatory drive summed over a pool of neurons [92]. In Reynolds-Heeger’s NMA [77], attention is modeled by attentional gain factors that multiplicatively modulate the excitatory drive of the neurons prior to normalization (Fig 3B). This computation predicts that attention exhibits a response gain (contrast gain) change when the size of the attentional field is small (large) relative to the stimulus size (Fig 3A, B). An intermediate attentional field size will lead to a mix of both response gain and contrast gain changes. Behavioral studies on covert attention have confirmed this prediction [84,96]. The additive shift by attention observed in human fMRI can also be explained by applying NMA to a neural population consisting of neurons with heterogeneous (position and orientation) tuning, and summing activity across many neurons to simulate the response of fMRI voxels [102].
Interestingly, unlike covert attention, presaccadic attention generates response gain changes regardless of the size of the attentional field (Fig 3A,C; Table 2; [99]). Model comparisons revealed that whereas modulations by covert attention are best fit by NMA, modulations by presaccadic attention are best fit by a response gain model, in which response gain factors modulate the neurons’ response after normalization (Fig 3C). These results indicate that the computations underlying presaccadic attention and covert attention are different [99].
Neural correlates of presaccadic and covert attention
Common brain areas but distinct subpopulations
The tight coupling between eye movements and attentional orienting raises the question of whether presaccadic and covert spatial attention are based on the same neural processes. At a broad scale, brain structures active during saccadic eye movements (the frontal eye fields (FEF), the precentral sulcus and the superior colliculus (SC)) are also selectively modulated during covert attention tasks in human- and non-human primates [105–108]. Feedback signals projecting from these areas to early visual cortices are assumed to enhance visual processing [109,110] and thereby account for the commonly observed presaccadic attentional effects.
In non-human primate studies, subthreshold microstimulation of FEF and SC—which would elicit a saccade if stimulated above threshold—modulates activity in visual cortex [111] and enhances visual sensitivity at the movement field of the stimulated neurons (Fig 1E) [46,112]. However, FEF and SC have distinct neuronal populations related to visual and motor activity [112–117]. Because microstimulation modulates both visual and motor cells, it cannot distinguish oculomotor and attentional orienting which are controlled by distinct populations within these overlapping neuronal circuits: Visual and visuomotor cells exhibit sustained activity during covert attentional orienting, but motor cells remain silent [114,115] and have disproportionately little feedback connections to early visual cortex [116]. Furthermore, covertly attended locations and eye movement goals are represented by different subsets of spatially tuned neurons in the frontal cortex, contradicting the idea that the control of spatial attention is dependent on oculomotor control circuits [117].
Likewise, in humans, the early laterality of visual evoked event related potentials (ERPs) correlates with covert, but not presaccadic attention [118], and transcranial magnetic stimulation (TMS) over FEF has revealed a clear temporal dissociation of saccade preparation and attention [119]. These results are consistent with the findings that saccade preparation and covert attention—despite involving overlapping neural regions—are neurophysiologically distinct phenomena [113,120]. In summary, neurophysiological evidence reveals that distinct neural populations in FEF and SC control covert attention and saccade preparation in human and non-human primates, potentially giving rise to differences in their perceptual correlates.
Response enhancement and noise reduction
Both endogenous and exogenous attention enhance neural response amplitude at the attended location throughout the visual system [76, 121]. Response enhancement by presaccadic attention has also been observed in macaque V1 [122] and V4 [123, 124], and throughout both ventral and dorsal visual streams in the human cortex [125]. The enhanced response is accompanied by an increment of decodable stimulus information (orientation) from the neural population just before saccade onset [126], matching the presaccadic attentional effects in behavioral studies. Likewise, in line with behavioral evidence [e.g., 44], response enhancement by covert attention and presaccadic attention coexist, as revealed by an anti-saccade experiment reporting qualitatively similar activity modulations corresponding to an endogenously attended location and the opposite saccade target location [127]. Recording in V4 has also revealed that both covert attention [128–130] and presaccadic attention [131] reduce the variability and noise correlation among visual neurons, which correlate with changes in behavioral performance [132].
Featural representation
How do presaccadic and covert attention modulate featural representations at the neural level? In line with psychophysical findings [51–53], endogenous spatial attention increases the gain (amplitude) on orientation tuning functions of visual neurons without affecting their tuning width [133,134]. To our knowledge, there is no parallel neurophysiological study for exogenous attention. Studies in V4 have shown that presaccadic attention enhances the response of visual neurons more to the preferred orientation than to non-preferred orientations [123] and increases the gain on orientation tuning functions, but did not report whether tuning width was affected [127]. Thus, whereas endogenous attention does not affect neural orientation tuning width, it is unknown whether presaccadic attention does, as its effects on gain and tuning have not been assessed independently.
Two (non-mutually exclusive) neural mechanisms may underlie the increment of sensitivity to high-SF information by spatial attention: First, receptive field (RF) shifts: the deployment of endogenous attention can shift visual neurons’ RF toward the attended location. In monkey single-unit recording, this RF shift occurs in both ventral [135] and dorsal extrastriate visual cortex [136]. Likewise, human neuroimaging studies measuring population receptive fields (pRF) reported a shift of pRFs toward the attended location in the visual cortex [137]. The RF shifts enhance the neural representation of the attended stimuli and are accompanied by a shrinkage of RF size at the attended location [136]. In principle, smaller RF sizes could benefit the discrimination of smaller targets in texture segmentation tasks, as a smaller RF would reduce the interference from background stimuli [2]. Similarly, just before monkeys make saccadic eye movements, RFs shift toward the saccade target in response to presaccadic visual stimuli in V4 [124,138] and prefrontal cortex [139]. In sum, RF shifts have been observed for endogenous and presaccadic attention but comparable studies for exogenous attention are lacking. However, at the behavioral level, only presaccadic attention and exogenous attention shift behavioral SF tuning toward higher SFs (Figure 2 and Table 2). Endogenous attention conceivably evokes additional mechanisms that benefit the processing of low-SF information, thus leaving the overall SF sensitivity tuning function unchanged.
The second potential neural mechanism to support an increment in high-SF sensitivity is a preferential enhancement of visual neurons tuned to high SFs. Neurons in the mouse primary visual cortex exhibit gain increments during a heightened attentional state, as behaviorally assessed by locomotion and a dilated pupil. Critically, neurons selective for higher SFs exhibit larger gain increments than those selective for lower frequencies [140]. In mice, the dorsal lateral geniculate nucleus of the thalamus also shows a preferential gain increment in locomotion for neurons selective for high SF [141]. These neural modulations could support the perceptual effects observed in behavioral attention research, but neurophysiological studies using a spatial attention protocol have yet to test this assumption. A critical distinction is that the effect of locomotion is global across the entire visual field whereas the effect of spatial attention is restricted to the attended location. In general, these observations are consistent with psychophysical experiments employing a selective adaptation protocol, which revealed that spatial attention tunes SF sensitivity via high-SF channels [59,62].
Concluding remarks
Although both presaccadic attention and covert spatial attention enable selective processing of visual information and facilitate perception, there are clear dissociations in their temporal dynamics, modulations of featural representation of basic visual dimensions, neural computations and neural correlates. Despite remaining questions about the specific origin, nature and functional significance of presaccadic attention and covert attention, converging evidence indicates that the notion that visual attention cannot be decoupled from eye movement programming should be revised. Presaccadic and covert attention are distinct neurocognitive processes that do not depend on one another.
The dissociations between covert attention and presaccadic attention provide important insight into brain function and call for further investigation. Our understanding of presaccadic attention—as well as of its similarities to covert spatial attention and differences from it —would be further advanced by integrating knowledge gathered from psychophysics, neuropsychology, neurophysiology, neuroimaging and computational models in human and non-human primates, while keeping experimental protocols as similar as possible (see Outstanding Questions). Moreover, it would be informative to isolate individual components of eye movements (planning, programming and execution), which may differentially influence the findings in studies with human participants and nonhuman primates. Depending on the time period of the analysis, different oculomotor components could contribute to the effects of presaccadic attention or conflate the effects attributed to covert attention.
Outstanding Questions.
Do presaccadic attentional modulations of featural representations, as suggested, support transsaccadic integration and visual stability around the time of saccades?
Behaviorally, when participants plan a saccade, presaccadic attention enhances gain, narrows orientation tuning and shifts SF selectivity at the saccade target. Is this also the case when participants saccade in response to a salient stimulus, without voluntarily planning to do so?
Presaccadic attention changes contrast appearance. Does it also change appearance of other static (e.g., spatial frequency, size) and dynamic (e.g., flicker, speed) visual dimensions, as covert attention does?
Presaccadic attention alters featural representations at the saccade target. What happens simultaneously to featural representations at other locations, e.g. at the fovea (where the gaze is about to leave) and at locations around the saccade target?
Presaccadic attention affects encoding of sensory information. Does it also affect other processes such as information readout or decision criteria?
Presaccadic attention alters featural representations at the saccade target. What neural correlates of these effects would neurophysiological and neuroimaging studies reveal?
Reaction time studies with special populations (e.g., Moebius syndrome) inform our knowledge of eye movement programming/execution and covert attention. Would discrimination tasks yield consistent findings? Would people with such oculomotor conditions show the same modulations on featural representations as people with intact oculomotor systems?
Transcranial magnetic stimulation (TMS) has shown a causal role of occipital cortex in exogenous attention. Does the occipital cortex also play a causal role in presaccadic attention, and how does it interact with higher brain areas (e.g., FEF)?
How would to-be-developed models encompassing both covert and presaccadic attention advance our knowledge of visual perception by taking into account the dissociations reviewed here?
The findings that covert and presaccadic attention lead to different modulations of featural representations suggest that they serve different functional roles. We have proposed that the sharpening of orientation tuning [18], enhancement of high SF [18,47], increase in perceived contrast [15], and the overall amplification of the contrast response [49] elicited by presaccadic attention may contribute to transsaccadic integration, serving perceptual continuity across saccades by making the peripheral saccade target more fovea-like before it is shifted to the fovea. Neurophysiologists have also suggested that the orientation gain increment observed in monkey visual cortex could aid transsaccadic integration [123]. This notion that presaccadic attention may contribute to transsaccadic integration is rooted in the idea of Bayesian integration that the sameness of two inputs makes them more likely to be considered to have a common source [142,143].
This review has focused primarily on the differences among presaccadic, covert exogenous and covert endogenous attention regarding their effects on sensory encoding, but their differences may manifest in other processes too. There is evidence, for instance, that covert endogenous attention also affects information processing after sensory encoding, as neural activity is also selectively modulated during readout of visual information in occipital cortex, TPJ (temporo-parietal junction) and precentral sulcus (FEF) [144–148]. Likewise, it is still unknown whether presaccadic attention modulates neural activity during readout and if so whether such modulation differs from that of exogenous or endogenous covert attention. To further our understanding of presaccadic attention and to compare it with endogenous and exogenous covert attention, it would be informative to conduct analogous studies assessing the modulatory effects of presaccadic attention on neural activity during encoding and readout.
In addition, transcranial magnetic stimulation (TMS) has been successfully used in human participants to identify brain regions causally related to visual attention. For example, by briefly disrupting cortical excitability of the occipital cortex with TMS the effects of covert exogenous attention at attended and unattended locations are extinguished [104]. Likewise, TMS to specific areas of the dorsal (rIPS, intraparietal sulcus) and ventral (rTPJ) attention networks abolish benefits from exogenous cueing, showing that both networks are implicated in exogenous attention [149]. Furthermore, TMS to FEF has been shown to affect presaccadic attention [150]. Future studies are required to test the effects of presaccadic and covert attention in multiple brain regions, for systematic comparisons of the necessary roles of specific brain regions and neural pathways in humans.
The perceptual consequences, featural representation and neural computations and correlates of covert attention have been well characterized, but more so for the endogenous than the exogenous type [for reviews see [1,2,56,65,76,121]. Likewise, the characterization of presaccadic attention has concentrated more on tasks in which participants prepare a saccade to a target location voluntarily (according to a central cue) than involuntarily (reflexively saccading to a peripheral target). Further investigating exogenous attention and involuntary eye movements, as well as comparing their perceptual consequences, featural modulations, computations and neural correlates with endogenous attention and voluntary eye movements, will advance our knowledge of how presaccadic attention and covert attention modulate visual selection and perception.
Highlights.
To effectively process visual information, individuals attend to relevant visual inputs by selectively processing information at some locations at the expense of information elsewhere. One can attend overtly, by making saccadic eye movements, or covertly, by prioritizing information without moving the eyes. Both processes improve visual performance, but with different temporal dynamics.
Behavioral evidence shows that covert spatial attention is not the consequence of oculomotor planning.
Presaccadic and covert exogenous (involuntary) and endogenous (voluntary) attention differentially alter featural representation of basic dimensions–orientation and spatial frequency.
Different neural computations mediate the effects of presaccadic and covert attention on contrast sensitivity.
Partially overlapping brain areas underlie presaccadic and covert attention, but different neuronal populations within each area subserve each type of attention.
FUNDING and ACKNOWLEDGMENTS:
This research was supported by National Institutes of Health National Eye Institute R01 EY019693 to M.C. and a Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation to N.M.H. We thank Antoine Barbot, Antonio Fernández, Marc Himmelberg, Michael Jigo and other members of the Carrasco lab, as well as Luca Wollenberg and Heiner Deubel, for useful comments.
Glossary
- Adaptation:
Reduced sensory responses to prolonged or repeated presentations of the same stimulus. In psychophysical experiments, visual performance for a test stimulus following an adaptor stimulus is lower when the stimuli share similar features (e.g., orientation).
- Anti-saccade task:
Participants are required to suppress a reflexive saccade to a salient visual stimulus and instead perform a voluntary saccade in the opposite direction.
- Brain regions FEF:
In macaque monkeys, the Frontal Eye Field occupies the rostral bank of the arcuate sulcus; its putative human homolog is located near the precentral sulcus and the dorsal-most portion of the superior frontal sulcus. It plays an important role in guiding attention and saccades.
- SC:
A structure in the midbrain, the superior colliculus is part of the brain circuit for the transformation of sensory input into movement output. It receives direct projections from retinal ganglion cells and conveys information to V1 and to extrastriate visual areas. It plays a major role in the control of eye movements and is involved in the control of covert attention.
- V1:
Located in the calcarine sulcus in the medial occipital lobe, V1 is the first visual processing area in the cortex, it codes for stimulus orientation, spatial frequency and contrast.
- V4:
A cortical area in the visual ventral stream, receiving strong feedforward input from V2 and V1, and sending connections to the inferior temporal cortex.
- Fovea:
A depression in the inner retinal surface, ~1.5 mm wide, whose photoreceptor layer entirely consists of cones and which is specialized for highest visual acuity.
- Global-saccade:
A saccade involuntarily landing in between two potential saccade targets presented at nearby locations.
- Moebius syndrome:
A rare congenital neurological condition, characterized by oculomotor disorders including gaze paralysis, due to hypoplasia of the lower brainstem containing cranial nerves involved in the execution of eye movements.
- Point of subjective equality (PSE):
In comparative judgement experiments, the level (e.g., contrast) of the test stimulus varies while the standard stimulus remains constant. PSE is the test stimulus level at which both stimuli are chosen with equal probability and judged as appearing equal.
- Population receptive field (pRF):
The spatial region of the visual field, usually modeled as a two-dimensional Gaussian, that drives the response of a voxel in fMRI recording.
- Reverse correlation:
A technique for identifying the image features that drive sensory responses by presenting random (or pseudo-random) noise to the participants and correlating the image features with behavioral or neural responses.
- Saccadic adaptation:
Experimental procedure to dissociate saccade target and saccade landing location. By systematically displacing the target during the eye movement, the saccadic amplitude and/or direction gradually adapts to the intra-saccadic shift.
- Saccadic eye movements:
Fast, ballistic movements of the eyes that shift gaze quickly toward a visual target.
- Transsaccadic integration:
The integration of the presaccadic (e.g., a peripheral saccade target) and post-saccadic (e.g., the foveal image after saccade landing) information to achieve visual stability across saccadic eye movements.
- Visual masking:
A phenomenon in which the visibility of a visual target is reduced by the presence of other visual stimuli (noise or mask), which overlap with or have a close proximity to the target in space and time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We focus on perceptual modulations preceding saccadic eye movements (see reviews on perceptual effects during the eye movement [3] and on modulations specific to the remapped location [4]).
Declaration of interests. The authors declare no competing interests in relation to this review.
REFERENCES
- 1.Carrasco M Visual attention: the past 25 years. Vision Res. 2011. ;51: 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton-Erxleben K, Carrasco M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci. 2013;14: 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binda P, Morrone MC. Vision During Saccadic Eye Movements. Annu Rev Vis Sci. 2018;4: 193–213. [DOI] [PubMed] [Google Scholar]
- 4.Marino AC, Mazer JA. Perisaccadic Updating of Visual Representations and Attentional States: Linking Behavior and Neurophysiology. Front Syst Neurosci. 2016;10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35: 1897–1916. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57: 787–795. [DOI] [PubMed] [Google Scholar]
- 7.Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36: 1827–1837. [DOI] [PubMed] [Google Scholar]
- 8.Hanning NM, Deubel H, Szinte M. Sensitivity measures of visuospatial attention. J Vis. 2019;19: 17. [DOI] [PubMed] [Google Scholar]
- 9.Ghahghaei S, Verghese P. Texture segmentation influences the spatial profile of presaccadic attention. J Vis. 2017;17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntiroli M, Kerzel D, Born S. Placeholder objects shape spatial attention effects before eye movements. J Vis. 2018;18: 1. [DOI] [PubMed] [Google Scholar]
- 11.Szinte M, Puntiroli M, Deubel H. The spread of presaccadic attention depends on the spatial configuration of the visual scene. Sci Rep. 2019;9: 14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castet E, Jeanjean S, Montagnini A, Laugier D, Masson GS. Dynamics of attentional deployment during saccadic programming. J Vis. 2006;6: 196–212. [DOI] [PubMed] [Google Scholar]
- 13.Deubel H The time course of presaccadic attention shifts. Psychol Res. 2008;72: 630–640. [DOI] [PubMed] [Google Scholar]
- 14.Filali-Sadouk N, Castet E, Olivier E, Zenon A. Similar effect of cueing conditions on attentional and saccadic temporal dynamics. J Vis. 2010;10: 21.1–13. [DOI] [PubMed] [Google Scholar]
- 15.Rolfs M, Carrasco M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. J Neurosci. 2012;32: 13744–52a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison WJ, Mattingley JB, Remington RW. Eye movement targets are released from visual crowding. J Neurosci. 2013;33: 2927–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White AL, Rolfs M, Carrasco M. Adaptive deployment of spatial and feature-based attention before saccades. Vision Res. 2013;85: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H-H, Barbot A, Carrasco M. Saccade Preparation Reshapes Sensory Tuning. Curr Biol. 2016;26: 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonikaitis D, Klapetek A, Deubel H. Spatial attention during saccade decisions. J Neurophysiol. 2017; 118: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt AR, Reuther J, Hilchey MD, Klein RM. The Relationship Between Spatial Attention and Eye Movements. Curr Top Behav Neurosci. 2019;41: 255–278. [DOI] [PubMed] [Google Scholar]
- 21.Montagnini A, Castet E. Spatiotemporal dynamics of visual attention during saccade preparation: Independence and coupling between attention and movement planning. J Vis. 2007;7: 8.1–16. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Gersch TM, Schnitzer BS, Dosher BA, Kowler E. Eye movements and attention: the role of presaccadic shifts of attention in perception, memory and the control of saccades. Vision Res. 2012;74: 40–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan AZ, Blohm G, Pisella L, Munoz DP. Saccade execution suppresses discrimination at distractor locations rather than enhancing the saccade goal location. Eur J Neurosci. 2015;41: 1624–1634. [DOI] [PubMed] [Google Scholar]
- 24.Kreyenmeier P, Deubel H, Hanning NM. Theory of visual attention (TVA) in action: Assessing premotor attention in simultaneous eye-hand movements. Cortex. 2020;133: 133–148. [DOI] [PubMed] [Google Scholar]
- 25.Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25: 31–40. [DOI] [PubMed] [Google Scholar]
- 26.Craighero L, Carta A, Fadiga L. Peripheral oculomotor palsy affects orienting of visuospatial attention. Neuroreport. 2001. ;12: 3283–3286. [DOI] [PubMed] [Google Scholar]
- 27.Smith DT, Rorden C, Jackson SR. Exogenous Orienting of Attention Depends upon the Ability to Execute Eye Movements. Current Biology. 2004; 14: 792–795. [DOI] [PubMed] [Google Scholar]
- 28.Smith DT, Ball K, Ellison A, Schenk T. Deficits of reflexive attention induced by abduction of the eye. Neuropsychologia. 2010;48: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 29.Smith DT, Schenk T, Rorden C. Saccade preparation is required for exogenous attention but not endogenous attention or IOR. Journal of Experimental Psychology: Human Perception and Performance. 2012. pp. 1438–1447. doi: 10.1037/a0027794 [DOI] [PubMed] [Google Scholar]
- 30.Casteau S, Smith DT. Covert attention beyond the range of eye-movements: Evidence for a dissociation between exogenous and endogenous orienting. Cortex. 2020;122: 170–186. [DOI] [PubMed] [Google Scholar]
- 31.Hunt AR, Kingstone A. Covert and overt voluntary attention: linked or independent? Brain Res Cogn Brain Res. 2003;18: 102–105. [DOI] [PubMed] [Google Scholar]
- 32.Belopolsky AV, Theeuwes J. When are attention and saccade preparation dissociated? Psychol Sci. 2009;20: 1340–1347. [DOI] [PubMed] [Google Scholar]
- 33.MacLean GH, Klein RM, Hilchey MD. Does oculomotor readiness mediate exogenous capture of visual attention? J Exp Psychol Hum Percept Perform. 2015;41: 1260–1270. [DOI] [PubMed] [Google Scholar]
- 34.Hanning NM, Szinte M, Deubel H. Visual attention is not limited to the oculomotor range. Proceedings of the National Academy of Sciences. 2019; 116: 9665–9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanning NM, Deubel H. Attention capture outside the oculomotor range. Curr Biol. 2020;30: R1353–R1355. [DOI] [PubMed] [Google Scholar]
- 36.Masson N, Andres M, Pereira SC, Pesenti M, Vannuscorps G. Exogenous covert shift of attention without the ability to plan eye movements. Curr Biol. 2020;30: R1032–R1033. [DOI] [PubMed] [Google Scholar]
- 37.Carrasco M, Hanning NM. Visual Perception: Attending beyond the Eyes’ Reach. Curr Biol. 2020;30: R1322–R1324. [DOI] [PubMed] [Google Scholar]
- 38.Ditterich J, Eggert T, Straube A. Relation between the metrics of the presaccadic attention shift and of the saccade before and after saccadic adaptation. J Neurophysiol. 2000;84: 1809–1813. [DOI] [PubMed] [Google Scholar]
- 39.Doré-Mazars K, Collins T. Saccadic adaptation shifts the pre-saccadic attention focus. Exp Brain Res. 2005;162: 537–542. [DOI] [PubMed] [Google Scholar]
- 40.Collins T, Heed T, Röder B. Visual target selection and motor planning define attentional enhancement at perceptual processing stages. Front Hum Neurosci. 2010;4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Stigchel S, de Vries JP. There is no attentional global effect: Attentional shifts are independent of the saccade endpoint. J Vis. 2015;15: 17. [DOI] [PubMed] [Google Scholar]
- 42.Wollenberg L, Deubel H, Szinte M. Visual attention is not deployed at the endpoint of averaging saccades. PLoS Biol. 2018;16: e2006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wollenberg L, Hanning NM, Deubel H. Visual attention and eye movement control during oculomotor competition. J Vis. 2020;20: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klapetek A, Jonikaitis D, Deubel H. Attention allocation before antisaccades. J Vis. 2016;16: 11. [DOI] [PubMed] [Google Scholar]
- 45.Mikula L, Jacob M, Tran T, Pisella L, Khan AZ. Spatial and temporal dynamics of presaccadic attentional facilitation before pro- and antisaccades. J Vis. 2018; 18: 2. [DOI] [PubMed] [Google Scholar]
- 46.Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91: 152–162. [DOI] [PubMed] [Google Scholar]
- 47.Li H-H, Pan J, Carrasco M. Presaccadic attention improves or impairs performance by enhancing sensitivity to higher spatial frequencies. Sci Rep. 2019;9: 2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker S, Heathcote A, Finkbeiner M. Establishing the separable contributions of spatial attention and saccade preparation across tasks with varying acuity demands. J Exp Psychol Hum Percept Perform. 2020. [DOI] [PubMed] [Google Scholar]
- 49.Li H-H, Pan J, Carrasco M. Different computations underlie overt presaccadic and covert spatial attention. Nature Human Behaviour. 2021. doi: 10.1038/s41562-021-01099-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohl S, Kuper C, Rolfs M. Selective enhancement of orientation tuning before saccades. J Vis. 2017;17: 2. [DOI] [PubMed] [Google Scholar]
- 51.Baldassi S, Verghese P. Attention to locations and features: different top-down modulation of detector weights. J Vis. 2005;5: 556–570. [DOI] [PubMed] [Google Scholar]
- 52.Paltoglou AE, Neri P. Attentional control of sensory tuning in human visual perception. J Neurophysiol. 2012;107: 1260–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyart V, Nobre AC, Summerfield C. Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proc Natl Acad Sci U S A. 2012;109: 3593–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández A, Li H-H, Carrasco M. How exogenous spatial attention affects visual representation. J Vis. 2019;19: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez A, Okun S, Carrasco M. Differential effects of endogenous and exogenous attention on sensory tuning. bioRxiv. 2021. doi: 10.1101/2021.04.03.438325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carrasco M, Barbot A. How Attention Affects Spatial Resolution. Cold Spring Harb Symp Quant Biol. 2014;79: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroell LM, Rolfs M. The peripheral sensitivity profile at the saccade target reshapes during saccade preparation. Cortex. 2021. doi: 10.1016/j.cortex.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 58.Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396: 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carrasco M, Loula F, Ho Y-X. How attention enhances spatial resolution: evidence from selective adaptation to spatial frequency. Percept Psychophys. 2006;68: 1004–1012. [DOI] [PubMed] [Google Scholar]
- 60.Yeshurun Y, Carrasco M. The effects of transient attention on spatial resolution and the size of the attentional cue. Percept Psychophys. 2008;70: 104–113. [DOI] [PubMed] [Google Scholar]
- 61.Talgar CP, Carrasco M. Vertical meridian asymmetry in spatial resolution: visual and attentional factors. Psychon Bull Rev. 2002;9: 714–722. [DOI] [PubMed] [Google Scholar]
- 62.Barbot A, Carrasco M. Attention Modifies Spatial Resolution According to Task Demands. Psychol Sci. 2017;28: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jigo M, Carrasco M. Attention alters spatial resolution by modulating second-order processing. J Vis. 2018;18: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jigo M, Carrasco M. Differential impact of exogenous and endogenous attention on the contrast sensitivity function across eccentricity. J Vis. 2020;20: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrasco M, Barbot A. Spatial attention alters visual appearance. Current opinion in psychology. 2019;29: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ling S, Carrasco M. Transient covert attention does alter appearance: a reply to Schneider (2006). Percept Psychophys. 2007;69: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrasco M, Fuller S, Ling S. Transient attention does increase perceived contrast of suprathreshold stimuli: A reply to Prinzmetal, Long, and Leonhardt (2008). Perception & Psychophysics. 2008;70: 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Störmer VS, McDonald JJ, Hillyard SA. Cross-modal cueing of attention alters appearance and early cortical processing of visual stimuli. Proc Natl Acad Sci U S A. 2009; 106: 22456–22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anton-Erxleben K, Abrams J, Carrasco M. Evaluating comparative and equality judgments in contrast perception: attention alters appearance. J Vis. 2010;10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cutrone EK, Heeger DJ, Carrasco M. Attention enhances contrast appearance via increased input baseline of neural responses. J Vis. 2014;14: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbot A, Carrasco M. Emotion and anxiety potentiate the way attention alters visual appearance. Sci Rep. 2018;8: 5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou L-F, Buetti S, Lu S, Cai Y-C. Attentional effect on contrast appearance: From enhancement to attenuation. J Exp Psychol Hum Percept Perform. 2018;44: 806–817. [DOI] [PubMed] [Google Scholar]
- 74.Liu T, Abrams J, Carrasco M. Voluntary attention enhances contrast appearance. Psychol Sci. 2009;20: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeshurun Y, Carrasco M. The locus of attentional effects in texture segmentation. Nat Neurosci. 2000;3: 622–627. [DOI] [PubMed] [Google Scholar]
- 76.Maunsell JHR. Neuronal Mechanisms of Visual Attention. Annu Rev Vis Sci. 2015; 1: 373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61: 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrone MC, Denti V, Spinelli D. Color and luminance contrasts attract independent attention. Curr Biol. 2002;12: 1134–1137. [DOI] [PubMed] [Google Scholar]
- 79.Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 2004;44: 1389–1401. [DOI] [PubMed] [Google Scholar]
- 80.Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 2005;45: 1201–1212. [DOI] [PubMed] [Google Scholar]
- 81.Ling S, Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res. 2006;46: 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pestilli F, Viera G, Carrasco M. How do attention and adaptation affect contrast sensitivity? J Vis. 2007;7: 9.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pestilli F, Ling S, Carrasco M. A population-coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data. Vision Res. 2009;49: 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herrmann K, Montaser-Kouhsari L, Carrasco M, Heeger DJ. When size matters: attention affects performance by contrast or response gain. Nat Neurosci. 2010;13: 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buracas GT, Boynton GM. The effect of spatial attention on contrast response functions in human visual cortex. J Neurosci. 2007;27: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murray SO. The effects of spatial attention in early human visual cortex are stimulus independent. J Vis. 2008;8: 2.1–11. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Lu Z-L, Tjan BS, Dosher BA, Chu W. Blood oxygenation level-dependent contrast response functions identify mechanisms of covert attention in early visual areas. Proc Natl Acad Sci U S A. 2008; 105: 6202–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bundesen C, Habekost T, Kyllingsbaek S. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol Rev. 2005;112: 291–328. [DOI] [PubMed] [Google Scholar]
- 89.Bhatt R, Carpenter GA, Grossberg S. Texture segregation by visual cortex: perceptual grouping, attention, and learning. Vision Res. 2007;47: 3173–3211. [DOI] [PubMed] [Google Scholar]
- 90.Womelsdorf T, Anton-Erxleben K, Treue S. Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. J Neurosci. 2008;28: 8934–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schneider WX. Selective visual processing across competition episodes: a theory of task-driven visual attention and working memory. Philos Trans R Soc Lond B Biol Sci. 2013;368: 20130060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2011. ;13: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J, Maunsell JHR. A normalization model of attentional modulation of single unit responses. PLoS One. 2009;4: e4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boynton GM. A framework for describing the effects of attention on visual responses. Vision Res. 2009;49: 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jigo M, Heeger DJ, Carrasco M. How voluntary and involuntary attention differentially shape spatial resolution. bioRxiv. 2021. Available: https://www.biorxiv.org/content/10.1101/2021.01.26.428173v2.abstract
- 96.Herrmann K, Heeger DJ, Carrasco M. Feature-based attention enhances performance by increasing response gain. Vision Res. 2012;74: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ni AM, Ray S, Maunsell JHR. Tuned normalization explains the size of attention modulations. Neuron. 2012;73: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwedhelm P, Krishna BS, Treue S. An Extended Normalization Model of Attention Accounts for Feature-Based Attentional Enhancement of Both Response and Coherence Gain. PLoS Comput Biol. 2016;12: e1005225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verhoef B-E, Maunsell JH. Attention operates uniformly throughout the classical receptive field and the surround. Elife. 2016;5. doi: 10.7554/eLife.17256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verhoef B-E, Maunsell JHR. Attention-related changes in correlated neuronal activity arise from normalization mechanisms. Nat Neurosci. 2017;20: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ni AM, Maunsell JHR. Neuronal Effects of Spatial and Feature Attention Differ Due to Normalization. J Neurosci. 2019;39: 5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hara Y, Pestilli F, Gardner JL. Differing effects of attention in single-units and populations are well predicted by heterogeneous tuning and the normalization model of attention. Front Comput Neurosci. 2014;8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Talgar CP, Pelli DG, Carrasco M. Covert attention enhances letter identification without affecting channel tuning. J Vis. 2004;4: 22–31. [DOI] [PubMed] [Google Scholar]
- 104.Fernández A, Carrasco M. Extinguishing Exogenous Attention via Transcranial Magnetic Stimulation. Current Biology. 2020;30: 4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11: 210–216. [DOI] [PubMed] [Google Scholar]
- 106.Bogadhi AR, Bollimunta A, Leopold DA, Krauzlis RJ. Brain regions modulated during covert visual attention in the macaque. Sci Rep. 2018;8: 15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bollimunta A, Bogadhi AR, Krauzlis RJ. Comparing frontal eye field and superior colliculus contributions to covert spatial attention. Nat Commun. 2018;9: 3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jonikaitis D, Moore T. The interdependence of attention, working memory and gaze control: behavior and neural circuitry. Curr Opin Psychol. 2019;29: 126–134. [DOI] [PubMed] [Google Scholar]
- 109.Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321: 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bisley JW, Mirpour K. The neural instantiation of a priority map. Curr Opin Psychol. 2019;29: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421: 370–373. [DOI] [PubMed] [Google Scholar]
- 112.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A. 2005;102: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Juan C-H, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004;101: 15541–15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7: 56–64. [DOI] [PubMed] [Google Scholar]
- 115.Gregoriou GG, Gotts SJ, Desimone R. Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron. 2012;73: 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merrikhi Y, Clark K, Albarran E, Parsa M, Zirnsak M, Moore T, et al. Spatial working memory alters the efficacy of input to visual cortex. Nat Commun. 2017;8: 15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Messinger A, Cirillo R, Wise SP, Genovesio A. Separable neuronal contributions to covertly attended locations and movement goals in macaque frontal cortex. Sci Adv. 2021. ;7. doi: 10.1126/sciadv.abe0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hilo-Merkovich R, Carrasco M, Yuval-Greenberg S. Task performance in covert, but not overt, attention correlates with early laterality of visual evoked potentials. Neuropsychologia. 2018; 119: 330–339. [DOI] [PubMed] [Google Scholar]
- 119.Juan C-H, Muggleton NG, Tzeng OJL, Hung DL, Cowey A, Walsh V. Segregation of visual selection and saccades in human frontal eye fields. Cereb Cortex. 2008;18: 2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huddleston WE, Swanson AN, Lytle JR, Aleksandrowicz MS. Distinct saccade planning and endogenous visuospatial attention maps in parietal cortex: A basis for functional differences in sensory and motor attention. Cortex. 2021. ;137: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore T, Zirnsak M. Neural Mechanisms of Selective Visual Attention. Annu Rev Psychol. 2017;68: 47–72. [DOI] [PubMed] [Google Scholar]
- 122.Super H, van der Togt C, Spekreijse H, Lamme VAF. Correspondence of presaccadic activity in the monkey primary visual cortex with saccadic eye movements. Proceedings of the National Academy of Sciences. 2004;101: 3230–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moore T, Tolias AS, Schiller PH. Visual representations during saccadic eye movements. Proc Natl Acad Sci U S A. 1998;95: 8981–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron. 2001. ;29: 757–767. [DOI] [PubMed] [Google Scholar]
- 125.Saber GT, Pestilli F, Curtis CE. Saccade planning evokes topographically specific activity in the dorsal and ventral streams. J Neurosci. 2015;35: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore T, Chang MH. Presaccadic discrimination of receptive field stimuli by area V4 neurons. Vision Res. 2009;49: 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Steinmetz NA, Moore T. Eye movement preparation modulates neuronal responses in area V4 when dissociated from attentional demands. Neuron. 2014;83: 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12: 1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rabinowitz NC, Goris RL, Cohen M, Simoncelli EP. Attention stabilizes the shared gain of V4 populations. Elife. 2015;4: e08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steinmetz NA, Moore T. Changes in the response rate and response variability of area V4 neurons during the preparation of saccadic eye movements. J Neurophysiol. 2010;103: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ni AM, Ruff DA, Alberts JJ, Symmonds J, Cohen MR. Learning and attention reveal a general relationship between population activity and behavior. Science. 2018;359: 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.David SV, Hayden BY, Mazer JA, Gallant JL. Attention to stimulus features shifts spectral tuning of V4 neurons during natural vision. Neuron. 2008;59: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Connor CE, Gallant JL, Preddie DC, Van Essen DC. Responses in area V4 depend on the spatial relationship between stimulus and attention. J Neurophysiol. 1996;75: 1306–1308. [DOI] [PubMed] [Google Scholar]
- 136.Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci. 2006;9: 1156–1160. [DOI] [PubMed] [Google Scholar]
- 137.Klein BP, Harvey BM, Dumoulin SO. Attraction of position preference by spatial attention throughout human visual cortex. Neuron. 2014; 84: 227–237. [DOI] [PubMed] [Google Scholar]
- 138.Hartmann TS, Zirnsak M, Marquis M, Hamker FH, Moore T. Two Types of Receptive Field Dynamics in Area V4 at the Time of Eye Movements? Front Syst Neurosci. 2017; 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T. Visual space is compressed in prefrontal cortex before eye movements. Nature. 2014; 507: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mineault PJ, Tring E, Trachtenberg JT, Ringach DL. Enhanced Spatial Resolution During Locomotion and Heightened Attention in Mouse Primary Visual Cortex. J Neurosci. 2016;36: 6382–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aydin Ç, Couto J, Giugliano M, Farrow K, Bonin V. Locomotion modulates specific functional cell types in the mouse visual thalamus. Nat Commun. 2018; 9: 4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Körding KP, Beierholm U, Ma WJ, Quartz S, Tenenbaum JB, Shams L. Causal inference in multisensory perception. PLoS One. 2007;2: e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Atsma J, Maij F, Koppen M, Irwin DE, Medendorp WP. Causal Inference for Spatial Constancy across Saccades. PLoS Comput Biol. 2016;12: e1004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. J Cogn Neurosci. 2004; 16: 363–373. [DOI] [PubMed] [Google Scholar]
- 145.Sergent C, Ruff CC, Barbot A, Driver J, Rees G. Top–Down Modulation of Human Early Visual Cortex after Stimulus Offset Supports Successful Postcued Report. Journal of Cognitive Neuroscience. 2011. pp. 1921–1934. doi: 10.1162/jocn.2010.21553 [DOI] [PubMed] [Google Scholar]
- 146.Pestilli F, Carrasco M, Heeger DJ, Gardner JL. Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron. 2011. ;72: 832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dugué L, Merriam EP, Heeger DJ, Carrasco M. Specific Visual Subregions of TPJ Mediate Reorienting of Spatial Attention. Cereb Cortex. 2018;28: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dugué L, Merriam EP, Heeger DJ, Carrasco M. Differential impact of endogenous and exogenous attention on activity in human visual cortex. Sci Rep. 2020; 10: 21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahrens M-M, Veniero D, Freund IM, Harvey M, Thut G. Both dorsal and ventral attention network nodes are implicated in exogenously driven visuospatial anticipation. Cortex. 2019;117: 168–181. [DOI] [PubMed] [Google Scholar]
- 150.Van Ettinger-Veenstra HM, Huijbers W, Gutteling TP, Vink M, Kenemans JL, Neggers SFW. fMRI-guided TMS on cortical eye fields: the frontal but not intraparietal eye fields regulate the coupling between visuospatial attention and eye movements. J Neurophysiol. 2009;102: 3469–3480. [DOI] [PubMed] [Google Scholar]


